Submitted:
20 July 2023
Posted:
21 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Patients who have a subjective complaint of memory decline that family members or an observer confirm.
- Patients with poorer memory than those of the same age and education level.
- Patients with no defects in most normal cognitive functions.
- Patients who have basic activities of daily living.
- Patients who do not have dementia.
- Mini-Cog
- SPMSQ
- MMSE
- SLUMS
- CDR
- CASI
- Only one test is required to obtain the test results for multiple cognitive impairment screening tools in a subject, reducing the issue of multiple testing in subjects.
- Integration of scenario-based image virtual situations to improve test willingness and acceptance in subjects.
- The test results will be presented in images so that the tester and subject can intuitively understand and explain the results.
2. Materials and Methods
2.1. Development of MCI forecasting system
2.1.1. System framework
2.1.2. System assessment method
2.1.3. MCI fuzzy forecasting module
2.1.4. System scenario design
- System startup
- Orientation test
- Identification test
- Memory test
- Attention test
- Construction test
- Judgment test
- Calculation test
- Control ability test
- Test completion screen
2.2. Experimental design
2.2.1. Subjects
2.2.2. Experimental procedure
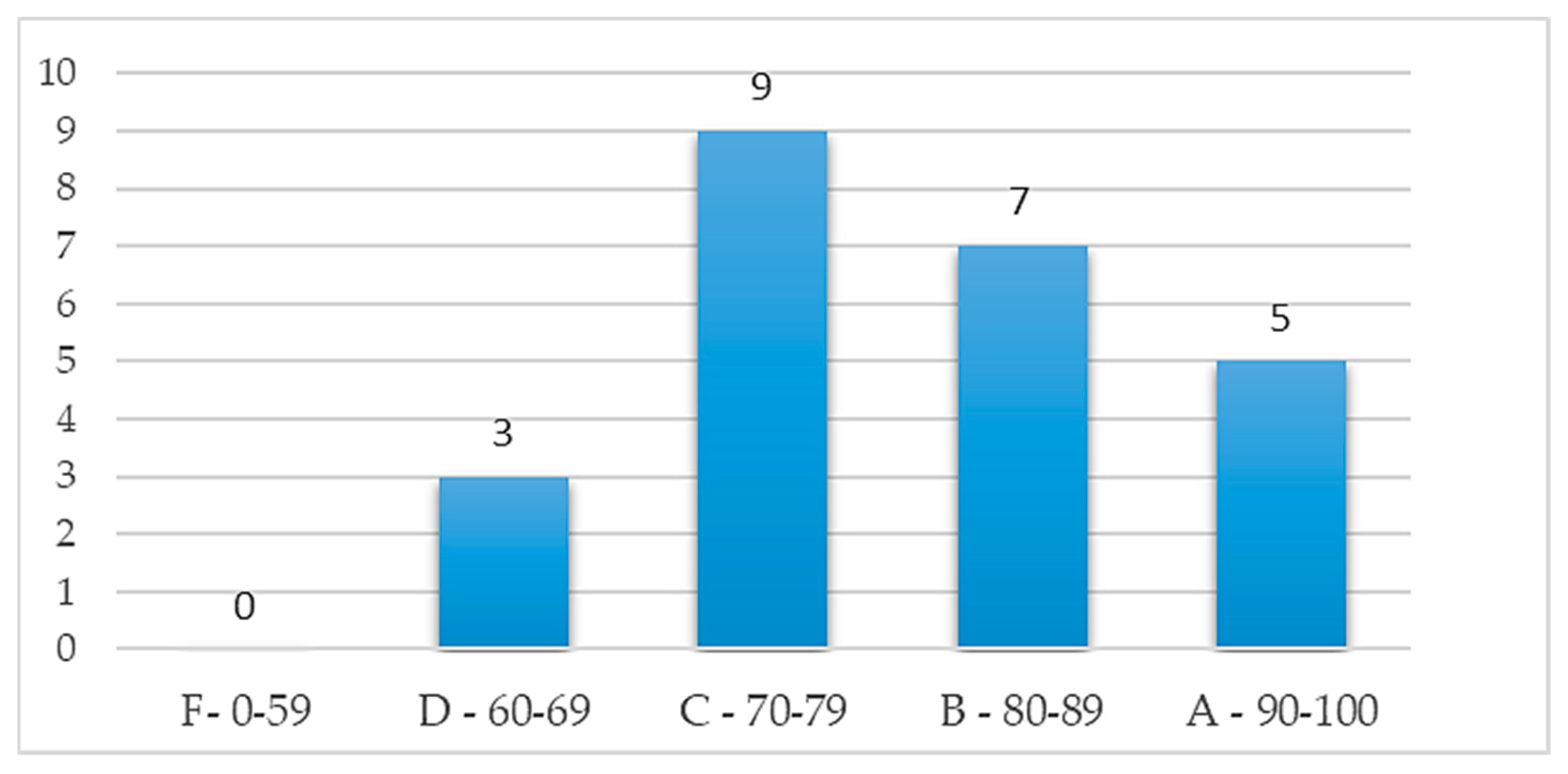
2.2.3. Effectiveness assessment
3. Results and discussion
3.1. Correlation analysis of MCI forecasting system
3.2. Effectiveness analysis of MCI forecasting system
3.3. Effectiveness analysis of fuzzy prediction
3.4. Effectiveness analysis of fuzzy prediction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nuckols, C.C. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. VA, United States: American Psychiatric Association Publishing 2013.
- World Alzheimer Report. The global impact of dementia: An analysis of prevalence, incidence, cost and trends. Alzheimer's Disease International, September, 2015: https://www.alzint.org/resource/world-alzheimer-report-2015/.
- Matej, R.; Tesar, A.; Rusina, R. Alzheimer's disease and other neurodegenerative dementias in comorbidity: A clinical and neuropathological overview. Clinical Biochemistry 2019, 73, 26–31. [Google Scholar] [CrossRef]
- Torres-Berrio, A.; Nava-Mesa, M.O. The opioid system in stress-induced memory disorders: From basic mechanisms to clinical implications in post-traumatic stress disorder and Alzheimer's disease. Progress in Neuro-Psychopharmacology & Biological Psychiatry 2019, 88, 327–338. [Google Scholar]
- WHO report. Dementia, 19 September 2019: https://www.who.int/news-room/fact-sheets/detail/dementia.
- Crook, T.; Bartus, R.T.; Ferris, S.H.; Whitehouse, P.; Cohen, G.D.; Gershon, S. Age-associated memory impairment: Proposed diagnostic criteria and measures of clinical change - report of a national institute of mental health work group. Developmental Neuropsychology 1986, 2, 261–276. [Google Scholar] [CrossRef]
- Levy, R. ; Working Party of the International Psychogeriatric Association. Aging-associated cognitive decline. International Psychogeriatrics 1994, 6, 63. [Google Scholar]
- Reisberg, B.; Ferris, S.H.; DeLeon, M.J.; Crook, T. The global deterioration scale for assessment of primary degenerative dementia. The American Journal of Psychiatry 1982, 139, 1136–1139. [Google Scholar]
- Petersen, R.C.; Roberts, R.O.; Knopman, D.S.; Boeve, B.F.; Geda, Y.E.; Ivnik, R.J.; Smith, G.E.; Jack Jr, C.R. Mild cognitive impairment: ten years later. Arch Neurol 2009, 66, 1447–1455. [Google Scholar] [CrossRef]
- Rossetto, F.; Castelli, I.; Baglio, F.; Massaro, D.; Alberoni, M.; Nemni, R.; Shamay-Tsoory, S.; Marchetti, A. Cognitive and affective theory of mind in mild cognitive impairment and parkinson's disease: preliminary evidence from the italian version of the yoni task. Developmental Neuropsychology 2018, 43, 764–780. [Google Scholar] [CrossRef]
- Qi, H.H.; Liu, H.; Hu, H.M.; He, H.J.; Zhao, X.H. Primary disruption of the memory-related subsystems of the default mode network in alzheimer's disease: resting-state functional connectivity MRI study. Frontiers in Aging Neuroscience 2018, 10. [Google Scholar] [CrossRef]
- Gosztolya, G.; Vincze, V.; Toth, L.; Pakaski, M.; Kalman, J.; Hoffmann, I. Identifying Mild Cognitive Impairment and mild Alzheimer's disease based on spontaneous speech using ASR and linguistic features. Computer Speech and Language 2019, 53, 181–197. [Google Scholar] [CrossRef]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, v.; Fratiglioni, L. Mild cognitive impairment: a concept in evolution. Journal of Internal Medicine 2014, 275, 214–228. [Google Scholar] [CrossRef]
- Huang, Y.H. What is Mild Cognitive Impairment? Chang Gung Medical News 2018, 39(10), 28–29. [Google Scholar]
- Wang, P.; Lirng, J.; Lin, K.; Chang, F.; Liu, H. Prediction of Alzheimer’s disease in mild cognitive impairment: a prospective study in Taiwan. Neurobiology of Aging 2006, 27, 1797. [Google Scholar] [CrossRef]
- Johnson, K.A.; Fox, N.C.; Sperling, R.A.; Klunk, W.E. Brain imaging in Alzheimer’s disease. Cold Spring Harbor Perspectives in Medicine 2012, 2, a006213. [Google Scholar] [CrossRef]
- Karas, G.B.; Burton, E.J.; Rombouts, S.A.R.B.; van Schijndel, R.A.; O’Brien, J.T.; Scheltens, P.; Barkhof, F. A comprehensive study of gray matter loss in patients with Alzheimer’s disease using optimized voxel-based morphometry. Neuroimage 2003, 18, 895–907. [Google Scholar] [CrossRef]
- Pantel, J.; Schonknecht, P.; Essing, M.; Schoder, J. Distribution of cerebral atrophy assessed by magnetic resonance imaging reflects patterns of neuropsychological deficits in Alzheimer’s dementia. Neuroscience Letters 2004, 361, 17–20. [Google Scholar] [CrossRef]
- National Institute for Health and Clinical Excellence. Dementia: Quick Reference Guide.
