Submitted:
01 August 2023
Posted:
03 August 2023
You are already at the latest version
Abstract
Keywords:
Introduction
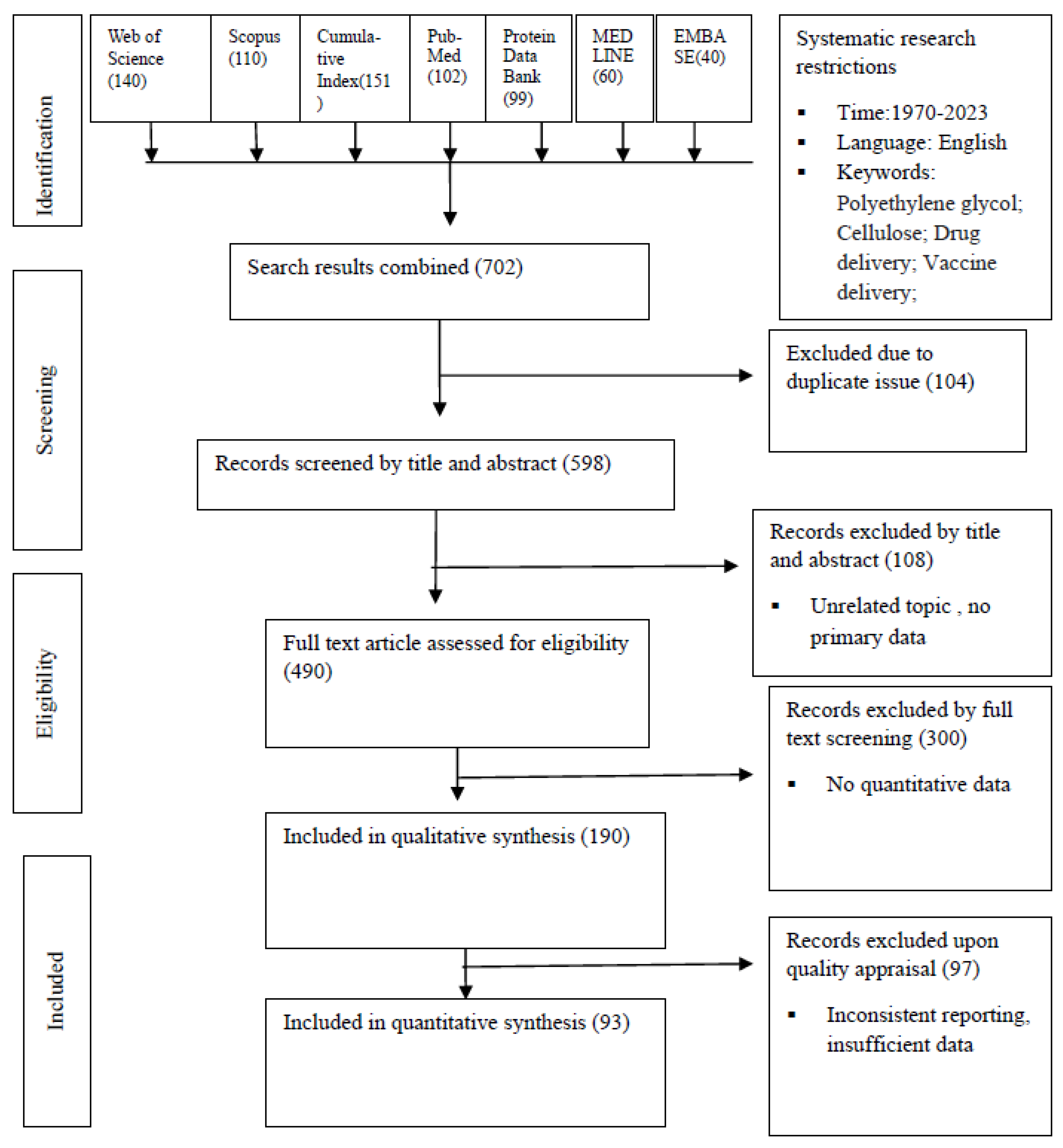
Methodology
Results and Discussion
Problems with PEG Polymers
Biocompatibility and biodegradability, properties of PEG-based and Cellulose-based polymer:
The pharmacokinetic properties of PEG and Cellulose-based polymer:
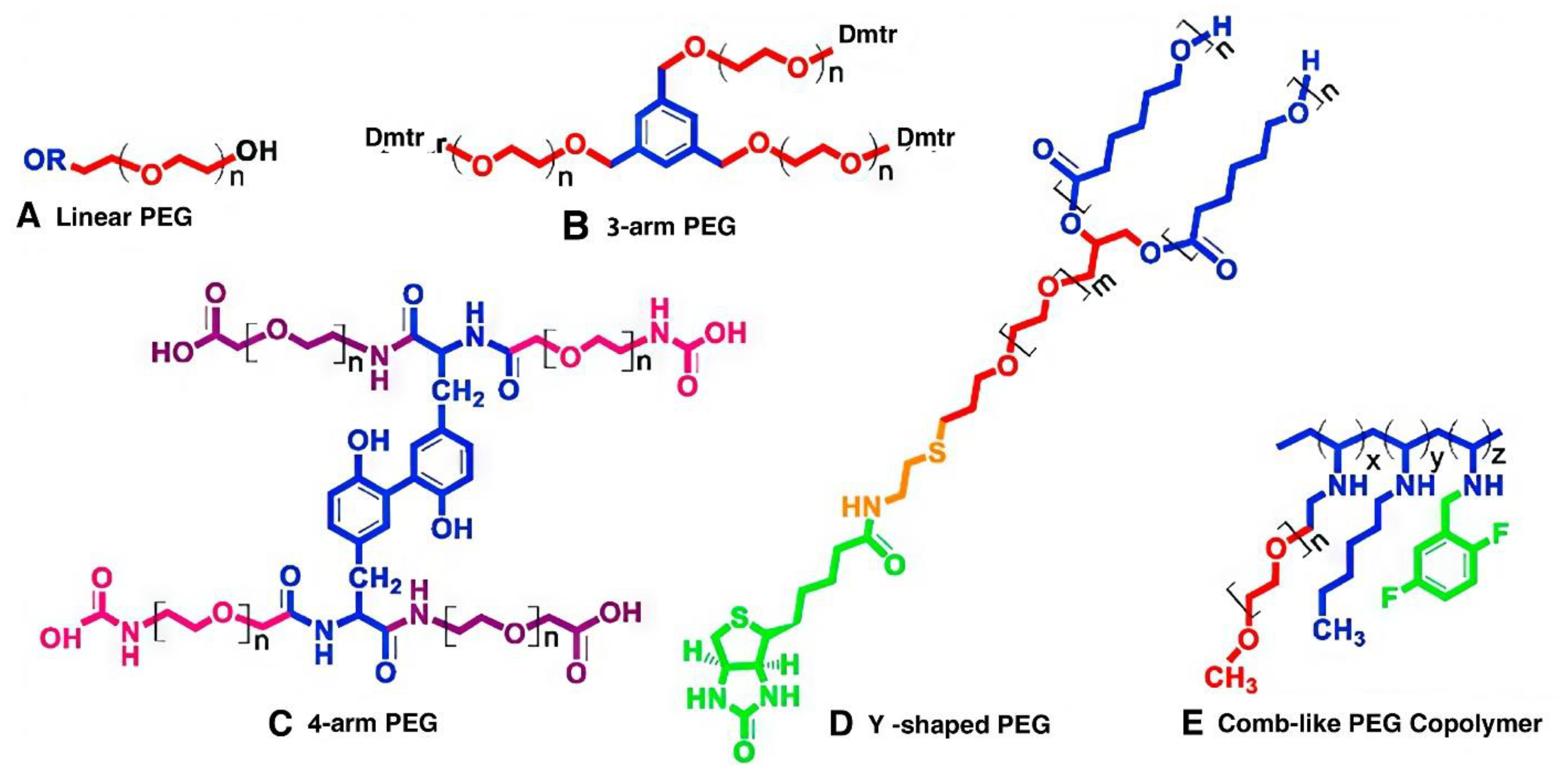
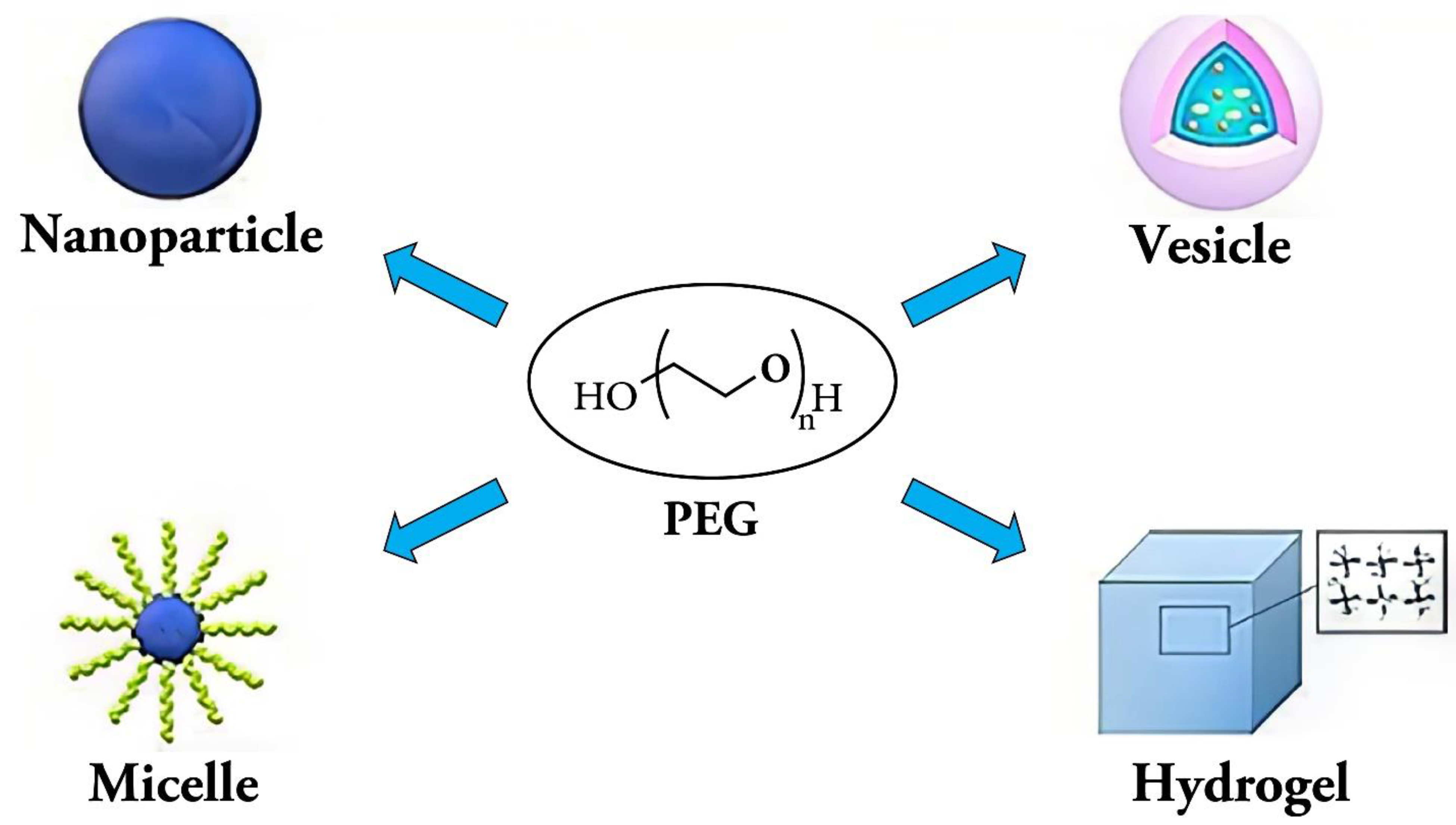
PEG-based polymer:
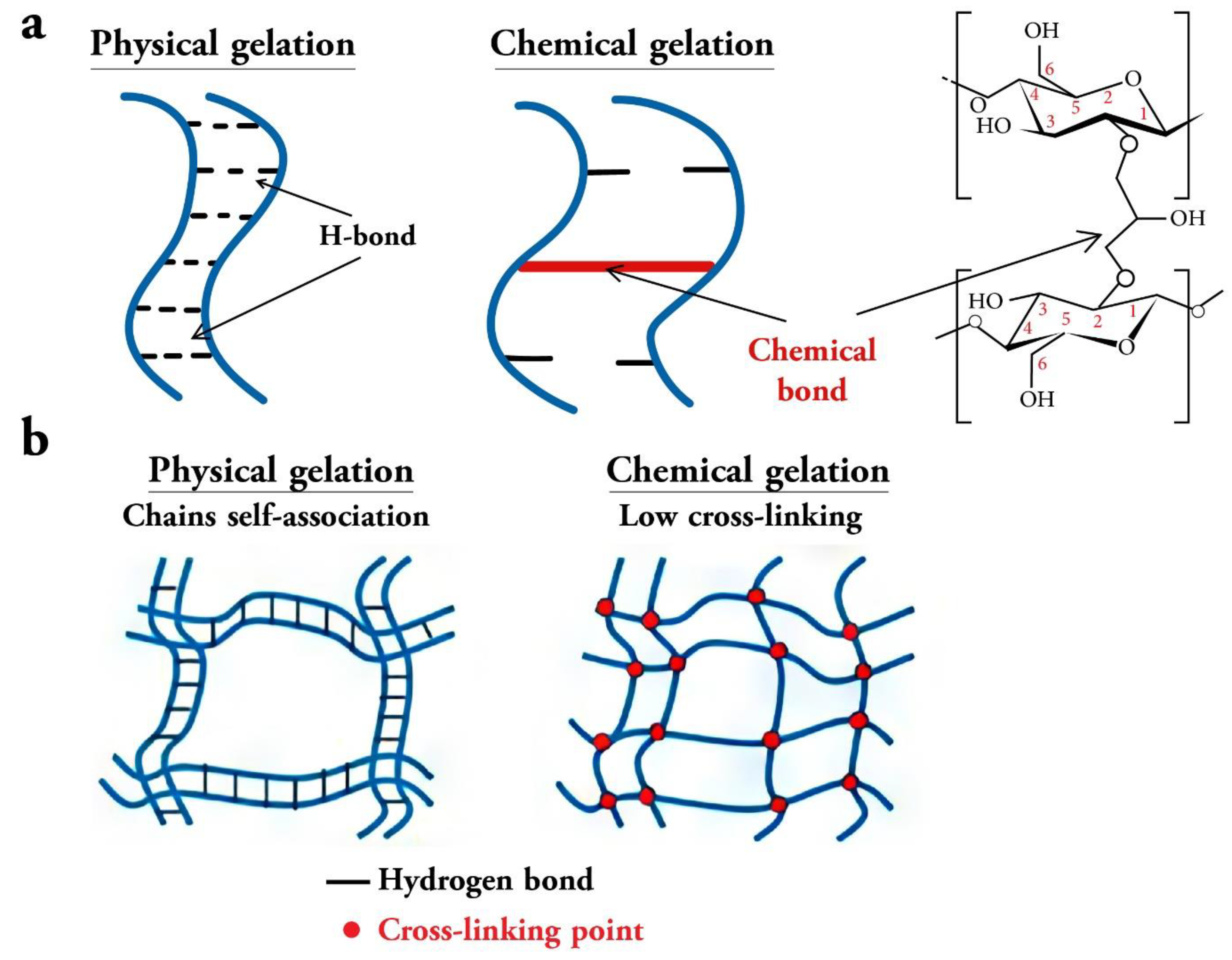
Cellulose-based Polymers:
Physicochemical properties of PEG and Cellulose Based Polymer:
Half-life, circulation time, maximum release, maximum percentage of loaded drug released, and the burst release properties of PEG and Cellulose based hydrogel:
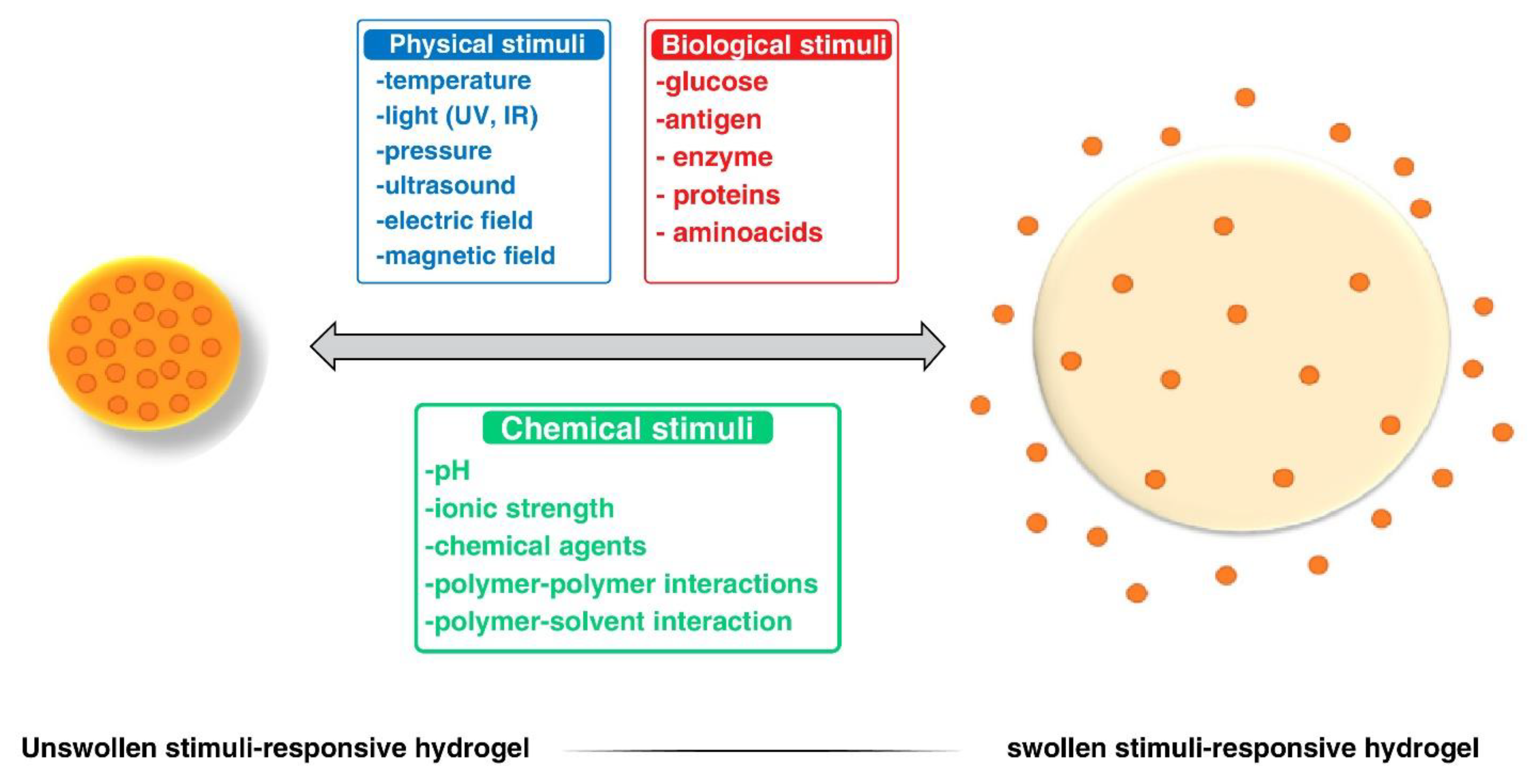
Double network hydrogels, injectable hydrogels, sliding hydrogels, conductive hydrogels, responsive hydrogels, and nanocomposite hydrogels:
Conclusion and Future Study Recommendation
Author Contributions
Funding
Conflicts of Interest
References
- Rappuoli, R.; Mandl, C.W.; Black, S.; De Gregorio, E. Vaccines for the twenty-first century society. Nat. Rev. Immunol. 2011, 11, 865–872. [Google Scholar] [CrossRef]
- Mamo, T.; Poland, G.A. Nanovaccinology: The next generation of vaccines meets 21st century materials science and engineering. Vaccine 2012, 30, 6609–6611. [Google Scholar] [CrossRef]
- Han, J.; Zhao, D.; Li, D.; Wang, X.; Jin, Z.; Zhao, K. Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers 2018, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Gale, E.C.; Alcántara-Hernández, M.; Luo, W.; Axpe, E.; Verma, R.; Yin, Q.; Yu, A.C.; Hernandez, H.L.; Maikawa, C.L.; et al. Injectable Hydrogels for Sustained Codelivery of Subunit Vaccines Enhance Humoral Immunity. ACS Central Sci. 2020, 6, 1800–1812. [Google Scholar] [CrossRef]
- Doherty, M.; Buchy, P.; Standaert, B.; Giaquinto, C.; Prado- Cohrs, D. Vaccine impact: Benefits for human health. Vaccine 2016, 34, 6707–6714. [Google Scholar] [CrossRef] [PubMed]
- Jahan, N.; Archie, S.R.; Al Shoyaib, A.; Kabir, N.; Cheung, K. Recent Approaches for Solid Dose Vaccine Delivery. Sci. Pharm. 2019, 87, 27. [Google Scholar] [CrossRef]
- Govindarajan, D.; Meschino, S.; Guan, L.; Clements, D.E.; ter Meulen, J.H.; Casimiro, D.R.; Coller, B.-A.G.; Bett, A.J. Preclinical development of a dengue tetravalent recombinant subunit vaccine: Immunogenicity and protective efficacy in nonhuman primates. Vaccine 2015, 33, 4105–4116. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, J.; Xu, S.; Shi, J.; Xu, S.; Wu, X.; Fu, F.; Peng, Z.; Zhang, L.; Zheng, S.; et al. Immunogenicity of porcine circovirus type 2 nucleic acid vaccine containing CpG motif for mice. Virol. J. 2016, 13, 185. [Google Scholar] [CrossRef]
