Submitted:
31 July 2023
Posted:
02 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Postoperative analgesia
Long-term outcomes
Statistical analysis
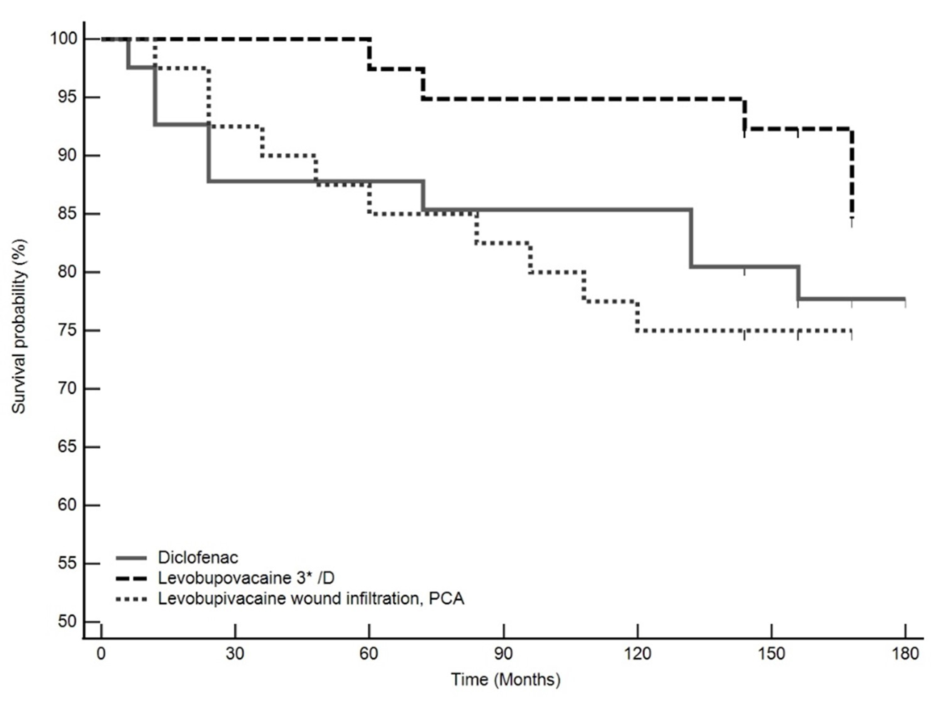
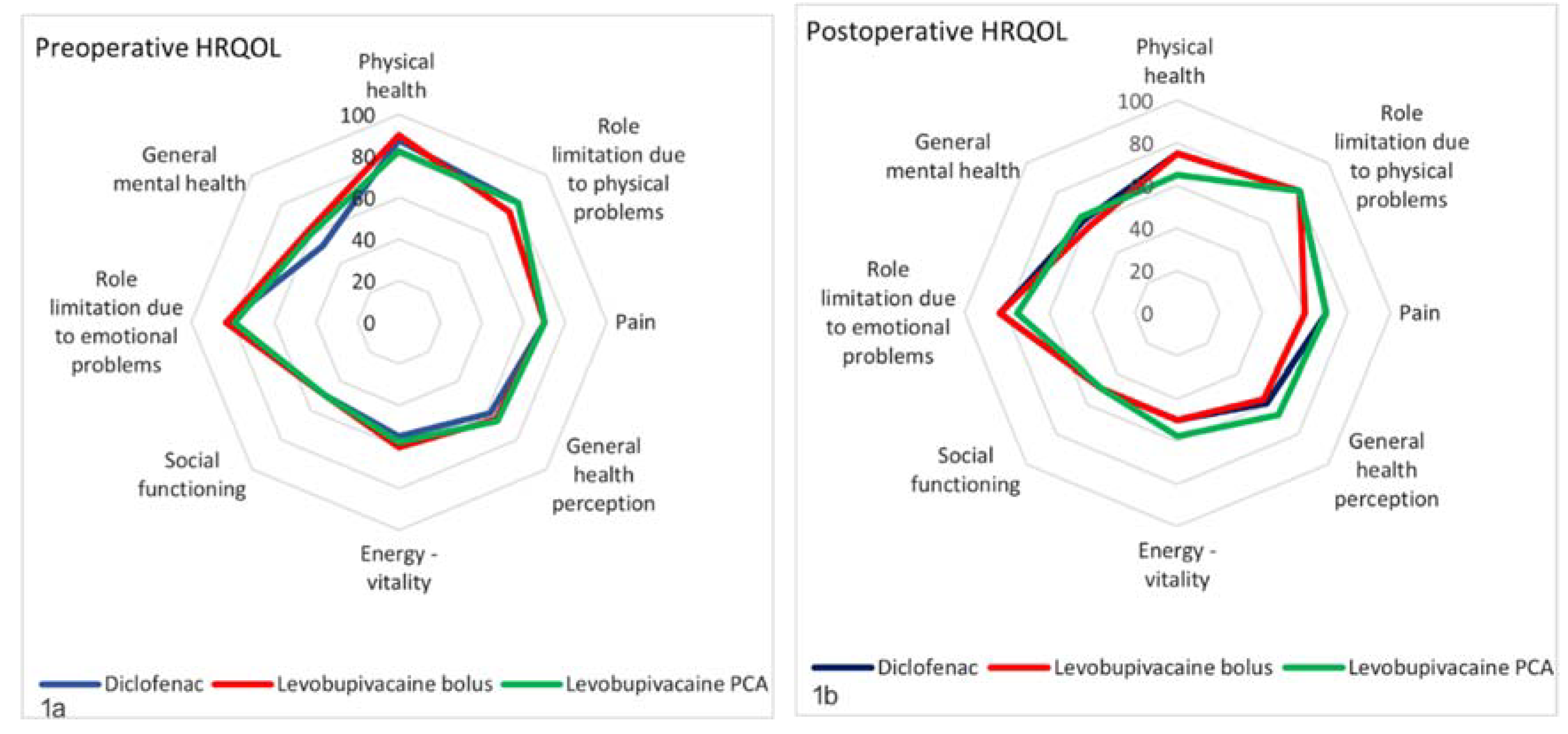
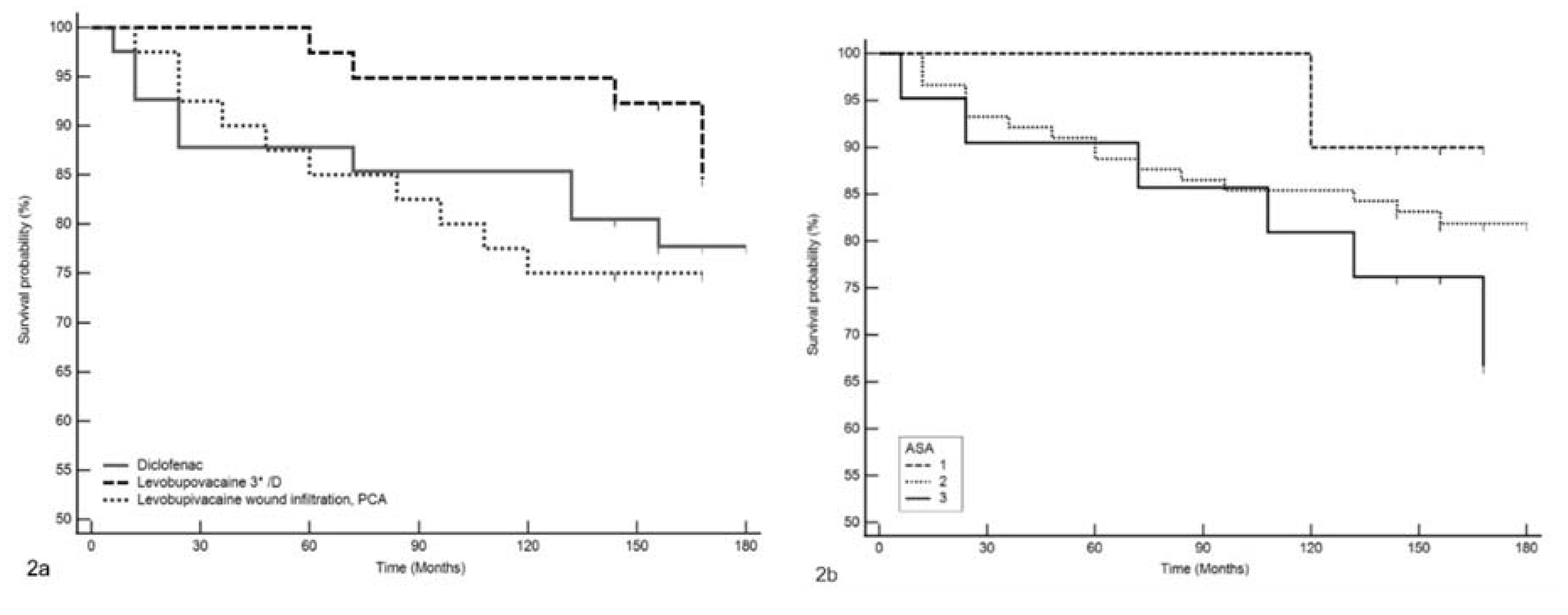
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Bundred, J.R.; Michael, S.; Stuart, B.; Cutress, R.I.; Beckmann, K.; Holleczek, B.; Dahlstrom, J.E.; Gath, J.; Dodwell, D.; Bundred, N.J. Margin status and survival outcomes after breast cancer conservation surgery: prospectively registered systematic review and meta-analysis. Bmj. 2022, 378, e070346. [Google Scholar] [CrossRef] [PubMed]
- Beguinot, M.; Monrigal, E.; Kwiatkowski, F.; Ginzac, A.; Joly, D.; Gayraud, G.; Le Bouedec, G.; Gimbergues, P. Continuous Wound Infiltration With Ropivacaine After Mastectomy: A Randomized Controlled Trial. J Surg Res 2020, 254, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Kaur, U.; Shamshery, C.; Agarwal, A.; Prakash, N.; Valiveru, R.C.; Mishra, P. Evaluation of postoperative pain in patients undergoing modified radical mastectomy with pectoralis or serratus-intercostal fascial plane blocks. Korean J Anesthesiol 2020, 73, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.E.; Greenberger, B.A.; Shi, Z.; Gajjar, S.; Shi, W.; Mourad, W.F.; Yan, W. Post-mastectomy and post-breast conservation surgery pain syndrome: a review of etiologies, risk prediction, and trends in management. Transl Cancer Res 2020, 9 (Suppl 1), S77–s85. [Google Scholar] [CrossRef]
- Park, S.; Park, J.; Choi, J.W.; Bang, Y.J.; Oh, E.J.; Park, J.; Hong, K.Y.; Sim, W.S. The efficacy of ultrasound-guided erector spinae plane block after mastectomy and immediate breast reconstruction with a tissue expander: a randomized clinical trial. Korean J Pain 2021, 34, 106–113. [Google Scholar] [CrossRef]
- Elsabeeny, W.Y.; Shehab, N.N.; Wadod, M.A.; Elkady, M.A. Perioperative Analgesic Modalities for Breast Cancer Surgeries: A Prospective Randomized Controlled Trial. J Pain Res 2020, 13, 2885–2894. [Google Scholar] [CrossRef]