- Schroeter, M.L.; Stein, T.; Maslowski, N.; Neumann, J. Neural correlates of Alzheimer's disease and mild cognitive impairment: A systematic and quantitative meta-analysis involving 1,351 patients. NeuroImage 2009, 47, 1196–206. [Google Scholar] [CrossRef]
- Flier, W.M.; Salloway, S.; Frisoni, G.B.; Strooper, B.; Breteler, M.M.B.; Blennow, K.; Scheltens, P. Alzheimer's disease. The Lancet 2016, 388, 505–517. [Google Scholar]
- Borson, S.; Scanlan, J.; Brush, M.; Vitaliano, P.; Dokmak, A. The Mini-Cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000, 15, 1021–1027. [Google Scholar] [CrossRef]
- Joyce, E.; Howell, E.H.; Senapati, A.; Starling, R.C.; Gorodeski, E.Z. Prospective assessment of combined handgrip strength and Mini-Cog identifies hospitalized heart failure patients at increased post-hospitalization risk. Esc Heart Failure 2018, 949–953. [Google Scholar] [CrossRef]
- Chan, C.C.H.; Fage, B.A.; Burton, J.K.; Smailagic, N.; Gill, S.S.; Herrmann, N.; Nikolaou, V.; Quinn, T.J.; Noel-Storr, A.H.; Seitz, D.P. Mini-Cog for the diagnosis of Alzheimer’s disease dementia and other dementias within a secondary care setting. Cochrane Database of Systematic Reviews 2019, CD011414. [Google Scholar] [CrossRef]
- Pfeiffer, F. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. Journal of the American Geriatrics Society 1975, 23, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, C.; Chan, A.; Matchar, D.; Seow, D.; Chuo, A.; Do, Y.K. Diagnostic performance of short portable mental status questionnaire for screening dementia among patients attending cognitive assessment clinics in Singapore. Annals Academy of Medicine Singapore 2013, 42, 315–319. [Google Scholar] [CrossRef]
- Folstein, M.; Folstein, S.E.; McHugh, P. Mini-mental state: a practical method for grading the cognitive state: a practical method for grading the cognitive state if patient for the clinical. Journal of Psychiatric Research 1975, 12, 12,189–198. [Google Scholar]
- Mahendran, R.; Chua, J.; Feng, L.; Kua, E.H.; Preedy, V.R. Chapter 109 - The Mini-Mental State Examination and Other Neuropsychological Assessment Tools for Detecting Cognitive Decline. In Diet and Nutrition in Dementia and Cognitive Decline; Martin, C.R., Preedy, V.R., Eds.; London: Academic Press 2015, Pages 1159-1174. [Google Scholar]
