1. Introduction
In 1950 S.S. Kutateladze was the first who substantiated in detail the hydrodynamic (hydromechanical) theory of boiling crisis. In [
1], he wrote: “Let us make the following four assumptions.
Changes in the boiling regime occur due to a change in the hydrodynamic regime of the two-phase boundary layer that exists during vaporization on the heating surface, and they are characterized by the certain critical values of the vaporization rate.
The boundary two-phase layer is so turbulent due to the vaporization process that molecular friction can be neglected both in the vapor and in the liquid flow components.
The velocity of a liquid component near the heating surface, due to deceleration created by the latter, is significantly less than the average vapor velocity.
The dimensions of the heating surface are so much larger than the bubbles and films freely formed in its immediate vicinity that the occurrence of a crisis in the vaporization regime is equally probable at all points of this surface. ”
In [
2], he adds the sentence: “The ideal model, which fully satisfies the last condition, is a very large horizontal plate with the heating surface facing upwards.”
As a result, using the method of dimensional analysis, Kutateladze obtained the relation for calculating the critical heat flux (CHF) in the process of pool boiling:
where the value of constant
k (called usually the Kutateladze constant) varies within 0.13 – 0.19; in [
1,
2] it is recommended to use its average value equal to 0.16;
is the latent heat of vaporization;
is the acceleration of gravity;
is the surface tension coefficient;
are the densities of liquid and vapor, respectively.
In [
3,
4], Zuber obtained a formula similar to (1) based on a physical model using the results of the analysis of Taylor and Helmholtz instabilities.
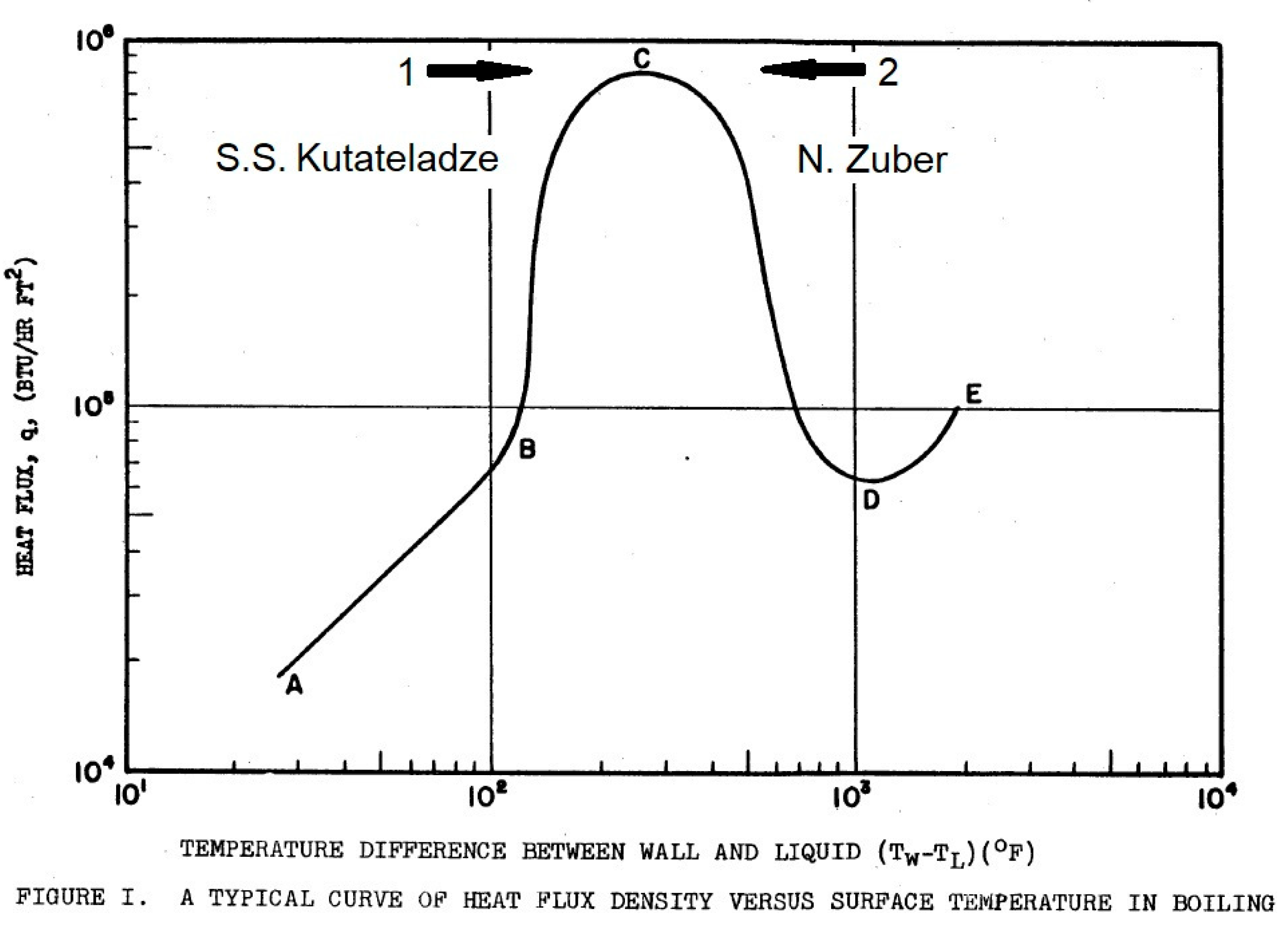
Figure 1 shows the boiling curve from [
3], where AB is the convective heat transfer regime, the no-boiling region; BC is the regime of nucleate boiling; CD is the regime of transitional boiling; DE is the film boiling regime; at point C, the boiling crisis begins. In this diagram, the branches BC and CD of the boiling curve in logarithmic coordinates are almost symmetrical relative to the vertical axis drawn through point C.
