1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are organic compounds formed by the incomplete combustion or pyrolysis of organic substances, including tobacco and fossil fuels, and are composed of two or more fused benzene rings. Various types of PAHs exist in particulate matter (PM), a significant air pollutant. Benzo[a]pyrene (BaP) is a representative PAH, and it has been reported that environmental or occupational exposure to BaP showed adverse effects on neurobehavioral function in adults and on neurodevelopment in children [1, 2]. Moreover, the International Agency for Research on Cancer (IARC) classifies BaP as a human carcinogen [
3]. BaP absorbed in the body induces cytochrome P450 1A1 activation and is metabolized to benzo[a]pyrene-7,8-diol-9,10-epoxide (BPDE) [
4]. Excessive reactive oxygen species (ROS) are generated during this metabolic process [
5].
Pyrene, one of the PAHs, is metabolized into 1-hydroxypyrene (1-OHP) by the cytochrome P450 enzyme and excreted into the urine. The 1-OHP urinary concentration is frequently used as an indicator of exposure levels to PAHs.
1-Nitropyrene (1-NP), one of the PAHs, is specifically produced during diesel fuel combustion and has been reported to cause genetic mutations and chromosomal aberration. The IARC classifies 1-NP as a Group 2A chemical [
3]. Exposure to 1-NP increases oxidative stress, inflammation, and endothelial dysfunction, thereby resulting in an increased risk of cardiovascular disease. To evaluate the exposure level to 1-NP, the concentration of 1-NP metabolites, including 6-hydroxy-1-nitropyrene (6-OHNP), 1-aminopyrene (1-AP), and N-acetyl-1-aminopyrene (1-NAAP), in the urine is measured.
BaP and 1-NP are believed to cause oxidative stress and harmful effects on the human body through these mechanisms; however, few studies have confirmed that ROS increases in the human body owing to BaP or 1-NP exposure.
PM concentration in Korea’s atmosphere is higher than those of in developed countries, particularly in winter. Cheongju, a city located in the center of Korea, has a large industrial complex and three waste incinerators; the PM brought across the border by the northwest wind in winter makes this city an area with a high PM concentration in Korea.
This study aimed to evaluate the relationship between exposure to PAHs, such as BaP and 1-NP, in the atmosphere and oxidative stress levels in the body of individuals living in Cheongju.
2. Materials and Methods
2.1. Study Participants
This study included 44 adults living around the industrial complex in Cheongju, a waste incinerator, or in suburban areas. Data on demographic factors, smoking habits, and daytime activity were collected using a questionnaire. They all provided the first urine in the morning of the day following atmospheric sampling. Participants received a thorough explanation of the study and provided written consent.
2.2. BaP and 1-NP Atmospheric Air Concentration Measurement
Atmospheric air was sampled using a personal air sampler (Apex Standard, SN0376420 Casella CEL, Bedford, UK) once in summer and once in winter. A 35-mm PTFE filter with a 2-μm pore size was attached to a personal air sampler and sampled for more than 24 h at a rate of approximately 3 L/min.
The filter was placed in a flask, 2 mL of dichloromethane was added, vortex mixed, and ultrasonically extracted. The extract was completely dried and re-dissolved in acetonitrile. Aliquots of the solution were subsequently injected into a two-dimensional high-performance liquid chromatography (HPLC) system with a fluorescence detector (FD) for the quantification of BaP and 1-NP, respectively [
6]. The injected sample was eluted through a clean-up column (Cosmosil, 5NPE, 150 × 4.6 mm i.d., 5 μm, Nacalai Tesque, Kyoto, Japan) with its guard column 1 (10 × 4.6 mm i.d.); subsequently, 1-NP was reduced to 1-aminopyrene by using a reduction column (NPpak-RS, 10 × 4.6 mm i.d., Jasco, Tokyo, Japan) at 80°C. The mobile phase for the clean-up and reduction columns was ethanol/acetate buffer (pH, 5.5) (95/5, v/v) at a 0.2-mL/min flow rate. A fraction of 1-AP and unchanged BaP eluted from the reduction column with the mobile phase was mixed with 30-mM ascorbic acid at a 1.6-mL/min flow rate and subsequently trapped on the concentration column (Spheri-5 RP-18, 30 × 4.6 mm i.d., 5 μm, Perkin Elmer, MA, USA). The concentrated fraction was passed through two separation columns (Inertsil ODS-P, 250 × 4.6 mm i.d., 5 μm, GL Sciences, Tokyo, Japan) with their guard column (10 × 4.6 mm i.d.) in tandem. All columns except the reduction column were maintained at 20°C. A programmed gradient elution of the separation columns was performed using 10-mM imidazole buffer (pH, 7.6) as eluent A and acetonitrile as eluent B. Finally, the separated analytes were detected the dual-channel FD. The excitation/emission wavelengths were 260/420 and 254/425 nm for BaP and 1-AP, respectively.