- Morley, J.E.; Tumosa, N. Saint Louis University Mental Status Examination (SLUMS). Aging Successfully 2002, XII, 4. [Google Scholar]
- Zheng, D.; Dong, X.; Sun, H.; Xu, Y.; Ma, Y.; Wang, X. The overall impairment of core executive function components in patients with amnestic mild cognitive impairment: a cross-sectional study. BioMed Central Neurology 2012, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Kaya, D.; Isik, A.T.; Usarel, C.; Soysal, P. Ellidokuz, H.; Grossberg, G.T. The saint louis university mental status examination is better than the mini-mental state examination to determine the cognitive impairment in turkish elderly people. Journal of The American Medical Directors Association 2016, 17, 370.e11-5. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.P.; Berg, L.; Danziger, W.L.; Coben, L.A.; Martin, R.L. A new clinical scale for the staging of dementia. Br J Psychiatry 1982, 140, 566–72. [Google Scholar] [CrossRef]
- Morris, J.C. The clinical dementia rating (CDR): current version and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef]
- Kim, J.W.; Byun, M.S.; Sohn, B.K.; Yi, D.; Seo, E.H.; Choe, Y.M.; Kim, S.G.; Choi, H.j.; Lee, J.H.; Chee, I.S.; Woo, J.I.; Lee, D.Y. Clinical dementia rating orientation score as an excellent predictor of the progression to alzheimer’s disease in mild cognitive impairment. Psychiatry Investing 2017, 14, 420–426. [Google Scholar] [CrossRef]
- Teng, E.L.; Hasegawa, K.; Homma, A.; Imai, Y.; Larson, E.; Graves, A.; Sugimoto, K.; Yamaguchi, T.; Sasaki, H.; Chiu, D. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr 1994, 6, 45–58. [Google Scholar] [CrossRef]
- Boletsis, C.; McCallum, S. Connecting the player to the doctor: utilising serious games for cognitive training & screening. DAIMI PB 2015, 597, 5–8. [Google Scholar]
- Zadeh, L.A. Fuzzy Sets. Information Control 1965, 8, 338–353. [Google Scholar] [CrossRef]
- Bellman, R.E.; Zadeh, L.A. Decision making in a fuzzy environment. Management Science 1970, 17, 141–164. [Google Scholar] [CrossRef]