- Li, R.; Xiao, C.; Liu, H.; Huang, Y.; Dilger, J.P.; Lin, J. Effects of local anesthetics on breast cancer cell viability and migration. BMC Cancer 2018, 18, 666. [Google Scholar] [CrossRef]
- Chen, J.L.; Liu, S.T.; Huang, S.M.; Wu, Z.F. ; Apoptosis, Proliferation, and Autophagy Are Involved in Local Anesthetic-Induced Cytotoxicity of Human Breast Cancer Cells. Int J Mol Sci, 2022; 23(24). [Google Scholar] [CrossRef]
- Castelli, V.; Piroli, A.; Marinangeli, F.; d'Angelo, M.; Benedetti, E.; Ippoliti, R.; Zis, P.; Varrassi, G.; Giordano, A.; Paladini, A.; <named-content content-type="background:red">Cimini, A. Local anesthetics counteract cell proliferation and migration of human triple-negative breast cancer and melanoma cells. J Cell Physiol 2020, 235, 3474–3484. [Google Scholar] [CrossRef]
- Kwakye, A.K.; Kampo, S.; Lv, J.; Ramzan, M.N.; Richard, S.A.; Falagán, A.A.; Agudogo, J.; Atito-Narh, E.; Yan, Q.; Wen, Q.P. Levobupivacaine inhibits proliferation and promotes apoptosis of breast cancer cells by suppressing the PI3K/Akt/mTOR signalling pathway. BMC Res Notes 2020, 13, 386. [Google Scholar] [CrossRef]
- Klein, I.; Kalichman, L.; Chen, N.; Susmallian, S. Effect of physical activity levels on oncological breast surgery recovery: a prospective cohort study. Sci Rep 2021, 11, 10432. [Google Scholar] [CrossRef]
- Savioli, F.; Edwards, J.; McMillan, D.; Stallard, S.; Doughty, J.; Romics, L. The effect of postoperative complications on survival and recurrence after surgery for breast cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol 2020, 155, 103075. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Kawai, A.; Wakisaka, M.; Sawada, S.; Shimoyama, M.; Yasuda, N.; Kin, T.; Arihiro, K. Outpatient breast-conserving surgery for breast cancer: Use of local and intravenous anesthesia and/or sedation may reduce recurrence and improve survival. Ann Med Surg (Lond) 2020, 60, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Legeby, M.; Sandelin, K.; Wickman, M.; Olofsson, C. Analgesic efficacy of diclofenac in combination with morphine and paracetamol after mastectomy and immediate breast reconstruction. Acta Anaesthesiol Scand 2005, 49, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Maslić Sersić, D.; Vuletić, G. Psychometric evaluation and establishing norms of Croatian SF-36 health survey: framework for subjective health research. Croat Med J 2006, 47, 95–102. [Google Scholar]
