Submitted:
29 July 2023
Posted:
31 July 2023
You are already at the latest version
Abstract
Keywords:
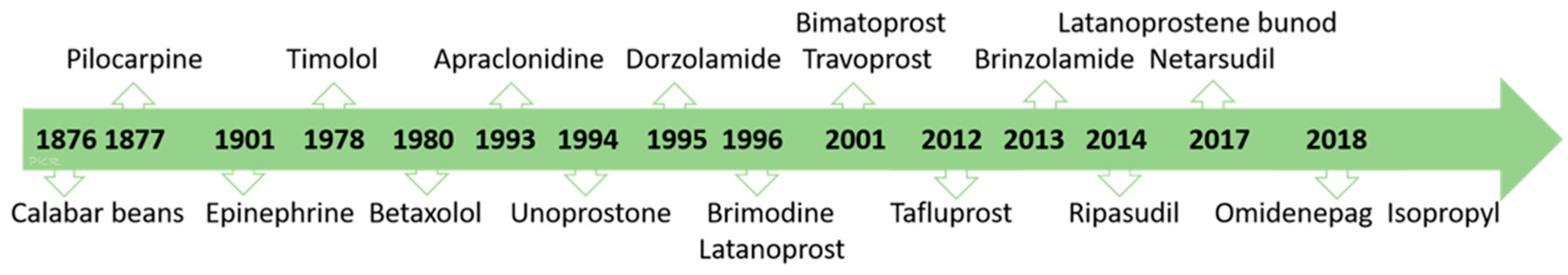
Introduction
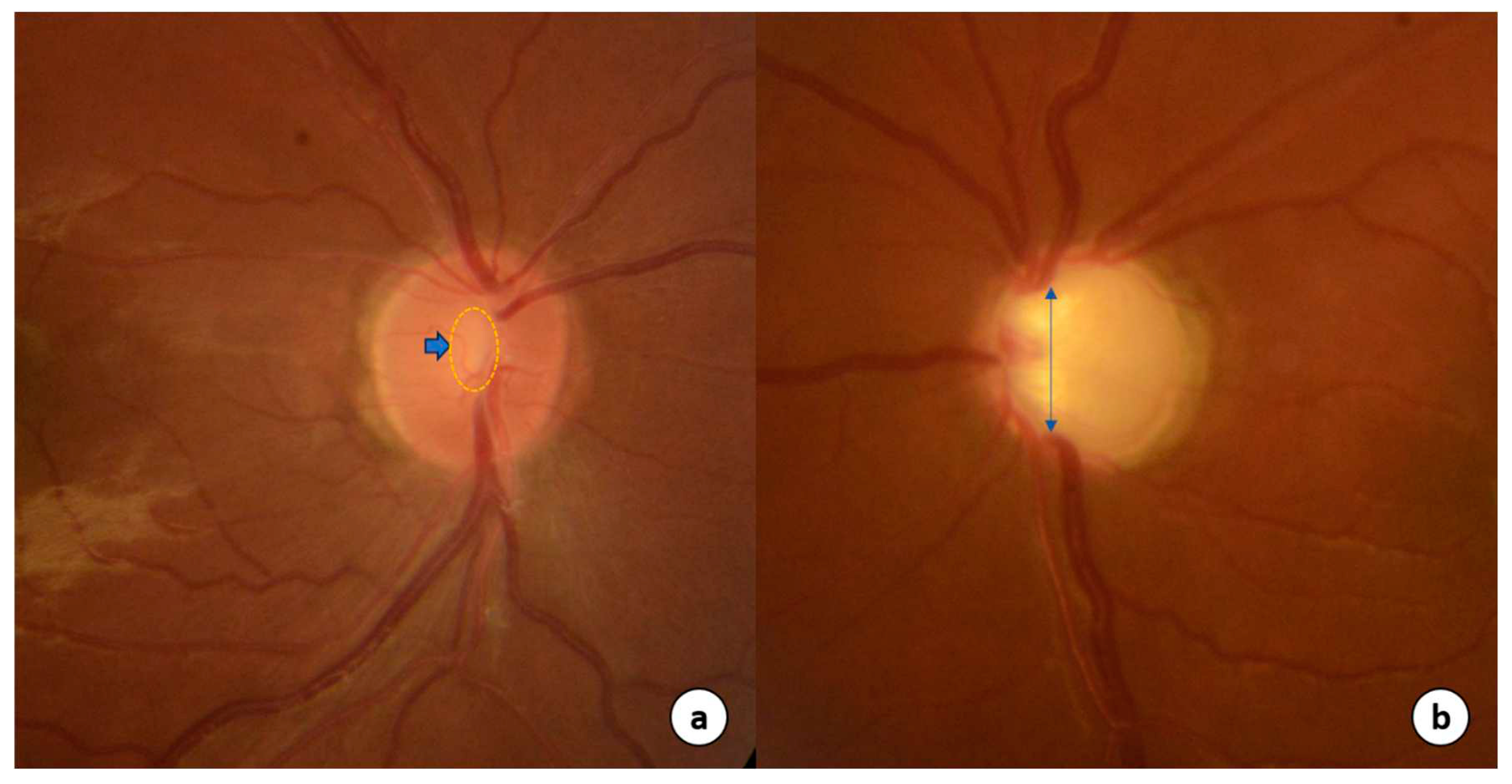
Pathophysiology of glaucoma
Targets for pharmacotherapy
Aqueous suppressants drugs
Beta-adrenergic antagonists
Adrenergic agonists
Carbonic anhydrase inhibitors
Uveoscleral outflow drugs
Prostaglandin analogues
Trabecular outflow drugs
Cholinergic
Nitic Oxide donors
Rho Kinase inhibitors
Therapeutic efficacy
IOP independent
Ocular blood flow
Neuroprotection
Choice of therapy
Tolerability and safety
Systemic side effects
Local side effects
Adjunctive therapy
Conclusions
References
- Ventura, L.M.; Sorokac, N.; Santos, R.D.L.; Feuer, W.J.; Porciatti, V. The Relationship between Retinal Ganglion Cell Function and Retinal Nerve Fiber Thickness in Early Glaucoma. Investig. Opthalmology Vis. Sci. 2006, 47, 3904–3911. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Blindness and Vision Impairment Collaborators.; Vision Loss Expert Group of the Global Burden of Disease Study.; Steinmetz J.D. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to vision 2020: the right to sight: an analysis for the global burden of disease study. Lancet. Glob. Health. 2021, 9, e144–e160.
- Kooner, K.S.; Al Bdoor, M.; Cho, B.J.; Adams-Huet, B. Risk factors for progression to blindness in high tension primary open-angle glaucoma: Comparison of blind and non-blind subjects. Clin. Ophthalmol. 2008, 2, 757–762. [Google Scholar] [CrossRef]
- Oliver, J.E.; Hattenhauer, M.G.; Herman, D.; Hodge, D.O.; Kennedy, R.; Fang-Yen, M.; Johnson, D.H. Blindness and glaucoma: a comparison of patients progressing to blindness from glaucoma with patients maintaining vision. Am. J. Ophthalmol. 2002, 133, 764–772. [Google Scholar] [CrossRef]
- Paula, J.S.; Furtado, J.M.; Santos, A.S.; Coelho, R.d.M.; Rocha, E.M.; Rodrigues, M.d.L.V. Risk factors for blindness in patients with open-angle glaucoma followed-up for at least 15 years. Arq. Bras. de Oftalmol. 2012, 75, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Vaswani, R.; Singh, A. Importance of defining a target intra-ocular pressure: A Meta-Analysis. Invest. Ophthalmol. Vis. Sci.2012, 53:5052. Invest. Ophthalmol. Vis. Sci. 2012, 53, 5052. [Google Scholar]
- Boland, M.V.; Ervin, A.-M.; Friedman, D.S.; Jampel, H.D.; Hawkins, B.S.; Vollenweider, D.; Chelladurai, Y.; Ward, D.; Suarez-Cuervo, C.; Robinson, K.A. Comparative Effectiveness of Treatments for Open-Angle Glaucoma: A Systematic Review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 158, 271–279. [Google Scholar] [CrossRef]
- Qu, J.; Wang, D.; Grosskreutz, C.L. Mechanisms of retinal ganglion cell injury and defence in glaucoma. Exp. Eye. Res. 2010, 91, 48–53. [Google Scholar]
- Burgoyne, C.F.; Downs, J.C.; Bellezza, A.J.; Suh, J.-K.F.; Hart, R.T. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog. Retin. Eye Res. 2005, 24, 39–73. [Google Scholar] [CrossRef]
- Quigley, H.A.; McKinnon, S.J.; Zack, D.J.; Pease, M.E.; Kerrigan-Baumrind, L.A.; Kerrigan, D.F.; Mitchell, R.S. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3460–3466. [Google Scholar]
- Brubaker, R.F. Goldmann’s equation and clinical measures of aqueous dynamics. Exp. Eye. Res. 2004, 78, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Bill, A. Uveoscleral drainage of aqueous humor: physiology and pharmacology. Prog. Clin. Biol. Res. 1989, 312, 417–427. [Google Scholar] [PubMed]
- Miyazaki, M.; Segawa, K.; Urakawa, Y. Age-related changes in the trabecular meshwork of the normal human eye. Jpn. J. Ophthalmol. 1987, 31, 558–569. [Google Scholar]
- Toris, C.B.; Camras, C.B.; E Yablonski, M. Acute versus chronic effects of brimonidine on aqueous humor dynamics in ocular hypertensive patients. Arch. Ophthalmol. 1999, 128, 8–14. [Google Scholar] [CrossRef]
- Acott, T.S.; Kelley, M.J.; Keller, K.E.; Vranka, J.A.; Abu-Hassan, D.W.; Li, X.; Aga, M.; Bradley, J.M. Intraocular Pressure Homeostasis: Maintaining Balance in a High-Pressure Environment. J. Ocul. Pharmacol. Ther. 2014, 30, 94–101. [Google Scholar] [CrossRef]
- Gedde, S.J.; Chen, P.P.; Muir, K.W.; Vinod, K.; Lind, J.T.; Wright, M.M.; Li, T.; Mansberger, S. L; American Academy of Ophthalmology Preferred Practice Pattern Glaucoma Panel. Primary Angle-Closure Disease Preferred Practice Pattern®. Ophthalmology. 2021, 128, P30–P70. [Google Scholar]
- Realini, T. A History of Glaucoma Pharmacology. Optom. Vis. Sci. 2011, 88, 36–38. [Google Scholar] [CrossRef]
- US Food and Drug Administration. New drug approval 215092; 22.09.2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215092Orig1s000ltr (accessed on 23 June 2023).
