Submitted:
14 July 2023
Posted:
17 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Strains and genome sequences
2.2. Gene cluster prediction and similarity network analysis
2.3. Cluster analysis based on evolutionary trees and sequence identity analysis
2.4. Structural analysis of multidomain-containing enzymes
2.5. Homology and similarity analysis of BGCs
2.6. Three-dimensional structure modelling and prediction of proteins
3. Results
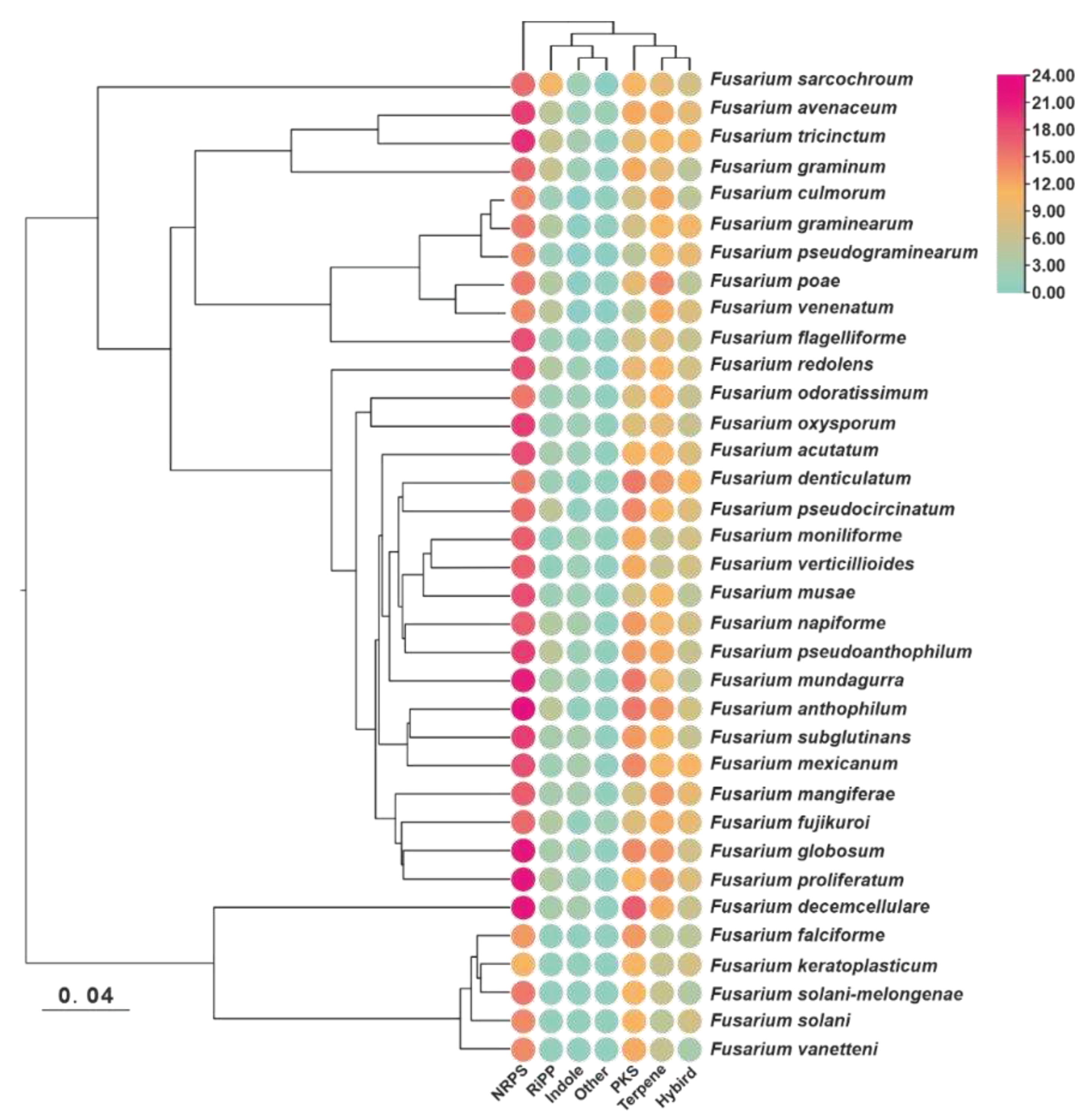
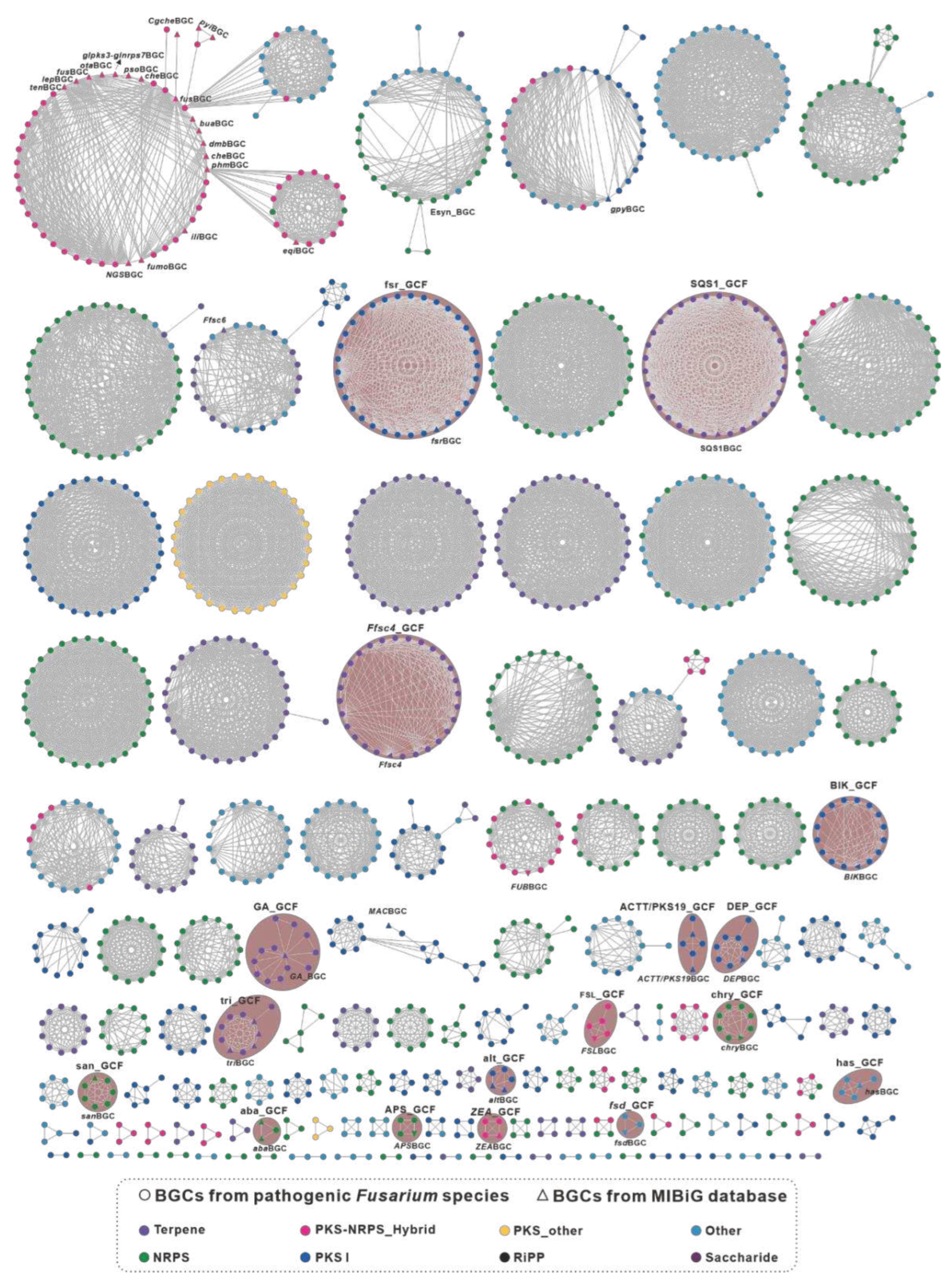
3.1. Biosynthetic classes and network analysis for the BGCs from pathogenic Fusarium species
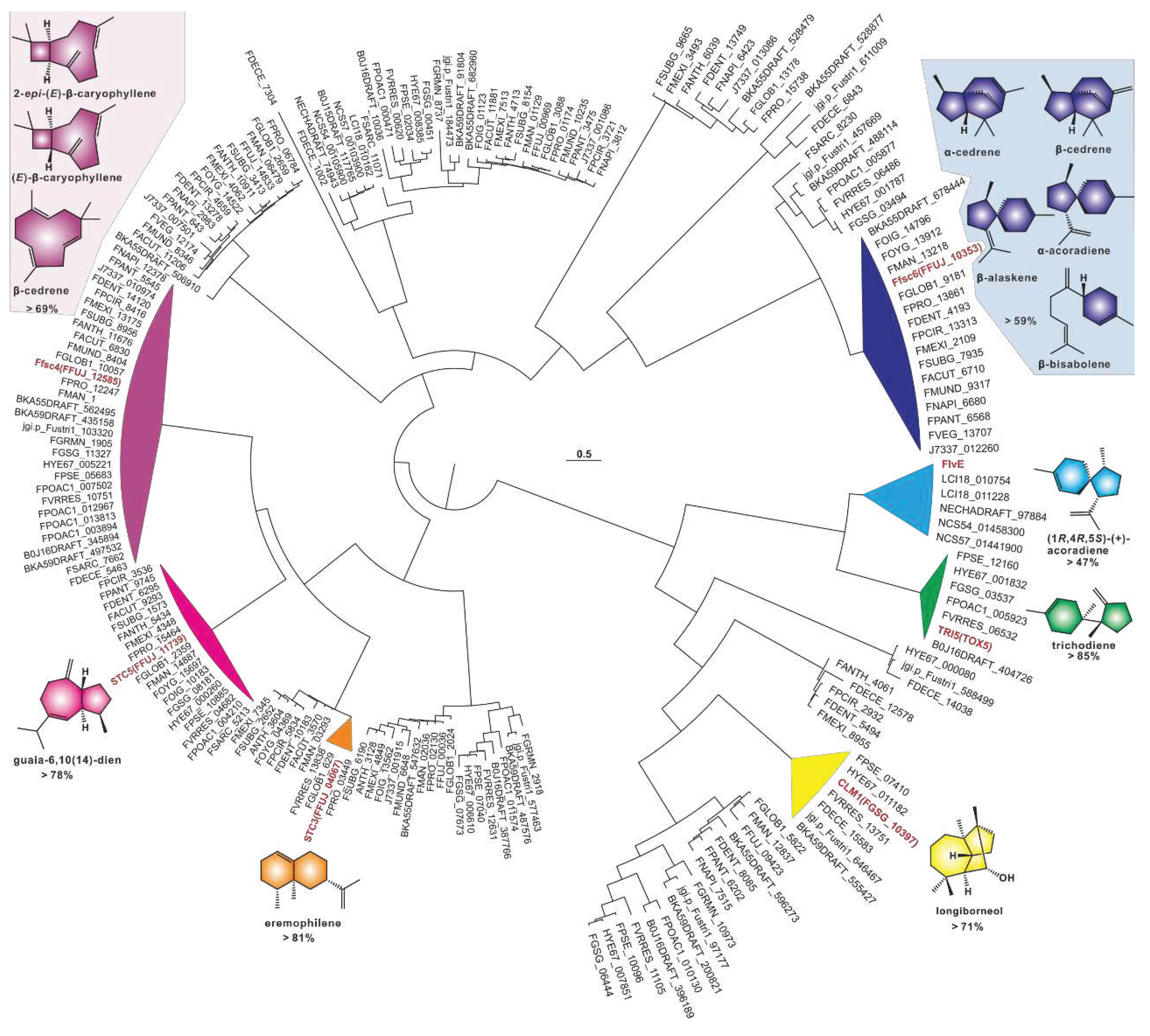
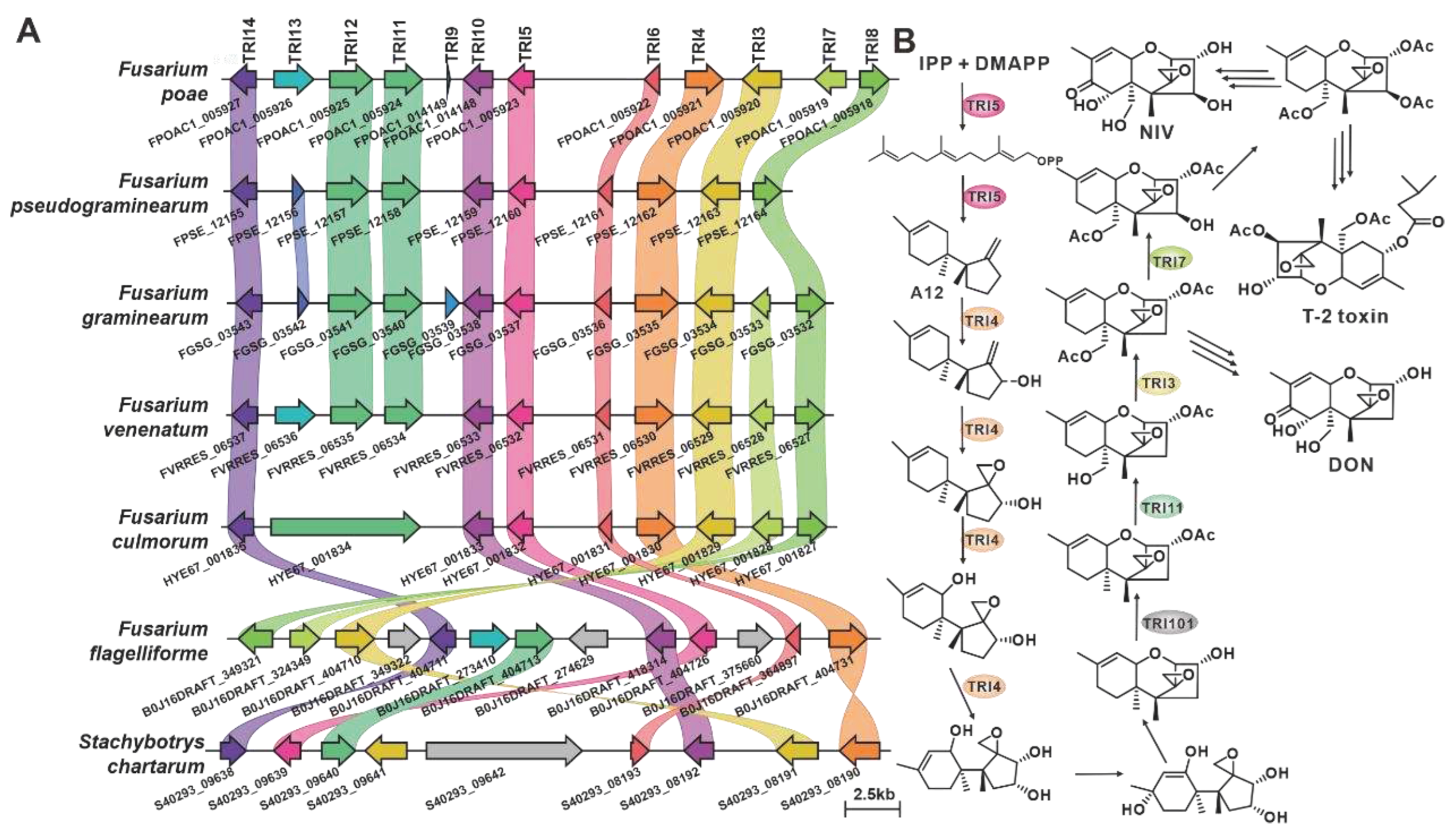
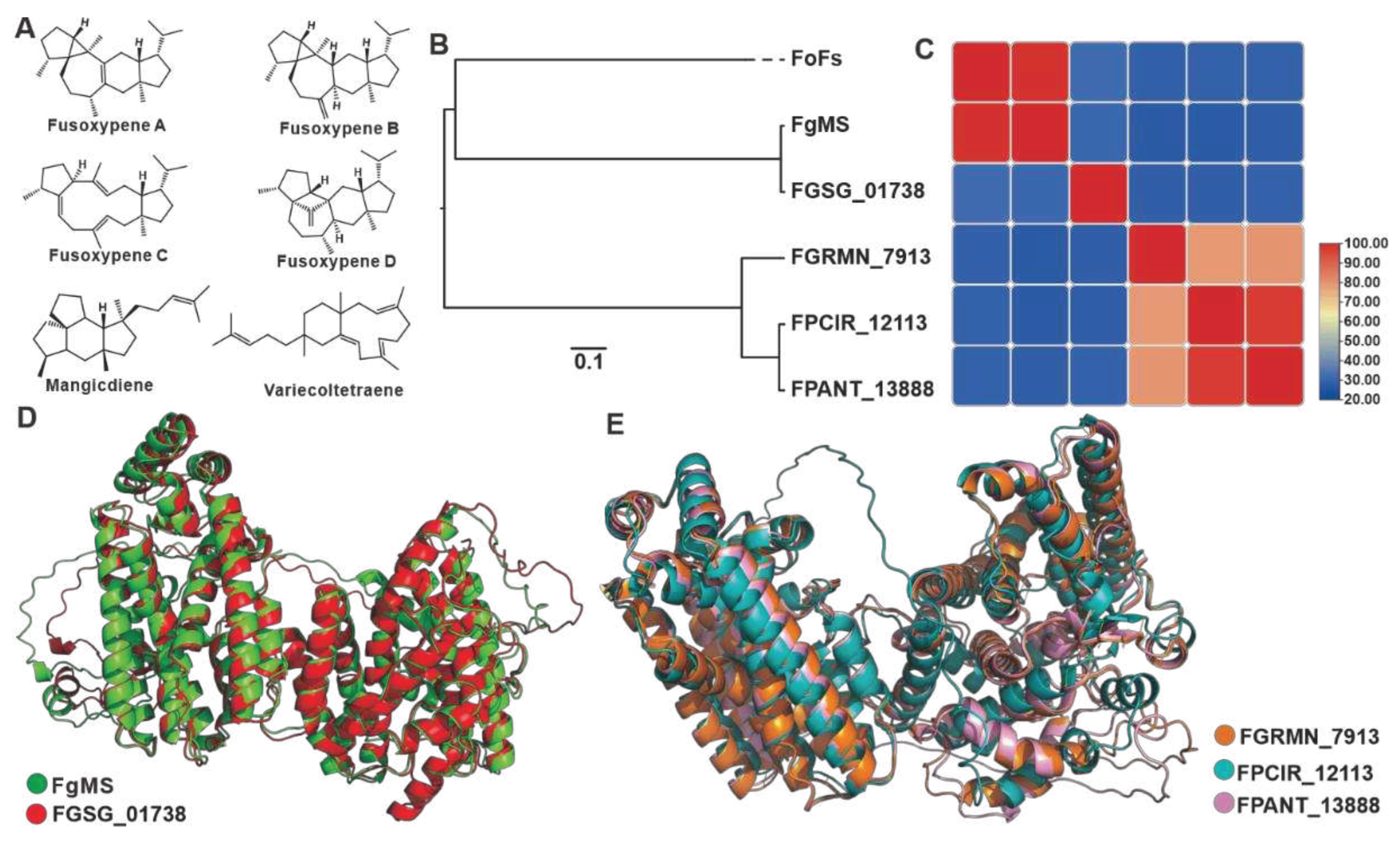
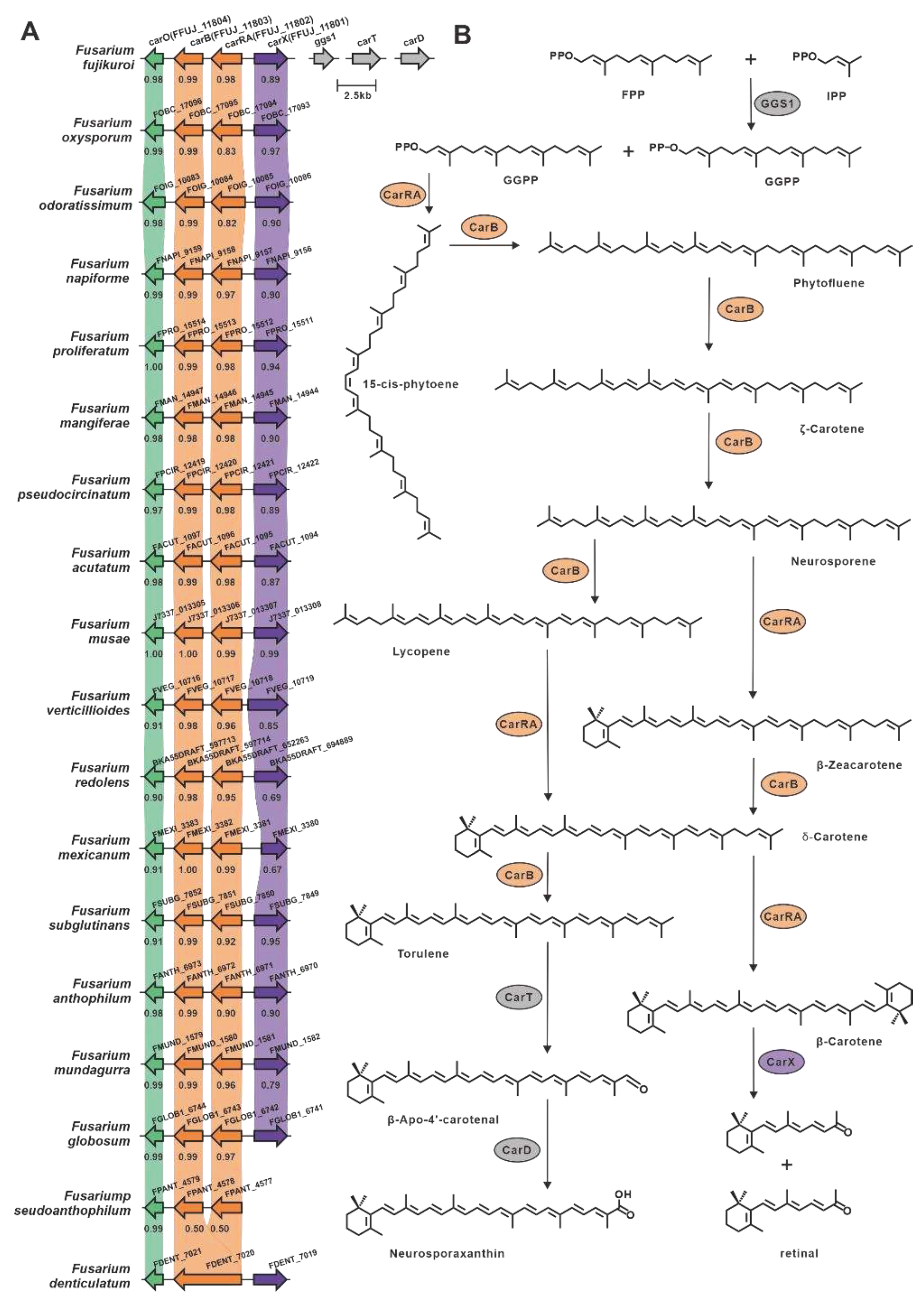
3.2. Terpene biosynthetic pathway of pathogenic Fusarium species
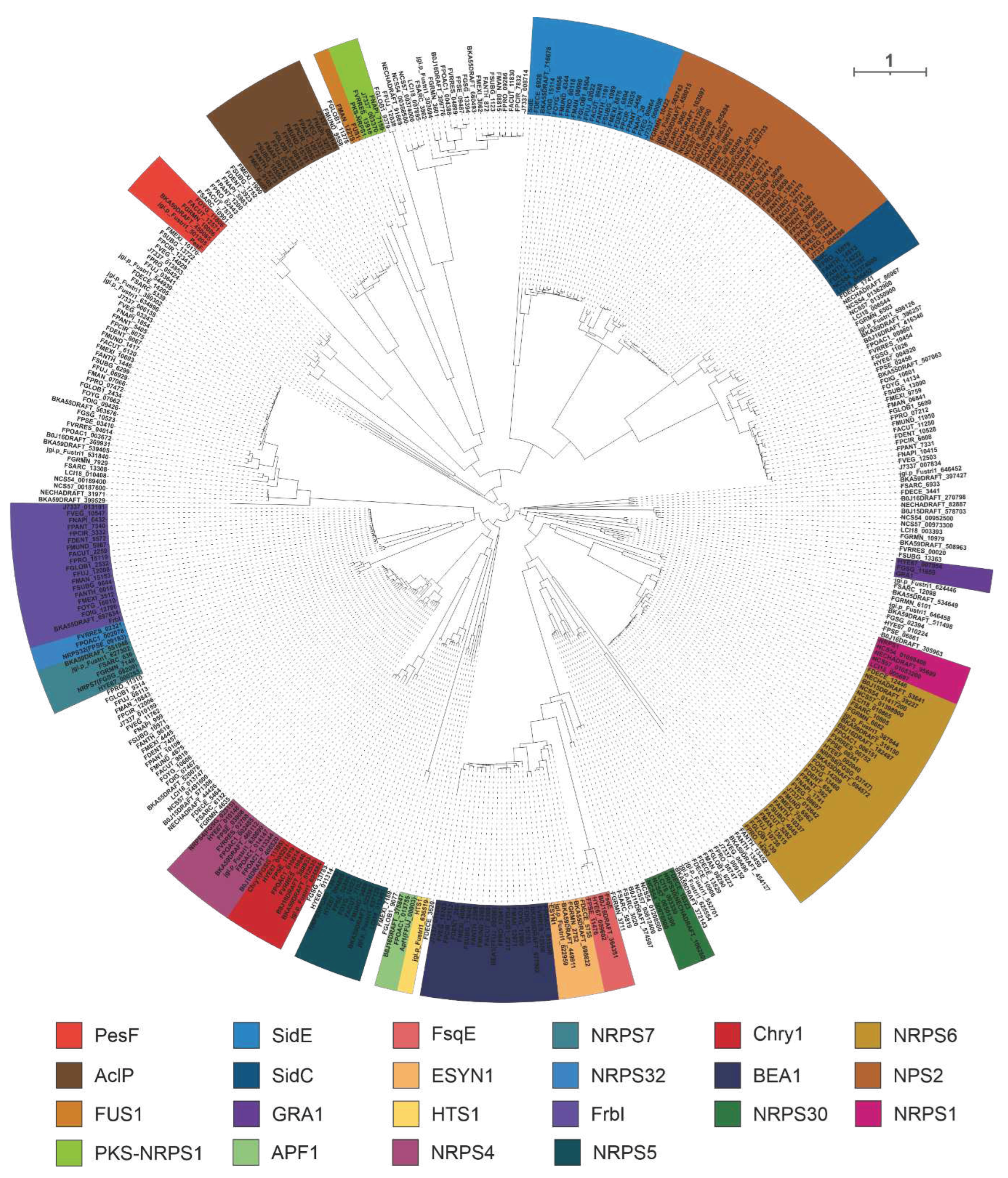
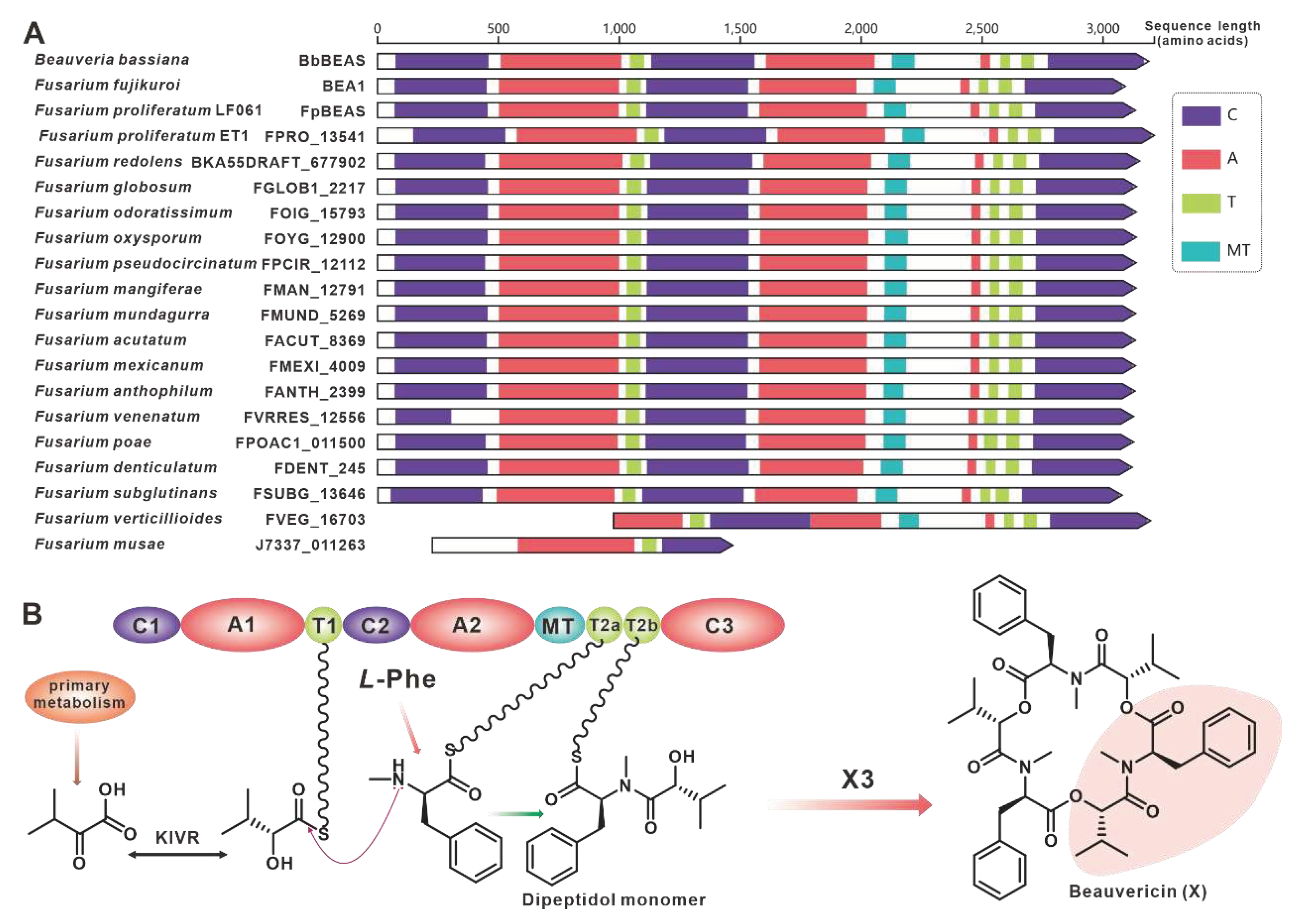
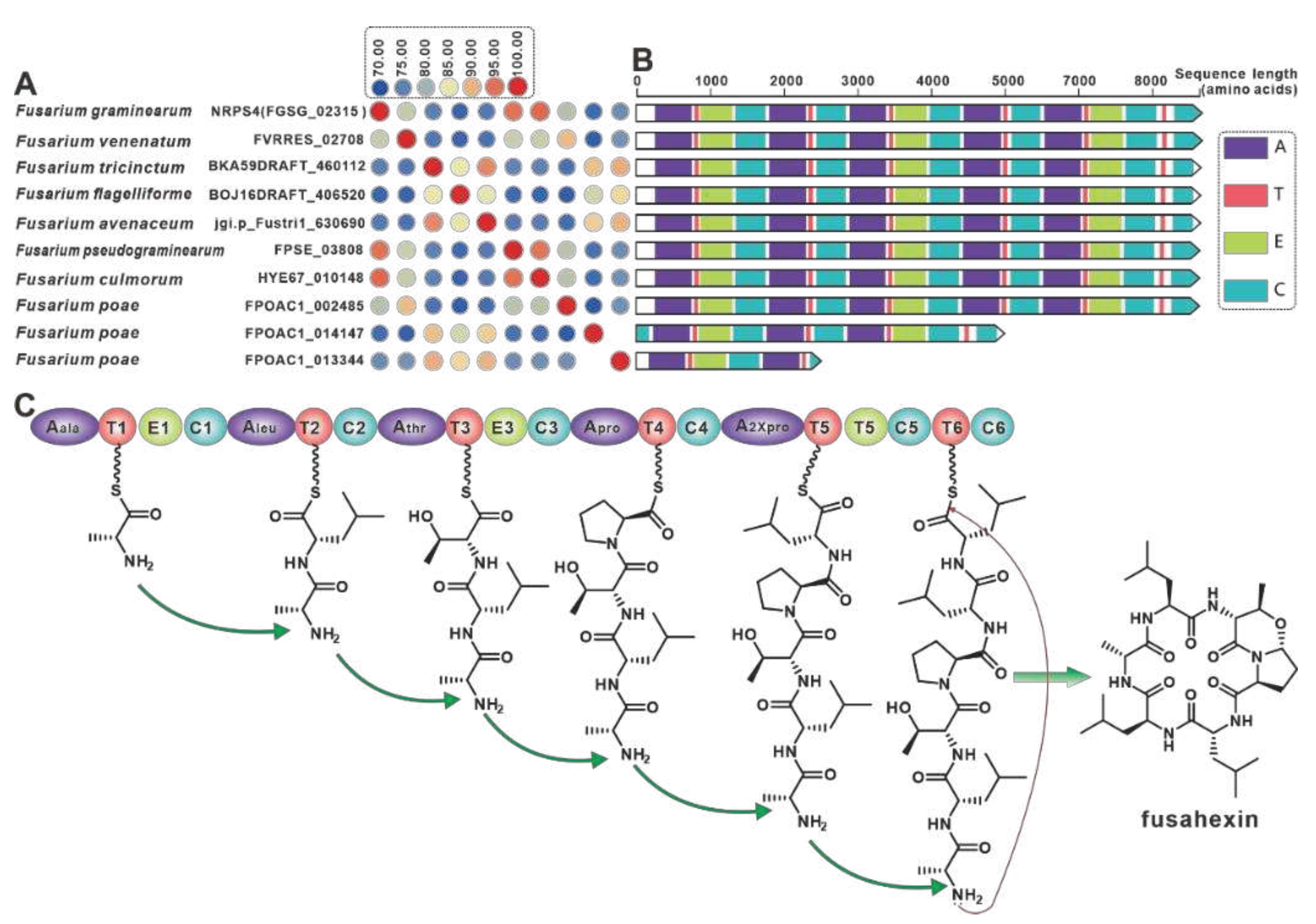
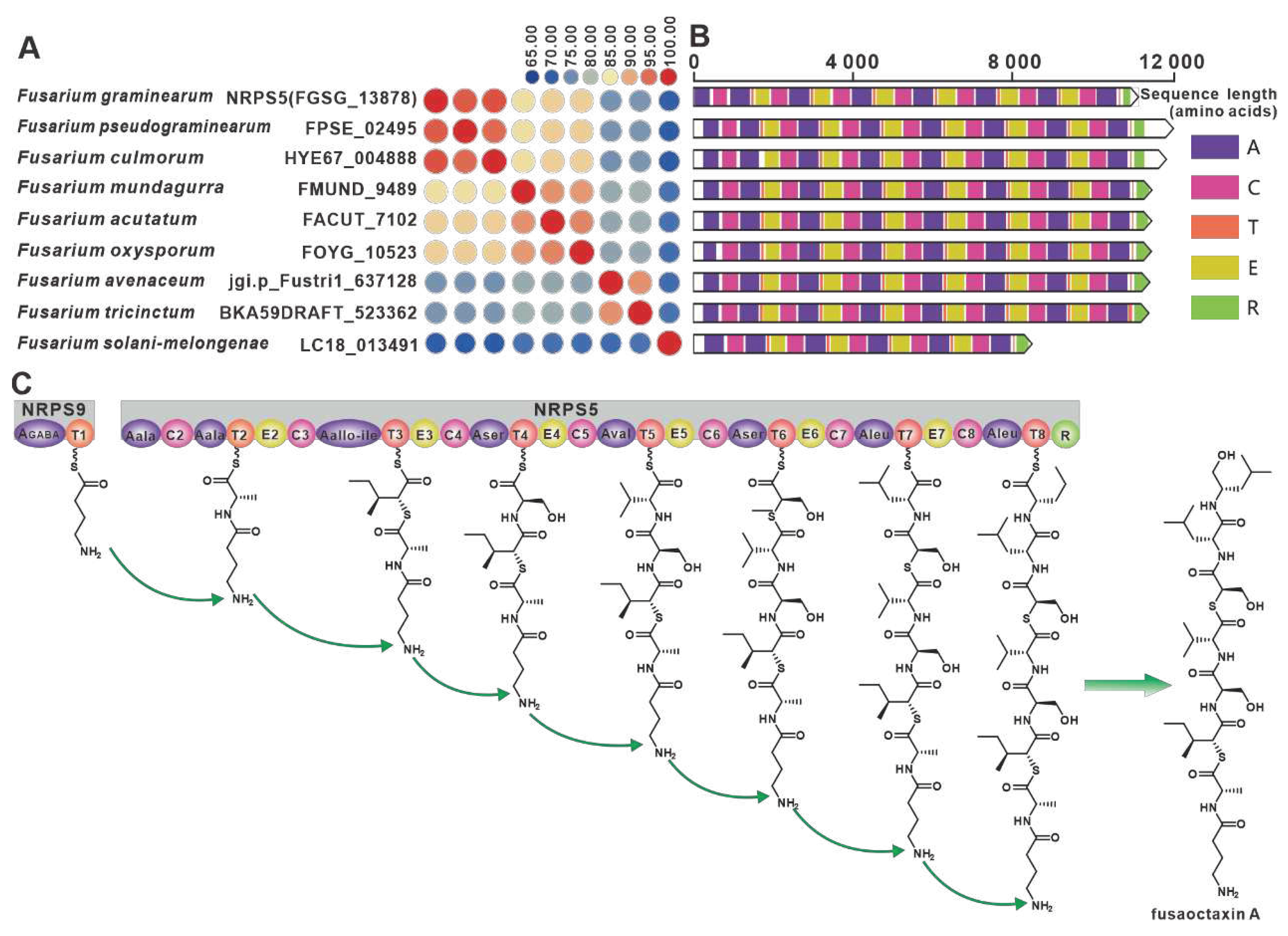
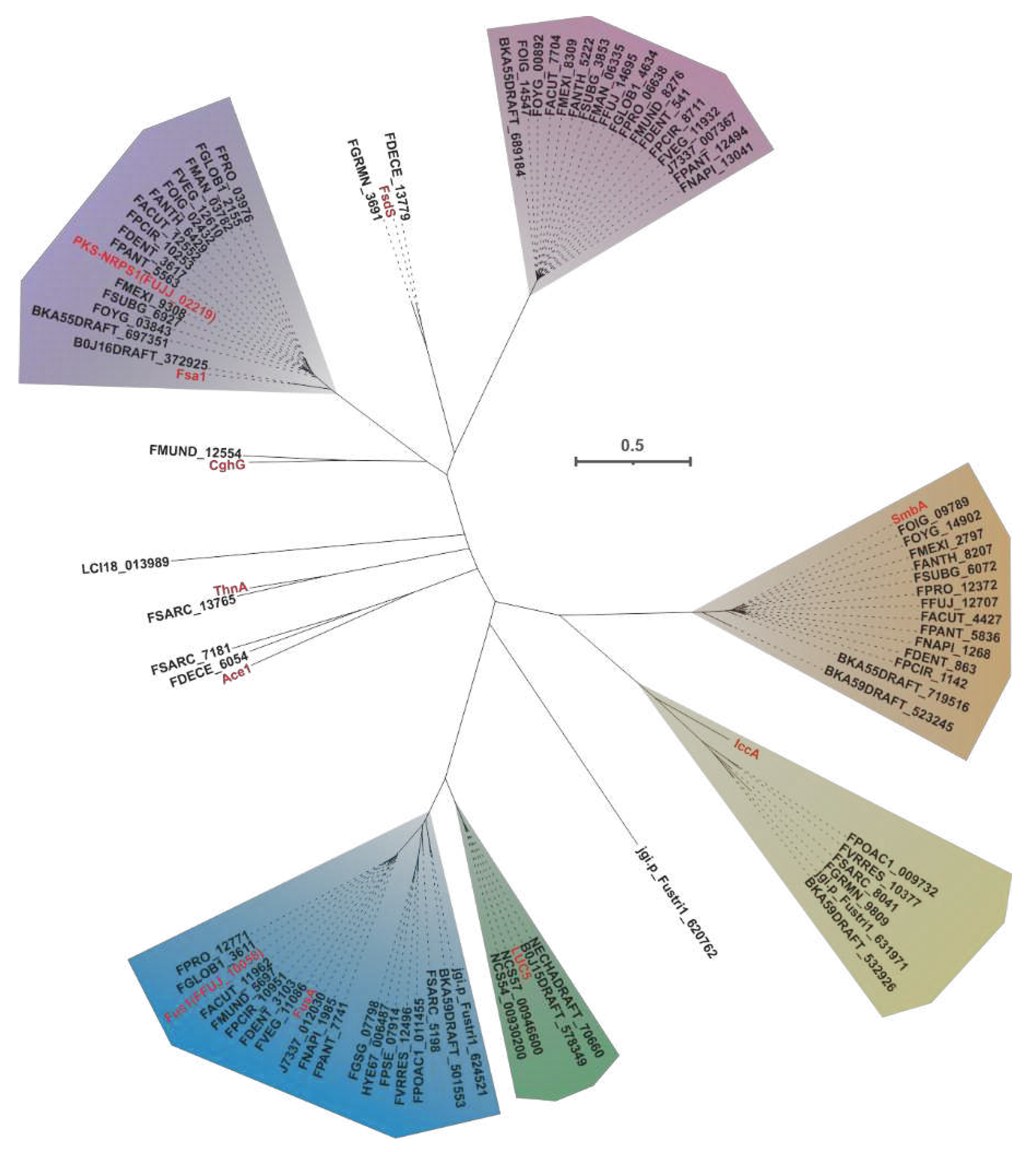
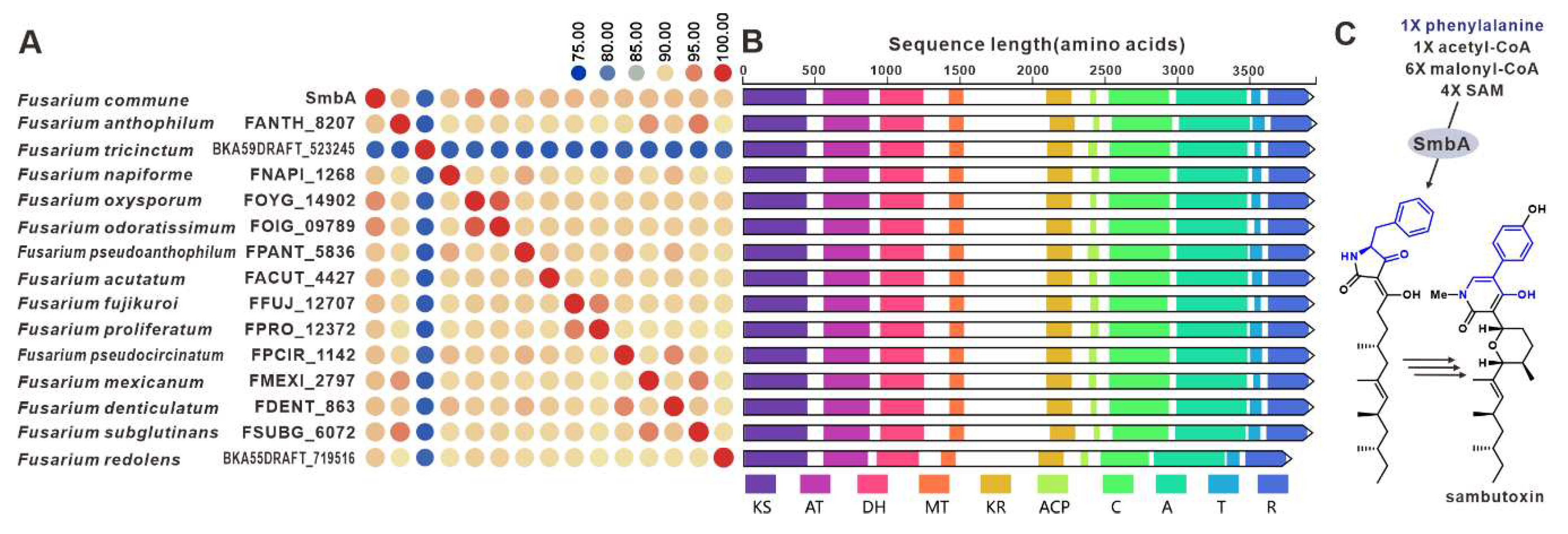
3.3. Nonribosomal peptide biosynthetic pathway of pathogenic Fusarium species
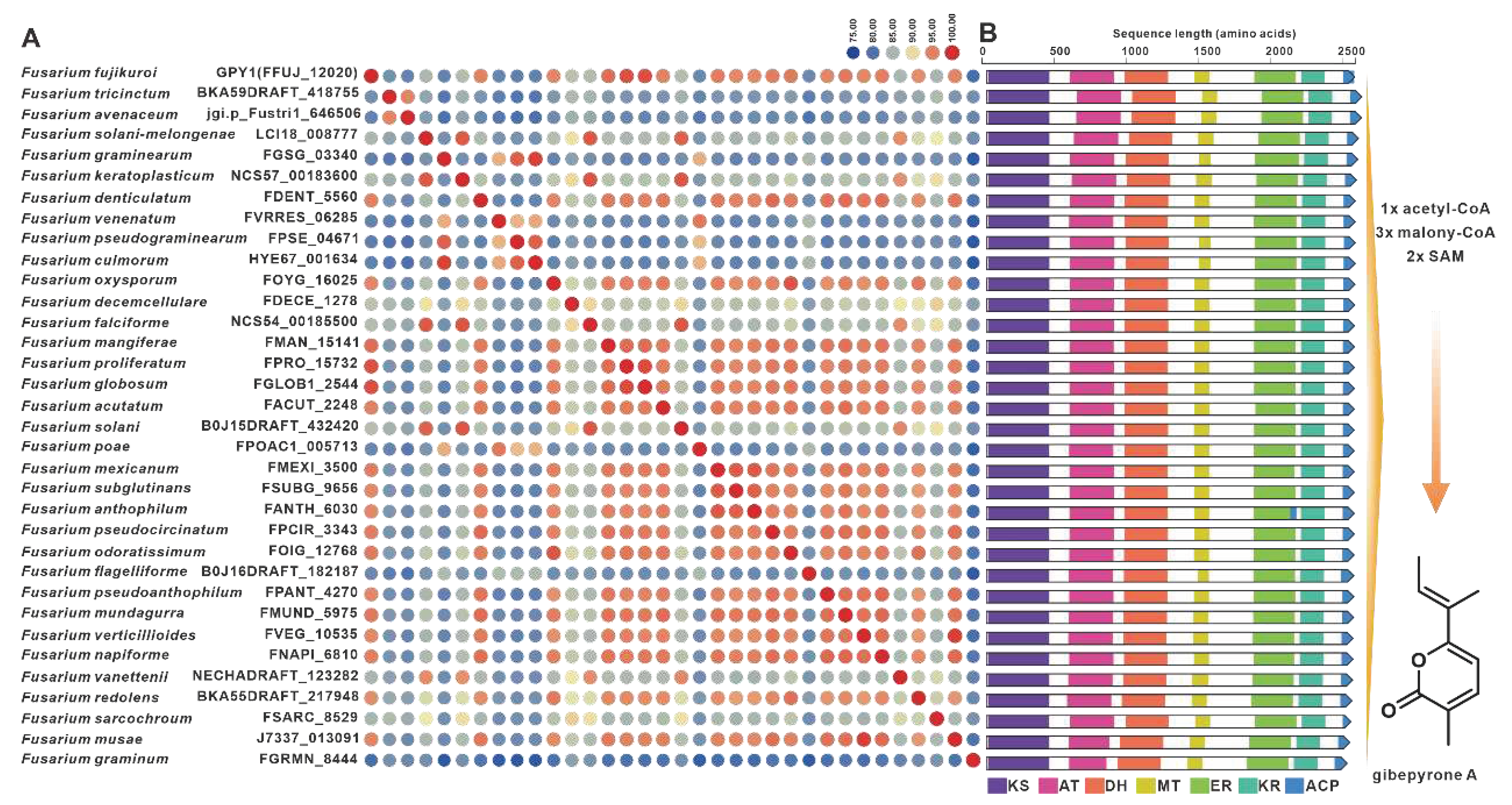
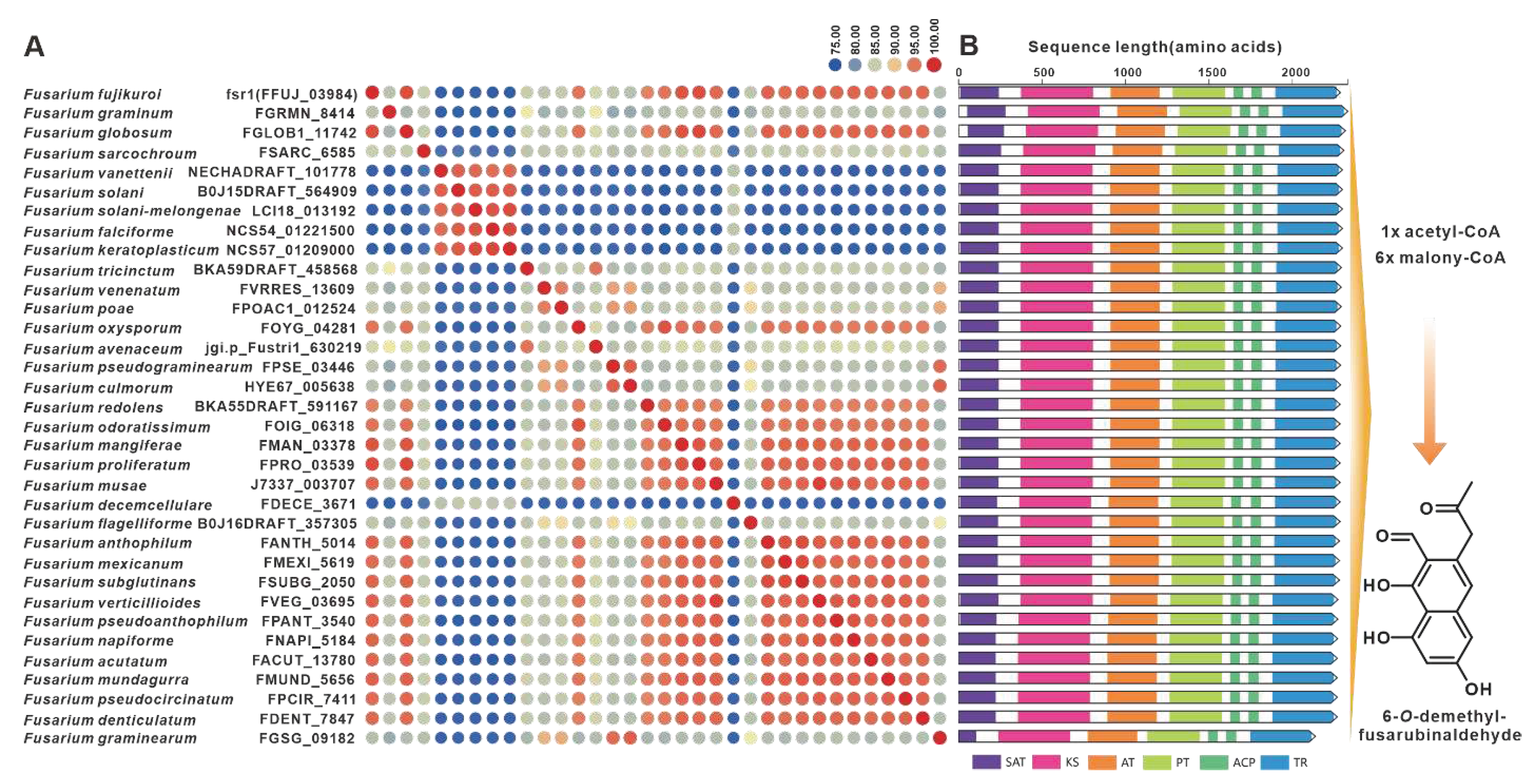
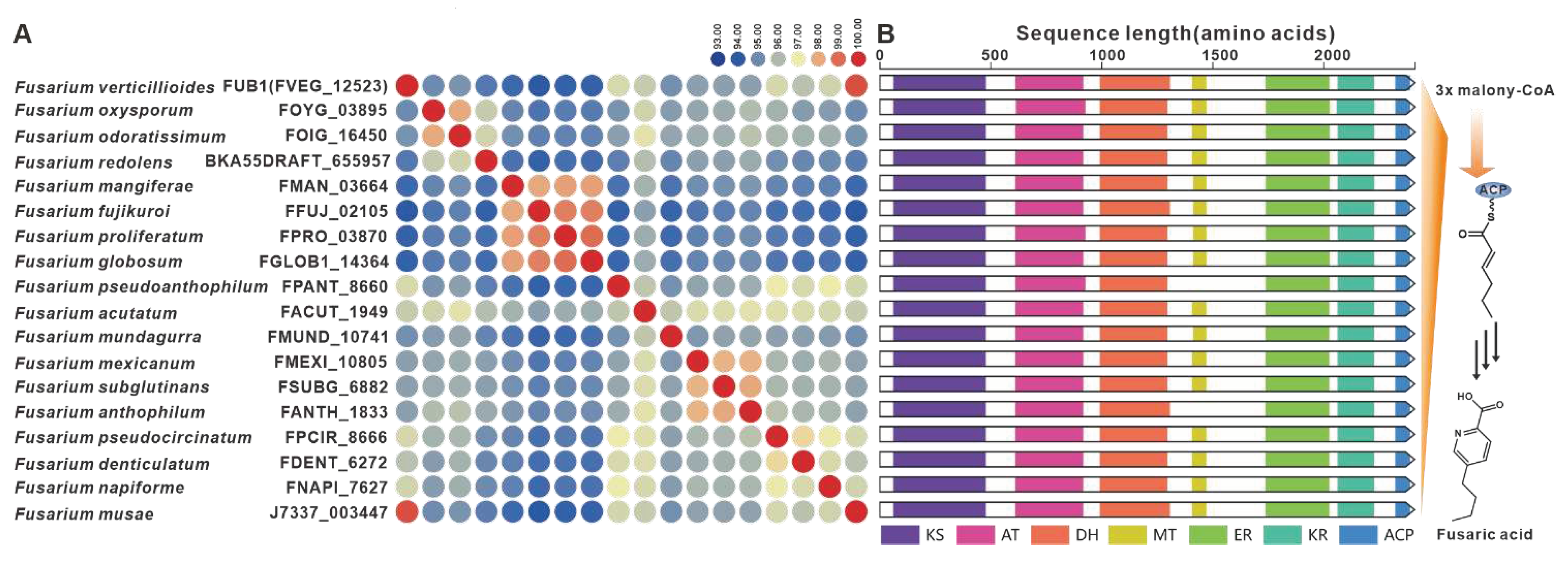
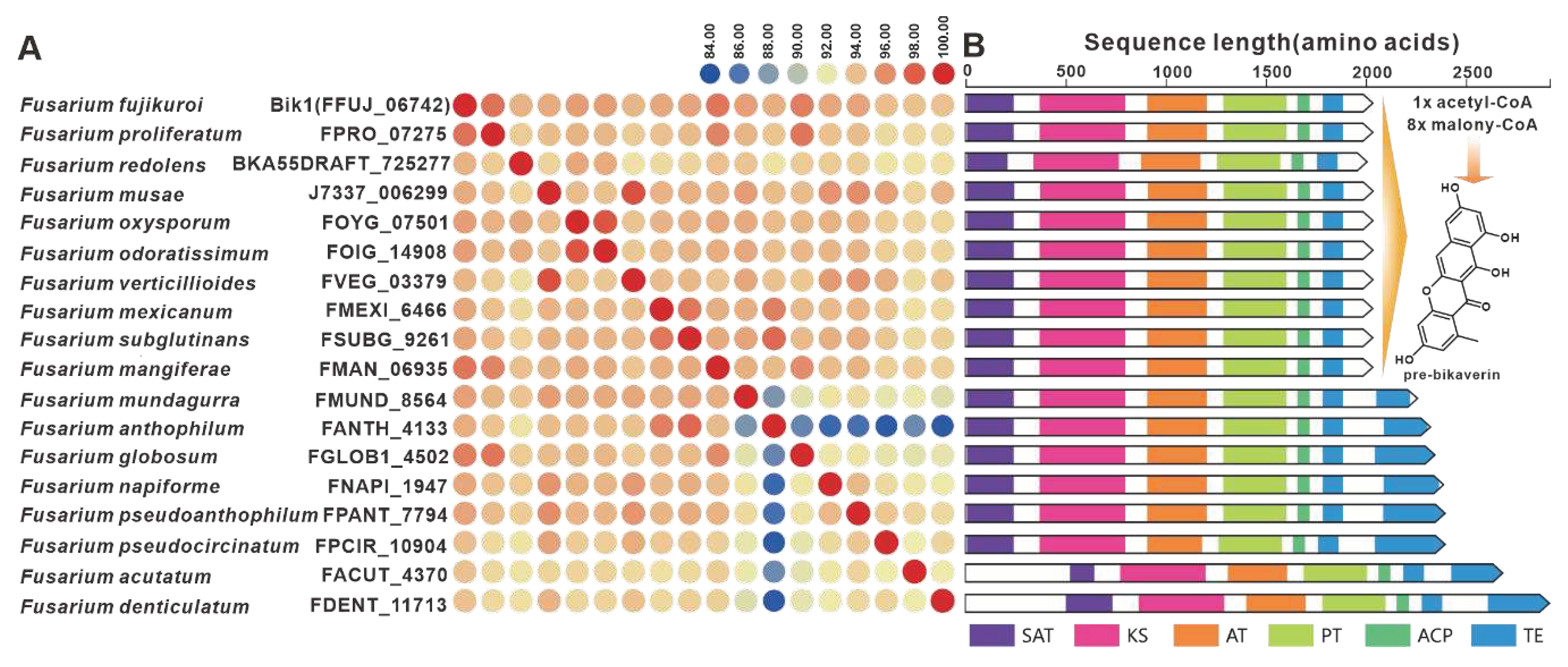
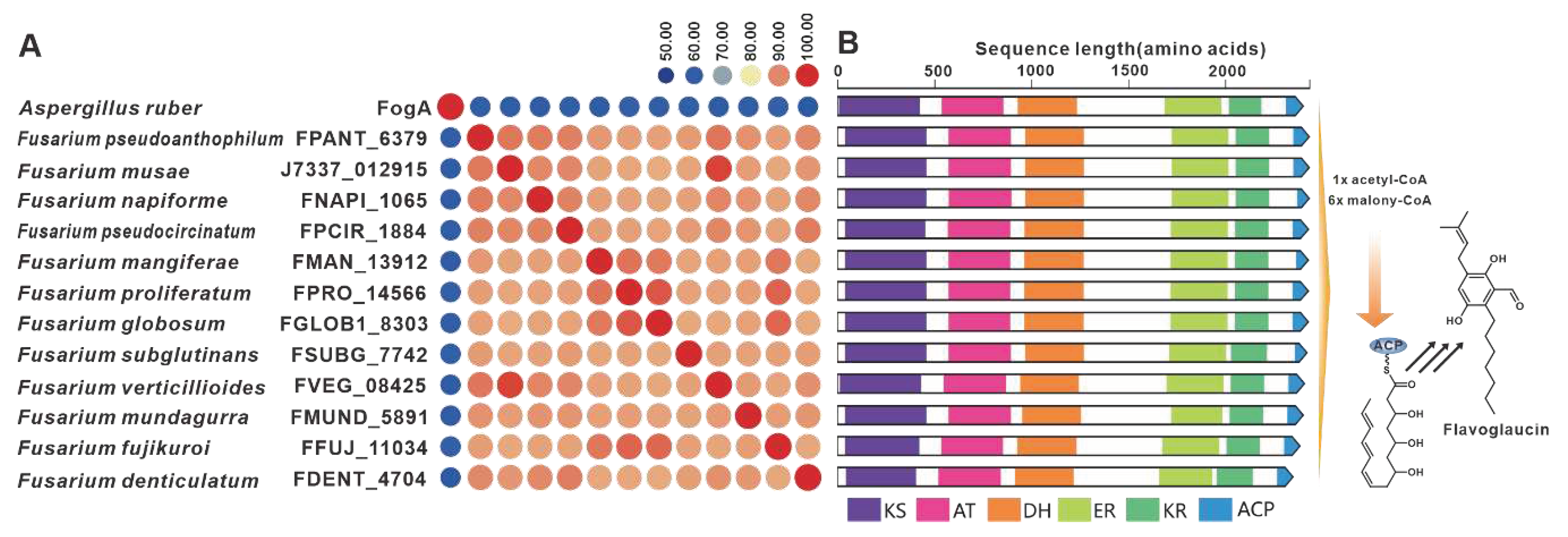
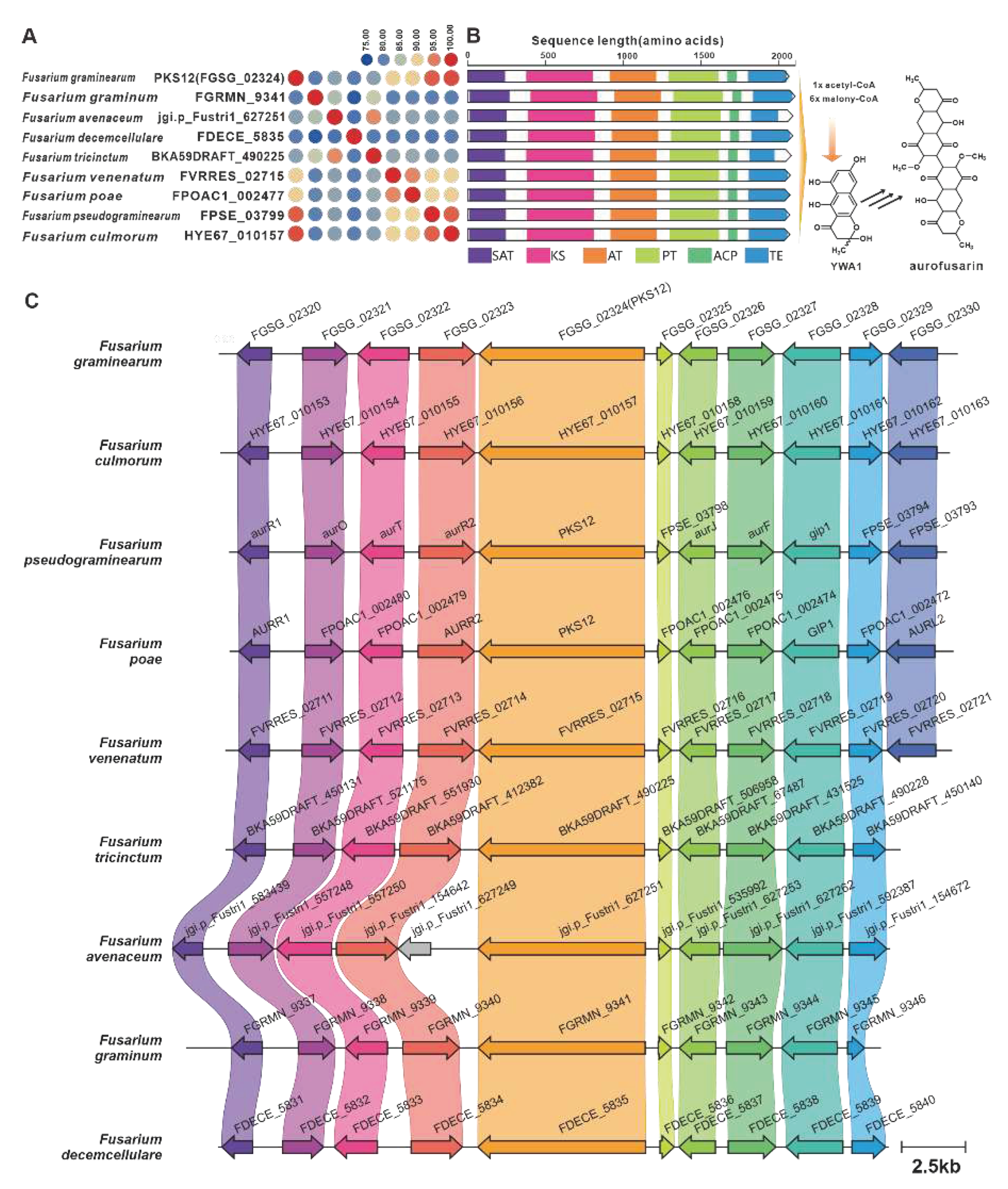
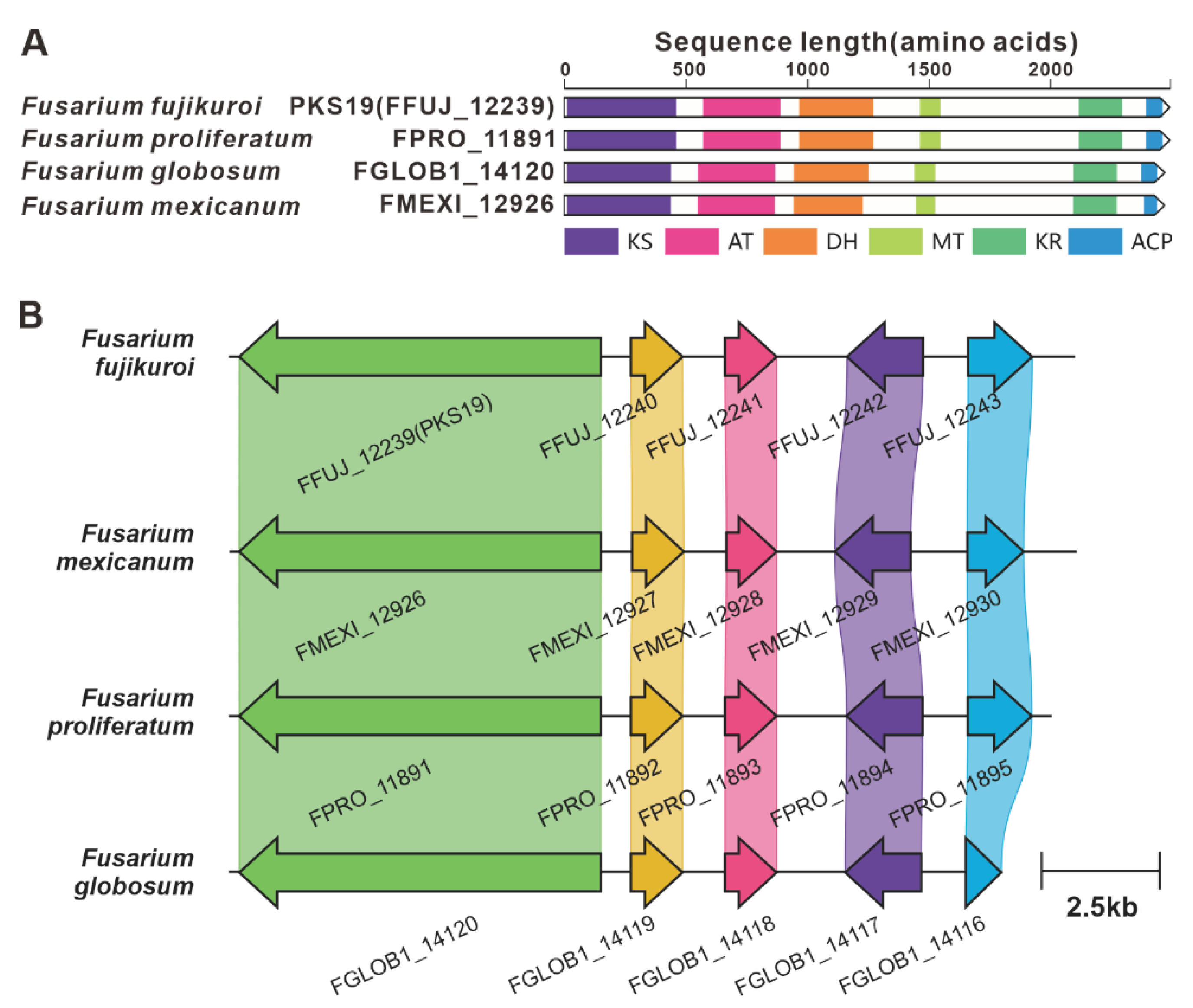
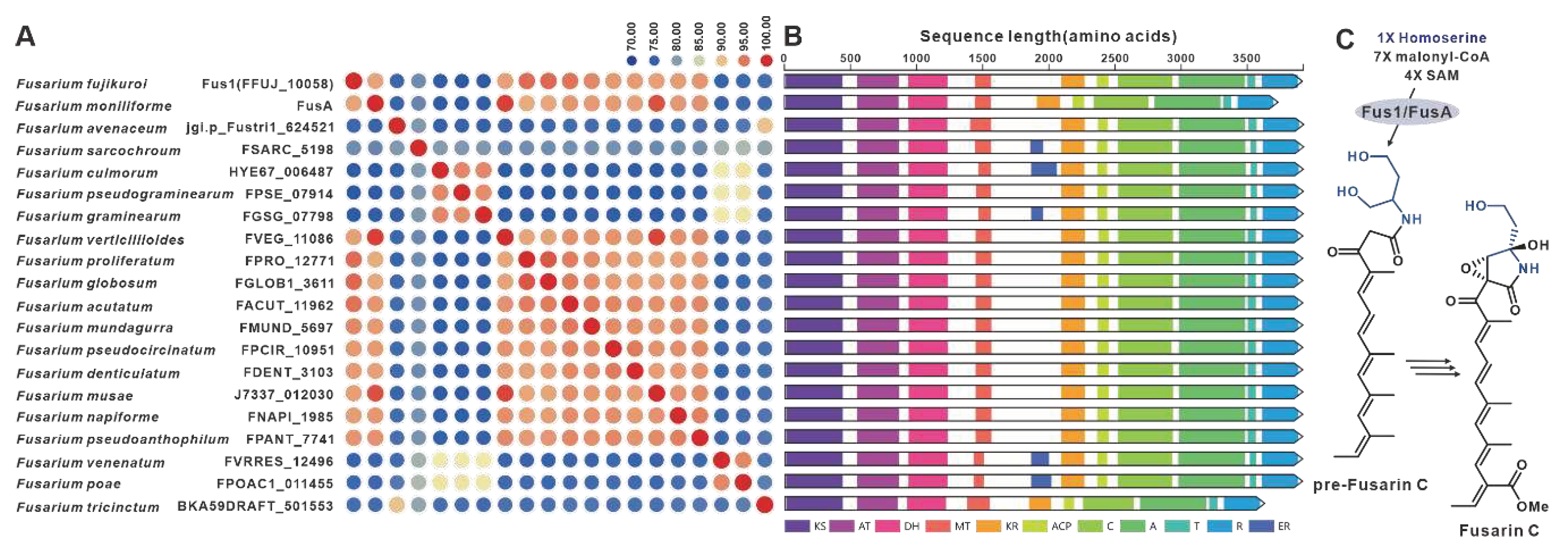
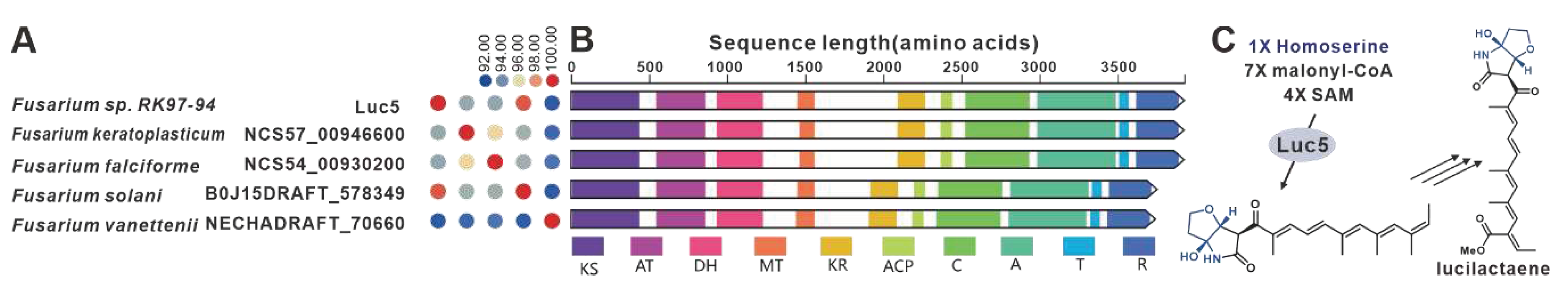
3.4. Polyketide biosynthetic pathway of pathogenic Fusarium species
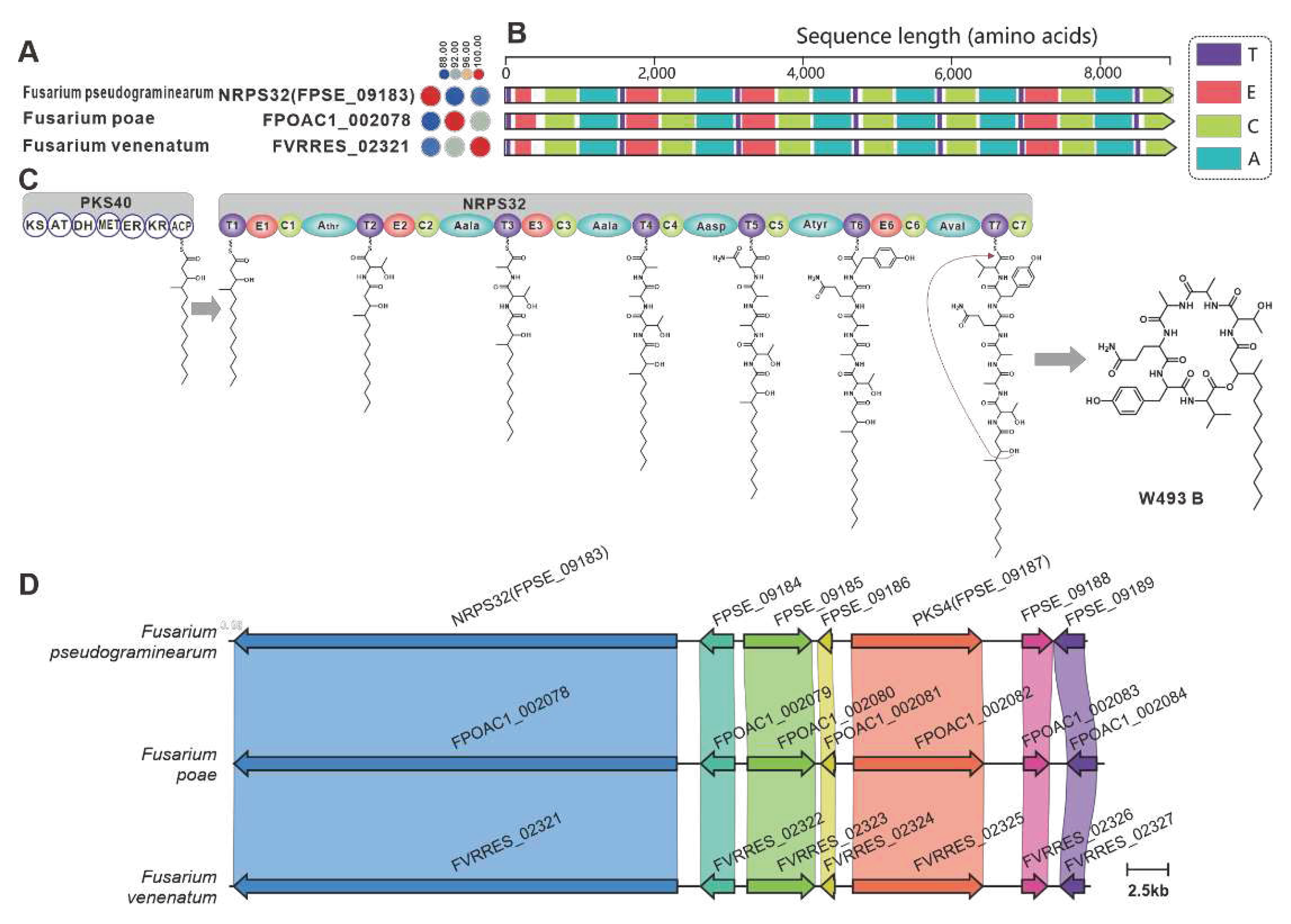
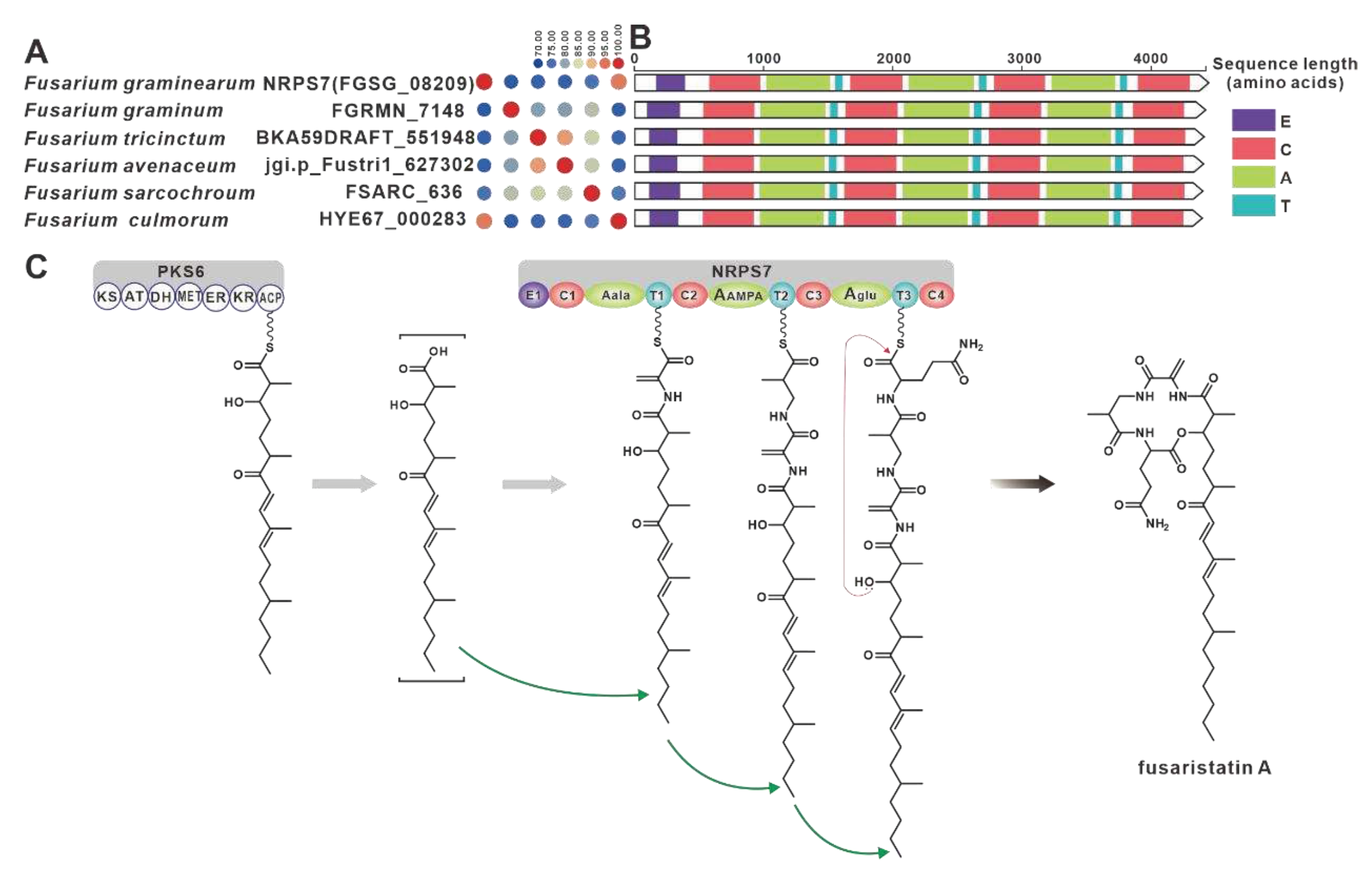
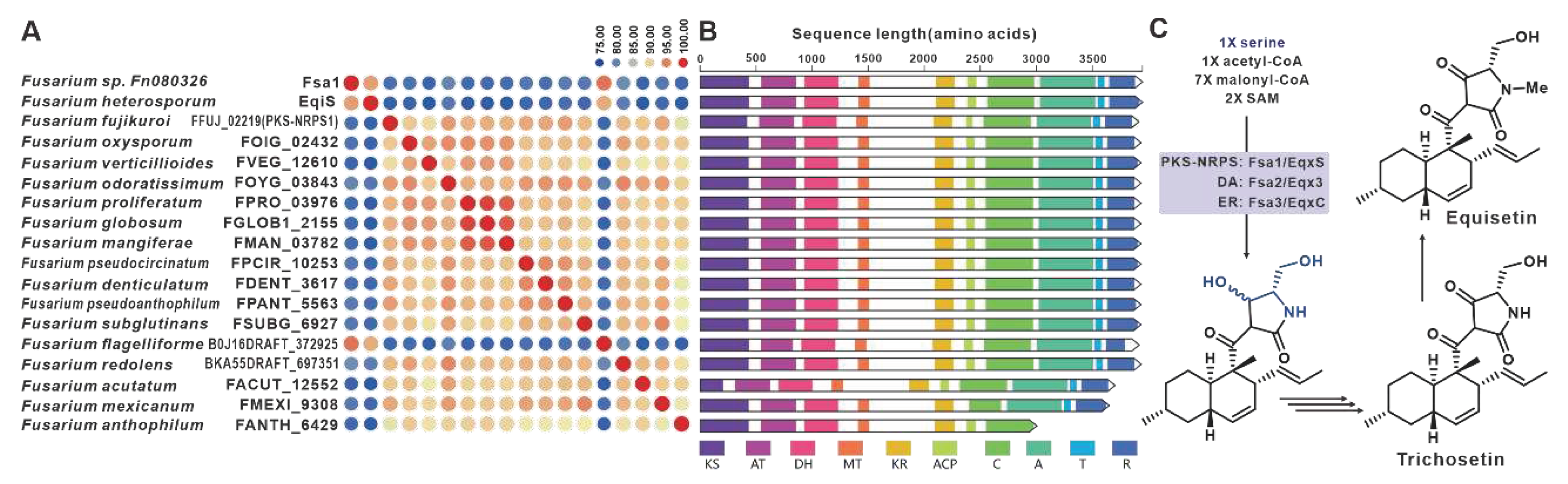
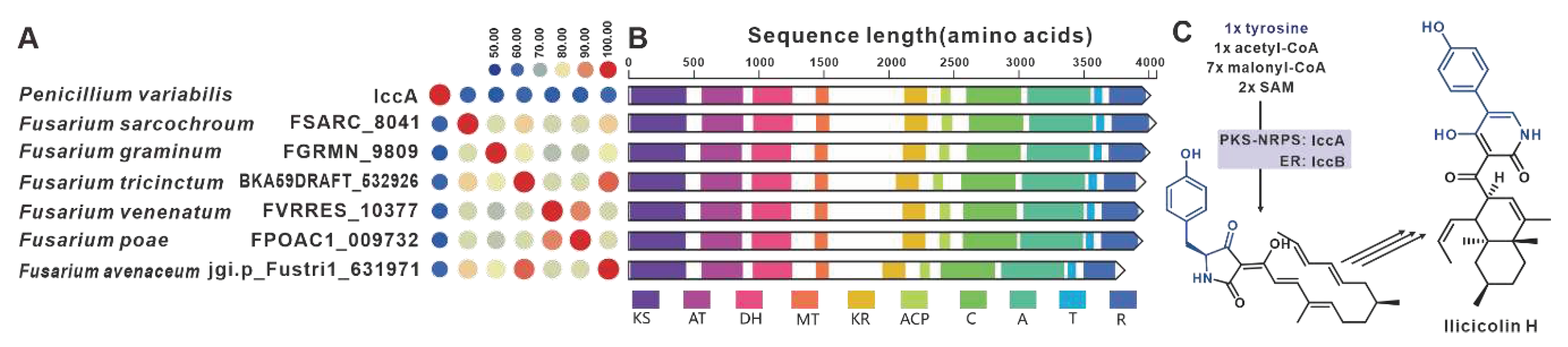
3.5. PKS-NRPS biosynthetic pathway of pathogenic Fusarium species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, L.-J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O'Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium Pathogenomics. Annual Review of Microbiology 2013, 67, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Rugbjerg, P.; Naesby, M.; Mortensen, U.H.; Frandsen, R.J.N. Reconstruction of the biosynthetic pathway for the core fungal polyketide scaffold rubrofusarin in Saccharomyces cerevisiae. MICROB CELL FACT 2013, 9, 31. [Google Scholar]
- Munkvold, G.P. Fusarium Species and Their Associated Mycotoxins. In Mycotoxigenic Fungi: Methods and Protocols, Moretti, A., Susca, A., Eds.; Springer New York: New York, NY, 2017; pp. 51–106. [Google Scholar]
- Bansal, Y.; Singla, N.; Kaistha, N.; Sood, S.; Chander, J. Molecular identification of Fusarium species complex isolated from clinical samples and its antifungal susceptibility patterns. Curr Med Mycol 2019, 5, 43–49. [Google Scholar]
- Zakaria, L. Fusarium Species Associated with Diseases of Major Tropical Fruit Crops. HORTICULTURAE 2023, 9, 322. [Google Scholar]
- Kuhnem, P.; Ward, T.; Silva, C.; Spolti, P.; Ciliato, M.; Tessmann, D.; Del Ponte, E. Composition and toxigenic potential of the Fusarium graminearum species complex from maize ears, stalks and stubble in Brazil. Plant Pathology 2016, 65, 1185–1191. [Google Scholar]
- Duan, C.; Qin, Z.; Yang, Z.; Li, W.; Sun, S.; Zhu, Z.; Wang, X. Identification of Pathogenic Fusarium spp. Causing Maize Ear Rot and Potential Mycotoxin Production in China. TOXINS 2016, 8, 186. [Google Scholar] [PubMed]
- Nganje, W.E.; Bangsund, D.A.; Leistritz, F.L.; Wilson, W.W.; Tiapo, N.M. Regional Economic Impacts of Fusarium Head Blight in Wheat and Barley. APPL ECON PERSPECT P 2004, 26, 332–347. [Google Scholar] [CrossRef]
