1. Introduction
The electrochemical oxygen reduction reaction (ORR) is a critical process in the air electrode in fuel cells and metal-air batteries [1, 2]. It determines the overall chemical-electricity conversion efficiency of these electrochemical devices due to the complicated 4e
- reaction pathways, sluggish reaction kinetics, and high overpotential [
3]. Platinum (Pt)-based composite materials, due to their high catalytic activity, are commonly recognized as state-of-the-art ORR electrocatalysts. However, their large-scale applications have been severely hindered by solemn drawbacks such as scarce reserves, prohibitive costs, insufficient long-term durability, and vulnerability to methanol crossover [
4]. Consequently, considerable efforts have been devoted to developing cost-effective electrocatalysts with competitive activity and excellent stability toward Pt-based materials.
Iron nitrides with rich N content in carbon matrix (Fe
xN-C) with active Fe-N-C sites, as a kind of newly developed non-noble metal electrocatalysts, present enhanced catalytic activity, low cost, and strong methanol tolerance, and have been recognized as promising alternatives to Pt-based catalysts [5, 6]. However, the Fe
xN nanoparticles are thermodynamically unstable and prone to migration and coalescence during the catalysis process because of their high surface energy, resulting in an apparent decrease in activity and stability [
7]. Encapsulating the Fe
xN nanoparticles in the nanoshells or nanopores of the carbon matrix is one of the most promising strategies to overcome the stability issue and benefit the generation of the Fe-N-C active site [
8]. Moreover, the FexN-C catalysts are generally connected to the gas diffusion layer to fabricate a gas electrode with ionomers such as Nafion. Li et al. recently found that the Nafion ionomer can be easily deactivated by the generating˙OH radicals, leading to fast performance degradation [
9]. By comprehensively evaluating costs, activity, and stability, developing an in-situ growth strategy of Fe
xN nanoparticles encapsulated carbon nanomaterials on gas diffusion electrodes (i.e., carbon cloth) is highly desirable, yet few researchers have been focused on this direction.
In this study, we report that Fe3N nanoparticles encapsulated N-doped carbon nanotubes in-situ grown on the surface of flexible carbon cloth (denoted as Fe3N@CNTs/CC) can be synthesized via simple one-step calcination of cotton cloth, one of the most commonly used biomass products. The Fe3N nanoparticles were produced during the carbonization process of cotton cloth under an ammonia (NH3) atmosphere and catalyzed the in-situ growth of N-doped carbon nanotubes on the generated carbon cloth, resulting in the overall fabrication of Fe3N@CNTs/CC hierarchical structure. The hierarchical structure is capable of enlarging the surface area, promotes the exposure of the active species, and gives rise to a remarkable ORR activity with a +50 mV higher half-wave potential (E1/2 = 0.91 V) than Pt/C (E1/2 = 0.86 V) in alkaline media. The long-term stability and methanol tolerance of Fe3N@CNTs/CC are also significantly improved compared to Pt/C. Meanwhile, as a flexible and free-standing electrocatalyst, Fe3N@CNTs/CC can be used directly as the air cathode for Zn-air batteries (ZIBs) and exhibits higher open circuit voltage (1.50 V vs. 1.42 V), power density (157 mW cm-2 vs. 124.5 mW cm-2) and specific capacity (814.9 mA h g−1 vs. 743.9 mA h g−1) than the Pt/C based gas electrode.
2. Materials and Methods
2.1. Materials and Chemicals
Ferric chloride hexahydrate (analytical pure), zinc acetate dihydrate ≥98%, potassium hydroxide (analytical pure), concentrated sulfuric acid 98%, ethanol 99% purchased from Sinopsin Group Chemical Reagent Co., LTD. Commercial Pt/C catalyst (20 wt%) from Sigma-Aldrich (China), Nafion solution (5%) and Teflon dispersion (60%) from Dupont (USA), Vulcan XC 72R (99.9%) from Johnson Matthey. Deionized water is made in the laboratory.
2.2. Preparation of Fe3N@CNT/CC
Cellulose cotton cloth (5g) was washed with deionized water thrice and dried in an oven at 80 °C. Next, an impregnation solution was prepared by dissolving 2.7g FeCl3•6H2O in 5 ml deionized water at 60 °C. The impregnation solution was then dropped evenly onto the cellulose cotton cloth and dried at 80 °C. The impregnated cotton cloth was then annealed in a tube furnace at 1000 °C for 1 hour in an NH3 atmosphere.
2.3. Physicochemical Characterization
Material Characterization. X-ray diffractometer (XRD, χPert Pro, Panaco, Netherlands) was used to characterize the phase composition of the samples. Field emission scanning electron microscope (SEM, JEOL JSM-7500F) and transmission electron microscope (TEM, JEM 2100F) were used to observe the surface morphology of the materials, and energy-dispersive X-ray spectroscopy (EDS) measurements were performed on an FEI Tecni G20 (USA) transmission electron microscope. X-ray photoelectron spectroscopy (XPS) was used to characterize the material’s composition, element content, and valence state. The pore structure and specific surface area of the materials were characterized by N2 adsorption−desorption isotherms (Quantachrome, USA Autosorb-iQ2) at 77 K. The pore-size-distribution plot was measured by Barrett Joyner-Halenda (BJH) model.
2.4. Electrochemical Measurements
The electrochemical measurements were conducted using a typical three-electrode system of CHI760E electrochemical workstation with 0.1 M KOH as the electrolyte at 25 °C. The platinum wire and Ag/AgCl electrodes were used as the opposite and reference electrodes, respectively. The catalyst-modified glassy carbon rotating disk electrode (RDE) was used as the working electrode (0.1256 cm2). To prepare the catalyst ink,4 mg catalyst is mixed with 500 µL complex solvent (The volume ratio of ethanol: deionized water: Nafion=9:36:5) and ultrasound for 30 min. The prepared 10 µL catalyst ink droplet was deposited on the turntable electrode (RDE) with a catalyst load of 0.31 mg cm-2.