It follows from the literature review given in [
3] that Zuber was well acquainted with the works of Kutateladze [
1,
2]. In [
3], he writes: “Because the flow configuration in nucleate boiling is not well defined, an analysis of the critical heat flux performed by considering nucleate boiling is reduced to dimensional analysis.” Kutateladze in his works [
1,
2] performed a dimensional analysis and considered the transition to CHF in terms of nucleate boiling (shown by arrow 1 in
Figure 1).
In his model [
3,
4], Zuber considered the transition to CHF in terms of transitional boiling (shown by arrow 2 in
Figure 1). The only quantitative experimental study of transitional boiling in the literature at the time of model development was [
5]. The process of methanol boiling on a horizontal copper tube with an outer diameter of 3/8 inch (~9.5 mm) and a working length of about 6 inches (~152 mm) was studied there. The tube was heated with steam. Zuber studied the photographs and description of transitional boiling on the horizontal tube, presented in [
5]. Further in [
3] he wrote: “… because of Taylor instability, a definite geometrical configuration can be expected in transitional boiling. We shall make use of this defined geometry and analyze, therefore, the critical heat flux by considering transitional boiling”, as well as “Thus, in the region of the critical heat flux a small increase of the heat flux density results in a large increase of the surface temperature. Consequently, in the
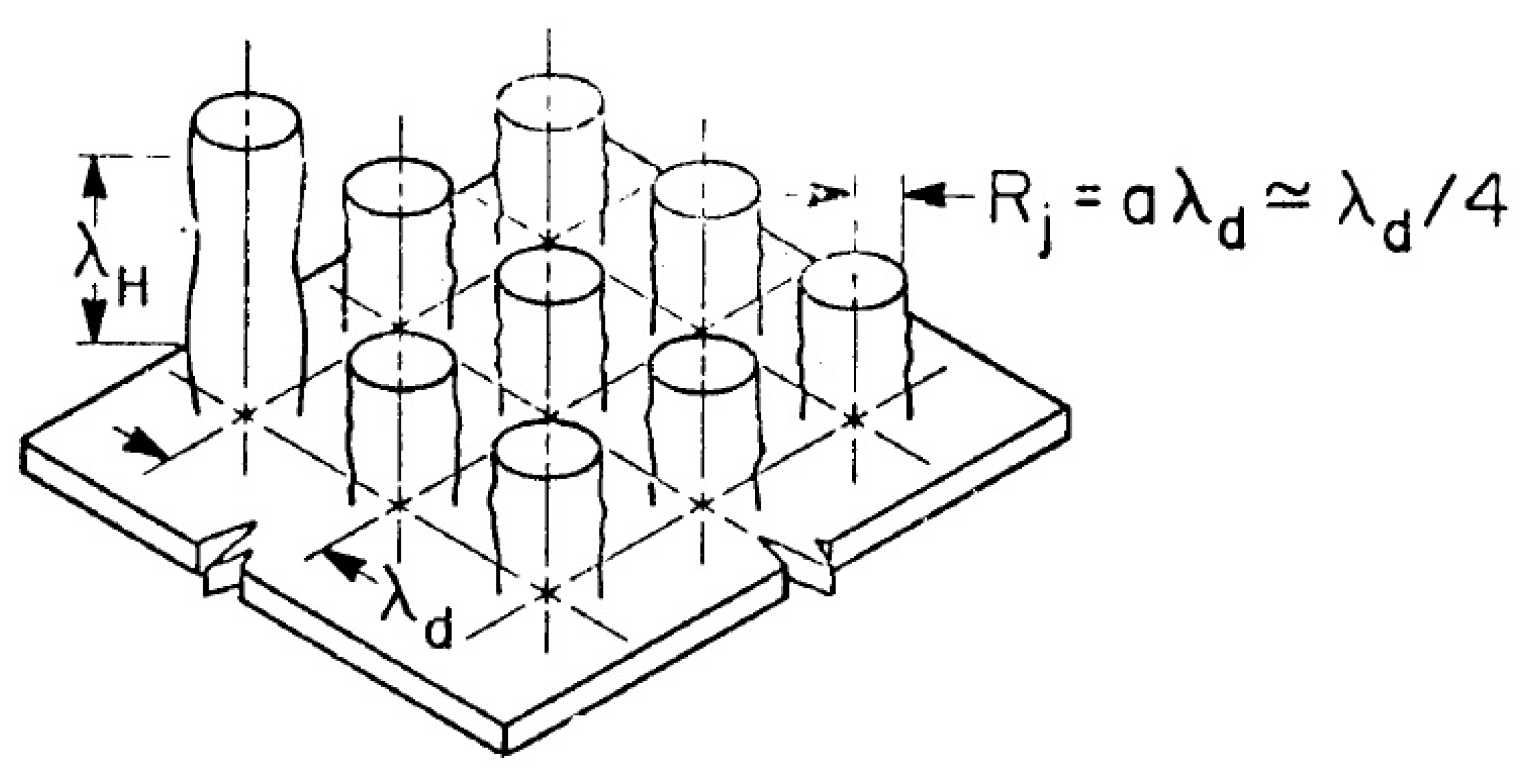
plane there exists a plateau across which boiling changes from the nucleate to the transitional boiling regime”. Based on the analysis of observations on a tube, he formulated the main theoretical provisions for calculating the boiling crisis on an infinite horizontal surface. The Zuber model considers an already formed vapor film on a plane under a liquid layer. Taylor in [
6] showed that the interface separating two liquids of different densities is unstable if the acceleration is directed from a heavier medium to a lighter one. Bellman and Pennington [
7] showed that interface perturbations can be stable or unstable depending on whether the critical wavelength is shorter or longer, given by the equation
, where
is the capillary constant. They also showed that the “most dangerous” instability wavelength, i.e., the wavelength at which the perturbation amplitude grows most rapidly, is determined by the expression
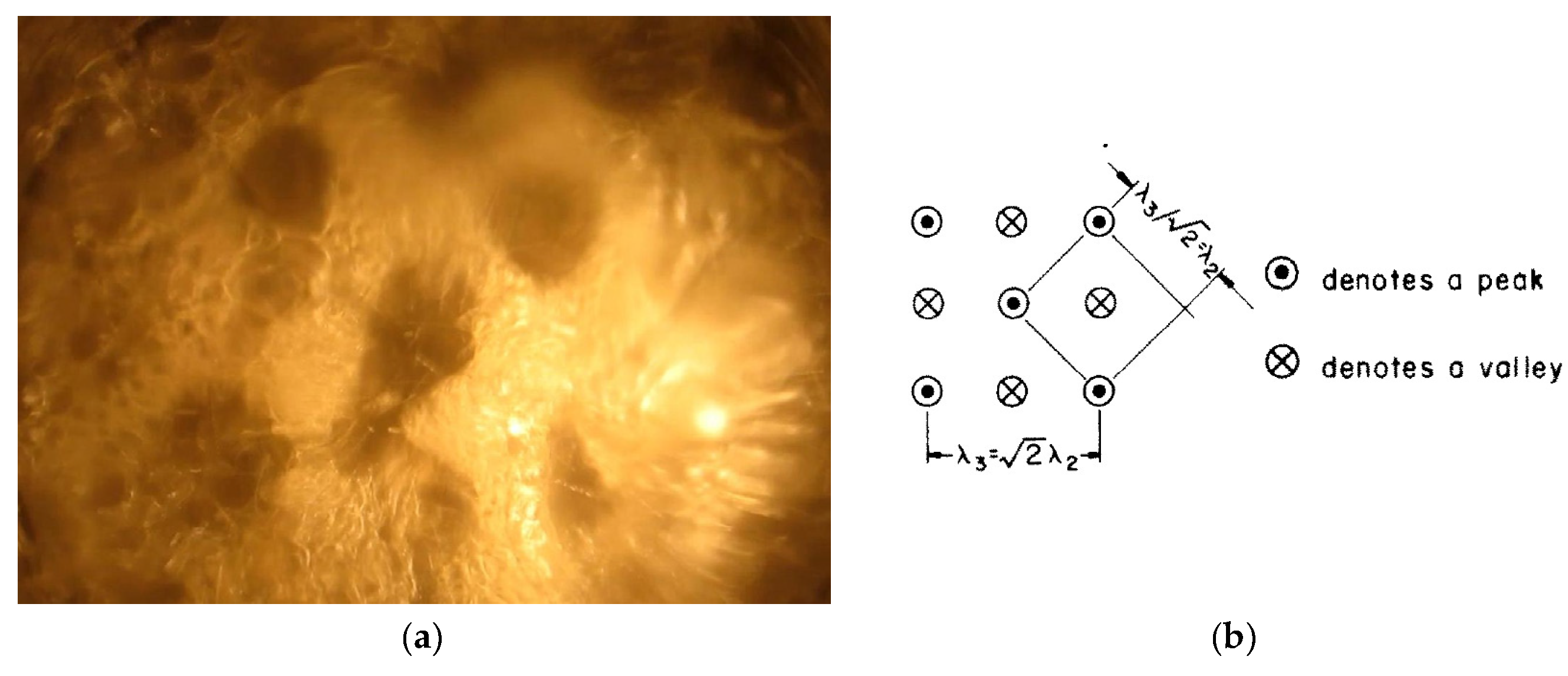
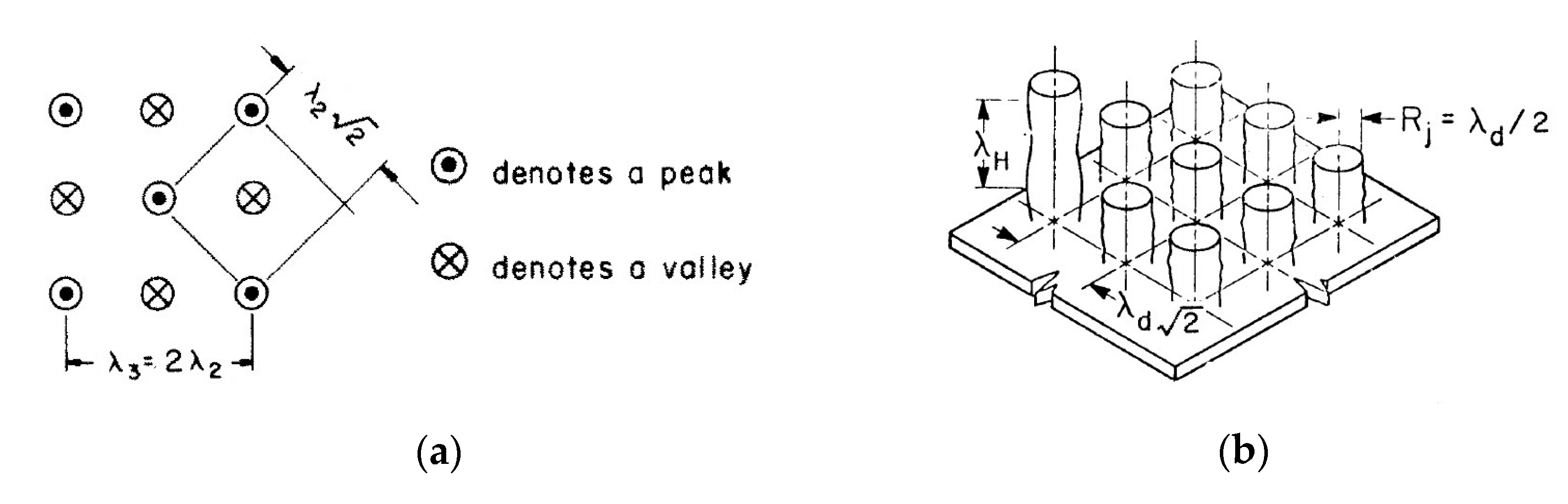
. In the Zuber model (
Figure 2), vapor jets rise up at the vertices of a square grid with a side corresponding to the characteristic Taylor instability wavelengths. The critical vapor velocity in jets is determined by the Helmholtz instability. The wavelength of the Helmholtz instability was taken equal to
, where
or
, where
is a vapor jet radius. For the critical heat flux, Zuber obtained the following expression:
where
, if
and
, if
. Zuber recommends taking
as the most appropriate value.
