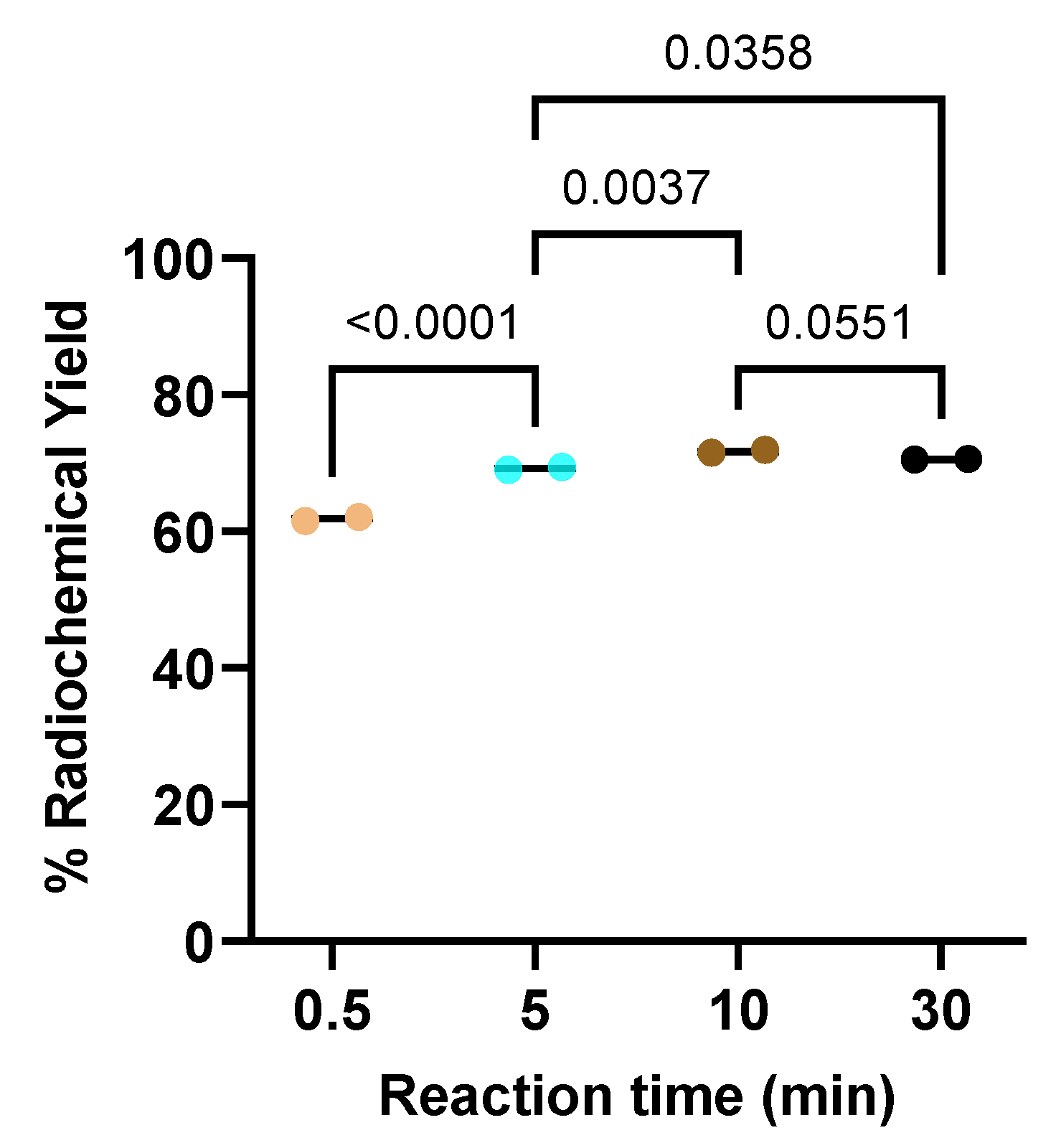
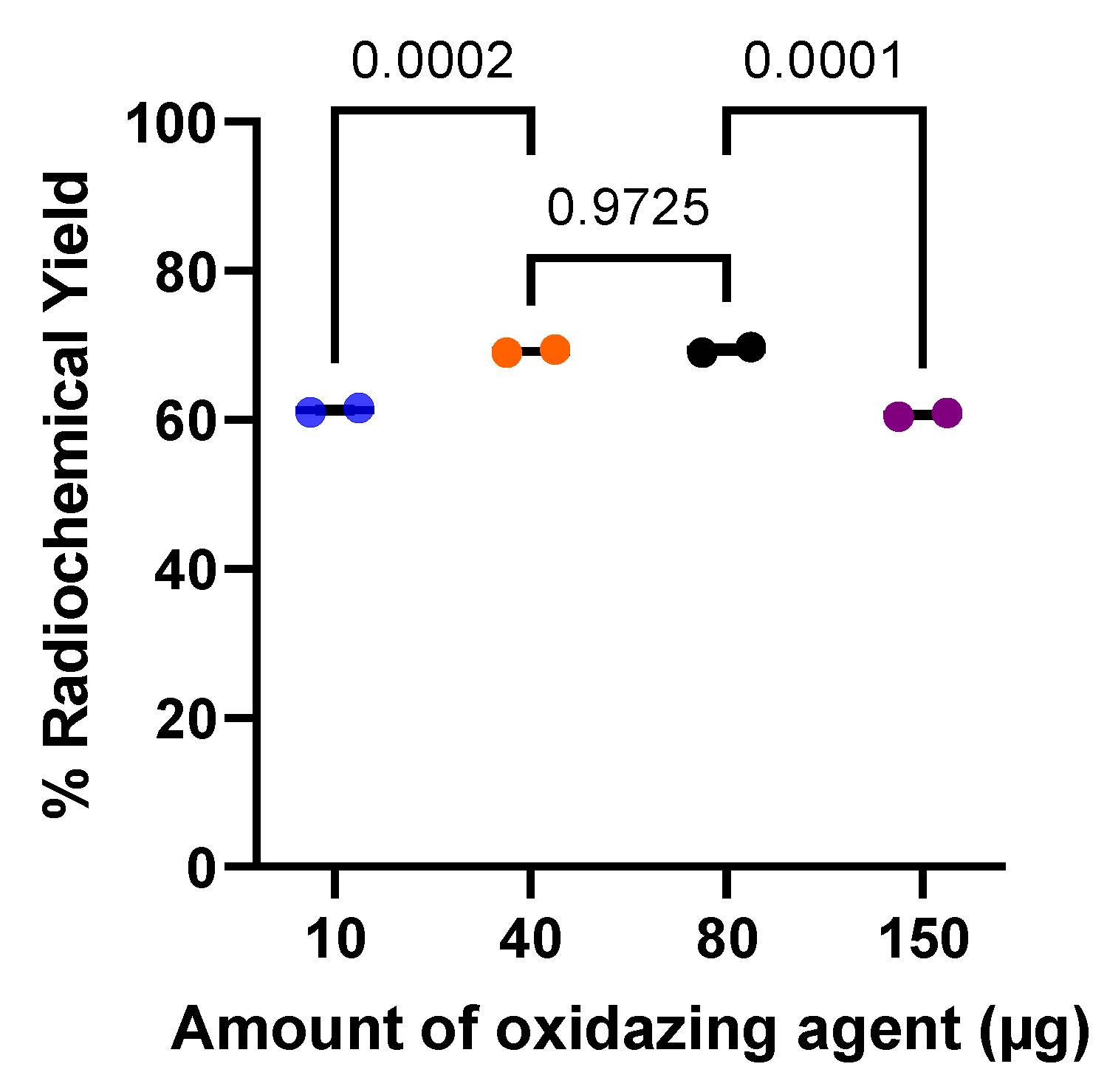
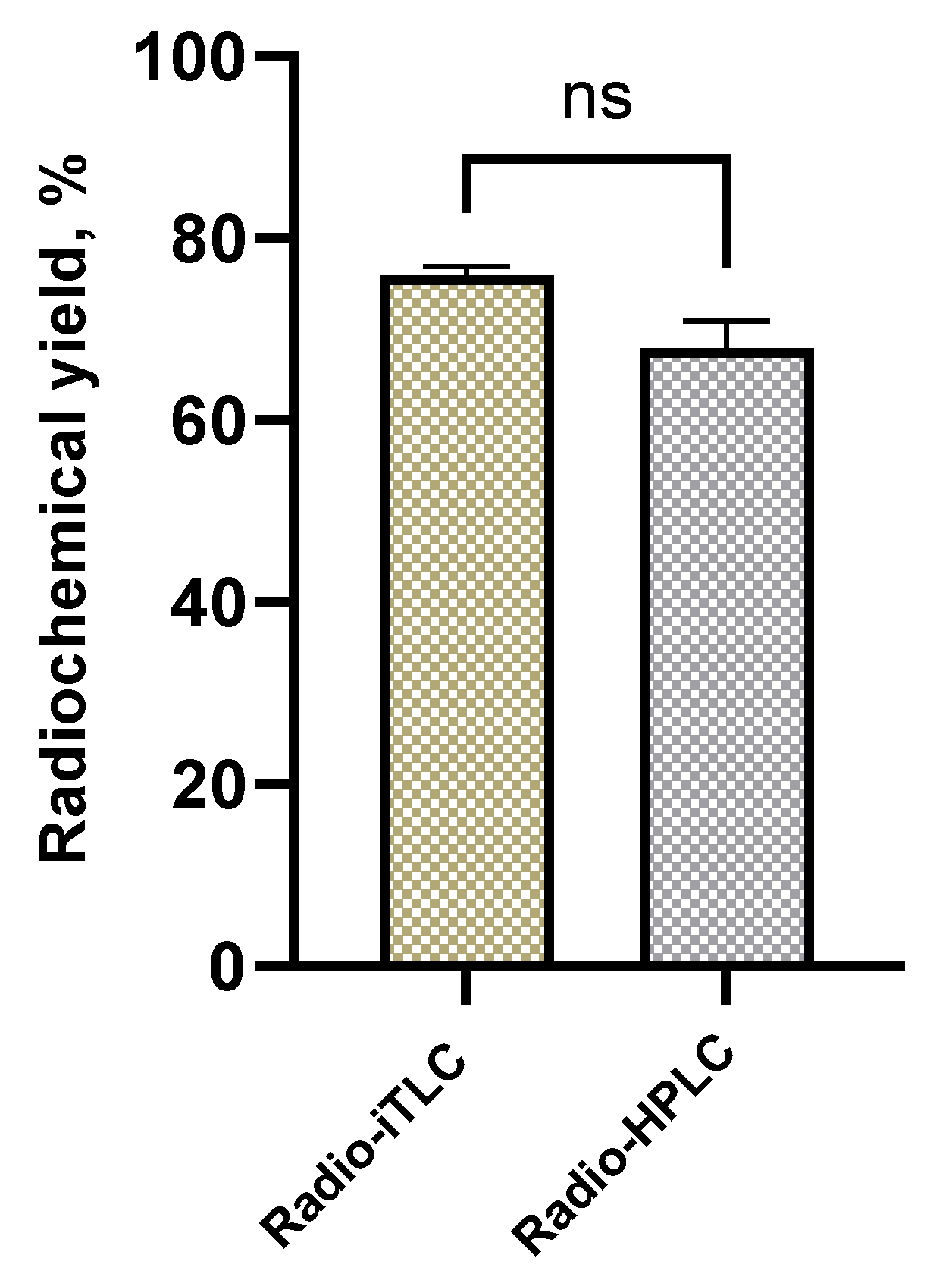
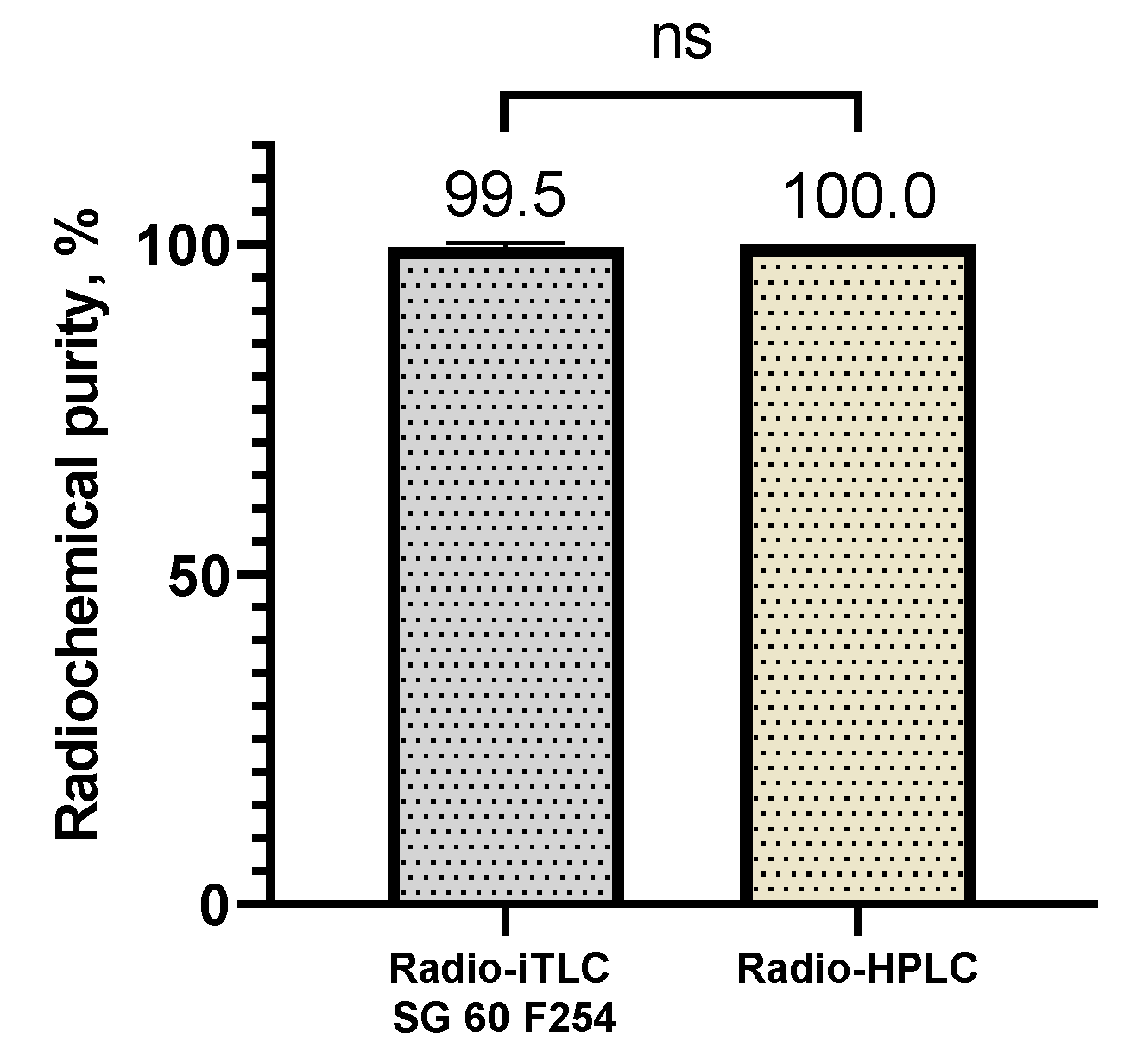
Purifications of the radiolabeling yield were performed by Sep-Pak® C18 cartridge, catalogue number WAT036815, (Waters, Milford, Massachusetts, USA). Thin-layer chromatography (TLC) analysis was performed using iTLC glass microfiber