Submitted:
23 June 2023
Posted:
05 July 2023
You are already at the latest version
Abstract
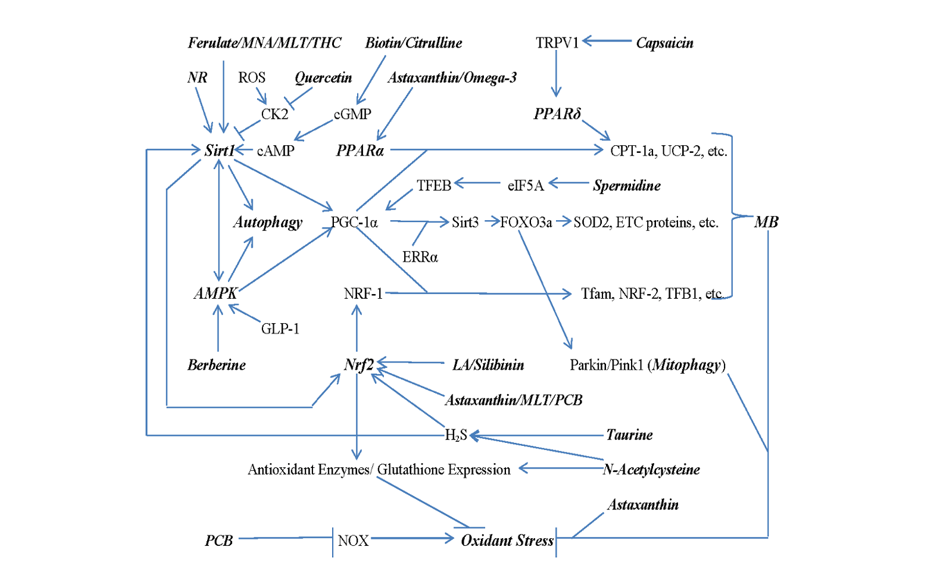
Keywords:
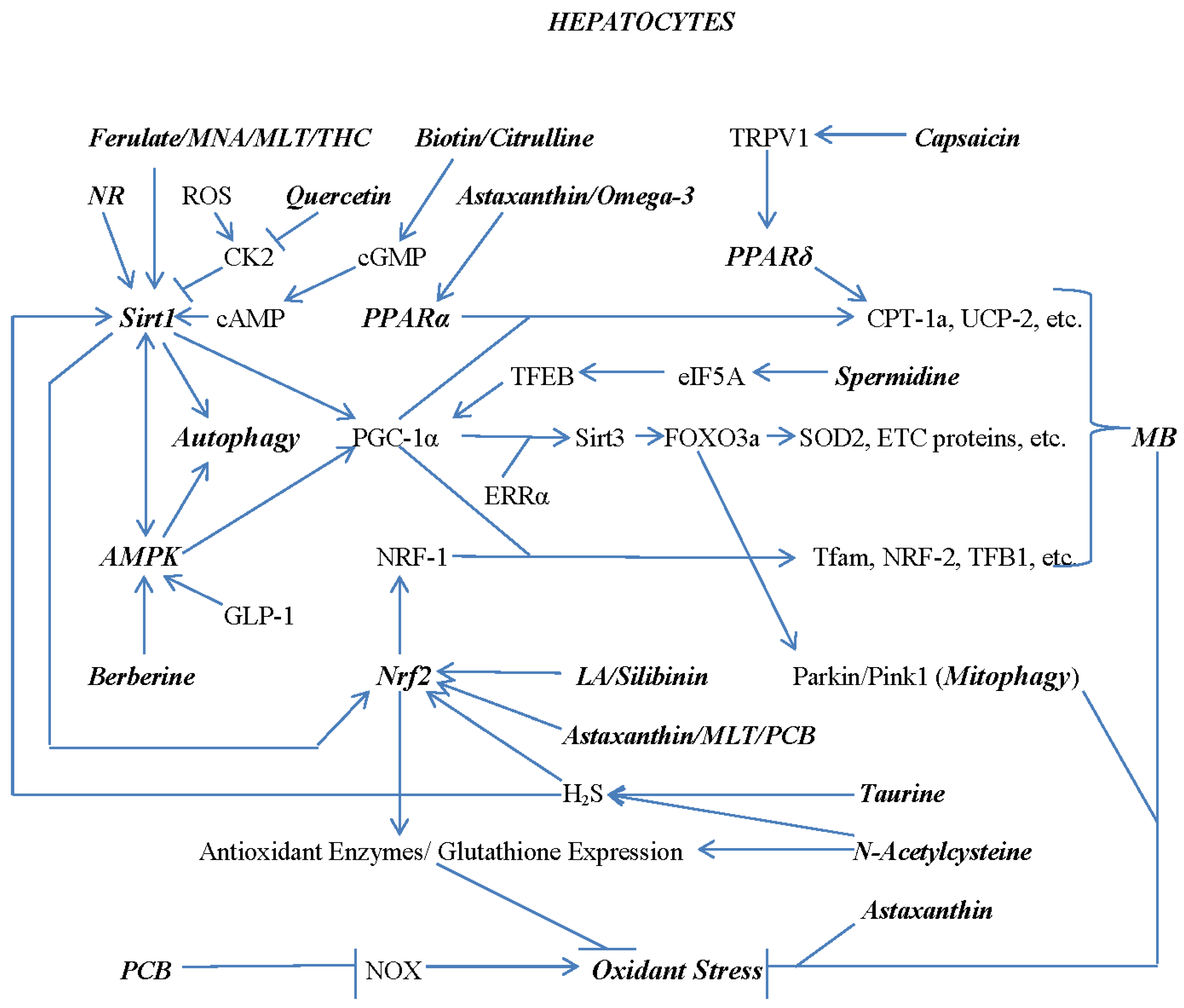
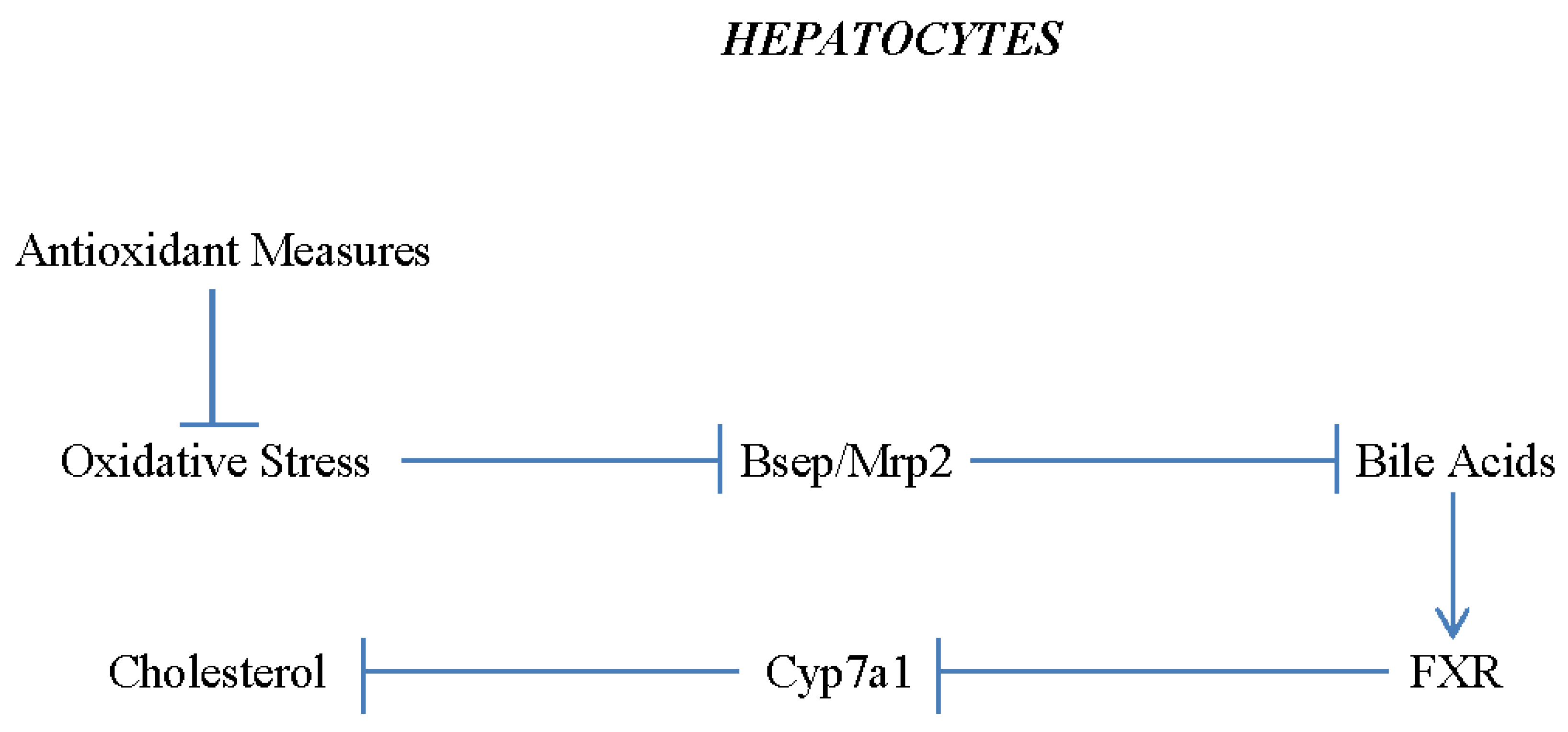
1. Pathogenesis of Non-Alcoholic Fatty Liver Disease and Its Complication – an Overview
2. Nutraceutical Promotion of Mitochondrial Biogenesis, Mitophagy, and Antioxidant Expression
3. Multiple Strategies for Sirt1 Activation
4. Activating AMPK, PPARα, and PPARδ
5. Boosting Nrf2 Activity
6. Spermidine Promotes TFEB Expression
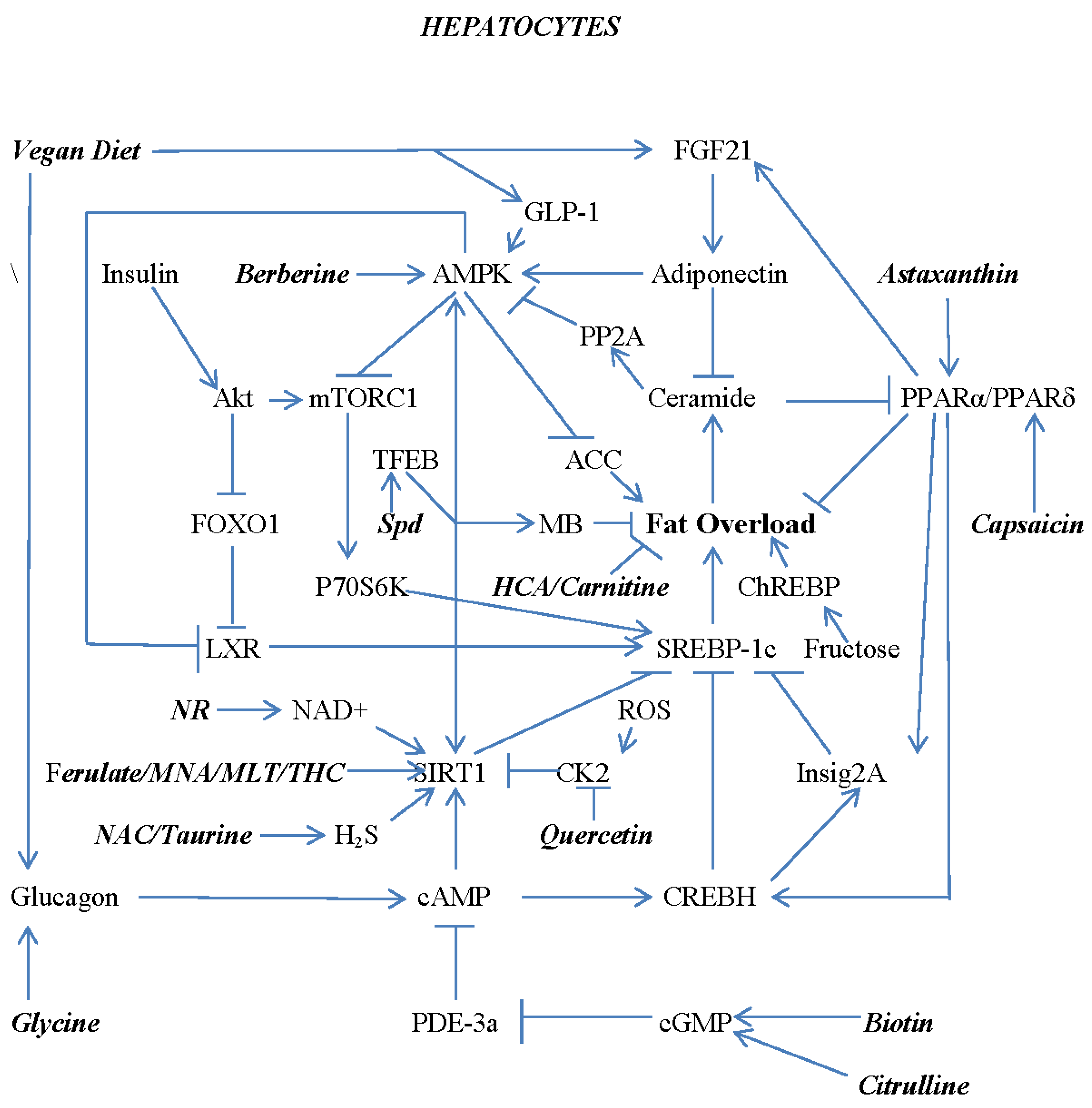
7. Combating Hepatocyte Lipid Overload with Nutraceuticals and Diet
8. Avoiding Progression of NAFLD to NASH and Fibrosis – Roles for Soy Isoflavones and Glycine
Funding
Conflicts of Interest
References
- Pafili, K.; Roden, M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol. Metab. 2021, 50, 101122. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, N.; Kato, M.; Tanaka, M.; Miyazaki, M.; Takao, S.; Kohjima, M.; Kotoh, K.; Enjoji, M.; Nakamuta, M.; Takayanagi, R. Effects of insulin resistance and hepatic lipid accumulation on hepatic mRNA expression levels of apoB, MTP and L-FABP in non-alcoholic fatty liver disease. Exp. Ther. Med. 2011, 2, 1077–1081. [Google Scholar] [CrossRef]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Denechaud, P.-D.; Dentin, R.; Girard, J.; Postic, C. Role of ChREBP in hepatic steatosis and insulin resistance. FEBS Lett. 2007, 582, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-X.; Pan, Q.; Liu, X.-L.; Zhou, D.; Xin, F.-Z.; Zhao, Z.-H.; Zhang, R.-N.; Zeng, J.; Qiao, L.; Hu, C.-X.; et al. Therapeutic effect and autophagy regulation of myriocin in nonalcoholic steatohepatitis. Lipids Heal. Dis. 2019, 18, 179. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Z.; Ru, J.H.; Lopes-Virella, M.F.; Lyons, T.J.; Huang, Y. Saturated fatty acid combined with lipopolysaccharide stimulates a strong inflammatory response in hepatocytes in vivo and in vitro. Am. J. Physiol. Metab. 2018, 315, E745–E757. [Google Scholar] [CrossRef]
- Morrison, M.C.; Mulder, P.; Stavro, P.M.; Suárez, M.; Arola-Arnal, A.; van Duyvenvoorde, W.; Kooistra, T.; Wielinga, P.Y.; Kleemann, R. Replacement of Dietary Saturated Fat by PUFA-Rich Pumpkin Seed Oil Attenuates Non-Alcoholic Fatty Liver Disease and Atherosclerosis Development, with Additional Health Effects of Virgin over Refined Oil. PLOS ONE 2015, 10, e0139196. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Sädevirta, S.; Zhou, Y.; Kayser, B.; Ali, A.; Ahonen, L.; Lallukka, S.; Pelloux, V.; Gaggini, M.; Jian, C.; et al. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care 2018, 41, 1732–1739. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.-S.; Roh, Y.S. Molecular insights into the role of mitochondria in non-alcoholic fatty liver disease. Arch. Pharmacal Res. 2019, 42, 935–946. [Google Scholar] [CrossRef]
- Xu, J.; Shen, J.; Yuan, R.; Jia, B.; Zhang, Y.; Wang, S.; Zhang, Y.; Liu, M.; Wang, T. Mitochondrial Targeting Therapeutics: Promising Role of Natural Products in Non-alcoholic Fatty Liver Disease. Front. Pharmacol. 2021, 12, 796207. [Google Scholar] [CrossRef]
- Gabbia, D.; Cannella, L.; De Martin, S. The Role of Oxidative Stress in NAFLD–NASH–HCC Transition—Focus on NADPH Oxidases. Biomedicines 2021, 9, 687. [Google Scholar] [CrossRef]
- Knorr, J.; Wree, A.; Tacke, F.; Feldstein, A.E. The NLRP3 Inflammasome in Alcoholic and Nonalcoholic Steatohepatitis. Semin. Liver Dis. 2020, 40, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Gaul, S.; Leszczynska, A.; Alegre, F.; Kaufmann, B.; Johnson, C.D.; Adams, L.A.; Wree, A.; Damm, G.; Seehofer, D.; Calvente, C.J.; et al. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J. Hepatol. 2021, 74, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Colak, Y.; Hasan, B.; Erkalma, B.; Tandon, K.; Zervos, X.; Menzo, E.L.; Erim, T. Pathogenetic mechanisms of nonalcoholic fatty liver disease and inhibition of the inflammasome as a new therapeutic target. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101710. [Google Scholar] [CrossRef] [PubMed]
- Calcagno D, Chu A, Gaul S et al. Nlrp3 activation causes spontaneous inflammation and fibrosis that mimics human NASH. Hepatology 2022.