- E Trope, G.; Clark, B. Beta adrenergic receptors in pigmented ciliary processes. Br. J. Ophthalmol. 1982, 66, 788–792. [Google Scholar] [CrossRef]
- Elena, P.-P.; Denis, P.; Kosina-Boix, M.; Saraux, H.; Lapalus, P.; Casini, G.; Monte, M.D.; Fornaciari, I.; Filippi, L.; Bagnoli, P.; et al. Beta Adrenergic Binding Sites in the Human Eye: An Autoradiographic Study. J. Ocul. Pharmacol. Ther. 1990, 6, 143–149. [Google Scholar] [CrossRef]
- Bartels, S.P.; O Roth, H.; Jumblatt, M.M.; Neufeld, A.H. Pharmacological effects of topical timolol in the rabbit eye. Investig. Ophthalmol. Vis. Sci. 1980, 19. [Google Scholar]
- Bylund, D.B.; Chacko, D.M. Characterization of alpha2 adrenergic receptor subtypes in human ocular tissue homogenates. Investig. Ophthalmol. Vis. Sci. 1999, 40. [Google Scholar]
- Chiou, G.C.; Chen, Y. Effects of Antiglaucoma Drugs on Ocular Blood Flow in Ocular Hypertensive Rabbits. J. Ocul. Pharmacol. Ther. 1993, 9, 13–24. [Google Scholar] [CrossRef]
- Gilsbach, R.; Röser, C.; Beetz, N.; Brede, M.; Hadamek, K.; Haubold, M.; Leemhuis, J.; Philipp, M.; Schneider, J.; Urbanski, M.; et al. Genetic Dissection of α2-Adrenoceptor Functions in Adrenergic versus Nonadrenergic Cells. Mol. Pharmacol. 2009, 75, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Stamer, W.D.; Huang, Y.; Seftor, R.E.; Svensson, S.S.; Snyder, R.W.; Regan, J.W. Cultured human trabecular meshwork cells express functional alpha 2A adrenergic receptors. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2426–2433. [Google Scholar]
- Yamagishi, R.; Honjo, M.; Aihara, M. Effect of IOP-lowering drugs on episcleral venous pressure in mouse eye. Invest. Ophthalmol. Vis. Sci. 2018, 59, 2712. [Google Scholar]
- Gharagozloo, N.Z.; Relf, S.J.; Brubaker, R.F. Aqueous Flow is Reduced by the Alpha-adrenergic Agonist, Apraclonidine Hydrochloride (ALO 2145). Ophthalmology 1988, 95, 1217–1220. [Google Scholar] [CrossRef]
- Toris, C.B.; Tafoya, M.E.; Camras, C.B.; Yablonski, M.E. Effects of Apraclonidine on Aqueous Humor Dynamics in Human Eyes. Ophthalmology 1995, 102, 456–461. [Google Scholar] [CrossRef]
- Goldberg, I.; Galanopoulos, A. Clinical efficacy and neuroprotective effects of brimonidine in the management of glaucoma and ocular hypertension. Clin. Ophthalmol. 2009, 3, 117–122. [Google Scholar] [CrossRef]
- Simsek, T.; Yanik, B.; Conkbayir, I.; Zilelioglu, O.; Eliacik, M.; Erdur, S.K.; Altıok, I.B.; Gulkilik, G.; Aslan, C.A.; Kaya, F.; et al. Comparative Analysis of the Effects of Brimonidine and Dorzolamide on Ocular Blood Flow Velocity in Patients with Newly Diagnosed Primary Open-Angle Glaucoma. J. Ocul. Pharmacol. Ther. 2006, 22, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Enz, T.J.; Bittner, M.; Tribble, J.R.; Williams, P.A.; Thiel, M.A.; Schmid, M.K.; Bachmann, L.M.; Bochmann, F. Comparative Assessment of Retinal Blood Flow Velocity Changes Following Brimonidine and Brinzolamide Administration Using Retinal Function Imaging. Transl. Vis. Sci. Technol. 2022, 11, 16. [Google Scholar] [CrossRef]
- Krupin, T.; Sly, W.S.; Whyte, M.P.; Dodgson, S.J. Failure of Acetazolamide to Decrease Intraocular Pressure in Patients with Carbonic Anhydrase II Deficiency. Am. J. Ophthalmol. 1985, 99, 396–399. [Google Scholar] [CrossRef]
- Maren, T.H. The rates of movement of Na+, Cl-, and HCO-3 from plasma to posterior chamber: effect of acetazolamide and relation to the treatment of glaucoma. Investig. Ophthalmol. 1976, 15, 356–364. [Google Scholar]
- Maus, T.L.; Larsson, L.-I.; McLaren, J.W.; Brubaker, R.F. Comparison of Dorzolamide and Acetazolamide as Suppressors of Aqueous Humor Flow in Humans. Arch. Ophthalmol. 1997, 115, 45–49. [Google Scholar] [CrossRef]
- Camras, C.B.; Bito, L.Z.; E Eakins, K. Reduction of intraocular pressure by prostaglandins applied topically to the eyes of conscious rabbits. Investig. Ophthalmol. Vis. Sci. 1977, 16, 1125–1134. [Google Scholar]
- Lindsey, J.D.; Kashiwagi, K.; Boyle, D.; Kashiwagi, F.; Firestein, G.S.; Weinreb, R.N. Prostaglandins increase proMMP-1 and proMMP-3 secretion by human ciliary smooth muscle cells. Curr. Eye Res. 1996, 15, 869–875. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Kashiwagi, K.; Kashiwagi, F.; Tsukahara, S.; Lindsey, J.D. Prostaglandins increase matrix metalloproteinase release from human ciliary smooth muscle cells. Investig. Ophthalmol. Vis. Sci. 1997, 38. [Google Scholar]
- Ocklind, A. Effect of Latanoprost on the Extracellular Matrix of the Ciliary Muscle. A Study on Cultured Cells and Tissue Sections. Exp. Eye Res. 1998, 67, 179–191. [Google Scholar] [CrossRef]
- Davies, S.S.; Ju, W.-K.; Neufeld, A.H.; Abran, D.; Chemtob, S.; Roberts, L.J. Hydrolysis of Bimatoprost (Lumigan) to its Free Acid by Ocular TissueIn Vitro. J. Ocul. Pharmacol. Ther. 2003, 19, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Ong, T.; Scassellati Sforzolini, B.; Vittitow, J.L.; Singh, K.; Kaufman, P.L. VOYAGER study group. A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open-angle glaucoma: the VOYAGER study. Br. J. Ophthalmol. 2015, 99, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Kirihara, T.; Taniguchi, T.; Yamamura, K.; Iwamura, R.; Yoneda, K.; Odani-Kawabata, N.; Shimazaki, A.; Matsugi, T.; Shams, N.; Zhang, J.-Z. Pharmacologic Characterization of Omidenepag Isopropyl, a Novel Selective EP2 Receptor Agonist, as an Ocular Hypotensive Agent. Investig. Opthalmology Vis. Sci. 2018, 59, 145–153. [Google Scholar] [CrossRef]
- Fuwa, M.; Toris, C.B.; Fan, S.; Taniguchi, T.; Ichikawa, M.; Odani-Kawabata, N.; Iwamura, R.; Yoneda, K.; Matsugi, T.; Shams, N.K.; et al. Effects of a Novel Selective EP2 Receptor Agonist, Omidenepag Isopropyl, on Aqueous Humor Dynamics in Laser-Induced Ocular Hypertensive Monkeys. J. Ocul. Pharmacol. Ther. 2018, 34, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Gil, D.W.; A Krauss, H.; Bogardus, A.M.; WoldeMussie, E. Muscarinic receptor subtypes in human iris-ciliary body measured by immunoprecipitation. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1434–1442. [Google Scholar]
- Wiederholt, M.; Schäfer, R.; Wagner, U.; Lepple-Wienhues, A. Contractile response of the isolated trabecular meshwork and ciliary muscle to cholinergic and adrenergic agents. Ger. J. Ophthalmol. 1996, 5, 146–153. [Google Scholar]
- Bleiman, B.S.; Schwartz, A.L. Paradoxical Intraocular Pressure Response to Pilocarpine. Arch. Ophthalmol. 1979, 97, 1305–1306. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, P.L.; Bárány, E.H. Loss of acute pilocarpine effect on outflow facility following surgical disinsertion and retrodisplacement of the ciliary muscle from the scleral spur in the cynomolgus monkey. Investig. Ophthalmol. 1976, 15, 793–807. [Google Scholar]
- Wiederholt, M.; Thieme, H.; Stumpff, F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog. Retin. Eye Res. 2000, 19, 271–295. [Google Scholar] [CrossRef]
- A Erickson, K.; Schroeder, A. Direct effects of muscarinic agents on the outflow pathways in human eyes. Investig. Ophthalmol. Vis. Sci. 2000, 41. [Google Scholar]
- Schneemann, A.; Dijkstra, B.G.; Berg, T.J.v.D.; Kamphuis, W.; Hoyng, P.F. Nitric oxide/guanylate cyclase pathways and flow in anterior segment perfusion. Graefe’s Arch. Clin. Exp. Ophthalmol. 2002, 240, 936–941. [Google Scholar] [CrossRef]
- Cavet, M.E.; Vittitow, J.L.; Impagnatiello, F.; Ongini, E.; Bastia, E. Nitric Oxide (NO): An Emerging Target for the Treatment of Glaucoma. Investig. Opthalmology Vis. Sci. 2014, 55, 5005–5015. [Google Scholar] [CrossRef]
