1. Introduction
Gastric acid-related diseases (ARDs) are primarily caused by the excessive production or imbalance of gastric acid in the stomach. The main gastric ARDs include gastroesophageal reflux disease (GERD), peptic ulcers, gastritis, and Zollinger–Ellison syndrome [
1]. ARD medications are designed to reduce gastric acid production, relieve symptoms, and promote tissue healing. Thus, antacids such as proton pump inhibitors (PPIs) and H
2 blockers have been used for treatment [
2,
3]. Potassium-competitive acid blockers (P-CABs), a new class of acid suppressants, have recently been developed as an alternative treatment [
4]. Among ARDs, gastritis is one of the most common clinically diagnosed diseases worldwide, and its prevalence is gradually increasing in Korea [
5]. Gastritis manifests as inflammation or irritation of the stomach lining and may be caused by many factors, including infection, alcohol, particular medications, and some allergic and immune conditions [
6]. Although empirical treatment is primarily performed using conventional drugs, and a few studies have reported the effects of H
2-receptor antagonists and PPIs on the endoscopic improvement of acute and chronic gastritis [
7], some patients may not respond well to these treatments [8–10]. More effective medications are required to provide better relief for patients not experiencing sufficient symptom improvement with the existing options.
Recently developed P-CABs (e.g., vonoprazan, tegoprazan, and fexuprazan) are significantly more efficient than PPIs in suppressing gastric acid secretion [
11]. Because P-CABs do not require acid activation, they show a faster onset than PPIs [
12]. Additionally, P-CABs efficiently reduce nocturnal acid breakthroughs because of their longer action duration [
13]. In addition, it is metabolized predominantly by cytochrome P450 (CYP) 3A4 with small inter-individual variability, whereas many PPIs, mainly metabolized by CYP2C19, may exhibit evident differences in drug exposure. The Ministry of Food and Drug Safety (MFDS) recently approved fexuprazan, a P-CAB developed by Daewoong Pharmaceutical Co., Ltd. (Seoul, Korea), for use in two formulations of 10 [
14] and 40 mg [
15]. Fexuprazan 40 mg once daily is indicated for therapeutic use in erosive GERD [
16], whereas fexuprazan 10 mg twice daily are prescribed for ameliorating gastric mucosal lesions associated with acute and chronic gastritis [
17]. Fexuprazan was the first P-CAB approved for acute and chronic gastritis treatment in South Korea [
18].
Phase 3 clinical trials in patients with gastritis have demonstrated the efficacy of fexuprazan 10 mg (twice daily). Daewoong Pharmaceutical Co., Ltd. developed a new formulation of fexuprazan 10 mg to improve patient medication compliance and market competitiveness by changing the shape of the tablet from round to oblong [
19]. Thus, information on the pharmacokinetics (PKs) of the new formulation of fexuprazan 10 mg is necessary to ensure comparable exposure to the previous formulation.
In this study, the PKs of the new fexuprazan 10 mg formulation were evaluated after single and multiple administrations in Part 1. In Part 2 of the comparative PK study, the bioequivalence between the new and approved formulations was investigated.
2. Results
2.1. Demographic characteristics
Eight male subjects were enrolled, and all subjects completed Part 1 of the study. Accordingly, the PK and safety profiles of the eight subjects from Part 1 were analyzed. Furthermore, their demographics were evaluated. The mean (± standard deviation) age, height, weight, and body mass index (BMI) were 32.63 (±9.74) years, 176.58 (±7.44) cm, 74.76 (±7.50) kg, and 23.95 (±1.55) kg/m2, respectively. Twenty-four subjects were enrolled in Part 2 and randomly assigned to Sequence A (nine males and three females) or B (10 males and two females). Of the subjects in Part 2, one was excluded because of loss to follow-up on day 3 of Period 2, and 23 subjects completed the clinical trial. Accordingly, the PK profiles of 23 subjects who completed the study and the safety profiles of 24 subjects who received at least one dose of investigational products (Ips) were analyzed. The mean (± standard deviation) age, height, weight, and BMI were 27.17 (±6.63) years, 172.24 (±8.50) cm, 72.48 (±10.90) kg, and 24.33 (±2.46) kg/m2, respectively. There were no significant differences between the sequence groups in terms of mean age, height, weight, and BMI (P-values = 0.1812, 0.7203, 0.4868, and 0.2141, respectively).
