Submitted:
21 July 2023
Posted:
21 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Sample Collection and Analysis
2.3. DNA Extraction
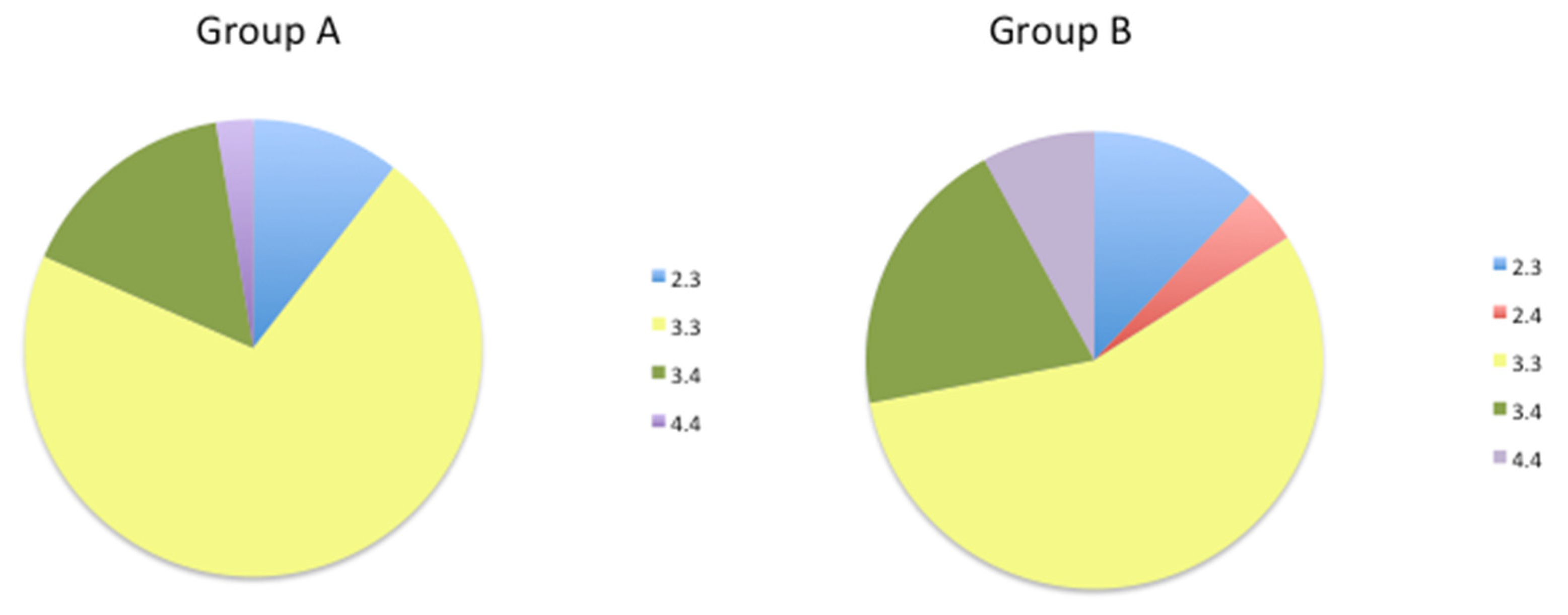
2.4. Quantification of Global DNA Methylation (5mC)
2.5. Genotyping
3. Results
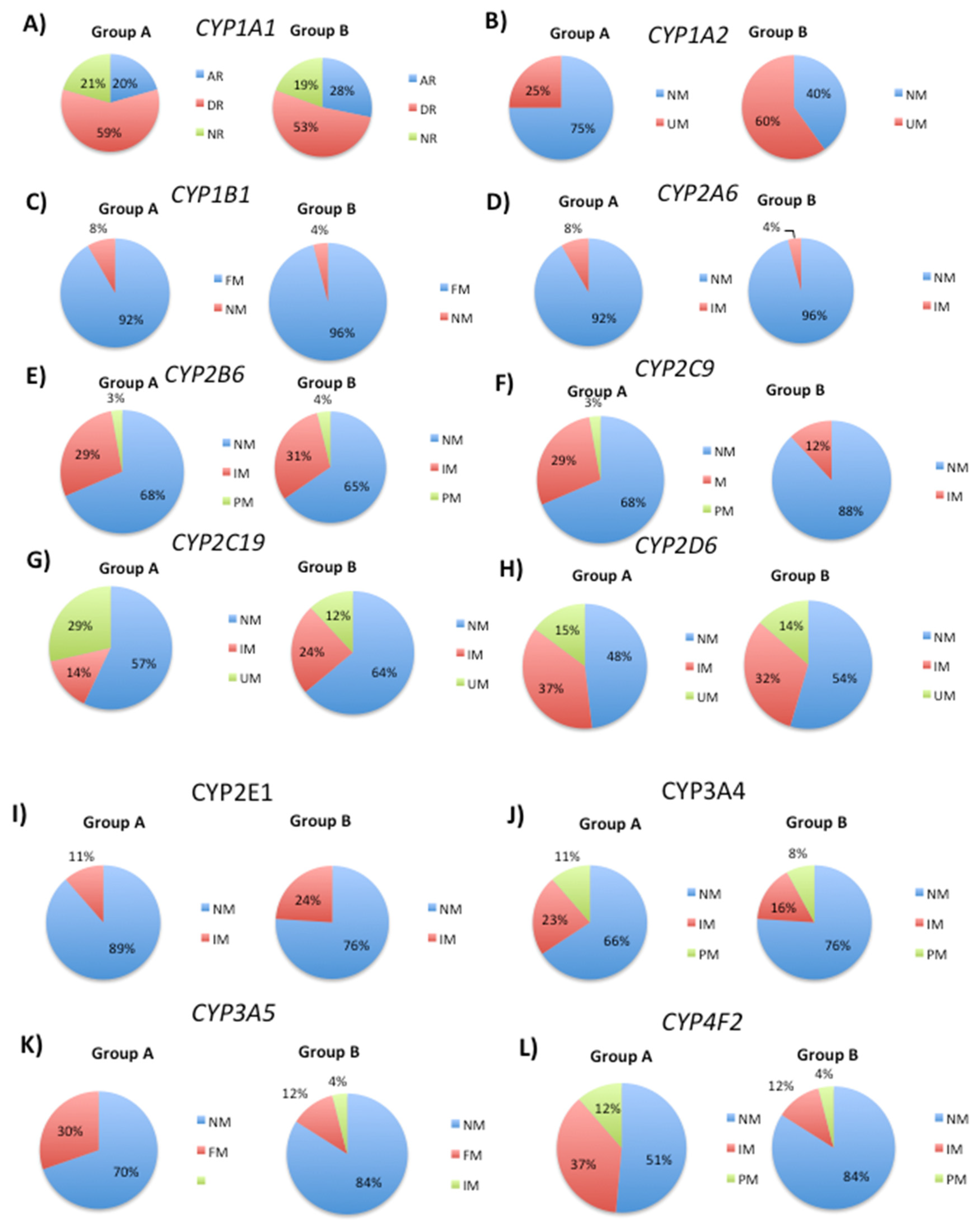
3.1. Genetic Variability in Drug Metabolizing Enzymes Influences 5mC Levels
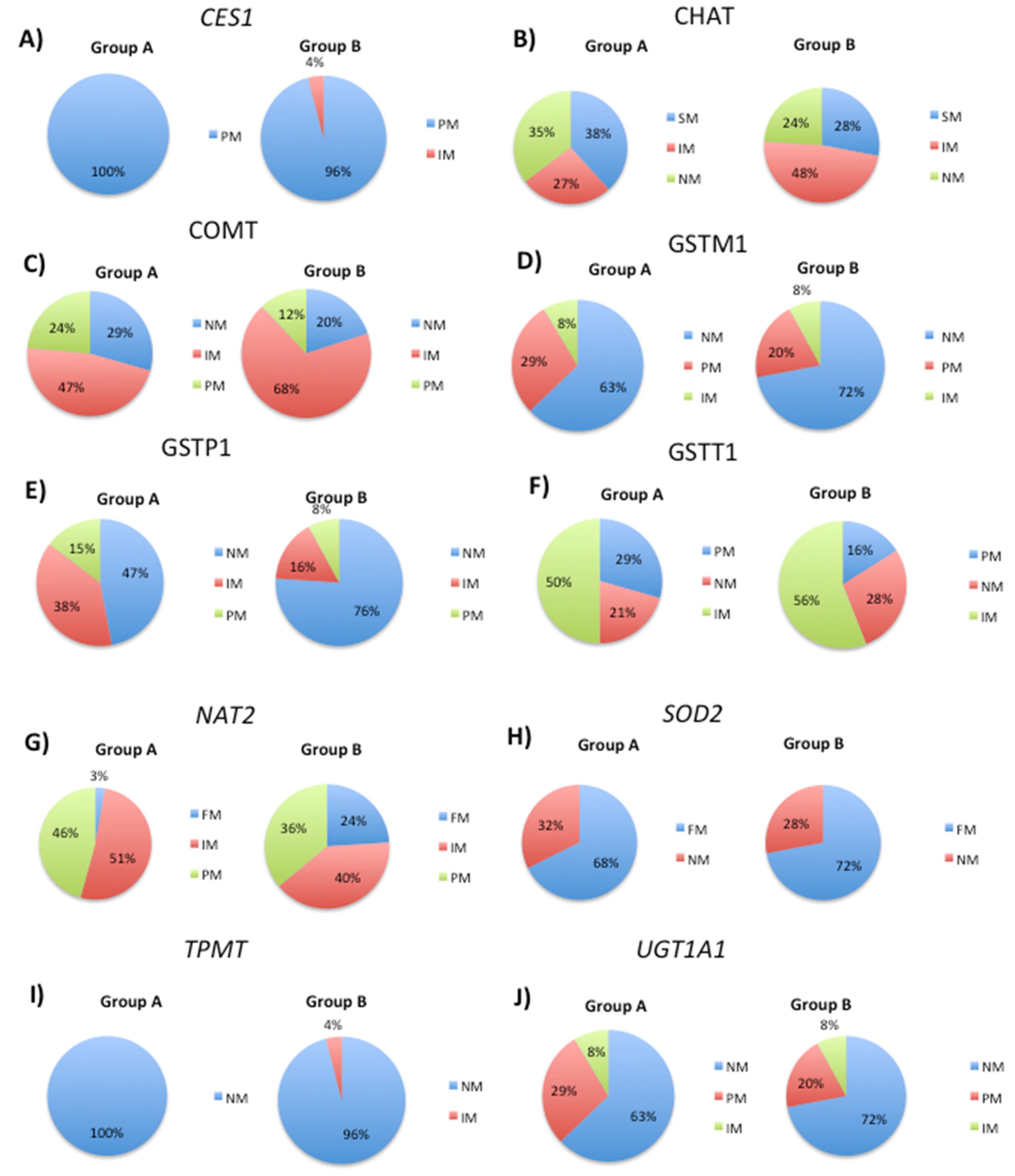
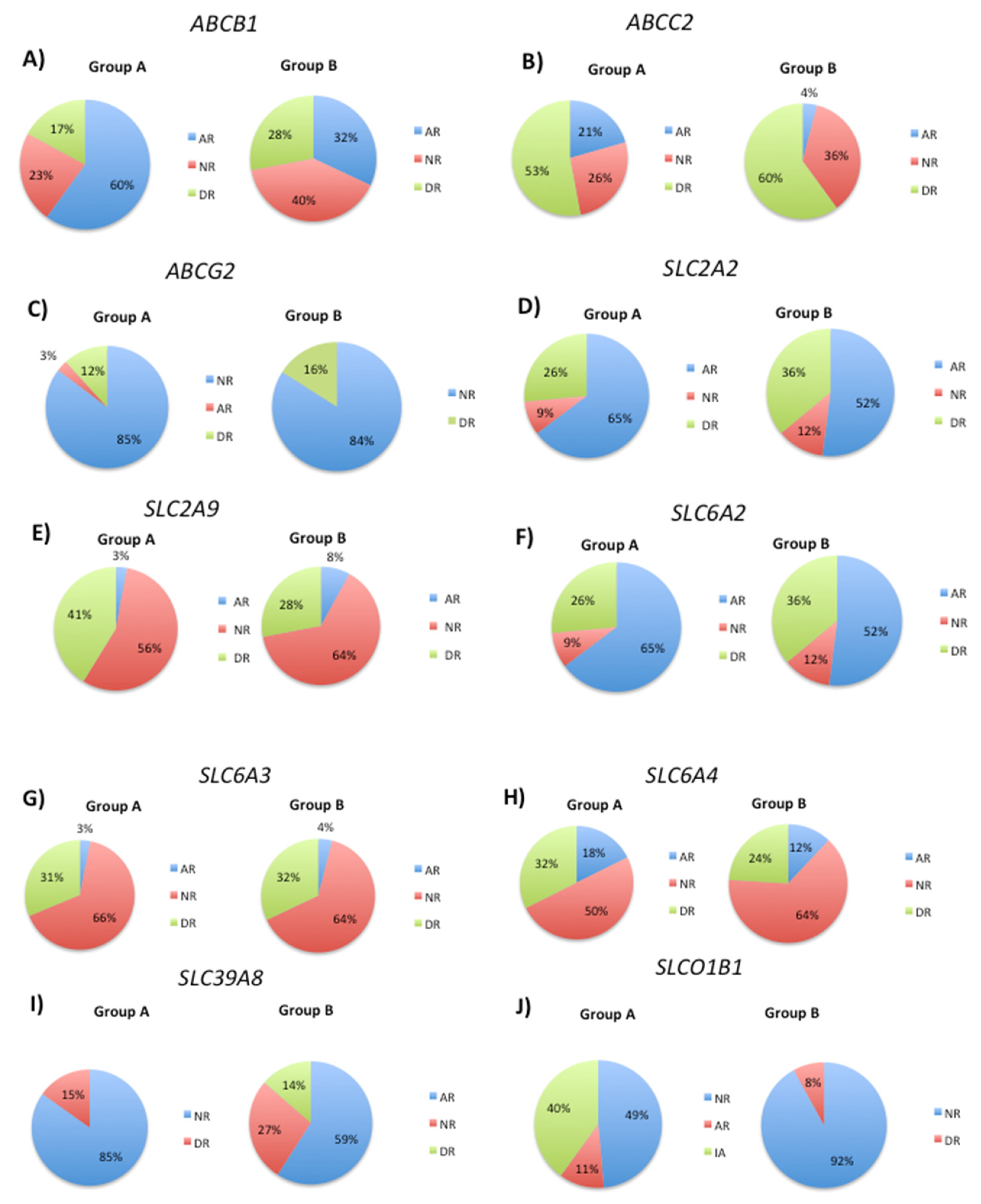
3.2. Modification of DNA Methylation Levels by Transport Gene Phenotypes
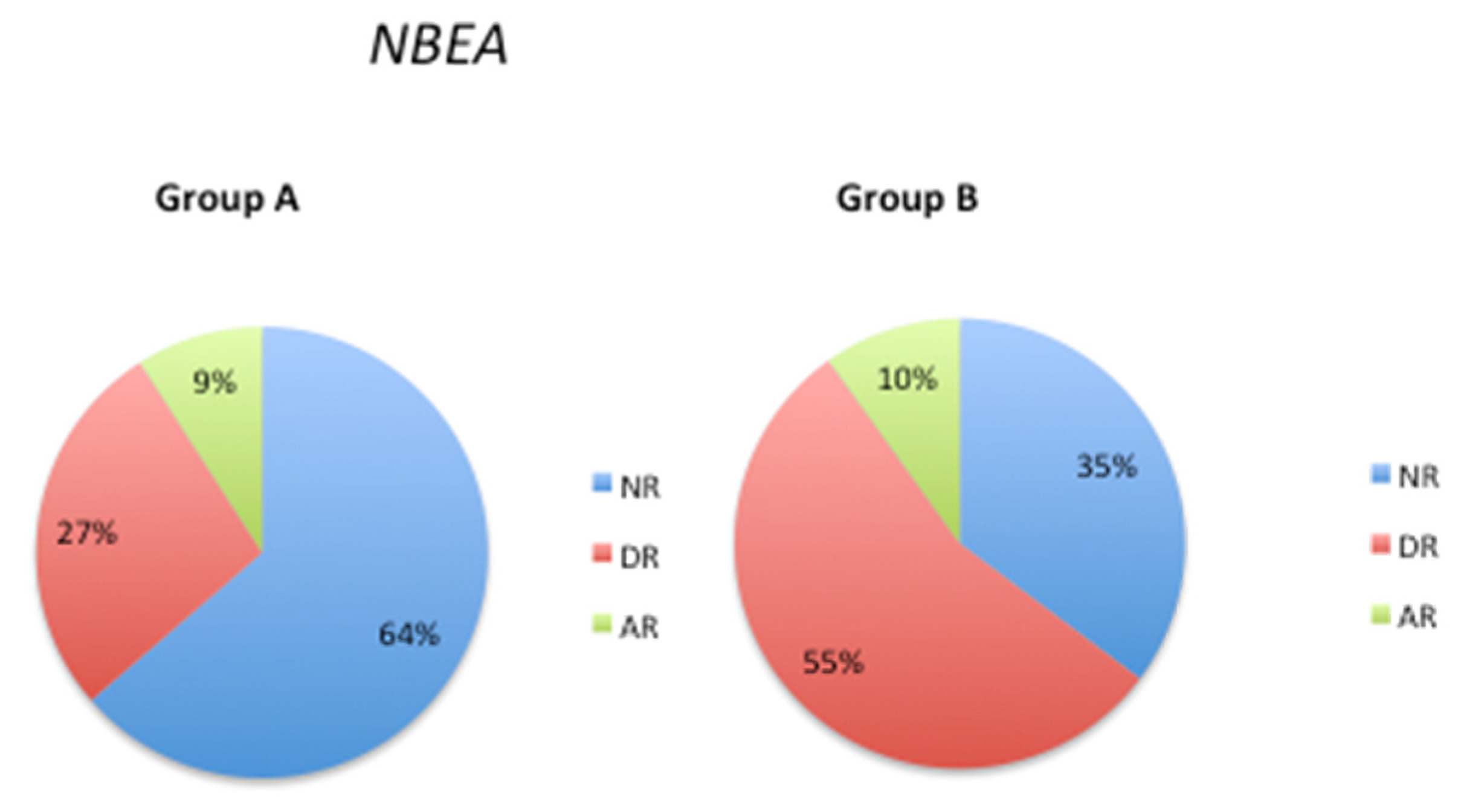