- Kaufman, P.L. Latanoprostene bunod ophthalmic solution 0.024% for IOP lowering in glaucoma and ocular hypertension. Expert Opin. Pharmacother. 2017, 18, 433–444. [Google Scholar] [CrossRef]
- Ishizaki, T.; Maekawa, M.; Fujisawa, K.; Okawa, K.; Iwamatsu, A.; Fujita, A.; Watanabe, N.; Saito, Y.; Kakizuka, A.; Morii, N.; et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996, 15, 1885–1893. [Google Scholar] [CrossRef]
- Rao, P.V.; Pattabiraman, P.P.; Kopczynski, C. Role of the Rho GTPase/Rho kinase signaling pathway in pathogenesis and treatment of glaucoma: Bench to bedside research. Exp. Eye Res. 2017, 158, 23–32. [Google Scholar] [CrossRef]
- 54. Buffault, J.; Brignole-Baudouin, F.; Reboussin, É.; Kessal, K.; Labbé, A.; Mélik Parsadaniantz, S.; Baudouin, C. The Dual Effect of Rho-Kinase Inhibition on Trabecular Meshwork Cells Cytoskeleton and Extracellular Matrix in an In Vitro Model of Glaucoma. J. Clin. Med. 2022, 11, 1001. [Google Scholar] [CrossRef]
- Wang, R.-F.; Williamson, J.E.; Kopczynski, C.; Serle, J.B. Effect of 0.04% AR-13324, a ROCK, and Norepinephrine Transporter Inhibitor, on Aqueous Humor Dynamics in Normotensive Monkey Eyes. Eur. J. Gastroenterol. Hepatol. 2015, 24, 51–54. [Google Scholar] [CrossRef]
- Toris, C.B.; McLaughlin, M.A.; Dworak, D.P.; Fan, S.; Havens, S.; Zhan, G.-L.; Horan, N.; Prasanna, G. Effects of Rho Kinase Inhibitors on Intraocular Pressure and Aqueous Humor Dynamics in Nonhuman Primates and Rabbits. J. Ocul. Pharmacol. Ther. 2016, 32, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.C.; Konstas, A.G.; Nelson, L.A.; Kruft, B. Meta-analysis of 24-Hour Intraocular Pressure Studies Evaluating the Efficacy of Glaucoma Medicines. Ophthalmology 2008, 115, 1117–1122.e1. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lindsley, K.; Rouse, B.; Hong, H.; Shi, Q.; Friedman, D.S.; Wormald, R.; Dickersin, K. Comparative effectiveness of first-line medications for primary open-angle glaucoma: A systematic review and network meta-analysis. Ophthalmology. 2016, 123, 129–140. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, R.; Webers, C.A.; Schouten, J.S.; Zeegers, M.P.; Hendrikse, F.; Prins, M.H. Intraocular Pressure–Lowering Effects of All Commonly Used Glaucoma Drugs: A Meta-analysis of Randomized Clinical Trials. Ophthalmology 2005, 112, 1177–1185. [Google Scholar] [CrossRef]
- Freiberg, J.C.; von Spreckelsen, A.; Khachatryan, N.; Kolko, M.; Azuara-Blanco, A.; Virgili, G. Rho kinase inhibitor for primary open-angle glaucoma and ocular hypertension. Cochrane Database Syst. Rev. 2022, 6, CD013817. [Google Scholar] [CrossRef]
- Moussa, W.G.E.H.; Farhat, R.G.; Nehme, J.C.; Sahyoun, M.A.; Schakal, A.R.; Jalkh, A.E.; Karam, M.P.A.; Azar, G.G. Comparison of Efficacy and Ocular Surface Disease Index Score between Bimatoprost, Latanoprost, Travoprost, and Tafluprost in Glaucoma Patients. J. Ophthalmol. 2018, 2018, 1319628. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, F.; Liu, K.; Duan, X. Efficacy, and safety of prostaglandin analogues in primary open-angle glaucoma or ocular hypertension patients: A meta-analysis. Medicine. (Baltimore) 2019, 98, e16597. [Google Scholar] [PubMed]
- Lin, L.; Zhao, Y.J.; Chew, P.T.; Sng, C.C.A.; Wong, H.-T.; Yip, L.W.; Wu, T.S.; Bautista, D.; Teng, M.; Khoo, A.L.; et al. Comparative Efficacy and Tolerability of Topical Prostaglandin Analogues for Primary Open-Angle Glaucoma and Ocular Hypertension. Ann. Pharmacother. 2014, 48, 1585–1593. [Google Scholar] [CrossRef]
- Matsuo, M.; Matsuoka, Y.; Tanito, M. Efficacy and Patient Tolerability of Omidenepag Isopropyl in the Treatment of Glaucoma and Ocular Hypertension. Clin. Ophthalmol. 2022, ume 16, 1261–1279. [Google Scholar] [CrossRef]
- Aihara, M.; Lu, F.; Kawata, H.; Iwata, A.; Odani-Kawabata, N.; Shams, N.K. Omidenepag isopropyl versus latanoprost in primary open-angle glaucoma and ocular hypertension: the Phase 3 AYAME Study. Am. J. Ophthalmol. 2020, 220, 53–63. [Google Scholar] [CrossRef]
- Cai, Z.; Cao, M.; Liu, K.; Duan, X. Analysis of the Responsiveness of Latanoprost, Travoprost, Bimatoprost, and Tafluprost in the Treatment of OAG/OHT Patients. J. Ophthalmol. 2021, 2021, 5586719. [Google Scholar] [CrossRef]
- Zimmerman, T.J.; Kaufman, H.E. Timolol, dose response and duration of action. Arch. Ophthalmol. 1977, 95, 605–607. [Google Scholar] [CrossRef]
- Berry, D.P. Jr.; Van Buskirk, E.M.; Shields, M.B. Betaxolol and timolol: A comparison of efficacy and side effects. Arch. Ophthalmol. 1984, 102, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Schadlu, R.; Maus, T.L.; Nau, C.B.; Brubaker, R.F. Comparison of the efficacy of apraclonidine and brimonidine as aqueous suppressants in humans. Arch. Ophthalmol. 1998, 116, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, A.; Singh, G. Efficacy of three different formulations of brimonidine for control of intraocular pressure in primary open-angle glaucoma: A 6-week randomized trial. Oman. J. Ophthalmol. 2018, 11, 140–143. [Google Scholar] [CrossRef]
- Stewart, W.C.; Konstas, A.G.; Kruft, B.; Mathis, H.M.; Stewart, J.A. Meta-Analysis of 24-h Intraocular Pressure Fluctuation Studies and the Efficacy of Glaucoma Medicines. J. Ocul. Pharmacol. Ther. 2010, 26, 175–180. [Google Scholar] [CrossRef]
- Harris, L.S.; Galin, M.A. Dose Response Analysis of Pilocarpine-Induced Ocular Hypotension. Arch. Ophthalmol. 1970, 84, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Diestelhorst, M. The additive intraocular pressure-lowering effect of latanoprost 0.005% daily once and pilocarpine 2% t.i.d. in patients with open-angle glaucoma or ocular hypertension. a 6-month, randomized, multicenter study. German Latanoprost Study Group. Graefes. Arch. Clin. Exp. Ophthalmol. 2000, 238, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Hartenbaum, D.; Maloney, S.; Vaccarelli, L.; Liss, C.; Wilson, H.; Gormley, G.J. Comparison of dorzolamide and pilocarpine as adjunctive therapy in patients with open-angle glaucoma and ocular hypertension. Clin. Ther. 1999, 21, 1533–1538. [Google Scholar] [CrossRef]
- Ikegami, K.; Shigeyoshi, Y.; Masubuchi, S. Circadian Regulation of IOP Rhythm by Dual Pathways of Glucocorticoids and the Sympathetic Nervous System. Investig. Opthalmology Vis. Sci. 2020, 61, 26. [Google Scholar] [CrossRef]
- Liu, J.H.K.; Weinreb, R.N. Asymmetry of Habitual 24-Hour Intraocular Pressure Rhythm in Glaucoma Patients. Investig. Opthalmology Vis. Sci. 2014, 55, 7398–7402. [Google Scholar] [CrossRef]
- Neroev, V.; Malishevskaya, T.; Weinert, D.; Astakhov, S.; Kolomeichuk, S.; Cornelissen, G.; Kabitskaya, Y.; Boiko, E.; Nemtsova, I.; Gubin, D. Disruption of 24-Hour Rhythm in Intraocular Pressure Correlates with Retinal Ganglion Cell Loss in Glaucoma. Int. J. Mol. Sci. 2020, 22, 359. [Google Scholar] [CrossRef] [PubMed]
- Matlach, J.; Bender, S.; König, J.; Binder, H.; Pfeiffer, N.; Hoffmann, E.M. Investigation of intraocular pressure fluctuation as a risk factor of glaucoma progression. Clin. Ophthalmol. 2018, ume 13, 9–16. [Google Scholar] [CrossRef]
- Drance, S.M. The Significance of the Diurnal Tension Variations in Normal and Glaucomatous Eyes. Arch. Ophthalmol. 1960, 64, 494–501. [Google Scholar] [CrossRef]
