Submitted:
21 July 2023
Posted:
21 July 2023
You are already at the latest version
Abstract
Keywords:
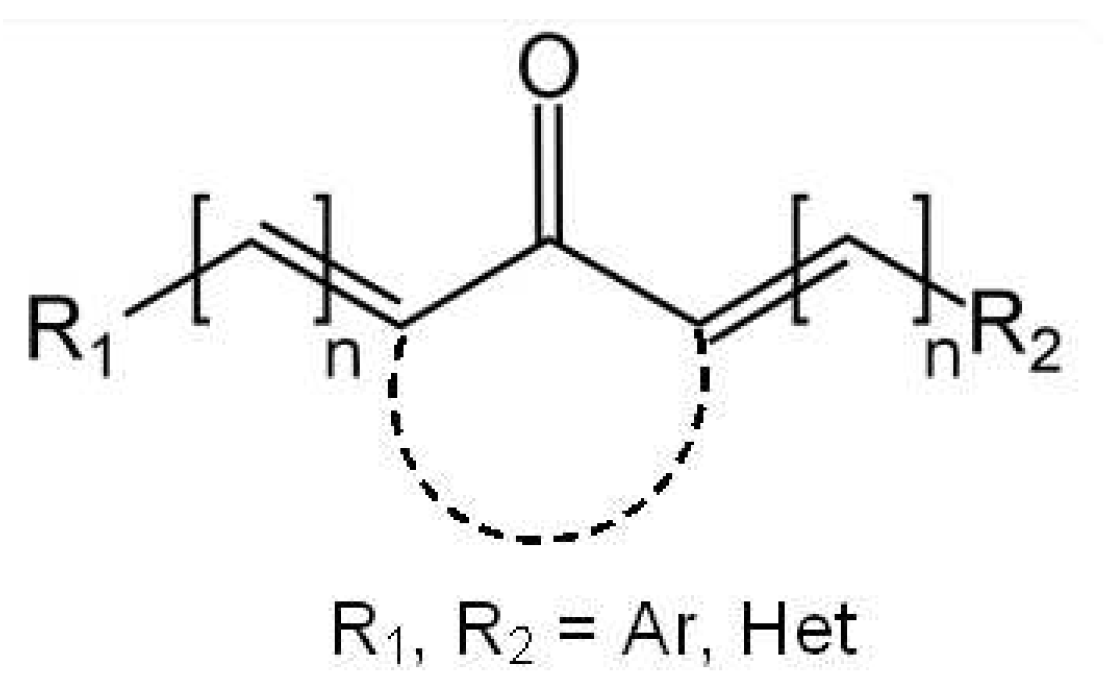
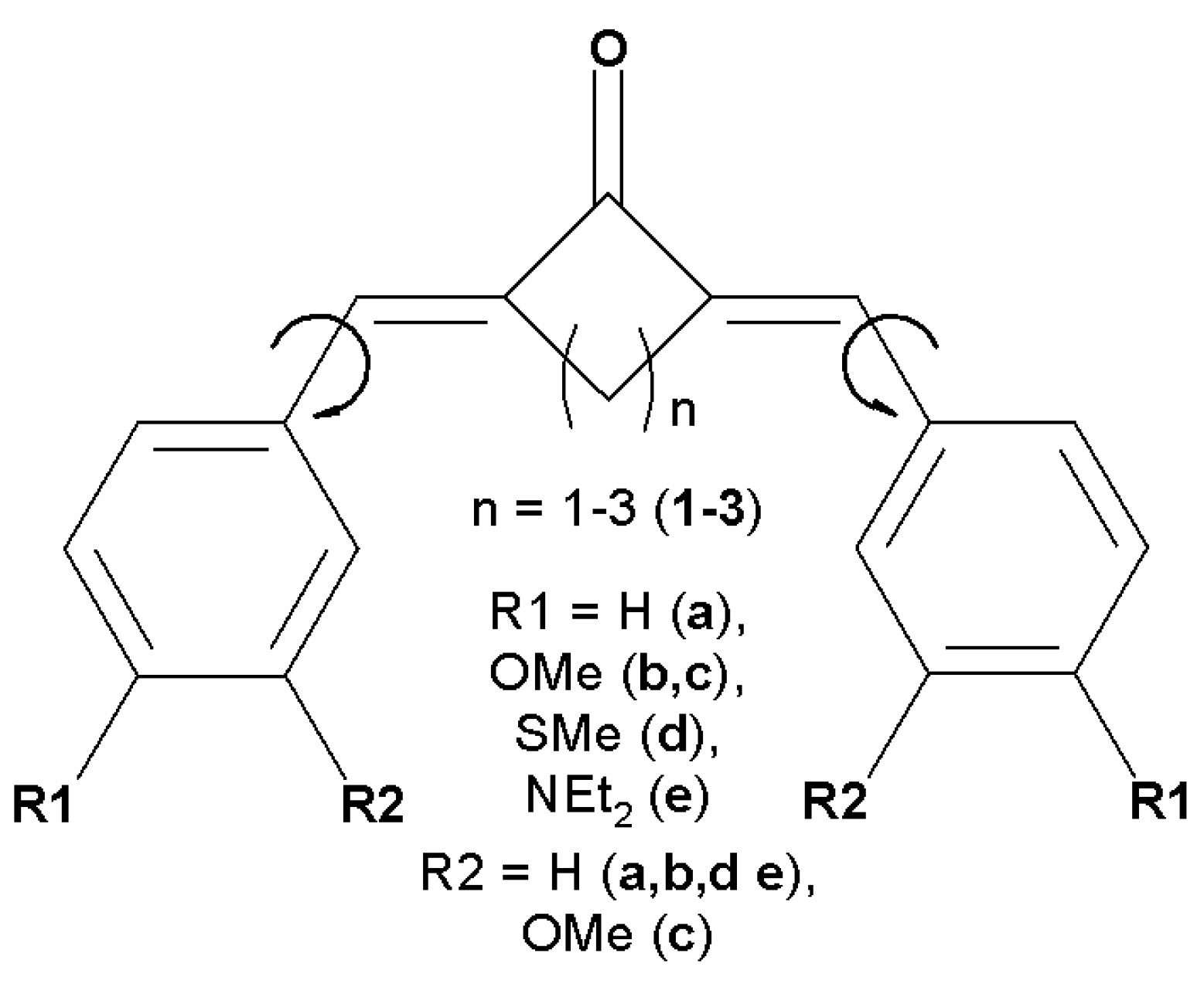
1. Introduction
2. Results
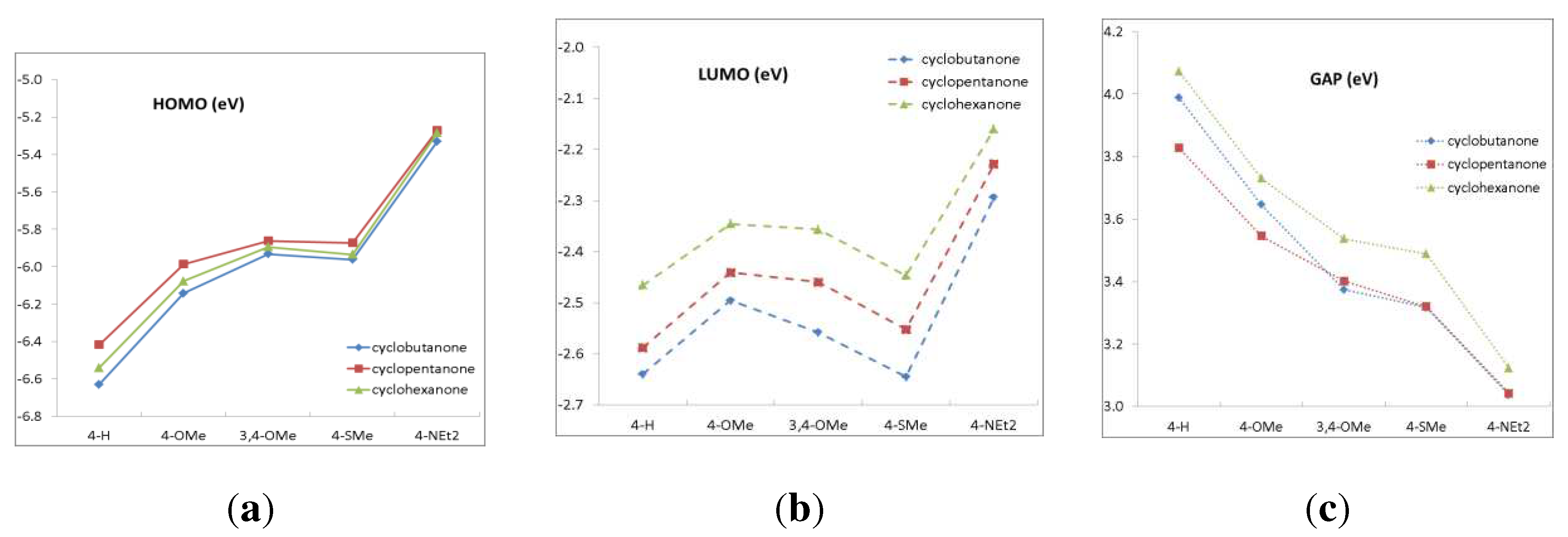
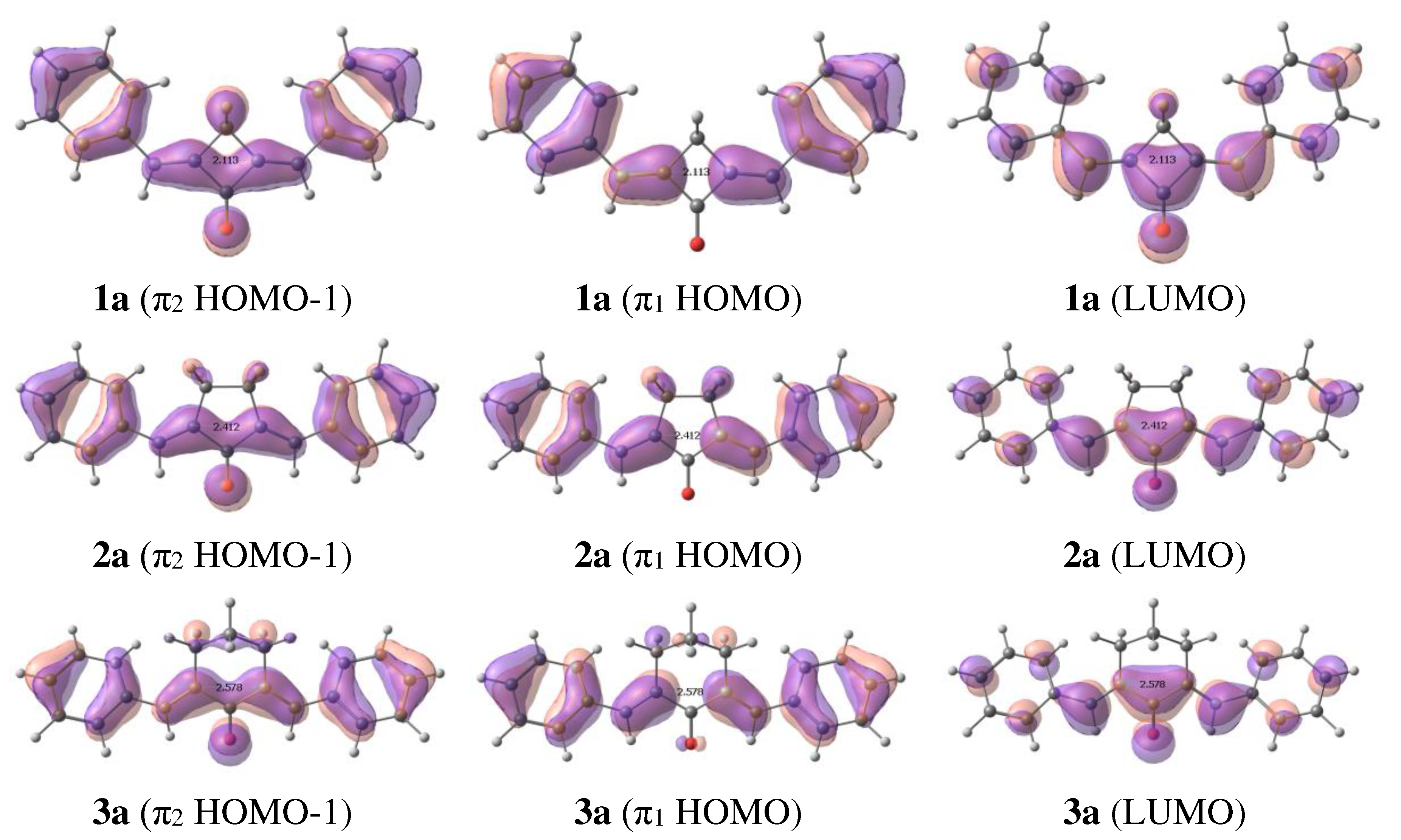
2.1. Ground state structures and molecular orbitals
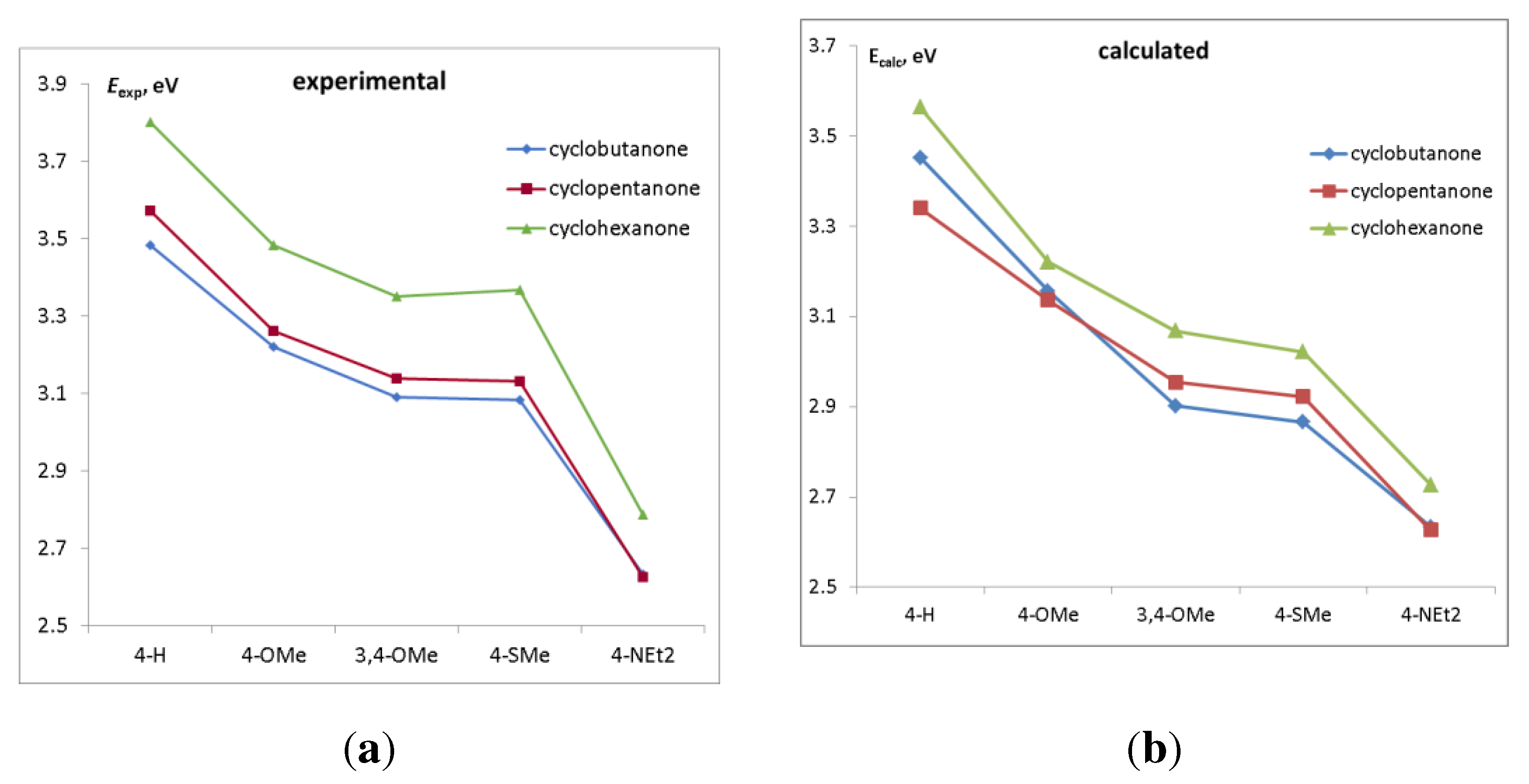
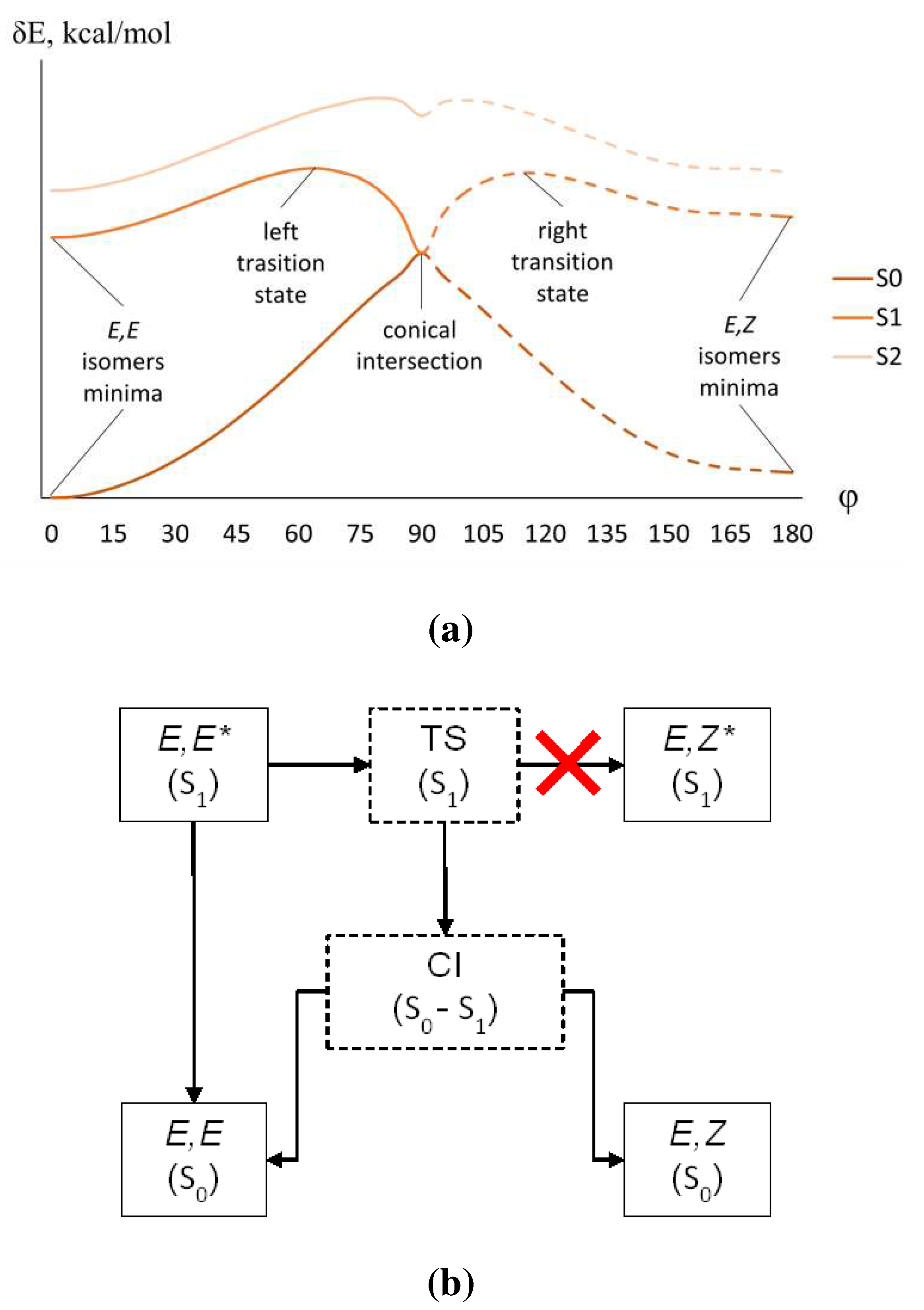
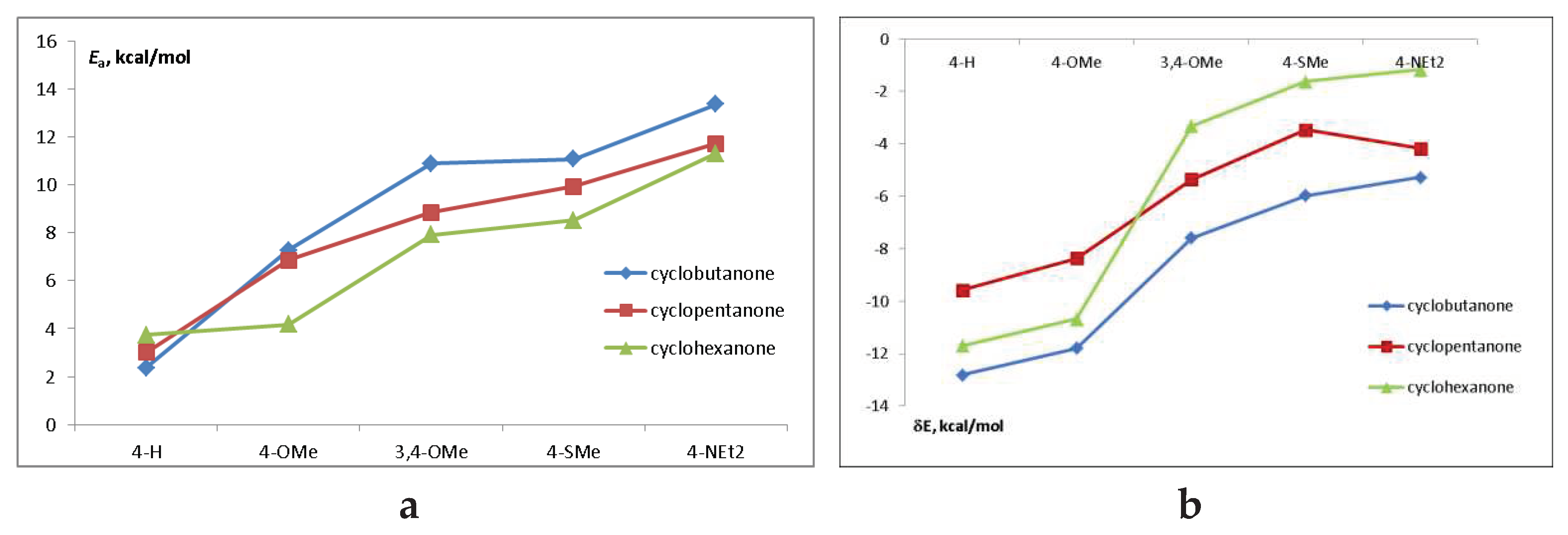
2.2. Absorption and emission spectra
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fomina, M.V.; Vatsadze, S.Z.; Freidzon, A.Y.; Kuz’mina, L.G.; Moiseeva, A.A.; Starostin, R.O.; Nuriev, V.N.; Gromov, S.P. Structure–Property Relationships of dibenzylidenecyclohexanones. ACS Omega 2022, 7, 10087–10099. [Google Scholar] [CrossRef]
