1. Introduction
The restoration of abdominal wall defects as a result of occurred trauma, infection, congenital conditions, complications of abdominal surgeries, neoplastic diseases and others belongs among the most routinely performed surgeries of the general population [
1]. Beneath them, incisional hernias that occurred after laparotomy, are exhibited in 5-20% of patients, and their repair represents a highly demanding reconstructive surgery [
2]. It is estimated that more than 700.000 surgeries for abdominal wall reconstruction are performed in the United States, annually and more than 20 million are performed globally, each year [
1,
2,
3,
4].
Mostly, abdominal wall surgery and herniation can lead to significant complications including muscle weakness and collagen ratio disturbance [
5]. Regarding the latter, it has been already described in the literature, that the amount of collagen I is reduced, while the amount of collagen III is increased, resulting in alterations in abdominal wall tensile strength and mechanical stability [
5,
6,
7,
8]. In accordance with the above-provided information, the proper reconstruction of abdominal wall defects requires the use of scaffolds characterized by specific biomechanical properties, to support tissue regeneration and deep wound healing [
9]. Nowadays, a great number of commercially produced synthetic scaffolds are used as implants for abdominal wall reconstruction [
1,
10,
11]. Synthetic scaffolds can be produced utilizing either biodegradable or non-biodegradable materials. Polyglycolic acid (PLGA) and polyglactin 910 (PLGA 910), are biodegradable materials, which are degraded through the hydrolysis process [
12,
13,
14]. In this way, tissue-resident macrophages can efficiently degrade these materials into monomers, which further can be deposited to the regenerated ECM. However, the abdominal wall reconstruction performed with biodegradable materials results mostly in scar tissue formation at the injury site [
15,
16,
17]. This in turn can result in complications due to scar tissue formation, impairing in this way tissue integrity and mechanical resistance. Unfortunately, new reconstructive surgery to replace the formed scar tissue is required [
18]. On the other hand, polypropylene (PP) and expanded tetrapolyfluorethylene (ePTFE) belong to the category of non-biodegradable materials, with good biomechanical properties and tissue reinforcement [
19,
20,
21]. Although these materials have good properties, severe adverse reactions such as calcification, and host immune system activation have been reported in the majority of the cases [
1].
For this purpose, scaffolds derived from different origins e.g. natural plant-based or animal-derived ECM must be evaluated as a potential new strategy for abdominal wall reconstruction. In the context of tissue regeneration, biological scaffolds composed of native or glutaraldehyde crosslinked ECM, have been used in the past, with limited success [
22,
23]. However, after long-term implantation, also aberrant host immune responses have been reported [
24,
25]. Immune system activation includes mostly the polarization of macrophages into the M1 phenotype, the activation of CD4 T cells, and the migration of Th1 and B cells to the implantation site, which further leads to calcification and tissue rejection [
24,
25,
26]. On the other hand, the production of biological scaffolds utilizing advanced tissue engineering approaches such as decellularization may overpass the aforementioned issues [
27]. Decellularization relies on the use of a combination of physical and chemical methods, leading to cellular loss and the production of an acellular matrix [
28]. The decellularized ECM can beneficially support cell adhesion, survival, proliferation, and differentiation, compared to the synthetic or cross-linked scaffolds [
29]. The latter is related also to better biomechanical properties, which can lead to improved tissue reconstruction at the injury site [
30,
31]. Currently, different research groups elaborate on protocols for the successful decellularization of abdominal wall scaffolds of animal origin [