- Tsironi, S.; Almaliotis, D.; Ntonti, P.; Sidiropoulos, G.; Theodoridou, E.; Theofrastou, E.; Karachrysafi, S.; Psimenidou, E.; Sarafi, A.; Kapourani, V.; et al. Clinical Outcomes of the Implementation of IOP Monitoring, in and out of Office Time, to 1500 Patients—A Cohort Study. Vision 2022, 6, 69. [Google Scholar] [CrossRef]
- Barkana, Y.; Anis, S.; Liebmann, J.; Tello, C.; Ritch, R. Clinical Utility of Intraocular Pressure Monitoring Outside of Normal Office Hours in Patients With Glaucoma. Arch. Ophthalmol. 2006, 124, 793–797. [Google Scholar] [CrossRef]
- Orzalesi, N.; Rossetti, L.; Invernizzi, T.; Bottoli, A.; Autelitano, A. Effect of timolol, latanoprost, and dorzolamide on circadian IOP in glaucoma or ocular hypertension. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2566–2573. [Google Scholar]
- Yildirim, N.; Sahin, A.; Gultekin, S. The Effect of Latanoprost, Bimatoprost, and Travoprost on Circadian Variation of Intraocular Pressure in Patients With Open-angle Glaucoma. Eur. J. Gastroenterol. Hepatol. 2008, 17, 36–39. [Google Scholar] [CrossRef]
- Orzalesi, N.; Rossetti, L.; Bottoli, A.; Fogagnolo, P. Comparison of the Effects of Latanoprost, Travoprost, and Bimatoprost on Circadian Intraocular Pressure in Patients with Glaucoma or Ocular Hypertension. Ophthalmology 2006, 113, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Konstas, A.G.; Stewart, W.C.; Topouzis, F.; Tersis, I.; Holmes, K.T.; Stangos, N.T. Brimonidine 0.2% given two or three times daily versus timolol maleate 0.5% in primary open-angle glaucoma. Arch. Ophthalmol. 2001, 131, 729–733. [Google Scholar] [CrossRef]
- Orzalesi, N.; Rossetti, L.; Bottoli, A.; Fumagalli, E.; Fogagnolo, P. The Effect of Latanoprost, Brimonidine, and a Fixed Combination of Timolol and Dorzolamide on Circadian Intraocular Pressure in Patients With Glaucoma or Ocular Hypertension. Arch. Ophthalmol. 2003, 121, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Medeiros, F.A.; Slight, J.R.; Weinreb, R.N. Diurnal and Nocturnal Effects of Brimonidine Monotherapy on Intraocular Pressure. Ophthalmology 2010, 117, 2075–2079. [Google Scholar] [CrossRef]
- Gulati, V.; Fan, S.; Zhao, M.; Maslonka, M.A.; Gangahar, C.; Toris, C.B. Diurnal and Nocturnal Variations in Aqueous Humor Dynamics of Patients With Ocular Hypertension Undergoing Medical Therapy. JAMA Ophthalmol 2012, 130, 677–684. [Google Scholar] [CrossRef]
- Lee, P.W.-Y.; Doyle, A.; Stewart, J.A.; Kristoffersen, C.J.; Stewart, W.C. Meta-analysis of timolol on diurnal and nighttime intraocular pressure and blood pressure. Eur. J. Ophthalmol. 2010, 20, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- E Topper, J.; Brubaker, R.F. Effects of timolol, epinephrine, and acetazolamide on aqueous flow during sleep. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1315–1319. [Google Scholar]
- Oddone, F.; Rossetti, L.; Tanga, L.; Berardo, F.; Ferrazza, M.; Michelessi, M.; Roberti, G.; Manni, G.; Centofanti, M. Effects of Topical Bimatoprost 0.01% and Timolol 0.5% on Circadian IOP, Blood Pressure and Perfusion Pressure in Patients with Glaucoma or Ocular Hypertension: A Randomized, Double Masked, Placebo-Controlled Clinical Trial. PLOS ONE 2015, 10, e0140601. [Google Scholar] [CrossRef]
- Liu, J.H.; Slight, J.R.; Vittitow, J.L.; Sforzolini, B.S.; Weinreb, R.N. Efficacy of Latanoprostene Bunod 0.024% Compared With Timolol 0.5% in Lowering Intraocular Pressure Over 24 Hours. Am. J. Ophthalmol. 2016, 169, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Walters, T.R.; DuBiner, H.B.; Carpenter, S.P.; Khan, B.; VanDenburgh, A.M. 24-Hour IOP control with once-daily bimatoprost, timolol gel-forming solution, or latanoprost: a 1-month, randomized, comparative clinical trial. Surv. Ophthalmol. 2004, 49, S26–S35. [Google Scholar] [CrossRef] [PubMed]
- 94. Shiratori, N.; Nishio, Y.; Takeda, A.; Sugimoto, S.; Takazawa, K.; Otsuka, N.; Ishida, N.; Shii, D.; Hori, K.; Nakamoto, K. Twenty-four-hour intraocular pressure control with omidenepag isopropyl 0.002% in patients with glaucoma and ocular hypertension. Clin. Ophthalmol. 2021, 15, 3997–4003. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, S.; Van Bergen, T.; Sijnave, D.; Hollanders, K.; Castermans, K.; Defert, O.; Leysen, D.; Vandewalle, E.; Moons, L.; Stalmans, I. AMA0076, a Novel, Locally Acting Rho Kinase Inhibitor, Potently Lowers Intraocular Pressure in New Zealand White Rabbits with Minimal Hyperemia. Investig. Opthalmology Vis. Sci. 2014, 55, 1006–1016. [Google Scholar] [CrossRef]
- Araujo, S.V.; Bond, J.B.; Wilson, R.P.; Moster, M.R.; Schmidt, C.M. Jr.; Spaeth, G.L. Long term effect of apraclonidine. Br. J. Ophthalmol. 1995, 79, 1098–1101. [Google Scholar] [CrossRef]
- Derick, R.J.; Robin, A.L.; Walters, T.R.; Barnebey, H.S.; Choplin, N.; Schuman, J.; Kelley, E.P.; Chen, K.; Stoecker, J.F. Brimonidine tartrate: a one-month dose response study. Ophthalmology. 1997, 104, 131–136. [Google Scholar] [CrossRef]
- Strahlman, E.; Tipping, R.; Vogel, R. A Double-Masked, Randomized 1-Year Study Comparing Dorzolamide (Trusopt), Timolol, and Betaxolol. Arch. Ophthalmol. 1995, 113, 1009–1016. [Google Scholar] [CrossRef]
- Bito, L.Z. A new approach to the medical management of glaucoma, from the bench to the clinic, and beyond: the Proctor Lecture. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1126–1133. [Google Scholar]
- Riva, I.; Katsanos, A.; Floriani, I.; Biagioli, E.; Konstas, A.G.; Centofanti, M.; Quaranta, L. Long-term 24-hour Intraocular Pressure Control With Travoprost Monotherapy in Patients With Primary Open-angle Glaucoma. Eur. J. Gastroenterol. Hepatol. 2014, 23, 535–540. [Google Scholar] [CrossRef]
- Steinert, R.F.; Thomas, J.V.; Boger, W.P. Long-term Drift and Continued Efficacy After Multiyear Timolol Therapy. Arch. Ophthalmol. 1981, 99, 100–103. [Google Scholar] [CrossRef]
- Piltz, J.; Gross, R.; Shin, D.H.; Beiser, J.A.; Dorr, D.A.; Kass, M.A.; Gordon, M.O. Contralateral effect of topical beta-adrenergic antagonists in initial one-eyed trials in the ocular hypertension treatment study. Am. J. Ophthalmol. 2000, 130, 441–453. [Google Scholar] [CrossRef]
- 103. Yuksel, N.; Karabas, L.; Altintas, O.; Yildirim, Y.; Caglar, Y. A Comparison of the Short-Term Hypotensive Effects and Side Effects of Unilateral Brimonidine and Apraclonidine in Patients with Elevated Intraocular Pressure. Ophthalmologica 2002, 216, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.L.; Senthil, S.; Garudadri, C.S. Contralateral intraocular pressure lowering effect of prostaglandin analogues. Indian J. Ophthalmol. 2014, 62, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Dunham, C.N.; Spaide, R.F.; Dunham, G. The contralateral reduction of intraocular pressure by timolol. Br. J. Ophthalmol. 1994, 78, 38–40. [Google Scholar] [CrossRef]
- Sit, A.J.; Gupta, D.; Kazemi, A.; McKee, H.; Challa, P.; Liu, K.C.; Lopez, J.; Kopczynski, C.; Heah, T. Netarsudil Improves Trabecular Outflow Facility in Patients with Primary Open Angle Glaucoma or Ocular Hypertension: A Phase 2 Study. Am. J. Ophthalmol. 2021, 226, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, V.; Cruz, M.; Pottenburgh, J.; Saeedi, O.J. Precise quantification of episcleral venous flow rates inhuman subjects before and after netarsudil 0. 02%. Invest. Ophthalmol. Vis. Sci. 2022, 63, 3497. [Google Scholar]
- Suzuki, M.; Suzuki, Y.; Komori, R.; Orii, Y.; Arimura, S.; Iwasaki, K.; Takamura, Y.; Inatani, M. Aqueous column changes in the episcleral veins after the instillation of ripasudil versus latanoprost: a randomized, double-blind, crossover clinical trial. Sci. Rep. 2022, 12, 15255. [Google Scholar] [CrossRef]