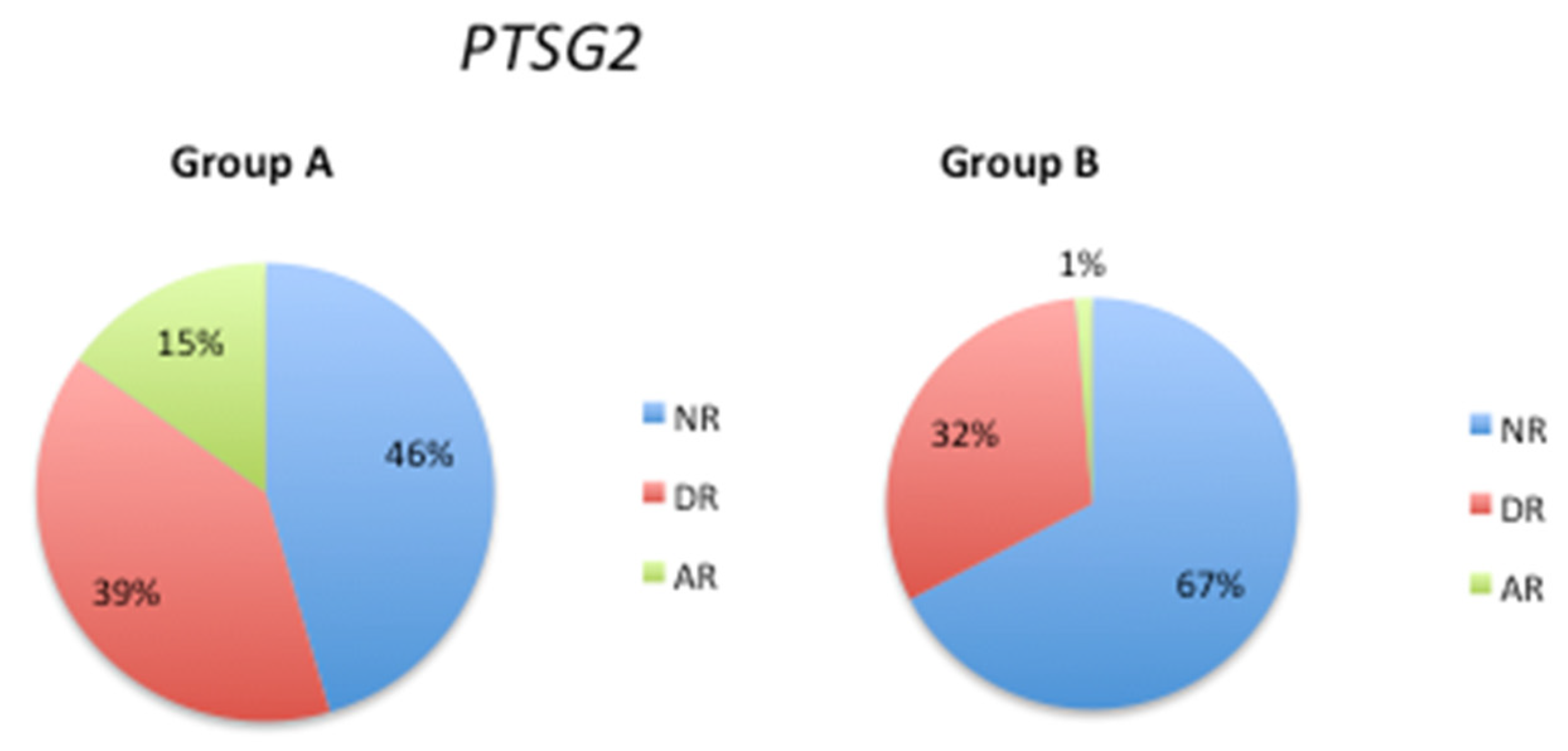
3.3. Pathological Gene Phenotypes Alter DNA Methylation Levels
4. Discussion
5. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- A., R.; M.M., R. Potential therapeutics against neurological disorders: Natural products-based drugs. Front Pharmacol 2022, 13, 950457. [CrossRef] [PubMed]
- A., F. Global, Regional, and Netional burden of 12 Mental Disorders in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study. Lancet Psychiatry 2022, 9, 137–150. [CrossRef] [PubMed]