chromatography sheet impregnated with a silica gel (iTLC SG fiber sheet) (Agilent Technologies, Inc., Folsom, CA, USA) and iTLC silica gel 60 F254 aluminium plates (iTLC SG 60 F254) (Merck KGaA, Darmstadt, Germany)
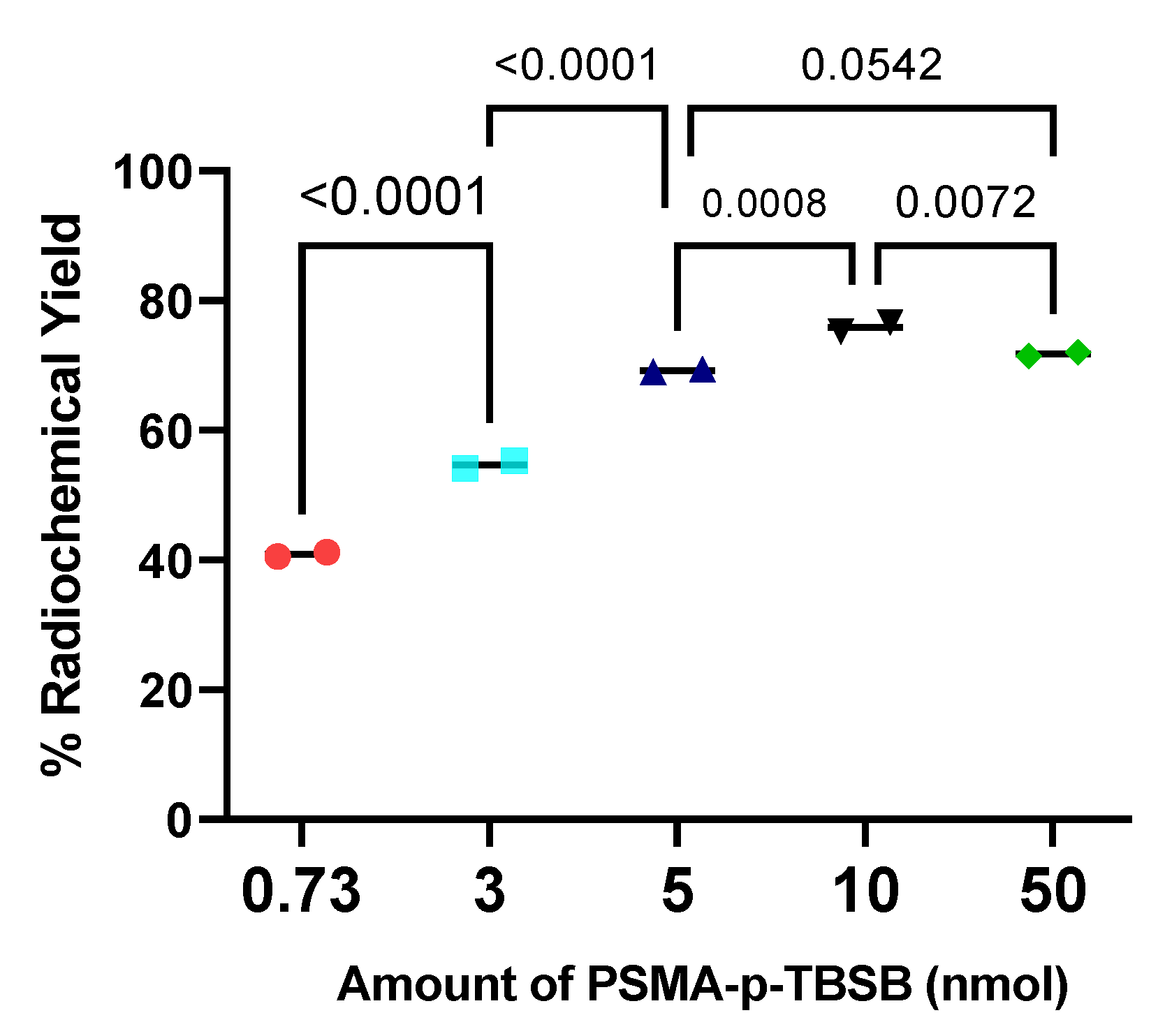
Data on radiolabeling, binding specificity, and biodistribution were analyzed by ANOVA test with Turkey’s post-hoc analysis and two-tailed