2.3. 8-Hydroxydeoxyguanosine (8-OHdG) Urinary Concentration Measurement
The 8-OHdG urinary concentration was measured using an ELISA kit (New 8-OHdG Check, JaICA, Fukuroi, Japan) according to the manufacturer’s instructions. A brief description of the measurement method is as follows. The urine samples and standards were added to the 8-OHdG–coated microtiter plate. After washing, an enzyme-labeled secondary antibody was added to the plate. Unbound HRP-conjugated secondary antibody was washed out, and the substrate solution was added. After the termination of the reaction, absorbance was read at 450 nm.
2.4. Thiobarbituric Acid Reactive Substance (TBARS) Urinary Concentration Measurement
TBARS were measured at three different wavelengths (fluorescence, λ-ex 530 nm and λ-ex 550 nm; λ-ex 515 nm and λ-ex 553 nm; and absorbance, 532 nm) using a microplate reader [
7]. The TBARS concentration was estimated using the following equation: TBARS level (μM) = −0.282 + 1.830 × (the TBARS level measured at the fluorescence wavelengths of λ-ex 530 nm and λ-em 550 nm, μM) −0.685 × (the TBARS level measured at the fluorescence wavelengths of λ-ex 515 nm and λ-em 553 nm, μM) + 0.035 × (the TBARS level measured at the absorbance wavelength of 532 nm, μM).
2.5. 1-OHP Urinary Concentration Measurement
To 45 mL of urine, 4.5 mL of 1-M sodium acetate buffer (pH, 5.0), 450 μL of β-glucuronidase/aryl sulfatase (type H-2, from Helix pomatia: β-glucuronidase activity, 100,000 units/mL; and sulfatase activity, 7,500 units/mL), and 50 mg of blue rayon were added, and the mixtures were incubated at 37oC for 16 h. Blue rayon was removed, washed with water, and eluted with 2 mL of methanol and 2 mL of methanol + 1% NH3 solution. After evaporating to dry with N2 gas, it was dissolved again in 400-μL methanol. Fifty microliters of this eluent were used for 1-OHP concentration measurement, and the rest of the eluent was used for 6-OHNP and 1-NAAP concentration measurement.
The 1-OHP concentration was measured using an HPLC (LC-20AD, Shimadzu, Kyoto, Japan) with a 250 × 4.6 mm reverse phase column (J'sphere ODS-H80, YMC, Kyoto, Japan) and a fluorescent detector (RF-20A, Shimadzu, Kyoto, Japan). The mobile phase was 65% acetonitrile, and the flow rate was 1 mL/min. The excitation/emission wavelength of the FD was 242 nm/388 nm [
8].
2.6. 6-OHNP and 1-NAAP Urinary Concentration Measurement
Nitroreduction reaction was performed by passing the remaining eluent through the online reduction column (NPpak-RS). 6-Hydroxy-1-aminopyrene concentrations were measured with the same HPLC system with column and mobile phase as those used for the measurement of 1-OHP concentration. The excitation/emission wavelength of the FD was 285 nm/428 nm.
Amide reaction was performed at room temperature for 1 h by adding 200 μL of acetic anhydride to the nitroreduced fraction. Since we used an amidification method of converting 1-AP to 1-NAAP, the 1-NAAP concentration measured in this study is the sum of the 1-AP and 1-NAAP concentrations. 1-NAAP concentration was measured using the same HPLC system with the column and mobile phase as those used for the measurement of 1-OHP concentration. The excitation/emission wavelength of the FD was 273 nm/385 nm [
9].
The urinary concentrations of those substances were corrected by the urinary creatinine concentration.
2.7. Statistical Analysis
Paired T-tests were used to compare summer and winter measurements. Regression analysis was performed to determine the relationship between BaP and 1-NP exposure levels, their urinary metabolite concentrations, and urinary 8-OHdG and TBARS concentrations. A general linear model was used to statistically analyze the effects of BaP or 1-NP exposure on 8-OHdG and TBARS urinary concentrations after controlling for age, sex, smoking habit and the levels of the other exposure markers.