Authors of [
8] proposed to improve the Zuber equation for CHF by assuming
and they obtained
. In [
9],
was obtained by considering the interfacial detachment mechanism.
Due to the fact that equations (1) and (2) almost coincide (the expression under the root on the right in formula (2) noticeably differs from one only at near-critical pressures), in some publications one can come across the terms “Kutateladze–Zuber theory” or “Kutateladze–Zuber equation”. There is a note on the difference in approach to the boiling crisis in Theofanous et al. [
10,
11]: “The commonality of the Kutateladze and Zuber ideas, expressing the external hydrodynamics control, boils down to scaling a critical vapor velocity in terms of the relevant fluid properties–densities and surface tension–and the body force field. There is no external length scale, and this implies a limitation to horizontal infinite flat plates. For both there is an ‘‘internal’’ length scale, the capillary length, and this provides an approximate measure of the dimensions above which the plate can be considered infinite… But there are also some basic differences in the two ways of thinking that have to be kept in mind in addressing issues of geometry, satisfying respectively self-similarity.
Zuber’s postulated Rayleigh–Taylor controlled counter-current flow is macroscopic, and it is easy to see that self-similarity for it requires strict absence of external length scales able to introduce departures from (on the average) one-dimensional (1D) behavior [
4]. In turn, this requires that the liquid pool contains no bypass regions (its cross-section is fully coincident with the heater’s cross-section), and that it is of small aspect ratio (height divided by the smallest lateral dimension)”.
He further explains that Gaertner experiment [
12] with a disk of the 5-cm diameter, placed on the bottom of a deep (20 cm) and wide (14 cm) reservoir with liquid, does not satisfy these requirements.
“Kutateladze’s idea of ‘‘repulsion’’ is more vaguely stated, and thus it allows a somewhat greater flexibility in satisfying self-similarity. It appears that he is focused in a boundary layer, right next to the heater surface, and he thinks of a hydrodynamic transition occurring within this layer. Following his experiments with Gogonin [
13], they assert that a width of even 2 capillary lengths (~2 mm in their ethanol experiments) is sufficient to eliminate the effect of an external length scale. Their water pool was deep (~15 cm) and much wider than their heater (10 cm). These experiments yielded the
Ku = 0.145 result (as in Eq. (1)). Excellent agreement with Eq. (1) was also reported by Kutateladze and Malenkov in later water boiling experiments [
14]. Unfortunately, no mention of the flow regimes observed was made in these papers, but Kutateladze had no doubt that the twophase flow above the heater was dispersed and highly chaotic (i.e., he disagreed with Zuber’s picture of a relatively regular array of vapor jets). What remains unclear is the extent of interplay between the external hydrodynamics and the transition within the boundary layer that was the focus of Kutateladze’s interest. In this interpretation both Gaertner’s and Kutateladze’s own experiments are open to question of such ‘‘external’’ influences. Remarkably, all other previous experiments are even further removed from these self-similarity requirements.”
Theofanous et al. [
11] propose to introduce the scale separation. They suppose that “Based on infrared thermometry, and aided by X-ray radiography data on void fraction, the case for a scale separation phenomenon in high heat flux pool boiling is argued. This indicates that boiling crisis is controlled by the microhydrodynamics and rupture of an extended liquid microlayer, sitting and vaporizing autonomously on the heater surface”. They conclude: “Thus we can conclude that boiling heat transfer is independent of the complex two-phase flow hydrodynamics above the heater, and in particular that the previous hydrodynamic theory of boiling crisis is not appropriate”. In the work of Yagov [
15], a new model of liquid boiling crisis was developed based on the physical estimates of the process of evaporation of the thin liquid film menisci, adjacent to “dry spots” on the heating surface. The crisis beginning is associated with violation of the balance of liquid supplied to the “dry spot” boundary and evaporated liquid. The boiling crisis is explained as a result of coalescence and growth of the “dry spots” area on the heated surface. Calculation relations are obtained for the CHF at pool boiling in the region of low reduced pressures
PS/Pcr < 0.001 (
PS and
Pcr are the saturation pressure and the critical pressure, respectively), for the region of high reduced pressures
PS/Pcr > 0.03, and the interpolation formula for CHF calculation at arbitrary pressure is derived. The calculated dependences are in good agreement with the experimental data. In most current experimental studies on heat transfer during boiling, the near-wall region, formation and growth of dry spots are investigated [
10,
11,
16,
17,
18].
In [
8], the authors specified the requirements for a setup to test the Zuber theory with a heater in the form of an infinite flat plate. For such a testing, to get rid of the horizontal inflow of liquid, which has a noticeable effect on CHF, it is necessary that the experimental conditions should be as close as possible to the theoretical model of the process on an infinite plate, i.e., the experimental heater should be a very clean plate with the dimensions much larger than
limited by vertical lateral walls. It is shown in [
20,
21] that with the ratio of horizontal dimensions for a square plate
, where
is the side of a square plate, the surface can be considered an infinite plane.
In [
22], Lienhard pointed out a number of erroneous assumptions, including the arbitrariness of some of relationships used in the Zuber model [
3,
4]. In [
22], a number of unresolved issues in the hydrodynamic theory of boiling crises are presented. One of the questions concerns mainly the properties of the heating surface that are not taken into account by the Zuber theory. The second unresolved issue, considered in [
22], is the question of where the Helmholtz instability occurs actually, on what scales from the heating surface. The problem with the Zuber crisis model is that in many cases it is difficult to identify the vapor jet behavior. As Lienhard writes in [
22], Haramura and Katto [
23] suggested that the Helmholtz process does not develop in the obvious vapor jets and columns at all, but rather in a small structure of mini-jets near the surface that feed the visible jets from below. At its core, the theory of Haramura and Katto is also a substantiation of the hydrodynamic theory. However, in this theory, in contrast to the Zuber theory, a liquid film under a mushroom-shaped bubble, directly adjacent to the heating surface, is considered.