t-test to determine any significant difference using GraphPad Prism (version 9.5.0 for Windows; GraphPad Software, La Jolla, CA, USA).
4.1. Chemistry
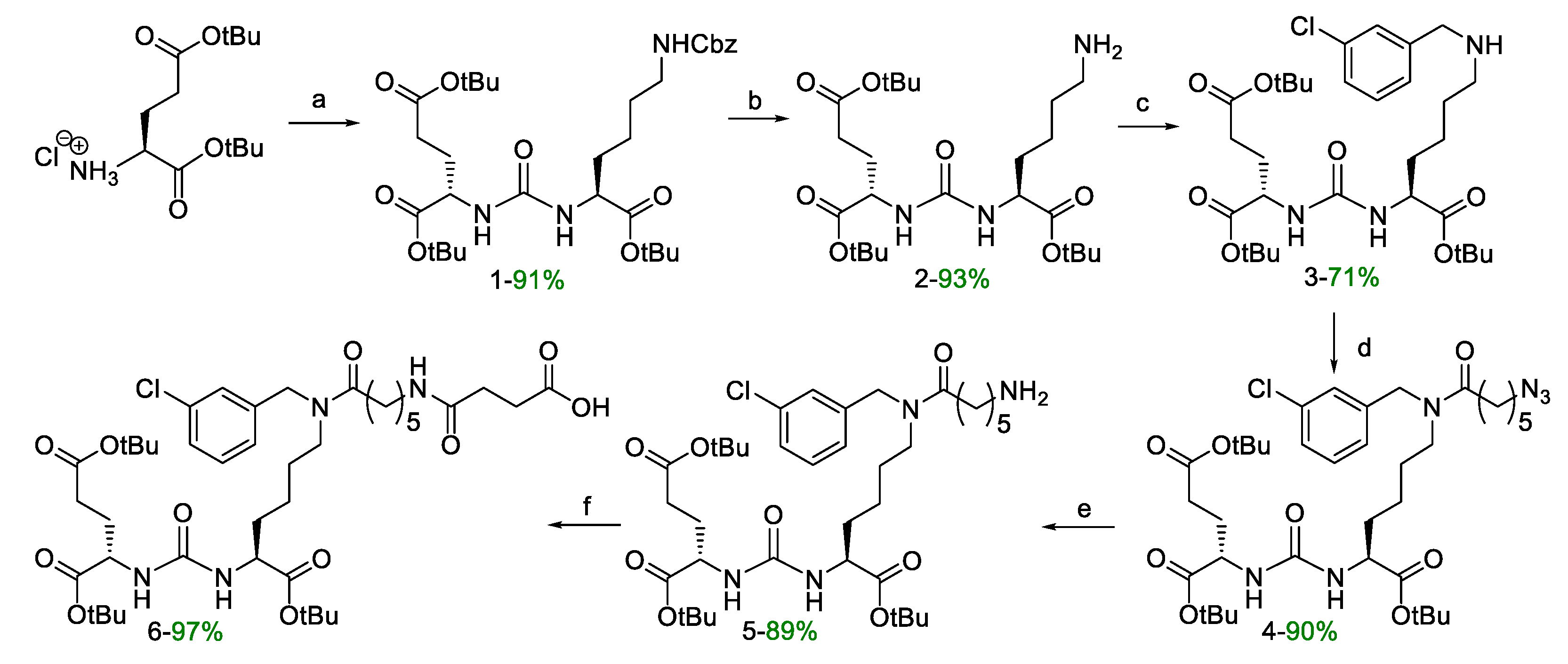
Compound 6. DIPEA (1.4 eq; 244 μL; 1.4 mmol) and succinic anhydride (1.02 eq; 102 mg; 1.02 mmol) were added to a solution of compound 5 (1 eq; 725 mg; 1.0 mmol) in 20 mL of DCM. The mixture was stirred for 12 h, then MeOH (2 eq.) was added and the resulting mixture was stirred for 1 h. Then the solvent was removed under reduced pressure, the residue was dissolved in DCM and extracted with (1) 0.1 M HCl (2 * 30 mL), (2) brine (2 * 30 mL). Then the organic fraction was dried over Na2SO4, and concentrated under reduced pressure to obtain final compound 6 as yellow oil (801 mg, yield 97%).