Statistical analysis was performed using SAS OnDemand (SAS Institute, Cary, NC, USA). A p-value of <0.05 was considered statistically significant.
3. Results
The general characteristics of the study participants are shown in
Table 1. The mean age was 63.8 years, and approximately 60% (26/44) of them were females. Current smokers were 16.0%. Residents who were living near the incinerator, those living near the industrial complex, and rural residents were 36.4%, 22.7%, and 40.9%, respectively.
BaP and 1-NP atmospheric air concentrations and TBARS, 8-OHdG, 1-OHP, 6-OHNP, and 1-NAAP urinary concentrations are presented in
Table 2. The concentrations of BaP and 1-NP in the atmospheric air were significantly higher in winter than those in summer. TBARS and 6-OHNP urinary concentrations were significantly different between winter and summer; however, 8-OHdG, 1-OHP and 1-NAAP urinary concentrations were not.
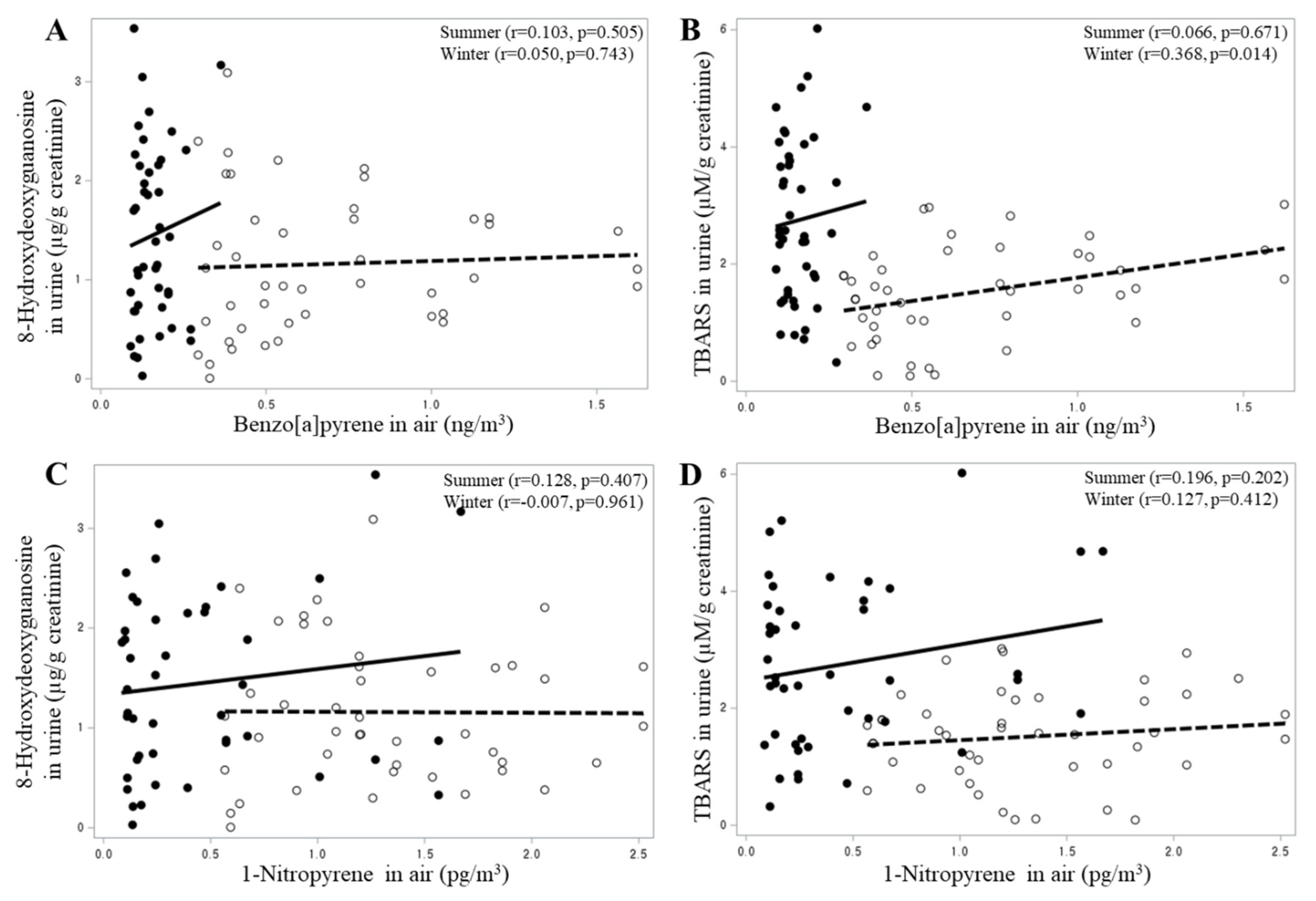
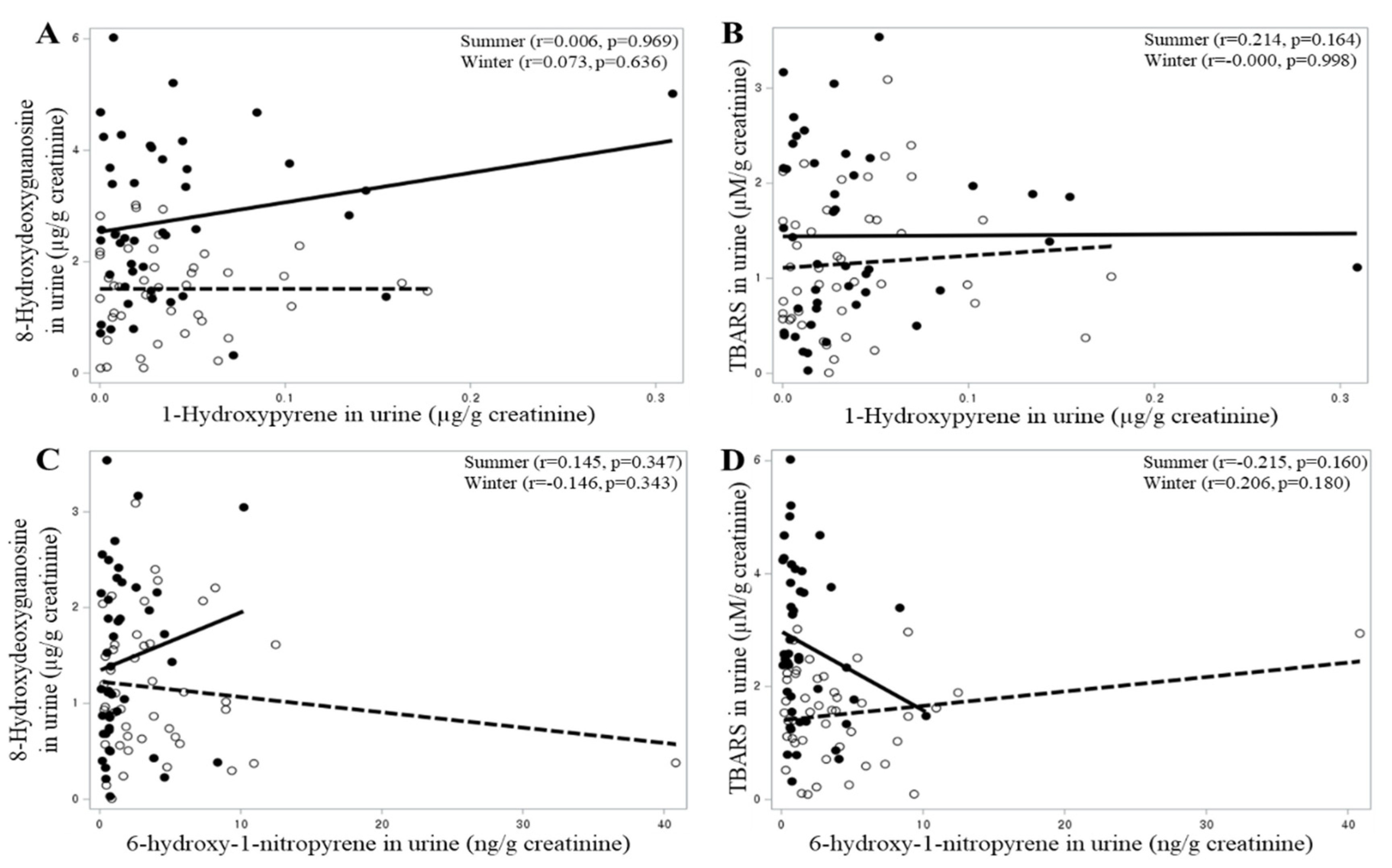
The results of the correlation analysis of PAHs for TBARS and 8-OHdG are shown in
Figure 1 and
Figure 2. BaP was significantly associated with the 8-OHdG urinary concentration in summer. The 1-NP atmospheric air concentration and 1-OHP and 6-OHNP urinary concentrations were not significant determinants for urinary TBARS and 8-OHdG concentrations. The 1-NAAP urinary concentration showed a significant positive association with the 8-OHdG urinary concentration in summer.