- Sergeyev, O.; Barinsky, I. , Synthetic peptide vaccines. Vopr. Virusol. 2016, 61, 5–8. [Google Scholar] [CrossRef]
- Apte, S.H.; Stephenson, R.J.; Simerska, P.; Groves, P.; Aljohani, S.; Eskandari, S.; Toth, I.; Doolan, D.L. Systematic evaluation of self-adjuvantinglipopeptidenano-vaccine platforms for the induction of potent CD8(+) T-cell responses. Nanomedicine 2016, 11, 137–152. [Google Scholar] [CrossRef]
- Haynes, B.F. New approaches to HIV vaccine development. Curr. Opin. Immunol. 2015, 35, 39–47. [Google Scholar] [CrossRef]
- Junter, G.-A.; Karakasyan, C. Polysaccharides against Viruses: Immunostimulatory Properties and the Delivery of Antiviral Vaccines and Drugs. Crit. Rev. Ther. Drug Carr. Syst. 2020, 37, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Ewald, A.; Lochner, B.; Gbureck, U.; Groll, J.; Krüger, R. Structural Optimization of Macroporous Magnesium Phosphate Scaffolds and their Cytocompatibility. Key Eng. Mater. 2011, 493-494, 813–819. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zhang, J.; Wang, L.; Ou, C.; Shu, Y.; Wu, Q.; Ma, G.; Gong, C. Gambogic acid-encapsulated polymeric micelles improved therapeutic effects on pancreatic cancer. Chin. Chem. Lett. 2019, 30, 885–888. [Google Scholar] [CrossRef]
- He, T.; Liang, X.; Li, L.; Gong, S.; Li, X.; Zhang, M.; Zhu, S.; Xiao, H.; Wu, Q.; Gong, C. A spontaneously formed and self-adjuvanted hydrogel vaccine triggers strong immune responses. Mater. Des. 2020, 197, 109232. [Google Scholar] [CrossRef]
- Lakshmi, P.; Bhaskaran, S.; Saroja, C. Recent trends in vaccine delivery systems: A review. Int. J. Pharm. Investig. 2011, 1, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Pagels, R.F.; Prud’Homme, R.K. Polymeric nanoparticles and microparticles for the delivery of peptides, biologics, and soluble therapeutics. J. Control. Release 2015, 219, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, C.; Thakur, A.; Krajišnik, D.; Guyon, R.; Longet, S.; Razim, A.; Górska, S.; Pantelić, I.; Ilić, T.; Nikolić, I.; et al. Technological Approaches for Improving Vaccination Compliance and Coverage. Vaccines 2020, 8, 304. [Google Scholar] [CrossRef]
- D souza AA, Shegokar R Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv 2016, 13, 1257–1275. [CrossRef]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discovery Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Alconcel, S.N.; Baas, A.S.; Maynard, H.D. FDA-approved poly (ethylene glycol)–protein conjugate drugs. Polymer Chemistry 2011, 2, 1442–1448. [Google Scholar] [CrossRef]
