1. Introduction
Titanium alloy has the advantages of low density, high specific strength and strong corrosion resistance, so it is widely used in aircraft engines [
1]. The high temperature components of modern aero engines are mostly made of nickel-based superalloys, which is not conducive to improving the thrust-weight ratio of aero engines. Therefore, replacing nickel-based superalloys with titanium alloys can significantly reduce weight and greatly improve the push-to-weight ratio. Traditional titanium and titanium alloys account for one-third of the weight of modern aircraft engines and are the second most commonly used engine materials after nickel-based superalloys [
2]. However, at temperatures above 500°C, titanium alloys will undergo severe oxidation, leading to deterioration of creep resistance and fatigue properties, which limits their use at high temperatures [
3]. For example, Ti-6Al-4V titanium alloy, due to its poor high-temperature oxidation performance, its maximum application temperature is below 400-500 °C [
4]. At high temperatures, due to the high affinity between titanium and oxygen, a non-dense oxide film is formed, resulting in the degradation of titanium alloy, and the high-temperature oxidation results in the embrittlement of the alloy by dissolved oxygen [
5]. On the other hand, when titanium alloys are operated in a salt-containing service environment (such as engine components), chloride-rich salt deposits inevitably condense on the alloy material, and NaCl is the main component of these salt layers deposited on the surface of the alloy. Generally, titanium alloy gas turbine compressors in the aerospace industry operate at high temperatures (100-600°C). Therefore, titanium alloy materials are often subjected to thermal corrosion caused by salt deposition, resulting in reduced engine efficiency, increased fuel consumption, and reduced service life [
6]. In addition, titanium alloys have the disadvantages of poor wear resistance and low thermal conductivity during use [
7], which lead to premature failure of high-temperature components and greatly reduced service life. Especially in a high temperature and complex environment, it is extremely harmful to the titanium alloy substrate, which greatly limits the application range of titanium alloys and its internal field application under sliding conditions. Based on the above performance defects of titanium alloy, it is very necessary to modify titanium alloy. Because TiAl alloys have superior properties such as high specific strength and Young's modulus, proper strength retention at high temperatures, and high creep resistance at temperatures up to 900°C [
8], aluminum based coatings are chosen to be prepared on the surface of titanium alloys. However, the thermal expansion coefficient of TiAl coating does not match the substrate, and with the increase of Ti content, the oxidation resistance and wear resistance of TiAl coating decrease with the number of defects in the cladding [
9]. In order to improve TiAl coating, it is a simple and effective method to add other elements with special properties to the coating. Therefore, the titanium alloy aluminum-based coating has been developed into Al-X or Ti-Al-X coating and has been widely used.
In recent years, the titanium alloy aluminum-based coating has been greatly developed, mainly in the preparation of the coating, the addition of elements in the coating and the heat treatment method of the coating three aspects. In order to improve the high temperature oxidation resistance, thermal corrosion resistance and wear resistance of titanium alloy, the aluminum-based coating of titanium alloy is constantly improved. In this paper, the oxidation behavior of titanium alloy at high temperature is analyzed from the perspective of thermodynamics and kinetics, and the mechanism of thermal corrosion of titanium alloy at high temperature and the friction mechanism of titanium alloy are analyzed. The research progress on improving high temperature oxidation resistance, thermal corrosion resistance and wear resistance of titanium alloys in recent years is also reviewed in this paper.
2. Aluminide coating
2.1. Coating preparation method
2.1.1. Powder aluminizing
In a heat-resistant container, the sample is packed in a diffusion-impregnating agent consisting of a mixture of metallic aluminum powder or aluminum-rich alloy powder, an activating substance (halogen compound), and a filler (such as alumina). Using hydrogen or argon as a protective gas, thermal diffusion treatment is carried out according to the specified time.
2.1.1.1. High activity aluminizing
At lower temperatures (700-850°C), aluminum diffuses inward through the initially formed surface layer faster than metal diffuses outward due to the higher activity of aluminum. Reactive diffusion forms an aluminum-rich phase on the surface of the alloy, and the growth mechanism of the coating is the inward diffusion of aluminum in the cementing agent [
10]. Coating growth is mainly due to the inward diffusion of aluminum in the infiltration agent, so no Kirkendal holes are produced, and the alumina particles of the infiltration agent do not remain in the coating. However, because the formed aluminide is brittle, the diffused coating still needs further diffusion treatment. Aluminized coatings contain a variety of precipitated phases and impurities, which is because during the inward growth of the coating, these impurities will be wrapped in the coating. High activity aluminizing requires two heating, many operating steps and high cost. Moreover, the surface is easy to contain inclusions, which is unfavorable to oxidation resistance and will accelerate the destruction of oxide film. Therefore, the aluminized coating can not guarantee the long-term high-temperature oxidation protection of the substrate.
2.1.1.2. Low activity aluminizing
At higher temperatures (950-1200°C), because the activity of aluminum is lower than that of metal, the rate of outward diffusion of metal through the initially formed surface is higher than the rate of inward diffusion of aluminum in the aluminizing process. Reactive diffusion forms an aluminum-rich phase on the surface of the alloy, and the growth mechanism of the coating is the outward diffusion of the metal [
11]. Aluminum and alloying elements react to form aluminide coatings, and the growth of the coatings is mainly achieved by the outward diffusion of alloying elements. Low activity aluminizing can cause holes on the side of coating and substrate due to Kirkendall effect. Inert packed alumina particles are also embedded in the outer surface area of the coating, becoming coating inclusions. However, aluminizing is widely used because of its simple process, low cost and good oxidation resistance.
2.1.2. Laser cladding aluminizing
Laser cladding is a surface modification technology with high processing efficiency. Alloy powders with different compositions and properties can be cured quickly with the substrate surface using a high energy density laser beam, which can improve the microstructure and properties of the substrate material [
12,
13]. Laser cladding has become an excellent method for preparing titanium alloy coatings due to its good compactness, low dilution rate, high bond strength, small heat-affected zone and fine grain size [
14,
15]. However, the high equipment cost of this method and the easy cracking of the prepared coating (due to the rapid heating and cooling rates) partially limit the application of this method.
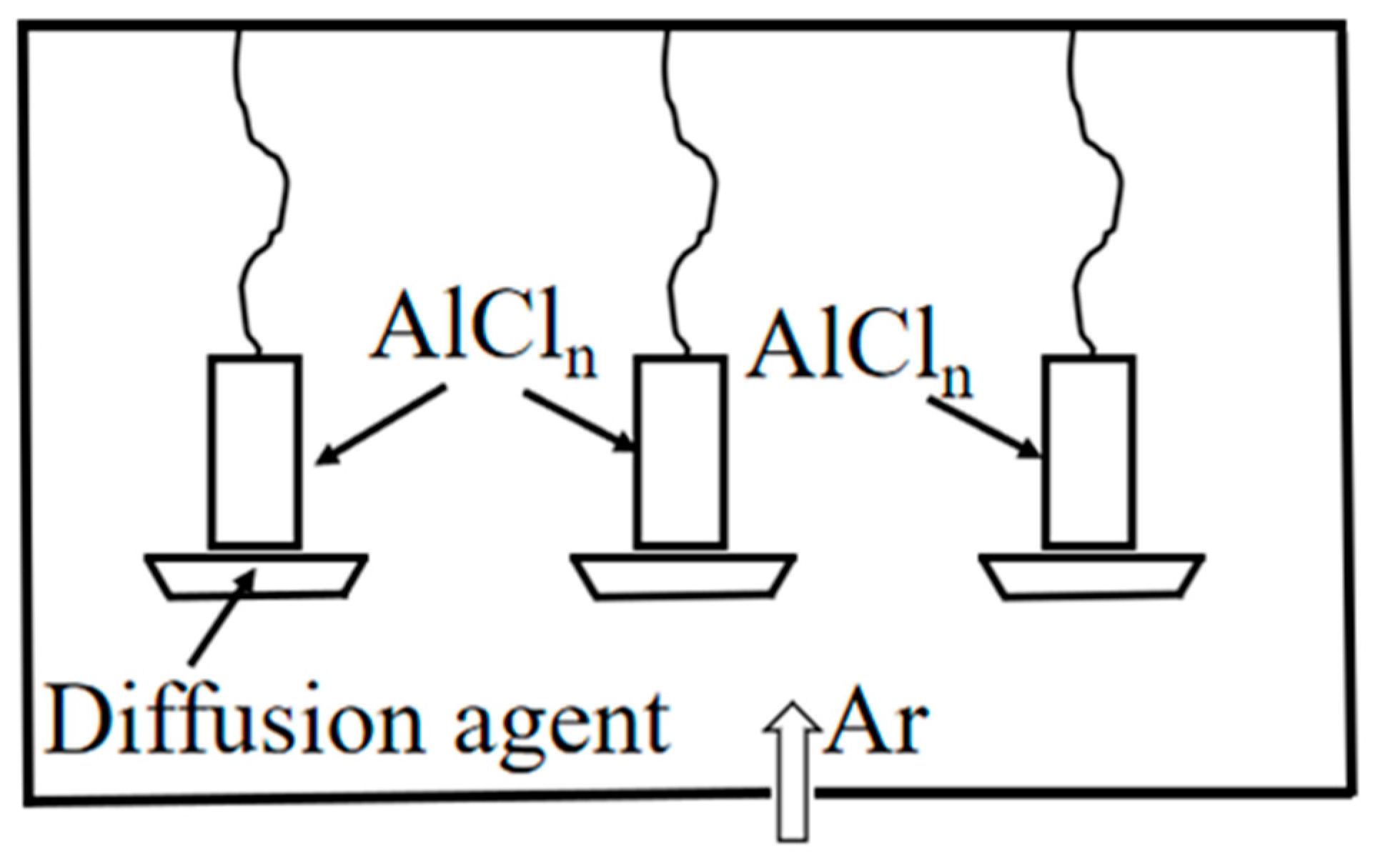
2.1.3. Gas phase aluminizing
Figure 1 shows the schematic diagram of gas phase aluminizing. Gas phase aluminizing is very similar to powder package aluminizing, but the sample is placed away from the powder mixture so that the formed coating can be formed from the gas phase. It minimizes the possibility of forming unwanted phases and compounds on the surface, and this method has minimal surface contamination of the specimen [
16]. Gas phase aluminizing is performed in a sealed reaction vessel where the sample is suspended in a tray of permeating agent and argon gas is injected. When heated to a given temperature, the aluminum in the infiltration agent reacts with the activator halide compound to form a volatile metal halide (AlCl
n). The gaseous halides diffuse to the alloy surface and further react to diffuse to form an aluminide coating [
17]. Compared with low activity aluminizing, gas phase aluminizing adopts non-contact method, which successfully avoids the possibility of the dispersant particles in the aluminizing agent inserting into the coating during the aluminizing process and maintains the cleanliness of the sample surface.
2.1.4. Slurry aluminizing
Slurry method is to spray or brush the slurry mixture made of aluminum powder or aluminum alloy powder with activator and binder at room temperature on the alloy surface, and then spread at 1000-1200°C. A slurry with high aluminum content is used to obtain an inward diffusion coating, and an outward diffusion aluminide coating is obtained when the aluminum activity is low [
18]. This method is convenient for preparing aluminide modified coatings with various reactive elements, such as YCrAl and CeCrAl coatings. It is convenient to play the special role of the active reaction element, so as to improve the high-temperature oxidation resistance of the alloy.