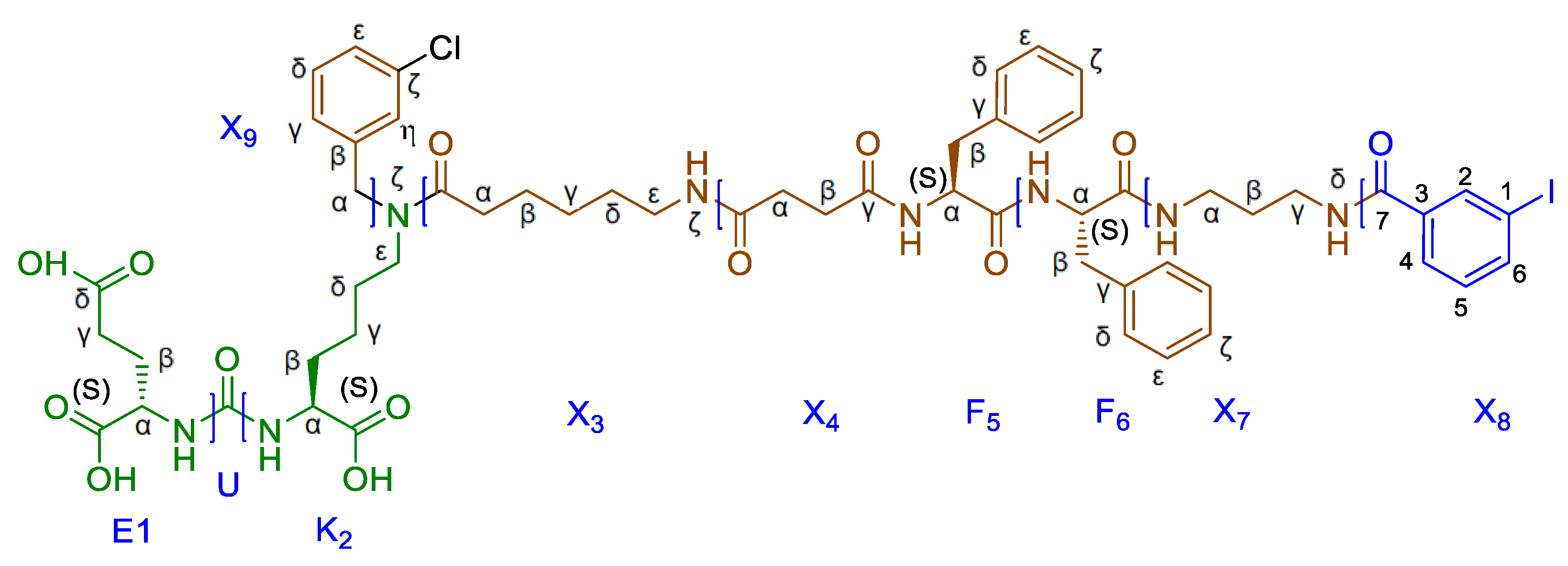
1HNMR (400 MHz, DMSO-d6, δ): 12.06 (br.s., 1Н, X4C(O)OH), 7.81 (t, J = 5.2Hz, m) &7.77 (t, J = 5.2Hz, n) (1H, X3NHk, m + n, m/n = 3/2), 7.40 (t, J = 7.7Hz, X8He, n), 7.37–7.27 (m, X8Hd + X8He(m)), 7.26-7.21 (m, 1H, X8Ht, m + n), 7.19–7.10 (m, 1H, X8Hg, m + n), 6.34–6.20 (m, 2Н, K2NH + E1NH, m + n), 4.56 (s, n) & 4.48 (s, m) (2H, X8Ha, m + n, m/n = 3/2), 4.07–4.00 (m, 1H, E1Ha, m + n), 4.00–3.90 (m, 1H, K2Ha, m + n), 3.22 (t, J = 7.3 Hz, n) & 3.19 (t, J = 7.3Hz, m) (2H, K2He, m + n, m/n = 3/2), 3.01 (q, J = 6.4, 12.7 Hz, m) & 2.96 (q, J = 6.4, 12.7 Hz, n) (2H, X3He, m + n, m/n = 3/2), 2.44–2.38 (m, 2H, X4Hb, m + n), 2.36 (t, J = 7.4Hz, X3Ha, m), 2.31–2.25 (m, 2H, X4Ha, m + n), 2.25–2.15 (m, E1Hg + X3Ha(n)), 1.91–1.80 (m, 1Н, E1Hb(a)), 1.72–1.63 (m, 1Н, E1Hb(b)), 1.63–1.56 (m, 1Н, K2Hb(a)), 1.40–1.35 (m, 27H, tBu), 1.56–1.15 (m, 11Н, K2Hb(b) + X3Hb + X3Hd+ K2Hd + K2Hg + X3Hg, m + n).