- Uno, M.; Kurita, S.; Misu, H.; Ando, H.; Ota, T.; Matsuzawa-Nagata, N.; Kita, Y.; Nabemoto, S.; Akahori, H.; Zen, Y.; et al. Tranilast, an antifibrogenic agent, ameliorates a dietary rat model of nonalcoholic steatohepatitis. Hepatology 2008, 48, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, P.; Ma, Z.; Li, M.; Teng, X.; Sun, L.; Wan, G.; Li, Y.; Guo, L.; Liu, H. Novel Interplay Between Sonic Hedgehog and Transforming Growth Factor-β1 in Human Nonalcoholic Steatohepatitis. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Huang, M.; Kim, H.-G.; Zhang, Y.; Chowdhury, K.; Cai, W.; Saxena, R.; Schwabe, R.F.; Liangpunsakul, S.; Dong, X.C. SIRT6 Protects Against Liver Fibrosis by Deacetylation and Suppression of SMAD3 in Hepatic Stellate Cells. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 341–364. [Google Scholar] [CrossRef]
- 19. Farrell GC, Haczeyni F, Chitturi S. Pathogenesis of NASH: How Metabolic Complications of Overnutrition Favour Lipotoxicity and Pro-Inflammatory Fatty Liver Disease. Adv Exp Med Biol 2018;1061:19-44. 44. [CrossRef]
- Samuel, V.T.; Shulman, G.I. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab. 2018, 27, 22–41. [Google Scholar] [CrossRef]
- Kakisaka, K.; Suzuki, Y.; Fujiwara, Y.; Suzuki, A.; Kanazawa, J.; Takikawa, Y. Caspase-independent hepatocyte death: A result of the decrease of lysophosphatidylcholine acyltransferase 3 in non-alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2018, 34, 1256–1262. [Google Scholar] [CrossRef]
- Xia, S.-F.; Le, G.-W.; Wang, P.; Qiu, Y.-Y.; Jiang, Y.-Y.; Tang, X. Regressive Effect of Myricetin on Hepatic Steatosis in Mice Fed a High-Fat Diet. Nutrients 2016, 8, 799. [Google Scholar] [CrossRef]
- Choi, H.-N.; Shin, J.-Y.; Kim, J.-I. Ameliorative Effect of Myricetin on Nonalcoholic Fatty Liver Disease in ob/ob Mice. J. Med. Food 2021, 24, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011, 93, 884S–890S. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Settembre, C.; De Cegli, R.; Mansueto, G.; Saha, P.K.; Vetrini, F.; Visvikis, O.; Huynh, T.; Carissimo, A.; Palmer, D.; Klisch, T.J.; et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat. Cell Biol. 2013, 15, 647–658. [Google Scholar] [CrossRef]
- Evans, T.D.; Zhang, X.; Jeong, S.-J.; He, A.; Song, E.; Bhattacharya, S.; Holloway, K.B.; Lodhi, I.J.; Razani, B. TFEB drives PGC-1α expression in adipocytes to protect against diet-induced metabolic dysfunction. Sci. Signal. 2019, 12. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 2011, 1813, 1269–1278. [Google Scholar] [CrossRef]
- Gleyzer, N.; Vercauteren, K.; Scarpulla, R.C. Control of Mitochondrial Transcription Specificity Factors (TFB1M and TFB2M) by Nuclear Respiratory Factors (NRF-1 and NRF-2) and PGC-1 Family Coactivators. Mol. Cell. Biol. 2005, 25, 1354–1366. [Google Scholar] [CrossRef]
- Kong, X.; Wang, R.; Xue, Y.; Liu, X.; Zhang, H.; Chen, Y.; Fang, F.; Chang, Y. Sirtuin 3, a New Target of PGC-1α, Plays an Important Role in the Suppression of ROS and Mitochondrial Biogenesis. PLoS ONE 2010, 5, e11707. [Google Scholar] [CrossRef]
- Gupta P, Sharma G, Lahiri A, Barthwal MK. FOXO3a acetylation regulates PINK1, mitophagy, inflammasome activation in murine palmitate-conditioned and diabetic macrophages. J Leukoc Biol 2021.
- Ding, D.; Ao, X.; Li, M.; Miao, S.; Liu, Y.; Lin, Z.; Wang, M.; He, Y.; Wang, J. FOXO3a-dependent Parkin regulates the development of gastric cancer by targeting ATP-binding cassette transporter E1. J. Cell. Physiol. 2020, 236, 2740–2755. [Google Scholar] [CrossRef] [PubMed]
- Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res 2008 ;103(11):1232-40.
- Jung, K.-A.; Kwak, M.-K. The Nrf2 System as a Potential Target for the Development of Indirect Antioxidants. Molecules 2010, 15, 7266–7291. [Google Scholar] [CrossRef]
- Nagasawa, T.; Inada, Y.; Nakano, S.; Tamura, T.; Takahashi, T.; Maruyama, K.; Yamazaki, Y.; Kuroda, J.; Shibata, N. Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARδ agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. Eur. J. Pharmacol. 2006, 536, 182–191. [Google Scholar] [CrossRef]
- Tsuboyama-Kasaoka, N.; Takahashi, M.; Kim, H.; Ezaki, O. Up-Regulation of Liver Uncoupling Protein-2 mRNA by either Fish Oil Feeding or Fibrate Administration in Mice. Biochem. Biophys. Res. Commun. 1999, 257, 879–885. [Google Scholar] [CrossRef]
- Grav HJ, Tronstad KJ, Gudbrandsen OA et al. Changed energy state and increased mitochondrial beta-oxidation rate in liver of rats associated with lowered proton electrochemical potential and stimulated uncoupling protein 2 (UCP-2) expression: evidence for peroxisome proliferator-activated receptor-alpha independent induction of UCP-2 expression. J Biol Chem 2003 ;278(33):30525-33.
- Du K, Fang X, Li Z. Ferulic acid suppresses interleukin-1Î2-induced degeneration of chondrocytes isolated from patients with osteoarthritis through the SIRT1/AMPK/PGC-1α signaling pathway. Immun Inflamm Dis 2021 September;9(3):710-20.