During the test, cyclic voltammetry (CV) was tested in 0.1 M KOH solution saturated with O2 or N2 at a scan rate of 10 mV s–1. Linear sweep voltammetry (LSV) was tested in 0.1 M KOH solution saturated with O2 or N2 at a scan rate of 5mV s–1.
The ORR kinetic currents and number of transferred electrons were calculated by the K-L equations:
where j is the measured current density (j), jK is the kinetic current and jd is diffusion-limiting current, ω is a rotating speed (revolutions per minute), n is the number of transferred electrons, F is the Faraday constant (96,485 C mol−1), C0 is the bulk concentration of O2 (1.2 × 10−6 mol cm−3), D0 is the diffusion coefficient of O2 (1.9 × 10−5 cm2 s−1), and ν is the kinetic viscosity (0.01 cm2 s−1).
In the rotating ring disk electrode (RRDE) test, the measured values of the ring and disk currents were used to calculate the H
2O
2 production and the number of electron transfers (n) using the following equation:
where I
d denotes the disk current; I
r represents the ring current; N is the current collection efficiency of the Pt ring with a value of 0.36 from the reduction of K
3[Fe(CN)
6]. LSV measurements through RRDE tests were carried out at a rotating rate of 1600 rpm and a scan speed of 5 mV s
-1. Chronoamperometry was conducted at 0.6 V (vs. RHE) for 10,000 s (1600 rpm) in O
2-saturated 0.1 M KOH to investigate the durability of the catalyst[
10].
2.5. Zn–air battery tests
Zn sheet polished with sandpaper was used as the anode. Fe3N@CNT/CC was applied as the air cathode. The aqueous electrolyte comprised 6 M KOH and 0.2 M zinc acetate. For comparison, Pt/C based air electrode was prepared as follows: 1 mg Pt/C catalyst, 400 μL anhydrous ethanol, and 10 μL Nafion were mixed evenly and then deposited on commercial carbon cloth with a Pt/C loading mass of 0.82 mg cm-2.
3. Results and discussion
3.1. Structural and Compositional Analyses
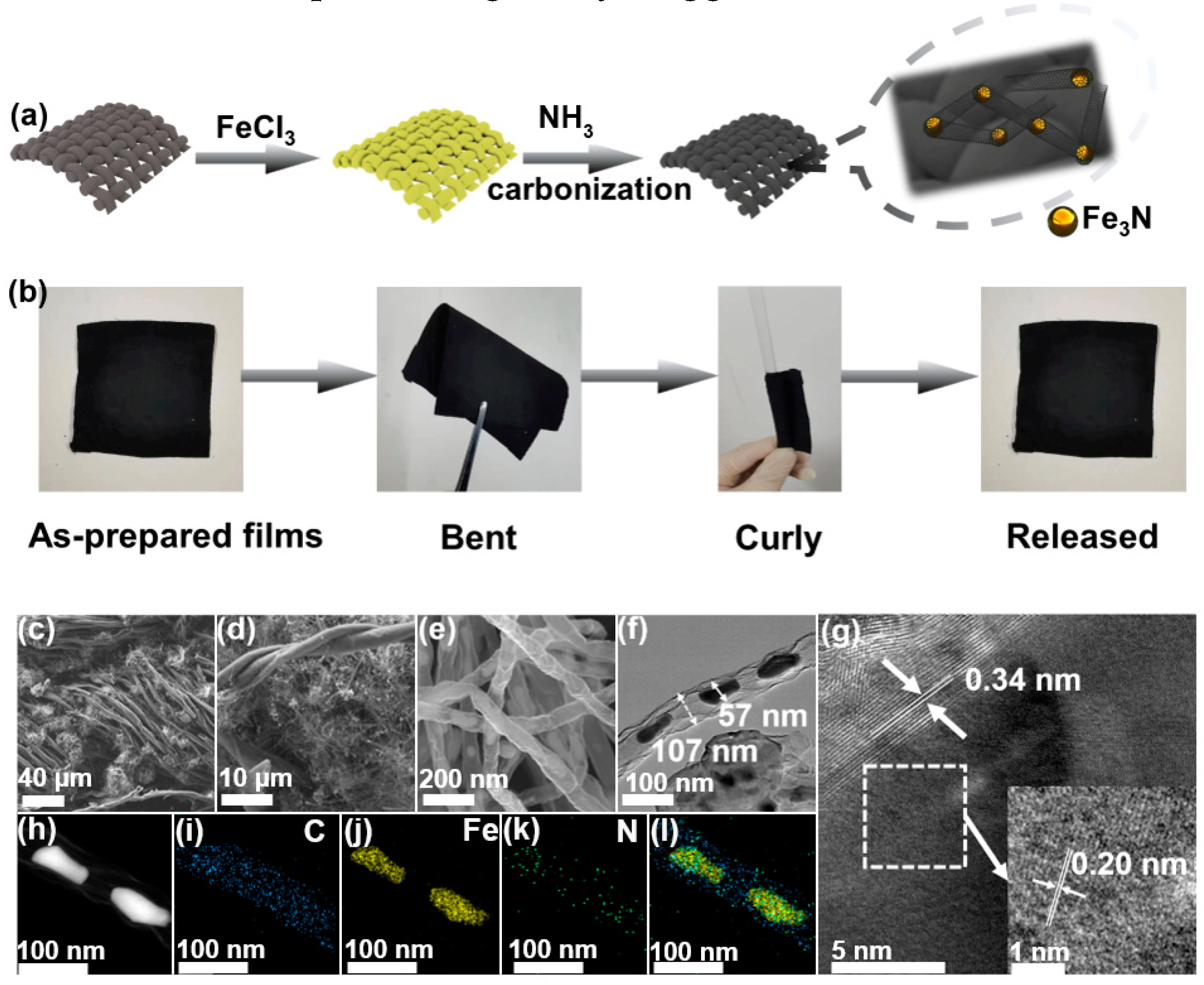