13C NMR (100 MHz, DMSO-d6, δ): 173.93 (X4Cg), 172.26 (K2C(n)), 172.23 (K2C(m)), 172.22 (X3C(n)), 172.19 (X3C(m)), 171.95 (E1C), 171.47 (E1Cg), 170.76 (X4C(m)), 170.73 (X4C(n)), 157.18 (U(m)), 157.16 (U(n)), 141.20 (X9Cb(m)), 140.80 (X9Cb(n)), 133.45 (X9Ce(n)), 133.10 (X9Ce(m)), 130.63 (X9Cd(n)), 130.26 (X9Cd(m)), 127.24 (X9Ct(m)), 127.17 (X9Ck(n)), 126.88 (X9Ck(m)), 126.34 (X9Ct(n)), 126.08 (X9Cg(m)), 124.99 (X9Cg(n)), 80.59 (E1tBu), 80.42 (K2tBu(m)), 80.33 (K2tBu(n)), 79.77 (E1dtBu), 53.01 (K2Ca(n)), 52.88 (K2Ca(m)), 52.20 (E1Ca(m)), 52.18 (E1Ca(n)) 49.63 (X9Ca(n)), 47.11 (X9Ca(m)), 46.83 (K2Ce(m)), 45.20 (K2Ce(n)),38.49 (X3Ce(m)), 38.43 (X3Ce(n)), 32.34 (X3Ca(n)), 31.95 (X3Ca(m)), 31.83 (K2Cb), 30.93 (E1Cg), 30.06 (X4Ca), 29.25 (X4Cb), 29.13 (X3Cd(m)), 29.04 (X3Cd(n)), 27.75 (tBuE1), 27.69 (K2Cd(m)), 27.66 (tBuK2), 27.64 (tBuE1g+E1Cb), 26.72 (K2Cd(n)), 26.23 (X3Cg(m)), 26.15 (X3Cg(n)), 24.76 (X3Cb(m)), 24.63 (X3Cb(n)), 22.45 (K2Cg(n)), 22.27 (K2Cg(m)).
ESI-MS C41H65ClN4O11: m/z calcd. for [M+H+]+: 825.44, found: 825.45
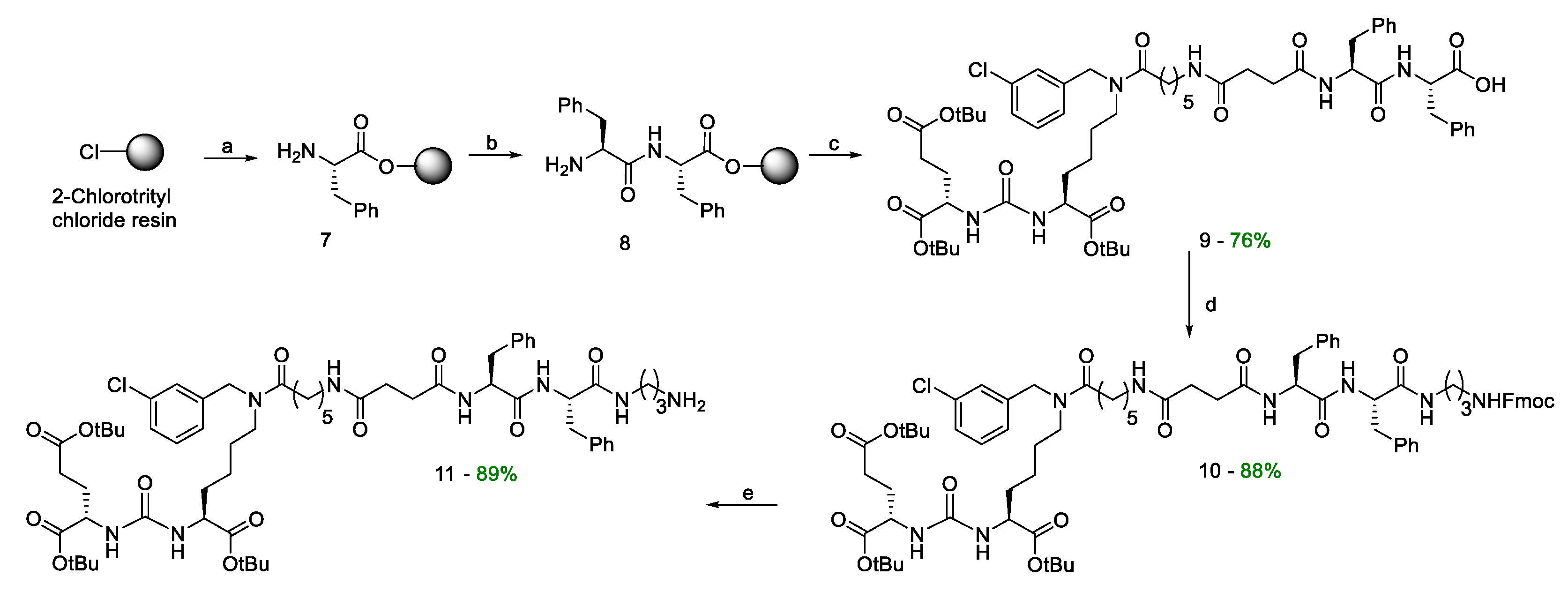
Compound 8. Activation of 2-CTC. The suspension of 2-СTC (1 eq.; 1g; 1.2–1.4 mmol/g; 100–200 mesh) in DCM (10 mL) was stirred for 10 min, after that the mixture was purged with Ar, then SOCl2 (3 eq.; 305 µL; 4.2 mmol) was added dropwise, and then DMF (16 µL; 5% V/V to SOCl2) was added and stirred at 40 °C for 4 h. After that the resin was filtered off and transferred to a polypropylene reactor, washed with DMF (3 * 10 mL, 1 min) and DCM (3 * 10 mL, 1 min).