- Fomina, M.V.; Freidzon, A.Y.; Kuz’mina, L.G.; Moiseeva, A.A.; Starostin, R.O.; Kurchavov, N.A.; Nuriev, V.N.; Gromov, S.P. Synthesis, Structure and Photochemistry of Dibenzylidenecyclobutanones. Molecules 2022, 27, 7602. [Google Scholar] [CrossRef] [PubMed]
- Vatsadze, S.Z.; Gavrilova, G.V.; Zyuz’kevich, F.S.; Nuriev, V.N.; Krut’ko, D.P.; Moiseeva, A.A.; Shumyantsev, A.V.; Vedernikov, A.I.; Churakov, A.V.; Kuz’mina, L.G.; et al. Synthesis, structure, electrochemistry, and photophysics of 2,5- dibenzylidenecyclopentanones containing in benzene rings substituents different in polarity. Russ. Chem. Bull. 2016, 65, 1761–1772. [Google Scholar] [CrossRef]
- Doroshenko, A.O.; Pivovarenko, V.G. Fluorescence quenching of the ketocyanine dyes in polar solvents: anti-TICT behavior. J. Photochem. Photobiol. A. 2003, 156, 55–64. [Google Scholar] [CrossRef]
- Gutrov, V.N.; Zakharova, G.V.; Fomina, M.V.; Nuriev, V.N.; Gromov, S.P.; Chibisov, A.K. Molecular photonics of dienones based on cycloalkanones and their derivatives. J. Photochem. Photobiol. A 2022, 425, 113678. [Google Scholar] [CrossRef]
- Homocianu, M.; Serbezeanu, D.; Tachita, V.B. Solvatochromism, Acidochromism and Photochromism of the 2,6-Bis(4-hydroxybenzylidene) Cyclohexanone Derivative. Int. J. Mol. Sci. 2023, 24, 5286. [Google Scholar] [CrossRef]