- Xu, T.; Song, Q.; Zhou, L.; Yang, W.; Wu, X.; Qian, Q.; Chai, H.; Han, Q.; Pan, H.; Dou, X.; et al. Ferulic acid alleviates lipotoxicity-induced hepatocellular death through the SIRT1-regulated autophagy pathway and independently of AMPK and Akt in AML-12 hepatocytes. Nutr. Metab. 2021, 18, 13. [Google Scholar] [CrossRef]
- Hou T, Zhang L, Yang X. Ferulic acid, a natural polyphenol, protects against osteoporosis by activating SIRT1 and NF-κB in neonatal rats with glucocorticoid-induced osteoporosis. Biomed Pharmacother 2019 December;120:109205.
- Chen X, Guo Y, Jia G, Zhao H, Liu G, Huang Z. Ferulic acid regulates muscle fiber type formation through the Sirt1/AMPK signaling pathway. Food Funct 2019 ;10(1):259-65.
- El-Mesallamy, H.O.; Gawish, R.; Sallam, A.-A.M.; Fahmy, H.A.; Nada, A.S. Ferulic acid protects against radiation-induced testicular damage in male rats: impact on SIRT1 and PARP1. Environ. Sci. Pollut. Res. 2017, 25, 6218–6227. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Sun, Y.; Cheng, L.; Jin, Z.; Yang, Y.; Zhai, M.; Pei, H.; Wang, X.; Zhang, H.; Meng, Q.; et al. Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1. J. Pineal Res. 2014, 57, 228–238. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, Y.; Zhang, F.; Xia, Y.; Liu, J.; Huang, R.; Wang, Y.; Hu, Y.; Wu, J.; Dai, C.; et al. CLOCK/BMAL1 regulates circadian change of mouse hepatic insulin sensitivity by SIRT1. Hepatology 2014, 59, 2196–2206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Liu, C.; Li, L.; Li, P. N1-Methylnicotinamide Improves Hepatic Insulin Sensitivity via Activation of SIRT1 and Inhibition of FOXO1 Acetylation. J. Diabetes Res. 2020, 2020, 1080152. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Moreno-Navarrete, J.M.; Wei, X.; Kikukawa, Y.; Tzameli, I.; Prasad, D.; Lee, Y.; Asara, J.M.; Fernández-Real, J.M.; Maratos-Flier, E.; et al. Nicotinamide N-methyltransferase regulates hepatic nutrient metabolism through Sirt1 protein stabilization. Nat. Med. 2015, 21, 887–894. [Google Scholar] [CrossRef]
- Chen Y, Zhang J, Li P, Liu C, Li L. N1‑methylnicotinamide ameliorates insulin resistance in skeletal muscle of type 2 diabetic mice by activating the SIRT1/PGC‑1α signaling pathway. Mol Med Rep 2021 April;23(4).
- Gebicki, J.; Sysa-Jedrzejowska, A.; Adamus, J.; Woźniacka, A.; Rybak, M.; Zielonka, J. 1-Methylnicotinamide: a potent anti-inflammatory agent of vitamin origin. Pol. J. Pharmacol. 2003, 55, 109–112. [Google Scholar]
- McCarty MF, Assanga SBI. Ferulic acid may target MyD88-mediated pro-inflammatory signaling - Implications for the health protection afforded by whole grains, anthocyanins, and coffee. Med Hypotheses 2018 September;118:114-20.
- Early, J.O.; Menon, D.; Wyse, C.A.; Cervantes-Silva, M.P.; Zaslona, Z.; Carroll, R.G.; Palsson-McDermott, E.M.; Angiari, S.; Ryan, D.G.; Corcoran, S.E.; et al. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc. Natl. Acad. Sci. USA 2018, 115, E8460–E8468. [Google Scholar] [CrossRef]
- Chen P, Chen F, Lei J, Li Q, Zhou B. Activation of the miR-34a-Mediated SIRT1/mTOR Signaling Pathway by Urolithin A Attenuates D-Galactose-Induced Brain Aging in Mice. Neurotherapeutics 2019 October;16(4):1269-82.
- Ghosh, N.; Das, A.; Biswas, N.; Gnyawali, S.; Singh, K.; Gorain, M.; Polcyn, C.; Khanna, S.; Roy, S.; Sen, C.K. Urolithin A augments angiogenic pathways in skeletal muscle by bolstering NAD+ and SIRT1. Sci. Rep. 2020, 10, 20184. [Google Scholar] [CrossRef] [PubMed]
- Liu J, Jiang J, Qiu J et al. Urolithin A protects dopaminergic neurons in experimental models of Parkinson’s disease by promoting mitochondrial biogenesis through the SIRT1/PGC-1α signaling pathway. Food Funct 2022 ;13(1):375-85.
- Shi PZ, Wang JW, Wang PC et al. Urolithin a alleviates oxidative stress-induced senescence in nucleus pulposus-derived mesenchymal stem cells through SIRT1/PGC-1α pathway. World J Stem Cells 2021 ;13(12):1928-46.
- Shan W, Gao L, Zeng W et al. Activation of the SIRT1/p66shc antiapoptosis pathway via carnosic acid-induced inhibition of miR-34a protects rats against nonalcoholic fatty liver disease. Cell Death Dis 2015 ;6(7):e1833.
- Wang T, Takikawa Y. Carnosic acid protects normal mouse hepatocytes against H2 O2 -induced cytotoxicity via sirtuin 1-mediated signaling. Hepatol Res 2016 February;46(2):239-46.
- Chen, S.-D.; Ji, B.-B.; Yan, Y.-X.; He, X.; Han, K.-Y.; Dai, Q.-X.; Zhang, M.-X.; Mo, Y.-C.; Wang, J.-L. Carnosic acid attenuates neuropathic pain in rat through the activation of spinal sirtuin1 and down-regulation of p66shc expression. Neurochem. Int. 2016, 93, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Tian X, Hu Y, Li M et al. Carnosic acid attenuates acute ethanol-induced liver injury via a SIRT1/p66Shc-mediated mitochondrial pathway. Can J Physiol Pharmacol 2016 April;94(4):416-25.
- Yu, M.-H.; Hung, T.-W.; Wang, C.-C.; Wu, S.-W.; Yang, T.-W.; Yang, C.-Y.; Tseng, T.-H.; Wang, C.-J. Neochlorogenic Acid Attenuates Hepatic Lipid Accumulation and Inflammation via Regulating miR-34a In Vitro. Int. J. Mol. Sci. 2021, 22, 13163. [Google Scholar] [CrossRef] [PubMed]
- Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A 2008 ;105(36):13421-6.
- Zhao, T.; Li, J.; Chen, A.F.; Lan, Y.; Li, Y.-J.; Li, D.-J.; Li, P.; Wang, J.-Y.; Diao, Y.-P.; Ye, G.-D.; et al. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. Am. J. Physiol. Metab. 2010, 299, E110–E116. [Google Scholar] [CrossRef]
- Pi, C.; Ma, C.; Wang, H.; Sun, H.; Yu, X.; Gao, X.; Yang, Y.; Sun, Y.; Zhang, H.; Shi, Y.; et al. MiR-34a suppression targets Nampt to ameliorate bone marrow mesenchymal stem cell senescence by regulating NAD+-Sirt1 pathway. Stem Cell Res. Ther. 2021, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-E.; Fu, T.; Seok, S.; Kim, D.-H.; Yu, E.; Lee, K.-W.; Kang, Y.; Li, X.; Kemper, B.; Kemper, J.K. Elevated microRNA-34a in obesity reduces NAD+levels and SIRT1 activity by directly targeting NAMPT. Aging Cell 2013, 12, 1062–1072. [Google Scholar] [CrossRef]
- Li, K.; Zhai, M.; Jiang, L.; Song, F.; Zhang, B.; Li, J.; Li, H.; Li, B.; Xia, L.; Xu, L.; et al. Tetrahydrocurcumin Ameliorates Diabetic Cardiomyopathy by Attenuating High Glucose-Induced Oxidative Stress and Fibrosis via Activating the SIRT1 Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 6746907. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Li, S.; Wang, Q.; Wang, H.; Xu, M.; An, Y. Tetrahydrocurcumin protects against sepsis-induced acute kidney injury via the SIRT1 pathway. Ren. Fail. 2021, 43, 1028–1040. [Google Scholar] [CrossRef]
- Zabihi, N.A.; Pirro, M.; Johnston, T.P.; Sahebkar, A. Is There a Role for Curcumin Supplementation in the Treatment of Non-Alcoholic Fatty Liver Disease? The Data Suggest Yes. Curr. Pharm. Des. 2017, 23, 969–982. [Google Scholar] [CrossRef]
- Jalali, M.; Mahmoodi, M.; Mosallanezhad, Z.; Jalali, R.; Imanieh, M.H.; Moosavian, S.P. The effects of curcumin supplementation on liver function, metabolic profile and body composition in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 48, 102283. [Google Scholar] [CrossRef]
- Pan, M.-H.; Chen, J.-W.; Kong, Z.-L.; Wu, J.-C.; Ho, C.-T.; Lai, C.-S. Attenuation by Tetrahydrocurcumin of Adiposity and Hepatic Steatosis in Mice with High-Fat-Diet-Induced Obesity. J. Agric. Food Chem. 2018, 66, 12685–12695. [Google Scholar] [CrossRef]
- Chen, J.-W.; Kong, Z.-L.; Tsai, M.-L.; Lo, C.-Y.; Ho, C.-T.; Lai, C.-S. Tetrahydrocurcumin ameliorates free fatty acid-induced hepatic steatosis and improves insulin resistance in HepG2 cells. J. Food Drug Anal. 2018, 26, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Wangpoengtrakul, C.; Tanaka, T.; Toyokuni, S.; Uchida, K.; Osawa, T. Curcumin and Especially Tetrahydrocurcumin Ameliorate Oxidative Stress-Induced Renal Injury in Mice. J. Nutr. 2001, 131, 2090–2095. [Google Scholar] [CrossRef]
- Gariani, K.; Menzies, K.J.; Ryu, D.; Wegner, C.J.; Wang, X.; Ropelle, E.R.; Moullan, N.; Zhang, H.; Perino, A.; Lemos, V.; et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology 2016, 63, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Gariani, K.; Ryu, D.; Menzies, K.J.; Yi, H.-S.; Stein, S.; Zhang, H.; Perino, A.; Lemos, V.; Katsyuba, E.; Jha, P.; et al. Inhibiting poly ADP-ribosylation increases fatty acid oxidation and protects against fatty liver disease. J. Hepatol. 2017, 66, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Horváth, B.; Rajesh, M.; Varga, Z.V.; Gariani, K.; Ryu, D.; Cao, Z.; Holovac, E.; Park, O.; Zhou, Z.; et al. PARP inhibition protects against alcoholic and non-alcoholic steatohepatitis. J. Hepatol. 2017, 66, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol 2014 August;24(8):464-71.
- Zhou, C.-C.; Yang, X.; Hua, X.; Liu, J.; Fan, M.-B.; Li, G.-Q.; Song, J.; Xu, T.-Y.; Li, Z.-Y.; Guan, Y.-F.; et al. Hepatic NAD+deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br. J. Pharmacol. 2016, 173, 2352–2368. [Google Scholar] [CrossRef]
- Han, X.; Bao, X.; Lou, Q.; Xie, X.; Zhang, M.; Zhou, S.; Guo, H.; Jiang, G.; Shi, Q. Nicotinamide riboside exerts protective effect against aging-induced NAFLD-like hepatic dysfunction in mice. PeerJ 2019, 7, e7568. [Google Scholar] [CrossRef]
- Choi, S.E.; Kwon, S.; Seok, S.; Xiao, Z.; Lee, K.-W.; Kang, Y.; Li, X.; Shinoda, K.; Kajimura, S.; Kemper, B.; et al. Obesity-Linked Phosphorylation of SIRT1 by Casein Kinase 2 Inhibits Its Nuclear Localization and Promotes Fatty Liver. Mol. Cell. Biol. 2017, 37, e00006-17. [Google Scholar] [CrossRef]
- Kim KJ, Cho KD, Jang KY et al. Platelet-activating factor enhances tumour metastasis via the reactive oxygen species-dependent protein kinase casein kinase 2-mediated nuclear factor-κB activation. Immunology 2014 September;143(1):21-32.