32,
33,
34]. The application of decellularization protocol can be easily performed in a great variety of tissues, although the preservation of the full-thickness ECM, comprises a highly demanding task. For this reason, the literature regarding the successful production of full-thickness abdominal wall scaffolds is limited [
32,
33,
34], yet the needs of reconstructive surgery demand the production of such structures. In the past, perfusion decellularization was proposed for the production of whole organ scaffolds, however, this process is laborious and requires advanced equipment and well-trained personnel [
35]. Although, the broad use of the above method, still serious issues concerning extensive ECM or capillary network damage, have been already reported, which may lead to unfavourable results regarding tissue implantation and cell repopulation either under
in vitro or
in vivo conditions [
32,
33,
34]. Based on our previous experience, we have validated a cost-effective perfusion-free decellularization method, which can potentially be applied as an alternative method for the production of full-thickness abdominal wall scaffolds [
33].
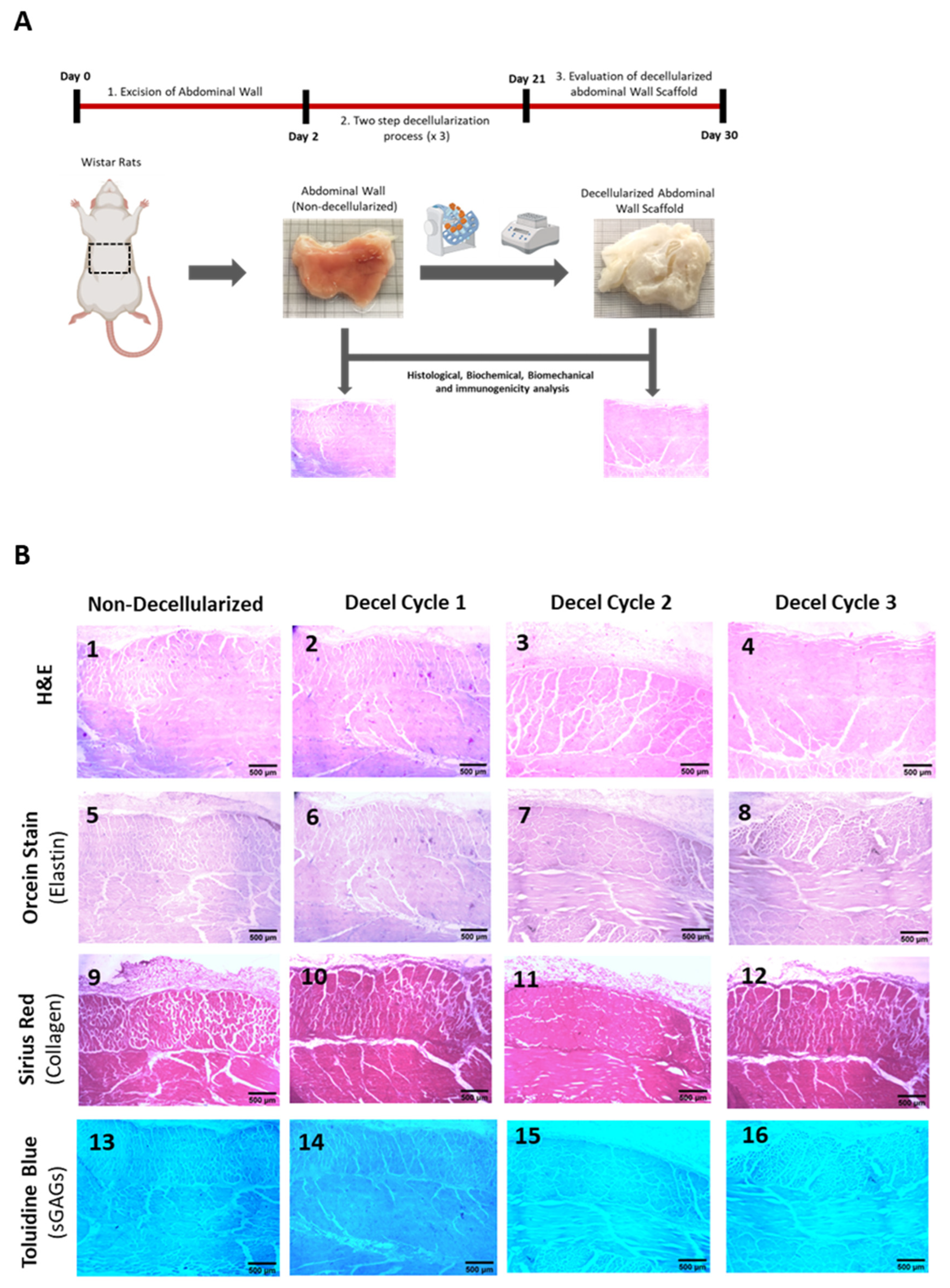
The aim of this study was to test the efficacy of the production of an acellular scaffold originating from full-thickness rat-derived abdominal wall samples. For this purpose, our optimized decellularization protocol consisting of 3 cycles, was applied to a full-thickness rat-derived abdominal wall. The evaluation of the decellularized abdominal wall ECM was performed using methods such as histological analysis, biochemical and DNA quantification. Furthermore, the biomechanical properties of the abdominal wall before and after the decellularization approach were also investigated. The obtained results may lead to significant conclusions regarding the full-thickness abdominal wall decellularization, to be used in large-scale experimental procedures.
2. Methods
2.1. Abdominal Wall Excision
Whole abdominal walls were harvested from Wistar rats (n=30), weighing 300-400 gr, under aseptic conditions. All animals were provided by the Animal Facility of the Biomedical Research Foundation Academy of Athens (BRFAA). Well-trained personnel of the animal facility handled and cared for the animals according to the international guidelines of animal care, and conformed with the Helsinki Declaration. In addition, this study was approved by the Bioethics Committee of BRFAA (ref 02-2021). Briefly, the animals were euthanatized and using sterile instruments a paramedian incision in the anterior abdominal wall was performed, in order to expose the external oblique muscles. The abdominal wall samples were extracted in dimensions of 4 x 5 cm and then stored in Phosphate Buffer Saline (PBS, Sigma-Aldrich, Darmstadt, Germany) 1x supplemented with 1% Penicillin-Streptomycin (P-S, Sigma-Aldrich, Darmstadt, Germany), until further processing.
2.2. Decellularization of whole rat abdominal wall samples
Whole abdominal wall samples (n=30) were decellularized using an already published protocol, validated previously in our lab [
33]. Whole rat abdominal wall samples were incubated with decellularization buffers under vigorous agitation at 150 rpm. The decellularization procedure involved the incubation of the samples in CHAPS buffer, pH:7 (8 mM CHAPS, 1 M NaCl and 25 mM EDTA, in PBS 1x, Sigma-Aldrich, Darmstadt, Germany) for 18 h at room temperature (RT), followed by subsequent incubation in SDS buffer, pH:7 (1.8 mM SDS, 1 M NaCl and 25 mM EDTA, in PBS 1x, Sigma-Aldrich, Darmstadt, Germany) for another 18h at RT. Finally, the samples were placed in a solution containing a-Minimum Essentials Medium (a-MEM, Sigma-Aldrich, Darmstadt, Germany) supplemented with 40% v/v Fetal Bovine Serum (FBS, Gibco, Thermo Fisher Scientific, Waltham, MA, USA) for 36 h at 37
o C. After each decellularization buffer, 3x washes with PBS 1x (Sigma-Aldrich, Darmstadt, Germany), at RT was performed. The whole decellularization procedure was repeated another 2 times (in total 3 decellularization cycles, n=5 samples/decellularization cycle).