2.1.5. Hot dip aluminizing
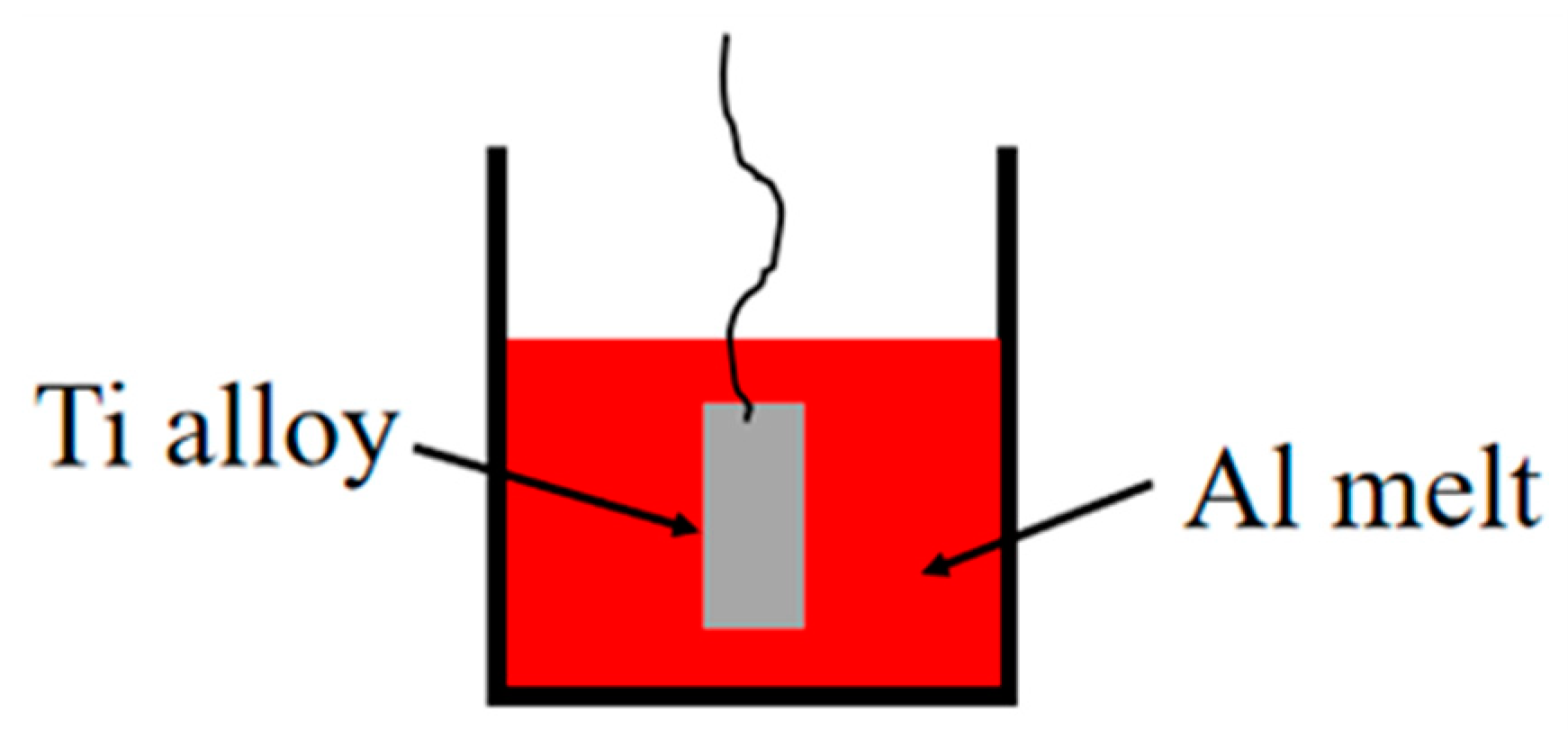
Hot dipping refers to the surface treatment technology in which the metal to be gilded is immersed in molten metal liquid at a certain temperature for a certain period of time, so that the surface of the metal to be gilded is coated with intermetallic compounds. After a series of physical and chemical reactions between the metallized metal and the metal liquid, the metal is removed and cooled in an appropriate manner. The main features of hot dipping are as follows: in the actual operation, the hot dipping has good controllability; The basic theory of hot dipping is relatively perfect, which can explain various phenomena better. Heating diffusion is the main reason for the formation of hot dip coating, so the obtained intermetallic coating will form metallurgical bonding, and there is no need to consider the bonding problem.
Figure 2 is the schematic diagram of hot dip aluminizing. Hot dip aluminum on the surface of alloy substrates, that is, the alloy substrate is placed in the aluminum melt at high temperature for a period of time, so that a series of reactions occur between aluminum and the alloy substrate. As an effective surface protection technology, this method can not only give a good appearance and a certain comprehensive mechanical properties of the alloy substrate surface, but also significantly improve the heat resistance and corrosion resistance of the substrate material. So this method has been more and more widely used.
However, the aluminized coating has some limitations such as easy cracking and peeling, difficult practical application and low oxidation resistance above 800°C [
8], so other elements are often added to the aluminum melt to improve it. Because this preparation method is easy to add other elements, hot dipping is often used to prepare coatings on the surface of titanium alloys to improve the performance of titanium alloys. For example, adding a small amount of Mn, Zn and Mo could improve the wettability and enhance the uniformity of the aluminized layer [
19].
Figure 2.
Schematic diagram of hot dip aluminizing.
Figure 2.
Schematic diagram of hot dip aluminizing.
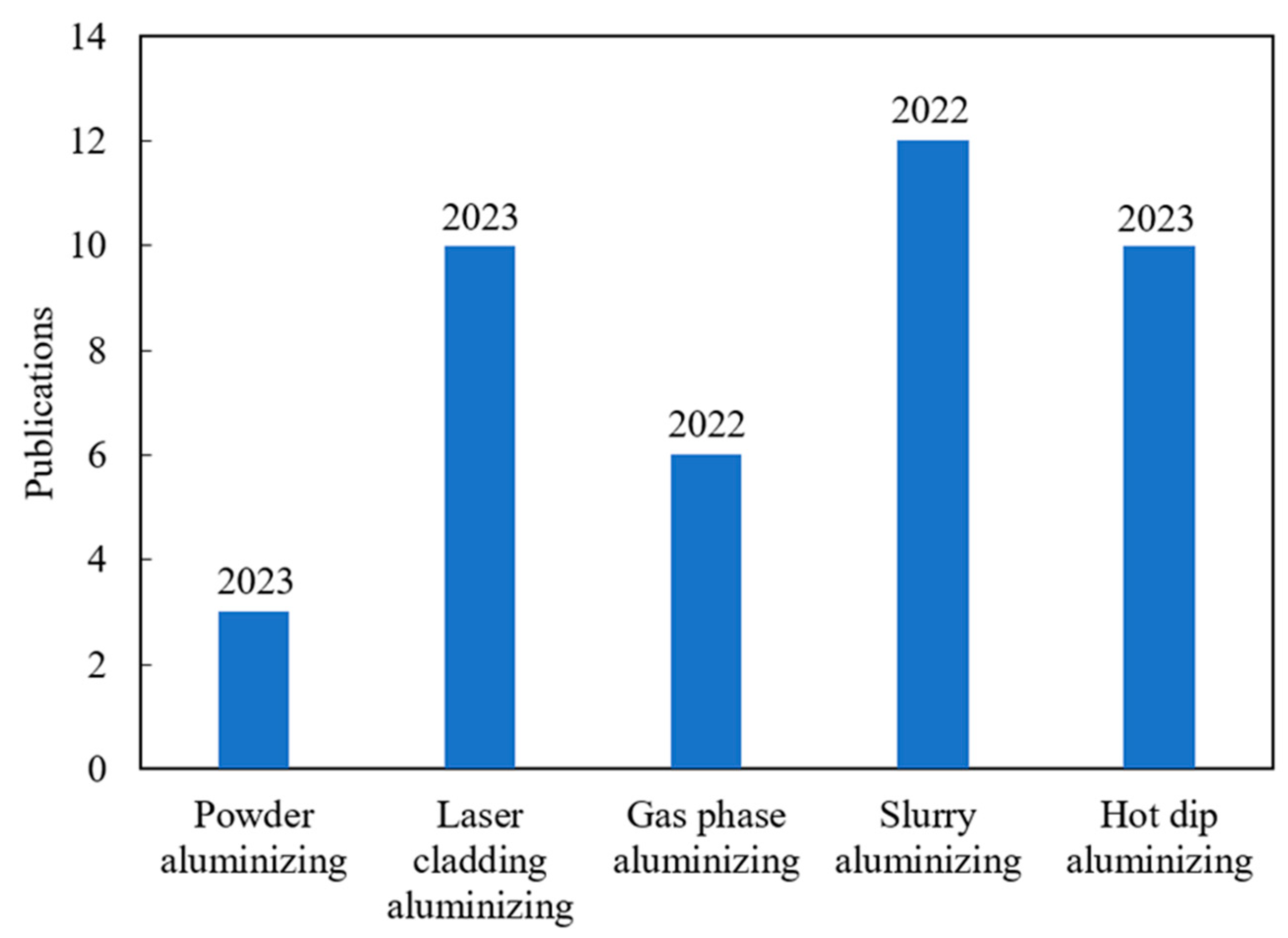
Figure 3.
Number of publications on different aluminide coating preparation methods in recent years (from the Science Direct database, the year of the last publication is indicated above the bar).
Figure 3.
Number of publications on different aluminide coating preparation methods in recent years (from the Science Direct database, the year of the last publication is indicated above the bar).
2.2. Improved aluminide coating
In various aluminum-based coating preparation methods that are easy to add other elements (such as hot dip aluminizing and slurry aluminizing), the aluminum-based coating is improved by adding a certain amount of other elements, so as to solve its insufficient performance. Commonly added elements are Cr, Si, Nb, and rare earth elements.
2.2.1. Cr improved aluminide coating
Al and Cr are easily oxidized rapidly in the air, forming a dense oxide film to prevent further oxidation of the surface. Al-Cr co-permeating layer has obvious advantages: (1) the rich chromium in the permeating layer can form a diffusion barrier reducing the mutual diffusion between the coating and the substrate. (2) Chromium is added to the coating to improve the adhesion between the coating and the substrate. Some people believed that the addition of chromium changed the TiAl
3 in the coating from the original DO22 structure to the LI2 structure Ti(Al,Cr)
3, which improved the plastic deformation ability of the coating [
20], and also improved the compatibility between the aluminized coating and the substrate [
21]. It was also believed that the addition of chromium refines the grain and improved the plastic deformation ability of the coating. (3) The addition of chromium to the coating improves the high-temperature oxidation resistance of the coating. On the one hand, due to the addition of chromium, the oxidation rate of the alloy is reduced.On the other hand, in the oxidation process, the addition of chromium is conducive to the formation of α-Al
2O
3, while inhibiting the formation of θ-Al
2O
3 [
22]. At present, the addition of chromium in chrome-improved aluminide coatings is achieved on the one hand by chromizing [
21], and on the other hand by alloying, that is, adding chromium into the substrate.