- Lolli, G.; Cozza, G.; Mazzorana, M.; Tibaldi, E.; Cesaro, L.; Donella-Deana, A.; Meggio, F.; Venerando, A.; Franchin, C.; Sarno, S.; et al. Inhibition of Protein Kinase CK2 by Flavonoids and Tyrphostins. A Structural Insight. Biochemistry 2012, 51, 6097–6107. [Google Scholar] [CrossRef]
- McCarty, M.F.; Assanga, S.I.; Lujan, L.L. Flavones and flavonols may have clinical potential as CK2 inhibitors in cancer therapy. Med Hypotheses 2020, 141, 109723. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, J.; Mei, G.; Chen, H.; Peng, S.; Zhao, Y.; Yao, P.; Tang, Y. Quercetin and non-alcoholic fatty liver disease: A review based on experimental data and bioinformatic analysis. Food Chem. Toxicol. 2021, 154, 112314. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.-F.; Le, G.-W.; Wang, P.; Qiu, Y.-Y.; Jiang, Y.-Y.; Tang, X. Regressive Effect of Myricetin on Hepatic Steatosis in Mice Fed a High-Fat Diet. Nutrients 2016, 8, 799. [Google Scholar] [CrossRef]
- Choi, H.-N.; Shin, J.-Y.; Kim, J.-I. Ameliorative Effect of Myricetin on Nonalcoholic Fatty Liver Disease in ob/ob Mice. J. Med. Food 2021, 24, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Liou CJ, Wei CH, Chen YL, Cheng CY, Wang CL, Huang WC. Fisetin Protects Against Hepatic Steatosis Through Regulation of the Sirt1/AMPK and Fatty Acid Î2-Oxidation Signaling Pathway in High-Fat Diet-Induced Obese Mice. Cell Physiol Biochem 2018;49(5):1870-84.
- Gaballah, H.H.; El-Horany, H.E.; Helal, D.S. Mitigative effects of the bioactive flavonol fisetin on high-fat/high-sucrose induced nonalcoholic fatty liver disease in rats. J. Cell. Biochem. 2019, 120, 12762–12774. [Google Scholar] [CrossRef]
- Choi, M.-S.; Choi, J.-Y.; Kwon, E.-Y. Fisetin Alleviates Hepatic and Adipocyte Fibrosis and Insulin Resistance in Diet-Induced Obese Mice. J. Med. Food 2020, 23, 1019–1032. [Google Scholar] [CrossRef]
- Yin, Y.; Gao, L.; Lin, H.; Wu, Y.; Han, X.; Zhu, Y.; Li, J. Luteolin improves non-alcoholic fatty liver disease in db/db mice by inhibition of liver X receptor activation to down-regulate expression of sterol regulatory element binding protein 1c. Biochem. Biophys. Res. Commun. 2017, 482, 720–726. [Google Scholar] [CrossRef]
- Liu, X.; Sun, R.; Li, Z.; Xiao, R.; Lv, P.; Sun, X.; Olson, M.A.; Gong, Y. Luteolin alleviates non-alcoholic fatty liver disease in rats via restoration of intestinal mucosal barrier damage and microbiota imbalance involving in gut-liver axis. Arch. Biochem. Biophys. 2021, 711, 109019. [Google Scholar] [CrossRef]
- Lv, Y.; Gao, X.; Luo, Y.; Fan, W.; Shen, T.; Ding, C.; Yao, M.; Song, S.; Yan, L. Apigenin ameliorates HFD-induced NAFLD through regulation of the XO/NLRP3 pathways. J. Nutr. Biochem. 2019, 71, 110–121. [Google Scholar] [CrossRef]
- Gerhart-Hines Z, Dominy JE, Jr., Blättler SM et al. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD(+). Mol Cell 2011 ;44(6):851-63.
- Banerjee, J.; Bruckbauer, A.; Thorpe, T.; Zemel, M.B. Biphasic Effect of Sildenafil on Energy Sensing is Mediated by Phosphodiesterases 2 and 3 in Adipocytes and Hepatocytes. Int. J. Mol. Sci. 2019, 20, 2992. [Google Scholar] [CrossRef]
- Vesely, D.L. Biotin Enhances Guanylate Cyclase Activity. Science 1982, 216, 1329–1330. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Méndez A, Fernández-Mejà a C. The hypotriglyceridemic effect of biotin supplementation involves increased levels of cGMP and AMPK activation. Biofactors 2012 September;38(5):387-94.
- McCarty, MF. Asymmetric Dimethylarginine Is a Well Established Mediating Risk Factor for Cardiovascular Morbidity and Mortality-Should Patients with Elevated Levels Be Supplemented with Citrulline? Healthcare (Basel) 2016 ;4(3).
- Jegatheesan, P.; Beutheu, S.; Freese, K.; Waligora-Dupriet, A.-J.; Nubret, E.; Butel, M.-J.; Bergheim, I.; De Bandt, J.-P. Preventive effects of citrulline on Western diet-induced non-alcoholic fatty liver disease in rats. Br. J. Nutr. 2016, 116, 191–203. [Google Scholar] [CrossRef]
- Darabi, Z.; Darand, M.; Yari, Z.; Hedayati, M.; Faghihi, A.; Agah, S.; Hekmatdoost, A. Inflammatory markers response to citrulline supplementation in patients with non-alcoholic fatty liver disease: a randomized, double blind, placebo-controlled, clinical trial. BMC Res. Notes 2019, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Suo R, Zhao ZZ, Tang ZH et al. Hydrogen sulfide prevents Hâ‚‚Oâ‚‚-induced senescence in human umbilical vein endothelial cells through SIRT1 activation. Mol Med Rep 2013 June;7(6):1865-70.
- Du, C.; Lin, X.; Xu, W.; Zheng, F.; Cai, J.; Yang, J.; Cui, Q.; Tang, C.; Cai, J.; Xu, G.; et al. Sulfhydrated Sirtuin-1 Increasing Its Deacetylation Activity Is an Essential Epigenetics Mechanism of Anti-Atherogenesis by Hydrogen Sulfide. Antioxidants Redox Signal. 2019, 30, 184–197. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Okeefe, J.H.; McCarty, M.F. Boosting endogenous production of vasoprotective hydrogen sulfide via supplementation with taurine and N-acetylcysteine: a novel way to promote cardiovascular health. Open Hear. 2017, 4, e000600. [Google Scholar] [CrossRef]
- Sun Q, Wang B, Li Y et al. Taurine Supplementation Lowers Blood Pressure and Improves Vascular Function in Prehypertension: Randomized, Double-Blind, Placebo-Controlled Study. Hypertension 2016 March;67(3):541-9.
- Zhao, H.; Qu, J.; Li, Q.; Cui, M.; Wang, J.; Zhang, K.; Liu, X.; Feng, H.; Chen, Y. Taurine supplementation reduces neuroinflammation and protects against white matter injury after intracerebral hemorrhage in rats. Amino Acids 2018, 50, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Guizoni, D.M.; Freitas, I.N.; Victorio, J.A.; Possebom, I.R.; Araujo, T.R.; Carneiro, E.M.; Davel, A.P. Taurine treatment reverses protein malnutrition-induced endothelial dysfunction of the pancreatic vasculature: The role of hydrogen sulfide. Metabolism 2021, 116, 154701. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.; Yin, M.; Zhang, Y.; Huang, L.; Chen, R.; Ni, J. Effects of berberine on blood glucose in patients with type 2 diabetes mellitus: a systematic literature review and a meta-analysis. Endocr. J. 2019, 66, 51–63. [Google Scholar] [CrossRef]
- Ju, J.; Li, J.; Lin, Q.; Xu, H. Efficacy and safety of berberine for dyslipidaemias: A systematic review and meta-analysis of randomized clinical trials. Phytomedicine 2018, 50, 25–34. [Google Scholar] [CrossRef]
- Guo, J.; Chen, H.; Zhang, X.; Lou, W.; Zhang, P.; Qiu, Y.; Zhang, C.; Wang, Y.; Liu, W.J. The Effect of Berberine on Metabolic Profiles in Type 2 Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Oxidative Med. Cell. Longev. 2021, 2021, 2074610. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.-M.; Lee, C.H.; Oh, W.K.; Kim, C.T.; et al. Berberine, a Natural Plant Product, Activates AMP-Activated Protein Kinase With Beneficial Metabolic Effects in Diabetic and Insulin-Resistant States. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef] [PubMed]
- Turner N, Li JY, Gosby A et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes 2008 May;57(5):1414-8.
- Hawley SA, Ross FA, Chevtzoff C et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab 2010 ;11(6):554-65.
- Fulco, M.; Cen, Y.; Zhao, P.; Hoffman, E.P.; McBurney, M.W.; Sauve, A.A.; Sartorelli, V. Glucose Restriction Inhibits Skeletal Myoblast Differentiation by Activating SIRT1 through AMPK-Mediated Regulation of Nampt. Dev. Cell 2008, 14, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Costford SR, Bajpeyi S, Pasarica M et al. Skeletal muscle NAMPT is induced by exercise in humans. Am J Physiol Endocrinol Metab 2010 January;298(1):E117-E126.
- Brandauer, J.; Vienberg, S.G.; Andersen, M.A.; Ringholm, S.; Risis, S.; Larsen, P.S.; Kristensen, J.M.; Frøsig, C.; Leick, L.; Fentz, J.; et al. AMP-activated protein kinase regulates nicotinamide phosphoribosyl transferase expression in skeletal muscle. J. Physiol. 2013, 591, 5207–5220. [Google Scholar] [CrossRef]
- Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem 2008 ;283(41):27628-35.
- Hou, X.; Xu, S.; Maitland-Toolan, K.A.; Sato, K.; Jiang, B.; Ido, Y.; Lan, F.; Walsh, K.; Wierzbicki, M.; Verbeuren, T.J.; et al. SIRT1 Regulates Hepatocyte Lipid Metabolism through Activating AMP-activated Protein Kinase. J. Biol. Chem. 2008, 283, 20015–20026. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Alimujiang, M.; Hu, L.; Liu, F.; Bao, Y.; Yin, J. Berberine alleviates lipid metabolism disorders via inhibition of mitochondrial complex I in gut and liver. Int. J. Biol. Sci. 2021, 17, 1693–1707. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, X.; Zhang, F.; Han, Y.; Chang, X.; Xu, X.; Li, Y.; Gao, X. Berberine ameliorates fatty acid-induced oxidative stress in human hepatoma cells. Sci. Rep. 2017, 7, 11340. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, C.; Hao, S.; Song, H.; Yang, L. The Therapeutic Effect of Berberine in the Treatment of Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Evidence-Based Complement. Altern. Med. 2016, 2016, 3593951. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Woo, S.-L.; Guo, X.; Li, H.; Zheng, J.; Botchlett, R.; Liu, M.; Pei, Y.; Xu, H.; Cai, Y.; et al. Berberine Ameliorates Hepatic Steatosis and Suppresses Liver and Adipose Tissue Inflammation in Mice with Diet-induced Obesity. Sci. Rep. 2016, 6, 22612. [Google Scholar] [CrossRef]
- Yan, H.-M.; Xia, M.-F.; Wang, Y.; Chang, X.-X.; Yao, X.-Z.; Rao, S.-X.; Zeng, M.-S.; Tu, Y.-F.; Feng, R.; Jia, W.-P.; et al. Efficacy of Berberine in Patients with Non-Alcoholic Fatty Liver Disease. PLOS ONE 2015, 10, e0134172. [Google Scholar] [CrossRef]
- Dang, Y.; An, Y.; He, J.; Huang, B.; Zhu, J.; Gao, M.; Zhang, S.; Wang, X.; Yang, B.; Xie, Z. Berberine ameliorates cellular senescence and extends the lifespan of mice via regulating p16 and cyclin protein expression. Aging Cell 2020, 19, e13060. [Google Scholar] [CrossRef]
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef]
- Choi, C.-I. Astaxanthin as a Peroxisome Proliferator-Activated Receptor (PPAR) Modulator: Its Therapeutic Implications. Mar. Drugs 2019, 17, 242. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wu, C.; Kim, J.; Kim, B.; Lee, S.-J. Astaxanthin reduces hepatic lipid accumulations in high-fat-fed C57BL/6J mice via activation of peroxisome proliferator-activated receptor (PPAR) alpha and inhibition of PPAR gamma and Akt. J. Nutr. Biochem. 2016, 28, 9–18. [Google Scholar] [CrossRef]
- Jia, Y.; Kim, J.-Y.; Jun, H.-J.; Kim, S.-J.; Lee, J.-H.; Hoang, M.H.; Hwang, K.-Y.; Um, S.-J.; Chang, H.I.; Lee, S.-J. The natural carotenoid astaxanthin, a PPAR-α agonist and PPAR-γ antagonist, reduces hepatic lipid accumulation by rewiring the transcriptome in lipid-loaded hepatocytes. Mol. Nutr. Food Res. 2012, 56, 878–888. [Google Scholar] [CrossRef]
- Choi, H.D.; Youn, Y.K.; Shin, W.G. Positive Effects of Astaxanthin on Lipid Profiles and Oxidative Stress in Overweight Subjects. Plant Foods Hum. Nutr. 2011, 66, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pham, T.X.; Wegner, C.J.; Kim, B.; Ku, C.S.; Park, Y.-K.; Lee, J.-Y. Astaxanthin lowers plasma TAG concentrations and increases hepatic antioxidant gene expression in diet-induced obesity mice. Br. J. Nutr. 2014, 112, 1797–1804. [Google Scholar] [CrossRef]
- Radice, R.P.; Limongi, A.R.; Viviano, E.; Padula, M.C.; Martelli, G.; Bermano, G. Effects of astaxanthin in animal models of obesity-associated diseases: A systematic review and meta-analysis. Free. Radic. Biol. Med. 2021, 171, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A 1997 ;94(9):4312-7.