- Lee, S.S.; Burke, J.; Shen, J.; Almazan, A.; Orilla, W.; Hughes, P.; Zhang, J.; Li, H.; Struble, C.; Miller, P.E.; et al. EMGT Group. Bimatoprost sustained-release intracameral implant reduces episcleral venous pressure in dogs. Veter- Ophthalmol. 2018, 21, 376–381. [Google Scholar] [CrossRef]
- Leske, M.C.; Heijl, A.; Hyman, L.; Bengtsson, B.; Dong, L.; Yang, Z. Predictors of Long-term Progression in the Early Manifest Glaucoma Trial. Ophthalmology 2007, 114, 1965–1972. [Google Scholar] [CrossRef]
- Leske, M.C.; Wu, S.-Y.; Hennis, A.; Honkanen, R.; Nemesure, B. Risk Factors for Incident Open-angle Glaucoma: The Barbados Eye Studies. Ophthalmology 2008, 115, 85–93. [Google Scholar] [CrossRef]
- Cherecheanu, A.P.; Garhofer, G.; Schmidl, D.; Werkmeister, R.; Schmetterer, L. Ocular perfusion pressure and ocular blood flow in glaucoma. Curr. Opin. Pharmacol. 2013, 13, 36–42. [Google Scholar] [CrossRef]
- Hayreh, S.S. Blood flow in the optic nerve head and factors that may influence it. Prog. Retin. Eye. Res. 2001, 20, 595—624. [Google Scholar]
- Kolli, A.; Toris, C.B.; Reed, D.M.; Gilbert, J.; Sit, A.J.; Gulati, V.; Kazemi, A.; Fan, S.; Musch, D.C.; Moroi, S.E. The effects of topical timolol and latanoprost on calculated ocular perfusion pressure in non-glaucomatous volunteers. J. Ocul. Pharmacol. Ther. 2021, 37, 565–574. [Google Scholar] [CrossRef]
- Ishibashi, S.; Hirose, N.; Tawara, A.; Kubota, T. Effect of Latanoprost on the Diurnal Variations in the Intraocular and Ocular Perfusion Pressure in Normal Tension Glaucoma. Eur. J. Gastroenterol. Hepatol. 2006, 15, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Costagliola, C.; Parmeggiani, F.; Virgili, G.; Lamberti, G.; Incorvaia, C.; Perri, P.; Campa, C.; Sebastiani, A. Circadian changes of intraocular pressure and ocular perfusion pressure after timolol or latanoprost in Caucasians with normal-tension glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 246, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, X.; Zhao, Y.; Yang, X.; Zhou, D.; Chen, B.; Duan, X. Effects of Tafluprost on Ocular Blood Flow. Ophthalmol. Ther. 2022, 11, 1991–2003. [Google Scholar] [CrossRef] [PubMed]
- Seibold, L.K.; DeWitt, P.E.; Kroehl, M.E.; Kahook, M.Y. The 24-Hour Effects of Brinzolamide/Brimonidine Fixed Combination and Timolol on Intraocular Pressure and Ocular Perfusion Pressure. J. Ocul. Pharmacol. Ther. 2017, 33, 161–169. [Google Scholar] [CrossRef]
- Liu, C.J.-L.; Ko, Y.-C.; Cheng, C.-Y.; Chiu, A.W.; Chou, J.C.; Hsu, W.-M.; Liu, J.-H. Changes in intraocular pressure and ocular perfusion pressure after latanoprost 0.005% or brimonidine tartrate 0.2% in normal-tension glaucoma patients. Ophthalmology 2002, 109, 2241–2247. [Google Scholar] [CrossRef]
- Pillunat, L.E.; Stodtmeister, R.; Vetrugno, M.; Cardascia, N.; Cantatore, F.; Sborgia, C.; Gugleta, K.; Costa, V.P.; Harris, A.; Stefánsson, E.; et al. Effect of Different Antiglaucomatous Drugs on Ocular Perfusion Pressures. J. Ocul. Pharmacol. Ther. 1988, 4, 231–242. [Google Scholar] [CrossRef]
- Siesky, B.; Harris, A.; Brizendine, E.; Marques, C.; Loh, J.; Mackey, J.; Overton, J.; Netland, P. Literature Review and Meta-Analysis of Topical Carbonic Anhydrase Inhibitors and Ocular Blood Flow. Surv. Ophthalmol. 2009, 54, 33–46. [Google Scholar] [CrossRef]
- Samaha, D.; Diaconu, V.; Bouchard, J.F.; Desalliers, C.; Dupont, A. Effect of Latanoprostene Bunod on Optic Nerve Head Blood Flow. Optom. Vis. Sci. 2022, 99, 172–176. [Google Scholar] [CrossRef]
- WoldeMussie, E.; Ruiz, G.; Wijono, M.; Wheeler, L.A. Neuroprotection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension. Investig. Ophthalmol. Vis. Sci. 2001, 42. [Google Scholar]
- Evans, D.W.; Hosking, S.L.; Gherghel, D.; Bartlett, J.D. Contrast sensitivity improves after brimonidine therapy in primary open angle glaucoma: a case for neuroprotection. Br. J. Ophthalmol. 2003, 87, 1463–1465. [Google Scholar] [CrossRef]
- Ichhpujani, P.; Rodrigues, A.M.; Kumar, S.; Singh, R.B. Analysing the change in contrast sensitivity post-travoprost treatment in primary open-angle glaucoma patients using Spaeth Richman contrast sensitivity test. Int. Ophthalmol. 2022, 43, 2037–2047. [Google Scholar] [CrossRef]
- Amanullah, S.; Okudolo, J.; Rahmatnejad, K.; Lin, S.-C.; Wizov, S.S.; Muhire, R.S.M.; Hark, L.A.; Zheng, C.X.; Zhan, T.; Spaeth, G.L. The relationship between contrast sensitivity and retinal nerve fiber layer thickness in patients with glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 2415–2422. [Google Scholar] [CrossRef]
- Tsai, J.-C.; Chang, H.-W.; Arthur, S.; Cantor, L.B.; Goldenberg-Cohen, N.; Dadon-Bar-El, S.; Hasanreisoglu, M.; Avraham-Lubin, B.C.R.; Dratviman-Storobinsky, O.; Cohen, Y.; et al. Comparison of the Effects of Brimonidine 0.2% and Timolol 0.5% on Retinal Nerve Fiber Layer Thickness in Ocular Hypertensive Patients: A Prospective, Unmasked Study. J. Ocul. Pharmacol. Ther. 2005, 21, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, R.; Aihara, M.; Araie, M. Neuroprotective effects of prostaglandin analogues on retinal ganglion cell death independent of intraocular pressure reduction. Exp. Eye Res. 2011, 93, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.M.; Nagrale, P. Rho Kinase Inhibitors as a Neuroprotective Pharmacological Intervention for the Treatment of Glaucoma. Cureus 2022, 14, e28445. [Google Scholar] [CrossRef]
- Tomita, G.; Araie, M.; Kitazawa, Y.; Tsukahara, S. A three-year prospective, randomized and open comparison between latanoprost and timolol in Japanese normal-tension glaucoma patients. Eye 2014, 18, 984–989. [Google Scholar] [CrossRef]
- Inoue, K.; Fujimoto, T.; Shiokawa, M.; Tomita, G. Effects of treatment with bimatoprost 0.03% for 3 years in patients with normal-tension glaucoma. Clin. Ophthalmol. 2014, 8, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, L.; Carpineto, P.; Ciancaglini, M.; Gallenga, P.E. A 12-month, randomized, double-masked study comparing latanoprost with timolol in pigmentary glaucoma. Ophthalmology 1999, 106, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Sihota, R.; Saxena, R.; Agarwal, H.C.; Gulati, V. Crossover Comparison of Timolol and Latanoprost in Chronic PrimaryAngle-closure Glaucoma. Arch. Ophthalmol. 2004, 122, 185–189. [Google Scholar] [CrossRef]
- Chen, M.-J.; Chen, Y.-C.; Chou, C.-K.; Hsu, W.-M. Comparison of the Effects of Latanoprost and Travoprost on Intraocular Pressure in Chronic Angle-Closure Glaucoma. J. Ocul. Pharmacol. Ther. 2006, 22, 449–454. [Google Scholar] [CrossRef]
- Konstas, A.G.P.; Hollo, G.; Irkec, M.; Tsironi, S.; Durukan, I.; Goldenfeld, M.; Melamed, S. Diurnal IOP control with bimatoprost versus latanoprost in exfoliative glaucoma: a crossover, observer-masked, three-centre study. Br. J. Ophthalmol. 2006, 91, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Konstas, A.G.; Kozobolis, V.P.; Katsimpris, I.E.; Boboridis, K.; Koukoula, S.; Jenkins, J.N.; Stewart, W.C. Efficacy and Safety of Latanoprost versus Travoprost in Exfoliative Glaucoma Patients. Ophthalmology 2007, 114, 653–657. [Google Scholar] [CrossRef]
- Aihara, M.; Ropo, A.; Lu, F.; Kawata, H.; Iwata, A.; Odani-Kawabata, N.; Shams, N. Intraocular pressure-lowering effect of omidenepag isopropyl in latanoprost non-/low-responder patients with primary open-angle glaucoma or ocular hypertension: the FUJI study. Jpn. J. Ophthalmol. 2020, 64, 398–406. [Google Scholar] [CrossRef]