2.2.2. Si improved aluminide coating
The aluminum-silicon coating is made by adding Si element on the basis of aluminizing coating. Due to the high viscosity of molten aluminum, adding 2-6% Si can improve the fluidity of molten aluminum and improve the brittleness of aluminizing layer [
23]. The research of Cammarota et al. [
24] showed that when Si was added as an alloying element, the life of Ti-Al protective layer could be extended. Moskal [
25] reported that Ti-Si and Ti-Al-Si silicide phases in silicon-rich titanium aluminide coatings formed an oxygen diffusion barrier and prevented the internal oxidation of Al and Ti, thereby improving the oxidation resistance of titanium alloys.
Ti-Al intermetallic compound, Ti
5Si
3 and (or)TiSi
2 phase alloy coatings were prepared on the surface of Ti-Al compound by Si thermal diffusion method. At high temperature, an oxide layer mainly composed of Al
2O
3 and SiO
2 was formed on the surface of the alloy. This could effectively prevent Ti
4+ and O
2- from forming scales in the oxide layer, improving the high-temperature oxidation resistance of the alloy. The surface of oxidized sample had a special oxide /Ti-Si compound/substrate structure. Since the coefficient of thermal expansion of Ti-Si compounds was similar to that of the substrate, the oxide skin had a good thermal cycle stripping ability [
26]. Hu Xiaoyuan et al. [
27] researched and invented Self-generated Gradient Hot-dipping Infiltration (SGHDI) method to prepare in-situ autogenous Ti-Al-Si gradient coating. This kind of coating solved the problem that the coating was easy to crack and fall off due to the large difference in thermal expansion coefficient between the coating and the substrate. The bonding surface of the coating and the substrate was transformed from the abrupt state of the interface into a transition surface, so that the performance of the contact surface lied between the substrate and the coating, forming a smooth connection to reduce the problem that the coating was easy to deform, crack and fall off the substrate. However, this method of coating preparation provided Si element through quartz glass tube, which was difficult to control the content of Si element added and the shape of the sample was limited by quartz glass tube.
2.2.3. Nb improved aluminide coating
By doping 15-27 at% Nb in Ti-Al alloy, the ductility and fracture toughness at room temperature could be improved [
28]. Nb element increased the activity of Al and promoted the formation of Al
2O
3-rich layer in the oxide layer. Dai Jingjie et al. [
2] used laser cladding technology to prepare Ti-Al and Ti-Al-
xNb (
x=10, 20, 30, 40, 50) coatings on the surface of Ti-6Al-4V respectively. It was found that Nb element could reduce the cracking tendency of Ti-Al-
xNb coating. In the process of high temperature oxidation, the addition of Nb promoted the formation of continuous Al
2O
3 layer in the oxide layer, and significantly improved the adhesion of the oxide layer on Ti-Al-
xNb coating.
2.2.4. Pt improved aluminate coating
Among many modified aluminide coatings, the effect of Pt-Al coating is more significant. Adding platinum to the coating can improve the anti-spalling and self-healing ability of Al
2O
3 on the coating surface. It can enhance the stability of the coating structure and reduce the mutual diffusion between the coating and the substrate, so that the coating can maintain a high aluminum concentration for a long time. S.P. Trivedi et al. [
29] prepared Pt-Al compound coating on titanium base alloy IMI-834. By observing various microstructure of the coating, it was found that the outer layer of the coating contained PtAl
2 and Al
3Ti phases, the middle layer was mainly composed of Al
3Ti phase, and the inner layer was an interdiffusion layer. The microstructure of the coating was affected by the content of Pt and Al. A large number of studies have shown that the addition of platinum to the platinum-modified aluminide coating caused a "pinning effect" between the coating and the substrate, which increased the bonding strength between the coating and the substrate and greatly improved the high-temperature oxidation resistance of the alloy. But there is no single explanation.
2.2.5. Rare earth elements improved aluminide coating
Rare earth elements are the lanthanides and scandium and yttrium in total 17 elements. China's rare earth content is very rich, accounting for more than one-third of the world's total rare earth content. Rare earth elements are divided into light rare earth and heavy rare earth according to the difference in atomic number, and the reserves of light rare earth are more than heavy rare earth. Different rare earths have different mechanisms of action and play different roles. Two rare earth elements, lanthanum and cerium, are mainly used in large quantities in China. Lanthanum is active and often plays a catalytic role in metal modification. Cerium is mainly used as an additive or reducing agent in the reaction. The addition of rare earth elements to the metal substrate had the functions of purification, modification and alloying. Trace amounts of rare earth elements would significantly improve the physical, chemical and mechanical properties of metal materials [
30].
Before the study of rare earth modified high-temperature titanium alloy coating, there have been a lot of studies on the modification of common metals such as rare earth modified magnesium alloy and rare earth modified aluminum alloy. However, in comparison, the properties of titanium alloy are more active and difficult to control than other metal elements under high temperature service environment or conditions, so the modification research of rare earth elements on high temperature titanium alloy is more difficult and difficult to promote [
31]. The addition of different rare earth elements or a variety of rare earth elements can play a different role in the modification of high temperature titanium alloy coating. The addition of cerium group rare earth elements is mostly to produce high temperature titanium alloys with high performance, so that titanium alloys have better strength. The main feature of praseodymium is to enhance the corrosion resistance of high temperature titanium alloys. After adding ytterbium element to high temperature titanium alloy, the grain of titanium alloy can be refined, and the comprehensive properties of titanium alloy can be improved.
After the rare earth element is added to the metal, because of its strong activity, it will react with impurity elements such as oxygen in the substrate and produce rare earth compounds. Rare earth compounds are insoluble substances with high melting point and tend to be isolated at grain boundaries or cracks. This will hinder the dislocation movement, so that the grain boundary can not move and lead to the grain can not grow, play a role in refining the grain. The addition of cerium element can purify the impurities in the substrate and reduce the internal stress of the substrate. Wang Zihan et al. [
32] prepared the Ti-Al-Si gradient coating with Ce on the surface of TC4 by using the Self-generated Gradient Hot-dipping Infiltration (SGHDI) method. Finally, a Ce rich quaternary phase (TiAl
6Si
2Ce
2) could be formed in the L-(Al, Si, Ce) alloy layer.It inhibited the formation of the loose τ2 phase: Ti(Al,Si)
2 phase which caused Al
2O
3 to be less dense. The Ti-Al-Si gradient coating with Ce had excellent resistance to high temperature oxidation because it could maintain a dense and continuous multiphase layer structure for a long time in the process of high temperature oxidation, so that the oxygen atoms could not be diffused to the substrate.
3. High temperature oxidation resistant coating
3.1. Thermodynamic analysis of high temperature oxidation of titanium alloy
Titanium alloys in high temperature oxidation atmosphere can occur the following reactions:
Since the affinity of TiO formed by Ti with oxygen is similar to the affinity of Al
2O
3 formed by Al with oxygen, this fact prevents the selective oxidation of Al to Al
2O
3. TiO is unstable and oxidizes to TiO
2 in practice.
3.2. Kinetics analysis of high temperature oxidation of titanium alloy
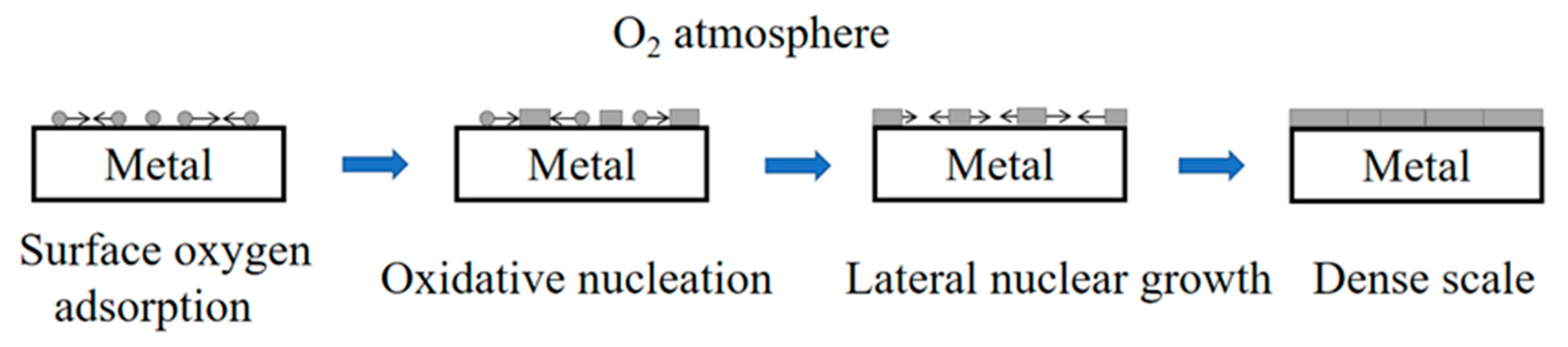
Figure 4 shows the process of oxide formation on the metal surface. The oxidation scale formation of pure metal surface included four steps: surface oxygen adsorption, oxidative nucleation, lateral nuclear growth and dense scale. When the lateral growth of the nucleus ended, the metal surface was completely covered by a thin oxide film, thus separating it from the gaseous environment. The properties of oxide film had obvious influence on the oxidation resistance of metal. For pure titanium, in an oxidizing atmosphere, at a lower temperature, the dense TiO
2 oxidation scale formed by Ti and oxygen could completely cover the alloy surface, thus separating the alloy from the gas environment. In this case, due to the small activity of oxygen, the diffusion rate was slow, and the oxidation kinetics followed the parabolic law. However, under high temperature conditions, the dense titanium dioxide oxide became loose and porous. The activity of oxygen was enhanced and the diffusion speed was accelerated. The oxygen atom entered the substrate, while the Ti atom diffused from the core and crack to the surface. The oxidation layer became thicker and the bonding strength with the substrate became worse. The oxidation kinetics followed a linear rule. As the temperature increased, the mass gain increased [
33,
34].