- van der Windt, D.A.; van der Heijden, G.J.; de Winter, A.F.; Koes, B.W.; Devillé, W.; Bouter, L.M. The responsiveness of the Shoulder Disability Questionnaire. Ann Rheum Dis 1998, 57, 82–87. [Google Scholar] [CrossRef]
- Chavez-MacGregor, M.; Mittendorf, E.A.; Clarke, C.A.; Lichtensztajn, D.Y.; Hunt, K.K.; Giordano, S.H. Incorporating Tumor Characteristics to the American Joint Committee on Cancer Breast Cancer Staging System. Oncologist 2017, 22, 1292–1300. [Google Scholar] [CrossRef]
- Meerkerk, C.D.A.; Chargi, N.; de Jong, P.A.; van den Bos, F.; de Bree, R. Sarcopenia measured with handgrip strength and skeletal muscle mass to assess frailty in older patients with head and neck cancer. J Geriatr Oncol 2021, 12, 434–440. [Google Scholar] [CrossRef]
- Hallet, J.; Tillman, B.; Zuckerman, J.; Guttman, M.P.; Chesney, T.; Mahar, A.L.; Chan, W.C.; Coburn, N.; Haas, B. Association Between Frailty and Time Alive and At Home After Cancer Surgery Among Older Adults: A Population-Based Analysis. J Natl Compr Canc Netw 2022, 20, 1223–1232. [Google Scholar] [CrossRef]
- Ewertz, M.; Jensen, A.B. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncol 2011, 50, 187–193. [Google Scholar] [CrossRef]
- Longo, U.G.; Mazzola, A.; Carotti, S.; Francesconi, M.; Catapano, S.; Magrì, F.; Perrone, G.; Morini, S.; De Salvatore, S.; Denaro, V. The role of estrogen and progesterone receptors in the rotator cuff disease: a retrospective cohort study. BMC Musculoskelet Disord 2021, 22, 891. [Google Scholar] [CrossRef]
- Irwin, M.L.; Cartmel, B.; Gross, C.P.; Ercolano, E.; Li, F.; Yao, X.; Fiellin, M.; Capozza, S.; Rothbard, M.; Zhou, Y.; Harrigan, M. ; Sanft, T.; Schmitz, K.; Neogi, T.; Hershman, D.; Ligibel, J. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol 2015, 33, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Najjar, H.; Easson, A. Age at diagnosis of breast cancer in Arab nations. Int J Surg 2010, 8, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.K.; Li, J.; Huang, R.; Fan, J.H.; Zheng, R.S.; Zhang, B.N.; Zhang, B.; Tang, Z.H.; Xie, X.M.; Yang, H.J.; <named-content content-type="background:red">He, J.J. ; Li, H.; Li, J.Y.; Qiao, Y.L.; Chen, W.Q. Age of diagnosis of breast cancer in China: almost 10 years earlier than in the United States and the European union. Asian Pac J Cancer Prev 2014, 15, 10021–10025. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; George, P.S.; Kunnambath, R.; Mathew, B.S.; Kumar, A.; Syampramod, R.; Booth, C.M. Educational Status, Cancer Stage, and Survival in South India: A Population-Based Study. JCO Glob Oncol 2020, 6, 1704–1711. [Google Scholar] [CrossRef]
- Li, Y.; Guo, J.; Sui, Y.; Chen, B.; Li, D.; Jiang, J. Quality of Life in Patients with Breast Cancer following Breast Conservation Surgery: A Systematic Review and Meta-Analysis. J Healthc Eng 2022, 2022, 3877984. [Google Scholar] [CrossRef]
- Joaquim, A.; Leão, I.; Antunes, P.; Capela, A.; Viamonte, S.; Alves, A.J.; Helguero, L.A.; Macedo, A. Impact of physical exercise programs in breast cancer survivors on health-related quality of life, physical fitness, and body composition: Evidence from systematic reviews and meta-analyses. Front Oncol 2022, 12, 955505. [Google Scholar] [CrossRef]
- Cramer, H.; Lauche, R.; Klose, P.; Lange, S.; Langhorst, J.; Dobos, G.J. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev 2017, 1, Cd010802. [Google Scholar] [CrossRef]
- Tao, M.H.; Shu, X.O.; Ruan, Z.X.; Gao, Y.T.; Zheng, W. Association of overweight with breast cancer survival. Am J Epidemiol 2006, 163, 101–107. [Google Scholar] [CrossRef]
- Riondino, S.; Formica, V.; Valenzi, E.; Morelli, C.; Flaminio, V.; Portarena, I.; Torino, F.; Roselli, M. Obesity and Breast Cancer: Interaction or Interference with the Response to Therapy? Curr Oncol 2023, 30, 1220–1231. [Google Scholar] [CrossRef]
- Caetano Dos Santos, F.L.; Michalek, I.M.; Wojciechowska, U.; Didkowska, J. Changes in the survival of patients with breast cancer: Poland, 2000-2019. Breast Cancer Res Treat 2023, 197, 623–631. [Google Scholar] [CrossRef]