- Harris, W.S. n-3 fatty acids and serum lipoproteins: Human studies. Am. J. Clin. Nutr. 1997, 65, 1645S–1654S. [Google Scholar] [CrossRef] [PubMed]
- Tsuboyama-Kasaoka, N.; Takahashi, M.; Kim, H.; Ezaki, O. Up-Regulation of Liver Uncoupling Protein-2 mRNA by either Fish Oil Feeding or Fibrate Administration in Mice. Biochem. Biophys. Res. Commun. 1999, 257, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.; Geohas, J.; Dicklin, M.; Huebner, M.; Udani, J. Safety and lipid-altering efficacy of a new omega-3 fatty acid and antioxidant-containing medical food in men and women with elevated triacylglycerols. Prostaglandins, Leukot. Essent. Fat. Acids 2015, 99, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, L.; Wang, F.; Chen, J.; Zhao, Y.; Wang, P.; Nilius, B.; Liu, D.; Zhu, Z. Dietary capsaicin prevents nonalcoholic fatty liver disease through transient receptor potential vanilloid 1-mediated peroxisome proliferator-activated receptor δ activation. 465, 1303. [Google Scholar] [CrossRef]
- Gao, F.; Liang, Y.; Wang, X.; Lu, Z.; Li, L.; Zhu, S.; Liu, D.; Yan, Z.; Zhu, Z. TRPV1 Activation Attenuates High-Salt Diet-Induced Cardiac Hypertrophy and Fibrosis through PPAR-δUpregulation. PPAR Res. 2014, 2014, 491963. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xie, X.; Yuan, L.; Qiu, J.; Duan, W.; Xu, B.; Chen, X. Resveratrol ameliorates rheumatoid arthritis via activation of SIRT1-Nrf2 signaling pathway. BioFactors 2019, 46, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-W.; Zhao, G.-J.; Li, X.-L.; Hong, G.-L.; Li, M.-F.; Qiu, Q.-M.; Wu, B.; Lu, Z.-Q. SIRT1 exerts protective effects against paraquat-induced injury in mouse type II alveolar epithelial cells by deacetylating NRF2 in vitro. Int. J. Mol. Med. 2016, 37, 1049–1058. [Google Scholar] [CrossRef]
- Xu, J.-J.; Cui, J.; Lin, Q.; Chen, X.-Y.; Zhang, J.; Gao, E.-H.; Wei, B.; Zhao, W. Protection of the enhanced Nrf2 deacetylation and its downstream transcriptional activity by SIRT1 in myocardial ischemia/reperfusion injury. Int. J. Cardiol. 2021, 342, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Ma F, Wu J, Jiang Z et al. P53/NRF2 mediates SIRT1’s protective effect on diabetic nephropathy. Biochim Biophys Acta Mol Cell Res 2019 August;1866(8):1272-81.
- Kawai Y, Garduño L, Theodore M, Yang J, Arinze IJ. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J Biol Chem 2011 ;286(9):7629-40.
- Yang H, Chen J, Chen Y, Jiang Y, Ge B, Hong L. Sirtuin inhibits M. tuberculosis -induced apoptosis in macrophage through glycogen synthase kinase-3Î2. Arch Biochem Biophys 2020 ;694:108612.
- Rojo AI, Sagarra MR, Cuadrado A. GSK-3beta down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. J Neurochem 2008 April;105(1):192-202.
- Jain AK, Jaiswal AK. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J Biol Chem 2007 ;282(22):16502-10.
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef]
- Bloom D, Dhakshinamoorthy S, Jaiswal AK. Site-directed mutagenesis of cysteine to serine in the DNA binding region of Nrf2 decreases its capacity to upregulate antioxidant response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. Oncogene 2002 ;21(14):2191-200.
- Kobayashi, A.; Kang, M.-I.; Watai, Y.; Tong, K.I.; Shibata, T.; Uchida, K.; Yamamoto, M. Oxidative and Electrophilic Stresses Activate Nrf2 through Inhibition of Ubiquitination Activity of Keap1. Mol. Cell. Biol. 2006, 26, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Valdecantos, M.P.; Prieto-Hontoria, P.L.; Pardo, V.; Módol, T.; Santamaría, B.; Weber, M.; Herrero, L.; Serra, D.; Muntané, J.; Cuadrado, A.; et al. Essential role of Nrf2 in the protective effect of lipoic acid against lipoapoptosis in hepatocytes. Free. Radic. Biol. Med. 2015, 84, 263–278. [Google Scholar] [CrossRef]
- Li, S.; Takahara, T.; Fujino, M.; Fukuhara, Y.; Sugiyama, T.; Li, X.-K.; Takahara, S. Astaxanthin prevents ischemia-reperfusion injury of the steatotic liver in mice. PLoS ONE 2017, 12, e0187810. [Google Scholar] [CrossRef] [PubMed]
- Shatoor, A.S.; Al Humayed, S.; Almohiy, H.M. Astaxanthin attenuates hepatic steatosis in high-fat diet-fed rats by suppressing microRNA-21 via transactivation of nuclear factor erythroid 2-related factor 2. J. Physiol. Biochem. 2021, 78, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Ma H, Chen S, Xiong H et al. Astaxanthin from Haematococcus pluvialis ameliorates the chemotherapeutic drug (doxorubicin) induced liver injury through the Keap1/Nrf2/HO-1 pathway in mice. Food Funct 2020 ;11(5):4659-71.
- Tripathi, D.; Jena, G. Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: Role of Nrf2, p53, p38 and phase-II enzymes. Mutat. Res. Toxicol. Environ. Mutagen. 2010, 696, 69–80. [Google Scholar] [CrossRef]
- Fang, J.; Yan, Y.; Teng, X.; Wen, X.; Li, N.; Peng, S.; Liu, W.; Donadeu, F.X.; Zhao, S.; Hua, J. Melatonin prevents senescence of canine adipose-derived mesenchymal stem cells through activating NRF2 and inhibiting ER stress. Aging 2018, 10, 2954–2972. [Google Scholar] [CrossRef]
- Joshi, A.; Upadhyay, K.K.; Vohra, A.; Shirsath, K.; Devkar, R. Melatonin induces Nrf2-HO-1 reprogramming and corrections in hepatic core clock oscillations in Non-alcoholic fatty liver disease. FASEB J. 2021, 35, e21803. [Google Scholar] [CrossRef] [PubMed]
- Early, J.O.; Menon, D.; Wyse, C.A.; Cervantes-Silva, M.P.; Zaslona, Z.; Carroll, R.G.; Palsson-McDermott, E.M.; Angiari, S.; Ryan, D.G.; Corcoran, S.E.; et al. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc. Natl. Acad. Sci. USA 2018, 115, E8460–E8468. [Google Scholar] [CrossRef] [PubMed]
- McCarty, MF. Clinical potential of Spirulina as a source of phycocyanobilin. J Med Food 2007 December;10(4):566-70.
- Lanone, S.; Bloc, S.; Foresti, R.; Almolki, A.; Taillé, C.; Callebert, J.; Conti, M.; Goven, D.; Aubier, M.; Dureuil, B.; et al. Bilirubin decreases NOS2 expression via inhibition of NAD(P)H oxidase: implications for protection against endotoxic shock in rats. FASEB J. 2005, 19, 1890–1892. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ishikawa, K.; Itabe, H.; Maruyama, Y. Carbon monoxide and bilirubin from heme oxygenase-1 suppresses reactive oxygen species generation and plasminogen activator inhibitor-1 induction. Mol. Cell. Biochem. 2006, 291, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Roberts, S.J.; Datla, S.R.; Dusting, G.J. NO Modulates NADPH Oxidase Function Via Heme Oxygenase-1 in Human Endothelial Cells. Hypertension 2006, 48, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Zheng J, Inoguchi T, Sasaki S et al. Phycocyanin and phycocyanobilin from Spirulina platensis protect against diabetic nephropathy by inhibiting oxidative stress. Am J Physiol Regul Integr Comp Physiol 2013 ;304(2):R110-R120.
- Das, S.; Alhasson, F.; Dattaroy, D.; Pourhoseini, S.; Seth, R.K.; Nagarkatti, M.; Nagarkatti, P.S.; Michelotti, G.A.; Diehl, A.M.; Kalyanaraman, B.; et al. NADPH Oxidase–Derived Peroxynitrite Drives Inflammation in Mice and Human Nonalcoholic Steatohepatitis via TLR4-Lipid Raft Recruitment. Am. J. Pathol. 2015, 185, 1944–1957. [Google Scholar] [CrossRef]
- Liang, S.; Kisseleva, T.; Brenner, D.A. The Role of NADPH Oxidases (NOXs) in Liver Fibrosis and the Activation of Myofibroblasts. Front. Physiol. 2016, 7, 17. [Google Scholar] [CrossRef]
- Matsumoto, M.; Zhang, J.; Zhang, X.; Liu, J.; Jiang, J.X.; Yamaguchi, K.; Taruno, A.; Katsuyama, M.; Iwata, K.; Ibi, M.; et al. The NOX1 isoform of NADPH oxidase is involved in dysfunction of liver sinusoids in nonalcoholic fatty liver disease. Free. Radic. Biol. Med. 2018, 115, 412–420. [Google Scholar] [CrossRef]
- Jiang JX, Fish SR, Tomilov A et al. Nonphagocytic Activation of NOX2 Is Implicated in Progressive Nonalcoholic Steatohepatitis During Aging. Hepatology 2020 October;72(4):1204-18.
- PentÃ3n-Rol G, Marà n-Prida J, McCarty MF. C-Phycocyanin-derived Phycocyanobilin as a Potential Nutraceutical Approach for Major Neurodegenerative Disorders and COVID-19- induced Damage to the Nervous System. Curr Neuropharmacol 2021;19(12):2250-75.
- Basdeo SA, Campbell NK, Sullivan LM et al. Suppression of human alloreactive TÂ cells by linear tetrapyrroles; relevance for transplantation. Transl Res 2016 December;178:81-94.
- PentÃ3n-Rol G, Martà nez-Sánchez G, Cervantes-Llanos M et al. C-Phycocyanin ameliorates experimental autoimmune encephalomyelitis and induces regulatory T cells. Int Immunopharmacol 2011 January;11(1):29-38.