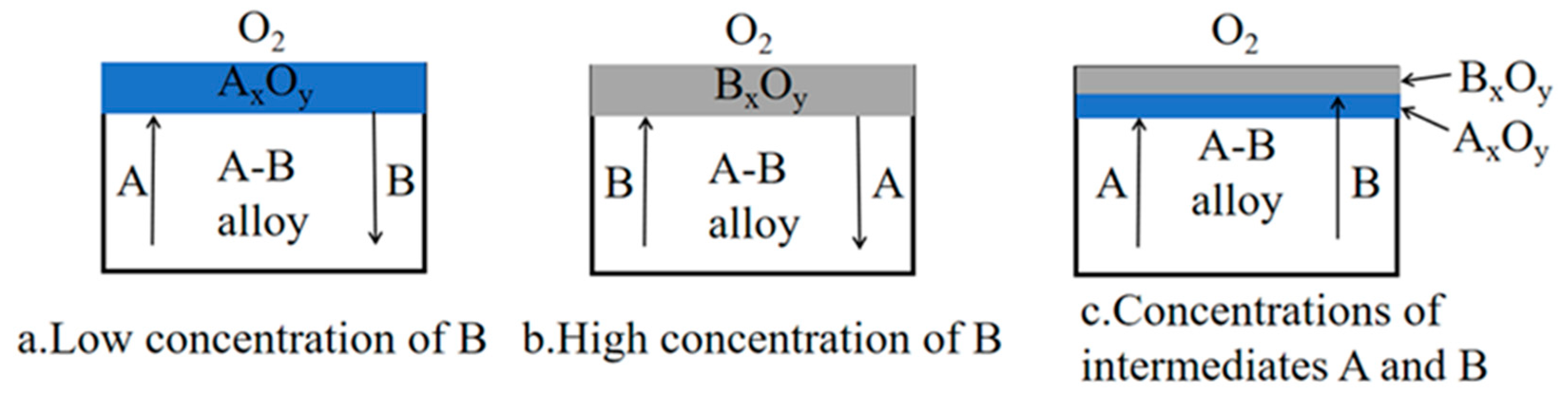
Figure 5 shows the oxidation process of binary A-B alloy. For A-B binary alloys that do not form mixed oxides or spinels, and B was a rarer element, there were three boundary cases. For low concentrations of B, only A formed oxides (
Figure 5a). B was enriched at the A-oxide interface of the alloy until a critical concentration of B oxide was reached. If the concentration of B was high enough, only B oxide was formed and A diffused into the alloy (
Figure 5b). For concentrations of intermediates A and B, both A and B were in oxide form (
Figure 5c).
3.3. Improve the high temperature oxidation resistance of titanium alloy
3.3.1. Surface coating technology
Surface coating technology uses coatings to change the surface properties of the substrate. The substrate components do not react with the coating, or react with only a small amount of the coating. Surface coating technologies include vapor deposition, hot dipping, ion plating, thermal spraying, laser cladding, sol-gel method, etc. The key of oxidation protection of coatings is the adhesion and long-term stability of coatings. The metal-based coatings used for oxidation protection on the surface of titanium alloy mainly include aluminum plating coating, Al-X series coating, Ti-Al-X series coating and rare earth element modified aluminide coating.
3.3.1.1. Aluminized coating
Aluminum plating on titanium alloys can produce TiAl
3 coatings. Continuous Al
2O
3 oxide film can be formed after high temperature oxidation in the air, which plays a role in improving the high-temperature oxidation resistance of the substrate. Based on the advantages of the hot dipping method, such as simplicity, operability and good controllability, Shiang-Cheng Jeng et al. [
35] prepared a simple hot dip aluminized coating on Ti-6Al-4V alloy and evaluated the oxidation resistance of the coating at high temperatures. After oxidation at 800 °C for 96h, the surface of aluminized sample was intact while the surface of non-aluminized sample was seriously spalling. The weight increase of aluminized samples was significantly lower than that of non-aluminized samples, indicating that the hot dip aluminized coating could effectively protect the oxidation resistance of Ti-6Al-4V alloy at high temperature.
3.3.1.2. Al-X series coating
Al-X coatings include Al-Si, Al-Cr, etc. Because of its high aluminum content and excellent thermal stability, Al-X series coatings are often used as one of the effective protective coatings to improve the high temperature oxidation of titanium alloys and titanium aluminides.
Mei Xuelin et al. [
36] prepared Al-Si coating on Ti-6Al-4V alloy by using low oxygen partial pressure fusion technology. The cyclic oxidation test results showed that continuous Al
2O
3 layer was formed on the surface of the alloy, which effectively improved the high-temperature oxidation resistance of the alloy. The presence of silicon-rich layer hindered the diffusion of antioxidant elements in the coating substrate and inhibited the degradation rate. Cheng Chen et al. [
37] successfully prepared a dense and continuous Al-Si coating on Ti-6Al-4V alloy substrate by mechanical alloying method, with a thickness of about 350 mm. The mass gain and oxidation rate of the substrate at 850°C were greatly reduced by the synthesis of the coating. The oxide layer had a multi-layer structure. It was successively composed of Al diffusion layer, Ti
5Si
4 intermediate layer, internal TiAl
3 layer, external Ti-Al-Si alloy layer and oxide layer from the inside of the substrate to the surface of the coating. This multi-layer structure could hinder and prevent the inward diffusion of oxygen at high temperature, so the synthesized Al-Si coating could effectively improve the oxidation resistance of Ti-6Al-4V substrate. Zhao Yuguang et al. [
38] prepared Al-Cr coating on the surface of Ti-6Al-4V alloy by low oxygen self-melting technology. The oxidation resistance of the coated sample at 600°C was almost 300 times that of the uncoated sample. Zhou Wen et al. [
39] successfully prepared Al+Cr coating on Ti-6Al-4V alloy by vacuum melting method, which improved the high-temperature oxidation resistance of the sample. This new coating preparation method was more simple, efficient and low-cost. Compared with the sample of Cr modified aluminide, the oxidation resistance of Al+Cr coated by vacuum melting was about 2 times that of Cr modified aluminide coated. This was because only a single α-alumina scaling was formed on the surface of the vacuum welding Al + Cr coating, and no metastable θ-alumina scaling was formed.
However, the coating of Al-X system is prone to cracking, spalling and rapid degra dation due to the large difference in thermal expansion coefficient between the coating and the substrate, which significantly reduces the service life of the coating and degrades the mechanical properties of the substrate. Therefore, it is necessary to seek the element-modified Al-X coating or prepare a coating that is well combined with the substrate to improve the anti-spalling property of the coating.
3.3.1.3. Ti-Al-X series coating
The composition of Ti-Al-X coating is similar to that of Ti and TiAl based alloys, and it has good compatibility and little difference in thermal expansion coefficient. These characteristics can reduce the mutual diffusion between the coating and the substrate, and reduce the cracking and peeling of the coating.
Jiang Huang et al. [
40] prepared a novel Si-Al diffusion coating consisting of uniform Ti (Al,Si)
3 phase on γ-TiAl alloy by post-heat treatment of cold-sprayed Al-40Si(wt.%) coating. The high temperature oxidation performance of the diffusion coating was evaluated at 950°C for 1000h. The results showed that the Ti
5Si
3 layer played a major role in the oxidation process, which acted as a diffusion barrier to inhibit the mutual diffusion between the coating and the substrate, thus improving the long-term oxidation resistance of γ-TiAl. In order to improve the surface properties of Ti-6Al-4V titanium alloy, Jingjie Dai et al. [
41] used laser surface alloying method to prepare Ti-Al-Si coatings with different Si contents on the surface of Ti-6Al-4V titanium alloy. The results showed that the coating had good metallurgical bonding with the substrate. There was no obvious crack in Ti-Al-Si coating. Ti-Al-Si coatings showed better high-temperature oxidation resistance at 800°C for 1000 h. The higher content of Al and Si in the coatings, the better the high-temperature oxidation resistance. The effect of Si on the oxidation resistance of Ti-Al-Si coating at high temperature was mainly to refine the oxidation grains and promote the formation of Al
2O
3. In order to improve the oxidation resistance of titanium substrate composites, X.T. Li et al. [
42] successfully prepared Ti-Al-Si coating with mesh structure on TiBw/Ti6Al4V composites with mesh structure by using hot dipping solidification and subsequent heat treatment techniques. The cyclic oxidation tests at 973K, 1073K and 1173K showed that Ti-Al-Si coating could significantly improve the oxidation resistance of the composite, which was mainly due to the protective oxide skin produced on the coating. In addition, due to the unique network structure of the coating, there was no crack in the oxidation coating. The presence of Si atoms in the coating could promote the formation of Al
2O
3 and inhibit the formation of TiO
2 at the same time. The Ti
5Si
4 layer in the interface could acted as a barrier layer, delaying the interface reaction between Ti(Al,Si)
3 coating and the composite substrate. It effectively improved the high temperature oxidation resistance of the coating. F. Oukati Sadeq et al. [
43] proposed a fast, simple and reflective method for the synthesis of Ti-Si-Al coating on the surface of Ti-6Al-4V alloy by hot impregnating silicon. The results showed that 850°C was the best process temperature. The appropriate thickness of titanium silicide was obtained under the appropriate soaking time, and the weight gain of the coating sample was the least in the isothermal oxidation experiment at 1000°C. The TiO
2-Al
2O
3-SiO
2, TiSi
2 and Ti
3Al continuous protective layers were formed in the initial oxidation stage, which made the coated Ti-6Al-4V alloy have higher high temperature oxidation resistance.
3.3.1.4. Rare earth modified aluminide coating
Many studies have shown that moderate amount of rare earth elements can improve the high temperature oxidation resistance of titanium alloys. In order to improve the high temperature oxidation resistance of Ti65 alloy, Zeng Siqi et al. [
44] prepared the Ti-Al-Si composite coating with Ce added on the surface of Ti65 alloy by two-step hot dipping method. The main components of the coating were (Ti,Ce)(Al,Si)
3 phases. The two-step hot dipping method could control the thickness of the coating by controlling the time and temperature of the hot dipping. After pre-oxidation treatment, the coating was transformed from a single phase to a multiphase layer structure composed of Ti-Al binary phase and Ti-Si binary phase. The results showed that the weight gain of the substrate after 23 days of constant temperature oxidation at 800°C was 26 times that of the plated sample, indicating that the oxidation resistance of Ti65 alloy at high temperature was obviously improved. The effects of common rare earth elements added to titanium alloys on their high-temperature oxidation resistance are shown in
Table 1:
3.3.2. Preoxidation treatment
It has been pointed out that the preoxidation treatment had great influence on the compatibility and oxidation resistance of titanium alloy aluminized coating. However, research in this area is quite limited. W.Zhou et al. [
49] pre-oxidized (4h under 1000°C inert environment) + aluminized and aluminized + pre-oxidized (4h under 1000°C inert environment) Ti-6Al-4V alloy, and compared the surface morphology and oxidation kinetics curves of the samples. It was concluded that pre-oxidation (4h under 1000°C inert environment) + aluminized titanium alloy had better oxidation resistance and crack resistance. The pre-oxidation of titanium alloy before aluminization had a great influence on the aluminization process: Al
5Ti
3, Al
3Ti and Al
2TiO
5 were formed, and the coating was thinner. Therefore, appropriate heat treatment could be carried out before aluminizing titanium alloy to improve the high temperature oxidation resistance of the aluminizing coating.
Table 2.
Comparative summary of the weight gain of substrate and coated sample after high temperature oxidation in the literature.
Table 2.