2.3. Histological Analysis
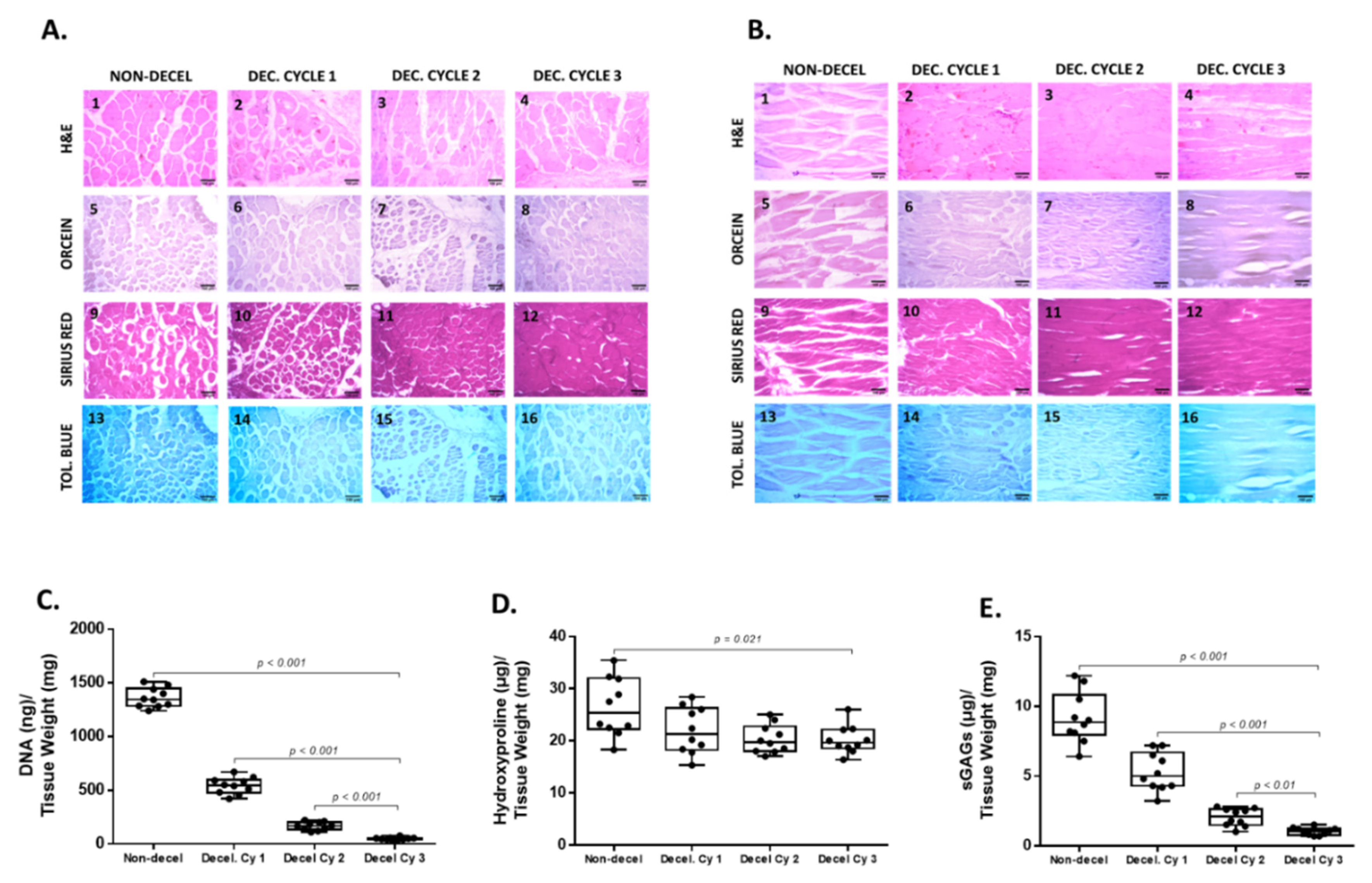
Non-decellularized (n=10) and decellularized (n=15) whole rat abdominal wall samples (obtained from the 1st, 2nd and 3rd decellularization cycle) were fixed overnight using the 10% v/v neutral formalin buffer (Sigma-Aldrich, Darmstadt, Germany), followed by washes with distilled water. After this step, all samples were dehydrated in an increasing series of alcohol buffers, paraffin-embedded and finally sectioned at 5 μm. The production of acellular abdominal wall scaffolds was evaluated with the performance of specific histological stains. For this purpose, hematoxylin and eosin (H&E, VWR, Avantor, Radnor, PA, USA), Sirius Red (SR, VWR, Avantor, Radnor, PA, USA), Orcein (OS, VWR, Avantor, Radnor, PA, USA) and Toluidine Blue (TB, VWR, Avantor, Radnor, PA, USA) were used for the evaluation of abdominal wall ECM, collagen fibers, elastin fibers and sulphated glycosaminoglycans (sGAGs), respectively. The sections of non-decellularized and decellularized abdominal wall samples were deparaffinized, rehydrated, stained with each stain, and mounted with a cover slide. Whole rat abdominal wall samples were characterized