- Gupta, V.; Bhavanasi, S.; Quadir, M.; Singh, K.; Ghosh, G.; Vasamreddy, K.; Ghosh, A.; Siahaan, T.J.; Banerjee, S.; Banerjee, S.K. Protein PEGylation for cancer therapy: bench to bedside. J. Cell Commun. Signal. 2019, 13, 319–330. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.; Boyce, A.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Aarstad, O.; Heggset, E.B.; Pedersen, I.S.; Bjørnøy, S.H.; Syverud, K.; Strand, B.L. Mechanical Properties of Composite Hydrogels of Alginate and Cellulose Nanofibrils. Polymers 2017, 9, 378. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J. Cellulose and Its Derivatives: Towards Biomedical Applications; Springer: Amsterdam, The Netherlands, 2021; Volume 28, ISBN 1057002003. [Google Scholar]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Jeong, D.; Joo, S.; Hu, Y.; Vilas, V.; Cho, E.; Jung, S. Carboxymethyl Cellulose-Based Superabsorbent Hydrogels Containing Carboxymehtyl _ -Cyclodextrin for Enhanced Mechanical Strength and e FfEctive Drug Delivery. Eur. Polym. J. 2018, 105, 17–25. [Google Scholar] [CrossRef]
- Barbucci, R.; Magnani, A.; Consumi, M. Swelling Behavior of Carboxymethylcellulose Hydrogels in Relation to Cross-Linking, pH, and Charge Density. Macromolecules 2000, 33, 7475–7480. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: a review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Suflet, D.M. 11—Cellulose-Based Hydrogels for Medical/Pharmaceutical Applications; Elsevier B.V.: Amsterdam, The Netherlands, 2019; ISBN 9780444637741. [Google Scholar]
- Basu, P.; Narendrakumar, U.; Arunachalam, R.; Devi, S.; Manjubala, I. Characterization and Evaluation of Carboxymethyl Cellulose-Based Films for Healing of Full-Thickness Wounds in Normal and Diabetic Rats. ACS Omega 2018, 3, 12622–12632. [Google Scholar] [CrossRef] [PubMed]
- Priya, G.; Madhan, B.; Narendrakumar, U.; Kumar, R.V.S.; Manjubala, I. In Vitro and In Vivo Evaluation of Carboxymethyl Cellulose Scaffolds for Bone Tissue Engineering Applications. ACS Omega 2021, 6, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Capanema, N.S.V.; Mansur, A.A.P.; de Jesus, A.C.; Carvalho, S.M.; de Oliveira, L.C.; Mansur, H.S. Superabsorbent CrosslinkedCarboxymethyl Cellulose-PEG Hydrogels for Potential Wound Dressing Applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef]
- Verbeke, C.S.; Mooney, D.J. Injectable, Pore-Forming Hydrogels for In Vivo Enrichment of Immature Dendritic Cells. Adv. Heal. Mater. 2015, 4, 2677–2687. [Google Scholar] [CrossRef] [PubMed]
- Falconer, J.L.; Christie, R.J.; Pollard, E.J.; Olsen, S.C.; Grainger, D.W. Live RB51 vaccine lyophilized hydrogel formulations with increased shelf life for practical ballistic delivery. Int. J. Pharm. 2016, 498, 187–194. [Google Scholar] [CrossRef]