- Waller, J.M.; Brayford, D. Fusarium diseases in the tropics. Tropical Pest Management 1990, 36, 181–194. [Google Scholar]
- Rampersad, S.N. Pathogenomics and Management of Fusarium Diseases in Plants. PATHOGENS 2020, 9, 340. [Google Scholar]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-Produced Mycotoxins in Plant-Pathogen Interactions. TOXINS 2019, 11, 664. [Google Scholar]
- Matic, S.; Gullino, M.L.; Spadaro, D. The puzzle of bakanae disease through interactions between Fusarium fujikuroi and rice. FRONT BIOSCI 2017, 9, 333–344. [Google Scholar]
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Stela, M.; Bijak, M. T-2 Toxin-The Most Toxic Trichothecene Mycotoxin: Metabolism, Toxicity, and Decontamination Strategies. MOLECULES 2021, 26, 6868. [Google Scholar]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdisciplinary Toxicology 2010, 3, 94–99. [Google Scholar] [PubMed]
- Aupanun, S.; Poapolathep, S.; Giorgi, M.; Imsilp, K.; Poapolathep, A. An overview of the toxicology and toxicokinetics of fusarenon-X, a type B trichothecene mycotoxin. J VET MED SCI 2017, 79, 6–13. [Google Scholar]
- Tudzynski, B.; Mihlan, M.; Rojas, M.C.; Linnemannstons, P.; Gaskin, P.; Hedden, P. Characterization of the final two genes of the gibberellin biosynthesis gene cluster of Gibberella fujikuroi: des and P450-3 encode GA4 desaturase and the 13-hydroxylase, respectively. J BIOL CHEM 2003, 278, 28635–28643. [Google Scholar] [CrossRef]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research Progress on Fumonisin B1 Contamination and Toxicity: A Review. MOLECULES 2021, 26, 5238. [Google Scholar]
- Lopez-Diaz, C.; Rahjoo, V.; Sulyok, M.; Ghionna, V.; Martin-Vicente, A.; Capilla, J.; Di Pietro, A.; Lopez-Berges, M.S. Fusaric acid contributes to virulence of Fusarium oxysporum on plant and mammalian hosts. MOL PLANT PATHOL 2018, 19, 440–453. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a Fusarium mycotoxin, zearalenone. Crit Rev Food Sci Nutr 2020, 60, 2710–2729. [Google Scholar]
- Juan-Garcia, A.; Ruiz, M.J.; Font, G.; Manyes, L. Enniatin A1, enniatin B1 and beauvericin on HepG2: Evaluation of toxic effects. FOOD CHEM TOXICOL 2015, 84, 188–196. [Google Scholar]
- Mallebrera, B.; Juan-Garcia, A.; Font, G.; Ruiz, M.-J. Mechanisms of beauvericin toxicity and antioxidant cellular defense. TOXICOL LETT 2016, 246, 28–34. [Google Scholar]
- Jin, J.M.; Lee, S.; Lee, J.; Baek, S.R.; Kim, J.C.; Yun, S.H.; Park, S.Y.; Kang, S.; Lee, Y.W. Functional characterization and manipulation of the apicidin biosynthetic pathway in Fusarium semitectum. MOL MICROBIOL 2010, 76, 456–466. [Google Scholar]
- Niehaus, E.M.; Kleigrewe, K.; Wiemann, P.; Studt, L.; Sieber, C.M.; Connolly, L.R.; Freitag, M.; Guldener, U.; Tudzynski, B.; Humpf, H.U. Genetic manipulation of the Fusarium fujikuroi fusarin gene cluster yields insight into the complex regulation and fusarin biosynthetic pathway. CHEM BIOL 2013, 20, 1055–1066. [Google Scholar] [PubMed]
- Sims, J.W.; Fillmore, J.P.; Warner, D.D.; Schmidt, E.W. Equisetin biosynthesis in Fusarium heterosporum. CHEM COMMUN 2005, 41, 186–188. [Google Scholar] [CrossRef]
- Go, E.B.; Kim, L.J.; Nelson, H.M.; Ohashi, M.; Tang, Y. Biosynthesis of the Fusarium mycotoxin (−)-sambutoxin. Organic letters 2021, 23, 7819–7823. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, C.L.M.; Chooi, Y.-H. Synthaser: a CD-Search enabled Python toolkit for analysing domain architecture of fungal secondary metabolite megasynth(et)ases. FUNGAL BIOL BIOTECH 2021, 8, 13. [Google Scholar]