- Bonner, M. F.; Price, A.R. Where is the anterior temporal lobe and what does it do? Journal of Neuroscience 2013, 33, 4213–4215. [Google Scholar] [CrossRef]
- Marquié, M.; Castilla-Martí, M.; Valero, S.; et al. Visual impairment in aging and cognitive decline: experience in a Memory Clinic. Scientific Report 2019, 9, 8698. [Google Scholar] [CrossRef] [PubMed]
- Claus, J. J.; Mohr, E. Attentional deficits in Alzheimer's, Parkinson's, and Huntington's diseases. Acta Neurol Scand 1996, 93, 346–51. [Google Scholar] [CrossRef] [PubMed]
- Ala, T.A; Hughes, L.F.; Kyrouac, G.A.; Ghobrial, M.W. Elble, R.J. The Mini-Mental State exam may help in the differentiation of dementia with Lewy bodies and Alzheimer's disease. International Journal of Geriatric PsychiatryVolume 2002, 17, 503–509. [Google Scholar] [CrossRef]
- Capucho, P.H.F.V.; Brucki, S.M.D. ; Judgment in Mild Cognitive Impairment and Alzheimer's disease. Dement Neuropsychol 2011, 5, 297–302. [Google Scholar] [CrossRef]
- Li, X.-Y.; Zhang, Y.-Y.; Feng, L.; Meng, Q.-H. Early event-related potentials changes during simple mental calculation in Chinese older adults with mild cognitive impairment: A case–control study. Neuroscience Letters 2010, 475, 29–32. [Google Scholar] [CrossRef]
- Chung, J.-Y.; Yoon, H.-J; Kim, H.; Choi, K.Y.; Lee, J.J.; Lee, K.H.; Seo, E.H. Reversion from mild cognitive impairment to normal cognition: false-positive error or true restoration thanks to cognitive control ability? Neuropsychiatric Disease and Treatment 2019, 15, 3021–3032. [Google Scholar] [CrossRef]
- Brooke, J. SUS: A "quick and dirty" usability scale. In Usability Evaluation in Industry; Jordan, P.W., Thomas, B., Weerdmeester, B.A., McClelland, A.L., Eds.; London: Taylor and Francis, 1986. [Google Scholar]
- Bangor, A.; Kortum, P.; Miller, J. Determining what individual SUS scores mean: Adding an adjective rating scale. Journal of usability studies 2009, 4, 114–123. [Google Scholar]
- Zucchella, C.; Sinforiani, E.; Tassorelli, C.; Cavallini, E.; Tost-Pardell, D.; Grau, S. Serious games for screening pre-dementia conditions: from virtuality to reality? A pilot project. Funct Neurol 2014, 29, 153–158. [Google Scholar] [PubMed]
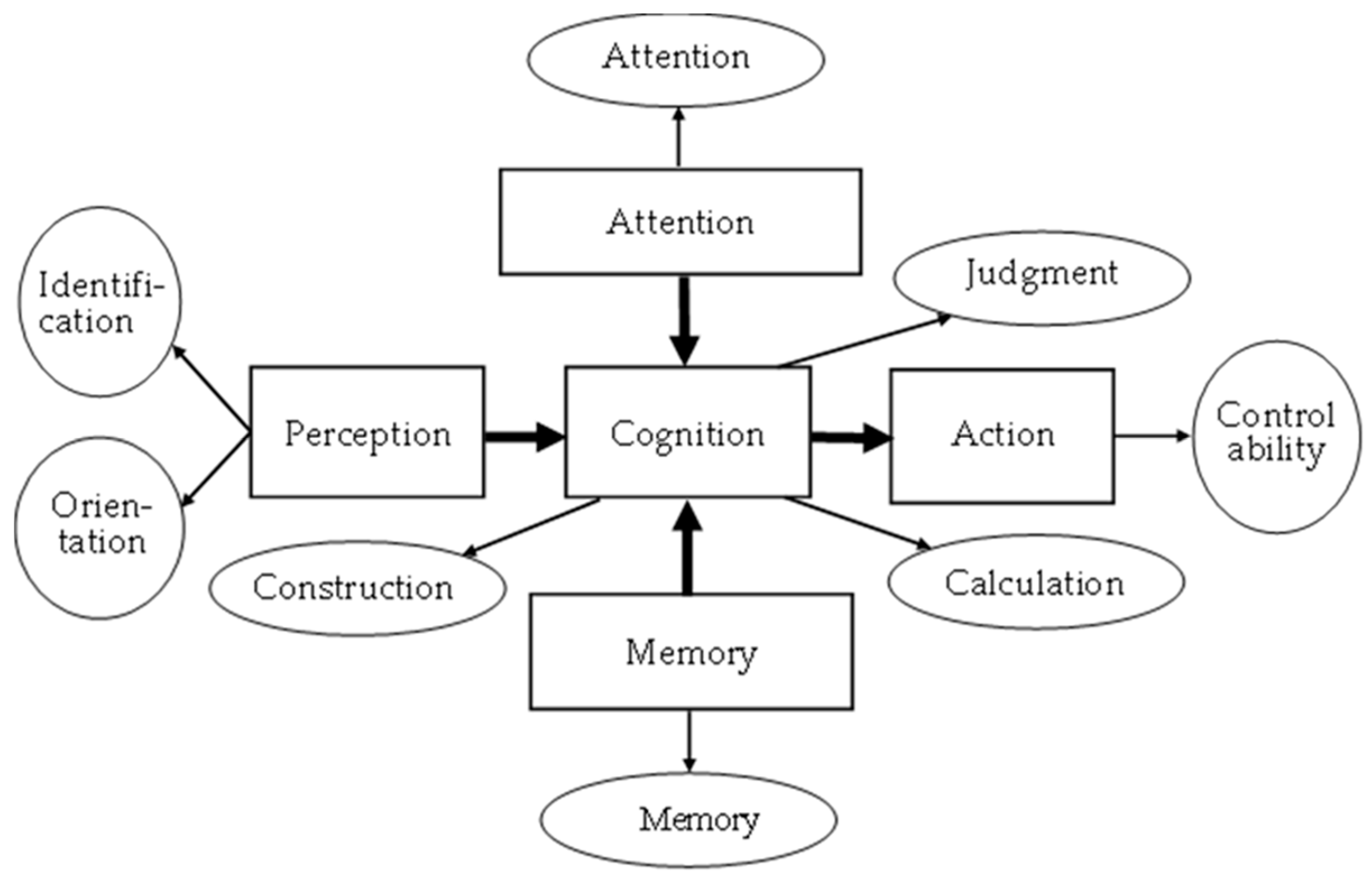