Fe3N@CNT/CC was prepared by a simple thermal treatment of FeCl
3 decorated cellulose cotton cloth in an NH
3 atmosphere, as is illustrated in
Figure 1a. The digital images of the Fe
3N@CNT/CC film in
Figure 1b reveal its excellent flexibility, as it can be bent and rolled without structural deterioration. SEM analyzed the morphology of Fe3N@CNT/CC. It is observed that the fiber-braided structure of the cotton cloth is well retained during the carbonization process(
Figure 1c). Carbon nanotubes are generated and connected to the fibers (
Figure 1d) with a diameter of ~100 nm (
Figure 1e). Shiny nanoparticles are observed to be encapsulated within the carbon nanotubes (
Figure 1e). As is shown in the TEM images (
Figure 1f), the nanoparticles have a width of 50-60 nm and a length in the range of 100-120 nm. The lattice spacing of the nanoparticles is 0.206 nm (
Figure 1g), corresponding to the (-1-11) crystal plane of Fe
3N [10, 11]. Additionally, clear lattice fringes are observed outside the nanoparticles with a lattice spacing of 0.34 nm, corresponding to the (002) crystal plane of graphite carbon. The elemental mapping reveals that C and N are evenly distributed throughout the carbon nanotubes, forming N-doped carbon nanotubes. In contrast, Fe was only distributed in the cores of the carbon nanotubes (
Figure 1h), indicating Fe
3N nanoparticles are encapsulated in the carbon nanotubes. In this manner, Fe
3N nanoparticles are trapped in the nanocavity of carbon nanotubes, preventing catalyst agglomeration [
12].
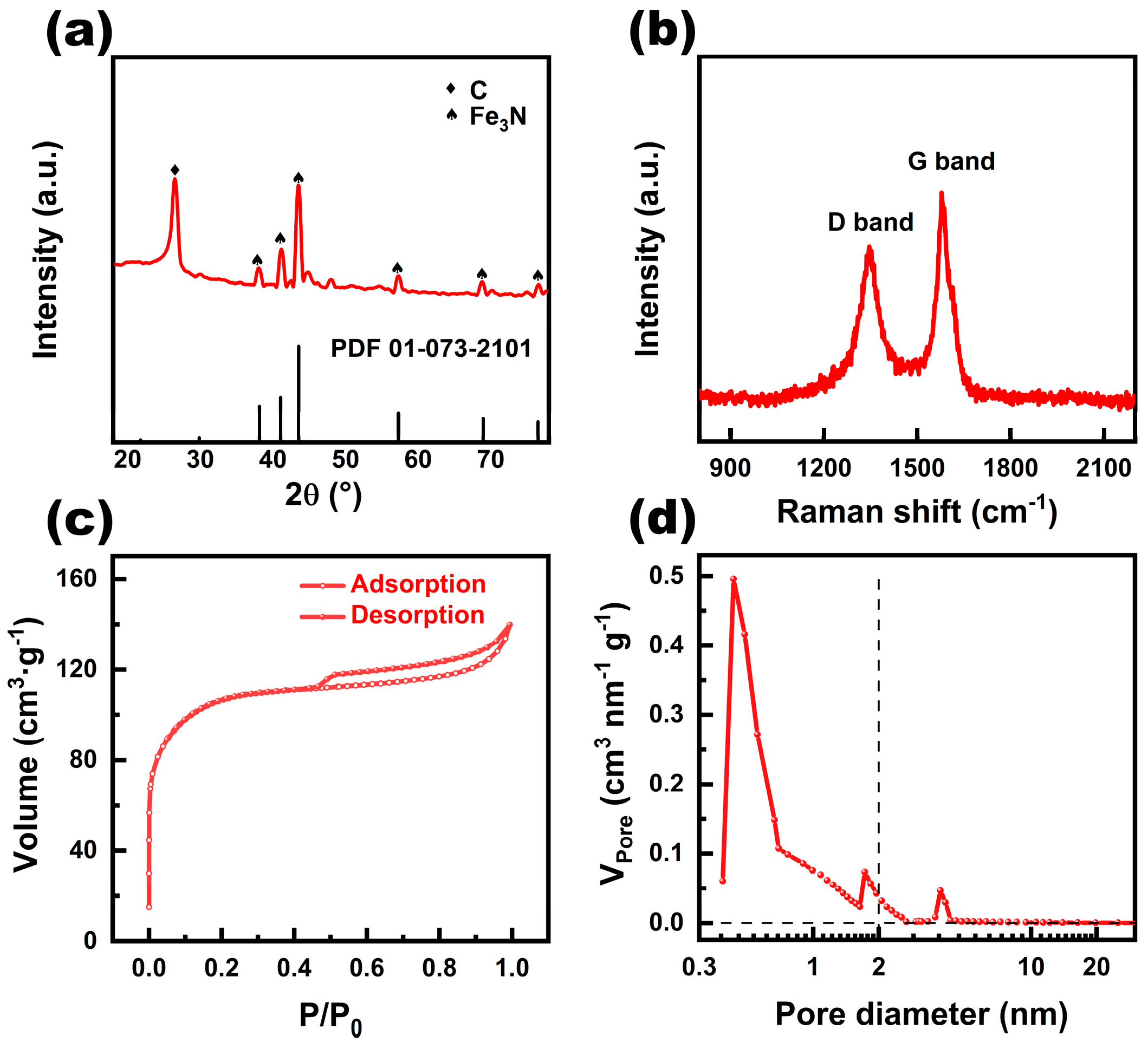
X-ray diffraction (XRD) spectrum was recorded to analyze the crystallinity of the obtained Fe
3N@CNT/CC (
Figure 2a). The diffraction peaks at 38.2°, 41.1°, 43.6°, 57.3°, 69°, and 76.5°correspond to the (110), (002), (-1-11), (-1-12), (300), and (-1-13) crystal planes of Fe
3N, respectively[
3]. The diffraction peak at 26.4° corresponds to the (002) facet of graphite carbon[
3], consistent with the HRTEM results.