2.2. PKs of fexuprazan 10 mg after single and multiple administration (Part 1)
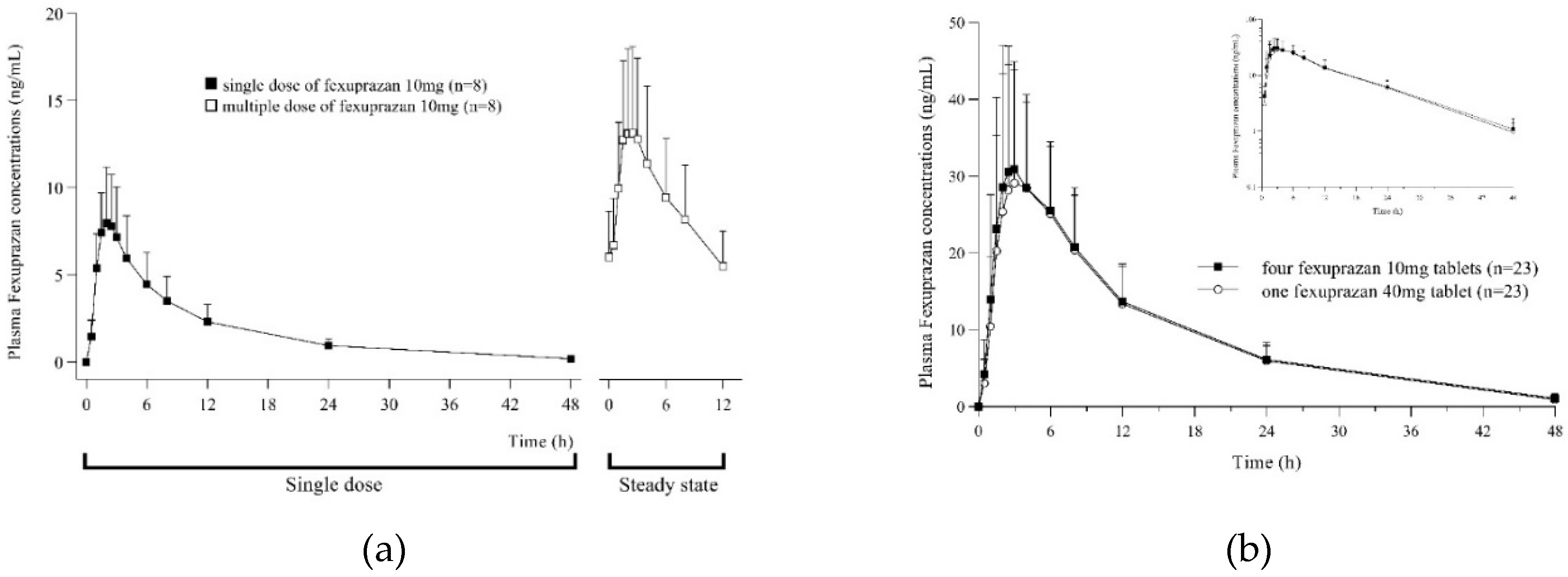
The PK parameters of single and multiple doses of fexuprazan are summarized in
Table 1. In period 1, after a single dose of fexuprazan 10 mg once daily, the plasma concentration of fexuprazan peaked at 2.25 h, and the mean maximum plasma concentration (C
max) and AUC
0-12h were 8.26 ng/mL and 53.47 h∙ng/mL, respectively. The concentration of fexuprazan in the plasma showed rapid absorption and then declined in a polyphasic manner (
Figure 1A).
Table 1.
Comparison of pharmacokinetic parameters of fexuprazan.
Table 1.
Comparison of pharmacokinetic parameters of fexuprazan.
| Part 1: Single dose vs. Multiple doses |
|---|
| Parameter |
Single dose1) (n = 8) |
Multiple dose1) (n = 8) |
| Cmax or Cmax,ss (ng/mL) |
8.26 ± 2.96 |
13.85 ± 4.96 |
| Cmin,ss (ng/mL) |
. |
5.36 ± 2.07 |
| Tmax or Tmax,ss (h) |
2.25 (1.50–3.00) |
2.00 (1.50–3.00) |
| AUC0-12h or AUC0-12h,ss (h∙ng/mL) |
53.47 ± 20.83 |
109.73 ± 40.52 |
| AUClast (h∙ng/mL) |
84.91 ± 36.82 |
. |
| accumulation ratio |
. |
2.11 |
| Part 2: four 10mg tablets vs. one 40mg tablet |
| Parameter |
4 × fexuprazan 10 mg2)
(n = 23) |
1 × fexuprazan 40 mg2)
(n = 23) |
| Cmax (ng/mL) |
34.62 ± 15.44 |
33.87 ± 15.04 |
| Tmax (h) |
2.50 (1.50–6.00) |
3.00 (2.00–6.00) |
| AUClast (h∙ng/mL) |
463.19 ± 169.78 |
446.24 ± 152.94 |
| AUCinf (h∙ng/mL) |
479.88 ± 175.31 |
459.82 ± 154.55 |
| Vz/F (L) |
1372.91 ± 697.78 |
1341.60 ± 601.42 |
| t1/2 (h) |
9.91 ± 2.00 |
9.44 ± 1.65 |
| CL/F (L/h) |
94.60 ± 36.74 |
96.81 ± 32.69 |
Figure 1.
Plasma concentration-time curve after administration of a single dose (black square) and multiple doses (white square) of fexuprazan 10mg (A), after administration of four fexuprazan 10mg tablets (T1, black square) and one fexuprazan 40mg tablet (T2, white circle) (B).