The works of Theofanous et al. [
10,
11] deny the influence of external (macro) hydrodynamics on the boiling crisis. They also question the influence of external hydrodynamics on the boiling crisis in the experiments of Gaertner [
12] and Kutateladze [
13,
14]. At the same time, Theofanous remarks that, unfortunately, the observed flow regimes were not mentioned in Kutateladze works. In Gaertner experiments, large mushroom-shaped bubbles were observed in pre-crisis boiling regimes. The theory of Haramura and Katto was constructed on the basis of these observations [
23]. It is believed that mushroom-shaped bubbles are formed by merging of several bubbles in a region of vapor (light phase) accumulation near the wall. The microhydrodynamics of the near-wall region affects the external (macro) hydrodynamics. To find out this influence, it is necessary to study the spatial distribution of such bubbles over the heating surface under the conditions of the infinite plane geometry proposed in [
8], when the horizontal inflow of liquid is excluded.
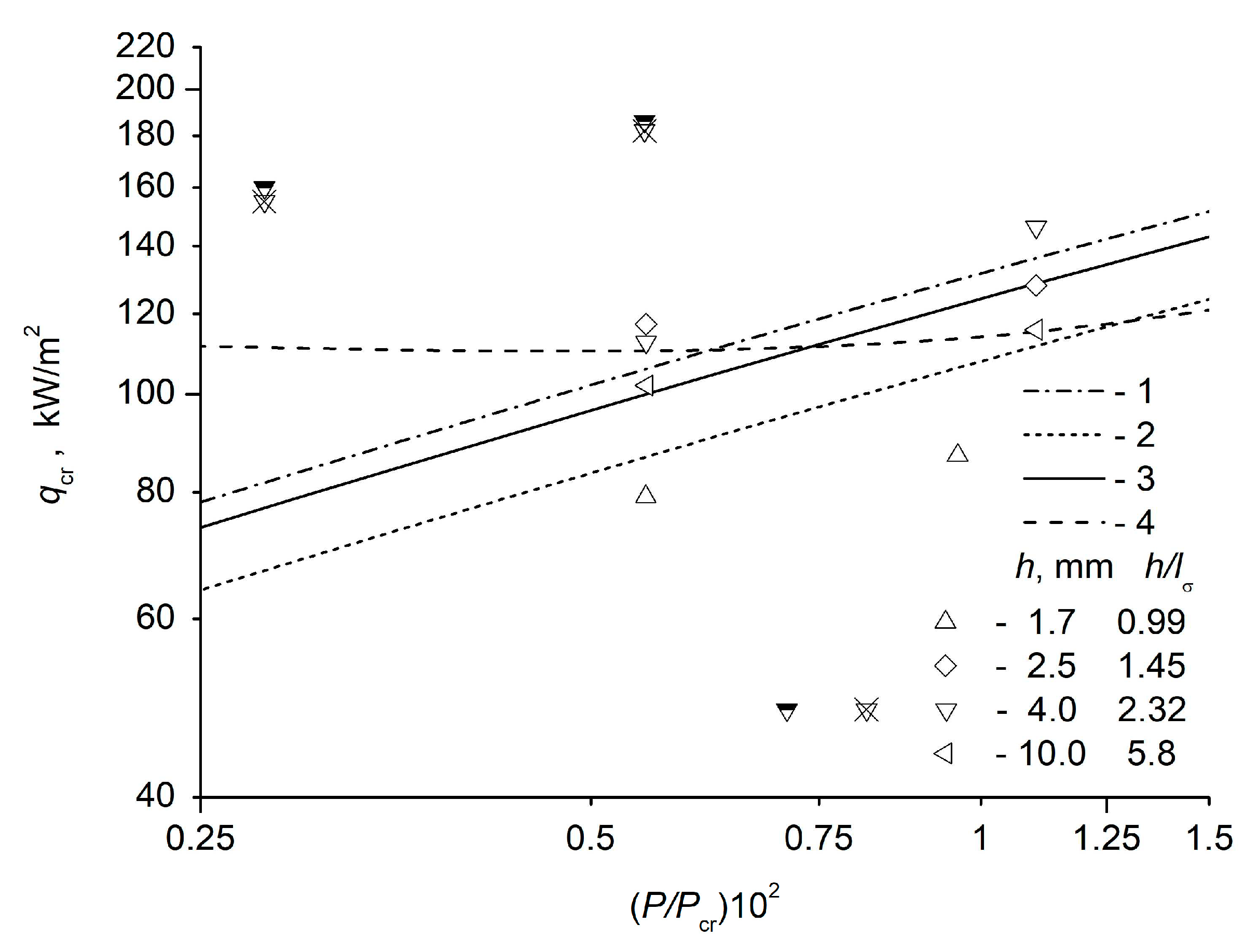
As it was noted by Theofanous et al. [
11], in the Zuber theory the ratio of the liquid layer height to the smallest transverse dimension must be small. The liquid layer must be of a finite height. Theofanous et al. [
11] present experimental data on the crisis of water boiling at atmospheric pressure in a parallelepiped volume with height
H = 70 mm and the smallest linear horizontal dimension
l = 35 mm, i.e.