- Ishii-Mizuno, Y.; Umeki, Y.; Onuki, Y.; Watanabe, H.; Takahashi, Y.; Takakura, Y.; Nishikawa, M. Improved sustained release of antigen from immunostimulatory DNA hydrogel by electrostatic interaction with chitosan. Int. J. Pharm. 2017, 516, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Z.; Mei, C.; Jiang, Z.; Feng, Y.; Gao, R.; Wang, Q.; Huang, J. Protein Enables Conformation Transition of a Hydrogel Based on Pentapeptide and Boosts Immune Response in Vivo. Bioconjugate Chem. 2018, 29, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Alford, A.; Tucker, B.; Kozlovskaya, V.; Chen, J.; Gupta, N.; Caviedes, R.; Gearhart, J.; Graves, D.; Kharlampieva, E. Encapsulation and Ultrasound-Triggered Release of G-Quadruplex DNA in Multilayer Hydrogel Microcapsules. Polymers 2018, 10, 1342. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Wu, X.; Olson, V.; D’Souza, M. Characterization of rabies pDNAnanoparticulate vaccine in poloxamer 407 gel. Int. J. Pharm. 2018, 545, 318–328. [Google Scholar]
- Nomura, D.; Saito, M.; Takahashi, Y.; Takahashi, Y.; Takakura, Y.; Nishikawa, M. Development of orally-deliverable DNA hydrogel by microemulsification and chitosan coating. Int. J. Pharm. 2018, 547, 556–562. [Google Scholar] [CrossRef]
- Gupta, D.; Gangwar, A.; Jyoti, K.; Jyothi, V.G.S.; Sodhi, R.K.; Mehra, N.K.; Singh, S.B.; Madan, J. Self healing hydrogels: A new paradigm immunoadjuvant for delivering peptide vaccine. Colloids Surfaces B: Biointerfaces 2020, 194, 111171. [Google Scholar] [CrossRef]
- Zou, Y.; Li, S.; Ngai, T.; Zhang, S.; Ma, G.; Wu, J. Green preparation of hydrogel particles-in-emulsions for simultaneous enhancement of humoral and cell-mediated immunity. Eng. Life Sci. 2020, 20, 514–524. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Ye, T.; Li, F.; Gao, X.; Wang, Y.; Ye, P.; Qing, S.; Wang, C.; Yue, H.; et al. Choice of Nanovaccine Delivery Mode Has Profound Impacts on the Intralymph Node Spatiotemporal Distribution and Immunotherapy Efficacy. Adv. Sci. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Gamal, W.; Menon, I.J.; Olson, V.; Wu, X.; D’Souza, M.J. Laser-assisted skin delivery of immunocontraceptive rabies nanoparticulate vaccine in poloxamer gel. Eur. J. Pharm. Sci. 2020, 155, 105560. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Gupta, R.K.; Deshpande, M.C.; Schwendeman, S.P. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv. Drug Deliv. Rev. 2005, 57, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.-F.; Sexton, A.; De Rose, R.; Kent, S.J.; Zelikin, A.N.; Caruso, F. A paradigm for peptide vaccine delivery using viral epitopes encapsulated in degradable polymer hydrogel capsules. Biomaterials 2009, 30, 5178–5186. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Hong, S.J.; Lee, Y.-K.; Cho, S. Oral Vaccine Delivery for Intestinal Immunity—Biological Basis, Barriers, Delivery System, and M Cell Targeting. Polymers 2018, 10, 948. [Google Scholar] [CrossRef]
- Nishiguchi, T. Taguchi, Sustained-immuno stimulatory nanocellulose scaffold to enhance vaccine efficacy, Biomed. Mater. Res. 108A (2020) 159–1170.