- Singh, G.; Sander, J.W. The global burden of epilepsy report: Implications for low- and middle-income countries. Epilepsy & behavior 2020, 105, 106949. [Google Scholar] [CrossRef]
- R., C.; V., N.; O., M.-I.; L., C.; N., C.; R., P.; J.C., C. Pharmacogenomics of Alzheimer´s Disease: Novel strategies for drug utilization and development. Pharmacogenomics in Drug Discov and Development 2022, 275–387. [CrossRef]
- O., O.; M., P.; A.K., D. Pharmacogenetics of adverse drug reactions. Adv Pharmacol 2018, 83, 155–190.
- R., C.; V., N.; L., C.; N., C.; J.C., C. Genophenotypoc factors and Pharmacogenomics in Adverse Drug Reactions. Int J Mol Sci 2021, 22, 13302. [CrossRef]
- J.C., E.; R., A. Pharmacogenomics: What the Doctor ordered? Mo Med 2019, 116, 217–225.
- L., B. Pharmacogenomics of adverse drug reactions: Practical applications and prespectives. Pharmacogenomics 2009, 10, 961–969. [CrossRef] [PubMed]
- R., C. Pharmacogenomi of drugs to treat brain disorders. Expert Rev Prec Med Drug Dev 2020, 5, 181–234. [CrossRef]
- J., J.; J., K. PG-path: Modeling and personalizing phramacogenomis-based pathways. PLoS ONE 2020, 15, e0230950. [CrossRef]
- R.A., W.; D.M., R.; J.H., M. Combinatorial pharmacogenetics. Nat Rev Drug Discovery 2005, 4, 911–918. [CrossRef] [PubMed]
- Kozyra, M.; Ingelman-Sundberg, M.; Lauschke, V.M. Rare genetic variants in cellular transporters, metabolic enzymes, and nuclear receptors can be important determinants of interindividual differences in drug response. Genet. Med. 2017, 19, 20–29. [Google Scholar] [CrossRef]
- R., C.; N., C.; J.C., C. The role of pharmacogenomics in adverse drug reactions. Expert Rev clin Pharmacol 2019, 12, 407–442. [CrossRef] [PubMed]
- Z, D.; DA., F. Pharmacogenetics of drug metabolism. Clin Transl Sci 2017, 327–345.