- McCarty, M.F. Clinical potential of phycocyanobilin for induction of T regulatory cells in the management of inflammatory disorders. Med Hypotheses 2011, 77, 1031–1033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Alsaleh, G.; Feltham, J.; Sun, Y.; Napolitano, G.; Riffelmacher, T.; Charles, P.; Frau, L.; Hublitz, P.; Yu, Z.; et al. Polyamines Control eIF5A Hypusination, TFEB Translation, and Autophagy to Reverse B Cell Senescence. Mol. Cell 2019, 76, 110–125. [Google Scholar] [CrossRef]
- Metur, S.P.; Klionsky, D.J. The curious case of polyamines: spermidine drives reversal of B cell senescence. Autophagy 2020, 16, 389–390. [Google Scholar] [CrossRef] [PubMed]
- Wolff, E.C.; Kang, K.R.; Kim, Y.S.; Park, M.H. Posttranslational synthesis of hypusine: evolutionary progression and specificity of the hypusine modification. Amino Acids 2007, 33, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Simon, A.K. Polyamines reverse immune senescence via the translational control of autophagy. Autophagy 2019, 16, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Alsaleh G, Panse I, Swadling L et al. Autophagy in T cells from aged donors is maintained by spermidine and correlates with function and vaccine responses. Elife 2020 ;9.
- Zhang, H.; Alsaleh, G.; Feltham, J.; Sun, Y.; Napolitano, G.; Riffelmacher, T.; Charles, P.; Frau, L.; Hublitz, P.; Yu, Z.; et al. Polyamines Control eIF5A Hypusination, TFEB Translation, and Autophagy to Reverse B Cell Senescence. Mol. Cell 2019, 76, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Kiechl, S.; Pechlaner, R.; Willeit, P.; Notdurfter, M.; Paulweber, B.; Willeit, K.; Werner, P.; Ruckenstuhl, C.; Iglseder, B.; Weger, S.; et al. Higher spermidine intake is linked to lower mortality: a prospective population-based study. Am. J. Clin. Nutr. 2018, 108, 371–380. [Google Scholar] [CrossRef]
- Zhao, E.; Czaja, M.J. Transcription factor EB: A central regulator of both the autophagosome and lysosome. Hepatology 2012, 55, 1632–1634. [Google Scholar] [CrossRef]
- Moon, Y.-A. The SCAP/SREBP Pathway: A Mediator of Hepatic Steatosis. Endocrinol. Metab. 2017, 32, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.P.; Cunningham, R.P.; Meers, G.M.; Johnson, S.A.; Wheeler, A.A.; Ganga, R.R.; Spencer, N.M.; Pitt, J.B.; Diaz-Arias, A.; Swi, A.I.A.; et al. Compromised hepatic mitochondrial fatty acid oxidation and reduced markers of mitochondrial turnover in human NAFLD. Hepatology 2022, 76, 1452–1465. [Google Scholar] [CrossRef]
- Longo M, Meroni M, Paolini E, Macchi C, Dongiovanni P. Mitochondrial dynamics and nonalcoholic fatty liver disease (NAFLD): new perspectives for a fairy-tale ending? Metabolism 2021 April;117:154708.
- Kersten, S. Integrated physiology and systems biology of PPARα. Mol Metab 2014 July;3(4):354-71.
- Velasco G, Geelen MJ, Guzmán M. Control of hepatic fatty acid oxidation by 5’-AMP-activated protein kinase involves a malonyl-CoA-dependent and a malonyl-CoA-independent mechanism. Arch Biochem Biophys 1997 ;337(2):169-75.
- Abolfathi, M.; Mohd-Yusof, B.-N.; Hanipah, Z.N.; Redzwan, S.M.; Yusof, L.M.; Khosroshahi, M.Z. The effects of carnitine supplementation on clinical characteristics of patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 48, 102273. [Google Scholar] [CrossRef] [PubMed]
- Jena BS, Jayaprakasha GK, Singh RP, Sakariah KK. Chemistry and biochemistry of (-)-hydroxycitric acid from Garcinia. J Agric Food Chem 2002 ;50(1):10-22.
- Oh SY, Park SK, Kim JW, Ahn YH, Park SW, Kim KS. Acetyl-CoA carboxylase beta gene is regulated by sterol regulatory element-binding protein-1 in liver. J Biol Chem 2003 ;278(31):28410-7.
- Ponugoti, B.; Kim, D.-H.; Xiao, Z.; Smith, Z.; Miao, J.; Zang, M.; Wu, S.-Y.; Chiang, C.-M.; Veenstra, T.D.; Kemper, J.K. SIRT1 Deacetylates and Inhibits SREBP-1C Activity in Regulation of Hepatic Lipid Metabolism. J. Biol. Chem. 2010, 285, 33959–33970. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Shimano, H.; Amemiya-Kudo, M.; Yahagi, N.; Hasty, A.H.; Matsuzaka, T.; Okazaki, H.; Tamura, Y.; Iizuka, Y.; Ohashi, K.; et al. Identification of Liver X Receptor-Retinoid X Receptor as an Activator of the Sterol Regulatory Element-Binding Protein 1c Gene Promoter. Mol. Cell. Biol. 2001, 21, 2991–3000. [Google Scholar] [CrossRef]
- Liu X, Qiao A, Ke Y et al. FoxO1 represses LXRα-mediated transcriptional activity of SREBP-1c promoter in HepG2 cells. FEBS Lett 2010 ;584(20):4330-4.
- Owen, J.L.; Zhang, Y.; Bae, S.-H.; Farooqi, M.S.; Liang, G.; Hammer, R.E.; Goldstein, J.L.; Brown, M.S. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc. Natl. Acad. Sci. USA 2012, 109, 16184–16189. [Google Scholar] [CrossRef]
- Dong Q, Majumdar G, O’Meally RN, Cole RN, Elam MB, Raghow R. Insulin-induced de novo lipid synthesis occurs mainly via mTOR-dependent regulation of proteostasis of SREBP-1c. Mol Cell Biochem 2020 January;463(1-2):13-31.
- Lee JH, Kang HS, Park HY et al. PPARα-dependent Insig2a overexpression inhibits SREBP-1c processing during fasting. Sci Rep 2017 ;7(1):9958.
- Gwinn DM, Shackelford DB, Egan DF et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 2008 ;30(2):214-26.
- Yang J, Craddock L, Hong S, Liu ZM. AMP-activated protein kinase suppresses LXR-dependent sterol regulatory element-binding protein-1c transcription in rat hepatoma McA-RH7777 cells. J Cell Biochem 2009 ;106(3):414-26.
- Wang, H.; Zhao, M.; Sud, N.; Christian, P.; Shen, J.; Song, Y.; Pashaj, A.; Zhang, K.; Carr, T.; Su, Q. Glucagon regulates hepatic lipid metabolism via cAMP and Insig-2 signaling: implication for the pathogenesis of hypertriglyceridemia and hepatic steatosis. Sci. Rep. 2016, 6, srep32246. [Google Scholar] [CrossRef] [PubMed]
- Danno H, Ishii KA, Nakagawa Y et al. The liver-enriched transcription factor CREBH is nutritionally regulated and activated by fatty acids and PPARalpha. Biochem Biophys Res Commun 2010 ;391(2):1222-7.
- Yang L, Jin GH, Zhou JY. The Role of Ceramide in the Pathogenesis of Alcoholic Liver Disease. Alcohol Alcohol 2016 May;51(3):251-7.
- Ma, H.; Guo, X.; Cui, S.; Wu, Y.; Zhang, Y.; Shen, X.; Xie, C.; Li, J. Dephosphorylation of AMP-activated protein kinase exacerbates ischemia/reperfusion-induced acute kidney injury via mitochondrial dysfunction. Kidney Int. 2021, 101, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-X.; Pan, Q.; Liu, X.-L.; Zhou, D.; Xin, F.-Z.; Zhao, Z.-H.; Zhang, R.-N.; Zeng, J.; Qiao, L.; Hu, C.-X.; et al. Therapeutic effect and autophagy regulation of myriocin in nonalcoholic steatohepatitis. Lipids Heal. Dis. 2019, 18, 179. [Google Scholar] [CrossRef]
- Holland, W.L.; Miller, R.A.; Wang, Z.V.; Sun, K.; Barth, B.M.; Bui, H.H.; Davis, K.E.; Bikman, B.T.; Halberg, N.; Rutkowski, J.M.; et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011, 17, 55–63. [Google Scholar] [CrossRef]
- Holland, W.L.; Xia, J.Y.; Johnson, J.A.; Sun, K.; Pearson, M.J.; Sharma, A.X.; Quittner-Strom, E.; Tippetts, T.S.; Gordillo, R.; Scherer, P.E. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol. Metab. 2017, 6, 267–275. [Google Scholar] [CrossRef]
- Yang, T.; Wang, X.; Zhou, Y.; Yu, Q.; Heng, C.; Yang, H.; Yuan, Z.; Miao, Y.; Chai, Y.; Wu, Z.; et al. SEW2871 attenuates ANIT-induced hepatotoxicity by protecting liver barrier function via sphingosine 1-phosphate receptor-1–mediated AMPK signaling pathway. Cell Biol. Toxicol. 2021, 37, 595–609. [Google Scholar] [CrossRef]
- McCarty, M.F. GCN2 and FGF21 are likely mediators of the protection from cancer, autoimmunity, obesity, and diabetes afforded by vegan diets. Med Hypotheses 2014, 83, 365–371. [Google Scholar] [CrossRef]
- Castaño-Martinez, T.; Schumacher, F.; Schumacher, S.; Kochlik, B.; Weber, D.; Grune, T.; Biemann, R.; Mccann, A.; Abraham, K.; Weikert, C.; et al. Methionine restriction prevents onset of type 2 diabetes in NZO mice. FASEB J. 2019, 33, 7092–7102. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Tian, H.; Lam, K.S.; Lin, S.; Hoo, R.C.; Konishi, M.; Itoh, N.; Wang, Y.; Bornstein, S.R.; Xu, A.; et al. Adiponectin Mediates the Metabolic Effects of FGF21 on Glucose Homeostasis and Insulin Sensitivity in Mice. Cell Metab. 2013, 17, 779–789. [Google Scholar] [CrossRef]
- Sanchez A, Hubbard RW. Plasma amino acids and the insulin/glucagon ratio as an explanation for the dietary protein modulation of atherosclerosis. Med Hypotheses 1991 September;36(1):27-32.
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein-Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Kim, Y.A.; Keogh, J.B.; Clifton, P.M. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr. Res. Rev. 2017, 31, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Y.-N.; Ye, C.-Y.; Feng, W.-B.; Zhou, Q.-T.; Yang, D.-H.; Wang, M.-W. GLP-1 mimetics as a potential therapy for nonalcoholic steatohepatitis. Acta Pharmacol. Sin. 2021, 43, 1156–1166. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Subramanian, S.; Chait, A.; Haigh, W.G.; Yeh, M.M.; Farrell, G.C.; Lee, S.P.; Savard, C. Cholesterol crystallization within hepatocyte lipid droplets and its role in murine NASH. J. Lipid Res. 2017, 58, 1067–1079. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Landis, C.S.; Jin, G.-Y; Haigh, W.G.; Farrell, G.C.; Kuver, R.; Lee, S.P.; Savard, C. Cholesterol Crystals in Hepatocyte Lipid Droplets Are Strongly Associated With Human Nonalcoholic Steatohepatitis. Hepatol. Commun. 2019, 3, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-W.; Yen, C.-C.; Kuo, L.-L.; Lo, C.-W.; Huang, C.-S.; Chen, C.-C.; Lii, C.-K. Benzyl isothiocyanate ameliorates high-fat/cholesterol/cholic acid diet-induced nonalcoholic steatohepatitis through inhibiting cholesterol crystal-activated NLRP3 inflammasome in Kupffer cells. Toxicol. Appl. Pharmacol. 2020, 393, 114941. [Google Scholar] [CrossRef]
- Pérez LM, Milkiewicz P, Elias E, Coleman R, Sánchez Pozzi EJ, Roma MG. Oxidative stress induces internalization of the bile salt export pump, Bsep, and bile salt secretory failure in isolated rat hepatocyte couplets: a role for protein kinase C and prevention by protein kinase A. Toxicol Sci 2006 May;91(1):150-8.