- Harris, J.M.; Martin, N.E.; Modi, M. Pegylation: a novel process for modifying pharmacokinetics. Clin. Pharmacokinet 2001, 40, 539–551. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Li, W.; Zhan, P.; De Clercq, E.; Lou, H.; Liu, X. Current drug research on PEGylation with small molecular agents. Prog. Polym. Sci. 2013, 38, 421–444. [Google Scholar] [CrossRef]
- Brandt, J.V.; Piazza, R.D.; dos Santos, C.C.; Chacon, J.V.; Amantéa, B.E.; Pinto, G.C.; Magnani, M.; Piva, H.L.; Tedesco, A.C.; Primo, F.L.; et al. Synthesis and colloidal characterization of folic acid-modified PEG-b-PCL Micelles for methotrexate delivery. Colloids Surf. B Biointerfaces 2019, 177, 228–234. [Google Scholar] [CrossRef]
- Veronese, F.M. Peptide and protein PEGylation: a review of problems and solutions. Biomaterials 2001, 22, 405–417. [Google Scholar] [CrossRef]
- Wennink, J.; Signori, F.; Karperien, M.; Bronco, S.; Feijen, J.; Dijkstra, P. Introducing small cationic groups into 4-armed PLLA–PEG copolymers leads to preferred micellization over thermo-reversible gelation. Polymer 2013, 54, 6894–6901. [Google Scholar] [CrossRef]
- Y. Yu, Y. Ma, J. Yin, C. Zhang, G. Feng, Y. Zhang, J. Meng, Tuning the microphase separation of the PES-g-PEG comb-like copolymer membrane for efficient CO2 separation, Separ. Purif.Technol. (2021). [CrossRef]
- J.Z. Wang, M.L. You, Z.Q. Ding, W.B. Ye, A review of emerging bone tissue engineering via PEG conjugated biodegradable amphiphilic copolymers, Mater SciEng C Mater BiolAppl 97 (2019) 1021–1035. [CrossRef]
- Wang, X.; Zhang, Y.; Xue, W.; Wang, H.; Qiu, X.; Liu, Z. Thermo-sensitive hydrogel PLGA-PEG-PLGA as a vaccine delivery system for intramuscular immunization. J. Biomater. Appl. 2017, 31, 923–932. [Google Scholar] [CrossRef]
- Christie, R.; Findley, D.; Dunfee, M.; Hansen, R.; Olsen, S.; Grainger, D. Photopolymerized hydrogel carriers for live vaccine ballistic delivery. Vaccine 2006, 24, 1462–1469. [Google Scholar] [CrossRef]
- A. S. Sawhney, C.P. Pathak, J.A. Hubbell, Bioerodible hydrogels based on photopolymerizedpoly(ethylene glycol)-co-poly(-hydroxy acid) diacrylatemacromers. Macromolecules 1993, 26, 581–587. [Google Scholar] [CrossRef]
- Lu, S.; Anseth, K.S. Release Behavior of High Molecular Weight Solutes from Poly(ethylene glycol)-Based Degradable Networks. Macromolecules 2000, 33, 2509–2515. [Google Scholar] [CrossRef]
- Bryant, S.J.; Anseth, K.S. The effects of scaffold thickness on tissue engineered cartilage in photocrosslinked poly (ethylene oxide) hydro-gels. Biomaterials 2001, 22, 619–626. [Google Scholar] [CrossRef]
- Wu, Q.; Gong, C.; Shi, S.; Wang, Y.; Huang, M.; Yang, L.; Zhao, X.; Wei, Y.; Qian, Z. Mannan Loaded Biodegradable and Injectable Thermosensitive PCL-PEG-PCL Hydrogel for Vaccine Delivery. Soft Mater. 2012, 10, 472–486. [Google Scholar] [CrossRef]
- Bobbala, S.; Tamboli, V.; McDowell, A.; Mitra, A.K.; Hook, S. Novel Injectable Pentablock Copolymer Based Thermoresponsive Hydrogels for Sustained Release Vaccines. AAPS J. 2016, 18, 261–269. [Google Scholar] [CrossRef]
- Song, H.; Yang, P.; Huang, P.; Zhang, C.; Kong, D.; Wang, W. Injectable polypeptide hydrogel-based co-delivery of vaccine and immune checkpoint inhibitors improves tumor immunotherapy. Theranostics 2019, 9, 2299–2314. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ding, Y.; Zhang, L.; Zhang, Y.; Liu, J.; et al. Polyethylene glycol (PEG)-related controllable and sustainable antidiabetic drug delivery systems. European Journal of Medicinal Chemistry 2021, 217, 113372. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Irani, M.; Esmaeilkhanian, A.; Bazli, L.; Asl, M.S.; Jang, H.W.; Kim, S.Y.; Ramakrishna, S.; Shokouhimehr, M.; Varma, R.S. Polymer incorporated magnetic nanoparticles: Applications for magnetoresponsive targeted drug delivery. Mater. Sci. Eng. B 2021, 272. [Google Scholar] [CrossRef]
- Ghauri, Z.H.; Islam, A.; Qadir, M.A.; Ghaffar, A.; Gull, N.; et al. Novel pH- responsive chitosan/sodium alginate/PEG based hydrogelsfor release of sodium ceftriaxone. Materials Chemistry and Physics 2022, 277, 125456. [Google Scholar] [CrossRef]
- Pervaiz, F.; Tanveer, W.; Shoukat, H.; Rehman, S. Formulation and evaluation of polyethylene glycol/Xanthan gum-co-poly (Acrylic acid) interpenetrating network for controlled release of venlafaxine. Polym. Bull. 2022, 80, 469–493. [Google Scholar] [CrossRef]
- Iovene, A.; Zhao, Y.; Wang, S.; Amoako, K. Bioactive Polymeric Materials for the Advancement of Regenerative Medicine. J. Funct. Biomater. 2021, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Dethe, M.R.; A, P.; Ahmed, H.; Agrawal, M.; Roy, U.; Alexander, A. PCL-PEG copolymer based injectable thermosensitive hydrogels. J. Control. Release 2022, 343, 217–236. [Google Scholar] [CrossRef]
- Lin, C.-C.; Anseth, K.S. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Tourné-Péteilh, C.; Barège, M.; Lions, M.; Martinez, J.; Devoisselle, J.-M.; Aubert-Pouessel, A.; Subra, G.; Mehdi, A. Encapsulation of BSA in hybrid PEG hydrogels: stability and controlled release. RSC Adv. 2021, 11, 30887–30897. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.E.; Graf, M.; Beck, S.; Goepferich, A.M. A novel anhydrous preparation of PEG hydrogels enables high drug loading with biologics for controlled release applications. Eur. Polym. J. 2021, 147, 110286. [Google Scholar] [CrossRef]
- Yadav, D.; Dewangan, H.K. PEGYLATION: an important approach for novel drug delivery system. J. Biomater. Sci. Polym. Ed. 2020, 32, 266–280. [Google Scholar] [CrossRef]
- Veronese FM (Ed.), PEGylated protein drugs: basic science and clinical applications. Springer Science & Business Media, Germany, (2009).
- Tong, X.; Lee, S.; Bararpour, L.; Yang, F. Long-Term Controlled Protein Release from Poly(Ethylene Glycol) Hydrogels by Modulating Mesh Size and Degradation. Macromol. Biosci. 2015, 15, 1679–1686. [Google Scholar] [CrossRef]
- Kolate, A.; Baradia, D.; Patil, S.; Vhora, I.; Kore, G.; Misra, A. PEG — A versatile conjugating ligand for drugs and drug delivery systems. J. Control. Release 2014, 192, 67–81. [Google Scholar] [CrossRef]
- Li, R.; Chen, Z.; Dai, Z.; Yu, Y. Nanotechnology assisted photo- and sonodynamic therapy for overcoming drug resistance. Cancer Biol. Med. 2021, 18, 388–400. [Google Scholar] [CrossRef]
- Sylvestre, M.; Lv, S.; Yang, L.F.; Luera, N.; Peeler, D.J.; Chen, B.-M.; Roffler, S.R.; Pun, S.H. Replacement of L-amino acid peptides with D-amino acid peptides mitigates anti-PEG antibody generation against polymer-peptide conjugates in mice. J. Control. Release 2021, 331, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Wang, Z.; Zhou, J.; Wang, Y. Additive-free preparation of hemodialysis membranes from block copolymers of polysulfone and polyethylene glycol. J. Membr. Sci. 2020, 618, 118690. [Google Scholar] [CrossRef]
- Grundler, J.; Shin, K.; Suh, H.-W.; Zhong, M.; Saltzman, W.M. Surface Topography of Polyethylene Glycol Shell Nanoparticles Formed from Bottlebrush Block Copolymers Controls Interactions with Proteins and Cells. ACS Nano 2021, 15, 16118–16129. [Google Scholar] [CrossRef]
- Leng C, Hung HC, Sun S, Wang D, Li Y, et al., Probing the surface hydration of nonfoulingzwitterionic and PEG materials in contact with proteins. ACS Appl Mater Interfaces 7(30) (2015) 16881-16888.
- Li, H.; Wang, Y.; Tang, Q.; Yin, D.; Tang, C.; He, E.; Zou, L.; Peng, Q. The protein corona and its effects on nanoparticle-based drug delivery systems. Acta Biomater. 2021, 129, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Elsewedy, H.S.; Al Dhubiab, B.E.; A Mahdy, M.; Elnahas, H.M. A Review Article on the Basic Concepts of Drug Delivery Systems as Targeting Agents. Int. J. Pharma Med. Biol. Sci. 2021, 10. [Google Scholar] [CrossRef]
- Fu, Y.; Ding, Y.; Zhang, L.; Zhang, Y.; Liu, J.; Yu, P. Poly ethylene glycol (PEG)-Related controllable and sustainable antidiabetic drug delivery systems. Eur. J. Med. Chem. 2021, 217, 113372. [Google Scholar] [CrossRef] [PubMed]
- (WHO International Programme on Chemical Safety), last accessed 07.10.2009. Available online: http://www.inchem.org/documents/jecfa/jecmono/v14je19.htm.