- Gilchrist, C.L.M.; Chooi, Y.-H. clinker & clustermap.js: automatic generation of gene cluster comparison figures. BIOINFORMATICS 2021, 37, 2473–2475. [Google Scholar]
- Navarro-Muñoz, J.C.; Selem-Mojica, N.; Mullowney, M.W.; Kautsar, S.A.; Tryon, J.H.; Parkinson, E.I.; De Los Santos, E.L.C.; Yeong, M.; Cruz-Morales, P.; Abubucker, S.; et al. A computational framework to explore large-scale biosynthetic diversity. NAT CHEM BIOL 2020, 16, 60–68. [Google Scholar] [PubMed]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: new and improved predictions for detection, regulation, chemical structures and visualisation. NUCLEIC ACIDS RES 2023, 51, W46–w50. [Google Scholar]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. Corrigendum to: IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. MOL BIOL EVOL 2020, 37, 2461–2461. [Google Scholar]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. NUCLEIC ACIDS RES 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Terlouw, B.R.; Blin, K.; Navarro-Muñoz, J.C.; Avalon, N.E.; Chevrette, M.G.; Egbert, S.; Lee, S.; Meijer, D.; Recchia, Michael J. J.; Reitz, Zachary L.; et al. MIBiG 3.0: a community-driven effort to annotate experimentally validated biosynthetic gene clusters. NUCLEIC ACIDS RES 2022, 51, D603–D610. [Google Scholar]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Brock, N.L.; Huss, K.; Tudzynski, B.; Dickschat, J.S. Genetic Dissection of Sesquiterpene Biosynthesis by Fusarium fujikuroi. CHEMBIOCHEM 2013, 14, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, I.; Siemon, T.; Henrot, M.; Studt, L.; Rösler, S.; Tudzynski, B.; Christmann, M.; Dickschat, J.S. Mechanistic Characterisation of Two Sesquiterpene Cyclases from the Plant Pathogenic Fungus Fusarium fujikuroi. ANGEW CHEM INT EDIT 2016, 55, 8748–8751. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P.; Alexander, N.J.; Harris, L.J. CLM1 of Fusarium graminearum encodes a longiborneol synthase required for culmorin production. Appl Environ Microbiol 2010, 76, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Lou, T.; Li, A.; Xu, H.; Pan, J.; Xing, B.; Wu, R.; Dickschat, J.S.; Yang, D.; Ma, M. Structural Insights into Three Sesquiterpene Synthases for the Biosynthesis of Tricyclic Sesquiterpenes and Chemical Space Expansion by Structure-Based Mutagenesis. J AM CHEM SOC 2023, 145, 8474–8485. [Google Scholar]
- Hohn, T.M.; Desjardins, A.E. Isolation and gene disruption of the Tox5 gene encoding trichodiene synthase in Gibberella pulicaris. MOL PLANT MICROBE IN 1992, 5, 249–256. [Google Scholar] [CrossRef]
- Hohn, T.M.; Beremand, P.D. Isolation and nucleotide sequence of a sesquiterpene cyclase gene from the trichothecene-producing fungus Fusarium sporotrichioides. GENE 1989, 79, 131–138. [Google Scholar]
- Gardiner, D.M.; McDonald, M.C.; Covarelli, L.; Solomon, P.S.; Rusu, A.G.; Marshall, M.; Kazan, K.; Chakraborty, S.; McDonald, B.A.; Manners, J.M. Comparative Pathogenomics Reveals Horizontally Acquired Novel Virulence Genes in Fungi Infecting Cereal Hosts. PLOS PATHOG 2012, 8, e1002952. [Google Scholar] [CrossRef]
- Varga, E.; Wiesenberger, G.; Hametner, C.; Ward, T.J.; Dong, Y.; Schöfbeck, D.; McCormick, S.; Broz, K.; Stückler, R.; Schuhmacher, R.; et al. New tricks of an old enemy: isolates of Fusarium graminearum produce a type A trichothecene mycotoxin. ENVIRON MICROBIOL 2015, 17, 2588–2600. [Google Scholar] [CrossRef]