Figure 1.
Plasma concentration-time curve after administration of a single dose (black square) and multiple doses (white square) of fexuprazan 10mg (A), after administration of four fexuprazan 10mg tablets (T1, black square) and one fexuprazan 40mg tablet (T2, white circle) (B).
In period 2, following multiple doses of fexuprazan 10 mg twice daily, the plasma fexuprazan concentration peaked at 2 h and mean Cmax,ss, AUC0-12h,ss, and Cmin,ss were 13.85 ng/mL, 109.73 h∙ng/mL, and 5.36 ng/mL, respectively. Following repeated administration of fexuprazan 10 mg twice daily, their plasma concentrations at 7 d 12 h, 8 d 0 h, and 8 d 12 h were compared to the slope (β1=0) using a regression equation to determine whether a steady state had been reached. At this point, the concentration gradient (90% CI) was −0.0004 (−0.0168–0.0160), indicating that a steady state was reached. Multiple doses accumulated twice as much as a single dose. The calculated mean accumulation ratio is 2.11. Through multiple doses of fexuprazan 10 mg twice daily, a steady state was achieved, and rapid absorption was detected up to the peak, followed by a rapid decline in the plasma concentration–time curves for up to 12 h.
2.3. PKs of single dose of four fexuprazan 10 mg tablets and one fexuprazan 40 mg tablet (Part 2)
The PK parameters of fexuprazan after a single dose of four fexuprazan 10 mg (T1) tablets and one fexuprazan 40 mg (T2) tablet are summarized in
Table 1. An overlap was observed in the mean plasma concentration–time curves between T1 and T2 (
Figure 1B). The rapid absorption of T1 and T2 was detected for up to 2.50 and 3.00 h, respectively. After reaching C
max (T1, 34.62 ng/mL; T2, 33.87 ng/mL), the plasma concentration–time curves exhibited a rapid decline up to 12 h, followed by a slow decline for up to 48 h, indicating similar half-life for T1 and T2 (T1, 9.91 h; T2, 9.44 h). The mean AUC
last and AUC
inf values for T1 were 463.19 and 479.88 h∙ng/mL, respectively, and those for T2 were 446.24 and 459.82 h∙ng/mL, respectively. When administered with T1 and T2, the primary PK parameters, C
max and AUC
last, showed comparability, and point estimates and 90% CIs of the geometric mean ratios (GMR = T1/T2) of C
max and AUC
last were 1.0290 (0.9352–1.1321) and 1.0290 (0.9476–1.1174), respectively (
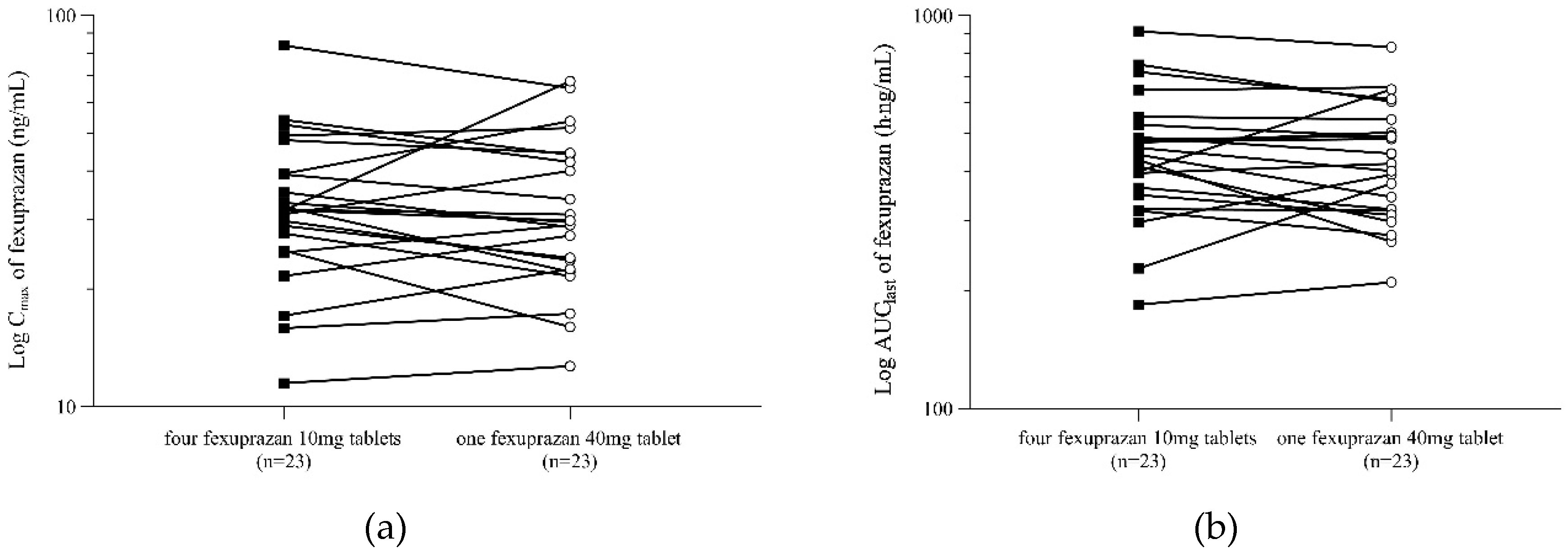
Table 2). The 90% CIs of the analyzed PK parameters met the bioequivalence standard of 0.80 to 1.25. The intra-subject coefficient of variance (CV, %) calculated using Equation 1 was <19% for both C
max and AUC
last. There was no noticeable within-subject difference between C
max and AUC
last for the reference and test formulations (
Figure 2).