- Chanan-Khan, J. Szebeni, S. Savay, L. Liebes, N. M. Rafique, C. R. Alving, F. M. Muggia, Ann. Oncol. 2003, 14, 1430–1437. [Google Scholar]
- J. Szebeni, Toxicology, 216 (2005) 106 – 121.
- E. T. M. Dams, P. Laverman, W. J. G. Oyen, G. Storm, G. L. Scherphof, J. W. M. van der Meer, F. H. M. Corstens, O. C. Boerman, J. Pharmacol. Exp. Ther., 292 (2000) 1071 – 1079.
- T. Ishida, R. Maeda, M. Ichihara, K. Irimura, H. Kiwada, J. Controlled Release, 88 (2003) 35 – 42.
- H. Koide, T. Asai, K. Hatanaka, T. Urakami, T. Ishii, E. Kenjo, M. Nishihara, M. Yokoyama, T. Ishida, H. Kiwada, N. Okua, Int. J. Pharm., 362 (2008) 197 – 200.
- T. Ishida, M. Harada, X. Y. Wang, M. Ichihara, K. Irimura, H. Kiwada, J. Controlled Release, 105 (2005) 305 – 317.
- F. H. M. Corstens, O. C. Berman, J. Pharmacol. Exp. Ther., 293( 2000) 996 – 100.
- Ciolacu, D.; Suflet, M.D. Cellulose-based hydrogels for medical/pharmaceutical applications. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Popa, V., Volf, I., Eds.; Elsevier: Amsterdam, The Netherlands, Chapter, 11 (2018) 401–439.
- Ciolacu, D.; Rudaz, C.; Vasilescu, M.; Budtova, T. Physically and chemically cross-linked cellulose cryogels: Structure, properties and application for controlled release. Carbohydr. Polym. 2016, 151, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D. Structure-property relationships. In Cellulose-Based Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing AG: Basel, Switzerland; Chapter, 3 (2018) 1–32.
- Ribeiro, A.M.; Magalhães, M.; Veiga, F.; Figueiras, A. Cellulose-Based Hydrogels in Topical Drug Delivery: A Challenge in Medical Devices. In Cellulose-Based Superabsorbent Hydrogels, 1st ed.; Mondal, M.I.H., Ed.; Springer International Publishing AG: Basel, Switzerland, Chapter, 40 (2018) 1–29 [CrossRef]
- Javanbakht, S.; Shaabani, A. Carboxymethyl cellulose-based oral delivery systems. Int. J. Biol. Macromol. 2019, 133, 21–29. [Google Scholar] [CrossRef] [PubMed]
- ProbalBasu,NabanitaSaha, Tomas Saha, PetrSaha Polymeric hydrogel based systems for vaccine delivery: A review, Polymer, 230 (2021) 1-10.
- Verma, A.; Dubey, J.; Verma, N.; Nayak, A.K. Chitosan-hydroxypropyl methylcellulose matrices as carriers for hydrodynamically balanced capsules of moxifloxacinHCl. Curr. Drug Deliv. 2016, 14, 83–90. [Google Scholar] [CrossRef]
- Abeer, M.M.; Amin, M.C.I.M.; Martin, C. A review of bacterial cellulose-based drug delivery systems: their biochemistry, current approaches and future prospects. J. Pharm. Pharmacol. 2014, 66, 1047–1061. [Google Scholar] [CrossRef]
- Majumder, T.; Biswas, G.R.; Majee, S.B. Hydroxy Propyl Methyl Cellulose: Di_erent Aspects in Drug Delivery. J. Pharm. Pharmacol. 2016, 4, 381–385. [Google Scholar]
- Zhang, Z.; Chen, L.; Deng, M.; Bai, Y.; Chen, X.; Jing, X. Biodegradable thermo- and pH-responsive hydrogels for oral drug delivery. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2941–2951. [Google Scholar] [CrossRef]
- Sezer, S. ; Şahin, ˙I.;Öztürk, K.; Şanko, V.; Koçer, Z.; Sezer, U.A. Cellulose-based hydrogels as biomaterials. In Cellulose-Based Superabsorbent Hydrogels, 1st ed.; Mondal, M.I.H., Ed.; Springer International Publishing AG: Basel, Switzerland, Chapter, 39; (2018) pp. 1–27.
- Edgar, K.J. Cellulose esters in drug delivery. Cellulose 2006, 14, 49–64. [Google Scholar] [CrossRef]
- Hasan, A.M.A.; El-Sayed Abdel-Raouf, M. Cellulose-Based Superabsorbent Hydrogels. In Cellulose-Based Superabsorbent Hydrogels, Polymers and Polymeric Composites: A Reference Series; Mondal, M.I.H., Ed.; Springer International Publishing AG: Basel, Swizterland, 2018; pp. 245–267. [Google Scholar]
- Chyzy, A.; Tomczykowa, M.; Plonska-Brzezinska, M.E. Hydrogels as Potential Nano-, Micro- and Macro-Scale Systems for Controlled Drug Delivery. Materials 2020, 13, 188. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-Based Hydrogels as Sustained Drug-Delivery Systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.-L. Review of Stimuli-Responsive Polymers in Drug Delivery and Textile Application. Molecules 2019, 24, 2547. [Google Scholar] [CrossRef]
- Yoshida, R.; Sakai, K.; Okano, T.; Sakurai, Y. Pulsatile drug delivery systems using hydrogels. Adv. Drug Deliv. Rev. 1993, 11, 85–108. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.L. Stimuli-responsive hydrogels: An interdisciplinary overview. In Hydrogels—Smart Materials for Biomedical Applications; Chapter 2; Popa, L., Ghica, M.V., Dinu-Pîrvu, C.E., Eds.; IntechOpen Limited: London, UK, 2018; pp. 1–23. [Google Scholar]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Onaciu, A.; Munteanu, R.A.; Moldovan, A.I.; Moldovan, C.S.; Berindan-Neagoe, I. Hydrogels Based Drug Delivery Synthesis, Characterization and Administration. Pharmaceutics 2019, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Caicco, M.J.; Cooke, M.J.; Wang, Y.; Tuladhar, A.; Morshead, C.M.; Shoichet, M.S. A hydrogel composite system for sustained epi-cortical delivery of Cyclosporin A to the brain for treatment of stroke. J. Control. Release 2013, 166, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cooke, M.J.; Morshead, C.M.; Shoichet, M.S. Hydrogel delivery of erythropoietin to the brain for endogenous stem cell stimulation after stroke injury. Biomaterials 2012, 33, 2681–2692. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Gupta, V.K.; Agarwal, S.; Dev, K.; Pathania, D. Controlled release of antibiotic amoxicillin drug using carboxymethyl cellulose-cl-poly(lactic acid-co-itaconic acid) hydrogel. Int. J. Biol. Macromol. 2017, 101, 612–620. [Google Scholar] [CrossRef]
- Perioli, L.; Ambrogi, V.; Rubini, D.; Giovagnoli, S.; Ricci, M.; Blasi, P.; Rossi, C. Novel mucoadhesivebuccal formulation containing metronidazole for the treatment of periodontal disease. J. Control. Release 2004, 95, 521–533. [Google Scholar] [CrossRef]
- Gupta, A.K. ENVIRONMENTAL RESPONSIVE HYDROGELS: A NOVEL APPROACH IN DRUG DELIVERY SYSTEM. J. Drug Deliv. Ther. 2012, 2. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Leu, Y.-L.; Wang, Y.-Y.; Tsai, Y.-H. In vitro topical application and in vivo pharmacodynamic evaluation of nonivamide hydrogels using Wistar rat as an animal model. Eur. J. Pharm. Sci. 2002, 15, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Huang, K.-Y.; Yang, B.-Y.; Ko, C.-H.; Huang, H.-M. Fabrication of Novel Hydrogel with Berberine-Enriched Carboxymethylcellulose and Hyaluronic Acid as an Anti-Inflammatory Barrier Membrane. BioMed Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Moreira, A.P.D.; da Silva Moreira Thiré, R.; Quilty, B.; Passos, T.M.; Simon, P.; Mancini, M.C.; McGuinness, G. Absorbent polyvinyl alcohol-sodium carboxymethyl cellulose hydrogels for propolis delivery in wound healing applications. Polym. Eng. Sci. 2017, 57, 1224–1233. [Google Scholar] [CrossRef]