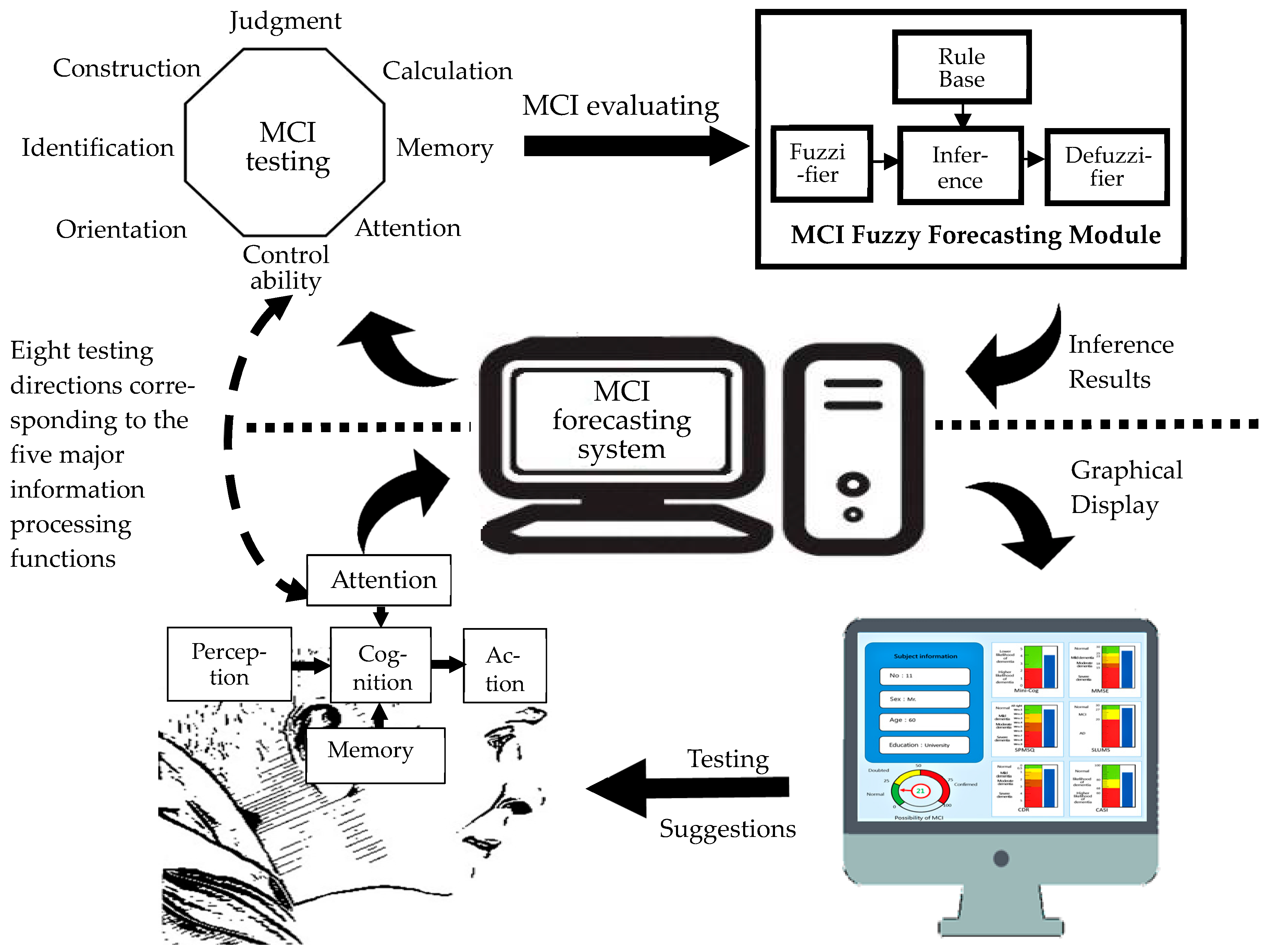
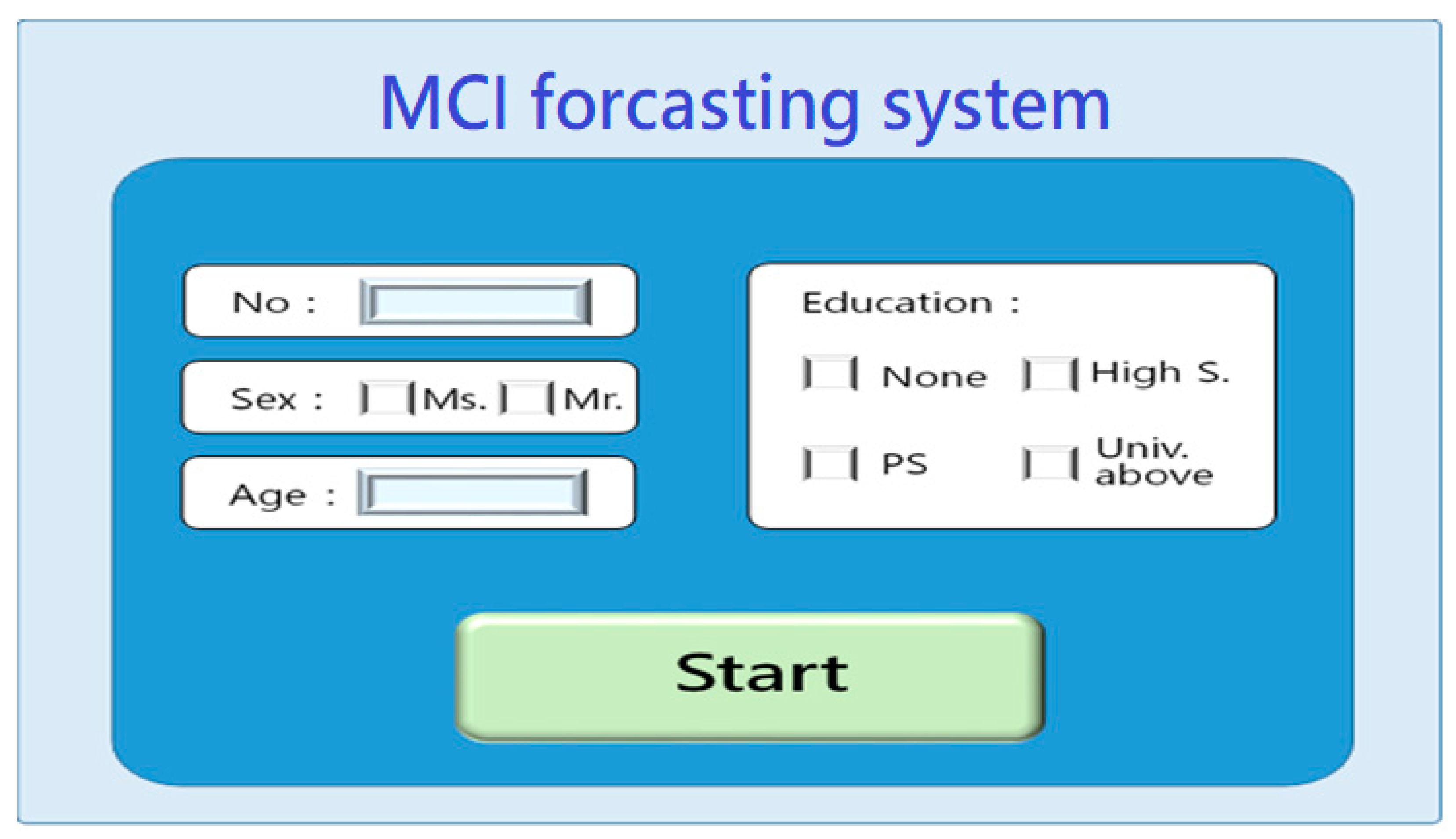


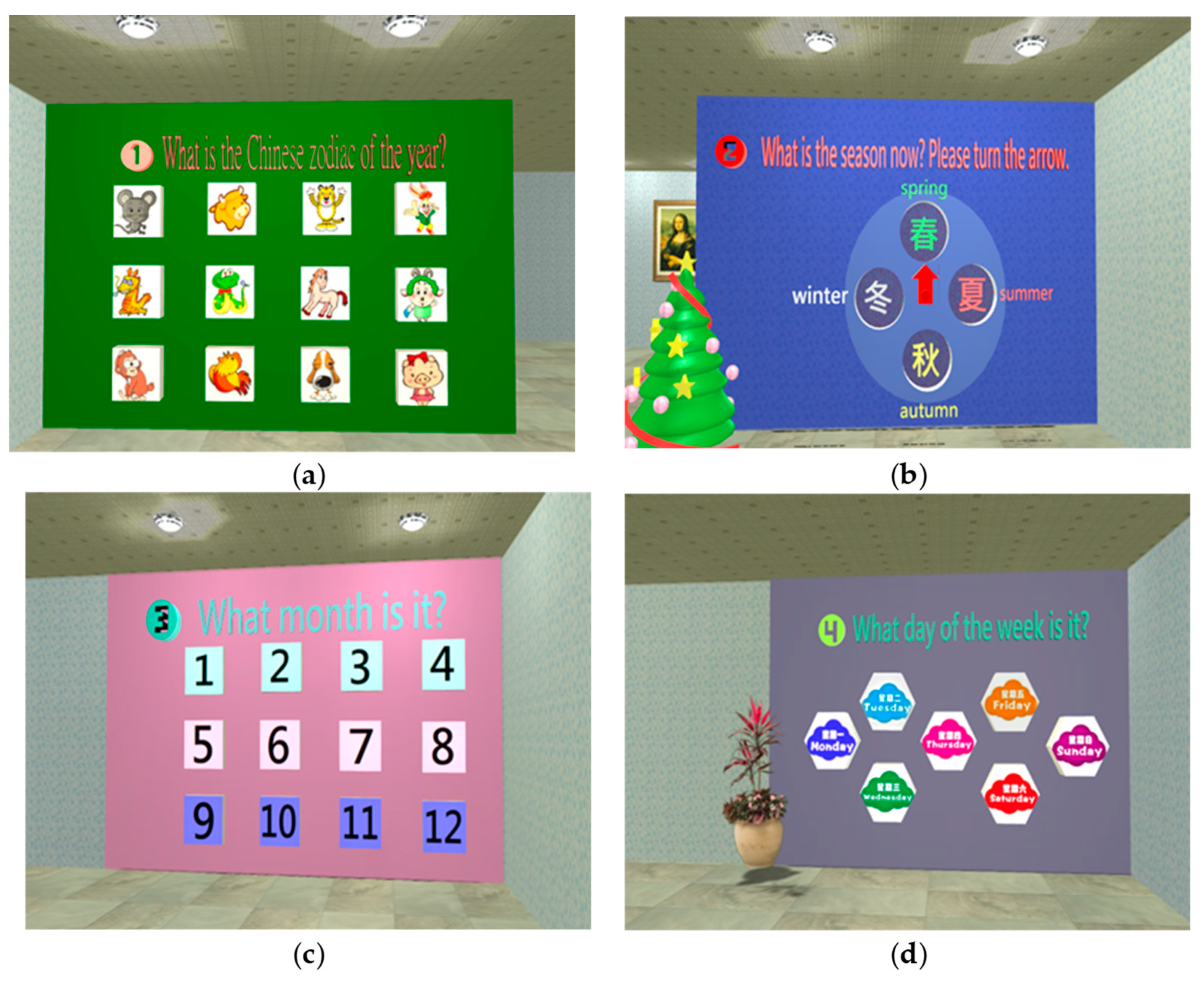





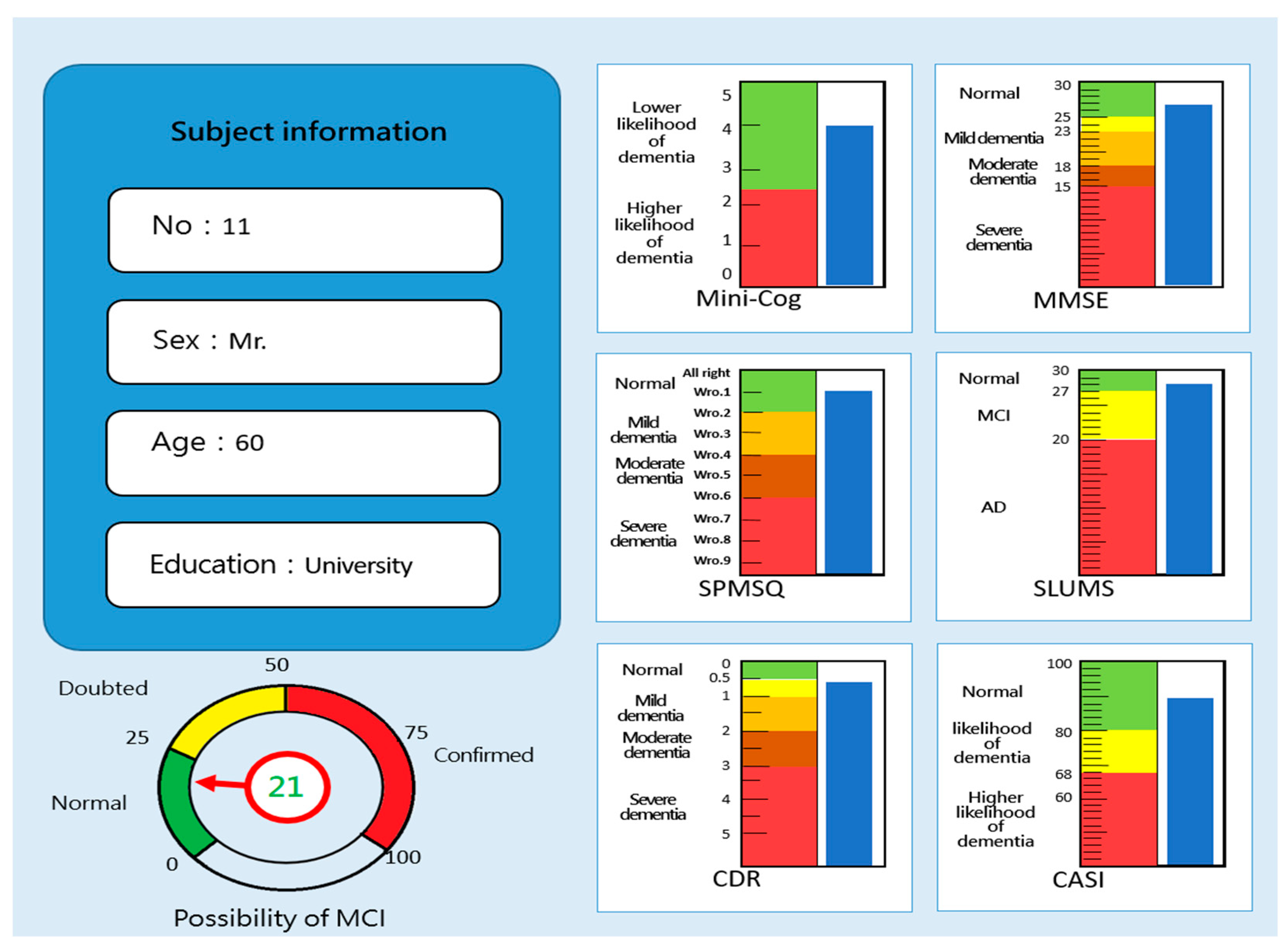
| Stage | Dimension | Sub-Item | Content | Mini-Cog | SPMSQ | MMSE | SLUMS | CDR | CASI |
|---|---|---|---|---|---|---|---|---|---|
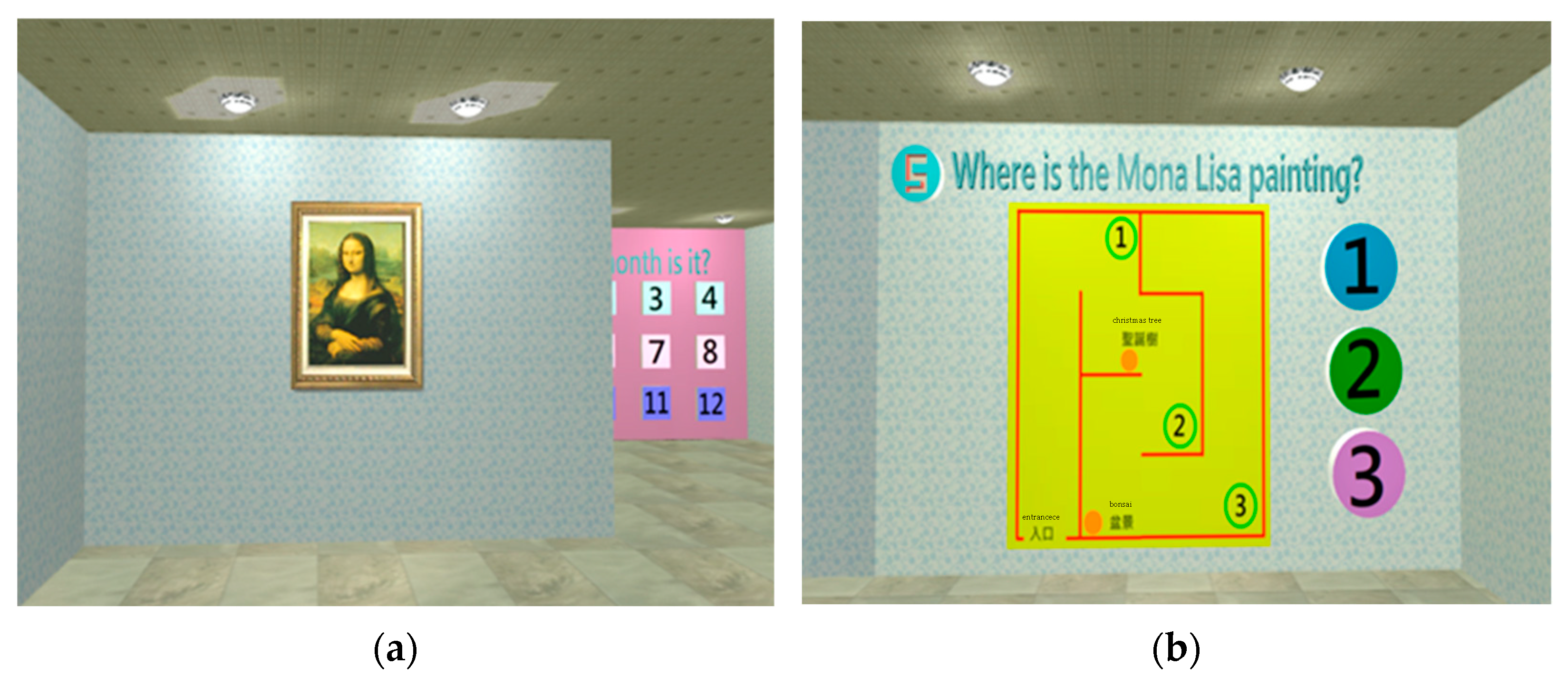
| Perception | Orientation | 1. Orientation | 1. Temporal orientation: (1) Zodiac sign? (2) What season is it? (3) What month is it? (4) What day of the week is it? 2. Spatial orientation: where is the Mona Lisa painting? |
Number of correct answers | 2–10 | 0.6–3 | 1. 1 point: 2 or 3 wrong answers; 2 points: 4 or 5 wrong answers 2. 0.5 points: wrong answer |
||
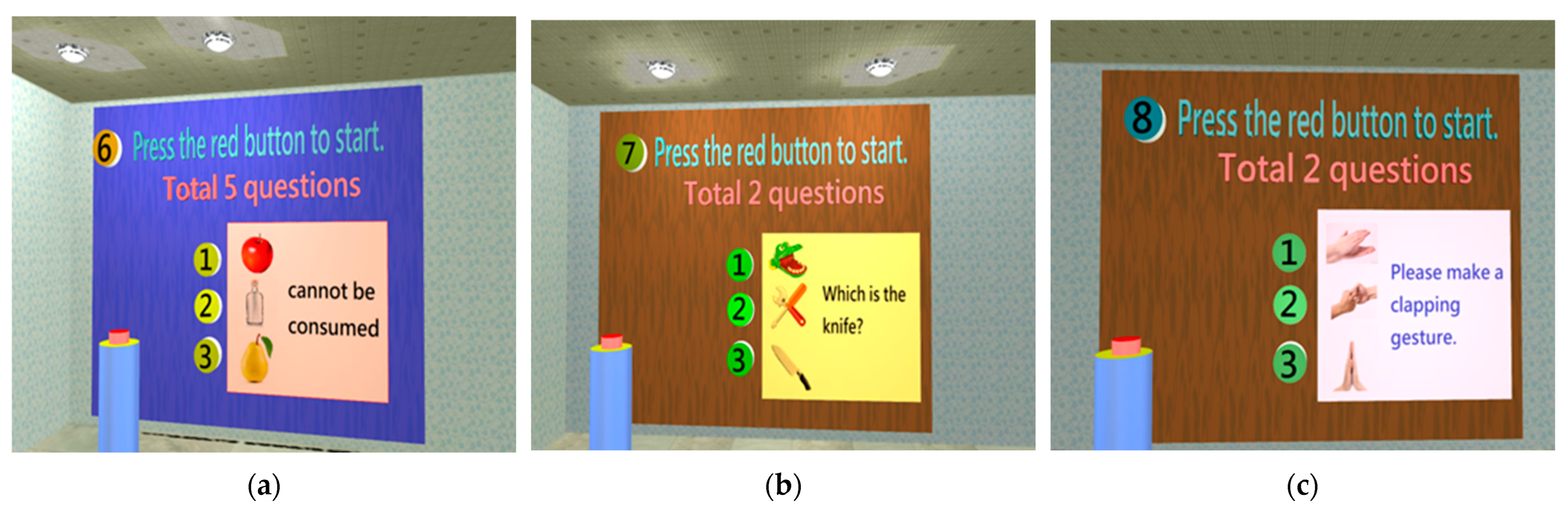
| Identification | 1. Language expression | 1. cannot be consumed (images: apple, bottle, pear) 2. Closed_(images: hands, eyes, pants) 3. I want to ride in a/an (images: mobile phone, airplane, camera) 4. I to eat (words: want, see, drink) 5. I walk (words: home, recollection, family) |
0.4 0.4 0.4 0.4 0.4 |
- | - | 1 1 1 1 1 |
|||
| 2. Image recognition | 1. Images (toys, knives, tools); select the knife 2. Images (television, fan, refrigerator); select the television |
- | 0.5 points: 1 or 2 wrong answers | 2.5 2.5 |
|||||
| 3. Language comprehension | 1. Clapping; select an image (clapping, applause, bringing the palms together) 2. Walking; select an image (jumping, walking, squatting) |
0.5 0.5 |
- | 0.5 points: 1 or 2 wrong answers | 1.5 1.5 |
||||
| Memory | Memory | 1. Short-term memory | 1. Images of banana, pineapple, watermelon, etc., will appear and the subject is required to select the banana, pineapple, and watermelon from the 5 objects for a total of 2 times. 2.     After memorizing, shape and color, matching is conducted twice. |
1. 1 point for correctly answering all questions 2. 1 point for correctly answering all questions |
1.5–6 | 1. 3–6 2. 3–6 |
0 points: 4 correct answers; 0.5 points: 3 correct answers; 1 point: 2 or fewer correct answers |
5–30 | |