Figure 2.
Subject profiles of Cmax (A) and AUClast (B) after administration of four fexuprazan 10mg tablets (T1, black square) and one fexuprazan 40mg tablet (T2, white circle).
Figure 2.
Subject profiles of Cmax (A) and AUClast (B) after administration of four fexuprazan 10mg tablets (T1, black square) and one fexuprazan 40mg tablet (T2, white circle).
2.4. Safety analysis
In Part 1, one subject experienced two treatment-emergent adverse events (TEAEs), both headaches. One TEAE occurred with a single fexuprazan 10 mg dose, whereas the other occurred with multiple fexuprazan 10 mg doses. Two TEAEs were assessed as adverse drug reactions (ADRs) and were mild and resolved over time without any action.
In Part 2, five subjects experienced eight TEAEs; three TEAEs were reported by two subjects in T1, and five TEAEs were reported by four subjects in T2. The details of the TEAEs are summarized in
Table 3. According to safety analysis, no significant differences in TEAE incidence between the two treatment groups were observed (P=0.3827). Seven TEAEs (T1, three TEAEs reported by two subjects; T2, four TEAEs reported by three subjects) reported by four subjects were assessed as ADRs. The reported ADRs included abdominal pain (two TEAEs in two subjects), diarrhea (one TEAE in one subject), hematochezia (one TEAE in one subject), nausea (one TEAE in one subject), headache (one TEAE in one subject). All TEAEs were mild and resolved spontaneously within a few hours or days. No TEAE leading to IP withdrawal, serious adverse events, or death were observed. No clinically significant findings were reported in terms of physical examination, vital signs, and 12-lead electrocardiogram (ECG). Safety data, including TEAEs, laboratory tests, vital signs, and ECG, confirmed that T1 was as safe and tolerable as T2 in healthy adults.
Table 2.
Bioequivalence comparison between four fexuprazan 10 mg tablets and one fexuprazan 40 mg tablet in Part 2.
Table 2.
Bioequivalence comparison between four fexuprazan 10 mg tablets and one fexuprazan 40 mg tablet in Part 2.
Pharmacokinetic
parameters
|
No. |
Geometric LS mean |
Geometric LS mean ratio (T1/T2) |
IntraCV(%) |
| T11)
|
T21)
|
Point Estimate |
90 % CI |
| Cmax (ng/mL) |
23 |
31.46 |
30.57 |
1.0290 |
0.9352 - 1.1321 |
19.0 |
| AUClast (h∙ng/mL) |
23 |
431.30 |
419.15 |
1.0290 |
0.9476 - 1.1174 |
16.3 |
Table 3.
Treatment-emergent adverse events following oral administration of four fexuprazan 10 mg tablets or one fexuprazan 40 mg tablet in Part 2.
Table 3.
Treatment-emergent adverse events following oral administration of four fexuprazan 10 mg tablets or one fexuprazan 40 mg tablet in Part 2.
| Adverse events |
4 × fexuprazan 10 mg1) (n=24) |
1 × fexuprazan 40 mg1) (n=24) |
Total (n=24) |
| Subjects with TEAEs |
2(8.3)[3] |
4(16.7)[5] |
5(20.8)[8] |
| Abdominal pain |
1(4.2)[1] |
1(4.2)[1] |
2(8.3)[2] |
| Diarrhoea |
1(4.2)[1] |
1(4.2)[1] |
1(4.2)[2] |
| Haematochezia |
1(4.2)[1] |
. |
1(4.2)[1] |
| Nausea |
. |
1(4.2)[1] |
1(4.2)[1] |
| Oedema peripheral |
. |
1(4.2)[1] |
1(4.2)[1] |
| Headache |
1(12.5)[1] |
1(4.2)[1] |
1(4.2)[2] |
3. Discussion
We successfully conducted a study comprising two parts according to the objectives of our research. In Part 1, the safety and PK profiles after single and multiple administrations of fexuprazan 10 mg were evaluated in eight subjects. After a single administration, PKs were evaluated for 3 d, with twice-daily administration following a 4-day washout period. Considering the observed terminal half-life of approximately 9 h after the single administration, the PKs after multiple administrations were assessed under steady-state conditions. The observed AUC
0-12,ss after administering fexuprazan 10 mg at 12 h intervals was 109.73 h∙ng/mL in the present study, corresponding to half of the AUC
0-24,ss (223.7 h∙ng/mL) after once-daily administration of fexuprazan 20 mg in the first-in-human study [
20]. This suggests that administering fexuprazan 10 mg twice daily and fexuprazan 20 mg once daily result in similar exposure over 24 h. After multiple fexuprazan dose administrations in the first-in-human study, a significant correlation was observed between gastric pH parameters and a dosage range of 20–160 mg [
20]. Thus, comparable values of AUC
0-24,ss after administering fexuprazan 10 mg twice daily and fexuprazan 20 mg once daily suggest that the gastric pH parameters, including the percentage of time that the gastric pH was ≥ 4.0, are also expected to be similar. Our findings support similar therapeutic effects observed in Phase 3 clinical trial conducted on patients with acute and chronic gastritis. The results indicate that both the once-daily dose of 20 mg and the twice-daily dose of 10 mg exhibited superior efficacy compared to the placebo [
17].