Figure 2b depicts the Raman spectrogram analysis of the Fe
3N@CNT/CC, which exhibits two distinct peaks at 1344 cm
-1 and 1574 cm
-1, corresponding to the typical D and G bands of carbon materials[
13]. The ratio of I
D/I
G indicates the degree of defect of the carbon material[
14]. The I
D/I
G value of Fe
3N@CNT/CC is determined to be about 0.89, which suggests that less defective or amorphous carbon exists in the as-prepared product. Nitrogen adsorption and desorption isotherm reveal that Fe
3N@CNT/CC possesses a specific surface area of 337.7 m
2·g
-1 (
Figure 2c). The pore volume is mainly contributed by the micropore, while a certain amount of mesopores also exist with an average size of 4 nm (
Figure 2d). The high surface area and pore volume can make the catalytically active sites accessible and ensure fast mass transport efficiency, which enables the catalyst to have significant advantages in electrocatalytic reactions[15, 16].
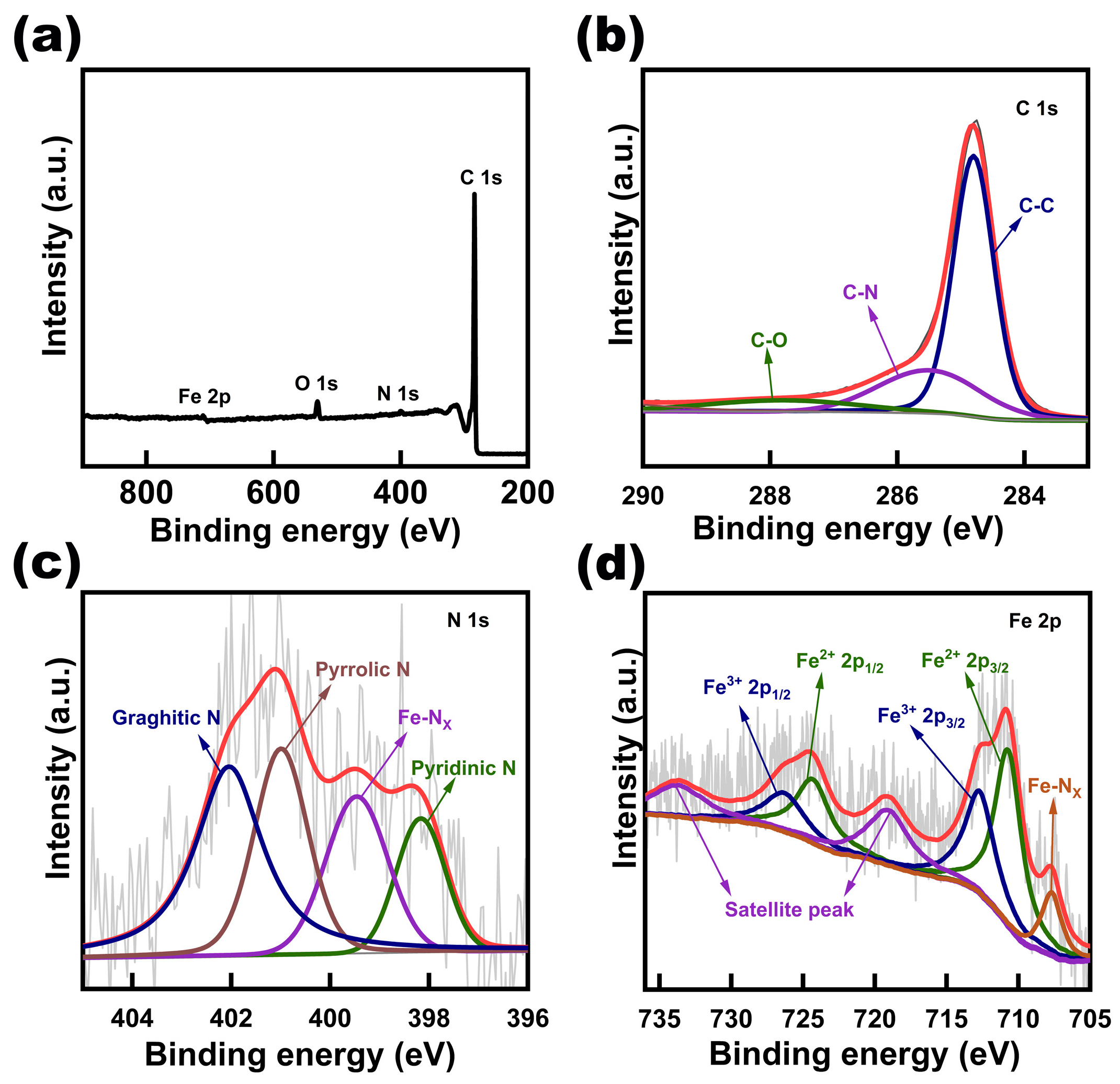
The XPS survey spectrum (
Figure 3a) reveals the presence of C, N, Fe, and O. In the C 1s spectrum (
Figure 3b), the peaks at 284.8 eV, 285.5 eV, and 287.8 eV correspond to C-C, C-N, and C-O signals, respectively [
17]. The N 1s (
Figure 3c) spectrum is divided into four peaks, including pyridinic nitrogen (398.2 eV), Fe-N
X (399.4 eV), pyrrolic nitrogen (401 eV), and graphite nitrogen (402.1 eV)[
18]. The peak corresponding to Fe-N bond can be observed at 707.8eV[