by a combination of longitudinally and circumferentially oriented fibers, and for this purpose, representative images were obtained. Images were acquired using the Leica DM L2 light microscope (Leica Microsystems, Weltzar, Germany) and processed with Image J v.1.46 (Wane Rasband, National Institute of Health, Bethesda, USA).
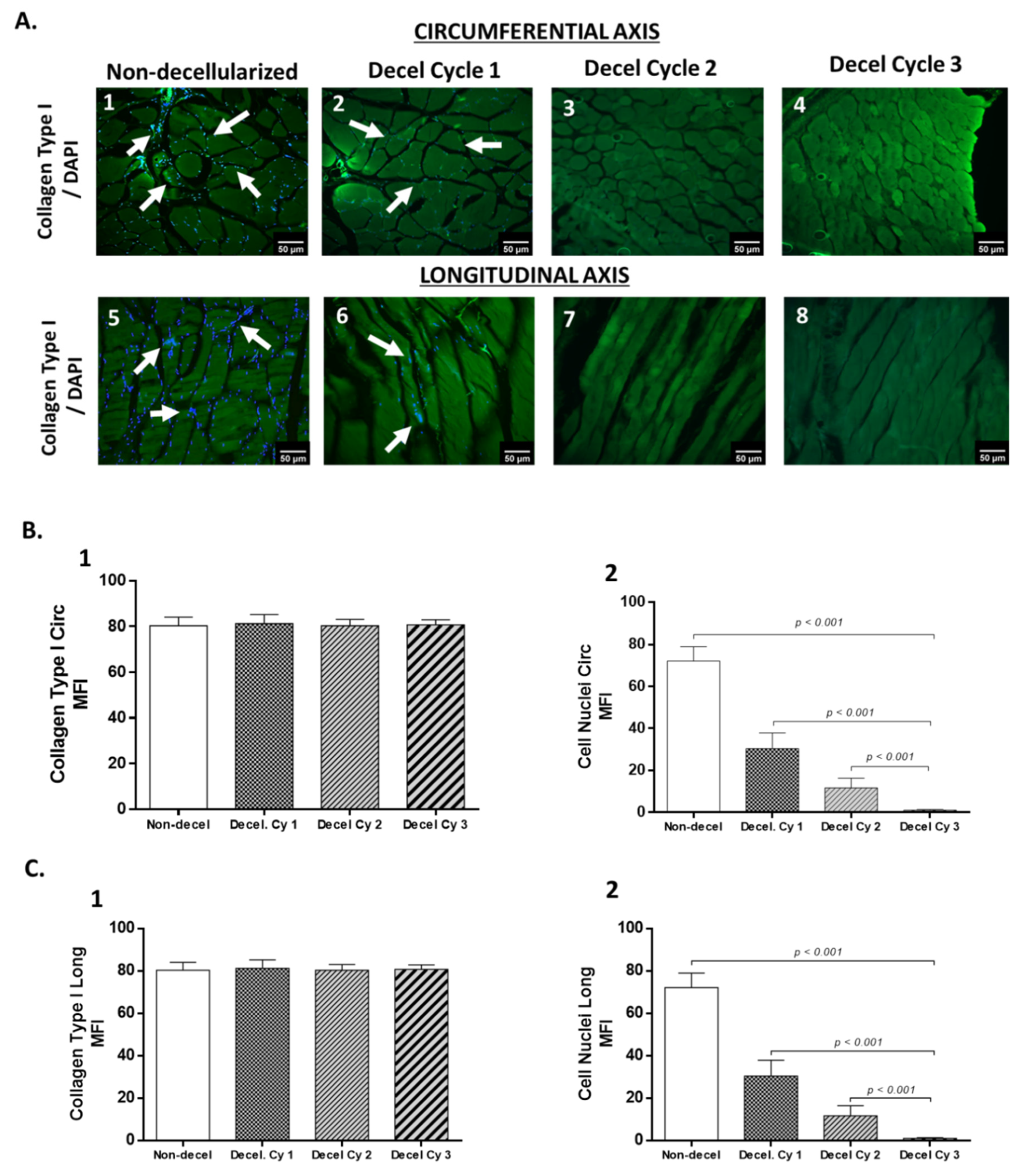
To further evaluate the impact of each decellularization cycle on collagen type I, indirect immunofluorescence was performed. Briefly, the sections of all study samples were deparaffinized, rehydrated, and blocked. Then, incubation with the primary monoclonal antibody against collagen type I (1:1000, Sigma-Aldrich, Darmstadt, Germany), was performed. Brief washes were performed and then incubation with the FITC-conjugated IgG secondary antibody (1:500, Sigma-Aldrich, Darmstadt, Germany) was performed. Finally, cell nuclei were stained with DAPI (Sigma-Aldrich, Darmstadt, Germany), and then the samples were dehydrated, and glycerin mounted. The samples were examined under the LEICA SP5 II confocal microscope and representative images were acquired with the LAS Suite v2 software (Leica Microsystems, Weltzar, Germany).