- Alexander, N.J.; Proctor, R.H.; McCormick, S.P. Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. TOXIN REV 2009, 28, 198–215. [Google Scholar]
- Tsukada, K.; Shinki, S.; Kaneko, A.; Murakami, K.; Irie, K.; Murai, M.; Miyoshi, H.; Dan, S.; Kawaji, K.; Hayashi, H.; et al. Synthetic biology based construction of biological activity-related library of fungal decalin-containing diterpenoid pyrones. NAT COMMUN 2020, 11, 1830. [Google Scholar]
- Mende, K.; Homann, V.; Tudzynski, B. The geranylgeranyl diphosphate synthase gene of Gibberella fujikuroi: isolation and expression. MOL GEN GENET 1997, 255, 96–105. [Google Scholar] [CrossRef]
- Wiemann, P.; Sieber, C.M.; von Bargen, K.W.; Studt, L.; Niehaus, E.M.; Espino, J.J.; Huss, K.; Michielse, C.B.; Albermann, S.; Wagner, D.; et al. Deciphering the cryptic genome: genome-wide analyses of the rice pathogen Fusarium fujikuroi reveal complex regulation of secondary metabolism and novel metabolites. PLOS PATHOG 2013, 9, e1003475. [Google Scholar]
- Jestoi, M. Emerging Fusarium -Mycotoxins Fusaproliferin, Beauvericin, Enniatins, And Moniliformin—A Review. Crit Rev Food Sci Nutr 2008, 48, 21–49. [Google Scholar] [PubMed]
- Bian, G.; Han, Y.; Hou, A.; Yuan, Y.; Liu, X.; Deng, Z.; Liu, T. Releasing the potential power of terpene synthases by a robust precursor supply platform. Metab Eng 2017, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Cheng, S.; Bian, G.; Yan, P.; Ma, Z.; Dai, W.; Chen, R.; Fu, S.; Huang, H.; Chi, H.; et al. Efficient exploration of terpenoid biosynthetic gene clusters in filamentous fungi. NAT CATAL 2022, 5, 277–287. [Google Scholar]
- Jiang, L.; Zhang, X.; Sato, Y.; Zhu, G.; Minami, A.; Zhang, W.; Ozaki, T.; Zhu, B.; Wang, Z.; Wang, X.; et al. Genome-Based Discovery of Enantiomeric Pentacyclic Sesterterpenes Catalyzed by Fungal Bifunctional Terpene Synthases. ORG LETT 2021, 23, 4645–4650. [Google Scholar]
- Liu, Z.; Jian, Y.; Chen, Y.; Kistler, H.C.; He, P.; Ma, Z.; Yin, Y. A phosphorylated transcription factor regulates sterol biosynthesis in Fusarium graminearum. NAT COMMUN 2019, 10, 1228. [Google Scholar]
- Ma, K.; Zhang, P.; Tao, Q.; Keller, N.P.; Yang, Y.; Yin, W.-B.; Liu, H. Characterization and Biosynthesis of a Rare Fungal Hopane-Type Triterpenoid Glycoside Involved in the Antistress Property of Aspergillus fumigatus. ORG LETT 2019, 21, 3252–3256. [Google Scholar]
- Prado-Cabrero, A.; Schaub, P.; Díaz-Sánchez, V.; Estrada, A.F.; Al-Babili, S.; Avalos, J. Deviation of the neurosporaxanthin pathway towards β-carotene biosynthesis in Fusarium fujikuroi by a point mutation in the phytoene desaturase gene. The FEBS Journal 2009, 276, 4582–4597. [Google Scholar] [CrossRef]
- Linnemannstons, P.; Prado, M.M.; Fernandez-Martin, R.; Tudzynski, B.; Avalos, J. A carotenoid biosynthesis gene cluster in Fusarium fujikuroi: the genes carB and carRA. MOL GENET GENOMICS 2002, 267, 593–602. [Google Scholar] [CrossRef]
- Arndt, B.; Janevska, S.; Schmid, R.; Hübner, F.; Tudzynski, B.; Humpf, H.-U. A Fungal N-Dimethylallyltryptophan Metabolite from Fusarium fujikuroi. CHEMBIOCHEM 2017, 18, 899–904. [Google Scholar] [CrossRef]
- Kremer, A.; Westrich, L.; Li, S.-M. A 7-dimethylallyltryptophan synthase from Aspergillus fumigatus: overproduction, purification and biochemical characterization. MICROBIOLOGY 2007, 153, 3409–3416. [Google Scholar] [CrossRef] [PubMed]
