1. Introduction
Advances in materials science, material engineering and printing technologies of smart electronic, optoelectronics and other devices are paving the way for development of extremely efficient devices which are improving quality of life rapidly [1–12]. Materials and their processes that improve the quality and enhance the performance of the devices while reducing the cost are most desirable. Inkjet printing, one such technology will continue to expand driven by the demand for smart devices, lightweight flexible materials, and environmentally sustainable technologies. Over the past years this technology has advanced from developing simple printable electronic elements like pressure and temperature sensors to state of the art applications including medical diagnostics, analytical applications, and development of optoelectronic systems. Inks used for printing have a wide array of properties ranging from electrical conducting and semi-conducting properties, thermal conductivity, electroluminescence etc. The Dupont, Sigma-Aldrich, Pedotinks are some leading manufacturers of conductive polymer-based inks. Companies like Xaar, FUJIFILM Dimatix and Global Inkjet Systems Ltd provide functional material printing. Functionality combined with easy processability are necessary to fabricate tailor made devices. Novel functional inks include but are not limited to carbon nanoparticles [13], redox responsive organometallic polymers like poly(ferrocenylsilane) (PFS) [14], photochromic dyes base on organic spiro compounds [16], polysiloxane based water repellant microemulsions [15] conductive polymer polyaniline (PANI) and its composite based inks [17], p-doped poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) [18,19] and n type poly(benzimidazobenzophenanthroline):poly(ethyleneimine) (BBL:PEI) electrically conductive polymers [20].
Inkjet printing offers rapid, reproducible, and low-cost ways to pattern conductive polymers on substrates and is an excellent tool for printing conductive polymers based completely functional products which include supercapacitors, sensors for different compounds, and energy storing electrochromic devices on different substrates. Advantages over other enabling technologies include scalability (small as well as large surface areas), high precision, simultaneous deposition of multicomponent layers [21,22], printing of delicate membranes without fear of contamination (non -contact printing where the substrate and the print head never come in contact) [11], and printing from aqueous solutions making it well suited for biological species [15,23].
Since their accidental discovery by H. Shirakawa et al. conjugated conductive polymers have been well researched due to their distinctive electronics properties[26,27]. These conductive polymers with C=C conjugated double bonds forming the backbone display interesting electrical properties which include low energy optical transitions, low ionization potentials and high electron affinities. These polymers can be oxidized and reduced thereby converting them into p and n doped materials with an increased conductivity [28]. Among the conjugated conductive polymers polyaniline, poly(phenylenevinyelene), polypyrrole and PSS have attracted much interest [29,30]. Conductive polymers fall in the class of smart polymer materials with dynamic electroactive structures and have been explored for use in electrochromic devices, electrophotocatalytic materials, sensors for pH and humidity, smart clothes and coatings, soft matter applications and solar cells to name a few [31–35]. Electrical conductive membranes are being studied as alternatives to conventional membranes with enhanced performance for water filtration and in wastewater treatment [36]. Conductive polymer based thermoelectric devises exhibit flexibility are inexpensive and potential candidates to convert waste heat to power [37,38]. Microfabrication which is important to a number of applications like microelectronics [39], microanalytical systems [40,41], microoptics and potable biomedical devices [42,43] and microfluidics [44,45] and can be aided by inkjet printing. As discussed above, due to excellent electronic and processability features of conductive polymer, the growing efforts have been devoted for ink formulation and fabrication of devices with conducting polymer via inkjet printing technologies. Hence, in this review we focus on formulation of the ink, fabrication, and the applications of conductive polymer-based inks for inkjet printing with special reference to tailor made supercapacitors, sensors, electrochromic devices, and patterning of conductive polymers on flexible substrates.
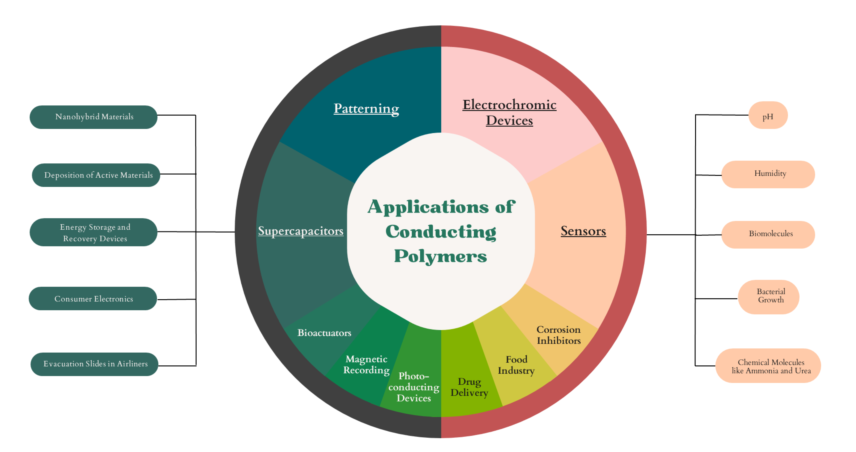
2. Applications of Inkjet Printer Conducting Polymers
The conductive polymers have been explored for various applications including supercapacitors, sensors, electrochromic devices etc. owing to their excellent properties such as mechanical, flexibility, electrical, and low-cost production etc. [46–50]. In this section are reviewed, conducting polymers for supercapacitors, sensors, electrochromic devices including formulation of the ink, inkjet printing process and keys performance highlights. Additionally, patterning of conductive polymers on flexible substrates is also reviewed in this section.
2.1. Conductive Polymer for Supercapacitors
An excellent choice as a sustainable device for energy storage, the supercapacitor has two electrodes, an electrolyte and a separator, the electrochemical material being of utmost importance. Supercapacitors are classified as electrochemical double layer capacitors (EDLC), pseudocapacitors or as a hybrid formed by a combination of both types [51]. The EDLC based on large surface area carbon materials like carbon nanotubes, activated carbon etc. are non-faradaic in nature, storing electrical energy by nanoscale charge separation at the electrode–electrolyte interface forming electrical double layers. The pseudocapacitors based on metal oxide, metal doped carbon and conductive polymers are faradaic in nature, based on reversible and quick surface redox reaction between the electrode and electrolyte. They provide a much higher energy density in comparison to EDLC. Polyaniline (PANI), polypyrrole (PPy), polythiophene (PTh) and poly 3,4-ethylenedioxythiophene (PEDOT) are being researched extensively for pseudocapacitive applications because of their large specific capacitance.
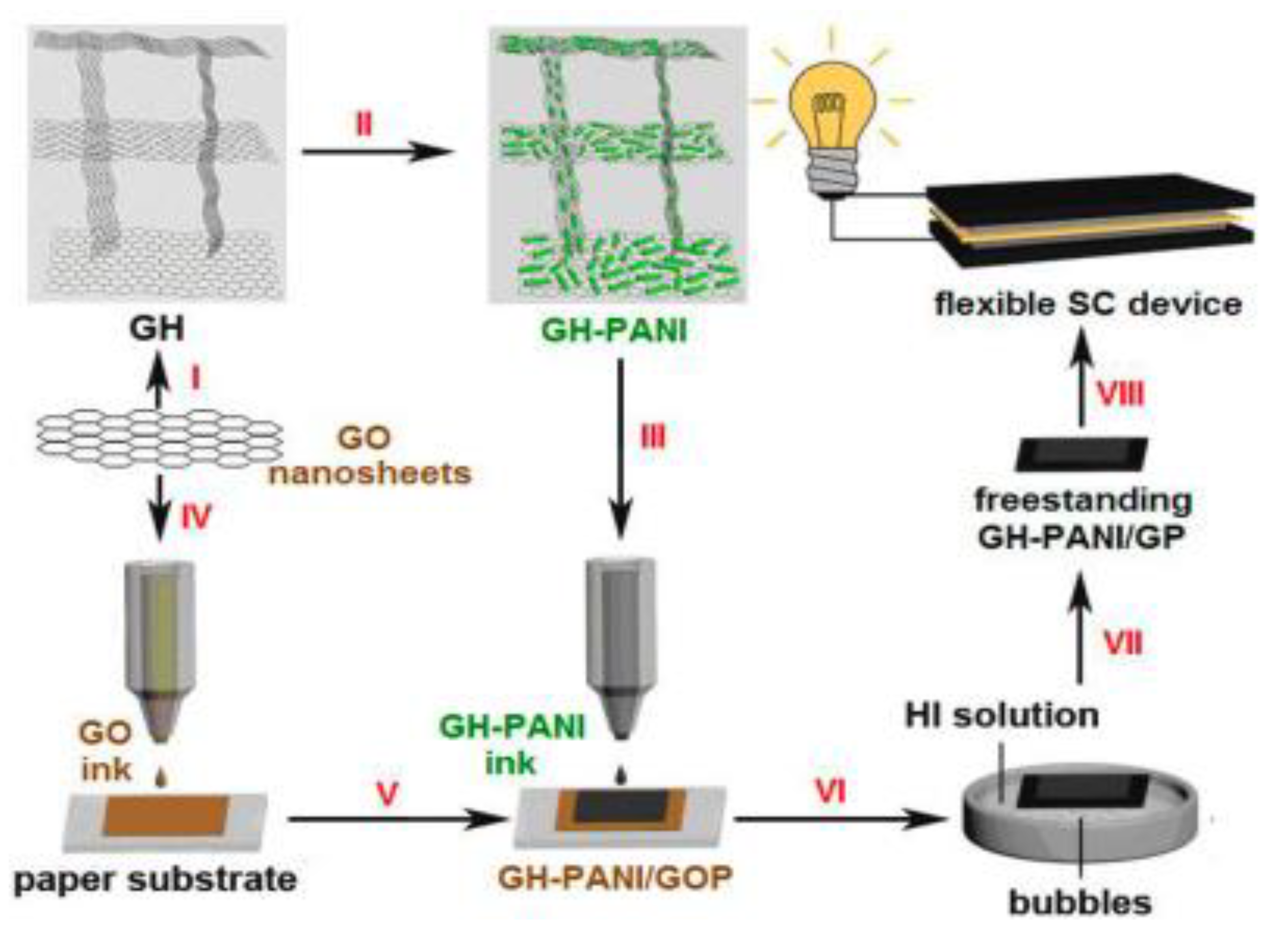
Inkjet printing known to deposit ink on demand helps greatly economize the consumption of material while also allowing printing of the material on various substrates like paper, glass, fabric of different designs and patterns helping make flexible supercapacitors. Thin paper like electrode material as well as free standing electrode materials which have the advantage of being free of insulating binders, conductive agents, current collectors and solvents are best suited for flexible and lightweight devices of the future. Development of a variety of methods by which polyaniline (PANI) can be synthesized has led to it being extensively researched for inkjet printed supercapacitors. A nanohybrid freestanding, electrode based on GP (Graphene paper) and GH-PANI (Graphene Hydrogel-PANI) π−π stacking interactions has been developed by inkjet printing. The various steps involved in fabrication of GP (Graphene paper) and GH-PANI (Graphene Hydrogel-PANI) based supercapacitor are illustrated in
Figure 1. The a symmetric supercapacitor device demonstrated good mechanical, electrochemical and capacitive properties, with a tolerable energy density of 24.02 Wh kg
-1 at a power density of 400.33 W kg
-1 [52].