The results of the general linear model analysis are shown in
Table 3. Current smoking status was associated with urinary TBARS and 8-OHdG concentrations in summer. The cumulative smoking amount was negatively associated with TBARS urinary concentration in summer. In the multivariate model, BaP was significantly associated with the TBARS urinary concentration in winter and the 8-OHdG urinary concentration in summer. The 1-NP atmospheric air concentration and 1-OHP and 6-OHNP urinary concentrations were not significantly associated with TBARS and 8-OHdG urinary concentrations in any season. The 1-NAAP urinary concentration was positively associated with the 8-OHdG urinary concentration in both seasons.
4. Discussion
The atmospheric air concentration of BaP or 1-NP was higher in winter than that in summer. This is not only because more fossil fuels are burned for heating in winter but also because considerable amounts of PM generated in foreign countries are brought across the border by the northwest wind. Nevertheless, the participants’ TBARS and 8-OHdG urinary concentrations were higher in summer than those in winter, and the TBARS urinary concentration was significantly different. Jacobs et al. (2020) reported that under high temperature environments, high temperature and dehydration increase oxidative damage and induce anti-oxidant defense [
10].
BaP exposure was significantly associated with oxidative stress marker concentrations. However, the urinary concentration of 1-OHP, which is widely used as a biomarker for PAH exposure, did not show a significant correlation with TBARS and 8-OHdG urinary concentrations. Since 1-OHP is a pyrene metabolite, which is neither carcinogenic nor mutagenic, the 1-OHP concentration would not show a significant relationship with those oxidative stress markers. We observed no significant correlation between the BaP atmospheric air concentration and 1-OHP urinary concentration of the participants (r = 0.059, p = 0.586). If the urinary concentration of BPDE, a specific exposure marker for BaP, was measured, a significant association was likely to be observed.
The level of exposure to 1-NP and the urinary concentration of 6-OHNP, which is a 1-NP metabolite, did not show a significant correlation with the urinary concentrations of those oxidative stress markers. However, the urinary concentration of 1-NAAP, another 1-NP metabolite, was significantly associated with the 8-OHdG urinary concentration. In the human body, absorbed 1-NP is mostly metabolized to 6-OHNP, 8-OHNP, or 3-OHNP, and <5% is metabolized to 1-AP or 1-NAAP. These results suggest that 8-OHdG is produced when 1-NP is metabolized to 1-AP or 1-NAAP but not when 1-NP is metabolized to 6-OHNP. The 1-NP atmospheric air concentration showed a significant association with the 6-OHNP urinary concentration (r = 0.291, p = 0.006) but not with that of 1-NAAP (r = −0.067, p = 0.534). Additionally, the relationship between 6-OHNP and 1-NAAP urinary concentrations was not significant (r = −0.109, p = 0.312). The proportion of 1-NP metabolized to 1-AP or 1-NAAP in the body is determined by the genetic polymorphism of the metabolic enzyme [
11]. Therefore, in an individual, if the exposure level to 1-NP increases, the urinary 1-AP or 1-NAAP concentration and, accordingly, the 8-OHdG urinary concentration increase; however, in comparison between individuals, a higher exposure level to 1-NP does not necessarily mean higher 1-AP, 1-NAAP, or 8-OHdG urinary concentrations.
While MDA and 8-OHdG are both biomarkers for oxidative stress, they reflect different aspects of oxidative damage. MDA is produced by lipid peroxidation, and high MDA levels are associated with cardiovascular disease, inflammation, and oxidative stress-related diseases. Since 8-OHdG is produced by ROS attacking DNA, 8-OHdG represents the degree of oxidative stress on DNA. Increased 8-OHdG concentrations in the body indicate increased oxidative damage to DNA, which is associated with various conditions, including aging, cancer, cardiovascular disease, and neurodegenerative disorders [
12]. BaP exposure showed a significant positive correlation with not only the 8-OHdG urinary concentration but also the TBARS urinary concentration; however, the 1-NAAP urinary concentration was statistically significant only with the 8-OHdG urinary concentration. These suggest that there may be a slight difference between the adverse health effects that can be caused by BaP exposure and those by 1-NP exposure.