19] in the Fe 2p spectrum (
Figure 3d). The existence of C-N in the C 1s spectrum, Fe-N
X in the N 1s spectrum, and the Fe-N bond in the Fe 2p spectrum verify the formation of Fe-N-C sites, which has been proved to be an efficient ORR active sites[
20]. Meanwhile, the graphitized N-doped carbon promotes the electron transfer process, further improving the electrical conductivity of the sample. [
3]
3.2. Electrocatalytic Activities of Fe3N@CNT/CC for ORR
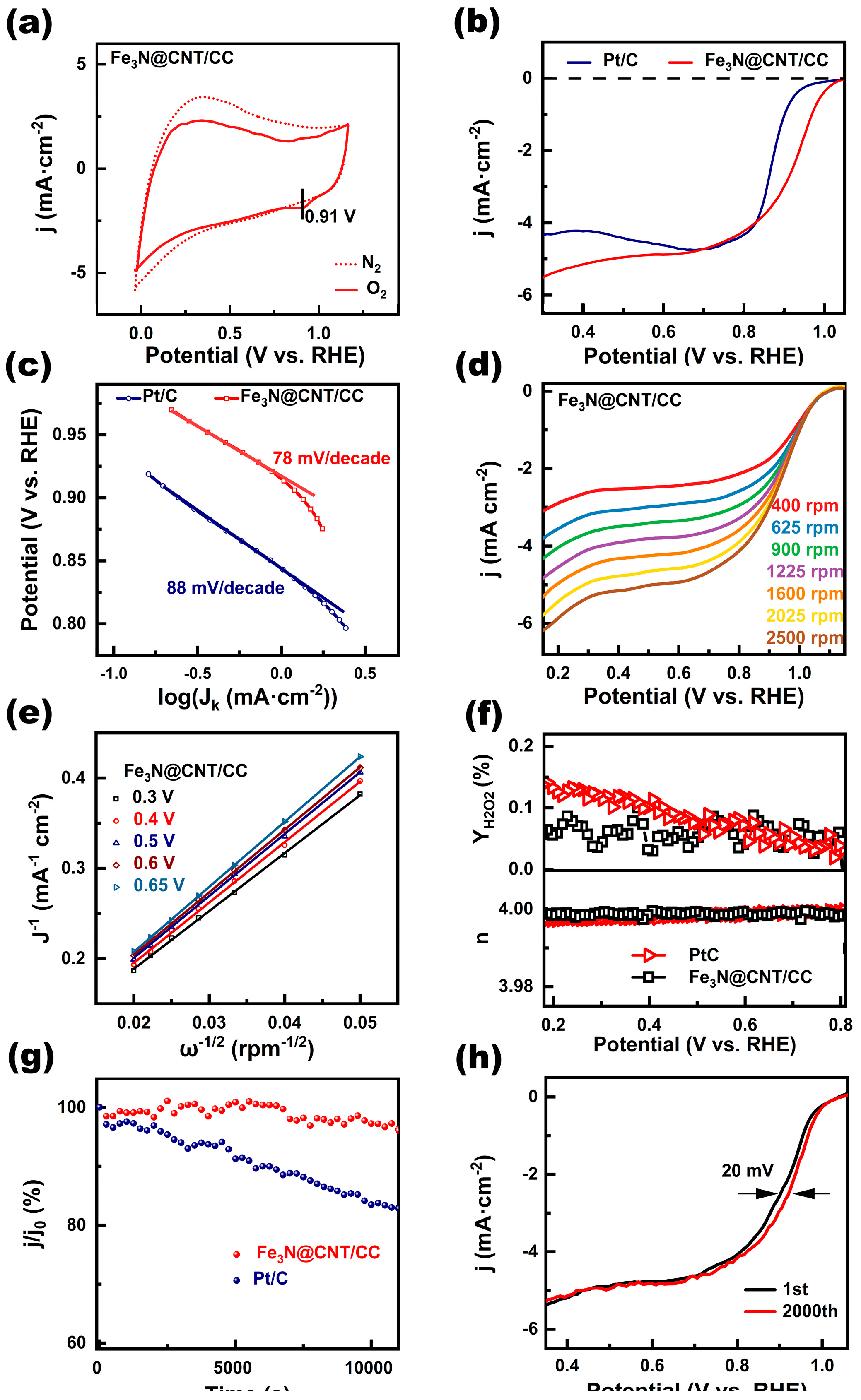
The ORR performances of Fe
3N@CNT/CC are compared with commercial Pt/C (
Figure 4). Fe
3N@CNT/CC demonstrated no ORR peaks in N
2-saturated electrolyte, while distinguished reduction peak for ORR appeared when the electrolyte was saturated with O
2 (
Figure 4a). The peak potentials corresponding to Fe
3N@CNT/CC was 0.91V, 60 mV positive than that corresponding to 20% Pt/C (0.85 V) (
Figure 4a,
Figure S1). The LSV curve of Fe
3N@CNT/CC (
Figure 4b) shows an onset potential of 1.0 V, a half-wave potential of 0.91 V, and a limit current density of 5.6 mA·cm
-1. In contrast, the initial potential (0.9 V), half-wave potential (0.86 V), and limit current (5 mA·cm
-1) of Pt/C catalyst were significantly worse than those of Fe
3N@CNT/CC. The corresponding Tafel slope of Fe
3N@CNT/CC (
Figure 4c) was 78 mV·dec
-1, lower than that of Pt/C (88 mV·dec
-1), indicating that Fe
3N@CNT/CC had a faster catalytic kinetic process[
3].