PANI production has traditionally been achieved by polymerizing the monomer aniline. A benign method where the aniline dimer (DANI) in an organic solvent is added to the oxidative solution of ammonium persulphate in aqueous medium has been used to prepare the emeraldine PANI (PANI-EB) as a fast reaction eliminating hazardous and toxic aniline monomer usage directly. Inks with extremely good jettability and geometric realization have been obtained by dedoping this salt doped with chloride ions to get PANI doped with trifluorosulphonic acid and camporsulphonic acid as counterions thereby increasing the conductivity of the resultant inks for producing inkjet-printed tracks of diverse geometry [53]. These inks are also of interest as they demonstrate that counterion displacement induced by measurement signal leads to negative resistance/capacitance and supercapacitance (-2.3mF @30Hz which corresponds to a mass capacity of -799 F g
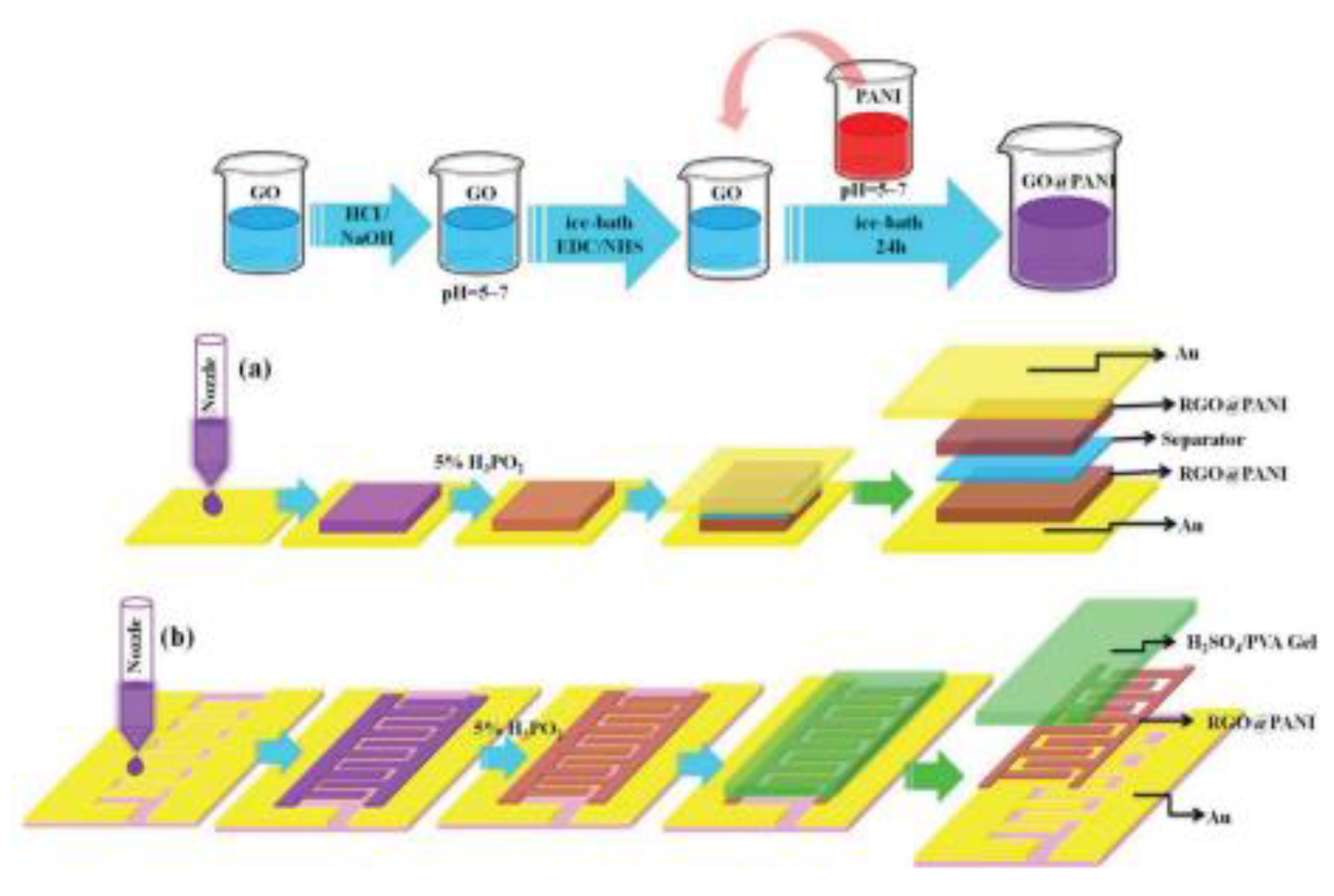
-1) ideal for application in low frequency range devices. An ink based on PANI nanoparticles covalently attached to the graphene oxide i.e. (GO) @ PANI composites has been synthesized by first synthesizing GO and PANI separately followed by mixing EDC/NHS (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride(EDC), and N-hydroxysulfosuccinimide (NHS)) activated GO and PANI in the ratio 9:1 to prepare the composite, which was inkjet printed on a gold coated polymer substrate (PET) and reduced by hypophosphorous acid to fabricate the desired electrodes [54]. Sandwiched and interdigitated supercapacitors were assembled (
Figure 2). Volumetric supercapacitance for both the sandwiched (258.5 F cm
-3 at 1 mV s
-1) and interdigitated supercapacitors (554 F cm
-3 at 1 mV s
-1), was high and an excellent cycling retention of over 90% after 2000 cycles of charging and discharging.
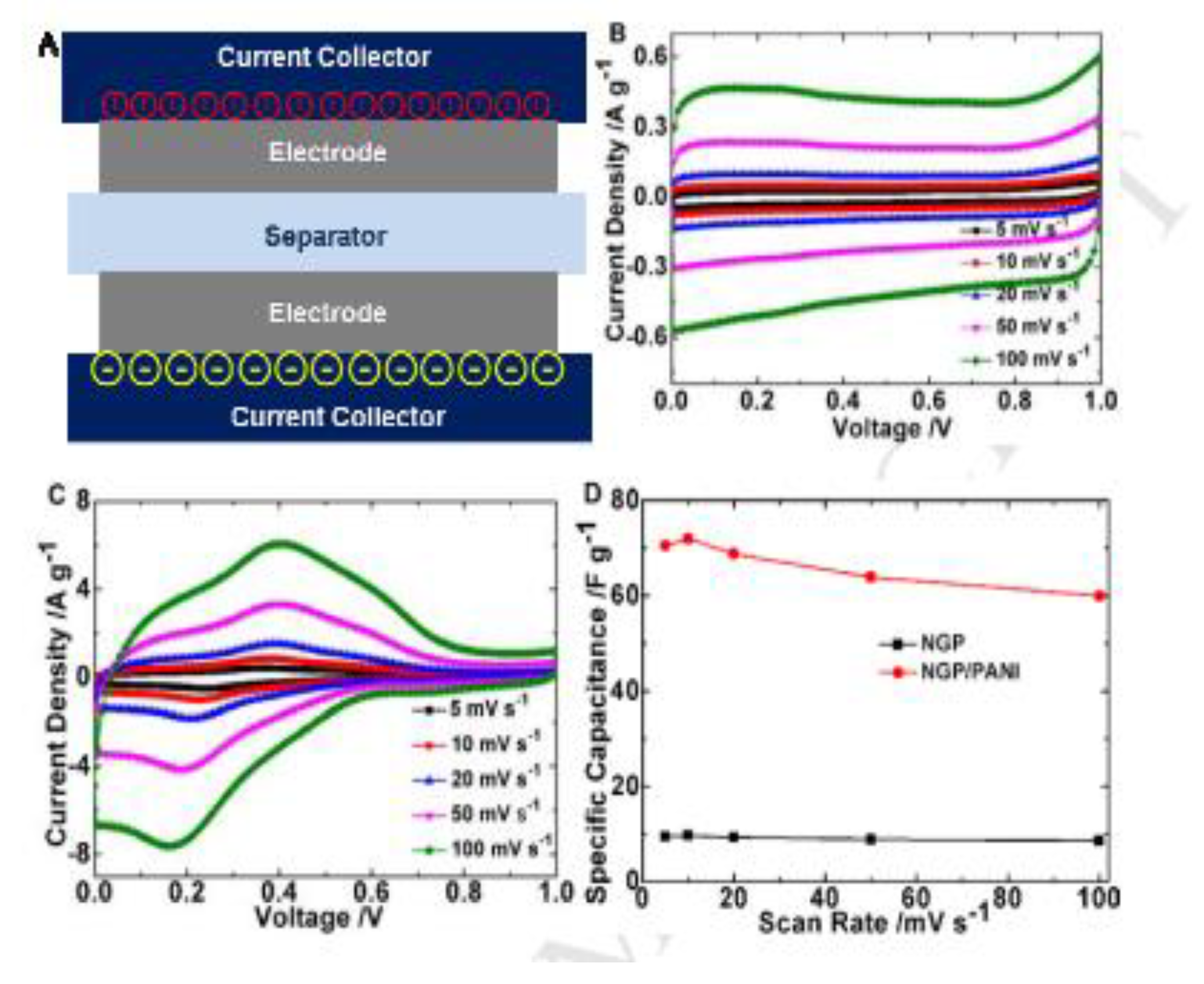
Xu and coworkers have formulated nanographene/polyaniline (NPG/PANI) inks of appropriate viscosity, surface tension and conductivity capable of being deposited as thin films on substrates by inkjet printing (
Figure 3A) [55]. Two NGP/PANI ink printed electrodes with carbon fabric as the substrate sandwiched together by a separator to form an electrochemical capacitor were studied using a 1 M H
2SO
4 electrolyte. They show a maximum specific capacitance of 82 F g
-1, a power density of 124 kW kg
-1, cycle life of 1000 cycles and energy density of 2.4 Wh kg
-1 with a scan rate of 20 mVs
-1 (
Figures 3B–D). On macro-porous graphene foam (ultrathin graphene sheets) a thin film of PANI was deposited by electrochemical deposition (polyaniline coated graphene), and the pores were further filled with aniline, phytic acid and ammonium persulphate by inkjet printing to allow oxidative polymerization to obtain PANI filled graphene foams. The PANI filled macro-porous graphene as an electrode for supercapacitors exhibited high areal capacitance of over 1700 mF cm
−2 more than two times the capacitance achievable by pure graphene or metal oxide /conductive polymer thin layer coated graphene [56]. Various conducting polymer such as PANI, PPy and PTh, have been widely used for the fabrication of supercapacitors via inkjet printing, some reported supercapacitors based on conducting polymer are tabulated in
Table 1.
2.2. Conductive Polymer for Sensors
Nanostructured conductive polymers are good candidates for sensor and biosensor design, a response being generated due to the electrical conductivity property, which can be modified by redox reactions and in some cases by protonation. They can be deposited on various surfaces with good thermal stability, chemical stability and at a low cost making them ideal for many applications. In addition, the conductivity can be tuned by doping or combining the conductive polymer with various additives, oxidants like ammonium persulfate, ammonium peroxy disulfate, aqueous copper(II) and iron (III) salts and halogen electron acceptors like bromine and iodine. Inkjet printing a solution or suspension containing the conductive polymer offers a simple and cost-effective method for preparing varied sensors having environment sensing, food testing and clinical applications. Among various sensor, pH sensors have received much attention in the food and healthcare industry, especially food borne pathogen detection and as personal wearable biochemical sensors to monitor sweat, blood and tumors as a complement to lab based equipment.
A paper based colorimetric sensor using Polyaniline-Pectin nanoparticles (PANI- PEC NPs) printed on Whatman filter paper grade 4 has been used for sensing the presence of E. coli. both qualitatively and quantitatively in milk and milk products. The acid metabolites produced by the bacterium (succinates, lactates, acetate, malates, etc) convert the blue emeraldine base form of PANI to a green-colored emeraldine salt form with increased conductivity. With a sensitivity of 0.52 ± 0.17 log CFU/mL E. coli within 10: 21 hours, it represents a practical system with good applicability and efficiency [57].
Aqueous-based PANI ink formulations with acrylic resin additives as binders were used to print pH-sensitive electrodes by Bilbao and coworkers. The working electrode was a screen-printed carbon ink layer onto valox® (Polybutylene Terephthalate) which was modified by inkjet printed PANI ink. The performance of the fabricated electrode was compared with electrodes prepared by electro-polymerization of PANI on carbon paste electrodes and found to be better. Response of these electrodes fabricated by inkjet printed PANI inks and acrylic resins on to screen printed carbon electrodes for synthetic sweat showed good sensitivity of up to 69.1 mV/pH [58].
PANI has long been used as a sensor for the toxic ammonia gas, ammonia deprotonating the emeraldine salt and converting it to the emeraldine base form with a corresponding drop in conductivity. A sensor for toxic ammonia gas has been developed by piezoelectric inkjet printing of polyaniline nanoparticle films on interdigitized silver electrode arrays (Ag IDAs) on a PET substrate. The sensor is further attached to polyimide-based flexible heating foils to facilitate operating at different temperatures. It has a stable logarithmic response of 1-100 ppm to ammonia with a t50 value of 15 s at room temperature with better performance at 80°C [59]. The response was also found to be largely independent of the relative humidity range of 35 to 98%.
Sensors for gaseous ammonia have also been developed by Duy Dam Le and coworkers. Silver has been inkjet printed on Si/SiO2 substrates and further drop coated with a blend film of emaraldine salt form of PANI and ethylene glycol (EG) to sense NH3. It showed good sensitivity as well as a linear response to ammonia concentration at a relative humidity of 3-70% [60]. The sensor could be regenerated for use by heat treatment at 60℃ in about 15 minutes. Inkjet printed layers (Dimatix materials printer) of polyaniline and copper (II) chloride on screen printed silver interdigitated electrode (IDE) on a flexible PET substrate have been studied to function as a chemiresistor for hydrogensulphide with sensitivity of 2.5 ppm by volume [61]. A linear relationship between measured current and concentration is observed in the 10-100ppmv region. The variation in current in the circuit is due to protonation of PANI to the emeraldine salt by H2S, as opposed to protonation of ammonia by PANI in ammonia sensors. A class of CuCl2/PANI chemiresistor sensors with inkjet printed silver electrodes on kaolin coated paper substrate has also been studied for sensing H2S. Exposure to H2S resulted in the formation of the copper sulphide and the acid HCl which protonates the EB PANI resulting in increase in conductivity. Concentrations as low as 10 ppm H2S could be sensed [62].
Inkjet printing provides a low consumption, cost effective method of preparing disposable diagnostic tools for medical applications. A disposable paper based electrochemical sensor for detection of ascorbic acid having good sensitivity of 17.7 lA/Mm with the limit-of-detection for ascorbic acid being 30 ± 3 lM in a concentration range of 30–270 lM. The assembly consists of screen printed conductive carbon graphite paste on Whatman Grade No.1 filter paper as the reference electrode and counter electrode and five layer PANI inkjet printed onto the screen printed carbon electrodes as the working electrode in acetate buffer at a pH of 5 [63].
The conducting emeraldine salt phase of PANI ink prepared by ammonium persulphate oxidation used for the printing of interdigitated patterns on the flexible untreated polymer substrate using an HP inkjet printer exhibited a change in resistance with change in relative humidity. The sensor exhibited a short response time of 5s, under low humidity conditions (6% RH) on exposure for 50 seconds, the electrical resistance exhibited an apparent increase, and when the sensor was exposed to high humidity conditions (97.3% RH) the resistance started decreasing [64]. The sensor has applications as low-cost RFID tags, polymer photovoltaic cells and in printed flexible electronics devices.