5. Conclusions
Oxidative stress in the body increases in proportion to inhalation exposure to BaP, and more 8-OHdG is produced in the body as the amount of 1-NP, which is metabolized to 1-AP or 1-NAAP, increases.
References
- Niu, Q., Zhang, H., Li, X. and Li, M. (2010): Benzo[a]pyrene-induced neurobehavioral function and neurotransmitter alterations in coke oven workers. Occup. Environ. Med., 67, 444-448. [CrossRef]
- Perera, F.P., Rauh, V., Whyatt, R.M., Tsai, W.Y., Tang, D., Diaz, D., Hoepner, L., Barr, D., Tu, Y.H., Camann, D. and Kinney, P. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect, 2006, 114, 1287-1292. [CrossRef]
- IARC. https://monographs.iarc.who.int/list-of-classifications.
- Michaelson, J.J., Trump, S., Rudzok, S., Gräbsch, C., Madureira, D.J.,Dautel, F., Mai, J., Attinger, S., Schirmer, K., von Bergen, M., Lehmann, I. and Beyer, A. Transcriptional signatures of regulatory and toxic responses to benzo-[a]-pyrene exposure. BMC Genomics, 2011, 12, 502. [CrossRef]
- Yang, F., Yang, H., Ramesh, A., Goodwin, J.S., Okoro, E.U. and Guo, Z. (Overexpression of Catalase Enhances Benzo(a)pyrene Detoxification in Endothelial Microsomes. PLoS One, 2016, 11, e0162561. [CrossRef]
- Boongla, Y, Orakij, W, Nagaoka, Y, Tang, N, Hayakawa, K, Toriba, A. Simultaneous determination of polycyclic aromatic hydrocarbons and their nitro-derivatives in airborne particulates by using two-dimensional high-performance liquid chromatography with on-line reduction and fluorescence detection. Asian J Atmos Environ 2017, 11:283–299. [CrossRef]
- Kil, H.N., Eom, S.Y., Park, J.D., Kawamoto, T., Kim, Y.D., Kim, H. A rapid method for estimating the levels of urinary thiobarbituric Acid reactive substances for environmental epidemiologic survey. Toxicol Res 2014, 30, 7-11. [CrossRef]
- Jongeneelen, F. J., Anzion, R. B. M., Scheepers, P. T. J., Bos, R. P., Henderson, P. TH., Nijenhuis, E. H., Veenstra, S. J., Brouns, R. M. E., Winkes, A. 1-Hydroxypyrene in urine as a biological indicator of exposure to polycyclic aromatic hydrocarbons in several work environments. Ann Occup Hyg 1988, 32, 35-43. [CrossRef]
- Toriba, A., Kitaoka, H., Dills, R.L., Mizukami, S., Tanabe, K., Takeuchi, N., Ueno, M., Kameda, T., Tang, N., Hayakawa, K., Simpson, C.D. Identification and quantification of 1-nitropyrene metabolites in human urine as a proposed biomarker for exposure to diesel exhaust. Chem Res Toxicol 2007, 20, 999-1007. [CrossRef]
- Jacobs, P.J., Oosthuizen, M.K., Mitchell, C., Blount, J.D., Bennett, N.C. Heat and dehydration induced oxidative damage and antioxidant defenses following incubator heat stress and a simulated heat wave in wild caught four-striped field mice Rhabdomys dilectus. PLoS One 2020, 15,e0242279. [CrossRef]
- Yun JK, Ochirpurev B, Eom SY, Toriba A, Kim YD, Kim H. Effects of gene polymorphisms of CYP1A1, CYP1B1, EPHX1, NQO1, and NAT2 on urinary 1-nitropyrene metabolite concentrations. Heliyon, 2022, 8:e10120. [CrossRef]
- Jelic, M.D., Mandic, A.D., Maricic, S.M., Srdjenovic, B.U. Oxidative stress and its role in cancer. J Cancer Res Ther 2021, 17, 22-28. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).