- Kedia, N.; Sarkar, A.; Shannigrahi, M.; Bagchi, S. Photophysics of representative ketocyanine dyes: Dependence on molecular structure. Spectrochim. Acta A 2011, 81, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.A.; Wolfbeis, O.S. Spectrochim. Acta A 1991, 47A, 187–192.
- Vatsadze, S.Z.; Gromov, S.P. Novel Linear Bis-Crown Receptors with Cross-Conjugated and Conjugated Central Cores. Macroheterocycles 2017, 10, 432–445. [Google Scholar] [CrossRef]
- Fomina, M.V.; Kurchavov, N.A.; Freidzon, A.Y.; Nuriev, V.N.; Vedernikov, A.I.; Strelenko, Y.A.; Gromov, S.P. Self-assembly involving hydrogen bonds. Spectral properties and structure of supramolecular complexes of bis-aza-18-crown-6-containing dienones with alkanediammonium salts. J. Photochem. Photobiol. A 2020, 402, 112801. [Google Scholar] [CrossRef]
- Volchkov, V.V.; Khimich, M.N.; Melnikov, M.Y.; Egorov, A.E.; Starostin, R.O.; Freidzon, A.Ya.; Dmitrieva, S.N.; Gromov, S.P. Hydrogen-Bonded Self-assembly of Supramolecular Donor–Acceptor Complexes of (E)-Bis(18-crown-6)azobenzene with Bis(ammoniopropyl) Derivatives of Bipyridine and Dipyridylethylene in Acetonitrile. J. Solution Chem. 2023. [Google Scholar] [CrossRef]
- Zou, Q.; Zhao, Y.; Makarov, N.S.; Campo, J.; Yuan, H.; Fang, D.C.; Perry, J.W.; Wu, F. Effect of alicyclic ring size on the photophysical and photochemical properties of bis(arylidene)cycloalkanone compounds. Phys. Chem. Chem. Phys 2012, 14, 11743–11752. [Google Scholar] [CrossRef]