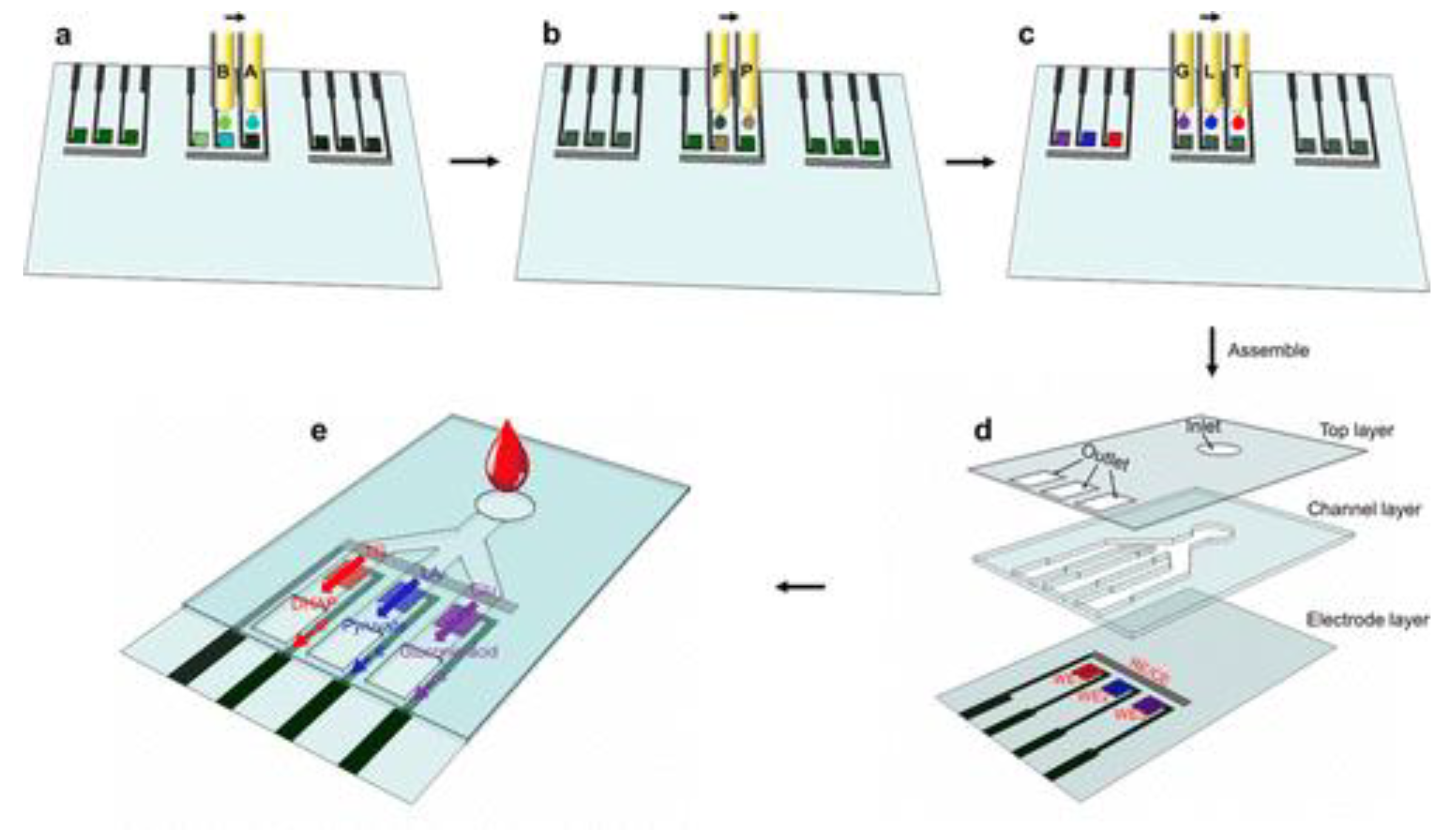
Multiplexed detection of various metabolites is useful for early and easy detection of diseases. It is a challenge to deposit electroactive materials and enzymes on selective electrodes in a precise fashion. By a ‘drop on demand’ selective inkjet printing process an assembly of a working electrode layer specific to each metabolite, a microfluidic channel layer, and a top cover has been fabricated (
Figure 4). It has been tested for analyzing biomolecules like triglycerides, glucose, and lactates in phosphate buffer saline as well as human serum samples [65]. The sensitivity with respect to triglycerides was found to be 7.49 μA/mM
-1cm
-2 between 0.1mM and 6mM, while that of lactate was 3.94 μAmM
-1cm
-2 between 0.08mM and 5mM. The glucose sensitivity exhibited by the sensor was 5.03 μAmM
-1cm
-2 between 1mM and 25mM.
Urea has been long known to function as an indicator for human kidney health. With the aim of developing a cheap disposable biosensor for urea, inkjet printed assemblies have been tried. Carbon paste electrodes have been screen printed onto pre-shrunk PET plates followed by sequential inkjet printing of PANI nanoparticles and Urease enzyme solution containing glycerol (0.1%, v/v) and Triton X-100 (0.01%, v/v) in phosphate buffer of pH 7.12 (0.1M) on the working electrodes. The decomposition of urea has been measured chronocoulometrically via the doping of ammonium at the polyaniline-modified i.e urease/nanoPANI biosensor electrode, surface at−0.3V vs. Ag/AgCl [66]. The sensor successfully measure ammonia in the 0.1-100 mM and urea in the 2–12 mM (r
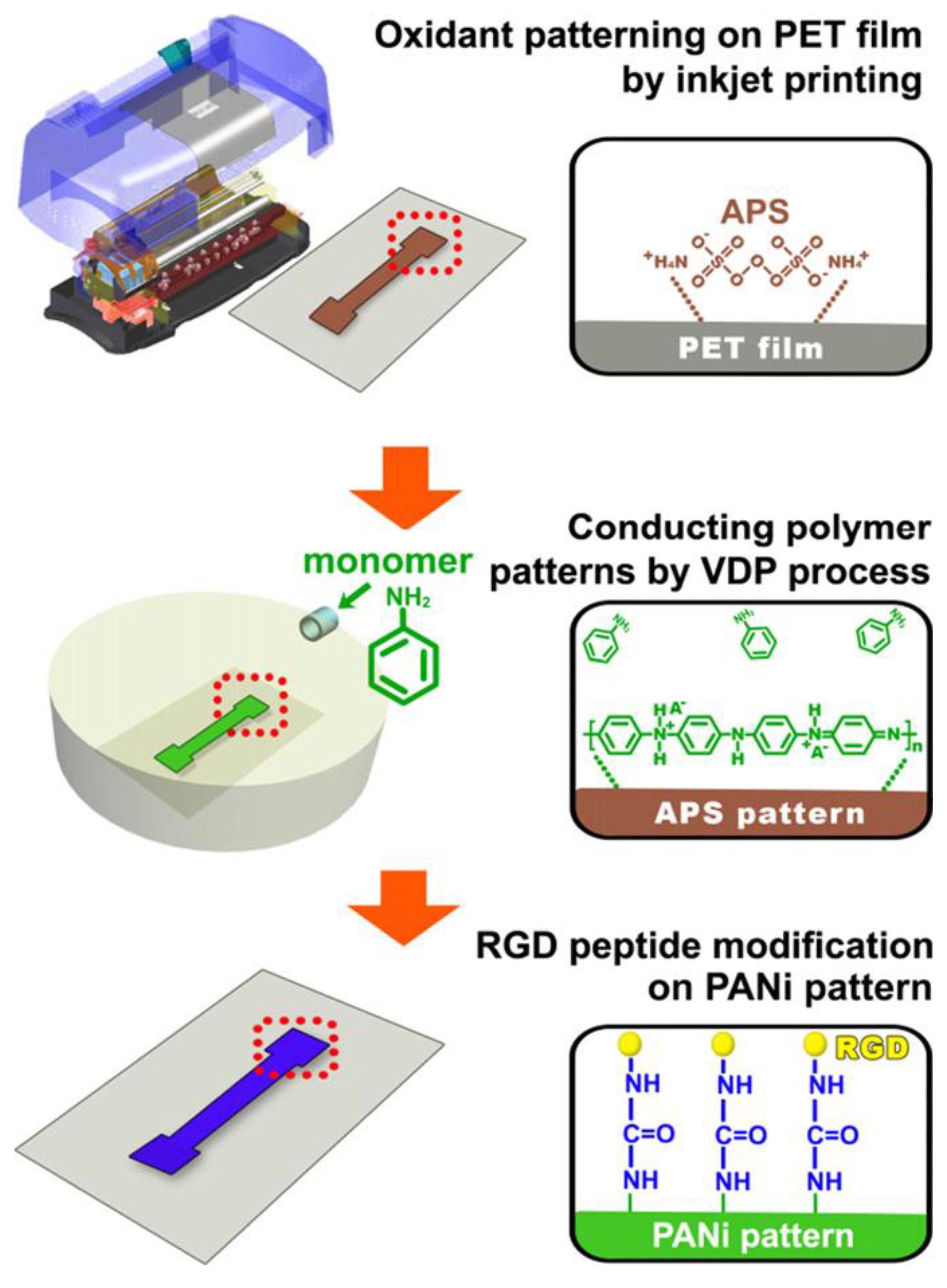
2=0.98) range. Inkjet printing has been used to fabricate a three-electrode configuration on a coated paper substrate. An assembly of inkjet printed gold nanoparticle based working and counter electrodes along with a quasi-reference inkjet printed silver nanoparticle electrode with an electrochemically deposited layer of Ag/AgCl has been deviced and studied as sensors after various modifications. Formation of self-assembled monolayers (SAM) of alkanethiols on the gold electrode surface have been studied as possible sensors for materials where terminally substituted thio groups are used. Electropolymerization of the working electrode with polyaniline (PANI) conductive polymer for pH sensing applications and glucose oxidase (GOx) entrapped poly- 3,4-ethylenedioxythiophene (PEDOT) films (PEDOT–GOx) to explore the possibility of forming amperometric glucose sensors has been explored [67]. Morrin and coworkers investigated PANI nanoparticles in an enzyme biosensing application. Aqueous nanoparticles of PANI along with horseradish peroxidase (HRP) enzyme were cast by a drop coating method onto the surface of the screen printed carbon working electrode simultaneously, the disadvantage being the inherent thickness of the film affecting its potential [68]. Oxidant patterning by inkjet printing on polyethylene terephthalate films followed by deposition of emeraldine salt form of PANI patterns and immobilization of RGD peptide over it by covalent linkages has been used for detection of biomolecules from live cells (
Figure 5). Rat pheochromocytoma PC12 cells were cultured on the RGD–immobilized PANI pattern and real-time electrical signal detection to track biomolecular release was studied [69].
To develop and study inkjet printing for printable chemical sensors, low sheet resistance elec- trodes of multi-wallled carbon nanotubes were inkjet printed on a transparency film followed by printing of randomly oriented PANI nanowires dispersed in aqueous medium resulting in a fully printed sensor assembly. The sensor is capable of detecting change in pH as PANI changes the resistivity with pH, further a H2O2 sensor has been fabricated by depositing silver nanoparticles (catalyze decomposition of H2O2 to produce OH-) over the PANI layer [70]. To explore the superior sensing of nanotubes and nanoparticles, carbon nanotubes as electrodes and chemiresistive polyaniline nanowire sensors were inkjet printed sequentially on the PET film to prepare a chemiresistive glucose sensor. Glucose oxidase and platinum nanoparticle catalysts were placed between the CNT layers. Glucose oxidase enzyme catalyzes glucose oxidation to produce gluconolactone and hydrogen peroxide, H2O2 is further catalyzed by platinum nanoparticles to produce OH-, the local change in pH is sensed by the PANI layer which changes resistivity [71]. A paper-based sensor was fabricated as a DNA biosensor and used for the determination of high-risk HPV type 16 by inkjet printing graphene polyaniline (G-PANI) conductive ink onto the screen-printed carbon ink working electrode. The AQ-PNA ( anthraquinone-labeled pyrro- lidinyl peptide nucleic acid (acpcPNA) probe) probe was immobilized on this electrode and modified to introduce negative charges to allow for electrostatic immobilization on the cationic G-PANI electrode. A linear response range of 10-200 nM was obtained and the detection limit of HPV type 16 DNA was found to be 2.3 nM. The sensor was successfully tested to detect the PCR amplified DNA from HPV type 16 positive SiHa cells successfully demonstrating that it functions as a highly sensitive ePAD DNA biosensor for the diagnostic screening and detection of cervical cancer [72].