- IS, L.; D., K. Polymorphic metabolism by functional alterations of human cytochrome P450 enzymes. Arch Pharm Res 2011, 34, 1799–1816. [CrossRef]
- J.D., S. The emerging field of neuroepigenetics. Neuron 2013, 80, 624–632. [CrossRef]
- B., M.; D.K., L. Epigenetics of dementia:understanding the disease as a transformation rather than a state. Lancet Neurol 2016, 15, 760–774. [CrossRef]
- A., B. The essentials of DNA methylation. Cell 1992, 70, 5–8. [CrossRef]
- A., H.; H., G.; A., J. Biochemistry and biology of mammal DNA methyltransferases. Cell Mol Life Sci 2004, 61, 2571–2587. [CrossRef] [PubMed]
- J., G.; I.M., M. Epigenetic codes in cognition and behaviour. Behav Brain Res 2008, 192, 70–87. [CrossRef]
- X., N.; F., V.; A., B. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. cell 1997, 88, 4471–4481. [CrossRef]
- J.U., G.; Y., S.; J.H., S.; J., S.; H., L.; al, e. Distribution recognition and regulation of non-CpG methylation in the adult mamamlian brain. Nat Neurosci 2013, 17, 215–222. [CrossRef]
- A., H.; R., G.; A., J. The Dnmt1 DNA-cytosine-c5-methyltransferase methylates DNA processively with high preference for hemimethylated target sites. J Boil Chem 2004, 279, 48350–48359. [CrossRef]
- M., O.; S., X.; E., L. Cloning and characterization of a family of novel mammalian DNMA (cytosine-5) methyltransferases. Nat genet 1998, 19, 219–220. [CrossRef] [PubMed]
- O., M.-I.; I., C.; J.C., C.; L., F.-N.; N., C.; R., C. DNA methylation in neurodegenrative and cerebrovascular disorders. Int J Mol Sci 2020, 21, 2220. [CrossRef]
- O., M.-I.; V., N.; N., C.; R., C. Epigenetic biomarkers as diagnostic tools for Neurodegenrative Disorders. Int J Mol Sci 2022, 23, 13. [CrossRef]
- E., M.; W., D.; D, G.; P., D. Distinctive patterns of DNA methylation associated with Parkinson disease: Identification of concordant epigenetic changes in brain and peripheral blood leukocytes. Epigenetics 2013, 1030–1038. [CrossRef]
- a., D.F.; B., A.; A., F.; M., D.B.; M., K.; al, e. Global changes in DNA methylation in Alzheimer´s Disease peripheral blood mononuclear cells. Brain Behav Immun 2015, 45, 139–144. [CrossRef] [PubMed]
- O., M.-I.; V., N.; L., C.; R., P.; S., S.; S., R.; M., A.; A., M.; N., C.; R., C. DNA methylation as a biomarker for monitoring disease outcome in patients with hypovitaminosis and Neurological Disorders. Genes 2023, 14, 365. [CrossRef] [PubMed]
- S., C.; N., P.; M., M. Pharmaciogenetics of pase I and phase II drug metabolism. Curr Pharm Des 2010, 16, 204–219. [CrossRef]
- M., M. Role of CYO pharmacogenetics and drug-drug interactions in the efficacy and safety of atypical and other antipsychotic agents. J Pharmacy & Pharmacology 2010, 58, 871–885. [CrossRef]
- S., P.-L.; V., L. Biochemistry, Biotransformation. StatPearls 2022.
- S., P.; M., H.; R., P.; M., D.; A., G.; S., P. Polymorphic Citochrome P450 enzymes (CYPs) and their role in personalized therapy. PLoS ONE 2013. [CrossRef]
- Siokas, V.; Stamati, P.; Pateraki, G.; Liampas, I.; Aloizou, A.M.; Tsirelis, D.; Nousia, A.; Sgantzos, M.; Nasios, G.; Bogdanos, D.P.; et al. Analysis of SOD2 rs4880 Genetic Variant in Patients with Alzheimer's Disease. Current issues in molecular biology 2022, 44, 4406–4414. [Google Scholar] [CrossRef] [PubMed]
- Abaji, R.; Krajinovic, M. Thiopurine S-methyltransferase polymorphisms in acute lymphoblastic leukemia, inflammatory bowel disease and autoimmune disorders: Influence on treatment response. Pharmacogenomics and personalized medicine 2017, 10, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Relling, M.V.; Schwab, M.; Whirl-Carrillo, M.; Suarez-Kurtz, G.; Pui, C.H.; Stein, C.M.; Moyer, A.M.; Evans, W.E.; Klein, T.E.; Antillon-Klussmann, F.G.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Updat. Clinical pharmacology and therapeutics 2019, 105, 1095–1105. [Google Scholar] [CrossRef]
- EEB, P.; LPC, L.; RB, A.; AAC, M.; BM, F.; RMR, B.; PP, A.; MR, F.; JF, G.; SEBD, S.; et al. UGT1A1 Gene Polymorphism Contributes as a Risk Factor for Lung Cancer: A Pilot Study with Patients from the Amazon. Genes 2022, 11, 493. [Google Scholar] [CrossRef]
- X, Z.; R, M.; X, M.; G., Y. Association of UGT1A1*6 polymorphism with irinotecan-based chemotherapy reaction in colorectal cancer patients: A systematic review and a meta-analysis. Biosci Rep 2020, 40, BSR20200576. [CrossRef] [PubMed]