H / l = 2, while the average void fraction ε = 40%, (where ε is the ratio of the vapor volume to the entire volume of the system). Hence it follows that the height of the initial liquid layer at a temperature of 100°C is 42 mm. The wavelength of the Helmholtz instability plotted from the most dangerous wavelength of the Taylor instability is
mm. Perhaps, by pure chance, a layer of water with a height almost equal to the wavelength of the Helmholtz instability in the Zuber theory was used in the experiments of [
11]. It is difficult to establish the upper limit of the layer height, when hydrodynamic boiling crisis is observed (the liquid is “pushed” from the heating surface by a vapor film). At the same time, there are thinner liquid films, when the boiling liquid film breaks down and the surface drying crisis is observed. In the geometry of an infinite plane [
8], it is possible to establish the minimum height of the liquid layer, above which the usual hydrodynamic boiling crisis is observed, and in the thinner layers, the surface drying crisis is observed.
In the geometry of an infinite plane [
8], when the heating surface is limited by vertical walls, the height of the two-phase layer of boiling liquid increases with an increase in its vapor content. One of the shortcomings of the Zuber theory is that, according to the theory, the vapor content of a liquid layer is π/16, while in experiments it is much higher. In the work of Kutateladze and Malenkov [
14] on establishing the analogy between the process of nucleate boiling and bubbling, the influence of this parameter is also directly indicated.
In their previous work [
24], the spatial distribution of mushroom-shaped bubbles over the heating surface is studied under the conditions of geometry of an infinite flat plate on a smooth heating surface. The effect of liquid layer height (n-dodecane) and void fraction on CHF and its mechanism is studied. Using the mathematical apparatus of the Zuber theory, the authors of this paper proposed a hydromechanical model of the nucleate boiling crisis, which takes into account the effect of void fraction on CHF. The theory of CHF is considered from the point of developed nucleate boiling using the mathematical apparatus of the Zuber theory.
In this paper, we present new experimental data on CHF during n-dodecane boiling on the surface of two 2D modulated capillary-porous coatings of the same morphology and geometry, made of different materials. Experiments are compared with theoretical calculations. New data on CHF for Novec – 7100 dielectric liquid on a smooth surface are also presented. A symmetric problem is considered: the Zuber problem in the traditional formulation with the involvement of new experimental data on observation of vapor columns in a liquid layer from the point of transitional boiling, in which the shortcomings of the known theory are eliminated. The numerical values of obtained solutions of one problem are compared, but with a symmetrical approach (in terms of nucleate boiling and in terms of transitional boiling). Attention is paid to the analysis of the arrangement symmetry of the observed structures and the results of solving the problems.
2. Materials and Methods
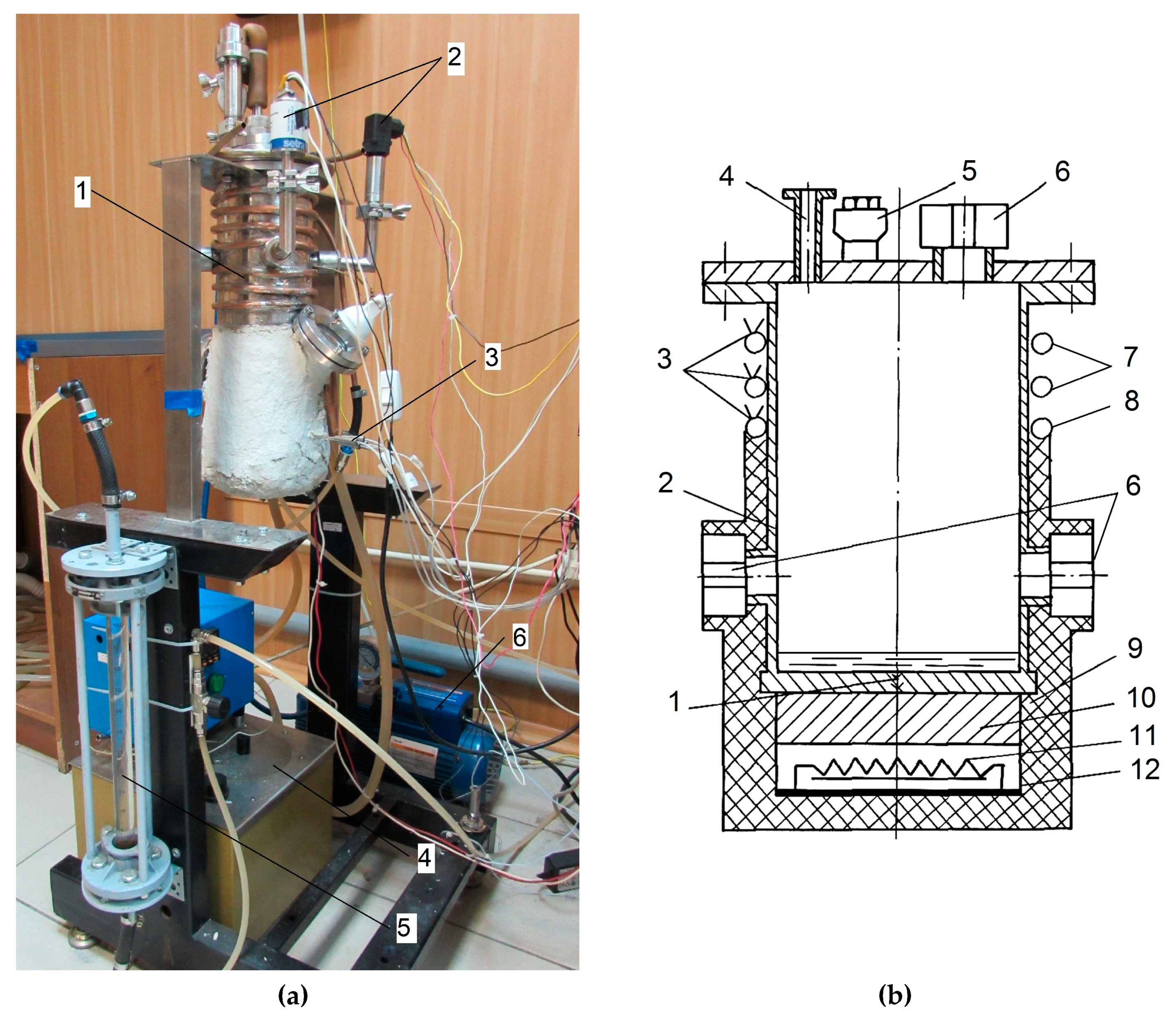
The studies were carried out on an experimental setup consisting of a working chamber, a cooling system, a pressure and temperature measurement system, a system for collecting and processing test data, and a system for controlling the heaters power and supply. In
Figure 3 (a), the working chamber was mounted on a frame; the pressure in the working chamber was measured by two pressure sensors. The temperature was measured with thermocouples; the reduced pressure was created by a vacuum pump. The water flow in the cooling system of the working chamber was regulated by a rotameter; the temperature in the coil for heating the working chamber was kept constant by means of a thermostat while pumping the liquid. The ionization-deformation pressure sensor Setra-730 is used in the pressure range from 33 to 1333 Pa. The measurement error of this sensor is ± 0.5% of the current reading. The measurement range of the deformation - membrane pressure sensor ASG Edwards is from 133 to 2·10
5 Pa. The measurement error is ± 0.2% of the total scale. This sensor was used to measure pressures above 1000 Pa. Before each series of measurements, the sensor was calibrated based on the readings of a more accurate sensor (Setra-730) at low pressure, and then appropriate corrections were added to the measured data. The signals of pressure and temperature sensors were recorded using the National Instruments equipment and processed in the LabVIEW application environment. A detailed description of the setup is given in [
25].