2.4. Biochemical analysis
The biochemical analysis included the evaluation of collagen (hydroxyproline) and sGAGs content. Moreover, representative areas of non-decellularized and decellularized whole rat abdominal wall samples (1 x 1 cm) were selected from each sample. Specifically, for the collagen content quantification, non-decellularized (n=10) and decellularized (n=30, n=10 / decellularization cycle) were digested in papain solution (125 μg / ml, Sigma-Aldrich, Darmstadt, Germany) at 60oC for 10, or until full digestion of the samples. Collagen content was estimated based on the determined hydroxyproline content with the hydroxyproline assay kit (MAK008, Sigma-Aldrich, Darmstadt, Germany) following the manufacturer’s instructions. SGAGs content in non-decellularized (n=10) and decellularized (n=30, n=10 / decellularization cycle) was performed, as has been previously described. Briefly, the samples were digested with 1 ml of lysis buffer (0.1 M Tris pH:8, 0.2 M NaCl and 5 mM EDTA in PBS 1x (Sigma Aldrich, Darmstadt, Germany) and 25 mg/ml proteinase K ( Sigma-Aldrich, Darmstadt, Germany) at 56oC for 12 h. Inactivation of proteinase K was performed at 95oC, for 5 min. The 1% w/v dimethylene blue stain (Sigma-Aldrich, Darmstadt, Germany) was used and the determination of sGAG content of each sample was performed photometrically at 525 nm. Finally, the sGAG concentration of each sample was estimated through interpolation to a standard curve, based on chondroitin sulphate standards of 3, 6, 12, 25, 50, 100 and 150 μg / ml.
2.5. DNA quantification
For the DNA quantification, non-decellularized (n=10) and decellularized (n=30, n=10 / decellularization cycle) samples, were initially digested using a Proteinase K ( 25 μg / ml, Sigma-Aldrich, Darmstadt, Germany) in PBS 1x (Sigma-Aldrich, Darmstadt, Germany) at 56o C for 12 h, followed by inactivation at 96oC for 5 min. Then, isolation of DNA of each sample was performed and finally eluted in 50 μl RNAse free water (Sigma-Aldrich, Darmstadt, Germany). The DNA content of each sample was spectrophotometrically determined using the Nanodrop (Thermo Fischer Scientific, Waltham, Massachusetts, USA) at 260 / 280 nm. Also, the DNA quantification results were verified using DNA electrophoresis on 1% w/v agarose gel. Images were acquired with the UVITEC Imaging System (Cleaver, Scientific, Warwickshire, USA).
2.6. Biomechanical Analysis
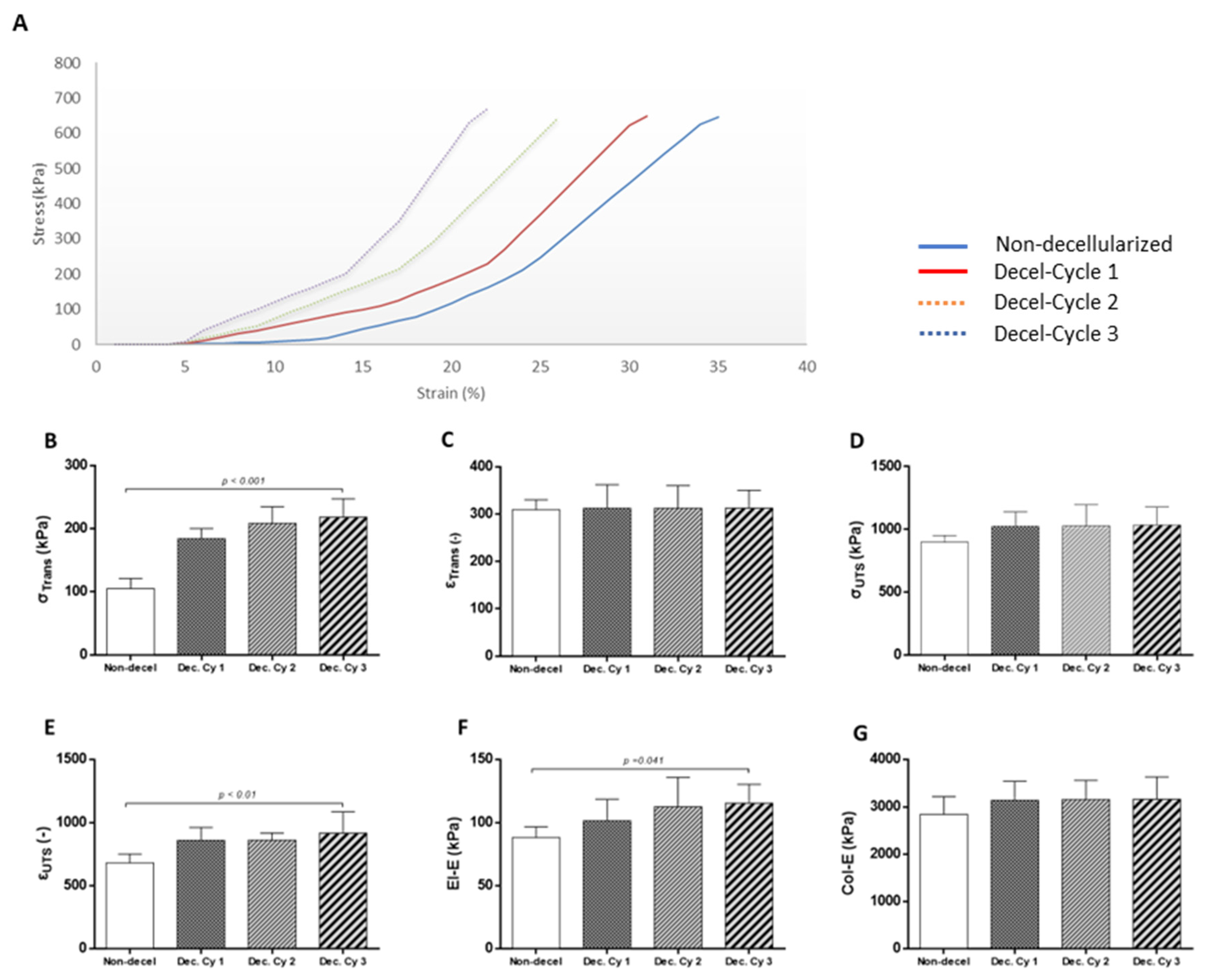
Biomechanical analysis of non-decellularized (n=5) and decellularized (n=5/ cycle) whole rat abdominal wall samples was performed to evaluate the mechanical properties. The biomechanical analysis involved the uniaxial testing of the samples which was performed in a Zwick/Roell tensile tester (model Z 0.5, Zwick GmbH & Co. KG, Ulm, Germany) equipped with a 100 N load cell. Non-decellularized and decellularized samples were cut into longitudinal strips (l=40 mm, w=10 mm) and placed before the analysis in prewarmed Krebs-Ringers (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) solution (at 37oC). During the biomechanical testing, the strips were continuously sprayed with PBS 1x (Sigma-Aldrich, Darmstadt, Germany). The strips derived from non-decellularized and decellularized samples were clamped at their ends, with sandpaper to avoid sample slippage, under zero strain on the mechanical device, which produced a preloading value of 0.005 N, before the data collection. All samples were preconditioned for 10 cycles at a rate of 10 mm/min. Sample extension (Δl, in mm) accompanying the generated load (F, in Newtons) were converted to engineering strain (ε), and engineering stress (σ in MPa). Elastin (El-E) and collagen (Col-E) phase slope, transition stress (σΤrans) and strain (εTrans), ultimate tensile strength (σUTS) and failure strain (εUTS) were used, to analyze the stress-strain behaviour of the studied samples.
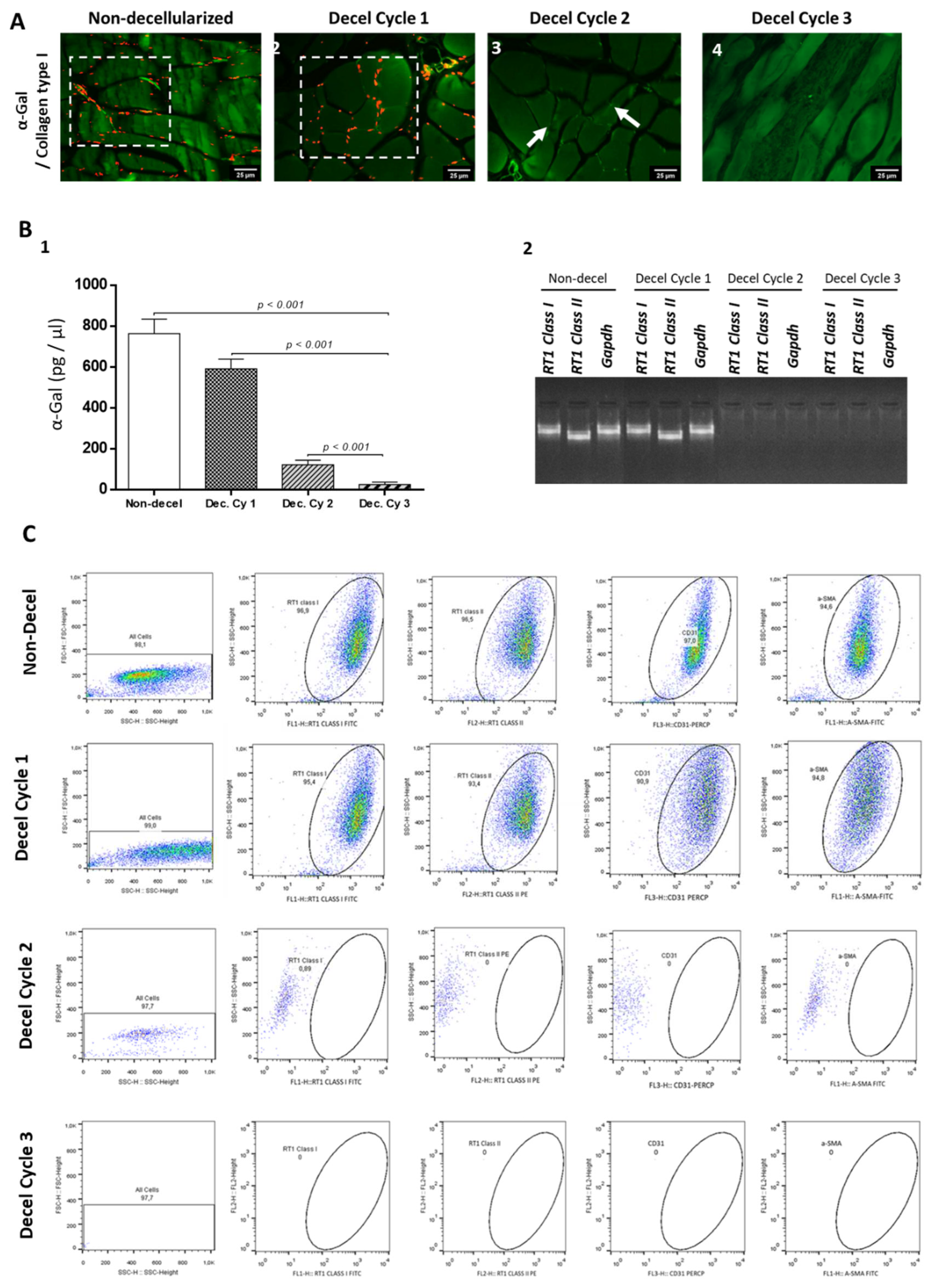
2.7. Determination of Acellular Whole Abdominal Wall Immunogenicity