- Sheng, Y.; Gao, J.; Yin, Z.-Z.; Kang, J.; Kong, Y. Dual-drug delivery system based on the hydrogels of alginate and sodiumcarboxymethyl cellulose for colorectal cancer treatment. Carbohydr. Polym. 2021, 269, 118325. [Google Scholar] [CrossRef]
- Sun, N.; Wang, T.; Yan, X. Self-assembled supermolecular hydrogel based on hydroxyethyl cellulose: Formation, in vitro release and bacteriostasis application. Carbohydr. Polym. 2017, 172, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.J.; Kim, A.; Park, S.N. Properties and in vitro drug release of hyaluronic acid-hydroxyethyl cellulose hydrogels for transdermal delivery of isoliquiritigenin. Carbohydr. Polym. 2016, 147, 473–481. [Google Scholar] [CrossRef]
- Furst, T.; Piette, M.; Lechanteur, A.; Evrard, B.; Piel, G. Mucoadhesive cellulosic derivative sponges as drug delivery system for vaginal application. Eur. J. Pharm. Biopharm. 2015, 95, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Sawant, P.D.; Luu, D.; Ye, R.; Buchta, R. Drug release from hydroethanolicgels.E_ect of drug’s lipophilicity (logP), polymer–drug interactions and solvent lipophilicity. Int. J. Pharm. 2010, 396, 45–52. [Google Scholar] [CrossRef]
- Qi, X.; Chen, H.; Rui, Y.; Yang, F.; Ma, N.; Wu, Z. Floating tablets for controlled release of ofloxacin via compression coating of hydroxypropyl cellulose combined with effervescent agent. Int. J. Pharm. 2015, 489, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P.R.; RaghavendraRao, N.G.; Soujanya, C. Formulation, evaluation and antiinflammatory activity of topical etoricoxibgel. Asian J. Pharm. Clin. Res. 2010, 3, 126–129. [Google Scholar]
- Bidkar, S.; Devendra, J.; Padsalg, A.; Patel, K.; Mokale, V. Formulation development and evaluation of fluconazole gel in various polymer bases formulation development and evaluation of fluconazole gel in various polymer bases. Asian J. Pharm. 2007, 1, 63–68. [Google Scholar]
- Cho, C.-W.; Choi, J.-S.; Shin, S.-C. Enhanced local anesthetic action of mepivacaine from the bioadhesive gels. Pak. J. Pharm. Sci. 2011, 24. [Google Scholar]
- Vlaia, L.; Olariu, I.; Coneac, G.; Mu¸t, A.M.; Vlaia, V.; Szabadai, Z.; Lupuleasa, D. Formulation, preparation and evaluation of HPMC-based hydroethanolic gels containing propranolol hydrochloride 3% and terpenes. Pract. Farm. 2015, 8, 5–15. [Google Scholar]
- Tanaka, A.; Furubayashi, T.; Tomisaki, M.; Kawakami, M.; Kimura, S.; Inoue, D.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Nasal drug absorption from powder formulations: The effect of three types of hydroxypropyl cellulose (HPC). Eur. J. Pharm. Sci. 2017, 96, 284–289. [Google Scholar] [CrossRef]
- Liakos, I.L.; D’autilia, F.; Garzoni, A.; Bonferoni, C.; Scarpellini, A.; Brunetti, V.; Carzino, R.; Bianchini, P.; Pompa, P.P.; Athanassiou, A. All natural cellulose acetate—Lemongrass essential oil antimicrobial nanocapsules. Int. J. Pharm. 2016, 510, 508–515. [Google Scholar] [CrossRef]
- Pastor, M.; Esquisabel, A.; Marquínez, I.; Talavera, A.; Pedraz, J.L. Cellulose acetate phthalate microparticles containing Vibriocholerae: Steps toward an oral cholera vaccine. J. Drug Target. 2014, 22, 478–487. [Google Scholar] [CrossRef]
- Zhoua, H.Y.; Jianga, L.J.; Caoa, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.F.; Zou, S.H.; Chen, X.G.; Liu, C.S.; Li, J.J.; Zhou, X.; Liu, Y.; Cheng, X.J. Character-izations of chitosan-based highly porous hydrogel—the effects of the solvent. J. Appl. Polym. Sci. 2012, 125, 88–98. [Google Scholar] [CrossRef]
- Christie, R.; Findley, D.; Dunfee, M.; Hansen, R.; Olsen, S.; Grainger, D. Photopolymerized hydrogel carriers for live vaccine ballistic delivery. Vaccine 2006, 24, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- M.D. Onofrei, A. Filimon, Cellulose-based hydrogels: designing concepts, properties, and perspectives for biomedical and environmental applications, in: A. M’endez-Vilas, A. Solano (Eds.), Polymer Science: Research Advances, Practical Applications and Educational Aspects, 2016, pp. 108–120.
- Chen, X.Y.; Butt, A.M.; Amin, M.C.I.M. Enhanced paracellular delivery of vaccine by hydrogel microparticles-mediated reversible tight junction opening for effective oral immunization. J. Control. Release 2019, 311-312, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, A.; Taguchi, T. Sustained-immuno stimulatory nanocellulose scaffold to enhance vaccine efficacy. Biomed. Mater. Res. 2020, 108A, 159–1170. [Google Scholar]
- Kim, E.; Erdos, G.; Huang, S.; Kenniston, T.W.; Balmert, S.C.; Carey, C.D.; Raj, V.S.; Epperly, M.W.; Klimstra, W.B.; Haagmans, B.L.; et al. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine 2020, 55, 102743. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Feng, X.; Wei, Y.; Zhang, Y. A comparative study of PEG and cellulose-based hydrogels for insulin delivery. Journal of Materials Science: Materials in Medicine 2018, 29, 17. [Google Scholar]
- Liu, Y.; Liu, C.; Zhang, X.; Zhou, Y.; Wang, X. Comparative study of PEG and cellulose-based hydrogels for drug delivery. Journal of Biomedical Materials Research Part A 2017, 105, 484–491. [Google Scholar]
- Sun, Y.; Zhang, Y.; Li, X.; Du, Y.; Li, Y.; Zhou, J.; Li, X. Double network hydrogels: Recent advances and future prospects. Journal of Materials Chemistry B 2021, 9, 3193–3215. [Google Scholar] [CrossRef]
- Gaihre, B.; Jayasuriya, A.C.; Zhang, M.; Farris, R.J. Double network cellulose hydrogels with improved mechanical properties for wound healing applications. Carbohydrate Polymers 2018, 181, 232–240. [Google Scholar]
- Wang, C.; Zhao, N.; Yuan, W. NIR/Thermoresponsive Injectable Self-Healing Hydrogels Containing Polydopamine Nanoparticles for Efficient Synergistic Cancer Thermochemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 9118–9131. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, Q.; Cui, Y.; Xu, L.; Ma, X. Nanocomposite hydrogels: Advances and challenges in biomedical applications. Nanoscale 2021, 13, 14–37. [Google Scholar] [CrossRef] [PubMed]