- Rossetti, L.; Gandolfi, S.; Traverso, C.; Montanari, P.; Uva, M.; Manni, G.; Carassa, R.; Mastropasqua, L.; Quaranta, L.; Marchini, G.; et al. An evaluation of the rate of nonresponders to latanoprost therapy. J. Glaucoma. 2006, 15, 238–243. [Google Scholar] [CrossRef]
- Inoue, K.; Inoue, J.; Kunimatsu-Sanuki, S.; Nozaki, N.; Shimizu, K.; Ishida, K.; Tomita, G. Short-Term Efficacy and Safety of Omidenepag Isopropyl in Patients with Normal-Tension Glaucoma. Clin. Ophthalmol. 2020, ume 14, 2943–2949. [Google Scholar] [CrossRef]
- Miki, A.; Miyamoto, E.; Ishida, N.; Shii, D.; Hori, K.; LESPOIR Research Group. Efficacy and safety of omidenepag isopropyl 0.002% ophthalmic solution: A retrospective analysis of real-world data in Japan. Adv. Ther. 2022, 39, 2085–2095. [Google Scholar] [CrossRef]
- Rouland, J.-F.; Morel-Mandrino, P.; Elena, P.-P.; Polzer, H.; Raj, P.S. Timolol 0.1% Gel (Nyogel 0.1%®) Once Daily versus Conventional Timolol 0.5% Solution Twice Daily: A Comparison of Efficacy and Safety. Ophthalmologica 2002, 216, 449–454. [Google Scholar] [CrossRef]
- Harris, A.; Arend, O.; Chung, H.S.; Kagemann, L.; Cantor, L.; Martin, B. A comparative study of betaxolol and dorzolamide effect on ocular circulation in normal-tension glaucoma patients. Ophthalmology 2000, 107, 430–434. [Google Scholar] [CrossRef]
- Gandolfi, S.; Cimino, L.; Mora, P. Effect of brimonidine on intraocular pressure in normal tension glaucoma: a short term clinical trial. Eur. J. Ophthalmol. 2003, 13, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Tsumura, T.; Yoshikawa, K.; Kimura, T.; Suzumura, H.; Kawashima, M.; Nanno, M.; Ishijima, K.; Takeda, R. The efficacy and safety of add-on 0. 1% brimonidine tartrate preserved with sodium chlorite in on-treatment Japanese normal-tension glaucoma patients. Clin. Ophthalmol. 2014, 8, 1681–1687. [Google Scholar] [PubMed]
- Ogata, N.; Kanda, T.; Kawahata, M.; Ichikawa, T.; Matsumoto, Y.; Morimitsu, W.; Nishino, Y.; Itoi, T.; Furumoto, K. Sedative and physiological effects of brimonidine tartrate ophthalmic solution in healthy cats. Veter- Anaesth. Analg. 2017, 44, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Enyedi, L.B.; Freedman, S.F. Safety and efficacy of brimonidine in children with glaucoma. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2001, 5, 281–284. [Google Scholar] [CrossRef]
- Greiner, R.C.; Beasley, H.M.; Bodhireddy, H.; Bouterse, C.R.; Eggleston, M.T.; Pfeiffer, D.C. Revisiting acidosis in acetazolamide treatment of severe glaucoma: A case report. Am. J. Ophthalmol. Case Rep. 2022, 27, 101658. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ishikawa, S.; Nakamura, Y.; Sakai, H.; Henzan, I.; Sawaguchi, S. 24-hour intraocular pressure in glaucoma patients randomized to receive dorzolamide or brinzolamide in combination with latanoprost. Clin. Ophthalmol. 2009, 3, 395–400. [Google Scholar]
- Ott, E.Z.; Mills, M.D.; Arango, S.; Getson, A.J.; Assaid, C.A.; Adamsons, I.A. A Randomized Trial Assessing Dorzolamide in Patients With Glaucoma Who Are Younger Than 6 Years. Arch. Ophthalmol. 2005, 123, 1177–1186. [Google Scholar] [CrossRef]
- Zimmerman, T.J.; Kooner, K.S.; Kandarakis, A.S.; Ziegler, L.P. Improving the Therapeutic Index of Topically Applied Ocular Drugs. Arch. Ophthalmol. 1984, 102, 551–553. [Google Scholar] [CrossRef]
- I Phillips, C.; Clark, C.V.; Levy, A.M. Posterior synechiae after glaucoma operations: aggravation by shallow anterior chamber and pilocarpine. Br. J. Ophthalmol. 1987, 71, 428–432. [Google Scholar] [CrossRef]
- Yoo, Y.-J.; Hwang, J.-M.; Yang, H.K. Dilute pilocarpine test for diagnosis of Adie’s tonic pupil. Sci. Rep. 2021, 11, 10089. [Google Scholar] [CrossRef]
- Tanasiewicz, M.; Hildebrandt, T.; Obersztyn, I. Xerostomia of Various Etiologies: A Review of the Literature. Adv. Clin. Exp. Med. 2016, 25, 199–206. [Google Scholar] [CrossRef]
- Tucker, T.; Early, J. Pilocarpine 1. 25% Ophthalmic Solution (Vuity) for the Treatment of Presbyopia.. 2023, 107, 659–660. [Google Scholar]
- Futakuchi, A.; Morimoto, T.; Ikeda, Y.; Tanihara, H.; Inoue, T.; Aihara, M.; Arimura, S.; Fukuchi, T.; Higashide, T.; Honjo, M.; et al. ROCK-S study group collaborators. Intraocular pressure-lowering effects of ripasudil in uveitic glaucoma, exfoliation glaucoma, and steroid-induced glaucoma patients: ROCK-S, a multicentre historical cohort study. Sci. Rep. 2020, 10, 10308. [Google Scholar] [CrossRef]
- Carstairs, J.R.; Nimmo, A.J.; Barnes, P.J. Autoradiographic visualization of beta-adrenoceptor subtypes in human lung. Am. Rev. Respir. Dis. 1985, 132, 541–547. [Google Scholar] [CrossRef]
- Avorn, J.; Glynn, R.J.; Gurwitz, J.H.; Bohn, R.L.; Monane, M.; Everitt, D.E.; Gilden, D.; Choodnovskiy, I. Adverse pulmonary effects of topical β-blockers used in the treatment of glaucoma. J. Glaucoma. 1995, 2, 158–165. [Google Scholar] [CrossRef]
- Sadiq, S.A.; Fielding, K.; Vernon, S.A. The effect of timolol drops on respiratory function. Eye 1998, 12, 386–389. [Google Scholar] [CrossRef]
- Kirwan, J.F.; Nightingale, J.A.; Bunce, C.; Wormald, R. Do selective topical beta antagonists for glaucoma have respiratory side effects? B.r J. Ophthalmol. 2004, 88, 196–198. [Google Scholar] [CrossRef]
- Müller, L.; Jensen, B.P.; Bachmann, L.M.; Wong, D.; Wells, A.P. New technique to reduce systemic side effects of timolol eye drops: The tissue press method—Cross-over clinical trial. Clin. Exp. Ophthalmol. 2019, 48, 24–30. [Google Scholar] [CrossRef]
- Ramdas, W.D.; van der Velde, N.; van der Cammen, T.J.; Wolfs, R.C. Evaluation of risk of falls and orthostatic hypotension in older, long-term topical beta-blocker users. Graefes. Arch. Clin. Exp. Ophthalmol. 2009, 247, 1235–1241. [Google Scholar]
- Farkouh, A.; Frigo, P.; Czejka, M. Systemic side effects of eye drops: a pharmacokinetic perspective. Clin. Ophthalmol. 2016, ume 10, 2433–2441. [Google Scholar] [CrossRef]
- Sridharrao, B.; Badrinath, S.S. Efficacy and safety of apraclonidine in patients undergoing anterior segment laser surgery. Br. J. Ophthalmol. 1989, 73, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, L.; Gandolfo, F.; Turano, R.; Rovida, F.; Pizzolante, T.; Musig, A.; Gandolfo, E. Effects of Topical Hypotensive Drugs on Circadian IOP, Blood Pressure, and Calculated Diastolic Ocular Perfusion Pressure in Patients with Glaucoma. Investig. Opthalmology Vis. Sci. 2006, 47, 2917–2923. [Google Scholar] [CrossRef] [PubMed]
- Mizoue, S.; Nitta, K.; Shirakashi, M.; Nitta, A.; Yamabayashi, S.; Kimura, T.; Ueda, T.; Takeda, R.; Matsumoto, S.; Yoshikawa, K. Multicenter, Randomized, Investigator-Masked Study Comparing Brimonidine Tartrate 0.1% and Timolol Maleate 0.5% as Adjunctive Therapies to Prostaglandin Analogues in Normal-Tension Glaucoma. Adv. Ther. 2017, 34, 1438–1448. [Google Scholar] [CrossRef]
- Zheng, Y.; Wong, T.Y.; Mitchell, P.; Friedman, D.S.; He, M.; Aung, T. Distribution of Ocular Perfusion Pressure and Its Relationship with Open-Angle Glaucoma: The Singapore Malay Eye Study. Investig. Opthalmology Vis. Sci. 2010, 51, 3399–3404. [Google Scholar] [CrossRef]
- Morris, S.; Geh, V.; Nischal, K.K.; Sahi, S.; Ahmed, M.A.S. Topical dorzolamide and metabolic acidosis in a neonate. Br. J. Ophthalmol. 2003, 87, 1052–1053. [Google Scholar] [CrossRef]