Comparative summary of the weight gain of substrate and coated sample after high temperature oxidation in the literature.
| Substrate |
Coating |
Oxidation condition |
Substrate weight gain /
Coated sample gain |
Lit. |
| Ti-6Al-4V |
Ti-Al-40Nb |
800°C /1000h |
15.02 |
[2] |
| Ti-6Al-4V |
Ti-Al-Si |
800°C /120h |
7.31 |
[27] |
| Ti-6Al-4V |
Al |
800°C /96h |
2.85 |
[35] |
| Ti-6Al-4V |
Al-Si |
850°C /100h |
5.56 |
[37] |
| Ti-6Al-4V |
Al-Cr |
600°C |
300.00 |
[38] |
| γ-TiAl |
Ti(Al,Si)3
|
950°C /1000h |
6.33 |
[40] |
Ti-6Al-4V
TiBw/Ti6Al4V
TiBw/Ti6Al4V
TiBw/Ti6Al4V
Ti-6Al-4V
Ti65 |
Ti-Al-Si
Ti-Al-Si
Ti-Al-Si
Ti-Al-Si
Ti-Al-Si
(Ti,Ce)(Al,Si)3
|
800°C /1000h
700°C /100h
800°C /100h
900°C /100h
1000°C /80h
800°C /552h |
24.80
11.14
16.68
27.38
6.60
26.00 |
[41]
[42]
[42]
[42]
[43]
[44] |
4. Thermal corrosion resistant coating
4.1. Mechanism of thermal corrosion under the action of molten salt
It is generally believed that thermal corrosion of gas turbine materials was mainly caused by fuel combustion products, which contained a large amount of sulfur, sodium, vanadium, chlorine, potassium, etc. [
50,
51]. Mixtures of these pollutants were highly corrosive at temperatures higher than the melting point of the pollutant, further accelerating the high-temperature corrosion process. High temperature corrosion was the main failure form of hot end components of important engineering systems such as aeroengine and land-based gas turbine. This corrosion was caused by condensation of thin films containing molten salts such as chloride, sulfate, or vanadate. Impurities absorbed from the air or present in the fuel led to the formation of low melting point salts such as NaCl and Na
2SO
4, which severely degraded the performance of the hot end components of the turbine [
52,
53].
The Marine environment is characterized by high humidity and high salinity, and its atmosphere is filled with a lot of salt. The hot end components of aero engines serving in Marine environment are susceptible to thermal corrosion due to high temperature caused by high-speed operation and the above environmental characteristics. When the engine works in the Marine environment, a large amount of water vapor and NaCl in the Marine atmosphere will enter the engine along with the gas and be deposited on the blade or coating surface. At the same time, NaCl reacts with sulfur oxide products of fuel combustion to generate Na
2SO
4, which is also deposited on the surface of the hot-end components, and these reaction equations are as follows:(4)(5)(6) [
54]. When the engine is running, because the air is compressed step by step, the pressure and temperature will gradually increase, and the temperature in the compressor part can reach 300-750°C. Although the temperature does not reach the melting point of the deposited salt on the surface, it causes thermal corrosion to the sealing coating of the compressor.
Thermal corrosion can be regarded as a process that accelerates the coexistence of thermal oxidation and vulcanization [
55]. Under high temperature conditions, even though part of the salt film may evaporate, there is still a certain amount of mixed sulfate that will be transformed into liquid state, and the decomposition reaction as shown in (7) will occur [
56].
For aluminum-silicon coatings, the general process of high-temperature thermal corrosion is that the anti-oxidation Al elements inside the coating first undergo selective oxidation (as shown in (3)), and then are decomposed by molten salt to O
2- rapid melting (as shown in (8)). The above process is repeated until the Al elements inside the coating are consumed and the coating basically fails. At this time, the Ti element in the substrate will react directly with the molten salt, as shown in (9).
4.2. Improve the heat resistance and corrosion performance of titanium alloy
4.2.1. Surface coating technology
4.2.1.1. Aluminized coating
Aluminized coating is the first high temperature protective coating used. Due to the cost effectiveness and simplicity of alumina diffusion coatings, it is also widely used for thermal corrosion resistance of Ti and Ni based alloys. The formation of dense Al
2O
3 layer can effectively prevent the entry of oxides. However, some studies have also shown that hot dip aluminized coating reduced the corrosion resistance of commercial purity titanium in 3.5 wt.%NaCl solution at room temperature, which was related to the difference in corrosion resistance between titanium and aluminum and the formation of galvanizing cells [
57]. In addition, the dissolution of Al
2O
3 in molten salt [
58] and the formation of holes in the aluminide coating during thermal cycling [
59] would lead to spalling and cracking of the aluminide coating, resulting in the failure of the coating. The aluminizing coating had defects such as high brittleness, easy cracking, easy mutual diffusion with substrate, poor thermal corrosion resistance and fatigue creep resistance, etc., resulting in the formation of cracks and falling off of the oxide layer of the coating at high temperature [
60], so the thermal corrosion resistance decreased. Therefore, in order to solve this series of defects, modified aluminide coatings began to develop. A large number of experiments have shown that the addition of some active elements can improve the thermal corrosion resistance of the coating [
61,
62].
4.2.1.2. Silicon modified aluminide coating
In recent years, although a large number of coatings have been developed, slurry method can prepare Al-Si coatings on the surface of special-shaped parts, and it has been widely used because of its simple operation, low cost and stable performance. The Si modified aluminide coating can slow down the degradation caused by the mutual diffusion between the coating and the substrate at high temperature, and has better high-temperature oxidation resistance and thermal corrosion resistance than the aluminizing coating [
63,
64]. Si element can increase the activity of Al element in the coating, promote the selective oxidation of Al element, reduce the consumption of Al element, and improve the bonding property between the oxide film and the coating [
65,
66,
67]. At the same time, the oxidation rate of superalloy can be reduced, and the isomild cyclic oxidation resistance of superalloy can be improved. A small amount of Si element added to the coating can play an isolating role in the solidification process of the molten alloy [
68]. Si elements gather on the surface of the coating to form Al-Si base, inhibit the diffusion of insoluble elements (Ti, Mo, W and V), strengthen the aluminide coating [
69], and extend the service time of the coating. However, the Si content in the coating should not be too much to avoid forming a harmful phase with low melting point with Ni in the substrate at high temperature. This makes the coating brittle and prone to peeling off during high-temperature oxidation and thermal corrosion [
70].
X.T. Li et al. [
71] prepared Ti-Al-Si coating on TiBw/Ti6Al4V composite material by a two-step method of hot dipping and heat treatment to improve its thermal corrosion resistance. The results showed that Ti-Al-3Si(wt%) coating and Ti-Al-20Si(wt%) coating could protect the substrate when exposed to NaCl at 800°C, 25% NaCl+ 75% Na
2SO
4 and Na
2SO
4 salt, and significantly improved the thermal corrosion resistance of the substrate. Among them, Ti-Al-20Si coating had better heat resistance and corrosion resistance, and the experimental results are shown in the following table (
Table 3). Dai Jingjie et al. [
72] prepared TiAl-
xSi coating on Ti-6Al-4V by laser surface alloying method, and exposed the sample to a salt mixture of 75wt.% Na
2SO
4 + 25 wt.% NaCl at 800°C for 300 h to explore the effect of Si element on the heat resistance and corrosion properties of the coating. The results showed that the addition of silicon significantly improved the heat resistance and corrosion of the coating. With the increase of Si content, the heat resistance and corrosion of the coating were improved. The mechanism of improving the heat resistance and corrosion of titanium alloy by preparing Ti-Al-Si coating was that it promoted the formation of Al
2O
3 and SiO
2, thereby increasing the density of oxide skin and hindering the internal diffusion of O
2.
4.2.1.3. Rare earth modified aluminide coating
In order to improve the thermal corrosion resistance of titanium alloy, people choose to add rare earth elements on the basis of titanium alloy aluminum-based coating. It is well known that rare earth elements can significantly reduce the oxidation rate of the alloy and greatly improve the adhesion of the oxide film. Lin Hao et al. [
73] deposited Al-Y gradient coating on γ-TiAl alloy by reactive magnetron sputtering (MS) system, and investigated the influence of Al-Y gradient coating on the heat resistance and corrosion properties of γ-TiAl alloy in Na
2SO
4 molten salt at 750°C, 850°C and 950°C. The experimental results showed that Y in Al-Y gradient coating was easy to react with S compared with a single aluminized coating, thus promoting the formation of Al
2O
3, which not only reduced the Al consumed by vulcanization in the initial stage of corrosion, but also facilitated the formation of oxidation protective layer. In conclusion, rare earth modified aluminide coating could improve the heat resistance and corrosion of TiAl alloy. At present, there are few researches on the modification of rare earth elements on the surface of titanium alloy to improve its high temperature corrosion resistance coating, but it has a certain application prospect.
4.2.2. Preoxidation treatment
Pre-oxidation is the operation of maintaining high temperature (generally greater than 900°C) for a period of time under a certain atmosphere and then cooling. Yuan-tao HU et al. [
74] pre-oxidized aluminized TiAl alloy (20h pre-oxidized in Muffle furnace at 900°C) before the thermal corrosion experiment, and then carried out thermal corrosion test at 700°C and 100h in eutectic salt mixture (75wt.% Na
2SO
4 + 25wt.% NaCl). The results showed that the pre-oxidation could promote the formation of dense alumina protective layer, effectively prevented the attack of corrosive elements on the substrate and the rapid consumption of aluminum coating. Moreover, the aluminized layer could provide enough aluminum source, so the aluminized TiAl alloy had good thermal corrosion resistance.
5. Wear-resistant coating
5.1. Analysis of friction mechanism of titanium alloy
Although titanium alloys have many advantages, their frictional properties are poor. The poor frictional properties of titanium alloys are characterized by high friction coefficient, severe adhesive wear, strong occlusal tendency and low wear resistance [
75]. Titanium and titanium alloys have low wear resistance due to two main reasons: low resistance to plastic shear and low work hardening; Low protection of surface oxides that are easily removed by spalling or microdebris due to high flash temperatures caused by friction during dry sliding, and fail to protect the subsurface layer from wear; In addition, dissolved oxygen from the atmosphere is prone to embrittlement of the substrate, which reduces the mechanical resistance of the material [
76]. The friction coefficient is related to d bond, and the smaller the percentage of d bond, the stronger the activity of the metal, and the greater the friction [
77]. Titanium has the lowest percentage of d-bonds (27%), so it has the most friction. It is well known that the use of lubricants can greatly reduce friction, but all conventional lubricants (mineral oils and greases) used on titanium alloys have been shown to be ineffective [
78]. And the low thermal conductivity of titanium exacerbates the problem of ineffective lubricants.
5.2. Improve wear resistance of titanium alloy
5.2.1. Surface coating technology
5.2.1.1. Aluminized coating
The low hardness and poor wear resistance of titanium alloys severely limit their ap- plication as key moving parts. Ti-Al intermetallic compounds have both metallic and covalent bonds, providing the final material with the toughness of metals and the high temperature properties of ceramics [
79,
80]. Therefore, Ti-Al intermetallic coating is a promising method to enhance the high temperature oxidation resistance and friction properties of titanium alloys. Liu Yang et al. [
81] used Ti6Al4V and AlSi10Mg powders as substrates to prepare a graded Ti-Al intermetallic compound coating by laser powder deposition (LPD) method, and explored the wear resistance of the coating through sliding wear evaluation experiments. The results showed that the hardness of each layer was obviously higher than that of Ti6Al4V substrate and the wear rate was much lower than that of Ti6Al4V substrate. The excellent wear resistance of Ti-Al intermetallic compound coating was due to the high hardness of Ti-Al intermetallic phase, which could effectively inhibit the corresponding penetration of the coating surface. Wenbin Zhang et al. [
82] prepared TiAl composite coating on Ti-6Al-4V alloy by laser cladding method, and studied the microstructure and tribological behavior of the composite coating at an estimated temperature (25-500°C). The results showed that the composite coating had enough toughness and no brittle spalling occurred on the wear surface.