- Basiglio, C.L.; Toledo, F.D.; Boaglio, A.C.; Arriaga, S.M.; Ochoa, J.E.; Pozzi, E.J.S.; Mottino, A.D.; Roma, M.G. Physiological concentrations of unconjugated bilirubin prevent oxidative stress-induced hepatocanalicular dysfunction and cholestasis. Arch. Toxicol. 2014, 88, 501–514. [Google Scholar] [CrossRef]
- Martín, P.L.; Ceccatto, P.; Razori, M.V.; Francés, D.E.; Arriaga, S.M.; Pisani, G.B.; Martínez, A.I.; Pozzi, E.J.S.; Roma, M.G.; Basiglio, C.L. Heme oxygenase-1 induction by hemin prevents oxidative stress-induced acute cholestasis in the rat. Clin. Sci. 2019, 133, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Kimmel, R.; Weinberger, C.; Stroup, D. Farnesoid X Receptor Responds to Bile Acids and Represses Cholesterol 7α-Hydroxylase Gene (CYP7A1) Transcription. J. Biol. Chem. 2000, 275, 10918–10924. [Google Scholar] [CrossRef]
- McCarty MF, Iloki Assanga SB, Lewis LnL, O’Keefe JH, DiNicolantonio JJ. Nutraceutical Strategies for Suppressing NLRP3 Inflammasome Activation: Pertinence to the Management of COVID-19 and Beyond. Nutrients 2020 ;13(1).
- DiNicolantonio JJ, McCarty MF, Barroso-Aranda J, Assanga S, Lujan LML, O’Keefe JH. A nutraceutical strategy for downregulating TGFÎ2 signalling: prospects for prevention of fibrotic disorders, including post-COVID-19 pulmonary fibrosis. Open Heart 2021 April;8(1).
- Yang, J.D.; Abdelmalek, M.F.; Pang, H.; Guy, C.D.; Smith, A.D.; Diehl, A.M.; Suzuki, A. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology 2014, 59, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I. Impact of oestrogens on the progression of liver disease. Liver Int. 2003, 23, 63–69. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Barroso-Aranda, J.; Contreras, F. Genistein and phycocyanobilin may prevent hepatic fibrosis by suppressing proliferation and activation of hepatic stellate cells. Med Hypotheses 2009, 72, 330–332. [Google Scholar] [CrossRef]
- Zhou Y, Shimizu I, Lu G et al. Hepatic stellate cells contain the functional estrogen receptor beta but not the estrogen receptor alpha in male and female rats. Biochem Biophys Res Commun 2001 ;286(5):1059-65.
- Cherlet, T.; Murphy, L.C. Estrogen receptors inhibit Smad3 transcriptional activity through Ap-1 transcription factors. Mol. Cell. Biochem. 2007, 306, 33–42. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Isoflavones made simple – Genistein’s agonist activity for the beta-type estrogen receptor mediates their health benefits. Med Hypotheses 2006, 66, 1093–1114. [Google Scholar] [CrossRef]
- Alisi, A.; Carpino, G.; Oliveira, F.L.; Panera, N.; Nobili, V.; Gaudio, E. The Role of Tissue Macrophage-Mediated Inflammation on NAFLD Pathogenesis and Its Clinical Implications. Mediat. Inflamm. 2017, 2017, 8162421. [Google Scholar] [CrossRef]
- Kazankov K, Jørgensen SMD, Thomsen KL et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol 2019 March;16(3):145-59.
- Alves, A.; Bassot, A.; Bulteau, A.-L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef]
- Gan, Z.; Zhang, M.; Xie, D.; Wu, X.; Hong, C.; Fu, J.; Fan, L.; Wang, S.; Han, S. Glycinergic Signaling in Macrophages and Its Application in Macrophage-Associated Diseases. Front. Immunol. 2021, 12, 762564. [Google Scholar] [CrossRef]
- Qu, W.; Stachlewitz, R.F.; Takashima, S.; Ikejima, K.; Arai, K.; Yokokawa, J.; Kon, K.; Watanabe, S.; Shumilina, E.; Huber, S.M.; et al. Kupffer cells contain a glycine-gated chloride channel. Am. J. Physiol. Liver Physiol. 1997, 272, G1581–G1586. [Google Scholar] [CrossRef]
- McCarty, M.F.; O’Keefe, J.H.; DiNicolantonio, J.J. Dietary Glycine Is Rate-Limiting for Glutathione Synthesis and May Have Broad Potential for Health Protection. Ochsner J. 2018, 18, 81–87. [Google Scholar] [PubMed]
- Gannon, M.C.; A Nuttall, J.; Nuttall, F.Q. The metabolic response to ingested glycine. Am. J. Clin. Nutr. 2002, 76, 1302–1307. [Google Scholar] [CrossRef]
- Yeung F, Hoberg JE, Ramsey CS et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 2004 ;23(12):2369-80.
- Naowaboot, J.; Piyabhan, P.; Munkong, N.; Parklak, W.; Pannangpetch, P. Ferulic acid improves lipid and glucose homeostasis in high-fat diet-induced obese mice. Clin. Exp. Pharmacol. Physiol. 2016, 43, 242–250. [Google Scholar] [CrossRef]
- Ma Y, Chen K, Lv L, Wu S, Guo Z. Ferulic acid ameliorates nonalcoholic fatty liver disease and modulates the gut microbiota composition in high-fat diet fed ApoE(-/-) mice. Biomed Pharmacother 2019 May;113:108753.
- Wei Z, Xue Y, Xue Y et al. Ferulic acid attenuates non-alcoholic steatohepatitis by reducing oxidative stress and inflammation through inhibition of the ROCK/NF-κB signaling pathways. J Pharmacol Sci 2021 September;147(1):72-80.
- Pan, M.; Song, Y.-L.; Xu, J.-M.; Gan, H.-Z. Melatonin ameliorates nonalcoholic fatty liver induced by high-fat diet in rats. J. Pineal Res. 2006, 41, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Pakravan, H.; Fani, A.; Aghaee, D.; Brumanad, S.; Pakzad, B. The Effects of Melatonin in Patients with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. Adv. Biomed. Res. 2017, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Akhavan RA, Ghasemi NM, Bondarsahebi Y et al. The effects of melatonin therapy on the treatment of patients with Non-alcoholic steatohepatitis: A systematic review and Meta-analysis on clinical trial studies. Eur J Pharmacol 2021 ;905:174154.
- Takeuchi, K.; Yokouchi, C.; Goto, H.; Umehara, K.; Yamada, H.; Ishii, Y. Alleviation of fatty liver in a rat model by enhancing N1-methylnicotinamide bioavailability through aldehyde oxidase inhibition. Biochem. Biophys. Res. Commun. 2018, 507, 203–210. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Z.; Zeng, W.; Zhao, J.; Zhou, X. Two sides of NNMT in alcoholic and non-alcoholic fatty liver development. J. Hepatol. 2020, 74, 1250–1253. [Google Scholar] [CrossRef]
- Noori, M.; Jafari, B.; Hekmatdoost, A. Pomegranate juice prevents development of non-alcoholic fatty liver disease in rats by attenuating oxidative stress and inflammation. J. Sci. Food Agric. 2017, 97, 2327–2332. [Google Scholar] [CrossRef]
- Liu, H.; Zhan, Q.; Miao, X.; Xia, X.; Yang, G.; Peng, X.; Yan, C. Punicalagin Prevents Hepatic Steatosis through Improving Lipid Homeostasis and Inflammation in Liver and Adipose Tissue and Modulating Gut Microbiota in Western Diet-Fed Mice. Mol. Nutr. Food Res. 2021, 65, e2001031. [Google Scholar] [CrossRef]
- Goodarzi R, Jafarirad S, Mohammadtaghvaei N, Dastoorpoor M, Alavinejad P. The effect of pomegranate extract on anthropometric indices, serum lipids, glycemic indicators, and blood pressure in patients with nonalcoholic fatty liver disease: A randomized double-blind clinical trial. Phytother Res 2021 October;35(10):5871-82.
- Ann, J.-Y.; Eo, H.; Lim, Y. Mulberry leaves (Morus alba L.) ameliorate obesity-induced hepatic lipogenesis, fibrosis, and oxidative stress in high-fat diet-fed mice. Genes Nutr. 2015, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-H.; Lin, H.-T.; Chung, D.-J.; Huang, C.-N.; Wang, C.-J. Mulberry Leaf Extracts prevent obesity-induced NAFLD with regulating adipocytokines, inflammation and oxidative stress. J. Food Drug Anal. 2018, 26, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Jegatheesan, P.; Beutheu, S.; Freese, K.; Waligora-Dupriet, A.-J.; Nubret, E.; Butel, M.-J.; Bergheim, I.; De Bandt, J.-P. Preventive effects of citrulline on Western diet-induced non-alcoholic fatty liver disease in rats. Br. J. Nutr. 2016, 116, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Sellmann, C.; Jin, C.J.; Engstler, A.J.; De Bandt, J.-P.; Bergheim, I. Oral citrulline supplementation protects female mice from the development of non-alcoholic fatty liver disease (NAFLD). Eur. J. Nutr. 2017, 56, 2519–2527. [Google Scholar] [CrossRef] [PubMed]
- Ouelaa W, Jegatheesan P, M’bouyou-Boungou J et al. Citrulline decreases hepatic endotoxin-induced injury in fructose-induced non-alcoholic liver disease: an ex vivo study in the isolated perfused rat liver. Br J Nutr 2017 June;117(11):1487-94.
- Darabi, Z.; Darand, M.; Yari, Z.; Hedayati, M.; Faghihi, A.; Agah, S.; Hekmatdoost, A. Inflammatory markers response to citrulline supplementation in patients with non-alcoholic fatty liver disease: a randomized, double blind, placebo-controlled, clinical trial. BMC Res. Notes 2019, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Rajcic, D.; Baumann, A.; Hernández-Arriaga, A.; Brandt, A.; Nier, A.; Jin, C.J.; Sánchez, V.; Jung, F.; Camarinha-Silva, A.; Bergheim, I. Citrulline supplementation attenuates the development of non-alcoholic steatohepatitis in female mice through mechanisms involving intestinal arginase. Redox Biol. 2021, 41, 101879. [Google Scholar] [CrossRef] [PubMed]
- Shatoor, A.S.; Al Humayed, S.; Almohiy, H.M. Astaxanthin attenuates hepatic steatosis in high-fat diet-fed rats by suppressing microRNA-21 via transactivation of nuclear factor erythroid 2-related factor 2. J. Physiol. Biochem. 2021, 78, 151–168. [Google Scholar] [CrossRef]
- Ota, T. Prevention of NAFLD/NASH by Astaxanthin and β-Cryptoxanthin. Adv. Exp. Med. Biol. 2021, 1261, 231–238. [Google Scholar] [CrossRef]
- Wu L, Mo W, Feng J et al. Astaxanthin attenuates hepatic damage and mitochondrial dysfunction in non-alcoholic fatty liver disease by up-regulating the FGF21/PGC-1α pathway. Br J Pharmacol 2020 August;177(16):3760-77.
- Yang, M.; Kimchi, E.T.; Staveley-O’carroll, K.F.; Li, G. Astaxanthin Prevents Diet-Induced NASH Progression by Shaping Intrahepatic Immunity. Int. J. Mol. Sci. 2021, 22, 11037. [Google Scholar] [CrossRef]
- Valenzuela, R.; Ortiz, M.; Hernández-Rodas, M.C.; Echeverría, F.; Videla, L.A. Targeting n-3 Polyunsaturated Fatty Acids in Non-Alcoholic Fatty Liver Disease. Curr. Med. Chem. 2020, 27, 5250–5272. [Google Scholar] [CrossRef]
- Lee, C.-H.; Fu, Y.; Yang, S.-J.; Chi, C.-C. Effects of Omega-3 Polyunsaturated Fatty Acid Supplementation on Non-Alcoholic Fatty Liver: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2769. [Google Scholar] [CrossRef]
- Cansanção K, Citelli M, Carvalho LN et al. Impact of Long-Term Supplementation with Fish Oil in Individuals with Non-Alcoholic Fatty Liver Disease: A Double Blind Randomized Placebo Controlled Clinical Trial. Nutrients 2020 ;12(11).