The ORR reactions have two types of electron transfer processes: 2e
- transfer process and 4e
- transfer process. In the 2e
- transfer process, oxygen reacts to produce H
2O
2 through the catalytic process[
21]. The reaction rate of this process is slow, which is not suitable for application in zinc-air batteries[
22]. Moreover, the generated H
2O
2 can cause damage to catalyst and battery components[23, 24]. The catalytic product of the 4e
- transfer process is H
2O, making it the required catalytic path for the ORR catalytic reaction[
25]. The number of transferred electrons (n) in the catalytic reactions was obtained by the Koutecký-Levich (K-L) calculation of the LSV curves at different speeds (
Figure 4d). As is shown in
Figure 4e, the average number of transferred electrons of Fe
3N@CNT/CC was determined to be 3.99, which is close to the theoretical value of 4 and comparable to that of Pt/C. In addition, rotating ring-disk electrode (RRDE) measurements were conducted to explore the ORR pathway of Fe
3N@CNT/CC (
Figure S2). The H
2O
2 yield of Fe
3N@CNT/CC was found to be only 0.14%–0.01% from 0.2 V to 0.8 V (
Figure 4f), further proving the 4- electron transfer process
.
Subsequently, the stability of Fe
3N@CNT/CC and Pt/C catalyst was assessed using timed amperometry at 0.8 V (vs. RHE). The current retention of Fe
3N@CNT/CC decreased by 2.75% during the test at 10000 s, whereas that of the Pt/C decreased by 16.5% (
Figure 4g). It was observed that Fe
3N@CNT/CC is more stable during the reaction.
Figure 4h depicts the durability test of Fe
3N@CNT/CC after 2000 CV cycle curves. It can be observed that the half-wave potential value of the catalyst only decreased by 20 mV after 2000 accelerated aging experiments, indicating its excellent stability as well. Methanol resistance is another essential index in ORR. It is seen from Fig.S3 that when methanol was added to the electrolyte, the current value of Fe
3N@CNT/CC decreased by 5%, whereas the current density of Pt/C decreased by 35% (
Figure S3), which suggests that Fe
3N@CNT/CC exhibits outstanding resistance to methanol.
3.3. Application of Fe3N@CNT/CC Catalyst in ZAB
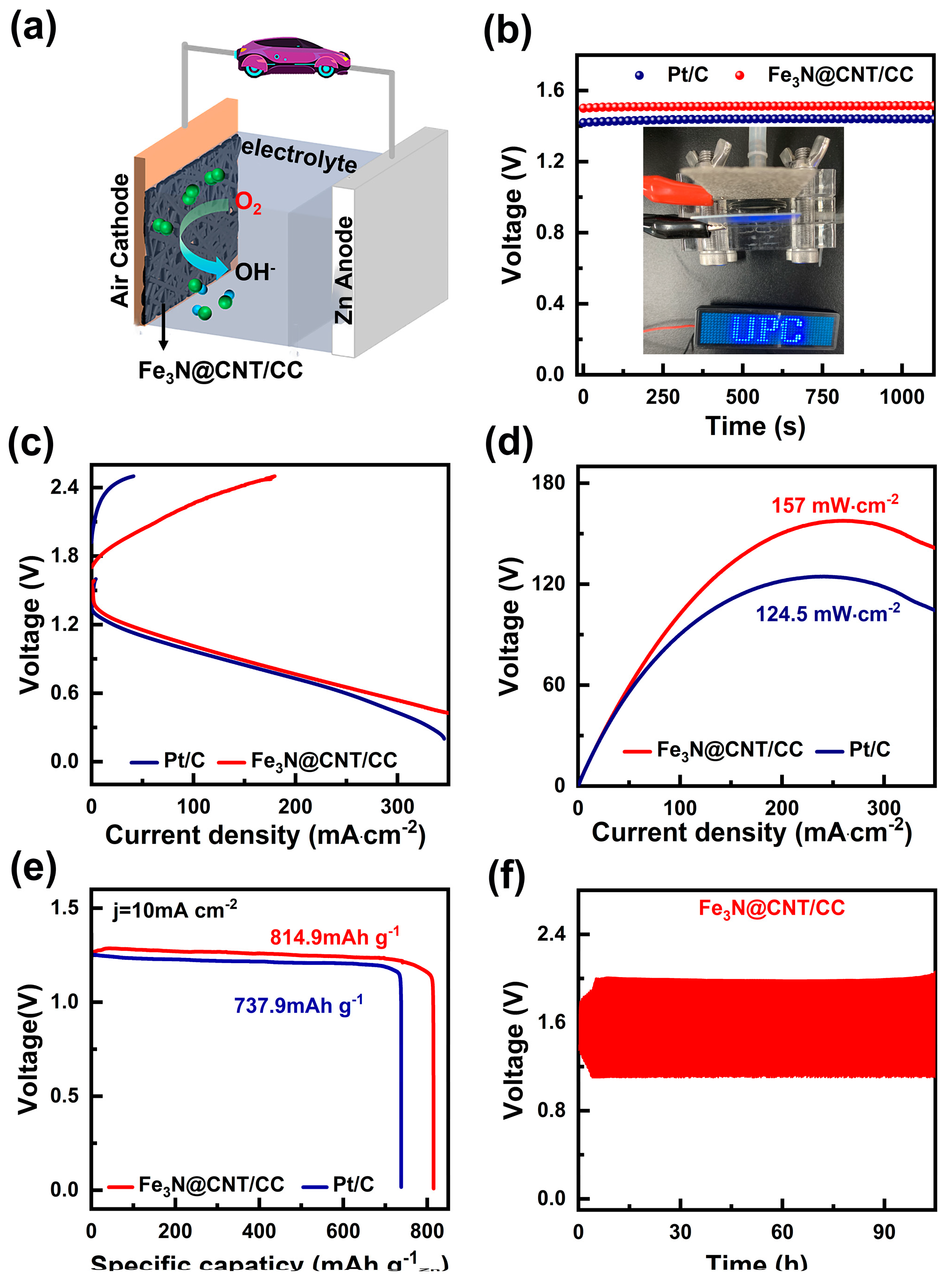
To further evaluate the practical application of Fe
3N@CNT/CC, it is essential to assemble it as the air cathode of a ZAB, as illustrated in
Figure 5a. The open circuit voltage of the carbon cloth composed of Fe
3N@CNT/CC is approximately 1.5 V (
Figure 5b), which is higher than that of Pt/C (1.43 V). As shown in
Figure 5c, Fe
3N@CNT/CC can supply a higher voltage at the same current density than Pt/C. The peak discharge power density of the ZAB with Fe
3N@CNT/CC as the air cathode is 157 mW cm
-2 at the current density of 300 mA cm
-2(
Figure 5c, 5d), which is higher than that of the Pt/C (124.5 mW cm
-2).