To determine the immunogenicity of decellularized whole abdominal wall scaffolds, the following assays were performed. Indirect immunofluorescence against α-gal (1:500, Sigma-Aldrich, Darmstadt, Germany) and collagen type I (1:1000, Sigma-Aldrich, Darmstadt, Germany) was performed as has been previously described. Secondary PE-conjugated monoclonal antibody (1:50) against α-gal epitope and FITC-conjugated monoclonal antibody (1:500) against collagen type I were used for the detection of the aforementioned proteins. Finally, all samples were dehydrated, glycerin mounted and examined using the LEICA SP5 II confocal microscope coupled with the LAS Suite v2 software (Leica Microsystems, Weltzar, Germany). In addition, the detection of α-gal epitopes was performed using the ELISA method, for additional verification of indirect immunofluorescence results. Initially, non-decellularized (n=5) and decellularized samples (n=5 / each cycle) were fully lysed in a 1% w/v papain medium (Sigma-Aldrich, Darmstadt, Germany) at 37
o C for a maximum of 10 hours. Then, the occurred lysates passed through 0.25 nm filters. After this step, the ELISA method was performed, following the manufacturer’s instructions (ab239716, Abcam, Cambridge, United Kingdom). Furthermore, gene expression profile in non-decellularized and decellularized samples was performed. Total mRNA was isolated from non-decellularized (n=5) and decellularized (n=5 / each cycle) using the TRI-reagent (Sigma-Aldrich, Darmstadt, Germany) according to the manufacturer’s instructions. The concentration of isolated mRNA was photometrically determined. Then, at least 400 ng of total mRNA was used as template for the cDNA production using the Omniscript reverse transcription kit (Qiagen, Hilden, Germany). PCR was performed using the Taq PCR master mix (Cat No 201443, Qiagen, Hilden, Germany) on Biometra T Gradient Thermoblock PCR Thermocycler (Biometra, Gottingen, Germany) with a final volume of 25 μl for each PCR reaction. The PCR program involved the below steps: 1) denaturation at 95
oC for 15 min, 2) denaturation at 94
o C for 30 s, 3) annealing at 60-62
o C for 90 s and 4) extension at 72
o C for 3 min. The number of cycles used in this program was 35. The primer pairs for each PCR reaction are listed in
Table 1. Finally, the PCR products were analyzed with electrophoresis on agarose gel (1% w / v, Sigma-Aldrich, Darmstadt, Germany). In addition, to evaluate the immunogenicity of non-decellularized and decellularized abdominal wall samples, flow cytometric analysis was also performed. Initially, non-decellularized (n=5) and decellularized samples (n=5 / each cycle) were fully digested in a 1% w/v collagenase medium at 37
o C for a maximum of 4 hours. The isolated cells were washed 3 times with PBS 1x (Sigma-Aldrich, Darmstadt, Germany) and then were submitted for the flow cytometric analysis. Specifically, cells obtained from all samples were analyzed for the expression of RT1 class I, RT1 class II, CD31 and a-SMA. Monoclonal antibodies against RT1 Class I, and a-SMA and a-SMA, were conjugated with fluorescein isothiocyanate (FITC), while RT1 class II and CD31 were conjugated with phycoerythrin (PE) and Peridinin-Chlorophyll-protein (PERCP), respectively. Anti-RT1 class I and class I were purchased from Biocompare (South San Francisco, USA). The rest of the monoclonal antibodies were purchased from BD Biosciences (New Jersey, USA) and analyzed at a minimum of 10000 all events in FACS Calibur ( BD Biosciences, New Jersey, USA).
2.8. Statistical Analysis
The statistical analysis of this study was performed using GraphPad Prism v 6.01 (GraphPad Software, San Diego, CA, USA). Comparisons regarding the hydroxyproline, sGAG, DNA content and also comprehensive biomechanical analysis were performed using the Kruskal-Wallis test. Furthermore, validation of our results involved the use of the unpaired nonparametric Mann-Whitney U test. Statistically significant differences between group values were considered when p value was less than 0.05. Indicated values were presented as mean ± standard deviation.