- Kakule, T.B.; Sardar, D.; Lin, Z.; Schmidt, E.W. Two Related Pyrrolidinedione Synthetase Loci in Fusarium heterosporum ATCC 74349 Produce Divergent Metabolites. ACS CHEM BIOL 2013, 8, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.H.; Mayfield, J.A.; Rine, J.; Sil, A. Histoplasma requires SID1, a member of an iron-regulated siderophore gene cluster, for host colonization. PLOS PATHOG 2008, 4, e1000044. [Google Scholar] [CrossRef]
- Tobiasen, C.; Aahman, J.; Ravnholt, K.S.; Bjerrum, M.J.; Grell, M.N.; Giese, H. Nonribosomal peptide synthetase (NPS) genes in Fusarium graminearum, F. culmorum and F. pseudograminearium and identification of NPS2 as the producer of ferricrocin. Curr Genet 2007, 51, 43–58. [Google Scholar] [CrossRef]
- Oide, S.; Moeder, W.; Krasnoff, S.; Gibson, D.; Haas, H.; Yoshioka, K.; Turgeon, B.G. NPS6, encoding a nonribosomal peptide synthetase involved in siderophore-mediated iron metabolism, is a conserved virulence determinant of plant pathogenic ascomycetes. PLANT CELL 2006, 18, 2836–2853. [Google Scholar] [CrossRef] [PubMed]
- Reiber, K.; Reeves, E.P.; Neville, C.M.; Winkler, R.; Gebhardt, P.; Kavanagh, K.; Doyle, S. The expression of selected non-ribosomal peptide synthetases in Aspergillus fumigatus is controlled by the availability of free iron. FEMS MICROBIOL LETT 2005, 248, 83–91. [Google Scholar] [CrossRef]
- Yasmin, S.; Alcazar-Fuoli, L.; Grundlinger, M.; Puempel, T.; Cairns, T.; Blatzer, M.; Lopez, J.F.; Grimalt, J.O.; Bignell, E.; Haas, H. Mevalonate governs interdependency of ergosterol and siderophore biosyntheses in the fungal pathogen Aspergillus fumigatus. Proc Natl Acad Sci U S A 2012, 109, E497–504. [Google Scholar] [CrossRef]
- Richter, L.; Wanka, F.; Boecker, S.; Storm, D.; Kurt, T.; Vural, Ö.; Süßmuth, R.; Meyer, V. Engineering of Aspergillus niger for the production of secondary metabolites. FUNGAL BIOL BIOTECH 2014, 1, 4. [Google Scholar] [CrossRef]
- Scott-Craig, J.S.; Panaccione, D.G.; Pocard, J.A.; Walton, J.D. The cyclic peptide synthetase catalyzing HC-toxin production in the filamentous fungus Cochliobolus carbonum is encoded by a 15.7-kilobase open reading frame. J BIOL CHEM 1992, 267, 26044–26049. [Google Scholar] [CrossRef]
- Xu, Y.; Orozco, R.; Wijeratne, E.M.K.; Gunatilaka, A.A.L.; Stock, S.P.; Molnár, I. Biosynthesis of the Cyclooligomer Depsipeptide Beauvericin, a Virulence Factor of the Entomopathogenic Fungus Beauveria bassiana. CHEM BIOL 2008, 15, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhuo, Y.; Jia, X.; Liu, J.; Gao, H.; Song, F.; Liu, M.; Zhang, L. Cloning and characterization of the gene cluster required for beauvericin biosynthesis in Fusarium proliferatum. SCI CHINA LIFE SCI 2013, 56, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, E.M.; Studt, L.; von Bargen, K.W.; Kummer, W.; Humpf, H.U.; Reuter, G.; Tudzynski, B. Sound of silence: the beauvericin cluster in Fusarium fujikuroi is controlled by cluster-specific and global regulators mediated by H3K27 modification. ENVIRON MICROBIOL 2016, 18, 4282–4302. [Google Scholar] [CrossRef] [PubMed]
- Chankhamjon, P.; Boettger-Schmidt, D.; Scherlach, K.; Urbansky, B.; Lackner, G.; Kalb, D.; Dahse, H.M.; Hoffmeister, D.; Hertweck, C. Biosynthesis of the halogenated mycotoxin aspirochlorine in koji mold involves a cryptic amino acid conversion. ANGEW CHEM INT EDIT 2014, 53, 13409–13413. [Google Scholar] [CrossRef] [PubMed]
- Westphal, K.R.; Bachleitner, S.; Severinsen, M.M.; Brundto, M.L.; Hansen, F.T.; Sorensen, T.; Wollenberg, R.D.; Lysoe, E.; Studt, L.; Sorensen, J.L.; et al. Cyclic, Hydrophobic Hexapeptide Fusahexin Is the Product of a Nonribosomal Peptide Synthetase in Fusarium graminearum. J Nat Prod 2021, 84, 2070–2080. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.J.; Tang, H.Y.; Wang, W.Q.; Yuan, T.L.; Wei, W.Q.; Pang, B.; Gong, X.M.; Wang, S.F.; Li, Y.J.; Zhang, D.; et al. A linear nonribosomal octapeptide from Fusarium graminearum facilitates cell-to-cell invasion of wheat. NAT COMMUN 2019, 10, 922. [Google Scholar] [CrossRef]