- Zou, Q.; Zhao, H.; Zhao, Y.; Wang, Y.; Gu, Y.; Wu, F. Polyethylene glycol-functionalized bis(arylidene)cycloalkanone photosensitizers for two-photon excited photodynamic therapy. Proc. SPIE 2012, Optics in Health Care and Biomedical Optics V, 85530J. [Google Scholar]
- Dumur, F. Recent advances on benzylidene cyclopentanones as visible light photoinitiators of polymerization. Eur. Polym. J. 2022, 181, 111639. [Google Scholar] [CrossRef]
- Sajan, D.; Lakshmi, K.U.; Erdogdu, Y.; Joe, I.H. Molecular structure and vibrational spectra of 2,6-bis(benzylidene)cyclohexanone: A density functional theoretical study. Spectrochim. Acta A 2011, 78, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Gutrov, V.N.; Zakharova, G.V.; Fomina, M.V.; Gromov, S.P.; Chibisov, A.K. Intermediates of the Photoinduced 2,4-Bis(4-Diethylaminobenzylidene)cyclobutanone Redox Reaction in Methanol. High Energy Chemistry 2020, 54, 436–440. [Google Scholar] [CrossRef]
- Zakharova, G.V.; Zyuz’kevich, F.S.; Nuriev, V.N.; Vatsadze, S.Z.; Plotnikov, V.G.; Gromov, S.P.; Chibisov, A.K. Photonics of Bis(diethylaminobenzylidene)cyclopentanone and Its Analogue with the Bisazacrown Moiety in Acetonitrile. High Energy Chemistry 2016, 50, 27–31. [Google Scholar] [CrossRef]
- Zakharova, G.V.; Zyuz’kevich, F.S.; Gutrov, V.N., Gavrilova G.V., Nuriev, V.N.; Vatsadze, S.Z.; Plotnikov, V.G.; Gromov, S.P.; Chibisov, A.K. Effect of Substituents on Spectral, Luminescent and Time-Resolved Char-acteristics of 2,5-Diarylidene Derivatives of Cyclopentanone. High Energy Chemistry 2017, 51, 113–117.