- Akbari, M.E.; Akbari, A.; Khayamzadeh, M.; Salmanian, R.; Akbari, M. Ten-Year Survival of Breast Cancer in Iran: A National Study (Retrospective Cohort Study). Breast Care (Basel) 2023, 18, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Kepp, K.P.; Björk, J.; Kontis, V.; Parks, R.M.; Bæk, K.T.; Emilsson, L.; Lallukka, T. Estimates of excess mortality for the five Nordic countries during the COVID-19 pandemic 2020-2021. Int J Epidemiol 2022, 51, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Beaubrun-Renard, M.M.; Veronique-Baudin, J.; Macni, J.; Ulric-Gervaise, S.; Almont, T.; Aline-Fardin, A.; Grossat, N.; Furtos, C.; Escarmant, P.; Vinh-Hung, V.; <named-content content-type="background:red">Bougas, S. ; Cabie, A.; Joachim, C. Overall survival of triple negative breast cancer in French Caribbean women. PLoS One 2022, 17, e0271966. [Google Scholar] [CrossRef] [PubMed]
- Chamaraux-Tran, T-N. ; Mathelin, C.; Aprahamian, M.; Joshi, G.P.; Tomasetto, C,; Diemunsch, P,; Akladios, C. Antitumor Effects of Lidocaine on Human Breast Cancer Cells: An In Vitro and In Vivo Experimental Trial. Anticancer Res 2018, 38, 95–105. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Wen, Y.; Wang, P.; Fan, L. Bupivacaine inhibits the malignant biological behavior of oral squamous cell carcinoma cells by inhibiting the activation of ERK1/2 and STAT3. Ann Transl Med, 2021; 9, 839. [Google Scholar] [CrossRef]
- Freeman, J.; Crowley, P.D.; Foley, A.G.; Gallagher, H.C.; Iwasaki, M.; Ma, D.; Buggy, D.J. Effect of Perioperative Lidocaine, Propofol and Steroids on Pulmonary Metastasis in a Murine Model of Breast Cancer Surgery. Cancers (Basel), 2019; 11(5). [Google Scholar] [CrossRef]
- Xing,; W. ; Chen, D.T.; Pan, J.H.; Chen, Y.H.; Yan, Y.; Li, Q,; Xue, R.F.; Yuan, Y.F.; Zeng, W.A. Lidocaine Induces Apoptosis and Suppresses Tumor Growth in Human Hepatocellular Carcinoma Cells In Vitro and in a Xenograft Model In Vivo. Anesthesiology 2017, 126, 868–881. [Google Scholar] [CrossRef]