| 2. Long-term memory | 1. Who is the current president of Taiwan? Photographs of Chen Shui-Bian, Ma Ying-Jeou, and Tsai Ing-Wen 2. Place of residence? Maps of Taiwan, Japan, and Hainan Island 3. How many minutes are there in 1 h? 4. How many months are there in 1 year? 5. In which direction does the sun set? |
Number of correct answers | 0.4–2 | 0 points: 4 correct answers; 0.5 points: 3 correct answers; 1 point: 2 or fewer correct answers |
2–10 | ||||
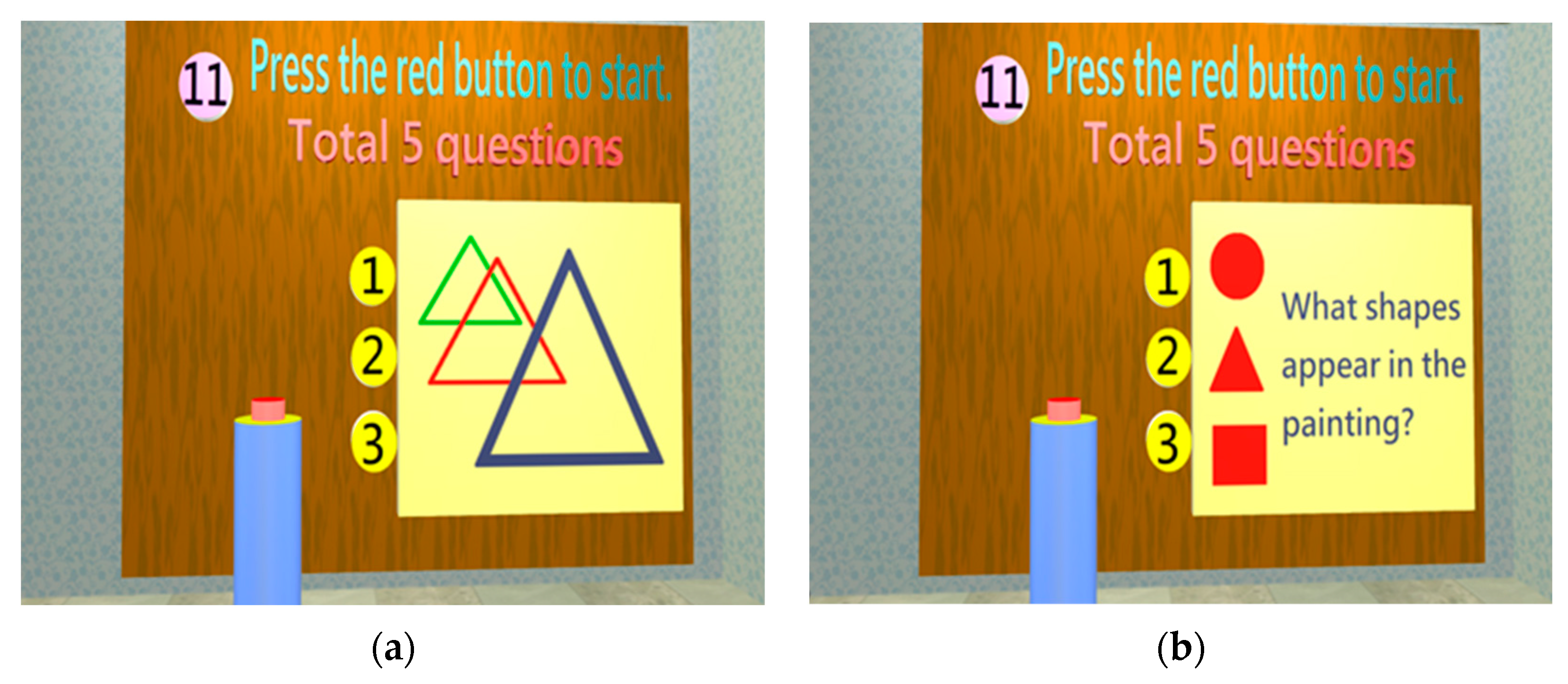
| Attention | Attention | 1. Attention | 1. Random shapes: determine the shape as △, □, and ○; 5 consecutive times | 1 point: 1 or more wrong answer | 1.2–6 | ||||
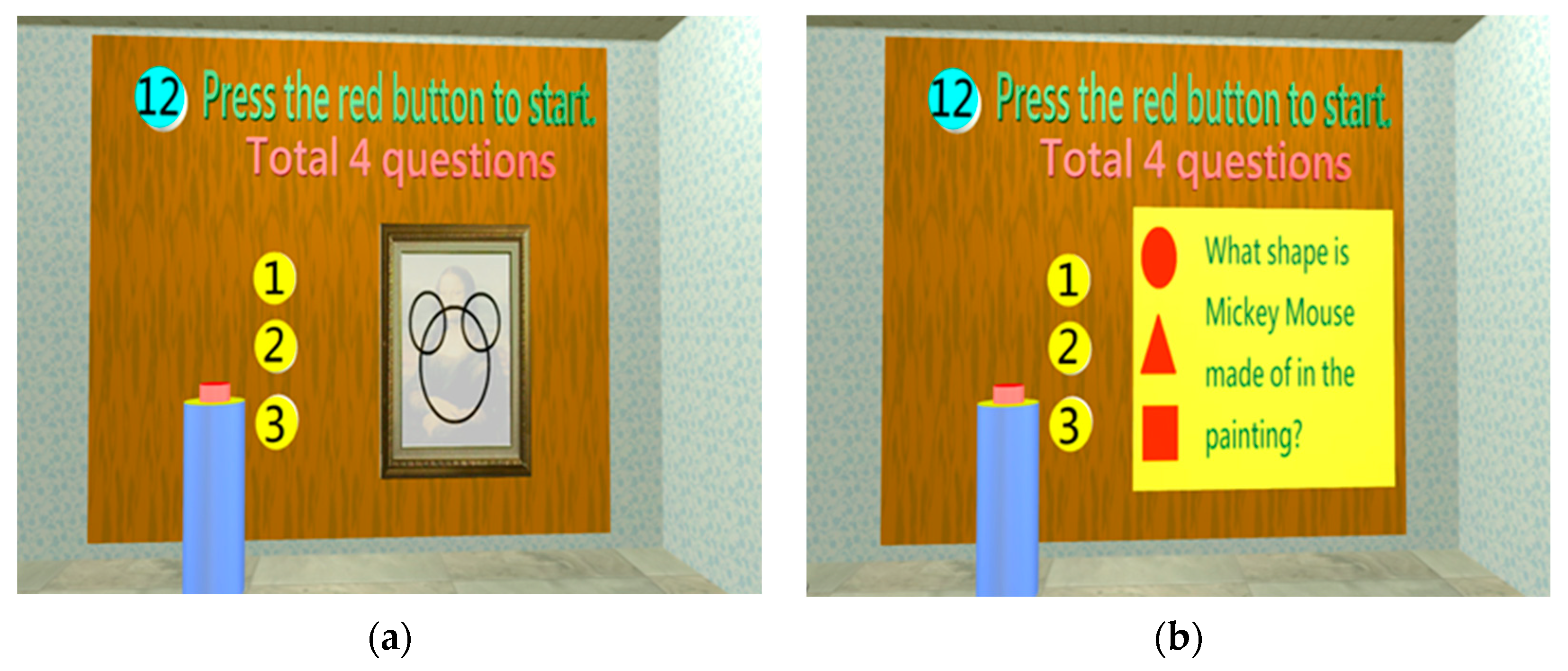
| Cognition | Construction | 1. Construction | 1. How do you draw Mickey Mouse using △, □, and ○? 2. How do you construct  using △, □, and ○? 3. How do you construct  using △, □, and ○? 4. Label the 2 o’clock and 8 o’clock positions in the image (2 positions). |
3 points: All correct answers 2 points: 1 wrong answer 1 point: 2 wrong answers 0 points: 3 or more wrong answers |
0.2–1 | 2.4–12 | 0 points: 4 correct answers; 0.5 points: 3 correct answers; 1 point: 2 or fewer correct answers |
3–15 | |
| Judgment | 1. Judgment | 1. Determine if the time is 10:10 2. What do physicians use? (Images: scalpel, bottle, and wooden rod) 3. What do teachers use? (Images: pot, books, and hammer) |
1 point: 2 wrong answers or more | 2–6 | |||||
| Calculation | 1. Calculations | 1. How many fish are there in total in the tank? 2. What is 3 + the previous answer? 3. What is 3 + the previous answer? 4. What is the previous answer + 3 twice? 5. You used 100 NTD to buy a 30 NTD apple and a 20 NTD orange. How much do you have left? |
Number of correct answers | 1 1 1 1 1 |
0.6 0.6 0.6 0.6 0.6 |
1 point: 3 wrong answers or more | 1 1 1 1 1 |
||
| Action | Control ability | 1. Control ability | 1. Rotate the cube to the front 2. Rotate the cube to the front  3. Rotate the cube to the front  |
1–3 | 1 point: 2 wrong answers or more | 1–3 |
| IF | MC | SP | MM | SL | CD | CA | CI | IF | MC | SP | MM | SL | CD | CA | CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Nor | Nor | Nor | Nor | Nor | Nor | Nor | 33 | Mild | Nor | Nor | Mild | Nor | Mild | Dou |
| 2 | Mild | Nor | Nor | Nor | Nor | Nor | Nor | 34 | Nor | Mild | Mild | Nor | Mild | Nor | Dou |