Figure 4e illustrates the constant current discharge test of the battery at a current density of 10 mA cm
-2. The specific capacity of Fe
3N@CNT/CC-based ZAB is 814.9 mAh g
-1, also higher than that of the Pt/C (737.9 mAh g
-1). In addition,
Figure 5f depicts the cycling stability of Fe
3N@CNT/CC-based ZAB. The voltage range does not widen during the 100-hour charge-discharge cycle, indicating the catalyst has excellent stability.
4. Conclusions
In this work, Fe3N nanoparticles encapsulated N-doped carbon nanotubes grown on the surface of flexible carbon cloth were synthesized via simple one-step calcination of cotton cloth. The structure feather and ORR performance of as-prepared catalysts were investigated in detail. The hierarchical structure can stable the active sites, promote the exposure of the active species, and possess satisfactory ORR performance, including excellent half-wave potential, higher long-term stability, and satisfactory resistance to methanol, overtopping commercial Pt/C catalysts. Benefiting from the unique structure, Fe3N@CNTs/CC can be used directly as a free-standing air cathode for ZABs, exhibiting higher open circuit voltage, power density, and specific capacity than the Pt/C-based gas electrode. This work paves the way for the construction of cost-effective, easy-preparation, and high-performance ORR catalysts and ZAB energy conversion devices.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1. Figure S1: CV curves of the Pt/C in N
2 and O
2 saturated 0.1 M KOH electrolyte with a scan rate of 10 mV·s
-1. Figure S2: RRDE voltammograms of Fe
3N@CNT/CC at a rotation rate of 1600 rpm; Figure S3: The chronoamperometric curves of Fe
3N@CNT/CC and Pt/C acquired by injecting 1.0 M methanol at 100 s.
Author Contributions
Conceptualization, D.L. and P.D.; methodology,Y.T.; validation, D.L.; formal analysis, C.T.; data curation, S.L.; writing—original draft preparation, Y.Z.; writing—review and editing, Y.Z.; visualization, Y.Z.; supervision, P.D.; funding acquisition, T.X. and Z.L. All authors have read and agreed to the published version of the manuscript. Y.Z. and D.L. contributed equally to this work.
Funding
This research was funded by the National Natural Science Foundation of China (51702365), the Natural Science Foundation of Shandong Province (ZR2022MB133), KeyResearch and Development Plan of Shandong Province (2019GGX102056, 2018GGX104018), New Faculty Start-up funding in China University of Petroleum (East China) (YJ201501029).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nie, Y.;Xu, X.;Wang, X.;Liu, M.;Gao, T.;Liu, B.;Li, L.;Meng, X.;Gu, P.;Zou, J. Coni alloys encapsulated in n-doped carbon nanotubes for stabilizing oxygen electrocatalysis in zinc-air battery. Nanomaterials. 2023, 13, 1788.
- Cebollada, J. ;Sebastián, D. ;Lázaro, M. J.;Martínez-Huerta, M. V. Carbonized polydopamine-based nanocomposites: The effect of transition metals on the oxygen electrocatalytic activity. Nanomaterials. 2023, 13, 1549. [Google Scholar] [PubMed]
- Li, T. ;Li, M. ;Zhang, M.;Li, X.;Liu, K.;Zhang, M.;Liu, X.;Sun, D.;Xu, L.;Zhang, Y.;Tang, Y. Immobilization of fe3n nanoparticles within n-doped carbon nanosheet frameworks as a high-efficiency electrocatalyst for oxygen reduction reaction in zn-air batteries. Carbon. 2019, 153, 364–371. [Google Scholar]
- Yu, X. ;Ye, S. Recent advances in activity and durability enhancement of pt/c catalytic cathode in pemfc : Part ii: Degradation mechanism and durability enhancement of carbon supported platinum catalyst. Journal of Power Sources. 2007, 172, 133–144. [Google Scholar]
- Yang, W. ;Chen, L. ;Liu, X.;Jia, J.;Guo, S. A new method for developing defect-rich graphene nanoribbons/onion-like carbon@co nanoparticles hybrid materials as an excellent catalyst for oxygen reactions. Nanoscale. 2017, 9, 1738–1744. [Google Scholar]
- Xiao, J. ;Xu, Y. ;Xia, Y.;Xi, J.;Wang, S. Ultra-small fe2n nanocrystals embedded into mesoporous nitrogen-doped graphitic carbon spheres as a highly active, stable, and methanol-tolerant electrocatalyst for the oxygen reduction reaction. Nano Energy. 2016, 24, 121–129. [Google Scholar]
- Ma, Q. ;Jin, H. ;Zhu, J.;Li, Z.;Xu, H.;Liu, B.;Zhang, Z.;Ma, J.;Mu, S. Stabilizing fe–n–c catalysts as model for oxygen reduction reaction. Advanced Science. 2021, 8, 2102209. [Google Scholar]
- Li, Z. ;Fang, Y. ;Zhang, J.;Lou, X. W. Necklace-like structures composed of fe3n@c yolk–shell particles as an advanced anode for sodium-ion batteries. Advanced Materials. 2018, 30, 1800525. [Google Scholar]