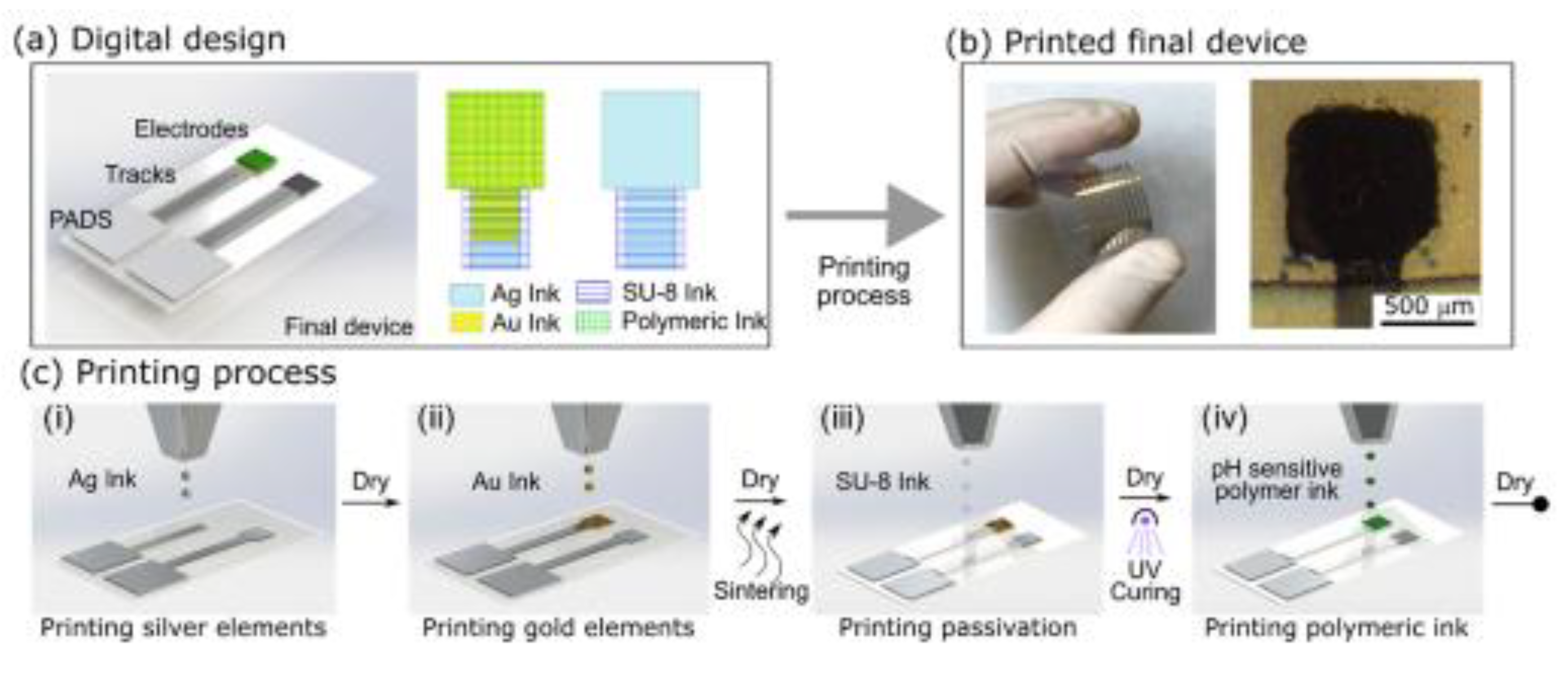
Soft photomasks have been printed on different substrates. Desired design patterns were inkjet printed onto transparent hybrid composite (biaxially oriented polypropylene coated with silica oxide (BOPP-SiOx) ) inorganic oxide film [73]. The inkjet printed soft photomasks were further used for depositing organic polymers (PANI)/inorganic materials on polymer films, photografting organic polymer materials like PAA onto polymer films and non-planar substrates. Inkjet printing was used by Zea et al. to create a printed pH sensor made of polyaniline, polypyrrole, and polyelectrolyte poly(sodium 4-styrenesulphonate) (PSS) based on PANI:PSS/PPy:PSS inks deposited on a gold microelectrode printed on a flexible substrate (
Figure 6). The gold ink functionalized with pthalocyanin as the substrate enhanced the conductivity by improving the electron mobility between the conductive polymer chain and gold nanoparticles. A liner super-Nernstian response (81.2 0.5 mV/ pH unit) over a wide pH range (pH 3–10) make it a promising sensor for various applications [74]. Furthered, various inkjet printed conducting polymer used sensing application are summarized in
Table 2.
2.3. Conductive Polymer for Electrochromic Devices
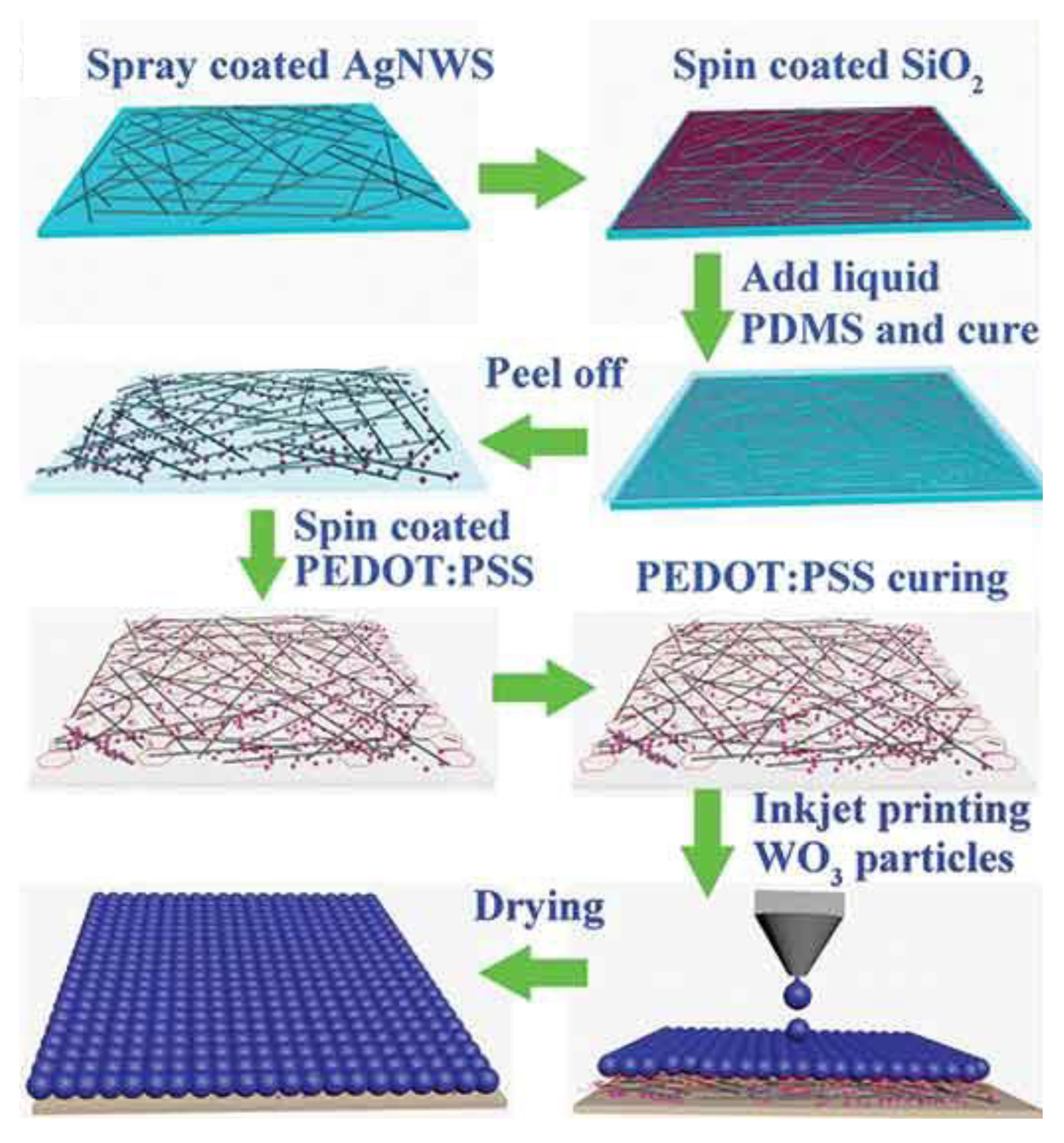
Electrochromic devices can change their colour reversibly and find use in display systems, smart windows and camouflage garments [75–78]. Deformable or flexible electrochromic devices without any deterioration in performance are capable for indicating stored energy levels by visual colour variation. An inkjet-printed stretchable transmissive electrochromic energy storage with WO
3 nanoparticles on a transparent electrode was fabricated [79]. The silver nanowires were spray coated on a glass slide and SiO
2 nanoparticles were spin coated on the AgNW/glass slide surface. PDMS (polydimethylsiloxane) was poured over the transparent conductive electrode, cured and the composite film was removed from the glass slide. PEDOT:PSS buffer layer was spin coated on to the ST AgNWs/PDMS followed by inkjet printing of the WO
3 nanoparticle with a few nanometer thickness (
Figure 7). The electrode demonstrated large optical modulation of 40%, fast switching speed (<4.5 s), high coloration efficiency (75.5 cm
2 C
−1), good stability and high specific capacity (32.3 mAh g
−1 and 44.8 mAh cm
−3). The electrode demonstrated good functionality when stretched up to 50% and electrochromic performance is maintained even on stretching further to 80% strain. Devices assembled with WO
3/PEDOT:PSS/AgNWs/PDMS negative hybrid electrode, PANI/MWCNT composite positive electrode, H
2SO
4/PVA gel electrolyte and 3M VHB tape as a separator showed good performance making it well suited for wearable devices.
Small et al. reported the synthesis and inkjet processing of polyaniline /MWCN water dispersable composite inks. These films allowed for the switching between yellow, green, and blue when printed onto photopaper, PET, Pt-ITO, and Au-PVDF substrates and exhibited excellent optical transparency, sheet resistance, and electrochromic activity. A change in sheet resistance (1000–5000 ohm sq
-1) and optical transmittance (30–70%) was reported with a change in the nanotube percentage [80]. PANI-silica and PEDOT-silica composites formed by oxidative polymerization of the monomers, aniline and the ethylenedioxythiophene (EDOT) on 20nm sized silica sol particles were converted by solvent exchange to intrinsically conductive polymer inks which were inkjet printed on indium tin oxide-coated poly(ethylene terephthalate) films. PANI–silica or PEDOT–silica/ITO-PET as well as a blend of the two composites were studied as active layers in electrochromic devices fabricated using polyethylene glycol methacrylate PEGMA-based polymeric electrolyte. Colour of the devices changed with change in potential. The colour could also be tuned by inkjet-printed PANI-silica and PEDOT-silica blended particles as an electro- chromic layer [81]. The various electronic/optoelectronics devices fabricated via inkjet printing of conductive polymers are summarized in
Table 3.
2.4. Patterning with Conductive Polymers
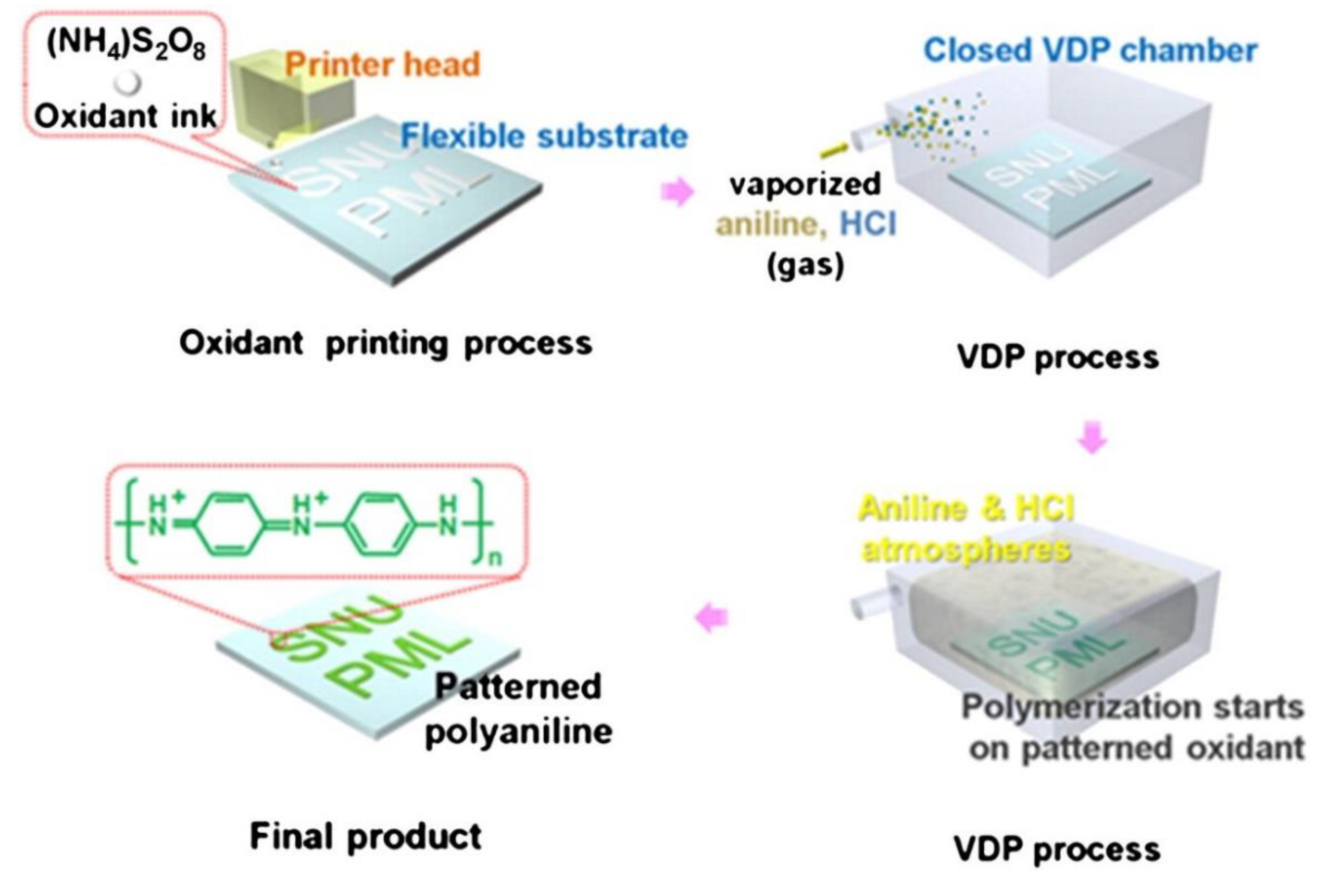
Conductive polymers have been patterned using various techniques like template-assisted synthesis, photolithography, soft-lithography, electrochemical deposition and dip-pen nanolithography [82–85]. Conductive polymers have also been ink jet printed directly onto substrates, but the method has certain limitations. The conductive polymers like polypyrrole, polyaniline (PANI), polythiophene and their derivatives are insoluble in common polar solvents and inks are usually prepared using stabilizers and surfactants to achieve the desired viscosity and surface tension. vapor deposition polymerization (VDP)-mediated inkjet printing (VDP-IJP) can overcome some of the processability issues. A facile method to pattern conductive polymers onto flexible substrates using vapor deposition polymerization (VDP)-mediated inkjet printing (VDP-IJP) was reported by Jang and coworkers wherein uniform well-defined patterns of PANI were formed via chemical oxidation polymerization of vaporized aniline on inkjet-printed oxidant patterns in the VDP chamber (
Figure 8). This process resulted in the formation of densely connected polyaniline nanofibers without use of surfactants and stabilizers resulting in patterned PANI being electrically active with low sheet resistance in the range of 103 Ω/□ on the polymeric substrate [86].