| 3 | Nor | Mild | Nor | Nor | Nor | Nor | Nor | 35 | Nor | Mild | Mild | Nor | Nor | Mild | Dou |
| 4 | Nor | Nor | Mild | Nor | Nor | Nor | Nor | 36 | Nor | Mild | Nor | Mild | Mild | Nor | Dou |
| 5 | Nor | Nor | Nor | Mild | Nor | Nor | Nor | 37 | Nor | Mild | Nor | Mild | Nor | Mild | Dou |
| 6 | Nor | Nor | Nor | Nor | Mild | Nor | Nor | 38 | Nor | Mild | Nor | Nor | Mild | Mild | Dou |
| 7 | Nor | Nor | Nor | Nor | Nor | Mild | Nor | 39 | Nor | Nor | Mild | Mild | Mild | Nor | Dou |
| 8 | Mild | Mild | Nor | Nor | Nor | Nor | Dou | 40 | Nor | Nor | Mild | Mild | Nor | Mild | Dou |
| 9 | Mild | Nor | Mild | Nor | Nor | Nor | Dou | 41 | Nor | Nor | Mild | Nor | Mild | Mild | Dou |
| 10 | Mild | Nor | Nor | Mild | Nor | Nor | Dou | 42 | Nor | Nor | Nor | Mild | Mild | Mild | Dou |
| 11 | Mild | Nor | Nor | Nor | Mild | Nor | Dou | 43 | Mild | Mild | Mild | Mild | Nor | Nor | Dou |
| 12 | Mild | Nor | Nor | Nor | Nor | Mild | Dou | 44 | Mild | Mild | Mild | Nor | Mild | Nor | Dou |
| 13 | Nor | Mild | Mild | Nor | Nor | Nor | Dou | 45 | Mild | Mild | Mild | Nor | Nor | Mild | Dou |
| 14 | Nor | Mild | Nor | Mild | Nor | Nor | Dou | 46 | Mild | Mild | Nor | Mild | Mild | Nor | Dou |
| 15 | Nor | Mild | Nor | Nor | Mild | Nor | Dou | 47 | Mild | Mild | Nor | Mild | Nor | Mild | Dou |
| 16 | Nor | Mild | Nor | Nor | Nor | Mild | Dou | 48 | Mild | Mild | Nor | Nor | Mild | Mild | Dou |
| 17 | Nor | Nor | Mild | Nor | Nor | Mild | Dou | 49 | Mild | Nor | Mild | Mild | Mild | Nor | Dou |
| 18 | Nor | Nor | Mild | Nor | Mild | Nor | Dou | 50 | Mild | Nor | Mild | Mild | Nor | Mild | Dou |
| 19 | Nor | Nor | Mild | Mild | Nor | Nor | Dou | 51 | Mild | Nor | Mild | Nor | Mild | Mild | Dou |
| 20 | Nor | Nor | Mild | Mild | Nor | Nor | Dou | 52 | Mild | Nor | Nor | Mild | Mild | Mild | Dou |
| 21 | Nor | Nor | Nor | Mild | Nor | Mild | Dou | 53 | Nor | Mild | Mild | Mild | Mild | Nor | Dou |
| 22 | Nor | Nor | Nor | Nor | Mild | Mild | Dou | 54 | Nor | Mild | Mild | Mild | Nor | Mild | Dou |
| 23 | Mild | Mild | Mild | Nor | Nor | Nor | Dou | 55 | Nor | Mild | Mild | Nor | Mild | Mild | Dou |