- Y, Y.; M, Z.; M, H.; Y, C.; Q, Z.; L, L.; F., H. UGT1A1*6 and UGT1A1*28 polymorphisms are correlated with irinotecan-induced toxicity: A meta-analysis. Asia Pac J Clin Oncol 2018, 14, e479–e489. [CrossRef]
- J.T., Y.; L., T.; J., H. Apolipoprotein E in Alzheiemr´s Disease: An update. Ann Rev Neurosci 2014, 37, 79–100. [CrossRef] [PubMed]
- E., A.; Y., p.; S., P.; C., P. APOE genotype and Alzheimer´s disease: The influence of lifestyle and environmental factors. ACS Chem Neurosci 2021, 12, 2749–2764. [CrossRef]
- F., M.-B.; G., G.; G., M.; G., B.; E., C.; al, e. Pharmacogenomics in Alzheimer´s disease: A genome-wide association study of response to cholinesterase inhibitors. Neurobiol Aging 2013, 34, e7–e13. [CrossRef]
- R., C. Pharmacogenomics of cognitive and neuropsychiatric disorders in Dementia. Int J Mol Sci 2020, 21, 3059. [CrossRef]
- Kuring, J.K.; Mathias, J.L.; Ward, L. Prevalence of Depression, Anxiety and PTSD in People with Dementia: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2018, 28, 393–416. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R. Pharmacogenomics of Cognitive Dysfunction and Neuropsychiatric Disorders in Dementia. Int J Mol Sci 2020, 21, 3059. [Google Scholar] [CrossRef] [PubMed]
- Z., C.; J., X.; C., W.; L., Q.; Y., F.; al., e. Population genetic differnece of pharmacigenomic VIP gene variants in the Lisu population from Yunnan Province. Medicine 2018, 97, e13674. [CrossRef]
- K., B.; P., C.; C., W.; P., W.; M., T.; al, e. Novel association between TGFA, TGFB1, IRF1, PTSG2 and IKBKB single-nucleotide polymorphisms and occurrence, severity and treatment response of major depressive disorder. Peer J 2020, 8, e8676. [CrossRef] [PubMed]
- X., T.; S., C. Epigenetic regulation of Cytochrome P450 enzymed and clinical implication. Curr Drug Metab 2015, 16, 86–96. [CrossRef] [PubMed]
- Y., S.; C., L.; G., L.; Y., C.; W., L.; al, e. Drug-metabolizing cytochrome P450 enzymes have multifarious influences on treatment outcomes. Clin Pharm 2021, 60, 585–601. [CrossRef] [PubMed]
- U.A., M. Pharmacogenetics and adverse drug reactions. Lancet 2000, 356, 1667–1671. [CrossRef] [PubMed]
- J., G.; X., Z.; S., B.; A., I.; Z., L.; C., X.; X., W. Metabolism and mechanism of human Cytochrome P450 Enzyme 1A2. Curr Drug Metab 2021, 22, 40–49. [CrossRef]
- R.A., P.; S.K., V.D.E.; C.M., T.; F., K.; D.M., U.; al, e. Coffee, ADORA2A, and CYP1A2: The caffeine connection in Parkinson´s disease. Eur J Neurology 2011, 18, 756–765. [CrossRef]
- L., v.T.; R., K.; H., V.; H-J., G. CYP1A2 activity is an important determinant of clozapine dosage in schizophrenic patients. Eur J Pharm Sci 2003, 20, 451–457. [CrossRef]
- L., D.; M., K. Clozapine therapy and CYP genotype. Med Genet Summaries 2021.
- R.C., G. Tacrine. Encyclopedia of Toxicology 2014, 466–467.
- R., C. Pharmacogenetic considerations when prescribing cholinesterase inhibitors for the treatment of Alzheimer´s disease. Expert Opin Drug Metab % Toxicology 2020, 16. [CrossRef]
- K., L.; K., R. Propanolol for migraine prophylasis. Cochrane Datanase Syst Rev 2017. [CrossRef]
- S., S.; A., v.W.; G., v.d.H.; R., v.W.; J., s.L.; A., d.J. Propanolol for the treatment of anxiety disorders: Systematic review and meta-analysis. J Phsychopharmacol 2016, 30, 128–139. [CrossRef]
- C., T.; E., A.; T., K.; R., A. Pharma GKB summary: Very important pharmacogene information for CYP1A2. Pharmacogenet Genom 2013, 22, 73–77.
- C.S., L. Microsomal ethanol-oxidizing system (MEOS): The first 30 years (1968-1998)- a review. Alcoholism: Clin % Exp Res 1999, 23, 991–1007. [CrossRef]
- W.A., G.-S.; L.A., R.-C.; M., R.-O.; M., C.-V.; J.A., A.-M.; J., G.; D., S.-A. The role of CYP2E1 in the drug metabolism or bioactivation in the brain. Oxid Med Cell Longev 2017, 2017, 4680732. [CrossRef]
- Y., B.; X., M. Chorzoxazone exhibits neuroprotection against AD by attenuating neuroinflammation and neurodegenration in vitro and in vivo. Int Immunopharmacol 2020, 88, 106790. [CrossRef] [PubMed]
- S., K.; B., S.; A., S.; S., T.-G.; K., Z.; U., S. hepatic. extrahepatic and extracellular vesicle Cytochrome P450 2E1 in alcohol and acetaminophen-mediated adverse intreactions and potential treatment options. Cells 2022, 11, 2620. [CrossRef]
- A., D. Pharmacogenomics of Warfarin. Stratified Medicine 2014, 14, 497–507.