- Hoffmanová, I.; Sánchez, D. Metabolic acidosis and anaemia associated with dorzolamide in a patient with impaired renal function. Br. J. Clin. Pharmacol. 2018, 84, 796–799. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Ling, X.C.; Tsai, W.-H.; Liu, J.-S.; Kuo, K.-L. Risks of Topical Carbonic Anhydrase Inhibitors in Glaucoma Patients With Chronic Kidney Disease: A Nationwide Population-Based Study. Arch. Ophthalmol. 2023, 253, 49–55. [Google Scholar] [CrossRef]
- Levy, B.; Ramirez, N.; Novack, G.D.; Kopczynski, C. Ocular Hypotensive Safety and Systemic Absorption of AR-13324 Ophthalmic Solution in Normal Volunteers. Am. J. Ophthalmol. 2015, 159, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Defert, O.; Boland, S. Rho kinase inhibitors: a patent review (2014 – 2016). Expert Opin. Ther. Patents 2017, 27, 507–515. [Google Scholar] [CrossRef]
- Rouland, J.F.; Le Pen, C.; Gouveia Pinto, C.; Berto, P.; Berdeaux, G. Cost-minimisation study of dorzolamide versus brinzolamide in the treatment of ocular hypertension and primary open-angle glaucoma: in four European countries. Pharmacoeconomics. 2003, 21, 201–213. [Google Scholar] [CrossRef]
- Michaud, J.-E.; Friren, B. Comparison of topical brinzolamide 1% and dorzolamide 2% eye drops given twice daily in addition to timolol 0.5% in patients with primary open-angle glaucoma or ocular hypertension. Am. J. Ophthalmol. 2001, 132, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Abramson, D.H.; Coleman, D.J.; Forbes, M.; Franzen, L.A. Pilocarpine. Effect on the anterior chamber and lens thickness. Arch. Ophthalmol. 1972, 87, 615–620. [Google Scholar] [CrossRef]
- Schuman, J.S.; Horwitz, B.; Choplin, N.T.; David, R.; Albracht, D.; Chen, K. A 1-Year Study of Brimonidine Twice Daily in Glaucoma and Ocular Hypertension. Arch. Ophthalmol. 1997, 115, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Aihara, M.; Lu, F.; Kawata, H.; Iwata, A.; Odani-Kawabata, N. Twelve-month efficacy and safety of omidenepag isopropyl, a selective EP2 agonist, in open-angle glaucoma and ocular hypertension: the RENGE study. Jpn. J. Ophthalmol. 2021, 65, 810–819. [Google Scholar] [CrossRef]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Araie, M. Phase 2 Randomized Clinical Study of a Rho Kinase Inhibitor, K-115, in Primary Open-Angle Glaucoma and Ocular Hypertension. Am. J. Ophthalmol. 2013, 156, 731–736. [Google Scholar] [CrossRef]
- Lopes, N.L.V.; Gracitelli, C.P.B.; Chalita, M.R.; De Faria, N.V.L. Ocular Surface Evaluation After the Substitution of Benzalkonium Chloride Preserved Prostaglandin Eye Drops by a Preservative-free Prostaglandin Analogue. Med hypothesis Discov. Innov. Ophthalmol. 2019, 8, 52–56. [Google Scholar] [PubMed]
- Nino, M.; Napolitano, M.; Scalvenzi, M. Allergic contact dermatitis due to the beta-blocker betaxolol in eyedrops, with cross-sensitivity to timolol. Contact. Dermatitis. 2010, 62, 319–320. [Google Scholar] [CrossRef]
- Cusano, F.; Luciano, S.; Capozzi, M.; Verrilli, D.A. Contact dermatitis from pilocarpine. Contact Dermat. 1993, 29, 99–99. [Google Scholar] [CrossRef]
- Sodhi, P.K.; Verma, L.; Ratan, J. Dermatological Side Effects of Brimonidine: A Report of Three Cases. J. Dermatol. 2003, 30, 697–700. [Google Scholar] [CrossRef]
- Napolitano, M.; Potestio, L.; Castagliola, C.; Fabbrocini, G.; Patruno, C. Allergic contact dermatitis probably due to brimonidine tartrate in eyedrops. Contact Dermat. 2021, 85, 382–384. [Google Scholar] [CrossRef]
- Mitsuyama, S.; Abe, F.; Higuchi, T. Allergic contact dermatitis due to dorzolamide eyedrops. Contact Dermat. 2021, 84, 58–59. [Google Scholar] [CrossRef]
- Navarro-Triviño, F.J.; Ruiz-Villaverde, R. Periocular allergic contact dermatitis caused by brinzolamide. Contact Dermat. 2020, 84, 274–276. [Google Scholar] [CrossRef]
- Sodhi, P.K.; Verma, L.; Ratan, S.K. Contact dermatitis from topical bimatoprost. Contact Dermat. 2004, 50, 50–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, T.H.; Kim, S.-C. Allergic Contact Dermatitis Caused by Topical Eye Drops Containing Latanoprost. Ann. Dermatol. 2014, 26, 269–270. [Google Scholar] [CrossRef]
- Corazza, M.; Virgili, A.; Maniovani, L.; Masieri, L.T. Allergic contact dermatitis from cross-reacting beta-blocking agents. Contact Dermat. 1993, 28, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.S.; Yun, S.J.; Lee, J.B.; Kim, S.J.; Won, Y.H.; Lee, S.C. Toxic Epidermal Necrolysis Induced by the Topical Carbonic Anhydrase Inhibitors Brinzolamide and Dorzolamide. Ann. Dermatol. 2008, 20, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Sakata, R.; Shirato, S.; Miyata, K.; Aihara, M. Recovery from deepening of the upper eyelid sulcus after switching from bimatoprost to latanoprost. Jpn. J. Ophthalmol. 2013, 57, 179–184. [Google Scholar] [CrossRef]
- Choi, H.Y.; Lee, J.E.; Lee, J.W.; Park, H.J.; Lee, J.E.; Jung, H.E. In vitro study of antiadipogenic profile of latanoprost, travoprost, bimatoprost, and tafluprost in human orbital preadipocytes. J. Ocul. Pharmacol. Ther. 2012, 28, 146–152. [Google Scholar] [CrossRef]
- Woodward, D.F.; Krauss, A.H.-P.; Chen, J.; Liang, Y.; Li, C.; Protzman, C.E.; Bogardus, A.; Chen, R.; Kedzie, K.M.; Krauss, H.A.; et al. Pharmacological Characterization of a Novel Antiglaucoma Agent, Bimatoprost (AGN 192024). Experiment 2003, 305, 772–785. [Google Scholar] [CrossRef]
- Tanimura, H.; Minamoto, A.; Narai, A.; Hirayama, T.; Suzuki, M.; Mishima, H.K. Corneal Edema in Glaucoma Patients After the Addition of Brinzolamide 1% Ophthalmic Suspension. Jpn. J. Ophthalmol. 2005, 49, 332–333. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.C.; Chen, T. Brinzolamide induced reversible corneal decompensation. Br. J. Ophthalmol. 2005, 89, 389–390. [Google Scholar] [CrossRef]
- Adamsons, I. Irreversible corneal decompensation in patients treated with topical dorzolamide. Am. J. Ophthalmol. 1999, 128, 774–775. [Google Scholar] [CrossRef]
- Sugrue, M.F; Johns, B. Concentrations of dorzolamide in the pigmented rabbit eye after repeated dosing with TRUSOPT. Invest. Ophthalmol. Vis. Sci. 1999, 40, S171. [Google Scholar]
- Baratz, K.H.; Nau, C.B.; Winter, E.J.; McLaren, J.W.; O Hodge, D.; Herman, D.C.; Bourne, W.M. Effects of Glaucoma Medications on Corneal Endothelium, Keratocytes, and Subbasal Nerves Among Participants in the Ocular Hypertension Treatment Study. Cornea 2006, 25, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Beltz, J.; Zamir, E. ; Mbbs; Franzco; Franzco Brimonidine Induced Anterior Uveitis. Ocul. Immunol. Inflamm. 2014, 24, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Hopf, S.; Mercieca, K.; Pfeiffer, N.; Prokosch-Willing, V. Brimonidine-associated uveitis – a descriptive case series. BMC Ophthalmol. 2020, 20, 489. [Google Scholar] [CrossRef]
- Hu, J.; Vu, J.T.; Hong, B.; Gottlieb, C. Uveitis and cystoid macular oedema secondary to topical prostaglandin analogue use in ocular hypertension and open angle glaucoma. Br. J. Ophthalmol. 2020, 104, 1040–1044. [Google Scholar] [CrossRef]
- Abraham, S.V.; Teller, J.J. Influence of various miotics on cataract formation. Br. J. Ophthalmol. 1969, 53, 833–838. [Google Scholar] [CrossRef]
- Beasley, H.; Fraunfelder, F.T. Retinal Detachments and Topical Ocular Miotics. Ophthalmology 1979, 86, 95–98. [Google Scholar] [CrossRef]
- American Psychological Association. APA dictionary of psychology-Adjunctive therapy. Available from- https://dictionary.apa. Available online: https://dictionary.apa.org/adjunctive-therapy (accessed on 6 July 2023).