5.2.1.2. Chromium modified aluminide coating
Due to the coexistence of Al, Cr and Ti elements, a multiphase structure is generated, and the hardness and wear resistance of titanium alloy are improved [
83,
84,
85]. Li Zhihao et al. [
86] prepared Ti-Al-Cr alloy layer on Ti-4Al-2V alloy by pulsed laser cladding technology, and the alloy layer had good metallurgical bonding with the substrate. The results showed that the microhardness of the Ti-Al-Cr alloy layer was about 2 times that of the titanium alloy substrate, and the wear rate was only 1/3 of the substrate. Therefore, Ti-Al-Cr alloy layer improved the wear resistance of titanium alloy substrate. The reason was the binding strengthening caused by strong solid solution and lattice distortion, as well as the Al/ Cr-induced difference from the substrate phase structure.
5.2.1.3. Nb modified aluminide coating
By hindering the diffusion of Ti and oxygen, Nb element can effectively improve the activity of Al element and reduce the concentration of oxygen vacancy in TiO
2 lattice, thus inhibiting TiO
2 growth and promoting the formation of Al
2O
3 protective layer. Wensheng Li et al. [
87] prepared Ti-Al-Nb intermetallic coating by in-situ synthesis of Ti-6Al-4Nb powder on the surface of Ti-25Al-17V alloy by laser. By measuring the friction coefficient curve and wear rate of the coating at 25°C, 200°C, 400°C, 600°C and 800°C, it was found that the friction coefficient and wear rate of the coating at 800 °C were lower. Therefore, Ti-25Al-17Nb coating had good wear resistance and anti-friction properties at 800°C. This was because at 800°C, the dense hardened Al
2O
3 oxide film gradually replaced the loose porous TiO
2 oxide film, hindering the diffusion of oxygen to the coating, reducing the possibility of brittle oxide formation, and the dense Al
2O
3 oxide film had good lubricity.
5.2.2. Preoxidation treatment
In order to form metallurgical bonding between the coating and the substrate through diffusion, Zhao, J.H.et al. [
88] adopted hot infiltration to first coat the surface of Ti-6Al-4V alloy with pure aluminum, and then carried out pre-oxidation treatment. After the sample was pre-oxidized, the Al coating was oxidized to alumina (Al
2O
3), and Ti-Al intermetallic compound was formed. The tribological properties of the sample were characterized by observing and analyzing the microstructure and composition of the sample and measuring the microhardness and friction coefficient of the sample. The results showed that when the preoxidation temperature was 750°C, the microhardness reached the maximum value, about 500 HV. The final optimized process parameters were hot dipping at 700°C for 15 minutes, followed by pre-oxidation at 750°C for 6 hours in a resistance furnace. This process improved the wear resistance of Ti-6Al-4V alloy by forming bulk TiAl
3 phase and oxide.
5.2.3. Carburizing
Yang Wenying et al. [
89] combined the hot dip aluminum process with the carburizing process. First, a layer of pure aluminum was hot dipped on the Ti65 alloy, and then it was solid carburized. After hot dipping of pure aluminum, the coating was successively TiAl
3 phase +L-(Ti,Al) mixed phase layer and L-(Ti,Al) layer from the substrate. After carburizing, the coating was successively TiAl
2, TiAl, Ti
3Al alloy phase layer and Ti-Al carburizing layer from the substrate. Finally, the hardness of substrate, aluminized sample and aluminized recarburized sample were measured by hardness meter and compared. The results showed that the hardness of aluminized recarburizing sample was about 960HV, which was obviously higher than that of substrate and aluminized sample. It also showed that the wear resistance of hot dip aluminized samples was improved by carburizing treatment. This was because the carbide particles formed in the Ti-Al carburizing layer played a strengthening role.
6. Conclusions
In this paper, the research on oxidation resistance, thermal corrosion resistance and wear resistance of titanium alloy aluminum-based coatings in recent years was described from four aspects: aluminide coating, high temperature oxidation resistance coating, thermal corrosion resistance coating and wear resistance coating. There are many ways to prepare aluminum-based coatings on titanium alloys, but each method has certain advantages and disadvantages. We should choose the specific preparation method according to the characteristics of the required preparation coating.
The high temperature oxidation resistance of the coating is mainly judged according to the mass gain. In general, the mass increase of coated samples during high-temperature oxidation is less than that of uncoated samples, because the coating can protect the substrate from oxidation. The Al coating on titanium alloy is oxidized by TiAl3 into continuous Al2O3 layer during high temperature oxidation process, which can effectively protect the substrate. The silicon-rich layer formed by adding Si element on the basis of Al coating reduces the degradation rate of antioxidant elements, and the multi-layer structure can hinder and prevent the inward diffusion of oxygen at high temperature. The Ti-Al-Si coating has good metallurgical bonding with the substrate. The addition of Cr element promotes the formation of single α-alumina scaling. The addition of rare earth elements promoted the formation of continuous Al2O3 and prevented crack propagation. Therefore, these modified aluminide coatings can further improve the high-temperature oxidation resistance of titanium alloys.
The heat resistance of the coating can be judged not only by the level of corrosion potential, but also by the quality gain. The higher the corrosion potential, the less the mass increase, and the better the corrosion resistance of the coating. The Ti-Al-Si coating was prepared by adding Si element to the titanium alloy aluminum coating. The coating generates and maintains a dense and continuous Al2O3 oxide film in a high-temperature molten salt environment, and promotes the formation of SiO2, thereby increasing the density of the oxide and slowing down the corrosion dissolution rate of the oxide. The addition of rare earth elements promotes the formation of Al2O3, which is conducive to the formation of oxidation protective layer.
The wear resistance of the coating is mainly related to microhardness, wear rate and friction coefficient. The higher the microhardness, the lower the wear rate, the lower the friction coefficient, the better the wear resistance of the coating. Ti-Al intermetallic compound coating has excellent wear resistance because it can effectively inhibit the corresponding penetration of the coating surface. The addition of Cr element to Ti-Al coating improves the friction resistance of titanium alloy due to the combination strengthening caused by strong solid solution and lattice distortion. The addition of Nb element promotes the formation of dense, hardened and lubricated Al2O3 oxide film, thus improving the wear resistance of the coating.
In addition, proper pre-oxidation treatment of titanium alloy before and after coating preparation can also greatly improve its high temperature oxidation resistance and other properties. In order to improve the properties of titanium alloys, new coating preparation methods are constantly developed on the basis of aluminum-based coatings, new elements are constantly tried to be added, and various heat treatment processes are constantly explored. In recent years, many good results have been achieved, but the cost, efficiency and weight gain of coating preparation should also be considered in combination with the actual application of titanium alloys. The existing studies reflect that rare earth elements are less used in titanium alloy aluminum-based coatings, which makes the advantages of abundant rare earth elements in China and the excellent role of rare earth elements not fully played. This problem needs to be thoroughly studied and solved by scientific and technological workers.
Author Contributions
Investigation, Data curation, and Writing – original draft, S.Z.; Supervision, Project administration, Funding acquisition, Methodology, and Writing – reviewed, F.L.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Project of Education Department of Hunan Province (No. 22A0100), Hunan Provincial Natural Science Foundation of China (No. 2021JJ30672), and College Students' innovation and entrepreneurship training program of Xiangtan University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the support provided by Materials Intelligent Design College Students' Innovation and Entrepreneurship Education Center, Xiangtan University, Xiangtan, Hunan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shao, L.; Li, W.S.; Li, D.Y.; Xie, G.L.; Zhang, C.Z.; Zhang, C.; Huang, J.F. A review on combustion behavior and mechanism of Ti alloys for advanced aero-engine. J. Alloys Compd. 2023, 960, 170584. [Google Scholar] [CrossRef]
- Dai, J.J.; Li, S.Y.; Zhang, H.X.; Yu, H.J.; Chen, C.Z.; Li, Y. Microstructure and high-temperature oxidation resistance of Ti-Al-Nb coatings on a Ti-6Al-4V alloy fabricated by laser surface alloying. Surf. Coat. Technol. 2018, 344, 479–488. [Google Scholar] [CrossRef]
- Li, S.; Yamaguchi, T. High-temperature oxidation performance of laser-cladded amorphous TiNiSiCrCoAl high-entropy alloy coating on Ti-6Al-4V surface. Surf. Coat. Technol. 2022, 433, 128123. [Google Scholar] [CrossRef]