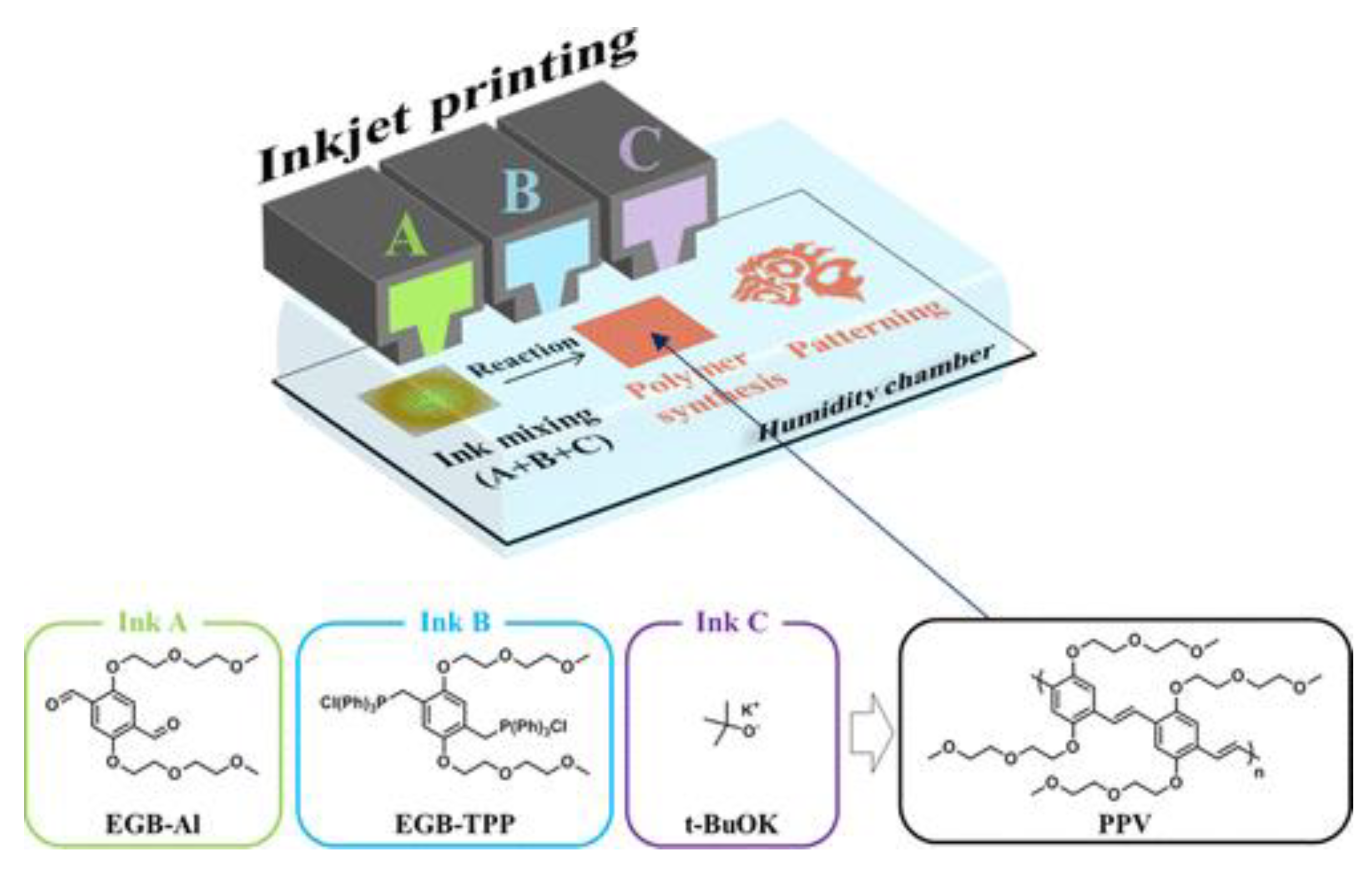
Reactive inkjet printing can be used to pattern functional organic materials on a solid substrate using small amount of reagent. A new strategy for formation of poly(phenylenevinylene) (PPV) patterns on paper by using the Reactive Inkjet Printing (RIJ) method has been reported by Jeon and coworkers (
Figure 9). Synthesized hydrophilic terephthaldehyde (ink A), bis(triphenylphosphonium salt) (ink B) and potassium t-butoxide (ink C) were printed using an inkjet printer under high humidity conditions in order (A, B and C) at the same location (overprinting), an in-situ Wittig reaction ensued resulting in the formation PPV patterns [87]. The unreacted reagents and byproducts could be efficiently removed by washing with water and chloroform as the prepared PPV was found to be insoluble in these solvents.
Inkjet printing of conductive polymer patterns on the surface of electrospun scaffolds using the cell stimulation has been explored. As a part of the study gelatin (Gel) modified with calcium phosphate nanoparticles (SG5) and polycaprolactone (PCL) modified with Osteogenon (Osteo) drug were utilized for the preparation of a bilayer scaffold by accumulating electrospun PCL/Osteo and Gel/SG5 fibers in a common collector [88]. Thin PANI patterns were subsequently deposited on the surface of the hybrid scaffold by inkjet printing. A bioactive hybrid scaffold system that can provide an electrically conductive environment for cells by bringing together conductive PANI, bioactive particles, and drugs in an electrospun scaffold was prepared. Combination of nanotemplating and inkjet printing have been used to prepare nano patterened thin polymer films. Nanoperforated TiO
2 (NP-TiO
2) and functionalized with hydrophobic perfluorinated phosphate, Zonyl FSE (ZFNP-TiO
2) fabricated on top of silicon wafers are used as substrates. Biotinylated polythiophene derivative (PBTL) ink, a biosensor material and dinonylnaphthalene sulfonic acid-doped polyaniline (PANI-DNNSA) ink, a pH and gas sensing material were inkjet printed on the onto NP-TiO
2 and ZFNP-TiO
2 substrates [89]. The non- functionalized polymer had higher surface energy and allows for deposition of uniform thin films. They have use in biosensing and electrical devices. Aniline dimer DANI was used to produce PANI by oxidative polymerization in aqueous medium using polystyrene sulphonate (PSS) as an emulsioning/doping agent. Controlling the amount of the oxidant resulted in mixed leucoemeraldine/emeraldine oxidation states. Solubilized in DMSO PANI/PSS was suitable as an ink for inkjet printing on flexible substrates and gave interesting negative capacitance effects [89]. Few other inkjet printed conductive polymer for nano pattering are summarized in
Table 4.
3. Conclusions and Future Perspectives
In summary, we reviewed various conducting polymer-based ink, their device fabrication using these conductive polymers via inkjet printing for variety of applications such as supercapacitors, sensors, and electrochromic devices. The ink formulation for inkjet printing and the keys improvement in devices are summarized in tables. The inkjet printing of conductive polymers for nano-pattering directly on substrates also briefly discussed in this review.
However, in past years various inkjet printable conductive polymers as PANI, PVV, Polyaniline. PEDOT etc and their composite were explored for various applications with a significant improvement of device performance [91]. But still there are several limitations which need to be address in the future. In case for supercapacitor the improvement in mechanical robustness, structural complexity, convenient fabrication, and device configuration is still critical task. For example, although conductive polymers have almost 10-fold stretchability capacity but final devices does not have this ability due to presence of other device components which are not stretchable [92]. Moreover, properties of conductive polymers need to be furthered tuned using optimized device configuration for better devices performance. Similarly, despite of excellent potential of inkjet printable conductive polymer, the conductive polymer-based sensors have some issues such as cross selectivity, short sensor lifetime, speed, instability which is a major drawback for conductive polymer based sensor technology [93,94]. The durability and sensitivity of sensor are highly depending upon conducting polymer-nanocomposites and interaction between organic and inorganic materials. The improving conducting polymer-nanocomposites via modifying surface and functionalization of inorganic particles and substrates, use of inorganic particles with high aspect ratio etc. will be possible approaches which could be adopted for improvement of conductive polymer-based sensor. The stability and conductivity of conductive polymer are major factor which decide the performance of electrochromic devices [95]. Although, conductive polymers based electrochromic devices are promising candidate for commercial scale use. But different challenges need to address such as well-desire, non-disperse and uniform morphology and size as compared to metal oxide electrochromic devices.
We believed this review provides overview about recent development inkjet printable conductive polymers for various applications and new direction to next generation conductive polymer-based devices for various applications. Consequently, inkjet printing ability of conductive polymers provided endless number of prospects to design highly efficient devices with higher reproducibility at low-cost which can be easily scale up at commercial level to make technologies commercially feasible.
Conflicts of Interest
The authors declare no competing financial interest.
References
- Oliveira, J.; Correia, V.; Castro, H.; Martins, P.; Lanceros-Mendez, S. Polymer-based smart materials by printing technologies: Improving application and integration. Addit. Manuf. 2018, 21, 269–283. [CrossRef]
- Wu, S.; Zeng, T.; Liu, Z.; Ma, G.; Xiong, Z.; Zuo, L.; Zhou, Z. 3D Printing Technology for Smart Clothing: A Topic Review. Materials 2022, 15, 7391. [CrossRef]
- Mendes-Felipe, C.; Oliveira, J.; Etxebarria, I.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. State-of-the-Art and Future Challenges of UV Curable Polymer-Based Smart Materials for Printing Technologies. Adv. Mater. Technol. 2019, 4. [CrossRef]
- Borghetti, M.; Cantù, E.; Sardini, E.; Serpelloni, M. Future Sensors for Smart Objects by Printing Technologies in Industry 4.0 Scenario. Energies 2020, 13, 5916. [CrossRef]
- Kim, A.; Oh, S.H.; Adhikari, A.; Sathe, B.R.; Kumar, S.; Patel, R. Recent advances in modified commercial separators for lithium–sulfur batteries. J. Mater. Chem. A 2023, 11, 7833–7866. [CrossRef]
- Meenu, M.; Padhan, B.; Patel, M.; Patel, R.; Xu, B. Antibacterial activity of essential oils from different parts of plants against Salmonella and Listeria spp.. Food Chem. 2023, 404, 134723. [CrossRef]
- Patel, M.; Patel, R.; Park, C.; Cho, K.; Kumar, P.; Park, C.; Koh, W.-G. Water-stable, biocompatible, and highly luminescent perovskite nanocrystals-embedded fiber-based paper for anti-counterfeiting applications. Nano Converg. 2023, 10, 1–11. [CrossRef]
- Kim, A.; Wert, N.A.; Gowd, E.B.; Patel, R. Recent Progress in PEG-Based Composite Phase Change Materials. Polym. Rev. 2023, 1–52. [CrossRef]
- Patel, M.; Meenu, M.; Pandey, J.K.; Kumar, P.; Patel, R. Recent development in upconversion nanoparticles and their application in optogenetics: A review. J. Rare Earths 2022, 40, 847–861. [CrossRef]
- Kim, A.; Dash, J.K.; Kumar, P.; Patel, R. Carbon-Based Quantum Dots for Photovoltaic Devices: A Review. ACS Appl. Electron. Mater. 2021, 4, 27–58. [CrossRef]
- Gupta, B.K.; Kumar, P.; Kedawat, G.; Kanika, K.; Vithayathil, S.A.; Gangwar, A.K.; Singh, S.; Kashyap, P.K.; Lahon, R.; Singh, V.N.; et al. Tunable luminescence from two dimensional BCNO nanophosphor for high-contrast cellular imaging. RSC Adv. 2017, 7, 41486–41494. [CrossRef]
- Gupta, B.K.; Kedawat, G.; Kumar, P.; Singh, S.; Suryawanshi, S.R.; (Garg), N.A.; Gupta, G.; Kim, A.R.; Gupta, R.K.; More, M.A.; et al. Field emission properties of highly ordered low-aspect ratio carbon nanocup arrays. RSC Adv. 2016, 6, 9932–9939. [CrossRef]
- Hrytsenko, O.; Hrytsenko, D.; Shvalagin, V.; Grodziuk, G.; Kompanets, M. The Use of Carbon Nanoparticles for Inkjet-Printed Functional Labels for Smart Packaging. J. Nanomater. 2018, 2018, 1–10. [CrossRef]
- Cirelli, M.; Hao, J.; Bor, T.C.; Duvigneau, J.; Benson, N.; Akkerman, R.; Hempenius, M.A.; Vancso, G.J. Printing “Smart” Inks of Redox-Responsive Organometallic Polymers on Microelectrode Arrays for Molecular Sensing. ACS Appl. Mater. Interfaces 2019, 11, 37060–37068. [CrossRef]
- Seipel, S.; Yu, J.; Periyasamy, A.P.; Viková, M.; Vik, M.; Nierstrasz, V.A. Inkjet printing and UV-LED curing of photochromic dyes for functional and smart textile applications. RSC Adv. 2018, 8, 28395–28404. [CrossRef]
- Yu, J.; Seipel, S.; Nierstrasz, V.A. Digital inkjet functionalization of water-repellent textile for smart textile application. J. Mater. Sci. 2018, 53, 13216–13229. [CrossRef]
- Bocchini, S.; Chiolerio, A.; Porro, S.; Accardo, D.; Garino, N.; Bejtka, K.; Perrone, D.; Pirri, C.F. Synthesis of polyaniline-based inks, doping thereof and test device printing towards electronic applications. J. Mater. Chem. C 2013, 1, 5101–5109. [CrossRef]
- Nair, N.M.; Pakkathillam, J.K.; Kumar, K.; Arunachalam, K.; Ray, D.; Swaminathan, P. Printable Silver Nanowire and PEDOT:PSS Nanocomposite Ink for Flexible Transparent Conducting Applications. ACS Appl. Electron. Mater. 2020, 2, 1000–1010. [CrossRef]
- Singh, A.; Katiyar, M.; Garg, A. Understanding the formation of PEDOT:PSS films by ink-jet printing for organic solar cell applications. RSC Adv. 2015, 5, 78677–78685. [CrossRef]
- Yang, C.-Y.; Stoeckel, M.-A.; Ruoko, T.-P.; Wu, H.-Y.; Liu, X.; Kolhe, N.B.; Wu, Z.; Puttisong, Y.; Musumeci, C.; Massetti, M.; et al. A high-conductivity n-type polymeric ink for printed electronics. Nat. Commun. 2021, 12, 1–8. [CrossRef]
- Liu, X.; Shen, Y.; Yang, R.; Zou, S.; Ji, X.; Shi, L.; Zhang, Y.; Liu, D.; Xiao, L.; Zheng, X.; et al. Inkjet Printing Assisted Synthesis of Multicomponent Mesoporous Metal Oxides for Ultrafast Catalyst Exploration. Nano Lett. 2012, 12, 5733–5739. [CrossRef]
- Negro, A.; Cherbuin, T.; Lutolf, M.P. 3D Inkjet Printing of Complex, Cell-Laden Hydrogel Structures. Sci. Rep. 2018, 8, 1–9. [CrossRef]
- Willert, A.; Tabary, F.Z.; Zubkova, T.; Santangelo, P.E.; Romagnoli, M.; Baumann, R.R. Multilayer additive manufacturing of catalyst-coated membranes for polymer electrolyte membrane fuel cells by inkjet printing. Int. J. Hydrogen Energy 2022, 47, 20973–20986. [CrossRef]
- Alamán, J.; Alicante, R.; Peña, J.I.; Sánchez-Somolinos, C. Inkjet Printing of Functional Materials for Optical and Photonic Applications. Materials 2016, 9, 910. [CrossRef]
- Hussain, A.; Abbas, N.; Ali, A. Inkjet Printing: A Viable Technology for Biosensor Fabrication. Chemosensors 2022, 10, 103. [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene, (CH) x. J. Chem. Soc. Chem. Commun. 1977, 578–580. [CrossRef]
- Twenty-Five Years of Conducting Polymers. Chem. Commun., 2003, No. 1, 1–4.