- M., B.; S., H.-Z.; K., S.; K., L.; D., K.; H., O.H.; B., S. Dietary antioxidants remodel DNA methylation patterns in chronid disease. Br J Pharmacol 2019, 177, 1382–1408. [CrossRef]
- H., U.; W., M.; S.W., L.; H.S., K.; S., K.; H.H., W.; B.J., C.; D.K., K. association of the cjoline acetyltransferase gene with responsiveness to acetylcholinesterase inhibitors in Alzheimer´s disease. Pharmacopsych 2015, 48. [CrossRef]
- K., H.; J., L.; T., K. System pharmacogenomics-gene, disease, drug and placebo interactions: A case study in COMT. Pharmacogenomics 2019, 20, 529–551. [CrossRef] [PubMed]
- J., A.; R., T. Enzymmatic O-methylation of epinephrine and other catechol. J Biol Chem 1958, 233, 702–705. [CrossRef]
- N., J.; P., B.; V., J.; M., B.; E., S. NAT gene polymorphisms and susceptibility to Alzheimer´s disease: Identification of a novel NAT1 allelic variant. BMC Med Genet 2004, 5, 6. [CrossRef]
- Borlak J; S., R.-B. N-acetyltransferase 2 (NAT2) gene polymorphisms in Parkinson´s disease. BMC Med Genet 2006, 7, 30. [CrossRef]
- R, C.; L, F.-N.; R, A.; al, e. EPodoFavalin-15999 (Atremorine ® )-induced dopamine response in Parkinson’s Disease: Pharmacogenetics-related effects. J Genomic Med Pharmacogenomics 2016, 1, 1–26.
- O., M.-I.; V., N.; J.C., C.; I., C.; L., C.; al, e. AtreMorine Treatment Regulates DNA Methylation in Neurodegenerative Disorders: Epigenetic and Pharmacogenetic Studies. Curr Pharmacogen & Pers Medicine 2021. [CrossRef]
- S., A.; Z., Z.; J., Z.; S-Q., C. Pharmacogenomics of drug metabolizing enzymes and transporters: Relevance to precision medicine. Genomics, Proteomics & Bioinformatis 2016, 14, 298–313.
- R., C.; C., T.; I., C. Opportinities in pharmacogenomics for the treatment of Alzheimer´s disease. Future Neurol 2015, 10, 229–252. [CrossRef]
- R., C.; I., C.; O., M.; R., A.; L., F.-N.; al, e. Atremorine in Parkisnon´s disease: From dopaminergic neuroprotection to pharmacogenomics. Medic Res Rev 2021, 1–46.
- R., C.; J.C., C.; L., C.; L., F.-N.; R., P.; al, e. Influence of pathogenic and metabolic genes on the pharmacogenetic of mood disorders in Alzheimer´s disease. Pharmaceuticals 2021, 14, 366. [CrossRef] [PubMed]
- R., F.-C.; S., K.; J., F.-R.; J., G.-R.; L., C.-F.; al, e. APOE in the bullseye of neurodegenrative diseases: Impact of the APOE genotype in Alzheimer´s disease pathology and brain disease. Mol Neurodegeneration 2022, 17, 62. [CrossRef]
- DW, D. ; al, e. E4 is associated with severity of Lewy body pathology independent of Alzheiemr pathology. Neurology 2018, 91, e1182–e1195. [CrossRef] [PubMed]
- N., Z.; al, e. APOE4 exacerbates a-synuclein pathology and related toxicity independent of amyloid. Sci Transl Med 2020, 12, eaay1809. [CrossRef]
- E., W.; al, e. APOE varepsilon4 carriers show delayed recovery of verbal memory and smaller enthrinal volume in the fisrt year after ischemic stroke. J Alzheim Dis 2019, 71, 245–259. [CrossRef]
- ST., P.; al, e. APOE-epsilon4 genotype and dementia before and after transient ischemic attack and stroke: Population based cohort study. Stroke 2020, 51, 751–758. [CrossRef] [PubMed]
- O., M.-I.; V., N.; J.C., C.; S., S.; N., C.; R., C. Gene expression profiling as a novel diagnostic tool for Neurodegenrative Disorders. Int J Mol Sci 2023, 24, 5746. [CrossRef] [PubMed]
- K., R.; al, e. Plasma levels of apolipoprotein E and risk of dementia in general population. Ann Neurol 2014, 77, 301–311.
- F., P.; E., C.; I., A.; C., F.; L., T. Predictors of response to acetylcholinesterase inhibitors: A systematic review. Front Neurosci 2022, 16, 998224. [CrossRef] [PubMed]
- R., C.; J.C., C.; L., C.; R., P.; N., C.; al, e. Pharmacogenetics of anxiety and depression in Alzheimer´s disease. Future Med 2023, 24.
| Metabolice (Phase I) | Metabolic (Phase II) | Transporter | Pathogenic |
|---|---|---|---|
| CYP1A2 | GSTP1 | ABCB1 | APOE |
| CYP2C9 | NAT2 | ABCC2 | NBEA |
| CYP4F2 | SLC2A9 | PTSG2 | |
| SLC39A8 | |||
| SLCO1B1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