4. Discussion
In the field of reconstructive surgery, the use of the appropriate prosthetics for the restoration of abdominal wall defects represents one of the greatest challenges of the last decade. Currently, a great number of biomaterials have been used for abdominal wall reconstruction, however, both synthetic biodegradable and non-biodegradable scaffolds are accompanied by severe adverse reactions such as scar tissue formation, altered mechanical properties, calcification and eventually graft loss [
1,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21]. Recently, animal-derived crosslinked biological scaffolds are also considered as an alternative and promising solution, for the proper restoration of abdominal wall defects [
22,
23]. Nevertheless, their natural origin crosslinked biological scaffolds are characterized by calcification occurrence and M1 macrophage-mediated tissue rejection [
24,
25]. More than 20 million abdominal wall reconstructive surgeries are performed annually at the international level, and for this reason, the search and utilization of alternative abdominal wall meshes, are highly important [
1,
2,
3,
4]. Taking into consideration the above data, the application of a non-immunogenic biological scaffold utilizing state-of-the-art tissue engineering methods for abdominal wall defect restoration may represent a promising alternative source of transplants. The present study aimed to the production of full-thickness abdominal wall scaffolds, utilizing a validated decellularization approach. For this purpose, rat-derived abdominal wall samples were decellularized appropriately to produce acellular non-immunogenic biological scaffolds, to serve as potential grafts for future abdominal wall tissue restoration. Initially, full-thickness abdominal wall samples were harvested, washed to remove any blood clots and submitted immediately to decellularization. Histological analysis revealed that complete decellularization of full-thickness abdominal wall scaffolds was achieved after the accomplishment of the 3
rd cycle. Cell removal was initiated after the 1
st decellularization cycle, however complete cell and nuclear remnants were eliminated by the end of the proposed protocol. In addition, decellularized abdominal wall scaffolds (after the 3
rd decellularization cycle) were characterized by the preservation of collagen, a significant ECM component. On the other hand, sGAGs appeared to be significantly removed after the application of the decellularization protocol. Regarding the elastin content, besides its low presence in the abdominal wall, no significant alteration between non-decellularized and decellularized samples was observed. The preservation of collagen in decellularized samples was further confirmed by the performance of indirect immunofluorescence (against collagen type I in combination with DAPI). Moreover, collagen type I fibers in decellularized samples retained their alignment both in the circumferential and longitudinal axis in the same way as the non-decellularized samples. The preservation of collagen type I in decellularized samples is of significant importance, comprising one of the major proteins of ECM structure, thus contributing to a great number of processes such as hemostasis performance and regulation of the wound healing process of the injured area [
38]. Besides its function in tissue healing, collagen type I plays a significant role in the recruitment and attachment of specific cellular populations to the wounded region through binding to arginine-glycine-aspartic acid (RGD) binding motifs [
39,
40,
41,
42,
43,
44]. Indeed, various cellular populations such as fibroblasts, pericytes and mesenchymal stromal cells can bind to the collagen fibers through the interaction of α1β1, α2β1 and ανβ1 with the RGD binding motifs of well-aligned collagen fibers, thus further can promote the wound healing and tissue regeneration [
39,
40,
41,
42,
43,
44]. Specifically, it has been shown that integrin-mediated binding with the RGD motifs can lead to an important intracellular signalling cascade through the upregulation of ILK-NF-kb, and GSK3β-AP1-CyclinD1, promoting in this way the cell survival, migration and proliferation to the injured site [
39,
40,
41,
42,
43,
44,
45,
46]. Besides, the collagen fibers also sGAGs are playing important role in collagen fibers orientation, and their loss after the decellularization process may affect the ECM structure and mechanical properties of the occurred scaffold [
47,
48]. However, the results of the present study seem to be in accordance with previously performed works, where also significant differences in sGAGs content were observed in decellularized samples. Importantly, in the study of Luo
et al. [
49] and Gui
et al. [
50] the levels of sGAG content in decellularized heart valves and umbilical arteries, respectively, were lower compared to the non-decellularized tissues. This can be explained by the already knowledge of the anionic nature of SDS, which is used as a basic detergent in all these studies [
28]. SDS can interfere with the negatively charged sites of the sGAGs, and thus can efficiently remove them from the decellularized tissue [
28,
49].
The above results regarding the collagen, sGAGs and cell removal were also confirmed by the quantification assays performed in the present study. Importantly, the cell removal was further confirmed by the low levels of the isolated DNA content from decellularized samples. Indeed, the performance of each decellularization cycle appeared to be lower compared to the previous one. Finally, after the 3
rd decellularization cycle, the DNA concentration was below 50 ng / μl dry tissue, thus confirming further the criteria for successful tissue decellularization as have been outlined by Gilbert
et al. [
28], and Crapo
et al. [
51]. In the present study, the DNA concentration was determined, through the measurement of 260 nm / 280 nm ratio with a spectrophotometer, avoiding in this way false quantification results occurring using commercial kits such as the Picogreen assay. Most of these commercial kits determine only the double-stranded (ds) DNA, without measuring the single-stranded (SS) DNA, which also may be present, especially in decellularized tissues [
49,
52]. Using the spectrophotometer, the genetic material quantification is most accurate, representing better the DNA content of the decellularized tissues. It is also widely known, that both ds and ss DNA can initiate a specialized immune response against the implanted graft, which could result in significant adverse reactions to the host [
49,
52,
53].
Biomechanical properties are playing important role in tissue mechanics and therefore were evaluated before and after the decellularization process applied to the full-thickness abdominal wall scaffolds. Interestingly, decellularized abdominal wall scaffolds characterized by higher values of σ
Trans, ε
Trans, σ
UTS, ε
UTS, El-E and Col-E compared to the non-decellularized samples, suggesting the adaptation of a stiffer behavior by the produced scaffolds. Also, it was observed that differences in biomechanical properties were observed after the performance of each decellularization cycle. Overall, the increase in abdominal wall scaffold strength is respective to the ECM structure. Considering that the collagen fibers are responsible for the preservation of tissue integrity during occurred overload differences in the corresponding anatomical area, changes in collagen orientation play important role in altered mechanical properties of the produced scaffolds. In addition, decellularized abdominal wall scaffolds were characterized by greater extensibility than the non-decellularized samples, which is further related to the disorganized collagen fibers alignment. To assess the relationship between the ECM structure and biomechanical properties, extensive histological analysis of all study samples both in circumferential and longitudinal directions was performed in the present study. However, histological analysis results did not reveal any change in the collagen fibers orientation (in both axis). Moreover, the indirect immunofluorescence results against collagen type I confirmed further the results obtained from the histological stains, thus confirming the preservation of collagen fibers alignment after the performance of the decellularization procedure. Besides collagen fibers, also elastin fibers are playing important role in tissue mechanical strength and extensibility. However, the elastin content in the abdominal wall is relatively low and the elastin fibers are mostly disorganized compared to other tissues such as elastic vessels (e.g. thoracic or abdominal aorta). However, no change in elastin content was observed between non-decellularized and decellularized abdominal wall samples. The explanation regarding the stiffer behavior of the decellularized scaffolds may be attributed to the cellular population elimination in the produced scaffolds. Indeed, SMCs and ECs, besides their important role in other biological processes, have the ability to retain the orientation of the collagen fibers, thus contributing further to the preservation of the ECM integrity. Based on the histological analysis, the collagen fibers of the non-decellularized samples are fully crimped, while in scaffolds are becoming uncrimp after the performance of each decellularization cycle. To this phenomenon, also the reduction of the sGAG content observed in decellularized scaffolds may play its role in the collagen crimp. It is known, that sGAGs are forming large polymers called proteoglycans which actively contribute to collagen cross-linking [
54]. The significant reduction of sGAGs in combination with the cellular elimination may lead to an important change in collagen crosslink, thus affecting further their crimp. Similar alterations in biomechanical properties have also been revealed in the past in other structures such as the aorta, intestine and elastic vessels, and have been extensively reported by Sexton
et. al. [
55], Stehbens
et. al. [
56]
, Sokolis
et. al. [
57] and Stergiopoulos
et. al. [
58]. Specifically, Stergiopoulos
et. al. [
58] indicated in their model, that the alteration of the biomechanical properties acquired by the decellularized scaffolds cannot be the mere result of cellular elimination only, but further structural ECM changes may provoke altered mechanical behavior. In a similar way, there is substantial evidence for the physical link between SMCs, ECs and ECM components, which further may contribute to the existence of residual forces in the abdominal wall. On the other hand, decellularization cause alterations at both the structural and cellular levels of ECM, thus the prestressed fibers no longer exist, and this may be the explanatory cause of the stiffer behavior of the decellularized abdominal wall scaffolds. Besides the above evidence for the altered mechanical properties explanation, stiffer abdominal wall scaffolds have been observed in other studies conducted with similar preparation methods. Specifically, Sanchez
et. al. [
32], Buell
et. al. [
33] and Chiu
et. al. [
34] demonstrated decellularized abdominal wall constructs with similar biomechanical properties, as those presented herein.