- Lichter, P.R.; Musch, D.C.; Gillespie, B.W.; Guire, K.E.; Janz, N.K.; Wren, P.A.; Mills, R.P.; CIGTS Study Group. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001, 108, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K. 2nd; Wilson, M.R.; Gordon, M.O. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 701–713, discussion 829-830. [Google Scholar] [CrossRef]
- Serle, J.B.; Wang, R.-F.; Mittag, T.W.; Shen, F.; Podos, S.M. Effect of Pilocarpine 4% in Combination With Latanoprost 0.005% or 8-iso Prostaglandin E2 0.1% on Intraocular Pressure in Laser-induced Glaucomatous Monkey Eyes. Eur. J. Gastroenterol. Hepatol. 2001, 10, 215–219. [Google Scholar] [CrossRef]
- Crawford, K.; Kaufman, P.L. Pilocarpine Antagonizes Prostaglandin F2α-Induced Ocular Hypotension in Monkeys. Arch. Ophthalmol. 1987, 105, 1112–1116. [Google Scholar] [CrossRef]
- Kent, A.R.; Vroman, D.T.; Thomas, T.J.; Hebert, R.L.; E Crosson, C. Interaction of pilocarpine with latanoprost in patients with glaucoma and ocular hypertension. J. Glaucoma 1999, 8, 257–262. [Google Scholar] [CrossRef]
- Yamagishi-Kimura, R.; Honjo, M.; Komizo, T.; Ono, T.; Yagi, A.; Lee, J.; Miyata, K.; Fujimoto, T.; Inoue, T.; Tanihara, H.; et al. Interaction Between Pilocarpine and Ripasudil on Intraocular Pressure, Pupil Diameter, and the Aqueous-Outflow Pathway. Investig. Opthalmology Vis. Sci. 2018, 59, 1844–1854. [Google Scholar] [CrossRef]
- Liu, J.H.; Medeiros, F.A.; Slight, J.R.; Weinreb, R.N. Comparing Diurnal and Nocturnal Effects of Brinzolamide and Timolol on Intraocular Pressure in Patients Receiving Latanoprost Monotherapy. Ophthalmology 2009, 116, 449–454. [Google Scholar] [CrossRef] [PubMed]
- O’connor, D.J.; Martone, J.F.; Mead, A. Additive intraocular pressure lowering effect of various medications with latanoprost. Am. J. Ophthalmol. 2002, 133, 836–837. [Google Scholar] [CrossRef]
- Feldman, R.M.; Tanna, A.P.; Gross, R.L.; Chuang, A.Z.; Baker, L.; Reynolds, A.; Prager, T.C. Additivity 398 Study Group. Comparison of the Ocular Hypotensive Efficacy of Adjunctive Brimonidine 0.15% or Brinzolamide 1% in Combination with Travoprost 0.004%. Ophthalmology 2007, 114, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Martinez-De-La-Casa, J.M.; Castillo, A.; Garcia-Feijoo, J.; Mendez-Hernandez, C.; Fernandez-Vidal, A.; Garcia-Sanchez, J. Concomitant administration of travoprost and brinzolamide versus fixed latanoprost/timolol combined therapy: three-month comparison of efficacy and safety. Curr. Med Res. Opin. 2004, 20, 1333–1339. [Google Scholar] [CrossRef]
- Reis, R.; Queiroz, C.F.; Santos, L.C.; Avila, M.P.; Magacho, L. A randomized, investigator-masked, 4-week study comparing timolol maleate 0.5%, brinzolamide 1%, and brimonidine tartrate 0.2% as adjunctive therapies to travoprost 0.004% in adults with primary open-angle glaucoma or ocular hypertension. Clin. Ther. 2006, 28, 552–559. [Google Scholar] [CrossRef]
- Cheng, J.W.; Cheng, S.W.; Yu, D.Y.; Wei, R.L.; Lu, G.C. Meta-analysis of α2-adrenergic agonists versus carbonic anhydrase inhibitors as adjunctive therapy. Curr. Med. Res. Opin. 2012, 28, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Konstas, A.G.P.; Holló, G.; Haidich, A.-B.; Mikropoulos, D.G.; Giannopoulos, T.; Voudouragkaki, I.C.; Paschalinou, E.; Konidaris, V.; Samples, J.R. Comparison of 24-Hour Intraocular Pressure Reduction Obtained with Brinzolamide/Timolol or Brimonidine/Timolol Fixed-Combination Adjunctive to Travoprost Therapy. J. Ocul. Pharmacol. Ther. 2013, 29, 652–657. [Google Scholar] [CrossRef]
- Öztürk, F.; Ermiş, S.S.; Inan. .; Idaǧ, A.A.; Yaman, S.; Craven, E.R.; Walters, T.R.; Williams, R.; Chou, C.; Cheetham, J.K.; et al. Comparison of the Efficacy and Safety of Dorzolamide 2% When Added to Brimonidine 0.2% or Timolol Maleate 0.5% in Patients with Primary Open-Angle Glaucoma. J. Ocul. Pharmacol. Ther. 2005, 21, 68–74. [Google Scholar] [CrossRef]
- Fuwa, M.; Shimazaki, A.; Odani-Kawabata, N.; Kirihara, T.; Taniguchi, T.; Iwamura, R.; Yoneda, K.; Kato, M.; Morishima, K.; Shams, N.K. Additive Intraocular Pressure-Lowering Effects of a Novel Selective EP2 Receptor Agonist, Omidenepag Isopropyl, Combined with Existing Antiglaucoma Agents in Conscious Ocular Normotensive Monkeys. J. Ocul. Pharmacol. Ther. 2021, 37, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Tanna, A.P.; Rademaker, A.W.; Stewart, W.C.; Feldman, R.M. Meta-analysis of the efficacy and safety of alpha2-adrenergic agonists, beta-adrenergic antagonists, and topical carbonic anhydrase inhibitors with prostaglandin analogs. Arch. Ophthalmol. 2010, 128, 825–833. [Google Scholar] [PubMed]

| Aqueous Suppressants Drugs |
|---|
| Alpha-adrenergic agonist |
| Apraclonidine 0.5% |
| Brimodine 0.1%, 0.15%, 0.2% |
| Beta-adrenergic antagonist |
| Betaxolol 0.5% |
| Timolol 0.5% |
| Carbonic anhydrase inhibitors (CAIs) |
| Brinzolamide 1% |
| Dorzolamide hydrochloride 2% |
| Aqueous Outflow Drugs |
| Trabecular meshwork outflow pathway |
| Cholinergic |
| Carbachol 0.75%,1.5%,3% |
| Demecarium 0.125%, 0.25% |
| Echothiophate 0.125% |
| Pilocarpine 1%,2% ,4% |
| Rho-Kinase inhibitors |
| Netarsudil 0.02% |
| Ripasudil 0.4% |
| Nitric acid donors |
| Latanoprostene bunod |
| Unconventional outflow pathway |
| Prostaglandin analogues |
| Bimatoprost 0.01%, 0.03% |
| Latanoprost 0.005% |
| Tafluprost 0.0015% |
| Travoprost 0.04% |
| Unoprostone Isopropyl 0.15% |
| Omidenepag Isopropyl 0.002% |
| Drugs | IOP related effects | IOP independent effects | ||||
|---|---|---|---|---|---|---|
| Aqueous secretion | Trabecular meshwork outflow | Uveoscleral pathway outflow | Episcleral venous pressure | Neuroprotection | Ocular blood flow | |
| Betaxolol | Decrease | No effect | No effect | |||
| Timolol | Decrease | No effect | No effect | No effect | Decrease [22] | |
| Apraclonidine | Decrease | Decrease [27] | Decrease [28] | |||
| Brimodine | Decrease | No effect | Increase [14] | Decrease [26] | + [29, 123, 124] | No effect [30,31] |
| Brinzolamide | Decrease | No effect | No effect | No effect | ? / No effect [31,121] | |
| Dorzolamide | Decrease | No effect | Increase [9, 110]/No effect [30] | |||
| Bimatoprost | Increase | Increase# | Increase | Increase/ Decrease [109] | ||
| Latanoprost | Increase | Increase# | Increase | Increase | Increase [11,13] | |
| Tafluprost | Increase# | Increase | Increase [117] | |||
| Travoprost | Increase | Increase# | Increase | Increase [13] | ||
| Unoprostone | Increase# | Increase | No effect | |||
| Omidenepag isopropyl | No effect | Increase | ||||
| Pilocarpine | No effect [46] | Increase | Decreases [206] | No effect | Increase [9,10] | |
| Latanoprostene bunod | Increase | Increase | Ongoing Trial | Increase [122] | ||
| Netarsudil | Decrease | Increase | No effect | Decrease [106, 107] | + [129, 130] | |
| Ripasudil | Increase | Decrease [108] | + [129, 130] | |||
| Drug | Time | IOP reduction (%) | Washout period | |||
|---|---|---|---|---|---|---|
| Onset | Peak effect | Duration | Peak | Trough | ||
| Betaxolol | 30 minutes | 2 hours | 12 hours | -23 | -20 | 1 week |
| Timolol | 30 minutes | 2 hours | 12-24 hours | -27 | -26 | 4 weeks |
| Brinzolamide | 1 hour | 2-3 hours | 8-12 hours | -17 | -17 | 1 week |
| Dorzolamide | 1 hour | 3 hours | 8 hours | -22 | -17 | 1 week |
| Apraclonidine | 1 hour | 45-90 minutes | 6-8 hours | -27 | -20 | 1-2 weeks |
| Brimonidine | 1 hour | 2-3 hours | 8-10 hours | -19 | -14 | 1-2 weeks |
| Bimatoprost | 4 hours | 8-12 hours | 24 hours | -33 | -28 | 4-6 weeks |
| Latanoprost | 3-4 hours | 8-12 hours | 24 hours | -31 | -28 | 4-6 weeks |
| Tafluprost | 2-4 hours | 12 hours | 24 hours | -31 | -27 | 4-6 weeks |
| Travoprost | 2 hours | 12 hours | >24 hours | -31 | -29 | 4-6 weeks |
| Unoprostone | 30-90 minutes | 2-3 hours | 2-5 hours | -25 | -10 | 2-4 weeks |
| Omidenepag isopropyl | 2-4 hours | 12 hours | >24 hours | -25 | -20 | 1 week |
| Pilocarpine | 60 minutes | 75 minutes | 4-6 hours | -25 | -15 | 48 hours |
| Netarsudil | 1-2 hours | - | >24 hours | -25 | -18 | - |
| Ripasudil | 1-2 hours | - | 12 hours | - | - | |
| Latanoprostene bunod | 1-3 hours | 11-13 hours | 24 hours | -32 | -30 | 4-6 weeks |
| Acetazolamide | 30 minutes | 2 hours | 6-8 hours | - | - | 3 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