The addition of FmocPhe(L)-OH. To the mixture of CTC-2 (1 eq.; 1g; 1.2–1.4 mmol/g; 100–200 mesh) in DMF (10 mL) FmocPhe(L)-OH (2 eq.; 1.085 g; 2.8 mmol) and DIPEA (10 eq.; 2.44 mL; 14 mmol) were added, and the mixture was stirred for 2 h. Then the resin was filtered off, washed with MeOH (3 * 10 mL, 5 min), DCM (3*10 mL, 1 min), DMF (3 * 10 mL, 1 min), and DCM (3 * 10 mL, 1 min).
Fmoc-deprotection. FmocPhe(L) on a 2-CTC resin (1 eq.) was washed with DMF (2*15 mL, 1min), then 4-methylpiperidine in DMF (20%/80% V/V, 15 mL) was added and the mixture was stirred for 15 min, then the resin was filtered off, washed with DMF (3 * 15 mL, 1 min), 4-methylpiperidine in DMF (20%/80% V/V, 15 mL) was added and the mixture stirred for 15 min. After the resin was filtered off, washed with DMF (3*15 mL, 1 min), and DCM (3 * 15 mL, 1 min).
The addition of FmocPhe(L)-OH. To the mixture of NH2-Phe(L) on a CTC-2 resin (1 eq.) in DMF (15 mL) FmocPhe(L)-OH (2 eq.; 1.085 g; 2.8 mmol), HOBt (0.5 eq.; 95 mg; 0.7 mmol), HBTU (2 eq.; 1.062 g; 2.8 mmol) and DIPEA (3 eq.; 0.73 mL; 4.2 mmol) were added and the mixture wasstirred for 2 h. Then the resin was filtered off, washed with DMF (3 * 15 mL, 1 min) and DCM (3 * 15 mL, 1 min).
Fmoc-deprotection. Fmoc-Phe(L)Phe(L) on a CTC-2 resin (1 eq.) was washed with DMF (2 * 15 mL, 1 min), then 4-methylpiperidineine in DMF (20%/80% V/V, 15 mL) was added and the mixture was stirred for 15 min, then the resin was filtered off, washed with DMF (3 * 15 mL, 1 min), 4-methylpiperidine in DMF (20%/80% V/V, 15 mL) was added and the mixture was stirred for 15 min. After the resin was filtered off, washed with DMF (3 * 15 mL, 1 min), DCM (3 * 15 mL, 1 min). Thus, the NH2-Phe(L)Phe(L) dipeptide was obtained on 2-CTC resin (~1.4 mmol).
Compound 9. To the NH2-Phe(L)Phe(L) dipeptide on a 2-CTC (1 eq; 0.54 mmol) in 7 ml DMF compound 6 (1.2 eq; 535 mg; 0.648 mmol), HOBt (0.5 eq; 37 mg; 0. 27 mmol), HBTU (2 eq; 410 mg; 1.08 mmol) and DIPEA (3 eq; 282 µl; 1.62 mmol) were added. The mixture was stirred for 4 hours. The solvent was removed by filtration and the resin was washed three times with DMF (7 ml), three times with DCM (7 ml), then dried from traces of solvents. DCM/TFA mixture (99.25%/0.75%, 11 ml) was added to the resin and left under stirring for 15 minutes, after which the resin was filtered off from the solution. The solvent was removed under reduced pressure and the residue was reevaporated with DCM and then purified by column chromatography (Puriflash on a PF-15C18AQ-F0025 column (15μ 40g): H2O(80%)/MeCN(20%) => H2O(0%)/MeCN(100%) for 15 minutes, after MeCN(100%) for 5 minutes Compound 9 was obtained as a white amorphous substance (460 mg, 76% yield).
1HNMR (400 MHz, DMSO-d6, δ): 12.71 (br.s., 1Н, F6COOH), 8.30-8.22 (br.d., 1Н, F5NHmn), 8.09-8.03 (br.d., 1Н, F6NHmn), 7.79 (t, J=5.4 Hz, m) & 7.75 (t, J=5.4 Hz, n) (1H, X3NHk), 7.40 (t, J=7.7Hz, X8Hdn), 7.36-7.29 (m, X8Hen+X8Hdm+X8Hem), 7.29-7.08 (m, 12H, F6He+F6Hd+X8Htmn+F5He+F6Hk+F5Hk+F5Hd +X8Hgmn), 6.37-6.18 (m, 2Н, K2NH+E1NH, m+n), 4.55 (s, X8Han), 4.53-4.44 (m, F6Ha+X8Ham), 4.44-4.36 (m, 1H, F5Ha), 4.08-3.99 (m, 1H, E1Ha), 3.99-3.90 (m, 1H, K2Ha), 3.22 (t, J=7.3 Hz, n) & 3.17 (t, J=7.3 Hz, m) (2H, K2He), 3.11-3.02 (m, 1Н, F6Hb(a)), 3.02-2.90 (m, 4Н, F6Hb(b)+X3Hemn+F5Hb(a)), 2.71-2.60 (m, 1Н, F5Hb(b)), 2.35 (t, J=7.4 Hz, X3Ham), 2.30-2.10 (m, X4Hbmn+E1Hg+X4Hamn+X3Han), 1.92-1.80 (m, 1Н, E1Hb(a)), 1.71-1.62 (m, 1Н, E1Hb(b)), 1.62-1.54 (m, 1Н, K2Hb(a)), 1.54-1.10 (m, 11Н, X3Hb+K2Hb(b)+X3Hd+K2Hd+K2Hg+X3Hg, m+n), 1.40-1.34 (m, 27H, tBu).