Figure 3.
Experimental facilities: (a) –photograph of experimental setup: 1 –process chamber of experimental setup, 2 – pressure sensors, 3 – thermocouples for measuring the temperature of the heated surface, 4 – thermostat, 5 – rotameter, 6 – vacuum pump; (b) –process chamber of experimental setup: 1 – bottom (smooth or with capillary-porous coating), 2 – case, 3 – thermocouples, 4 – branch pipe for fixing the pumping system, 5 – vacuum inlet, 6 – inspection windows, 7 – cooling coil, 8 – heating coil, 9 – thermal insulation layer, 10 – brass plate, 11 – electric heater, 12 – electric heater cover.
Figure 3.
Experimental facilities: (a) –photograph of experimental setup: 1 –process chamber of experimental setup, 2 – pressure sensors, 3 – thermocouples for measuring the temperature of the heated surface, 4 – thermostat, 5 – rotameter, 6 – vacuum pump; (b) –process chamber of experimental setup: 1 – bottom (smooth or with capillary-porous coating), 2 – case, 3 – thermocouples, 4 – branch pipe for fixing the pumping system, 5 – vacuum inlet, 6 – inspection windows, 7 – cooling coil, 8 – heating coil, 9 – thermal insulation layer, 10 – brass plate, 11 – electric heater, 12 – electric heater cover.
The working chamber was made in the form of a thermosyphon (
Figure 3 (b)). It is a cylindrical vessel made of steel 12Kh18N10T with an inner diameter of 120 mm, a height of 300 mm, and a wall thickness of 1 mm. To achieve uniform distribution of the heat flux over the heated surface, a highly thermally conductive brass plate 30 mm thick was placed between the chamber bottom and a heater with a power of 2 kW. To reduce the contact thermal resistance, the gap between the chamber bottom and the plate was filled with a special highly thermally conductive paste. There is a cooling coil on the outer surface of the upper part of the chamber. The chamber is cooled by water flowing through a coil. To reduce heat loss due to leakage along the chamber walls from the bottom to the cooling coil and to ensure uniform temperature distribution at the chamber bottom, an additional coil for heating the chamber walls was mounted below the cooling coil. There were three inspection windows on the working chamber top and on its wall to perform visual observations. Two windows were used for observation and one window was used for illumination. The results of visual observations were recorded by a video camera with a shooting frequency of 24 fps and 240 fps. To measure the heating surface temperature, the copper-constantan thermocouples in stainless capillaries were inserted into five holes of 1.5-mm diameter in the bottom at different heights. The heat flux was determined from the temperature gradient measured along the bottom centerline using a linear Fourier approximation of the output signal of five thermocouples. The calculated error in determining the heat flux was about ± 16% for a heat flux
q = 10
3 W/m
2, about ± 10% for a heat flux
q = 10
4 W/m
2, and ± 4% for a heat flux
q = 10
5 W/m
2. The surface temperature was determined by linear extrapolation of the measured temperature profile to the surface. The total error in measuring the surface temperature was no more than ± 0.4°C at 200°C. The pressure in the working chamber was kept constant. The lower horizontal surface (bottom) of the working chamber 12 mm thick, which is a horizontal smooth surface 120 mm in diameter with roughness Rz = 3.2 μm, bounded by the vertical walls of the chamber, was used as the heating surface. Two surfaces with capillary-porous coatings were also used. Coatings were applied to the working chamber bottom using SLM/SLS (Selective Laser Melting/Selective Laser Sintering) 3D laser printing technology.
N-dodecane was used as the working fluid. The physical properties of n-dodecane were calculated according to [
26]. The experiments were carried out at pressure
PS (
PS /
Pcr): 5 kPa (2.8∙10
-3), 10 kPa (5.5∙10
-3), and 20 kPa (1.1∙10
-2). When the pressure changed from 10 kPa to 20 kPa, the saturation temperature changed from 138.4°C to 158.3°C. The value of the capillary constant for n-dodecane varied from 1.53 mm to 1.46 mm; accordingly, the most dangerous length of the Taylor instability
varied from 16.6 to 15.9 mm. There was the ratio
L /
λd ~ 7.2, that is, in terms of configuration and size, the heating surface is an infinite flat plate under the conditions of [
8]. In this work, the layers of n-dodecane with a height of 1.7, 2.5, 4, and 10 mm were used. Some experiments on a smooth surface were carried out using the layer of Novec – 7100 dielectric liquid 10 mm high at a pressure of 100 kPa. The physical properties of Novec – 7100 were calculated according to [
27,
28]. The capillary constant of Novec – 7100 dielectric liquid at these pressures varies from 0.9 mm to 0.85 mm; therefore, the conditions of infinite flat plate geometry [
8] can also be satisfied for this liquid.