| 24 | Mild | Mild | Nor | Mild | Nor | Nor | Dou | 56 | Nor | Mild | Nor | Mild | Mild | Mild | Dou |
| 25 | Mild | Mild | Nor | Nor | Mild | Nor | Dou | 57 | Nor | Nor | Mild | Mild | Mild | Mild | Dou |
| 26 | Mild | Mild | Nor | Nor | Nor | Mild | Dou | 58 | Mild | Mild | Mild | Mild | Mild | Nor | Dou |
| 27 | Mild | Nor | Mild | Mild | Nor | Nor | Dou | 59 | Mild | Mild | Mild | Mild | Nor | Mild | Dou |
| 28 | Mild | Nor | Mild | Mild | Nor | Nor | Dou | 60 | Mild | Mild | Mild | Nor` | Mild | Mild | Dou |
| 29 | Mild | Nor | Mild | Nor | Nor | Mild | Dou | 61 | Mild | Mild | Nor` | Mild | Mild | Mild | Dou |
| 30 | Mild | Nor | Nor | Mild | Mild | Nor | Dou | 62 | Mild | Nor | Mild | Mild | Mild | Mild | Dou |
| 31 | Mild | Nor | Nor | Mild | Nor | Mild | Dou | 63 | Nor | Mild | Mild | Mild | Mild | Mild | Dou |
| 32 | Mild | Nor | Nor | Mild | Nor | Mild | Dou | 64 | Mild | Mild | Mild | Mild | Mild | Mild | Dou |
| Test Method | The Corresponding Scores Generated by “MCI Assessment System” Test | ||||||
|---|---|---|---|---|---|---|---|
| Mini-Cog | SPMSQ | MMSE | SLUMS | CDR | CASI | ||
| Traditional screening tests | Mini-Cog | 0.7894 | - | - | - | - | - |
| SPMSQ | - | 0.8020 | - | - | - | - | |
| MMSE | - | - | 0.8875 | - | - | - | |
| SLUMS | - | - | - | 0.7715 | - | - | |
| CDR | - | - | - | - | 0.6892 | - | |
| CASI | - | - | - | - | - | 0.9141 | |
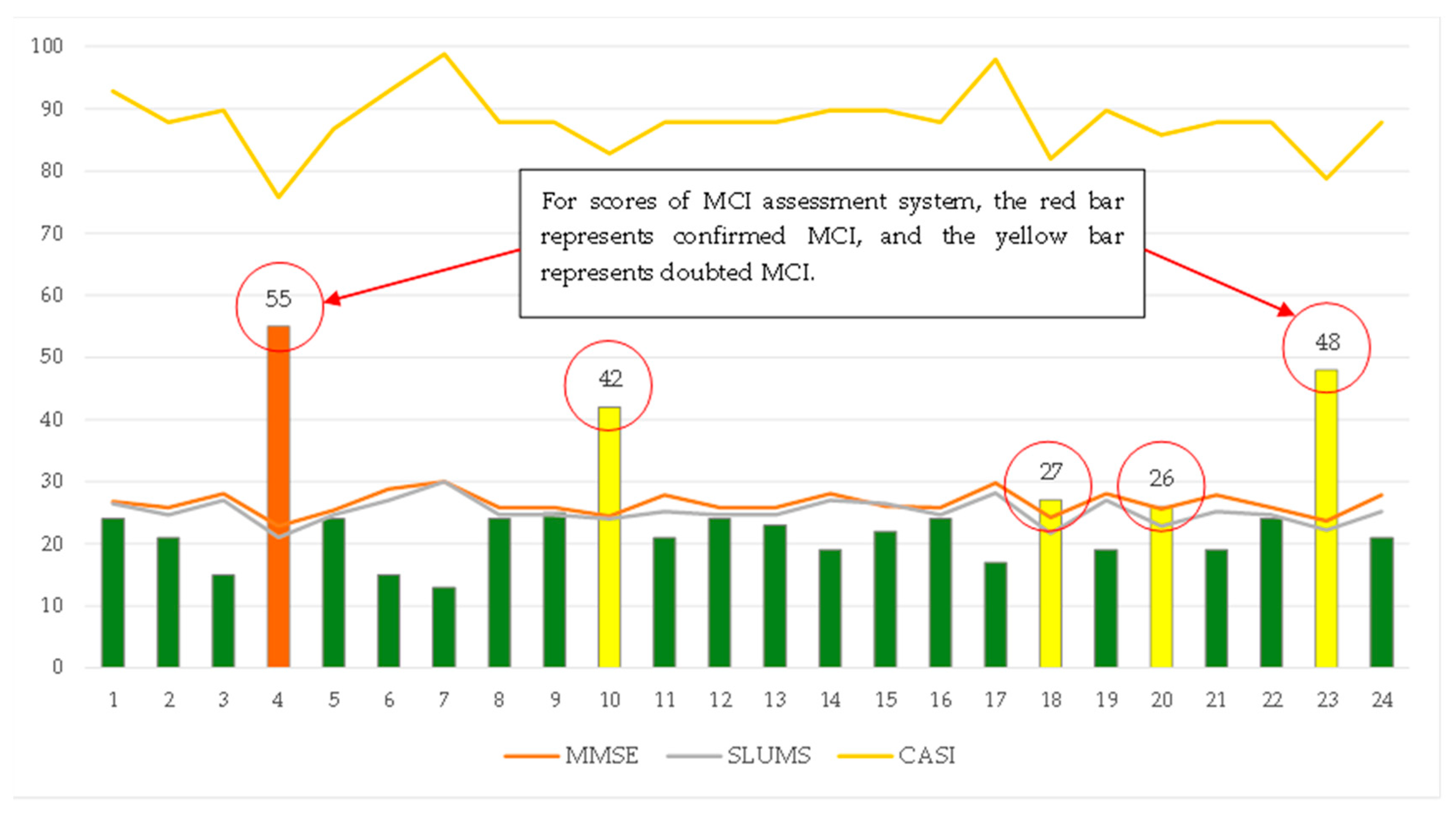
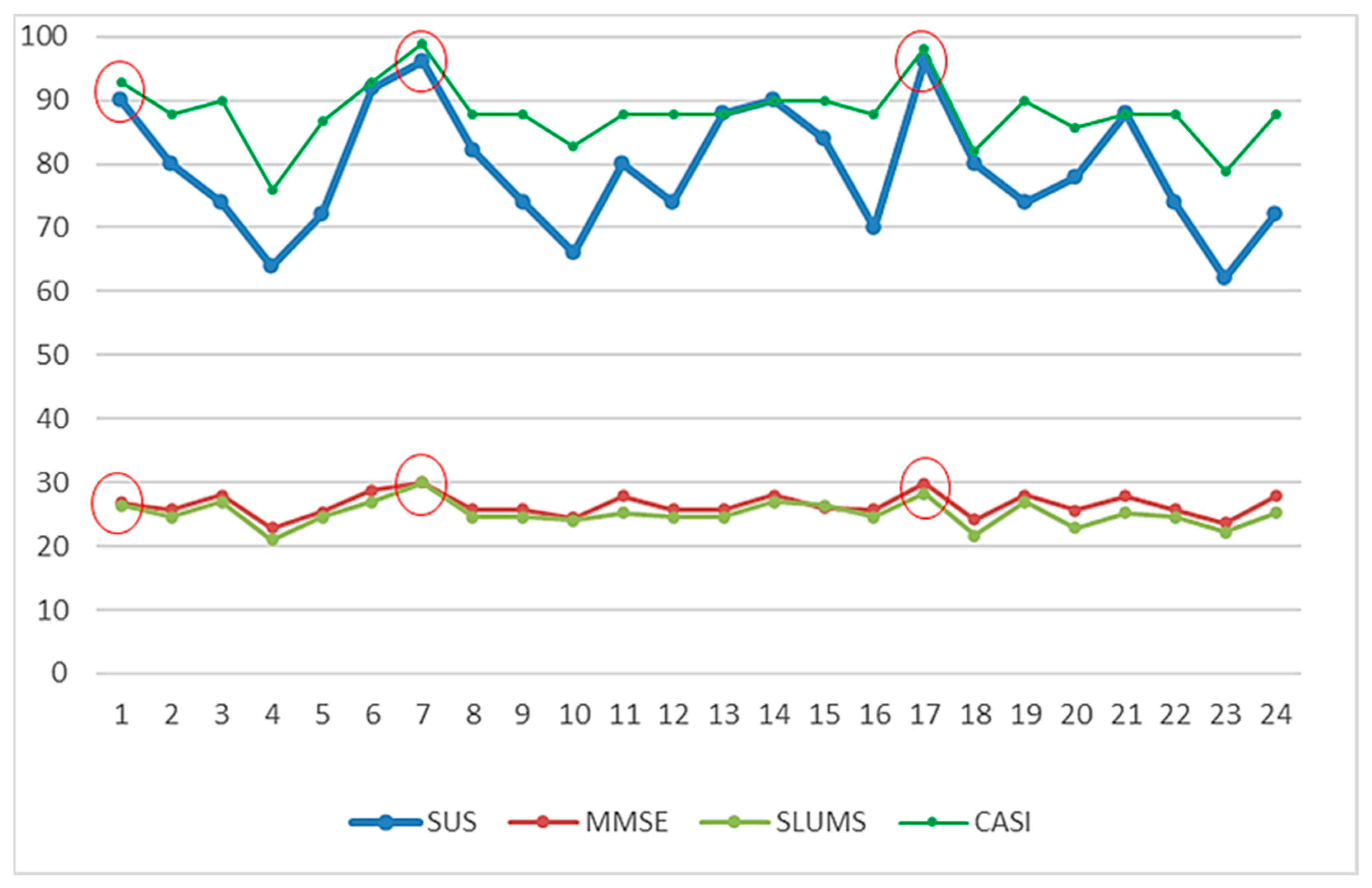
| No | Traditional Screening Tests | The Corresponding Scores Generated by the “MCI Assessment System” Test | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mini-Cog | SPMSQ | MMSE | SLUMS | CDR | CASI | Mini-Cog | SPMSQ | MMSE | SLUMS | CDR | CASI | |
| 1 | ||||||||||||
| 2 | ||||||||||||
| 3 | ||||||||||||
| 4 | ||||||||||||
| 5 | ||||||||||||
| 6 | ||||||||||||
| 7 | ||||||||||||
| 8 | ||||||||||||
| 9 | ||||||||||||
| 10 | ||||||||||||
| 11 | ||||||||||||
| 12 | ||||||||||||
| 13 | ||||||||||||
| 14 | ||||||||||||
| 15 | ||||||||||||
| 16 | ||||||||||||
| 17 | ||||||||||||
| 18 | ||||||||||||
| 19 | ||||||||||||
| 20 | ||||||||||||
| 21 | ||||||||||||
| 22 | ||||||||||||
| 23 | ||||||||||||
| 24 | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