- Conducting Polymers Forward. Nat. Mater., 2020, 19 (9), 921.
- Woloshun, R.R.; Yu, Y.; Xu, X.; Lee, J.K.; Zhu, S.; Shine, J.S.; Ebea, P.; Stevens, B.R.; Vidyasagar, S.; Collins, J.F. Four AAs increase DMT1 abundance in duodenal brush-border membrane vesicles and enhance iron absorption in iron-deprived mice. Blood Adv. 2022, 6, 3011–3021. [CrossRef]
- El-bery, H. M.; Salah, M. R.; Ahmed, S. M.; Soliman, S. A. E Ffi Cient Non-Metal Based Conducting Polymers for Photocatalytic Hydrogen Production: Comparative Study between Polyaniline, Polypyrrole And. 2021, 13229–13244.
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Shoaie, I.S.; Khalilzadeh, M.A.; Asl, M.S.; Van Le, Q.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent developments in conducting polymers: applications for electrochemistry. RSC Adv. 2020, 10, 37834–37856. [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: a comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [CrossRef]
- Mattam, L.B.; Bijoy, A.; Thadathil, D.A.; George, L.; Varghese, A. Conducting Polymers: A Versatile Material for Biomedical Applications. ChemistrySelect 2022, 7. [CrossRef]
- Atkare, S.; Hambir, S.; Jagtap, S.; Adhikari, A.; Singh, S.K.; Patel, R. Role of polyaniline/molybdenum trioxide nanocomposites in tuning the characteristics of humidity sensors. Polym. Adv. Technol. 2023, 34, 2585–2596. [CrossRef]
- Kumaravel, S.; Kim, E.; Kale, B. B.; Adhikari, A.; Patel, R.; Kundu, S. Recent Developments in Conductive Polymer-Based Electro-/Photoelectrocatalytic Materials for Effective Hydrogen/Oxygen Evolution Reactions: A Review. ChemElectroChem, 2022, 9 (19).
- Park, S.; Patel, R. Recent Progress in Conductive Polymer-based Membranes. Membr. J. 2021, 31, 101–119. [CrossRef]
- Sahu, D.; Wu, T.-J.; Wang, S.-C.; Huang, J.-L. Electrochromic behavior of NiO film prepared by e-beam evaporation. J. Sci. Adv. Mater. Devices 2017, 2, 225–232. [CrossRef]
- Li, J.; Huckleby, A.B.; Zhang, M. Polymer-based thermoelectric materials: A review of power factor improving strategies. J. Materiomics 2021, 8, 204–220. [CrossRef]
- Guo, X.; Xue, Z.; Zhang, Y. Manufacturing of 3D multifunctional microelectronic devices: challenges and opportunities. NPG Asia Mater. 2019, 11, 29. [CrossRef]
- Satoh, Y.; Ding, H.; Yang, H.; Deng, Y.; Hsueh, A.-J.; Shimizu, T.; Qiao, M.; Ma, C.; Kariya, K.; Kurihara, T.; et al. Wired Microfabricated Electrochemical Systems. Anal. Chem. 2021, 93, 12655–12663. [CrossRef]
- He, B.; Tan, L.; Regnier, F. Microfabricated Filters for Microfluidic Analytical Systems. Anal. Chem. 1999, 71, 1464–1468. [CrossRef]
- Grenci, G.; Bertocchi, C.; Ravasio, A. Integrating Microfabrication into Biological Investigations: the Benefits of Interdisciplinarity. Micromachines 2019, 10, 252. [CrossRef]
- Wang, J.; Chatrathi, M.P.; Tian, B.; Polsky, R. Microfabricated Electrophoresis Chips for Simultaneous Bioassays of Glucose, Uric Acid, Ascorbic Acid, and Acetaminophen. Anal. Chem. 2000, 72, 2514–2518. [CrossRef]
- Tiwari, S.K.; Bhat, S.; Mahato, K.K. Design and Fabrication of Low-cost Microfluidic Channel for Biomedical Application. Sci. Rep. 2020, 10, 9215. [CrossRef]
- Su, W.; Cook, B.S.; Fang, Y.; Tentzeris, M.M. Fully inkjet-printed microfluidics: a solution to low-cost rapid three-dimensional microfluidics fabrication with numerous electrical and sensing applications. Sci. Rep. 2016, 6, 35111. [CrossRef]
- Kumaravel, S.; Kim, E.; Kale, B.B.; Adhikari, A.; Patel, R.; Kundu, S. Recent Developments in Conductive Polymer-Based Electro-/Photoelectrocatalytic Materials for Effective Hydrogen/Oxygen Evolution Reactions: A Review. ChemElectroChem, 2022, 9(19) e202200724.
- Park, J.T.; Patel, R.; Jeon, H.; Kim, D.J.; Shin, J.-S.; Kim, J.H. Facile fabrication of vertically aligned TiO2 nanorods with high density and rutile/anatase phases on transparent conducting glasses: high efficiency dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 6131–6138. [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Development of molecularly imprinted polymer based phase boundaries for sensors design (review). Adv. Colloid Interface Sci. 2022, 305, 102693. [CrossRef]
- Gerard, M.; Chaubey, A.; Malhotra, B. Application of conducting polymers to biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [CrossRef]
- Cho, S.; and Lee, S. B. Fast Electrochemistry of Conductive Polymer Nanotubes: Synthesis, Mechanism, and Application, Acc. Chem. Res. 2008, 41, 6, 699–707.
- Ahmad, K.; Raza, W. Current State and Prospective of Supercapacitors BT - Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O. V., Torres-Martínez, L. M., Kharisov, B. I., Eds.; Springer International Publishing: Cham, 2021; pp 1835–1853.
- Chi, K.; Zhang, Z.; Xi, J.; Huang, Y.; Xiao, F.; Wang, S.; Liu, Y. Freestanding Graphene Paper Supported Three-Dimensional Porous Graphene–Polyaniline Nanocomposite Synthesized by Inkjet Printing and in Flexible All-Solid-State Supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 16312–16319. [CrossRef]
- Chiolerio, A.; Bocchini, S.; Porro, S. Inkjet Printed Negative Supercapacitors: Synthesis of Polyaniline-Based Inks, Doping Agent Effect, and Advanced Electronic Devices Applications. Adv. Funct. Mater. 2014, 24, 3375–3383. [CrossRef]
- Diao, J.; Yuan, J.; Ding, A.; Zheng, J.; Lu, Z. Flexible Supercapacitor Based on Inkjet-Printed Graphene@Polyaniline Nanocomposites with Ultrahigh Capacitance. Macromol. Mater. Eng. 2018, 303, 1–7. [CrossRef]
- Xu, Y.; Hennig, I.; Freyberg, D.; Strudwick, A.J.; Schwab, M.G.; Weitz, T.; Cha, K.C.-P. Inkjet-printed energy storage device using graphene/polyaniline inks. J. Power Sources 2014, 248, 483–488. [CrossRef]
- Zhang, J.; Wang, J.; Yang, J.; Wang, Y.; Chan-Park, M.B. Three-Dimensional Macroporous Graphene Foam Filled with Mesoporous Polyaniline Network for High Areal Capacitance. ACS Sustain. Chem. Eng. 2014, 2, 2291–2296. [CrossRef]
- Anjali, M.; Bharath, G.; Rashmi, H.; Avinash, J.; Naresh, K.; Raju, P.; Raghu, H. Polyaniline-Pectin nanoparticles immobilized paper based colorimetric sensor for detection of Escherichia coli in milk and milk products. Curr. Res. Food Sci. 2022, 5, 823–834. [CrossRef]
- Bilbao, E.; Kapadia, S.; Riechert, V.; Amalvy, J.; Molinari, F. N.; Escobar, M. M.; Baumann, R. R.; Monsalve, L. N. Functional Aqueous-Based Polyaniline Inkjet Inks for Fully Printed High-Performance PH-Sensitive Electrodes. Sensors Actuators B Chem., 2021, 346 (June).
- Crowley, K.; Morrin, A.; Hernandez, A.; Omalley, E.; Whitten, P.G.; Wallace, G.G.; Smyth, M.R.; Killard, A.J. Fabrication of an ammonia gas sensor using inkjet-printed polyaniline nanoparticles. Talanta 2008, 77, 710–717. [CrossRef]
- Le, D.D.; Nguyen, T.N.N.; Doan, D.C.T.; Dang, T.M.D.; Dang, M.C. Fabrication of interdigitated electrodes by inkjet printing technology for apllication in ammonia sensing. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 25002. [CrossRef]
- Crowley, K.; Morrin, A.; Shepherd, R.L.; Panhuis, M.I.H.; Wallace, G.G.; Smyth, M.R.; Killard, A.J. Fabrication of Polyaniline-Based Gas Sensors Using Piezoelectric Inkjet and Screen Printing for the Detection of Hydrogen Sulfide. IEEE Sensors J. 2010, 10, 1419–1426. [CrossRef]
- Sarfraz, J.; Tobjork, D.; Osterbacka, R.; Linden, M. Low-Cost Hydrogen Sulfide Gas Sensor on Paper Substrates: Fabrication and Demonstration. IEEE Sensors J. 2011, 12, 1973–1978. [CrossRef]
- Kit-Anan, W.; Olarnwanich, A.; Sriprachuabwong, C.; Karuwan, C.; Tuantranont, A.; Wisitsoraat, A.; Srituravanich, W.; Pimpin, A. Disposable paper-based electrochemical sensor utilizing inkjet-printed Polyaniline modified screen-printed carbon electrode for Ascorbic acid detection. J. Electroanal. Chem. 2012, 685, 72–78. [CrossRef]
- Kulkarni, M.V.; Apte, S.K.; Naik, S.D.; Ambekar, J.D.; Kale, B.B. Ink-jet printed conducting polyaniline based flexible humidity sensor. Sensors Actuators B: Chem. 2013, 178, 140–143. [CrossRef]
- Li, L.; Pan, L.; Ma, Z.; Yan, K.; Cheng, W.; Shi, Y.; Yu, G. All Inkjet-Printed Amperometric Multiplexed Biosensors Based on Nanostructured Conductive Hydrogel Electrodes. Nano Lett. 2018, 18, 3322–3327. [CrossRef]
- Suman; O’Reilly, E.; Kelly, M.; Morrin, A.; Smyth, M. R.; Killard, A. J. Chronocoulometric Determination of Urea in Human Serum Using an Inkjet Printed Biosensor. Anal. Chim. Acta, 2011, 697 (1–2), 98–102.