- Atanasoff-Kardjalieff, A.K.; Lünne, F.; Kalinina, S.; Strauss, J.; Humpf, H.-U.; Studt, L. Biosynthesis of fusapyrone depends on the H3K9 methyltransferase, FmKmt1, in Fusarium mangiferae. FRONGT FUNG BIOL 2021, 2, 671796. [Google Scholar] [CrossRef]
- Sorensen, J.L.; Sondergaard, T.E.; Covarelli, L.; Fuertes, P.R.; Hansen, F.T.; Frandsen, R.J.; Saei, W.; Lukassen, M.B.; Wimmer, R.; Nielsen, K.F.; et al. Identification of the biosynthetic gene clusters for the lipopeptides fusaristatin A and W493 B in Fusarium graminearum and F. pseudograminearum. J Nat Prod 2014, 77, 2619–2625. [Google Scholar] [CrossRef]
- Romans-Fuertes, P.; Sondergaard, T.E.; Sandmann, M.I.; Wollenberg, R.D.; Nielsen, K.F.; Hansen, F.T.; Giese, H.; Brodersen, D.E.; Sorensen, J.L. Identification of the non-ribosomal peptide synthetase responsible for biosynthesis of the potential anti-cancer drug sansalvamide in Fusarium solani. Curr Genet 2016, 62, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, R.D.; Saei, W.; Westphal, K.R.; Klitgaard, C.S.; Nielsen, K.L.; Lysoe, E.; Gardiner, D.M.; Wimmer, R.; Sondergaard, T.E.; Sorensen, J.L. Chrysogine Biosynthesis Is Mediated by a Two-Module Nonribosomal Peptide Synthetase. J Nat Prod 2017, 80, 2131–2135. [Google Scholar] [CrossRef] [PubMed]
- Bahadoor, A.; Brauer, E.K.; Bosnich, W.; Schneiderman, D.; Johnston, A.; Aubin, Y.; Blackwell, B.; Melanson, J.E.; Harris, L.J. Gramillin A and B: Cyclic Lipopeptides Identified as the Nonribosomal Biosynthetic Products of Fusarium graminearum. J Am Chem Soc 2018, 140, 16783–16791. [Google Scholar] [CrossRef]
- Jin, J.-M.; Lee, S.; Lee, J.; Baek, S.-R.; Kim, J.-C.; Yun, S.-H.; Park, S.-Y.; Kang, S.; Lee, Y.-W. Functional characterization and manipulation of the apicidin biosynthetic pathway in Fusarium semitectum. MOL MICROBIOL 2010, 76, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Yokoyama, T.; Nemoto, K.; Kumagai, T.; Terai, G.; Tamano, K.; Machida, M.; Shibata, T. Identification of a putative FR901469 biosynthesis gene cluster in fungal sp. No. 11243 and enhancement of the productivity by overexpressing the transcription factor gene frbF. J BIOSCI BIOENG 2017, 123, 147–153. [Google Scholar] [CrossRef]
- Niehaus, E.-M.; Kleigrewe, K.; Wiemann, P.; Studt, L.; Sieber, Christian M. K.; Connolly, Lanelle R.; Freitag, M.; Güldener, U.; Tudzynski, B.; Humpf, H.-U. Genetic Manipulation of the Fusarium fujikuroi Fusarin Gene Cluster Yields Insight into the Complex Regulation and Fusarin Biosynthetic Pathway. CHEM BIOL 2013, 20, 1055–1066. [Google Scholar] [CrossRef]
- Janevska, S.; Arndt, B.; Niehaus, E.M.; Burkhardt, I.; Rosler, S.M.; Brock, N.L.; Humpf, H.U.; Dickschat, J.S.; Tudzynski, B. Gibepyrone Biosynthesis in the Rice Pathogen Fusarium fujikuroi Is Facilitated by a Small Polyketide Synthase Gene Cluster. J BIOL CHEM 2016, 291, 27403–27420. [Google Scholar] [CrossRef] [PubMed]
- Studt, L.; Wiemann, P.; Kleigrewe, K.; Humpf, H.U.; Tudzynski, B. Biosynthesis of fusarubins accounts for pigmentation of Fusarium fujikuroi perithecia. Appl Environ Microbiol 2012, 78, 4468–4480. [Google Scholar] [CrossRef]
- Hai, Y.; Chen, M.; Huang, A.; Tang, Y. Biosynthesis of Mycotoxin Fusaric Acid and Application of a PLP-Dependent Enzyme for Chemoenzymatic Synthesis of Substituted l-Pipecolic Acids. J AM CHEM SOC 2020, 142, 19668–19677. [Google Scholar] [CrossRef]