Meanwhile, in the subgroup analyses, regardless of
Helicobacter pylori infection, the group receiving fexuprazan 10 mg twice daily showed a significantly higher erosion improvement rate than the placebo group [
17]. In patients with
H. pylori infection, the group receiving a twice-daily dose of fexuprazan 10 mg showed a significantly higher erosion improvement rate than that in patients without
H. pylori infection (patients with vs. without
H. pylori infection: 81.8% vs. 61.3%). In addition, the twice-daily fexuprazan 10 mg group showed significantly higher erosion improvement rates in patients with chronic gastritis (66.7% [10 mg b.i.d.] vs. 43.3% [20 mg q.d.]) [
7]. A comprehensive review of this study and previous clinical trials suggests that fexuprazan can be used for various ARD based on comparable exposure and efficacy, not only with a once-daily dose of 20 mg but also with a twice-daily dosage regimen.
Part 2 was conducted to compare the PK and safety profiles of the two formulations containing fexuprazan 40 mg. The test formulation (four tablets of fexuprazan 10 mg) had PK characteristics bioequivalent to those of the reference formulation (one tablet of fexuprazan 40 mg). This conclusion was supported by the finding that the C
max and AUC
last GMRs (90% CI) were within the conventional bioequivalence criteria following the administration of each formulation. In addition, the mean plasma concentration–time profiles of the two formulations were superimposable from pre-dosing to 48 hours after dosing (
Figure 1B). Moreover, the safety profiles of the two formulations did not differ (
Table 3). Finally, these results indicate that the test formulation of fexuprazan can be used as an alternative to the reference formulation without significant differences in systemic exposure and safety. The sample size and design of this study were appropriate for evaluating the bioequivalence and safety profiles of the two drugs. Based on this study regarding the intra-subject variability of C
max of fexuprazan 40 mg (19%), the minimum sample size was 18 subjects. Twenty-four subjects were enrolled, and 23 completed the study. The number of subjects was sufficient to minimize β-errors (type II), and the randomization of the study groups was sufficiently balanced to avoid bias associated with sequence allocation. The PK sampling time points were well established to observe the T
max and systemic exposure, which was supported by the fact that the AUC
extra (%) was < 5% in the test and reference formulations.
Fexuprazan has been approved for the treatment of various conditions including GERD, and erosive gastritis. In addition, among the P-CAB class of drugs, fexuprazan is the only drug approved in South Korea for gastritis. It has a longer half-life than other P-CABs, such as vonoprazan (6.95 h) and tegoprazan (3.65–5.39 h) [
21]. In preclinical studies, gastric acid secretion was similar to or greater than that of drugs of the same class [
20]. Therefore, efforts are being made to develop different formulations and strengths of fexuprazan to enhance clinical practice, expand indications, and improve medication convenience. The 10 mg formulation used in this study is a newly developed formulation that differs from the formulation used in the first-in-human study [
20], which was changed from a white, round film-coated tablet weighing 156 mg to an orange, oblong film-coated tablet weighing 157.5 mg (
Table S1). Oblong-shaped tablets are easier to swallow and have faster transit times in the esophagus than round-shaped tablets of the same weight [
19]. Considering that chronic ARDs can cause frequent irritation to the esophagus, leading to complications such as dysphagia [
22,
23], shape-changed formulations might help improve medication compliance in patients with gastritis.
This study had several limitations. First, PKs, safety, and tolerability were evaluated in relatively fewer healthy volunteers. Bioequivalent drug exposure and pharmacokinetic–pharmacodynamic (PKPD) relationships suggest a favorable study, effectiveness, and better compliance in a large number of patients with gastritis. Second, this study was conducted only with healthy Korean subjects. However, Hwang et al. showed that PK profiles, PKPD relationships, gastric acid suppression, and safety profiles were similar among Korean, Caucasian, and Japanese subjects after single and multiple dose administrations of fexuprazan [
24].