The immunogenicity of the decellularized scaffolds constitutes another important parameter, to serve as potential biological grafts. To assess if the proposed decelluarization protocol could be submitted for the preparation of acellular scaffolds of animal origin, the determination of α-gal epitopes was performed. Complete elimination of α-gal epitope was achieved after the performance of the 3
rd decellularization cycle, as it was indicated by histological and quantification analysis results. A-gal epitopes are considered as main immunogenic antigens and related to hyper-acute rejection when xeno-transplantation was performed in the past [
59,
60,
61]. Currently, there is a great amount of performing efforts to produce α-Gal epitope free knockout pigs, which can offer a solution to a global shortage of organs, however, this attempt is costly [
62,
63]. In the context of biological scaffold production originating from animal models, the decellularization method could potentially assist with this issue. The SDS, a detergent of decellularization, is known for its ability to break the hydrophobic interactions, and ionic and hydrogen bonds, thus can successfully remove negatively charged proteins and genetic material. In such a way, SDS has been shown that potential may interfere with the α-gal epitopes, forcing their elimination by the tissue’s ECM. Although efforts in removing animal-derived antigens from biological scaffolds were performed, α-Gal epitopes have been found in transplants such as heart valves, SIS-ECM and vessel grafts, impairing the graft longevity after transplantation [
59,
60,
61,
62,
63,
64,
65,
66]. In the present study, the proposed decellularization protocol successfully eliminated the α-gal epitopes from the produced scaffolds, thus decreasing their potential immunogenicity upon implantation [
64,
65,
66]. Moreover, molecular and cytometry analysis revealed the absence of both cellular populations e.g. ECs and SMCs and other antigenic epitopes e.g. the
RT1 class I and
II. RT1 class I and class II comprise the analogue genes of histocompatibility in rats [
67,
68]. Histocompatibility antigens are actively implicated in transplantation and graft survival, and their removal may be related to the production of universal biological transplants. In a previous study conducted by our lab, the same decellularization protocol was applied in human umbilical arteries, where also the elimination of the HLA class I and II antigens was confirmed after the accomplishment of a comprehensive shotgun quantitative proteomic analysis [
69]. The above results suggest further that the proposed decellularization protocol successfully produced low immunogenic full-thickness abdominal wall scaffolds derived from Wistar rats. The study presented herein, demonstrated a proof-of-concept protocol for the production of acellular full-thickness abdominal wall scaffolds, for potential use as transplants. In this study, an already established decellularization protocol, which has been validated by our research team in several tissues and organs, including the human umbilical cord artery, Wharton’s jelly tissue, oesophagus and kidney, was evaluated for the proposed tissue [
38,
52,
69,
70,
71,
72]. In the past, also other research groups tried to produce full-thickness abdominal wall scaffolds, with contradictory results. Specifically, Chiu
et. al. [
34] demonstrated the production of the porcine acellular dermal matrix (ADM) decellularized with the use of supercritical carbon dioxide (SCCO
2). Chiu
et. al. [
34] successfully produced ADM with this method, however, both ECM ultrastructure and main components appeared to be significantly affected by the SCCO
2. There was a significant difference between non-decellularized and decellularized samples regarding the histological stain intensity. In addition, ADM samples in higher magnification are characterized by alterations in ECM integrity. On the other hand, in our study, no alteration in histological stain intensity between non-decellularized and decellularized samples (either from 1
st, the 2
nd or 3
rd cycle) was observed. Only sGAG content was affected by the proposed decellularization protocol as it was confirmed by both histological and quantification results. However, in the majority of the studies focused on the production of decellularized scaffolds, a similar loss of sGAGs has been observed [
38,
49,
50,
69,
70,
71,
72]. Moreover, the sGAG content loss and cellular population elimination are responsible for the altered mechanical properties of the acellular scaffolds. In addition, although the studies conducted by the Buell
et. al. [
33] and Sanchez
et. al. [
32] indicated the production of acellular abdominal wall scaffolds, the tissue ECM was affected in a similar way as in the study of Chiu
et. al. [
34]. Taking into account the available data from the literature, in our study, a comprehensive evaluation of the decellularized scaffold histology (circumferential vs longitudinal axis) was performed, in order to acquire safe conclusions.
In conclusion, the results of this study clearly showed the successful production of a full-thickness rat-derived abdominal wall scaffold. Moreover, major immunogenic epitopes such as the α-gal or the RT1 class I and II antigens were removed, thus the produced scaffolds characterized by low immunogenicity. Considering our results, in the near future a second study is primarily prepared, where implantation of the produced full-thickness decellularized abdominal wall scaffold will be performed in an animal model, in order to investigate deeper the behavior of the transplant in terms of mechanical strength, capability for tissue integration, non-immunogenic nature and also the underlying tissue remodelling mechanisms. The proposed decellularization protocol efficiently could be used for the production of abdominal wall scaffolds derived from large animal models (porcine origin) or human cadaveric donors. Furthermore, this study exploited the possibility of the production of non-immunogenic acellular meshes, with good tissue integrity and mechanical properties, suitable to be used as transplants in abdominal wall defects. The production of such acellular biologic scaffolds, with in vivo tissue remodelling properties can be considered superior to the current gold-standard methods (e.g. synthetic scaffolds). Hence comprehensive evaluation of the properties of the decellularized biologic scaffolds is required to be performed in the future, to ensure the appropriate FDA clearance. Under these circumstances, these scaffolds could be used more efficiently by clinicians, bringing them one step closer to their clinical utility.