- Arndt, B.; Studt, L.; Wiemann, P.; Osmanov, H.; Kleigrewe, K.; Kohler, J.; Krug, I.; Tudzynski, B.; Humpf, H.U. Genetic engineering, high resolution mass spectrometry and nuclear magnetic resonance spectroscopy elucidate the bikaverin biosynthetic pathway in Fusarium fujikuroi. FUNGAL GENET BIOL 2015, 84, 26–36. [Google Scholar] [CrossRef]
- Wiemann, P.; Willmann, A.; Straeten, M.; Kleigrewe, K.; Beyer, M.; Humpf, H.U.; Tudzynski, B. Biosynthesis of the red pigment bikaverin in Fusarium fujikuroi: genes, their function and regulation. Molecular microbiology 2009, 72, 931–946. [Google Scholar] [CrossRef] [PubMed]
- Nies, J.; Ran, H.; Wohlgemuth, V.; Yin, W.B.; Li, S.M. Biosynthesis of the Prenylated Salicylaldehyde Flavoglaucin Requires Temporary Reduction to Salicyl Alcohol for Decoration before Reoxidation to the Final Product. ORG LETT 2020, 22, 2256–2260. [Google Scholar] [CrossRef] [PubMed]
- Kudo, F.; Matsuura, Y.; Hayashi, T.; Fukushima, M.; Eguchi, T. Genome mining of the sordarin biosynthetic gene cluster from Sordaria araneosa Cain ATCC 36386: characterization of cycloaraneosene synthase and GDP-6-deoxyaltrose transferase. J Antibiot 2016, 69, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, R.J.; Schutt, C.; Lund, B.W.; Staerk, D.; Nielsen, J.; Olsson, S.; Giese, H. Two novel classes of enzymes are required for the biosynthesis of aurofusarin in Fusarium graminearum. J BIOL CHEM 2011, 286, 10419–10428. [Google Scholar] [CrossRef]
- Wight, W.D.; Kim, K.-H.; Lawrence, C.B.; Walton, J.D. Biosynthesis and Role in Virulence of the Histone Deacetylase Inhibitor Depudecin from Alternaria brassicicola. MOL PLANT MICROBE IN 2009, 22, 1258–1267. [Google Scholar] [CrossRef]
- Fujii, I.; Yoshida, N.; Shimomaki, S.; Oikawa, H.; Ebizuka, Y. An iterative type I polyketide synthase PKSN catalyzes synthesis of the decaketide alternapyrone with regio-specific octa-methylation. CHEM BIOL 2005, 12, 1301–1309. [Google Scholar] [CrossRef]
- Kasahara, K.; Miyamoto, T.; Fujimoto, T.; Oguri, H.; Tokiwano, T.; Oikawa, H.; Ebizuka, Y.; Fujii, I. Solanapyrone synthase, a possible Diels-Alderase and iterative type I polyketide synthase encoded in a biosynthetic gene cluster from Alternaria solani. CHEMBIOCHEM 2010, 11, 1245–1252. [Google Scholar] [CrossRef]
- Sorensen, J.L.; Hansen, F.T.; Sondergaard, T.E.; Staerk, D.; Lee, T.V.; Wimmer, R.; Klitgaard, L.G.; Purup, S.; Giese, H.; Frandsen, R.J. Production of novel fusarielins by ectopic activation of the polyketide synthase 9 cluster in Fusarium graminearum. ENVIRON MICROBIOL 2012, 14, 1159–1170. [Google Scholar] [CrossRef]
- Ugai, T.; Minami, A.; Fujii, R.; Tanaka, M.; Oguri, H.; Gomi, K.; Oikawa, H. Heterologous expression of highly reducing polyketide synthase involved in betaenone biosynthesis. CHEM COMMUN 2015, 51, 1878–1881. [Google Scholar] [CrossRef]
- Kato, S.; Motoyama, T.; Uramoto, M.; Nogawa, T.; Kamakura, T.; Osada, H. Induction of secondary metabolite production by hygromycin B and identification of the 1233A biosynthetic gene cluster with a self-resistance gene. J Antibiot 2020, 73, 475–479. [Google Scholar] [CrossRef]