- Määttänen, A.; Vanamo, U.; Ihalainen, P.; Pulkkinen, P.; Tenhu, H.; Bobacka, J.; Peltonen, J. A low-cost paper-based inkjet-printed platform for electrochemical analyses. Sens. Actuators B Chem. 2013, 177, 153–162. [CrossRef]
- Morrin, A.; Wilbeer, F.; Ngamna, O.; Moulton, S.E.; Killard, A.J.; Wallace, G.G.; Smyth, M.R. Novel biosensor fabrication methodology based on processable conducting polyaniline nanoparticles. Electrochem. Commun. 2005, 7, 317–322. [CrossRef]
- Oh, W.-K.; Kim, S.; Shin, K.-H.; Jang, Y.; Choi, M.; Jang, J. Inkjet-printed polyaniline patterns for exocytosed molecule detection from live cells. Talanta 2013, 105, 333–339. [CrossRef]
- Song, E.; Tortorich, R.P.; Da Costa, T.H.; Choi, J.W. Inkjet printing of conductive polymer nanowire network on flexible substrates and its application in chemical sensing. Microelectron. Eng. 2015, 145, 143–148. [CrossRef]
- Song, E.; Da Costa, T.H.; Choi, J.W. A chemiresistive glucose sensor fabricated by inkjet printing. Microsyst. Technol. 2017, 23, 3505–3511. [CrossRef]
- Teengam, P.; Siangproh, W.; Tuantranont, A.; Henry, C.S.; Vilaivan, T.; Chailapakul, O. Electrochemical paper-based peptide nucleic acid biosensor for detecting human papillomavirus. Anal. Chim. Acta 2017, 952, 32–40. [CrossRef]
- Wang, L.; Ma, Y.; Chen, M.; Yao, H.; Zheng, X.; Yang, W. An inkjet printing soft photomask and its application on organic polymer substrates. Sci. China Chem. 2010, 53, 1695–1704. [CrossRef]
- Zea, M.; Texidó, R.; Villa, R.; Borrós, S.; Gabriel, G. Specially Designed Polyaniline/Polypyrrole Ink for a Fully Printed Highly Sensitive pH Microsensor. ACS Appl. Mater. Interfaces 2021, 13, 33524–33535. [CrossRef]
- Zea, M.; Texidó, R.; Villa, R.; Borrós, S.; Gabriel, G. Specially Designed Polyaniline/Polypyrrole Ink for a Fully Printed Highly Sensitive pH Microsensor. ACS Appl. Mater. Interfaces 2021, 13, 33524–33535. [CrossRef]
- Zhang, S.; Cao, S.; Zhang, T.; Fisher, A.; Lee, J. Y. Al 3+ Intercalation/de-Intercalation-Enabled Dual-Band Electrochromic Smart Windows with a High Optical Modulation, Quick Response and Long Cycle Life. Energy Environ. Sci., 2018, 11 (10), 2884–2892.
- Lee, H.J.; Lee, C.; Song, J.; Yun, Y.J.; Jun, Y.; Ah, C.S. Electrochromic devices based on ultraviolet-cured poly(methyl methacrylate) gel electrolytes and their utilisation in smart window applications. J. Mater. Chem. C 2020, 8, 8747–8754. [CrossRef]
- Cong, S.; Tian, Y.; Li, Q.; Zhao, Z.; Geng, F. Single-Crystalline Tungsten Oxide Quantum Dots for Fast Pseudocapacitor and Electrochromic Applications. Adv. Mater. 2014, 26, 4260–4267. [CrossRef]
- Cai, G.; Park, S.; Cheng, X.; Lee-Sie, A.; Pooi, E. &; Lee, S.; Lee-Sie Eh, A.; Lee, P. S. Inkjet-Printed Metal Oxide Nanoparticles on Elastomer for Strain-Adaptive Transmissive Electrochromic Energy Storage Systems. Sci. Technol. Adv. Mater., 2018, 19 (1), 759–770.
- Small, W.R.; Masdarolomoor, F.; Wallace, G.G.; Panhuis, M.I.H. Inkjet deposition and characterization of transparent conducting electroactive polyaniline composite films with a high carbon nanotube loading fraction. J. Mater. Chem. 2007, 17, 4359–4361. [CrossRef]
- Shim, G. H.; Han, M. G.; Sharp-Norton, J. C.; Creager, S. E.; Foulger, S. H. Inkjet-Printed Electrochromic Devices Utilizing Polyaniline-Silica and Poly(3,4-Ethylenedioxythiophene)-Silica Colloidal Composite Particles. J. Mater. Chem., 2008, 18 (5), 594–601.
- Huang, C.; Dong, B.; Lu, N.; Yang, B.; Gao, L.; Tian, L.; Qi, D.; Wu, Q.; Chi, L. A Strategy for Patterning Conducting Polymers Using Nanoimprint Lithography and Isotropic Plasma Etching. Small 2009, 5, 583–586. [CrossRef]
- Ouyang, S.; Xie, Y.; Wang, D.; Zhu, D.; Xu, X.; Tan, T.; Fong, H.H. Surface Patterning of PEDOT:PSS by Photolithography for Organic Electronic Devices. J. Nanomater. 2015, 2015, 603148. [CrossRef]
- Acikgoz, C.; Hempenius, M.A.; Huskens, J.; Vancso, G.J. Polymers in conventional and alternative lithography for the fabrication of nanostructures. Eur. Polym. J. 2011, 47, 2033–2052. [CrossRef]
- Abargues, R.; Rodríguez-Cantó, P.J.; García-Calzada, R.; Martínez-Pastor, J. Patterning of Conducting Polymers Using UV Lithography: The in-Situ Polymerization Approach. J. Phys. Chem. C 2012, 116, 17547–17553. [CrossRef]
- Cho, J.; Shin, K.-H.; Jang, J. Polyaniline micropattern onto flexible substrate by vapor deposition polymerization-mediated inkjet printing. Thin Solid Films 2010, 518, 5066–5070, . [CrossRef]
- Jeon, S.; Park, S.; Nam, J.; Kang, Y.; Kim, J.-M. Creating Patterned Conjugated Polymer Images Using Water-Compatible Reactive Inkjet Printing. ACS Appl. Mater. Interfaces 2016, 8, 1813–1818, . [CrossRef]
- Rajzer, I.; Rom, M.; Menaszek, E.; Pasierb, P. Conductive PANI patterns on electrospun PCL/gelatin scaffolds modified with bioactive particles for bone tissue engineering. Mater. Lett. 2015, 138, 60–63, . [CrossRef]
- Xu, Q.; Ihalainen, P.; Smått, J. H.; Määttänen, A.; Sund, P.; Wilén, C. E.; Peltonen, J. Template-Induced Fabrication of Nanopatterned Polymeric Films by Inkjet Printing. Appl. Surf. Sci., 2014, 313, 237–242.
- Bocchini, S.; Chiolerio, A.; Porro, S.; Accardo, D.; Garino, N.; Bejtka, K.; Perrone, D.; Pirri, C.F. Synthesis of polyaniline-based inks, doping thereof and test device printing towards electronic applications. J. Mater. Chem. C 2013, 1, 5101–5109, . [CrossRef]
- Li, Y.; Zhou, X.; Sarkar, B.; Gagnon-Lafrenais, N.; Cicoira, F. Recent Progress on Self-Healable Conducting Polymers. Adv. Mater. 2022, 34, 2108932, . [CrossRef]
- Sardana, S.; Gupta, A.; Singh, K.; Maan, A.; Ohlan, A. Conducting polymer hydrogel based electrode materials for supercapacitor applications. J. Energy Storage 2022, 45, 103510, . [CrossRef]
- Verma, A.; Gupta, R.; Verma, A. S.; Kumar, T. A review of composite conducting polymer-based sensors for detection of industrial waste gases. Sensors and Actuators Reports, 2023, 5,100143.
- Liu, X.; Zheng, W.; Kumar, R.; Kumar, M.; Zhang, J. Conducting polymer-based nanostructures for gas sensors. Co-ord. Chem. Rev. 2022, 462, 214517, . [CrossRef]
- Nguyen, T. V.; Le, Q.; V.; Peng, S.; Dai, Z,; Ahn, S. H.; Kim, S. Y. Exploring Conducting Polymers as a Promising Alternative for Electrochromic Devices. Adv. Mater. Technol., 2023, 2300474.
Figure 1.
Fabrication process for GH-PANI based supercapacitor: (Step I) Self-assembly of GO nanosheets into 3D GH. (Step II) Insitu polymerization of PANI on GH. (Step III) Ball milling and ultrasonic treatment of 3D GH−PANI composite to form GH−PANI ink. (Step IV) Printing GO ink on paper substrate to form GOP; (Step V) Overprinting the GH−PANI inks on GOP; (Step VI) Soaking GH−PANI/GOP in the HI solution. (Step VII) Simultaneous reducing GH−PANI/GOP by HI and peeling it off from the commercial paper substrate to form freestanding GH−PANI/GP. (Step VIII) Fabrication of flexible SC device based on GH−PANI/GP electrode and gel electrolyte. Reproduced with permission from [52].
Figure 1.
Fabrication process for GH-PANI based supercapacitor: (Step I) Self-assembly of GO nanosheets into 3D GH. (Step II) Insitu polymerization of PANI on GH. (Step III) Ball milling and ultrasonic treatment of 3D GH−PANI composite to form GH−PANI ink. (Step IV) Printing GO ink on paper substrate to form GOP; (Step V) Overprinting the GH−PANI inks on GOP; (Step VI) Soaking GH−PANI/GOP in the HI solution. (Step VII) Simultaneous reducing GH−PANI/GOP by HI and peeling it off from the commercial paper substrate to form freestanding GH−PANI/GP. (Step VIII) Fabrication of flexible SC device based on GH−PANI/GP electrode and gel electrolyte. Reproduced with permission from [52].
Figure 2.
The fabrication of (a) sandwich-structured and (b) interdigitated SCs on flexible gold films. Reproduced with permission from [54].
Figure 2.
The fabrication of (a) sandwich-structured and (b) interdigitated SCs on flexible gold films. Reproduced with permission from [54].
Figure 3.
(A) Supercapacitor device structure. (B) CV curves of NGP electrodes measured at different scan rates, in 1M H2SO4 electrolyte, two-electrode system. (C) CV curves of NGP/PANI electrodes measured at different scan rates, in 1M H2SO4 electrolyte, two-electrode system. (D) Specific capacitance as a function of scan rate in two electrode system, with 1M H2SO4 aqueous electrolyte. Reproduced with permission from [55].
Figure 3.
(A) Supercapacitor device structure. (B) CV curves of NGP electrodes measured at different scan rates, in 1M H2SO4 electrolyte, two-electrode system. (C) CV curves of NGP/PANI electrodes measured at different scan rates, in 1M H2SO4 electrolyte, two-electrode system. (D) Specific capacitance as a function of scan rate in two electrode system, with 1M H2SO4 aqueous electrolyte. Reproduced with permission from [55].
Figure 4.
Schematic illustration of the design and fabrication of the inkjet-printed multiplexed biosensor based on conductive hydrogels. (a) Precursor solutions A and B were printed on the predefined areas to form a PAni hydrogel on the working electrode (WE). (b) Chloroplatinic acid (P) and formic acid (F) solutions were printed to generate platinum nanoparticles (PtNPs) on the PAni hydrogel film. (c) Enzyme solutions G (glucose oxidase solution, GOx), L (lactic oxidase solution, LOx), and T (mixed solution of lipase/glycerol kinase/L-α-glycerophosphate oxidase, LP/GK/GPO) were then sequentially printed onto their corresponding electrodes one by one. (d) The multiplexed biosensor was assembled by integrating the top layer, channel layer, and electrode layer. (e) Schematic of the multiplexed detection of metabolites in human blood with the multiplex assay. Reproduced with permission from [65].
Figure 4.
Schematic illustration of the design and fabrication of the inkjet-printed multiplexed biosensor based on conductive hydrogels. (a) Precursor solutions A and B were printed on the predefined areas to form a PAni hydrogel on the working electrode (WE). (b) Chloroplatinic acid (P) and formic acid (F) solutions were printed to generate platinum nanoparticles (PtNPs) on the PAni hydrogel film. (c) Enzyme solutions G (glucose oxidase solution, GOx), L (lactic oxidase solution, LOx), and T (mixed solution of lipase/glycerol kinase/L-α-glycerophosphate oxidase, LP/GK/GPO) were then sequentially printed onto their corresponding electrodes one by one. (d) The multiplexed biosensor was assembled by integrating the top layer, channel layer, and electrode layer. (e) Schematic of the multiplexed detection of metabolites in human blood with the multiplex assay. Reproduced with permission from [65].
Figure 5.
Schematic illustration of strategy for selective cell patterning via modification of cell binding peptide (RGD peptide) on inkjet-printed PANI. Reproduced with permission from [69].
Figure 5.
Schematic illustration of strategy for selective cell patterning via modification of cell binding peptide (RGD peptide) on inkjet-printed PANI. Reproduced with permission from [69].
Figure 6.
(a) Digital design of the pH sensor. (b) Final printed platform with 9 IE and 1 pRE and microscopic image of the printed polymeric electrodes. (c) Inkjet printing steps: (i) printing of Ag RE, tracks, and pads, followed by a dry step; (ii) printing of the Au IE, followed by a dry and thermal sintering of both metallic Au and Ag layers; (iii) printing of the dielectric SU-8, drying, and UV cross-linking; and (iv) printing of CP inks, followed by a drying step. Reproduced with permission from [74].
Figure 6.
(a) Digital design of the pH sensor. (b) Final printed platform with 9 IE and 1 pRE and microscopic image of the printed polymeric electrodes. (c) Inkjet printing steps: (i) printing of Ag RE, tracks, and pads, followed by a dry step; (ii) printing of the Au IE, followed by a dry and thermal sintering of both metallic Au and Ag layers; (iii) printing of the dielectric SU-8, drying, and UV cross-linking; and (iv) printing of CP inks, followed by a drying step. Reproduced with permission from [74].
Figure 7.
Schematic illustration of fabrication procedure of the inkjet-printed stretchable WO3 transparent electrode. Reproduced with permission from [79].
Figure 7.
Schematic illustration of fabrication procedure of the inkjet-printed stretchable WO3 transparent electrode. Reproduced with permission from [79].
Figure 8.
The patterning conducting polymer using vapor deposition polymerization-mediated inkjet printing. Reproduced with permission from [86].
Figure 8.
The patterning conducting polymer using vapor deposition polymerization-mediated inkjet printing. Reproduced with permission from [86].
Figure 9.
Schematic representation for the reactive inkjet printing method and chemical structures of ink components and target conjugated polymer PPV [87].
Figure 9.