- Guo, R., Mao, J., Yan, L., Ma, Y., Liu, J., Shen, J., & Liu, Y.. Injectable hydrogels for drug delivery: Progress and challenges. Journal of Controlled Release, 330, (2021) 1088-1109. [CrossRef]
- Yang, F.; Shi, K.; Jia, Y.; Hao, Y.; Peng, J.; Yuan, L.; Chen, Y.; Pan, M.; Qian, Z. A biodegradable thermosensitive hydrogel vaccine for cancer immunotherapy. Appl. Mater. Today 2020, 19. [Google Scholar] [CrossRef]
- Huang, Y.; Jayathilaka, P.B.; Islam, S.; Tanaka, C.B.; Silberstein, M.N.; Kilian, K.A.; Kruzic, J.J. Structural aspects controlling the mechanical and biological properties of tough, double network hydrogels. Acta Biomater. 2022, 138, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Patel, M.; Koh, W.-G. Incorporation of Conductive Materials into Hydrogels for Tissue Engineering Applications. Polymers 2018, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Huang, S.; Yang, Y.; Zhang, J.; Ma, J. Recent progress in conductive hydrogels for tissue engineering applications. Journal of Materials Chemistry B 2021, 9, 3031–3051. [Google Scholar] [CrossRef]
- Xu, X.; Lu, Y.; Liu, S.; Wang, Y.; Wu, Q.; Zhang, B. Sliding hydrogels: Advances and challenges. Biomaterials Science 2021, 9, 4241–4256. [Google Scholar] [CrossRef]
- Jung, J.P.; Shim, J.H.; Lee, J.S.; Park, H.; Cho, D.W. PEG-based double network hydrogel with a low modulus for the enhancement of stem cell differentiation. Biomaterials Science 2020, 8, 4421–4432. [Google Scholar]
- Wei, X.; Liu, C.; Wang, Z.; Luo, Y. 3D printed core-shell hydrogel fiber scaffolds with NIR-triggered drug release for localized therapy of breast cancer. Int. J. Pharm. 2020, 580, 119219. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Huang, Y.; Liu, Y.; Zhang, J.; Wu, Z.; Cao, X. A Sliding Hydrogel with Enhanced Wound-Healing Effect. ACS Applied Materials & Interfaces 2021, 13, 6972–6982. [Google Scholar]
- Zheng, L.; Li, C.; Huang, X.; Lin, X.; Lin, W.; Yang, F.; Chen, T. Thermosensitive hydrogels for sustained-release of sorafenib and selenium nanoparticles for localized synergistic chemoradiotherapy. Biomaterials 2019, 216, 119220. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Yang, Q.; Huang, L.; Chen, L.; Ni, Y.; Xiao, H. Temperature and pH responsive cellulose filament/poly (NIPAM-co-AAc) hybrids as novel adsorbent towards Pb(II) removal. Carbohydr. Polym. 2018, 195, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, W.; Mao, Y. Responsive hydrogels for drug delivery and tissue engineering: A review. Journal of Materials Chemistry B 2021, 9, 3603–3625. [Google Scholar] [CrossRef]

| Derivatives of Cellulose | Drugs | Action/Treatment | Ref. |
|---|---|---|---|
| Methylcellulose | Cyclosporine | Continuous brain delivery | [115] |
| Erythropoietin | After a stroke, delivery to the brain for endogenous stem cell activation | [116] | |
| Carboxymethylcellulose | Amoxicillin | Excellent gram-positive bacterial antibioticStaphylococcus aureus | [117] |
| Acyclovir | regulated medication delivery systems | [118] | |
| Diclofenac | Skin injuries | [119] | |
| Nonivamide | Enhanced skin distribution and permeability | [120] | |
| Berberine | Safeguard healing tissue after surgery while executing a controlled medication release | [121] | |
| Propolis | Wound remedial | [122] | |
| methotrexate | Colorectal cancer | [123] | |
| Hydroxyethyl cellulose | Eugenol | E_cientbacteriostasis against Escherichia coli |
[124] |
| Isoliquiritigenin | System for transdermal distribution | [125] | |
| Cellulose based sponges | Rehydration of vaginal cavity | [126] | |
| Hydroxypropyl cellulose | Lidocaine | Encourage the use of a planned, regulated drug | [127] |
| Ofloxacin | Gastro-retentive | [128] | |
| Hydroxypropyl methylcellulose |
Etoricoxib | Acute or chronic sickness | [129] |
| Fluconazole | Fungus skin infections | [130] | |
| Mepivacaine | Relieve localized discomfort while executing a controlled medication release | [131] | |
| Propranolol | Boost percutaneous penetration | [132] | |
| Sodium carboxymethyl cellulose |
Fluorescein isothiocyanate-labeled dextran | Nasal treatment | [133] |
| Cellulose acetate | Nanocapsules | antimicrobial activity against Escherichia and Staphylococcus species | [134] |
| Cellulose acetate phthalate |
Powder-form Cholera vaccination | Cholera | [135] |
| Property | PEG-based hydrogels | Cellulose-based hydrogels |
|---|---|---|
| Half-life | Longer half-life due to resistance to degradation | Shorter half-life due to susceptibility to degradation |
| Circulation time | Longer circulation time | Shorter circulation time |
| Maximum release | Higher maximum release | Lower maximum release |
| Maximum % loaded drug released. | Higher maximum % loaded drug released. | Lower maximum % loaded drug released. |
| Burst release properties | Lower burst release properties | Higher burst release properties |
| Temperature properties | Good stability over a wide range of temperatures | Susceptible to temperature changes |
| pH properties | Good stability over a wide range of pH values | Susceptible to changes in pH |
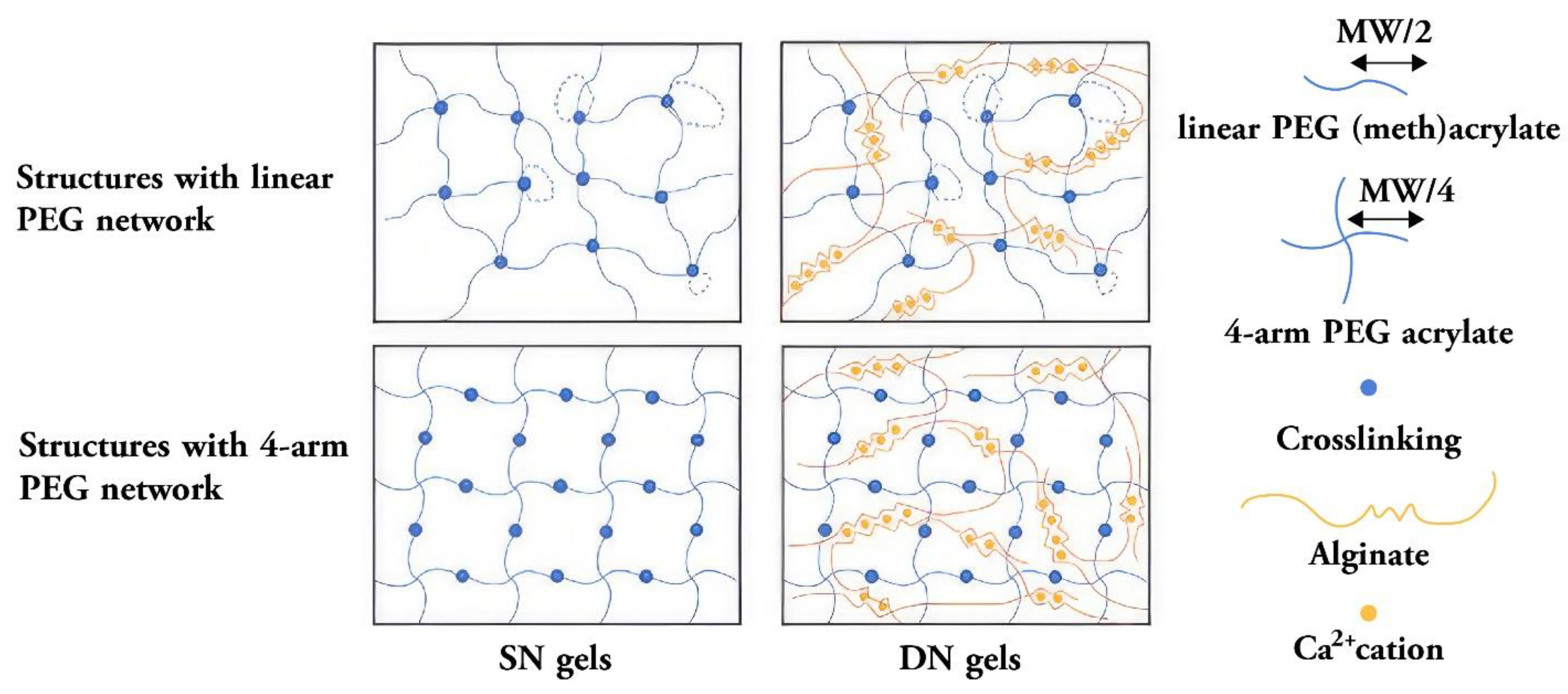
| Double network hydrogels | PEG-based double network hydrogels suitable for tissue engineering[155] | Cellulose-based double-network hydrogels suitable for wound healing [146] |
| Injectable hydrogels | PEG-based injectable hydrogels suitable for sustained drug delivery [156]. | Cellulose-based injectable hydrogels suitable for wound healing. |
| Sliding hydrogels | PEG-based sliding hydrogels suitable for cartilage tissue engineering. | Cellulose-based sliding hydrogels suitable for skin tissue engineering [157]. |
| Conductive hydrogels | PEG-based conductive hydrogels suitable for cardiac tissue engineering. | Cellulose-based conductive hydrogels suitable for neural tissue engineering. |
| Responsive hydrogels | PEG-based thermo-responsive hydrogels are suitable for sustained drug delivery [158]. | Cellulose-based pH-responsive hydrogels suitable for controlled drug delivery [159]. |
| Nanocomposite hydrogels | PEG-based nanocomposite hydrogels suitable for drug delivery. | Cellulose-based nanocomposite hydrogels suitable for sensing of heavy metal ions [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