- Xu, Y.J.; Yao, Z.P.; Jia, F.Z.; Wang, Y.L.; Jiang, Z.H.; Bu, H.T. Preparation of PEO ceramic coating on Ti alloy and its high temperature oxidation resistance. Curr. Appl. Phys. 2010, 10, 698–702. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Peng, Y.P.; Mao, Y.L.; Pang, C.J.; Lu, L.Y. Effect of hot-dip aluminizing on the oxidation resistance of Ti–6Al–4V alloy at high temperatures. Corros. Sci. 2012, 55, 187–193. [Google Scholar] [CrossRef]
- Yao, Z.; Marek, M. NaCl-induced hot corrosion of a titanium aluminide alloy. Mater. Sci. Eng. A 1995, 192–193, 994–1000. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, B.; Ye, W.T.; Zhang, T.; Wan, J.; Zhou, Q.; Shen, J.H.; Li, J.S.; Lu, W.F.; Wang, H. Simultaneously improving mechanical, thermal, and anti-wear properties of Ti alloys using 3D-networked graphene as reinforcement. Carbon 2023, 213, 118152. [Google Scholar] [CrossRef]
- Moskal, G.; Migas, D.; Mendala, B.; Kałamarz, P.; Mikuśkiewicz, M.; Iqbal, A.; Jucha, S.; Góral, M. The Si influence on the microstructure and oxidation resistance of Ti-Al slurry coatings on Ti-48Al-2Cr-2Nb alloy. Mater. Res. Bull. 2021, 141, 111336. [Google Scholar] [CrossRef]
- Lazurenko, D.V.; Bataev, I.A.; Laptev, I.S.; Ruktuev, A.A.; Maliutina, I.N.; Golkovsky, M.G.; Bataev, A.A. Formation of Ti-Al intermetallics on a surface of titanium by non-vacuum electron beam treatment. Mater. Charact. 2017, 134, 202–212. [Google Scholar] [CrossRef]
- Gurrappa, I.; Gogia, A.K. High performance coatings for titanium alloys to protect against oxidation. Surf. Coat. Technol. 2001, 139, 216–221. [Google Scholar] [CrossRef]
- Izumi, T.; Nishimoto, T.; Narita, T. Formation of nickel aluminide coating on γ-TiAl alloy. Intermetallics 2003, 11, 841–848. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Dubey, A.K. Recent trends in laser cladding and surface alloying. Opt. Laser Technol. 2021, 134, 106619. [Google Scholar] [CrossRef]
- Liu, Y.n.; Yang, L.J.; Yang, X.J.; Zhang, T.G.; Sun, R.L. Optimization of microstructure and properties of composite coatings by laser cladding on titanium alloy. Ceram. Int. 2021, 47, 2230–2243. [Google Scholar] [CrossRef]
- Kathuria, Y.P. Some aspects of laser surface cladding in the turbine industry. Surf. Coat. Technol. 2000, 132, 262–269. [Google Scholar] [CrossRef]
- Kwok, C.T.; Man, H.C.; Cheng, F.T.; Lo, K.H. Developments in laser-based surface engineering processes: with particular reference to protection against cavitation erosion. Surf. Coat. Technol. 2016, 291, 189–204. [Google Scholar] [CrossRef]
- Pourmohammad, H.; Bahrami, A.; Eslami, A.; Nazemi Harandi, A. Gas-phase aluminizing of HP40Nb steel used in reformer tubes: Synthesis, characterization, and its implications for the microstructure and high-temperature creep resistance of the base alloy. Int. J. Pres.Ves. Pip. 2022, 200, 104833. [Google Scholar] [CrossRef]
- Taghipour, M.; Eslami, A.; Bahrami, A. High temperature oxidation behavior of aluminide coatings applied on HP-MA heat resistant steel using a gas-phase aluminizing process. Surf. Coa. Technol. 2022, 434, 128181. [Google Scholar] [CrossRef]
- Wang, F.H.; Lou, H.Y.; Bai, L.X.; Wu, W.T. Hot corrosion of yttrium-modified aluminide coatings. Mater. Sci. Eng. A 1989, 120–121, 387–389. [Google Scholar] [CrossRef]
- Xiong, H.P.; Mao, W.; Xie, Y.H.; Ma, W.L.; Chen, Y.F.; Li, X.H.; Li, J.P.; Cheng, Y.Y. Liquid-phase siliconizing by Al–Si alloys at the surface of a TiAl-based alloy and improvement in oxidation resistance. Acta Mater. 2004, 52, 2605–2620. [Google Scholar] [CrossRef]
- Bai, C.Y.; Luo, Y.J.; Koo, C.H. Improvement of high temperature oxidation and corrosion resistance of superalloy IN-738LC by pack cementation. Surf. Coat. Technol. 2004, 183, 74–88. [Google Scholar] [CrossRef]
- Zhou, C.G.; Xu, H.B.; Gong, S.K.; Young Kim, K. A study of aluminide coatings on TiAl alloys by the pack cementation method. Mater. Sci. Eng. A 2003, 341, 169–173. [Google Scholar] [CrossRef]
- Gauthier, V.; Dettenwanger, F.; Schütze, M.; Shemet, V.; Quadakkers, W.J. Oxidation-Resistant Aluminide Coatings on γ-TiAl. Oxid. Met. 2003, 59, 233–255. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yakou, T. Control of intermetallic compound layers at interface between steel and aluminum by diffusion-treatment. Mater. Sci. Eng. A 2002, 338, 44–53. [Google Scholar] [CrossRef]
- Cammarota, G.P.; Casagrande, A.; Sambogna, G. Effect of Ni, Si and Cr in the structural formation of diffusion aluminide coatings on commercial-purity titanium. Surf. Coat. Technol. 2006, 201, 230–242. [Google Scholar] [CrossRef]
- Grzegorz, M. Microstructure and oxidation behaviour of TiAlSi coatings on TiAlCrNb alloy. J. Ach. Mater. Manuf. Eng. 2007, 20, 1–2. [Google Scholar]
- Zheng, M.H.; Rapp, R.A. Simultaneous Aluminizing and Chromizing of Steels to Form (Fe, Cr)3Al Coatings. Oxid. Met. 1998, 49, 19–31. [Google Scholar] [CrossRef]
- Hu, X.Y.; Li, F.G.; Shi, D.M.; Xie, Y.; Li, Z.; Yin, F.C. A design of self-generated Ti–Al–Si gradient coatings on Ti–6Al–4V alloy based on silicon concentration gradient. J. Alloys Compd. 2020, 830. [Google Scholar] [CrossRef]
- Gogia, A.K.; Nandy, T.K.; Banerjee, D.; Carisey, T.; Strudel, J.L.; Franchet, J.M. Microstructure and mechanical properties of orthorhombic alloys in the Ti-Al-Nb system. Intermetallics 1998, 6, 741–748. [Google Scholar] [CrossRef]
- Trivedi, S.P.; Das, D.K. Microstructural aspects of plain aluminide and Pt-aluminide coatings on Ti-base alloy IMI-834. Intermetallics 2005, 13, 1122–1133. [Google Scholar] [CrossRef]
- He, Y.M.; Lu, C.Y.; Ni, C.Y.; Chen, Q.X.; Zheng, W.J.; Wang, D.H.; Wei, L.F.; Wang, L.M.; Sun, Y.; Zou, H.; Gao, Z.L.; Yang, J.G. Tailoring microstructure and mechanical performance of the TC4 titanium alloy brazed joint through doping rare-earth element Dy into Ti-Cu-Ni filler alloy. J. Manuf. Process 2020, 50, 255–265. [Google Scholar] [CrossRef]
- Pint, P.A. On the formation of interfacial and internal voids in α-Al2O3 scales. Oxid. Met. 1997, 48, 303–328. [Google Scholar] [CrossRef]
- Wang, Z.H.; Li, F.G.; Hu, X.Y.; He, W.; Liu, Z.; Tan, Y. Preparation of Ti-Al-Si Gradient Coating Based on Silicon Concentration Gradient and Added-Ce. Coatings 2022, 12, 683. [Google Scholar] [CrossRef]
- Li, X.; Peng, X.Y.; Duan, Y.L.; Zhang, L.G.; Xu, G.F. Thermal oxidation behavior of commercial purity titanium at high temperature. Chin. J. Nonferrous Metals 2013, 23, 2190–2199. [Google Scholar]
- Zhao, A.L.; Wang, D.Y.; Wang, Y.; Gao, X.Y.; Hu, J. The comparison of thermal oxidation kinetics for pure titanium and titanium alloy. Titan. Ind. Prog. 2013, 30, 16–19. [Google Scholar]
- Jeng, S.C. Oxidation behavior and microstructural evolution of hot-dipped aluminum coating on Ti-6Al-4V alloy at 800 °C. Surf. Coat. Technol. 2013, 235, 867–874. [Google Scholar] [CrossRef]
- Mei, X.L. Fabrication of the Low Oxygen Partial Pressure Fusing Coatings Formed on Ti-6Al-4V Alloy and the Performances Analysis.
- Chen, C.; Feng, X.M.; Shen, Y.F. Oxidation behavior of a high Si content Al–Si composite coating fabricated on Ti–6Al–4V substrate by mechanical alloying method. J. Alloys and Compd. 2017, 701, 27–36. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Zhou, W.; Peng, X.; Liang, Y.H.; Qin, Q.D. Low oxygen pressure self-fused Al-Cr coatings formed on surface of Ti alloy and their oxidation resistance. J. Jilin Univ. Eng. Technol. Ed. 2004, 34, 521–526. [Google Scholar]
- Zhou, W.; Zhao, Y.G.; Qin, Q.D.; Li, W.; Xu, B. A new way to produce Al + Cr coating on Ti alloy by vacuum fusing method and its oxidation resistance. Mater. Sci. Eng. A 2006, 430, 254–259. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, F.; Cui, X.Y.; Wang, J.Q.; Xiong, T.Y. Long-term oxidation behavior of silicon-aluminizing coating with an in-situ formed Ti5Si3 diffusion barrier on γ-TiAl alloy. Appl. Surf. Sci. 2022, 582, 152444. [Google Scholar] [CrossRef]
- Dai, J.J.; Zhang, F.Y.; Wang, A.M.; Yu, H.J.; Chen, C.Z. Microstructure and properties of Ti-Al coating and Ti-Al-Si system coatings on Ti-6Al-4V fabricated by laser surface alloying. Surf. Coat. Technol. 2017, 309, 805–813. [Google Scholar] [CrossRef]
- Li, X.T.; Huang, L.J.; Jiang, S.; Gao, Y.N.; An, Q.; Wang, S.; Zhang, R.; Geng, L. Microstructure and super oxidation resistance of the network structured Ti-Al-Si coating. J. Alloys Compd. 2019, 807, 151679. [Google Scholar] [CrossRef]
- Oukati Sadeq, F.; Sharifitabar, M.; Shafiee Afarani, M. Synthesis of Ti–Si–Al coatings on the surface of Ti–6Al–4V alloy via hot dip siliconizing route. Surf. Coat. Technol. 2018, 337, 349–356. [Google Scholar] [CrossRef]
- Zeng, S.Q.; Li, F.G. Study on the Influence Law of Ce on Microstructure and High-Temperature Oxidation Resistance of Ti-Al-Si Composite Coating. Coatings 2023, 13(7), 1244. [Google Scholar] [CrossRef]
- Zhang, C.J.; Zhang, S.Z.; Liu, Z.G.; Chen, Y.Y.; Chai, L.H.; Wang, X.P. Improvement of cyclic oxidation resistance of Y-containing Ti–6Al–2.5Sn–4Zr–0.7Mo–0.3Si alloys. J. Alloys Compd. 2015, 624, 108–115. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Zhou, B.; Liu, N.; Chen, L.Q. Effects of Microstructure and Rare-Earth Constituent on the Oxidation Behavior of Ti–5.6Al–4.8Sn–2Zr–1Mo–0.35Si–0.7Nd Titanium Alloy. Oxid. Met. 2014, 81, 373–382. [Google Scholar] [CrossRef]