- Yang, J.; Sáinz, N.; Félix-Soriano, E.; Gil-Iturbe, E.; Castilla-Madrigal, R.; Fernández-Galilea, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Effects of Long-Term DHA Supplementation and Physical Exercise on Non-Alcoholic Fatty Liver Development in Obese Aged Female Mice. Nutrients 2021, 13, 501. [Google Scholar] [CrossRef] [PubMed]
- Antraco, V.J.; Hirata, B.K.S.; de Jesus Simão, J.; Cruz, M.M.; da Silva, Viviane, S. ; da Cunha de Sá, R.D.C.; Abdala, F.M.; Armelin-Correa, L.M.; Alonso-Vale, M.I.C. Omega-3 polyunsaturated fatty acids prevent nonalcoholic steatohepatitis (NASH) and stimulate adipogenesis. Nutrients 2021, 13, 622. [Google Scholar] [CrossRef] [PubMed]
- Sangouni, A.A.; Orang, Z.; Mozaffari-Khosravi, H. Effect of omega-3 supplementation on fatty liver and visceral adiposity indices in diabetic patients with non-alcoholic fatty liver disease: A randomized controlled trial. Clin. Nutr. ESPEN 2021, 44, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Valdecantos, M.P.; Pérez-Matute, P.; González-Muniesa, P.; Prieto-Hontoria, P.L.; Moreno-Aliaga, M.J.; Martínez, J.A. Lipoic Acid Improves Mitochondrial Function in Nonalcoholic Steatosis Through the Stimulation of Sirtuin 1 and Sirtuin 3. Obesity 2012, 20, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.S.; Kim, S.K.; Shin, H.J.; Jeon, B.T.; Hahm, J.R.; Roh, G.S. α-lipoic acid prevents non-alcoholic fatty liver disease in OLETF rats. Liver Int. 2012, 32, 1565–1573. [Google Scholar] [CrossRef]
- Kathirvel, E.; Morgan, K.; French, S.W.; Morgan, T.R. Acetyl-l-carnitine and lipoic acid improve mitochondrial abnormalities and serum levels of liver enzymes in a mouse model of nonalcoholic fatty liver disease. Nutr. Res. 2013, 33, 932–941. [Google Scholar] [CrossRef]
- 259. Trushina EN, Riger NA, Mustafina OK et al. [Effect of carnosine and α-lipoic acid on hepatocyte apoptosis and the cytokine profile in induced fatty liver disease in Wistar rats]. Vopr Pitan 2020;89(5):6-16.
- Ko, C.; Lo, Y.M.; Xu, J.; Chang, W.; Huang, D.; Wu, J.S.; Yang, C.; Huang, W.; Shen, S. Alpha-lipoic acid alleviates NAFLD and triglyceride accumulation in liver via modulating hepatic NLRP3 inflammasome activation pathway in type 2 diabetic rats. Food Sci. Nutr. 2021, 9, 2733–2742. [Google Scholar] [CrossRef]
- Zhong S, Fan Y, Yan Q et al. The therapeutic effect of silymarin in the treatment of nonalcoholic fatty disease: A meta-analysis (PRISMA) of randomized control trials. Medicine (Baltimore) 2017 December;96(49):e9061.
- Ou Q, Weng Y, Wang S et al. Silybin Alleviates Hepatic Steatosis and Fibrosis in NASH Mice by Inhibiting Oxidative Stress and Involvement with the Nf-κB Pathway. Dig Dis Sci 2018 December;63(12):3398-408.
- Sahin, E.; Bagci, R.; Aykanat, N.E.B.; Kacar, S.; Sahinturk, V. Silymarin attenuated nonalcoholic fatty liver disease through the regulation of endoplasmic reticulum stress proteins GRP78 and XBP-1 in mice. J. Food Biochem. 2020, 44, e13194. [Google Scholar] [CrossRef]
- Samuhasaneeto, S.; Thong-Ngam, D.; Kulaputana, O.; Patumraj, S.; Klaikeaw, N. Effects of N-acetylcysteine on oxidative stress in rats with non-alcoholic steatohepatitis. J. Med Assoc. Thail. 2007, 90, 788–797. [Google Scholar]
- Khoshbaten, M.; Aliasgarzadeh, A.; Masnadi, K.; Tarzamani, M.K.; Farhang, S.; Babaei, H.; Kiani, J.; Zaare, M.; Najafipoor, F. N-Acetylcysteine Improves Liver Function in Patients with Non-Alcoholic Fatty Liver Disease. Zahedan J. Res. Med Sci. 2010, 10, 12–16. [Google Scholar]
- Tsai, C.-C.; Chen, Y.-J.; Yu, H.-R.; Huang, L.-T.; Tain, Y.-L.; Lin, I.-C.; Sheen, J.-M.; Wang, P.-W.; Tiao, M.-M. Long term N-acetylcysteine administration rescues liver steatosis via endoplasmic reticulum stress with unfolded protein response in mice. Lipids Heal. Dis. 2020, 19, 105. [Google Scholar] [CrossRef]
- Abd Elwahab AH, Ramadan BK, Schaalan MF, Tolba AM. A Novel Role of SIRT1/FGF-21 in Taurine Protection Against Cafeteria Diet-Induced Steatohepatitis in Rats. Cell Physiol Biochem 2017;43(2):644-59.
- Murakami, S.; Ono, A.; Kawasaki, A.; Takenaga, T.; Ito, T. Taurine attenuates the development of hepatic steatosis through the inhibition of oxidative stress in a model of nonalcoholic fatty liver disease in vivo and in vitro. Amino Acids 2018, 50, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Funaki, A.; Fukuhara, C.; Sumiya, Y.; Sugiura, Y. Taurine attenuates hepatic steatosis in a genetic model of fatty liver disease. J. Toxicol. Sci. 2020, 45, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Liang, G.; Lv, Z.L.; Lan, L.C.; Zhu, F.L.; Tang, Q.; Huang, L.; Chen, X.Q.; Yang, M.X.; Shan, Q.W. Taurine Reduces Liver Damage in Non-Alcoholic Fatty Liver Disease Model in Rats by Down-Regulating IL-9 and Tumor Growth Factor TGF-β. Bull. Exp. Biol. Med. 2021, 171, 638–643. [Google Scholar] [CrossRef]
- Gao, M.; Zhao, W.; Li, C.; Xie, X.; Li, M.; Bi, Y.; Fang, F.; Du, Y.; Liu, X. Spermidine ameliorates non-alcoholic fatty liver disease through regulating lipid metabolism via AMPK. Biochem. Biophys. Res. Commun. 2018, 505, 93–98. [Google Scholar] [CrossRef]
- Ni, Y.; Hu, Y.; Lou, X.; Rong, N.; Liu, F.; Yang, C.; Zheng, A.; Yang, S.; Bao, J.; Fu, Z. Spermidine Ameliorates Nonalcoholic Steatohepatitis through Thyroid Hormone-Responsive Protein Signaling and the Gut Microbiota-Mediated Metabolism of Bile Acids. J. Agric. Food Chem. 2022, 70, 6478–6492. [Google Scholar] [CrossRef]
- 273. Li L, Chen J, Ni Y et al. TRPV1 activation prevents nonalcoholic fatty liver through UCP2 upregulation in mice. Pflugers Arch 2012 April;463(5):727-32. [CrossRef]
- Karimi-Sales, E.; Mohaddes, G.; Alipour, M.R. Hepatoprotection of capsaicin in alcoholic and non-alcoholic fatty liver diseases. Arch. Physiol. Biochem. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.K.; Yang, S.-M.; Han, I.-S. Capsaicin suppresses liver fat accumulation in high-fat diet-induced NAFLD mice. Anim. Cells Syst. 2020, 24, 214–219. [Google Scholar] [CrossRef]
- Fujimoto, M.; Tsuneyama, K.; Fujimoto, T.; Selmi, C.; Gershwin, M.E.; Shimada, Y. Spirulina improves non-alcoholic steatohepatitis, visceral fat macrophage aggregation, and serum leptin in a mouse model of metabolic syndrome. Dig. Liver Dis. 2012, 44, 767–774. [Google Scholar] [CrossRef]
- Ferreira-Hermosillo, A.; Torres-Duran, P.V.; Juarez-Oropeza, M.A. Hepatoprotective effects of Spirulina maxima in patients with non-alcoholic fatty liver disease: A case series. J. Med Case Rep. 2010, 4, 103. [Google Scholar] [CrossRef]
- Mazokopakis, E.E.; Papadomanolaki, M.G.; Fousteris, A.A.; Kotsiris, D.A.; Lampadakis, I.M.; Ganotakis, E.S. The hepatoprotective and hypolipidemic effects of Spirulina (Arthrospira platensis) supplementation in a Cretan population with non-alcoholic fatty liver disease: a prospective pilot study. Ann. Gastroenterol. 2014, 27, 387–394. [Google Scholar] [PubMed]
- Pham, T.X.; Lee, Y.; Bae, M.; Hu, S.; Kang, H.; Kim, M.-B.; Park, Y.-K.; Lee, J.-Y. Spirulina supplementation in a mouse model of diet-induced liver fibrosis reduced the pro-inflammatory response of splenocytes. Br. J. Nutr. 2019, 121, 748–755. [Google Scholar] [CrossRef]
- Abolfathi, M.; Mohd-Yusof, B.-N.; Hanipah, Z.N.; Redzwan, S.M.; Yusof, L.M.; Khosroshahi, M.Z. The effects of carnitine supplementation on clinical characteristics of patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 48, 102273. [Google Scholar] [CrossRef]
- Liu, H.; Zhong, H.; Leng, L.; Jiang, Z. Effects of soy isoflavone on hepatic steatosis in high fat-induced rats. J. Clin. Biochem. Nutr. 2017, 61, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Chen, C.; Hu, Y.-Y.; Feng, Q. Protective effect of genistein on nonalcoholic fatty liver disease (NAFLD). BioMedicine 2019, 117, 109047. [Google Scholar] [CrossRef]
- Ustundag, B.; Bahcecioglu, I.H.; Sahin, K.; Duzgun, S.; Koca, S.; Gulcu, F.; Ozercan, I.H. Protective Effect of Soy Isoflavones and Activity Levels of Plasma Paraoxonase and Arylesterase in the Experimental Nonalcoholic Steatohepatitis Model. Dig. Dis. Sci. 2007, 52, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Han, D.; Xu, R.; Wu, H.; Qu, C.; Wang, F.; Wang, X.; Zhao, Y. Glycine protects against high sucrose and high fat-induced non-alcoholic steatohepatitis in rats. Oncotarget 2016, 7, 80223–80237. [Google Scholar] [CrossRef]
- Yang X, Han D, Wang XX, Liu LK, Zhou X. Glycine protects against non-alcoholic hepatitis by downregulation of the TLR4 signaling pathway. Int J Clin Exp Pathol 2017;10(10):10261-8.
- Takashima, S.; Ikejima, K.; Arai, K.; Yokokawa, J.; Kon, K.; Yamashina, S.; Watanabe, S. Glycine prevents metabolic steatohepatitis in diabetic KK-Ay mice through modulation of hepatic innate immunity. Am. J. Physiol. Liver Physiol. 2016, 311, G1105–G1113. [Google Scholar] [CrossRef]
- Tarantino G, Balsano C, Santini SJ et al. It Is High Time Physicians Thought of Natural Products for Alleviating NAFLD. Is There Sufficient Evidence to Use Them? Int J Mol Sci 2021 ;22(24).
- Mazidi, M.; Kengne, A.P. Higher adherence to plant-based diets are associated with lower likelihood of fatty liver. Clin. Nutr. 2019, 38, 1672–1677. [Google Scholar] [CrossRef]
- Chiarioni, G.; Popa, S.L.; Dalbeni, A.; Senore, C.; Leucuta, D.C.; Baroni, L.; Fantin, A. Vegan Diet Advice Might Benefit Liver Enzymes in Nonalcoholic Fatty Liver Disease: an Open Observational Pilot Study. J. Gastrointest. Liver Dis. 2021, 30, 81–87. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