Schematic representation for the reactive inkjet printing method and chemical structures of ink components and target conjugated polymer PPV [87].
Table 1.
Conductive polymer supercapacitors inks for inkjet printing . .
Table 1.
Conductive polymer supercapacitors inks for inkjet printing . .
| Conductive Polymer |
Ink formulation and device fabrication |
Applications |
Highlights |
Reference |
| Polyaniline |
Reduction of GH-PANI/GOP by hydroiodic acid and simultaneously peeling off from the commercial paper substrate to give the freestanding electrode. (GH−PANI/GP electrode). |
Fabrication of graphene-based nanohybrid materials for use in many electronic systems. |
Maximum energy density- 24.02 Wh kg-1 at a power density of 400.33 W kg-1. and a power density of 3202.4 W kg-1 at energy density of 13.29 Wh kg-1 are achievable at an operating voltage of 0.8 V. |
[51] |
| Polyaniline |
Two distinct doped PANI inks were prepared by dissolving the emeraldine salt of PANI in dimethylsuphoxide followed by the addition of trifluorosulphonic acid and camporsulphonic acid. |
Materials with negative capacitance in low frequency range can be used in devices working at nominal grid conditions (50–60 Hz) and up to short wave radio frequencies. |
Negative capacitance has been reported. The highest negative supercapacitance achieved is -2.3 mF @ 30 Hz, corresponding to a specific mass capacity of -799 F g-1
|
[52] |
| Polyaniline |
Electrodes were fabricated by printing GO@PANI composites on gold-coated polymer substrates and further reduced. Sandwiched and interdigitated supercapacitors were developed. |
This method allows the end users to precisely deposit active materials according to their designs for miniature and wearable electronics. |
Devices fabricated have high volumetric capacities of 258.5 F cm-3 at 1 mV s-1 for sandwich structured and 554 F cm-3 at 1 mV s-1 for interdigitated ones. Even after 2000 cycles of charging and discharging over 90% capacitance retention could be achieved. |
[53] |
| Polyaniline |
Graphene polyaniline (NGP/PANI) inks of appropriate surface tension and viscosity are formulated and then inkjet printed to produce thin film supercapacitor electrodes. |
This preparation method allowed good control over pattern geometry and location in thin films. Manufacturing energy storage devices in printable electronics. |
In 1 M H2SO4 solution as the electrolyte a maximum specific capacitance of 82 Fg-1, power density of 124 kW kg-1 and energy density of 2.4 Wh kg-1 when a scan rate of 20 mV s-1 is applied is observed. ⇒A long life cycle of over 1000 cycles. |
[54] |
| Polyaniline |
Graphene foam made up of few layers of graphene electrodeposited with a thin layer of PANI with subsequent filling of the submillimeter size pores with PANI by using inkjet printing. |
Good option for high performance supercapacitors. |
The synergistic effect of graphene and PANI provides large areal capacitance of over 1700 mF cm-2. |
[39] |
Table 2.
Conductive polymer Sensors Inks for Inkjet printing.
Table 2.
Conductive polymer Sensors Inks for Inkjet printing.
| Conductive Polymer |
Ink formulation and device fabrication |
Applications |
Highlights |
Reference |
| Polyaniline |
PANI-PEC dispersion
in ethanol was printed
on a whatman filter
paper followed by
UV sterilization. |
Qualitative
and
quantitative detection
of E. coli in
each solution |
Incredible sensitivity of 0.52 ± 0.17 log CFU/mL.
Simple and cost effective |
[57] |
| Polyaniline |
Acrylic resin-based
inks were applied
onto SDS
stabilized
PANi lattices maintaining a PANI:resin weight ratio of 1:1 (dry basis). |
Compatible for
analysis
in wearable systems |
Improved viscosity as well as the wear resistance
Stable electrodes with reproducible
pH sensing ability. Great pH sensitivity (upto 69.1 mV/pH). |
[58] |
| Polyaniline |
Silver and carbon
IDA’s were.
screen-printed
while nano-
PANI suspension
was inkjet-printed. |
Detection of ammonia
in air |
Thermally stable sensor with high sensitivity to gaseous ammonia.
Unaffected by moisture and volatile organic compounds.
Can be used at elevated temperatures.
Very responsive in the analytically important (1-100 ppm) range. |
[59] |
| Polyaniline |
Inkjet-printed
silver electrodes
on Si/SiO2 were
further drop coated
with a blend film
of emaraldine salt form of PANI and ethylene glycol (EG). |
Detection of ammonia
|
Reliable output in the range of 0 to 100ppm of ammonia gas.
Fast recovery time of about 15 minutes |
[60] |
| Polyaniline |
Alternate PANI
and CuCl2
layers were
inkjet-printed
on silver
and carbon
interdigitated electrodes. |
Hydrogen-sulphide sensors for
short-term
analyses |
High sensitivity to hydrogen sulphide of upto 2.5 ppmv (parts per million by volume) |
[60] |
| Polyaniline |
Inkjet printed
silver electrodes
were drop coated
with dispersions
of PANI/CuCl2 t
o form films. |
Food
quality monitoring.
|
Appreciable sensitivity of upto 10 ppm due to protonation of PANI by H2S
Low cost H2S gas detector and can be used for food quality monitoring. he relatively low absolute resistance values allow for switching on of a LED using a low voltage battery in a simple sensor circuit |
[62] |
| Polyaniline |
Screen-Printed
Carbon
Electrode (SPCE)
was subjected to
inkjet-printing
with PANI to
form a
working electrode. |
Efficient sensor
for Ascorbic acid |
Good sensitivity of 17.7 lA/Mm for ascorbic acid
Low-cost, disposable and point-of-care sensor |
[63] |
| Polyaniline |
The PANI ink
was s
ynthesized
via
oxidative
polymerization . |
Low-cost RFID
tags,
polymer based
photovoltaic cells
and in printed
flexible electronics devices.
|
Efficient at room temperature |
[64] |
| Polyaniline |
The substrate
contains three
carbon
working electrodes
and a Ag/AgCl
shared counter
and reference
electrode.
PANI hydrogel
was synthesized
from phytic
acid,
ammonium persulphate and aniline. |
The biosensor is
efficient
and multipurpose
with capability
of detecting
glucose, lactates,
and triglycerides
with high
accuracy.
Integrated
multiplexed
biosensors
for monitoring
of parameters
in humans can
be mass produced. |
Easy multiple analyte detection by multiplexing multiple sensors on a chip.
The sensitivity with respect to triglycerides was found to be 7.49 μA/mM-1cm-2 between 0.1mM and 6mM, while that of lactate was 3.94 μAmM-1cm-2 between 0.08mM and 5mM.
The glucose sensitivity exhibited by the sensor was 5.03 μAmM-1cm-2 between 1mM and 25mM.
|
[65] |
| Polyaniline |
Screen-printing
of carbon electrodes
onto PET plates
followed by
inkjet-printing of
PANI NPs and
Urease enzyme
solution on the
working electrodes. |
Efficient urea
detector in
human serum
samples. |
Efficient to measure ammonia in the 0.1-100 mM and urea in the 2–12 mM (r2=0.98) range.
|
[66] |
| PEDOT, polyaniline |
Inkjet printed gold
NPs formed the
working and
counter electrodes.
and inkjet printed silver nanoparticle electrode functions as the reference electrode. |
Good pH and
glucose sensors.
|
Reusable after rinsing the aqueous samples
Sensitive to pH even after five weeks of storage
Paper-chip allows fast and on-site analysis.
Cost-effective and easy
Functions with low sample volume |
[67] |
| Polyaniline |
PANI was used
as NPS and
the enzymes were
drop-coated
parallely on
the electrodes. |
Enzyme biosensing |
Mass production is possible as no electrochemical processes are involved in fabrication. |
[68] |
| Polyaniline |
Inkjet-printing on
PET films by
deposition of
PANI patterns
and immobilization
of RGD peptide
over it by
covalent linkages |
Sensing on biomolecules in live cells. Neurotransmittor detection from live cells, tracking biomolecular release and detection of exocytosed biomolecules.
|
Good ability to translate and amplify exocytosis molecules into a detectable signal
|
[69] |
| Polyaniline |
Inkjet printing of multiwallled carbon nanotubes electrodes was followed by printing of randomly oriented PANI nanowires dispersed in aqueous medium |
pH, H2O2 sensor |
200 micrometre minimum printing resolution
Point of care diagnostics |
[70] |
| Polyanailine |
Sequential inkjet-printing of carbon nanotubes and polyaniline nanowires along with the glucose oxidase and platinum nanoparticle layers between the CNT layers. |
Excellent glucose sensor having the potential to be an on-demand printable point-of-care diagnostic kit for glucose measurement.
|
Quick and disposable
Linear relationship between current measured and glucose concentration with a detection limit of 2mM of glucose |
[71] |
| Polyaniline |
The AQ-PNA probe was immobilized on the working electrode, i.e. inkjet printed G-PANI conductive ink onto the screen printed carbon ink. |
Cost effective sensor which can be incinerated for screening and monitoring of the amount of HPV-DNA type 16 to to diagnose cervical cancer. |
Linear response range of 10-200 nM was obtained
Detection limit of HPV type 16 DNA was found to be 2.3 nM.
Highly sensitive ePAD DNA biosensor |
[72] |
| biaxially oriented polypropylene covered with silica oxide (BOPP-SiO4). |
The inkjet printed soft photomasks were used for depositing organic polymers/inorganic materials on polymer films. |
⇒Depositing organic polymers and inorganic material on polymer films.
⇒Photograft organic polymers onto a polymer film.
⇒Patterning on non planar substrates. |
Utilized for making intricate patterns on non-planar substrates, microsensors, optical structures, and other devices that don't need to be extremely durable or dimensionally stable
|
[73] |
| Polyaniline, polypyrrole and PSS |
Inkjet printing of polyaniline, polypyrrole, and poly(sodium 4-styrenesulphonate) (PSS) based inks deposited on gold microelectrode |
Sensitive pH sensor stable over a wide pH range |
⇒Low-cost and disposable
⇒A liner super-Nernstian response (81.2 0.5 mV/ pH unit) over a wide pH range (pH 3–10) is obtained. |
[74] |
Table 3.
Conductive polymer Electrochromic Inks for Inkjet printing .
Table 3.
Conductive polymer Electrochromic Inks for Inkjet printing .
| Conductive Polymer |
Ink composition and device fabrication |
Applications |
Highlights |
Reference |
| WO3 nanoparticles |
PEDOT:PSS buffer layer was spin coated onto the ST AgNWs /PDMS followed by inkjet printing of the WO3 nanoparticle layer. |
Deformable and wearable electronics, STEESDs with novel features. |
Large optical modulation of 40%, fast switching speed (<4.5 s), high coloration efficiency (75.5 cm2 C−1), good stability and high specific capacity (32.3 mAh g−1 and 44.8 mAh cm−3) observed.
Good functionality and maintenance of electrochromic performance even when stretched up to 50%-80% strain |
[79] |
| Polyaniline |
Water-soluble polyaniline composite material with MWNT were dispersed in water and deposited by inkjet printing yielding transparent conductive electroactive films. |
Development of inkjet printing as a viable tool for the fabrication of transparent conductive electroactive materials. |
These films allowed for the switching between yellow, green, and blue when printed onto photopaper, PET, Pt-ITO, and Au-PVDF substrates. A change in sheet resistance (1000–5000 ohm sq-1) and optical transmittance (30–70%) was reported with a change in the nanotube percentage. |
[80] |
| PANI-silica and PEDOT-silica |
PANI-silica and PEDOT-silica composites converted by solvent exchange to intrinsically conductive polymer inks which were inkjet printed on indium tin oxide-coated poly(ethylene terephthalate) films. |
Electrochromic display device fabrication with various intrinsically conductive polymer colloidal solutions. |
Colour of the devices changed with change in potential. The colour could also be tuned by inkjet-printed PANI-silica and PEDOT-silica blended particles as an electro- chromic |
[81] |
Table 4.
Conductive polymer Pattern Inks for Inkjet printing .
Table 4.
Conductive polymer Pattern Inks for Inkjet printing .
Conductive
Polymer |
Ink formulation and device fabrication |
Applications |
Highlights |
Reference |
| Polyaniline |
Vapor deposition polymerization (VDP)-mediated inkjet printing (VDP-IJP).
The substrate undergoes chemical oxidation polymerization at elevated temperatures that forms emeraldine salt PANI patterns. |
Micro-range accuracy
Efficient PANI synthesis
|
Minimum width of patterned line = 80 μm
Average sheet resistance = 3.8×103 Ω/□
Does of surfactants or stabilizers |
[86] |
| Poly(phenylenevinyelene) (PPV) |
Reactive inkjet printing
An in-situ Wittig reaction results in the formation PPV patterns. |
Readily generated PPV microarrays.
Patterning of functional organic materials on a solid substrate. |
High processability.
Easy removal of unreacted reagents and byproducts by dissolution in organic media. |
[87] |
| Polyaniline |
Electrospinning to obtain a bilayer biodegradable scaffold for PANI printing |
Bone tissue engineering. |
Stimulation of cellular functions like attachment, proliferation, migration and differentiation. |
[88] |
| Polyaniline |
Combination of Nanotemplating and inkjet printing
PBTL and PANI-DNNSA inks were inkjet printed onto NP-TiO2 and ZFNP-TiO2 substrates |
Biosensing and electronic devices |
Low cost and efficient |
[89] |
| Polyaniline |
Oxidative polymerization in aqueous medium using polystyrene sulphonate (PSS) as an emulsioning/doping agent. Controlling the amount of the oxidant resulted in mixed leucoemeraldine/emeraldine oxidation states |
Ideal for devices where positive parasitic capacitances have to be compensated.
|
Synthesis from the dimer DANI
Negative capacitance
Cost-effective and simple |
[90] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).