- Wang, L.; Guo, W.; Guan, H.; Yan, N.; Cai, X.; Zhu, L. Local anesthetic bupivacaine inhibits proliferation and metastasis of hepatocellular carcinoma cells via suppressing PI3K/Akt and MAPK signaling. J Biochem Mol Toxicol 2021, 35, e22871. [Google Scholar] [CrossRef]
| Parameters | Diclofenac (N=41) |
Levobupivacaine bolus (N=39) |
Levobupivacaine PCA (N=40) | p† |
|---|---|---|---|---|
| Age (yr) | 57 (49.5 – 65.5) | 56 (52 – 65) | 55.5 (49.5 – 60.5) | 0.522 |
| Body mass index (kg/m2) | 29 (23 – 31.4) | 27.8 (24.8 – 30.6) | 26 (22.7 – 30.8) | 0.639 |
| ASA status | 2 (2 – 2) | 2 (2 – 2) | 2 (2 – 2) | 0.716 |
| Hand grip strength (bar) | 0.38 (0.34 – 0.44) | 0.37 (0.31 – 0.44) | 0.40 (0.34 – 0.47) | 0.452 |
| Stage of disease | 4 (2 - 6) | 4 (2.5 – 4.5) | 4.5 (2.75 – 6.25) | 0.214 |
| Five -year survival bivariate regression analysis |
ß | Wald | P value | HR | 95% CI |
|---|---|---|---|---|---|
| Age at diagnosis | 0.04 | 1.35 | 0.25 | 104 | 0.97 do 1.11 |
| BMI | -0.01 | 0.04 | 0.84 | 0.99 | 0.88 do 1.11 |
| ASA | 0.38 | 0.41 | 0.52 | 1.46 | 0.46 do 4.65 |
| Hand grip strength | -6.92 | 4.89 | 0.03 | 0.001 | 0.001 do 0.45 |
| Stage of disease | 0.28 | 2.62 | 0.11 | 1.33 | 0.94 do 1.86 |
| Groups (diclofenac*) | |||||
| Levobupivacaine bolus | -1.63 | 2.21 | 0.14 | 0.20 | 0.02 do 1.68 |
| Levobupivacaine PCA | -0.005 | 0 | 0.99 | 0.99 | 0.29 do 3.44 |
| Drugs (diclofenac*) | |||||
| Any levobupivacaine group | -0.52 | 0.75 | 0.39 | 0.59 | 0.18 do 1.94 |
| Multivariate regression analysis | |||||
| Hand grip strength | -8.964 | 5.81 | 0.02 | 0.0002 | 0 do 0.20 |
| Constant | 0.44 | 4.45 | 0.03 | 1.56 | 1.03 do 2.36 |
| Time from surgery to outcome | Diseases stage at diagnosis | |||||
|---|---|---|---|---|---|---|
| n | Median (IQR) | P* | n | Median (IQR) | P* | |
| Current status | ||||||
| Died | 23 | 60 (24 – 120) | <0,001† | 23 | 5 (3 – 7) | 0.03‡ |
| Active disease, current treatment | 10 | 156 (155 – 168)(min 144 - max 168) | 10 | 6 (4 – 7) | ||
| No signs of disease | 87 | 156 (156 – 168)(min 144 - max 180) | 87 | 4 (2 – 5) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