2.1. Capillary-porous coatings
Capillary-porous coatings of stainless steel LPW 155 (15-5PH) and bronze powder AISI C836000 were applied to the lower base (bottom) of geometrically similar chambers specially made for each coating using laser 3D printing technology by the SLM/SLS method (selective laser melting / selective laser sintering). When applying coatings, special attention was paid to creating coatings of the same profile, with the same porosity and particle size, which remain in the porous structure. The coating technology is described in detail in [
29,
30].
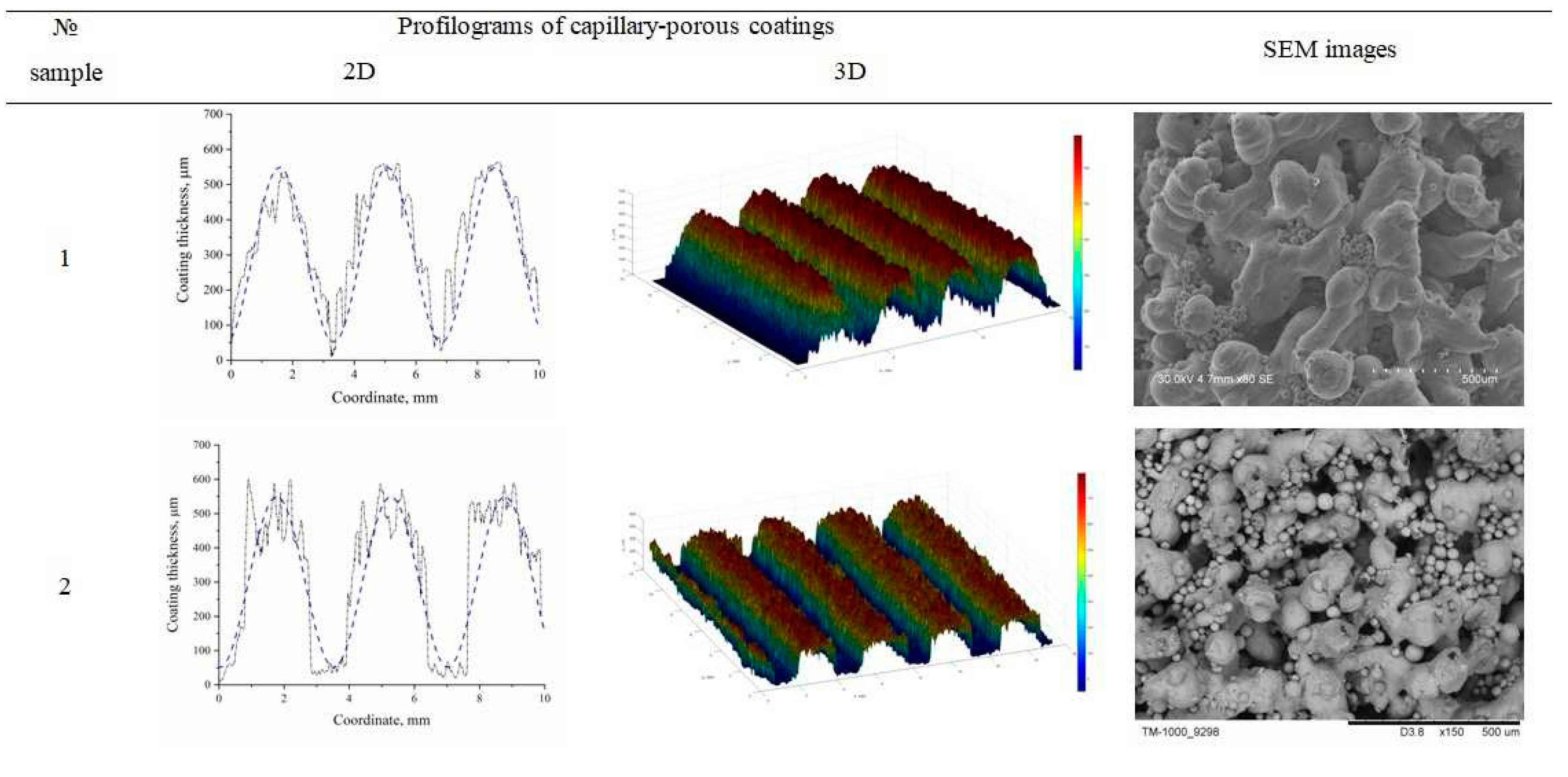
The morphology of the stainless steel sample was analyzed using a Hitachi S-3400N scanning electron microscope, and the morphology of the bronze samples was analyzed using a Hitachi TM1000 microscope. A BRUKER Contour GT-K1 optical microscope profilometer was used to plot coating profilograms. Topographic maps with thickness distribution and SEM-images of the developed capillary-porous coatings No. 1, 2 are presented in
Figure 4. According to the data obtained with the help of an optical microscope profilometer, the following characteristics of the coatings were determined: the height of ridges, the thickness of the residual layer between the ridges, and modulation wavelength
λm. Porosity measurements were carried out by measuring the density of samples of 10 × 10 × 5 mm and comparing the results with the density of the coating material.
Figure 4.
Profilograms and SEM images of capillary-porous coatings.
Figure 4.
Profilograms and SEM images of capillary-porous coatings.
The parameters of capillary-porous coatings are presented in
Table 1. The effective thermal conductivity of the coating was calculated using the formula:
where ϕ is the coating porosity;
λ is the thermal conductivity of the coating material. In [
31], formula (3) was derived for the cases, when the thermal conductivity is much smaller than the thermal conductivity of the coating material.