- Zhou, L. ;Li, Y. ;Chen, X.;Yang, Z.;Yang, S.;Wang, Q.;Liu, X.-Y.;Lu, S. New insights into degradation of fe–n–c catalyst layers: Ionomer decomposition. Journal of Materials Chemistry A. 2022, 10, 20323–20330. [Google Scholar]
- Hu, Y. ;Huang, D. ;Zhang, J.;Huang, Y.;Balogun, M.-S. J. T.;Tong, Y. Dual doping induced interfacial engineering of fe2n/fe3n hybrids with favorable d-band towards efficient overall water splitting. ChemCatChem. 2019, 11, 6051–6060. [Google Scholar]
- Chen, Z. Y. ;Li, Y. N.;Lei, L. L.;Bao, S. J.;Wang, M. Q.;Heng, L.;Zhao, Z. L.;Xu, M. W. Investigation of fe2n@carbon encapsulated in n-doped graphene-like carbon as a catalyst in sustainable zinc–air batteries. Catalysis Science & Technology. 2017, 7, 5670–5676. [Google Scholar]
- Zhang, N. ;Xie, S. ;Wang, W.;Xie, D.;Zhu, D.;Cheng, F. Ultra-small fe2n/n-cnts as efficient bifunctional catalysts for rechargeable zn-air batteries. Journal of The Electrochemical Society. 2020, 167, 020505. [Google Scholar]
- Compagnini, G. ;Puglisi, O. ;Foti, G. Raman spectra of virgin and damaged graphite edge planes. Carbon. 1997, 35, 1793–1797. [Google Scholar]
- Li, L. ;Qing, M. ;Liu, X.;Wang, H.;Liu, S.;Zhang, Y.;Wan, H.;Wen, X.;Yang, Y.;Li, Y. Efficient one-pot synthesis of higher alcohols from syngas catalyzed by iron nitrides. ChemCatChem. 2020, 12, 1939–1943. [Google Scholar]
- Zhu, C. ;Fu, S. ;Song, J.;Shi, Q.;Su, D.;Engelhard, M. H.;Li, X.;Xiao, D.;Li, D.;Estevez, L.;Du, D.;Lin, Y. Self-assembled fe–n-doped carbon nanotube aerogels with single-atom catalyst feature as high-efficiency oxygen reduction electrocatalysts. Small. 2017, 13, 1603407. [Google Scholar]
- Du, P. ;Bao, Y. ;Guo, C.;Wu, L.;Pan, J.;Zhao, C.;Ma, F.-X.;Lu, J.;Li, Y. Y. Design of fe,n co-doped multi-walled carbon nanotubes for efficient oxygen reduction. Chemical Communications. 2020, 56, 14467–14470. [Google Scholar]
- Zhu, G. ;Ma, L. ;Lv, H.;Hu, Y.;Chen, T.;Chen, R.;Liang, J.;Wang, X.;Wang, Y.;Yan, C.;Tie, Z.;Jin, Z.;Liu, J. Pine needle-derived microporous nitrogen-doped carbon frameworks exhibit high performances in electrocatalytic hydrogen evolution reaction and supercapacitors. Nanoscale. 2017, 9, 1237–1243. [Google Scholar]
- Guo, C. ;Li, L. ;Zhang, T.;Xu, Y.;Huo, Y.;Li, S. Space-confined iron nanoparticles in a 3d nitrogen-doped rgo-cnt framework as efficient bifunctional electrocatalysts for rechargeable zinc–air batteries. Microporous and Mesoporous Materials. 2020, 298, 110100. [Google Scholar]
- Liu, S. ;Zheng, W. ;Xie, W.;Cui, H.;Li, Y.;Zhang, C.;Ji, Z.;Liu, F.;Chen, R.;Sun, H.;Xu, J. Synthesis of three-dimensional honeycomb-like fe3n@nc composites with enhanced lithium storage properties. Carbon. 2022, 192, 162–169. [Google Scholar]
- Ma, J. ;Li, J. ;Wang, R.;Yang, Y.;Yin, P.;Mao, J.;Ling, T.;Qiao, S. Hierarchical porous s-doped fe–n–c electrocatalyst for high-power-density zinc–air battery. Materials Today Energy. 2021, 19, 100624. [Google Scholar]
- Zhou, X. ;Gao, J. ;Hu, Y.;Jin, Z.;Hu, K.;Reddy, K. M.;Yuan, Q.;Lin, X.;Qiu, H.-J. Theoretically revealed and experimentally demonstrated synergistic electronic interaction of cofe dual-metal sites on n-doped carbon for boosting both oxygen reduction and evolution reactions. Nano Letters. 2022, 22, 3392–3399. [Google Scholar]
- Wang, B. ;Tang, J. ;Zhang, X.;Hong, M.;Yang, H.;Guo, X.;Xue, S.;Du, C.;Liu, Z.;Chen, J. Nitrogen doped porous carbon polyhedral supported fe and ni dual-metal single-atomic catalysts: Template-free and metal ligand-free sysnthesis with microwave-assistance and d-band center modulating for boosted orr catalysis in zinc-air batteries. Chemical Engineering Journal. 2022, 437, 135295. [Google Scholar]
- Ye, H. ;Li, L. ;Liu, D.;Fu, Q.;Zhang, F.;Dai, P.;Gu, X.;Zhao, X. Sustained-release method for the directed synthesis of zif-derived ultrafine co-n-c orr catalysts with embedded co quantum dots. ACS Applied Materials & Interfaces. 2020, 12, 57847–57858. [Google Scholar]
- Hu, C. ;Dai, L. Carbon-based metal-free catalysts for electrocatalysis beyond the orr. Angewandte Chemie International Edition. 2016, 55, 11736–11758. [Google Scholar] [PubMed]
- Zhang, J. ;Wang, M. ;Wan, T.;Shi, H.;Lv, A.;Xiao, W.;Jiao, S. Novel (pt-o(x) )-(co-o(y) ) nonbonding active structures on defective carbon from oxygen-rich coal tar pitch for efficient her and orr. Adv Mater. 2022, 34, e2206960. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).