- Zakharova, G.V.; Gutrov, V.N.; Nuriev, V.N.; Zyuz’kevich, F.S.; Vatsadze, S.Z.; Gromov, S.P.; Chibisov, A.K. Effect of Substituents on Spectral, Luminescent, and Time-Resolved Spectral Properties of 2,6-Diarylidene De-rivatives of Cyclohexanone. High Energy Chemistry 2017, 51, 424–426. [Google Scholar] [CrossRef]
- L.A. Huck, W.J. Leigh. A Better Sunscreen: Structural Effects on Spectral Properties. Journal of Chemical Education 2010, 87, 1384–1387. [CrossRef]
- Granovsky, A.A. Firefly Version 8.2.0. Available online: http://classic.chem.msu.su/gran/firefly/index.html (accessed on 28 October 2022).
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.J.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Freidzon, A.Y.; Safonov, A.A.; Bagaturyants, A.A.; Alfimov, M.V. Solvatofluorochromism and Twisted Intramolecular ChargeTransfer State of the Nile Red Dye. Int. J. Quantum Chem. 2012, 112, 3059–3067. [Google Scholar] [CrossRef]
- Quentin, C.; Gerasimaite, R.; Freidzon, A.; Atabekyan, L.S.; Lukinaviˇcius, G.; Belov, V.N.; Mitronova, G.Y. Direct Visualization of ˙ Amlodipine Intervention into Living Cells by Means of Fluorescence Microscopy. Molecules 2021, 26, 2997. [Google Scholar] [CrossRef] [PubMed]
| a | b | c | d | e | |
|---|---|---|---|---|---|
| 1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.4 |
| 2 | 7.0 | 1.0 | 0.1 | 0.5 | 0.2 |
| 3 | 30.3 | 26.9 | 23.9 | 26.6 | 21.0 |
| a | b | c | d | e | |
|---|---|---|---|---|---|
| 1 | 0.11 | 0.14 | 0.14 | 0.13 | 0.20 |
| 2 | 0.10 | 0.13 | 0.12 | 0.11 | 0.19 |
| 3 | 0.07 | 0.10 | 0.10 | 0.09 | 0.16 |
| Cyclobutanone | |||
| Dienone | τr, ns | k, s-1 | ttc, ns |
| 1a | - | 1.12·1010 | 0.09 |
| 1b | 2.28 | 4.97·106 | 201 |
| 1c | 3.05 | 4.40·103 | 227400 |
| 1d | 2.62 | 4.06·103 | 2465000 |
| 1e | 3.07 | 1.22·102 | 8182600 |
| Cyclopentanone | |||
| 2a | - | 4.66·109 | 0.21 |
| 2b | 1.83 | 8.28·106 | 121 |
| 2c | 2.23 | 8.13·104 | 12300 |
| 2d | 1.97 | 3.34·104 | 29900 |
| 2e | 1.90 | 2.55·104 | 39200 |
| Cyclohexanone | |||
| 3a | - | 1.41·109 | 0.71 |
| 3b | 2.90 | 5.47·108 | 1.83 |
| 3c | 2.26 | 7.12·105 | 1404 |
| 3d | 2.02 | 4.92·105 | 2033 |
| 3e | 2.46 | 3.40·103 | 294000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