- Kato, S.; Motoyama, T.; Futamura, Y.; Uramoto, M.; Nogawa, T.; Hayashi, T.; Hirota, H.; Tanaka, A.; Takahashi-Ando, N.; Kamakura, T.; et al. Biosynthetic gene cluster identification and biological activity of lucilactaene from Fusarium sp. RK97-94. Biosci Biotechnol Biochem 2020, 84, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Bojja, R.S.; Cerny, R.L.; Proctor, R.H.; Du, L. Determining the Biosynthetic Sequence in the Early Steps of the Fumonisin Pathway by Use of Three Gene-Disruption Mutants of Fusarium verticillioides. J Agric Food Chem 2004, 52, 2855–2860. [Google Scholar] [CrossRef]
- Proctor, R.H.; Brown, D.W.; Plattner, R.D.; Desjardins, A.E. Co-expression of 15 contiguous genes delineates a fumonisin biosynthetic gene cluster in Gibberella moniliformis. FUNGAL GENET BIOL 2003, 38, 237–249. [Google Scholar] [CrossRef]
- Kim, Y.T.; Lee, Y.R.; Jin, J.; Han, K.H.; Kim, H.; Kim, J.C.; Lee, T.; Yun, S.H.; Lee, Y.W. Two different polyketide synthase genes are required for synthesis of zearalenone in Gibberella zeae. MOL MICROBIOL 2005, 58, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Gaffoor, I.; Trail, F. Characterization of two polyketide synthase genes involved in zearalenone biosynthesis in Gibberella zeae. Appl Environ Microbiol 2006, 72, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, M.; Chiang, Y.M.; Chang, S.L.; Praseuth, M.B.; Entwistle, R.; Sanchez, J.F.; Lo, H.C.; Yeh, H.H.; Oakley, B.R.; Wang, C.C. Illuminating the diversity of aromatic polyketide synthases in Aspergillus nidulans. J Am Chem Soc 2012, 134, 8212–8221. [Google Scholar] [CrossRef] [PubMed]
- WIEBE, L.A.; BJELDANES, L.F. Fusarin C, A Mutagen from Fusarium Moniliforme Grown on Corn. J FOOD SCI 1981, 46, 1424–1426. [Google Scholar] [CrossRef]
- Song, Z.; Cox, R.J.; Lazarus, C.M.; Simpson, T.T. Fusarin C biosynthesis in Fusarium moniliforme and Fusarium venenatum. CHEMBIOCHEM 2004, 5, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-C.; Lee, Y.-W.; Tamura, H.; Yoshizawa, T. Sambutoxin: A new mycotoxin isolated from Fusarium sambucinum. TETRAHEDRON LETT 1995, 36, 1047–1050. [Google Scholar] [CrossRef]
- Kato, N.; Nogawa, T.; Hirota, H.; Jang, J.H.; Takahashi, S.; Ahn, J.S.; Osada, H. A new enzyme involved in the control of the stereochemistry in the decalin formation during equisetin biosynthesis. Biochem Biophys Res Commun 2015, 460, 210–215. [Google Scholar] [CrossRef]
- Janevska, S.; Arndt, B.; Baumann, L.; Apken, L.H.; Mauriz Marques, L.M.; Humpf, H.-U.; Tudzynski, B. Establishment of the Inducible Tet-On System for the Activation of the Silent Trichosetin Gene Cluster in Fusarium fujikuroi. TOXINS 2017, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jamieson, C.S.; Zhao, Y.-L.; Li, D.; Ohashi, M.; Houk, K.N.; Tang, Y. Enzyme-Catalyzed Inverse-Electron Demand Diels–Alder Reaction in the Biosynthesis of Antifungal Ilicicolin H. J AM CHEM SOC 2019, 141, 5659–5663. [Google Scholar] [CrossRef]
- Song, Z.; Bakeer, W.; Marshall, J.W.; Yakasai, A.A.; Khalid, R.M.; Collemare, J.; Skellam, E.; Tharreau, D.; Lebrun, M.-H.; Lazarus, C.M.; et al. Heterologous expression of the avirulence gene ACE1 from the fungal rice pathogen Magnaporthe oryzae. CHEM SCI 2015, 6, 4837–4845. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J.; Mou, P.; Yan, Y.; Chen, M.; Tang, Y. Genome mining of cryptic tetronate natural products from a PKS-NRPS encoding gene cluster in Trichoderma harzianum t-22. ORG BIOMOL CHEM 2021, 19, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Yagishita, F.; Mino, T.; Uchiyama, N.; Patel, A.; Chooi, Y.-H.; Goda, Y.; Xu, W.; Noguchi, H.; Yamamoto, T.; et al. Involvement of Lipocalin-like CghA in Decalin-Forming Stereoselective Intramolecular [4+2] Cycloaddition. CHEMBIOCHEM 2015, 16, 2294–2298. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