- Zhong, X.Y.; Deng, T.S.; Xiao, W.L.; Zhong, M.; Lai, Y.H.; Ojo, O.A. Effect of minor Sc modification on the high-temperature oxidation behavior of near-α Ti alloy. Corros. Sci. 2023, 217, 111122. [Google Scholar] [CrossRef]
- Tan, Y.M.; Fang, H.Z.; Chen, R.R.; Liu, Y.L.; Su, Y.Q.; Guo, J.J.; Cui, H.Z.; Zhang, S.Y.; Fu, H.Z. Microalloying effects of Ho on microstructure evolution and high temperature properties of Ti46Al4Nb1Mo alloy. Intermetallics 2020, 126, 106883. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, Y.G.; Qin, Q.D.; Li, W.; Xu, B. Effect of pre-oxidation on aluminized coating and their oxidation resistance of Ti alloy. Mater. Lett. 2006, 60, 414–417. [Google Scholar] [CrossRef]
- Goward, G.W.; Boone, D.H. Mechanisms of formation of diffusion aluminide coatings on nickel-base superalloys. Oxid. Met. 1971, 3, 475–495. [Google Scholar] [CrossRef]
- Das, D.K.; Joshi, S.V.; Singh, V. Evolution of aluminide coating microstructure on nickel-base cast superalloy CM-247 in a single-step high-activity aluminizing process. Metall. Mater. Trans. A 1998, 29, 2173–2188. [Google Scholar] [CrossRef]
- Jacobson, N.S. Corrosion of Silicon-Based Ceramics in Combustion Environments. J. Am. Ceram. Soc. 1993, 76, 3–28. [Google Scholar] [CrossRef]
- Say, W.C.; Wu, J.K.; Chen, W.L. Hot corrosion ofα-SiC ceramics by V2O5 melt. J. Mater. Sc. 1990, 25, 1614–1617. [Google Scholar] [CrossRef]
- Steinmetz, P.; Duret, C.; Morbioli, R. Laboratory tests for hot-corrosion studies. Mater. Sci. Technol. 1986, 2, 262–271. [Google Scholar] [CrossRef]
- Xu, H.B.; Guo, H.B.; Liu, F.S.; Gong, S.K. Development of gradient thermal barrier coatings and their hot-fatigue behavior. Surf. Coat. Technol. 2000, 130, 133–139. [Google Scholar] [CrossRef]
- Molins, R.; Hou, P.Y. Characterization of chemical and microstructural evolutions of a NiPtAl bondcoat during high temperature oxidation. Surf. Coat. Technol. 2006, 201, 3841–3845. [Google Scholar] [CrossRef]
- Wang, Y.S.; Xiong, J.; Yan, J.; Fan, H.Y.; Wang, J. Oxidation resistance and corrosion behavior of hot-dip aluminized coatings on commercial-purity titanium. Surf. Coat. Technol. 2011, 206, 1277–1282. [Google Scholar] [CrossRef]
- Rhys-Jones, T.N.; Swindells, N. The high temperature corrosion of a commercial aluminide coating on IN738-LC and MarMOO2 at 700°C and 830°C. Corros. Sci. 1985, 25, 559–576. [Google Scholar] [CrossRef]
- Shirvani, K.; Saremi, M.; Nishikata, A.; Tsuru, T. Electrochemical study on hot corrosion of Si-modified aluminide coated In-738LC in Na2SO4–20wt.% NaCl melt at 750 °C. Corros. Sci. 2003, 45, 1011–1021. [Google Scholar] [CrossRef]
- Hou, P.Y.; McCarty, K.F. Surface and interface segregation in β-NiAl with and without Pt addition. Scripta Mater. 2006, 54, 937–941. [Google Scholar] [CrossRef]
- Khan, A.; Song, P.; Huang, T.H.; Zhou, Y.; Xiong, X.P.; Li, C.; Lü, J.G.; Chen, R.; Lu, J.S. Diffusion characteristics and structural stability of Pt modified β-NiAl/γ′-Ni3Al within NiCoCrAl alloy at high temperature. Appl. Surf. Sci. 2019, 476, 1096–1107. [Google Scholar] [CrossRef]
- Li, C.; Song, P.; Feng, J.; Huang, T.H.; Lü, K.Y.; Li, Q.L.; Duan, W.H.; Khan, A.; Zhai, R.X.; Lu, J.S. Alumina growth behaviour on the surface-modified NiCoCrAl alloy by Pt and Hf at high temperature. Appl. Surf. Sci. 2019, 479, 1178–1191. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, P.S. High-resolution, real-time three-dimensional shape measurement. Opti. Eng. 2006, 45, 123601. [Google Scholar] [CrossRef]
- Montero, X.; Galetz, M.C.; Schütze, M. Sulphidation Behavior of a Non Harmful Water-Based Al and Al–Si Slurry Coating on CM247 Superalloy. Oxid. Met. 2013, 80, 635–649. [Google Scholar] [CrossRef]
- Zang, J.J.; Song, P.; Feng, J.; Xiong, X.P.; Chen, R.; Liu, G.L.; Lu, J.S. Oxidation behaviour of the nickel-based superalloy DZ125 hot-dipped with Al coatings doped by Si. Corros. Sci. 2016, 112, 170–179. [Google Scholar] [CrossRef]
- Fu, C.; Kong, W.K.; Cao, G.H. Microstructure and oxidation behavior of Al + Si co-deposited coatings on nickel-based superalloys. Surf. Coat. Technol. 2014, 258, 347–352. [Google Scholar] [CrossRef]
- Meng, X.X.; Yuwen, P.; Shao, W.; Qu, W.T.; Zhou, C.G. Cyclic oxidation behaviour of Co/Si co-doped β-NiAl coating on nickel based superalloys. Corros. Sci. 2018, 133, 112–119. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, Y.J.; Liang, J.; Zhang, X.Y.; Zha, X.Q.; Zhang, L.J. Fatigue crack propagation of copper alloy ZCuAl8Mnl4Fe3Ni2 for propeller. Develop. Appli. Mater. 2010, 5. [Google Scholar]
- Dai, P.C.; Wu, Q.; Ma, Y.; Li, S.S.; Gong, S.K. The effect of silicon on the oxidation behavior of NiAlHf coating system. Appl. Surf. Sci. 2013, 271, 311–316. [Google Scholar] [CrossRef]
- Lee, D.B.; Kim, D.J. The oxidation of Ni3Al containing decomposed SiC-particles. Intermetallics 2001, 9, 51–56. [Google Scholar] [CrossRef]
- Li, X.T.; An, Q.; Huang, L.J.; Zhang, R.; Chen, X.; Chen, R.; Sun, F.B.; Geng, L. The hot corrosion behaviors and mechanisms of hot-dipped Ti-Al-Si coatings on TiBw/Ti6Al4V composite against NaCl and Na2SO4. J. Alloys Compd. 2022, 921, 166151. [Google Scholar] [CrossRef]
- Dai, J.J.; Zhang, H.X.; Sun, C.X.; Li, S.Y.; Chen, C.Z.; Yang, Y. The effect of Nb and Si on the hot corrosion behaviors of TiAl coatings on a Ti-6Al-4V alloy. Corros. Sci. 2020, 168, 108578. [Google Scholar] [CrossRef]
- Lin, H.; Liang, W.P.; Jia, Y.L.; Miao, Q.; Hu, R.Y.; Ding, Z.; Yu, L.J. Effect of Al -Y gradient coating on hot corrosion resistance of γ-TiAl alloy at different temperatures. Appl. Surf. Sci. 2019, 487, 868–875. [Google Scholar] [CrossRef]
- Hu, Y.T.; Zheng, L.; Yan, H.J.; Wu, L.K.; Lin, X.J.; Cao, F.H.; Jiang, M.Y. Improving hot corrosion resistance of aluminized TiAl alloy by anodization and pre-oxidation. Trans. Nonferrous Met. Soc. China 2021, 31, 193–206. [Google Scholar] [CrossRef]
- Ceschini, L.; Lanzoni, E.; Martini, C.; Prandstraller, D.; Sambogna, G. Comparison of dry sliding friction and wear of Ti6Al4V alloy treated by plasma electrolytic oxidation and PVD coating. Wear 2008, 264, 86–95. [Google Scholar] [CrossRef]
- Molinari, A.; Straffelini, G.; Tesi, B.; Bacci, T. ; Dry sliding wear mechanisms of the Ti6Al4V alloy. Wear 1997, 208, 105–112. [Google Scholar] [CrossRef]
- Buckley, D.H.; Miyoshi, K. Friction and wear of ceramics. Wear 1984, 100, 333–353. [Google Scholar] [CrossRef]
- Dong, H.; Bell, T. Enhanced wear resistance of titanium surfaces by a new thermal oxidation treatment. Wear 2000, 238, 131–137. [Google Scholar] [CrossRef]
- Du, Y.J.; Rao, K.P.; Chung, J.C.Y.; Han, X.D. Phase transitions in reactive formation of Ti5Si3/TiAl in situ composites. Metall. Mater. Trans. A 2000, 31, 763–771. [Google Scholar] [CrossRef]
- Gizynski, M.; Miyazaki, S.; Sienkiewicz, J.; Kuroda, S.; Araki, H.; Murakami, H.; Pakiela, Z.; Yumoto, A. Formation and subsequent phase evolution of metastable Ti-Al alloy coatings by kinetic spraying of gas atomized powders. Surf. Coat. Technol. 2017, 315, 240–249. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.S.; Ma, Y.Z.; Liang, C.P.; Liu, C.; Zhang, C.; Cai, Q.S. Microstructure and wear resistance of compositionally graded Ti Al intermetallic coating on Ti6Al4V alloy fabricated by laser powder deposition. Surf. Coat. Technol. 2018, 353, 32–40. [Google Scholar] [CrossRef]
- Zhang, W.B.; Li, W.S.; Zhai, H.M.; Wu, Y.R.; Wang, S.C.; Liang, G.; Wood, R.J.K. Microstructure and tribological properties of laser in-situ synthesized Ti3Al composite coating on Ti-6Al-4V. Surf. Coat. Technol. 2020, 395, 125944. [Google Scholar] [CrossRef]
- Lazurenko, D.; Golkovsky, M.; Stark, A.; Pyczak, F.; Bataev, I.; Ruktuev, A.; Petrov, I.; Laptev, I. Structure and Properties of Ti-Al-Ta and Ti-Al-Cr Cladding Layers Fabricated on Titanium. Metals 2021, 11, 1139. [Google Scholar] [CrossRef]
- Huang, T.-d.; Wu, S.-y.; Jiang, H.; Lu, Y.-p.; Wang, T.-m.; Li, T.-j. Effect of Ti content on microstructure and properties of TixZrVNb refractory high-entropy alloys. Int. J. Miner., Metall. Mater. 2020, 27, 1318–1325. [Google Scholar] [CrossRef]
- Xiang, K.; Chen, L.-Y.; Chai, L.J.; Guo, N.; Wang, H. Microstructural characteristics and properties of CoCrFeNiNbx high-entropy alloy coatings on pure titanium substrate by pulsed laser cladding. Appl. Surf. Sci. 2020, 517, 146214. [Google Scholar] [CrossRef]
- Li, Z.H.; Chai, L.J.; Qi, L.; Wang, Y.Y.; Liu, Y.Z.; Yang, T.; Wang, H.; Guo, N.; Zhao, Y.X. Laser-cladded Al-Cr-Ti ternary alloy coatings on Ti-4Al-2V alloy: Specific microstructure and enhanced surface performance. Surf. Coat. Technol. 2023, 452, 129073. [Google Scholar] [CrossRef]
- Li, W.S.; Zhang, W.B.; Zhai, H.M.; Wang, S.C.; Song, Q.; Wood, R.J.K.; Cheng, B.; He, D.Q.; Zhang, C.Z. Microstructure evolution and elevated temperature wear performance of in-situ laser-synthesized Ti-25Al-17Nb coating on Ti-6Al-4V. Tribology Int. 2022, 175, 107807. [Google Scholar] [CrossRef]
- Zhao, J.H.; Shangguan, J.J.; Gao, L.S.; Gu, C.; Wang, Y.J.; Shi, Y. New Insights into Microstructure Characteristics and Tribological Property of Ti Alloy Processed by Hot-Dip Aluminizing and Heat Treatment. Metall. Mater. Trans. A 2022, 53, 1035–1050. [Google Scholar] [CrossRef]
- Yang, W.Y.; Li, F.G. Study on the Effect of Carburizing on the Microstructure and High-Temperature Oxidation Properties of Hot-Dip Aluminum Coating on Titanium Alloy. Coatings 2023, 13, 1336. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).