4. Materials and Methods
4.1. Subjects and study design
This study was conducted at the Global Clinical Trials Center of CHA Bundang Medical Center, CHA University, Seongnam, Republic of Korea, with strict adherence to the key ethical principles outlined in the Declaration of Helsinki, Good Clinical Practice Guidelines of the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use, and local laws and regulations. The study protocol was reviewed and approved by the MFDS and Institutional Review Board (IRB) of CHA University (IRB no. CHAMC 2021-06-021). Furthermore, the study has been registered and can be found on ClinicalTrials.gov (
https://clinicaltrials.gov) under the identifier NCT05149274.
Before initiating the clinical trial, the investigators provided all subjects with the study information and relevant details. Screening procedures were conducted exclusively for individuals who voluntarily consented to participate in the clinical trial. The inclusion criteria were healthy adults aged 19–45 years, assessed for eligibility based on medical history, physical examination, vital signs, clinical laboratory tests, and a 12-lead ECG. The exclusion criteria included individuals with a clinically significant medical history, those who had participated in another clinical trial within six months preceding the screening, and those taking prescribed medications that could not be temporarily discontinued for at least two weeks before the screening.
This study included two parts: Part 1 and 2. Part 1 followed an open-label, single- or multiple-dose, 1-sequence, and 2-period design and aimed to evaluate the PKs of a single-dose and multiple-dose oral administration of fexuprazan 10 mg tablets in healthy subjects. During Period 1, the subjects in Part 1 received a single dose of fexuprazan 10 mg (T0), and blood samples were collected at various time points, including pre-dosing (0 h) and post-dosing (0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, and 48 h). In Period 2, the subjects received multiple doses of fexuprazan 10 mg twice daily for 3 d (T0’), and blood sampling was conducted at the same time points as in Period 1 up to 12 h in the steady state. Part 1 was conducted with eight subjects to explore their PK characteristics after single- and multiple-dose fexuprazan 10 mg administration.
Part 2 employed a randomized, open-label, single-dose, 2-sequence, 2-period, crossover design and aimed to confirm the bioequivalence between a single dose of four fexuprazan 10 mg tablets (T1) and a single dose of one fexuprazan 40 mg tablet (T2). The subjects were randomly assigned to Sequence A or B. Sequence A subjects received T1 in Period 1 and T2 in Period 2. Sequence B subjects received T2 in Period 1 and T1 in Period 2. Blood samples were collected at pre-dosing (0 h) and post-dosing (0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, and 48 h) during each period. According to a previous study on fexuprazan, the intra-subject CV for C
max and area under the concentration–time curve from zero to the time of the last quantifiable concentration (AUC
last) was approximately 19.8% and 16.8%, respectively [
12]. To assume an actual (four fexuprazan 10 mg tablets)/(one fexuprazan 40 mg tablet) ratio of 1.1 and an equivalence range of 0.8–1.25, a minimum of 18 subjects would be required to achieve a statistical power of at least 80% at a significance level of 0.05. In addition, considering a dropout rate of 25%, the target sample size was set at 24 subjects.
The fasted study subjects were administered IPs (either fexuprazan 10 mg or 40 mg) with 150 mL of water. The IP used in this study was developed by Daewoong Pharmaceutical Co., Ltd. Meanwhile, the blood samples were promptly collected in heparinized tubes for each blood sampling time point, and the plasma was separated through centrifugation at 1,900 × g for 10 min at 4 °C. The plasma aliquots were then stored at −70 °C until further analysis.
4.2. Determination of plasma fexuprazan concentration
Human plasma was analyzed using validated liquid chromatography–tandem mass spectrometry (LC-MS/MS). The chromatographic separation of fexuprazan and internal standard (IS) was achieved using an ACE C18, 2.1×50 mm, 3 μm column (Aberdeen, Scotland) under isocratic conditions with a flow rate of 0.5 mL/min. Fexuprazan and IS were detected using an AB SCIEX API 5000 mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA, USA) using multiple reaction monitoring in the positive electrospray ionization mode. Mass transitions for fexuprazan and IS were m/z 411.3 → 380.0 and m/z 414.3 → 380.2, respectively, and the method was validated over a concentration range of 0.1 to 100 ng/mL. The lower limit of quantitation was 0.1 ng/mL. The within-run precision and accuracy were 1.0 to 5.7% and −7.3 to 5.0%, respectively, and the between-run precision and accuracy were 1.0 to 6.4% and −1.0 to 1.0%, respectively.
4.3. PK assessment
Non-compartmental analysis (NCA) was performed using the Phoenix WinNonlin software version 8.3 (Certara Co., Princeton, NJ, USA) to determine the PK parameters of fexuprazan. In Part 1, the PK parameters were Cmax, Cmax at steady-state (Cmax,ss), the minimum observed concentration at steady-state during the dosing interval (Cmin,ss), time to reach Cmax (Tmax), time to reach Cmax,ss (Tmax,ss), AUC from 0 to 12 h (AUC0-12h), AUC0-12h at steady-state (AUC0-12h,ss), and AUClast. The accumulation ratio based on AUC0-12h was calculated as AUC0-12,ss multiple dose/AUC0-12 after a single dose.
In Part 2, the PK parameters included Cmax, Tmax, AUClast, the AUC from zero to infinity (AUCinf), apparent volume of distribution (Vz/F), elimination half-life (t1/2), and apparent clearance (CL/F); of which, Cmax, Cmax,ss, Tmax, Tmax,ss, and Cmin,ss were the actual observed values. AUC0-12h, AUC0-12h,ss, AUClast, and AUCinf were calculated using the linear trapezoidal rule. The elimination rate constant (ke) was estimated by linear regression of the terminal declining phase in the logarithmic plasma concentration–time profile, and t1/2 was calculated from the ratio of the natural logarithm of 2 to ke. AUCinf was determined by adding AUClast to the extrapolated area beyond the last observed concentration (Clast), AUClast + Clast / ke. CL/F was calculated as Dose/AUCinf, and Vz/F was calculated as (CL/F)/ke.
4.4. Safety and tolerability assessment
Safety was evaluated based on TEAEs, physical examinations, vital signs, 12-lead ECGs, and clinical laboratory tests. TEAEs were either spontaneously reported by subjects or identified through data collected during scheduled interviews throughout the study period. All TEAEs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) version 24.0 and summarized by treatment, severity, and relationships with IPs.
4.5. Statistical analysis
Descriptive statistics were used to summarize baseline demographics, such as age, weight, height, and BMI. The PK parameters and safety profiles were evaluated using descriptive statistics. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). In Part 2, the primary PK endpoints (Cmax and AUClast) were log-transformed to develop a mixed-effects model with treatment effects as fixed effects and subject effects as random effects. GMRs with 90% CIs of the primary PK parameters between the two treatment groups (T1 vs. T2) were estimated to evaluate bioequivalence. Bioequivalence for T1 vs. T2 was determined by assessing whether the 90% CI of the GMRs of the primary PK parameters were within the bioequivalence range of 0.8 to 1.25. Numerical data between the two treatments were compared using an independent t-test or the Wilcoxon rank-sum test. Categorical data were compared using the chi-square or Fisher’s exact test.
5. Conclusions
The new fexuprazan 10 mg formulation demonstrated favorable safety and tolerability when administered in single or multiple doses and exhibited bioequivalence compared to fexuprazan 40 mg. Therefore, the new fexuprazan 10 mg formulation can be effectively used in developing dosing regimens for various gastric ARDs, including acute and chronic gastritis.
Supplementary Materials
Table S1: Comparison between a newly developed fexuprazan 10mg and the formulation used in first in-human study.
Author Contributions
Conceptualization, W.S., A.-Y.Y. and A.K.; Methodology, W.S. and A.K.; Validation, H.P, H.L and A.K.; Formal analysis, W.S. and A.-Y.Y.; Investigation, W.S., A.-Y.Y., H.Y., and A.K.; Resources, A.K.; Data curation, W.S. and A.-Y.Y.; Writing – Original Draft Preparation, W.S. and A.-Y.Y.; Writing – Review & Editing, W.S., A.-Y.Y., H.P, H.L, H.Y., and A.K.; Visualization, W.S. and A.-Y.Y.; Supervision, H.P, H.L; Project administration, A.K.; Funding Acquisition, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Daewoong Pharmaceutical Co., Ltd.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice (ICH-GCP), as well as the Korean Good Clinical Practice guidelines, and approved by the Institutional Review Board of CHA University, Bundang Medical Center located in Seongnam, Korea (IRB No. CHAMC 2021-06-021).
Informed Consent Statement
Informed consent was obtained from all subjects previously to start the studies.
Data Availability Statement
The data are not publicly available due to confidentiality reasons.
Acknowledgments
The authors would like to express their gratitude to all the patients and staff who participated in the study.
Conflicts of Interest
Hyung Park and Hyejung Lee are employees of Daewoong Pharmaceutical Co., Ltd. The other authors declare that they have no conflict of interest.
References
- Welage LS, Berardi RR. Evaluation of omeprazole, lansoprazole, pantoprazole, and rabeprazole in the treatment of acid-related diseases. J Am Pharm Assoc (Wash). 2000;40(1):52-62; quiz 121-3. [CrossRef]
- Baldwin CM, Keam SJ. Rabeprazole: a review of its use in the management of gastric acid-related diseases in adults. Drugs. 2009;69(10):1373-401. [CrossRef]
- Shin JM, Vagin O, Munson K, Kidd M, Modlin IM, Sachs G. Molecular mechanisms in therapy of acid-related diseases. Cell Mol Life Sci. 2008;65(2):264-81. [CrossRef]
- Oshima T, Miwa H. Potent Potassium-competitive Acid Blockers: A New Era for the Treatment of Acid-related Diseases. J Neurogastroenterol Motil. 2018;24(3):334-44. [CrossRef]
- Kim MK, Ko BJ, Kim EY, Han BD, Cho KH. Fast Eating Speed Increases the Risk of Endoscopic Erosive Gastritis in Korean Adults. Korean J Fam Med. 2015;36(6):300-4. [CrossRef]
- Azer SA, Akhondi H. Gastritis. StatPearls. Treasure Island (FL)2023.
- den Hollander WJ, Kuipers EJ. Current pharmacotherapy options for gastritis. Expert Opin Pharmacother. 2012;13(18):2625-36. [CrossRef]
- Megha R, Farooq U, Lopez PP. Stress-Induced Gastritis. StatPearls. Treasure Island (FL)2023.
- Hanafy AS, Seleem WM. Refractory Helicobacter pylori gastritis: The hidden predictors of resistance. J Glob Antimicrob Resist. 2019;19:194-200. [CrossRef]
- Kashiwagi H. Ulcers and gastritis. Endoscopy. 2005;37(2):110-5. [CrossRef]
- Sachs G, Shin JM, Hunt R. Novel approaches to inhibition of gastric acid secretion. Curr Gastroenterol Rep. 2010;12(6):437-47. [CrossRef]
- Yang AY, Yoo H, Shin W, Lee YS, Lee H, Kim SE, et al. Size-reduced fexuprazan 20 mg demonstrated the optimal bioavailability and bioequivalence with the reference formulation. Transl Clin Pharmacol. 2023;31(1):40-8. [CrossRef]
- Katzka DA, Kahrilas PJ. Potassium-Competitive Acid Blocker Suppression of Gastric Acid in Erosive Esophagitis: Is Stronger and Longer Better? Gastroenterology. 2023;164(1):14-5. [CrossRef]
- 2022 Drug Approval Report: Ministry of Food and Drug Safety; 2023 [Available from: https://www.mfds.go.kr/eng/brd/m_19/view.do?seq=70438&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1.
- 2021 Drug Approval Report: Ministry of Food and Drug Safety; 2022 [Available from: https://www.mfds.go.kr/eng/brd/m_19/down.do?brd_id=eng0004&seq=70437&data_tp=A&file_seq=1.
- Lee KN, Lee OY, Chun HJ, Kim JI, Kim SK, Lee SW, et al. Randomized controlled trial to evaluate the efficacy and safety of fexuprazan compared with esomeprazole in erosive esophagitis. World J Gastroenterol. 2022;28(44):6294-309. [CrossRef]
- Kim GH, Choi MG, Kim JI, Lee ST, Chun HJ, Lee KL, et al. Efficacy and Safety of Fexuprazan in Patients with Acute or Chronic Gastritis. Gut Liver. 2023. [CrossRef]
- Daewoong Pharmaceutical’s ’Fexuclue’: First Domestic P-CAB Agent to Obtain Indication for Gastritis Korea Biomedical Review2022 [Available from: http://www.docdocdoc.co.kr/news/articleView.html?idxno=2026314.
- Size, shape, and other physical attributes of generic tablets and capsules guidance for industry: US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research; 2015 [Available from: https://www.fda.gov/files/drugs/published/Size--Shape--and-Other-Physical-Attributes-of-Generic-Tablets-and-Capsules.pdf?next=/answers/six-tips-to-avoid-getting-pill-stuck-in-your-throat/avoid-pill-getting-stuck-in-throat/.
- Sunwoo J, Oh J, Moon SJ, Ji SC, Lee SH, Yu KS, et al. Safety, tolerability, pharmacodynamics and pharmacokinetics of DWP14012, a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2018;48(2):206-18. [CrossRef]
- Shibli F, Kitayama Y, Fass R. Novel Therapies for Gastroesophageal Reflux Disease: Beyond Proton Pump Inhibitors. Curr Gastroenterol Rep. 2020;22(4):16. [CrossRef]
- Vakil NB, Traxler B, Levine D. Dysphagia in patients with erosive esophagitis: prevalence, severity, and response to proton pump inhibitor treatment. Clin Gastroenterol Hepatol. 2004;2(8):665-8. [CrossRef]
- Chiocca JC, Olmos JA, Salis GB, Soifer LO, Higa R, Marcolongo M, et al. Prevalence, clinical spectrum and atypical symptoms of gastro-oesophageal reflux in Argentina: a nationwide population-based study. Aliment Pharmacol Ther. 2005;22(4):331-42. [CrossRef]
- Hwang JG, Jeon I, Park SA, Lee A, Yu KS, Jang IJ, et al. Pharmacodynamics and pharmacokinetics of DWP14012 (fexuprazan) in healthy subjects with different ethnicities. Aliment Pharmacol Ther. 2020;52(11-12):1648-57.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).