13C NMR (100 MHz, DMSO-d6, δ): 172.77 (F6C), 172.25 (K2C(n)), 172.21 (K2C(m)), 172.14 (X3C(n)), 172.12 (X3C(m)), 171.93 (E1C), 171.45 (E1Cd), 171.42 (X4Cg(mn)), 171.37 (F5C(mn)), 171.16 (X4C(m)), 171.15 (X4C(n)) 157.15 (U(m)), 157.14 (U(n)), 141.19 (X8Cb(m)), 140.78 (X8Cb(n)), 138.13 (F5Cg), 137.57 (F6Cg), 133.44 (X8Ck(n)), 133.09 (X8Ck(m)), 130.61 (X8Cd(n)), 130.25 (X8Cd(m)), 129.18 (F6Cd+F5Cd), 128.23 (F6Ce), 127.99 (F5Ce), 127.22 (X8Ct(m)), 127.16 (X8Ce(n)), 126.87 (X8Ce(m)), 126.45 (F6Ck), 126.32 (X8Ct(n)), 126.18 (F5Ck), 126.07 (X8Cg(m)), 124.96 (X8Cg(n)), 80.58 (E1tBu), 80.41 (K2tBu(m)), 80.32 (K2tBu(n)), 79.76 (E1dtBu), 53.67 (F5Ca), 53.63 (F6Ca), 53.00 (K2Ca(n)), 52.87 (K2Ca(m)), 52.19 (E1Ca), 49.61 (X8Ca(n)), 47.10 (X8Ca(m)), 46.80 (K2Ce(m)), 45.20 (K2Ce(n)), 38.53 (X3Ce(m)), 38.47 (X3Ce(n)), 37.34 (F5Cb), 36.61 (F6Cb), 32.33 (X3Ca(n)), 31.95 (X3Ca(m)), 31.83 (K2Cb), 30.93 (E1Cg), 30.82 (X4Ca), 30.75 (X4Cb), 29.12 (X3Cd(m)), 29.02 (X3Cd(n)), 27.75 (tBuE1), 27.66 (tBuK2+K2Cd(m)), 27.63 (tBuE1d+E1Cb), 26.72 (K2Cd(n)), 26.29 (X3Cg(m)), 26.19 (X3Cg(n)), 24.76 (X3Cb(m)), 24.62 (X3Cb(n)), 22.44 (K2Cg(n)) 22.27 (K2Cg(m)).
ESI-MS C59H83ClN6O13: m/z calcd. for [M+H+]+: 1119.58, found: 1119.45.
HRMS (m/z, ESI): calcd. for C59H83ClN6O13 - [M+H+]+: 1119.5779, found: 1119.5746.
Compound 10. To a solution of compound 9 (1 eq.; 300 mg; 0.268 mmol) in 10 ml of DMF TFA*NH2(CH2)3NHFmoc (1.1 eq.; 121 mg; 0.294 mmol), DIPEA (2.5 eq.; 117 μl; 0.67 mmol) in 10 ml DMF, followed by HOBt (1 eq.; 36 mg; 0.268 mmol) and HBTU (1.5 eq.; 152 mg; 0.402 mmol) were added. The mixture was stirred for 12 hours under an inert atmosphere. The solvent was then removed under reduced pressure and the residue was reevaporated twice with DCM, dissolved in 30 ml of DCM and the extraction was carried out: 1) H2O (2*30 ml), 2) saturated NaCl solution (2*30 ml). Then the organic fraction was dried over Na2SO4, the solvent was removed, and the residue was purified using a column chromatography method (Puriflash on a column (15μ 40g)); DCM(100%)/MeOH(0%) => DCM(90%)/MeOH(10%) for 30 minutes, after MeOH (100%) for 5 minutes. Compound 10 was obtained as a pale-yellow amorphous substance (330 mg, 88% yield).
1HNMR (400 MHz, DMSO-d6, δ): 8.35-8.25 (br.d., 1Н, F5NHmn), 8.22-8.13 (m, 1Н, F6NHmn), 7.93 (t, J=5.4 Hz, X3NHkm), 7.91-7.84 (m, X3NHkn+FmocHt), 7.67 (d, J = 7.5 Hz, 2H, FmocHd), 7.60-7.52 (m, 1H, X7NH), 7.45-7.35 (m, FmocHk +X8Hdn), 7.35-7.08 (m, X8Hen+FmocHe+X8Hdm +X8Hem+X7NHd+F6He+F6Hd+X8Htmn+F5He+F6Hk +F5Hk+F5Hd +X8Hgmn), 6.36-6.21 (m, 2Н, K2NH+E1NH, m+n), 4.54 (s, n) & 4.47 (s, m) (2H, X8Ha, m+n), 4.44-4.36 (m, 1H, F6Ha), 4.36-4.25 (m, 3H, F5Ha+FmocHa), 4.20 (t, J = 6.9 Hz, 1H, FmocHb), 4.08-3.99 (m, 1H, E1Ha), 3.99-3.90 (m, 1H, K2Hamn), 3.21 (t, J=7.3 Hz, n) & 3.16 (t, J=7.3 Hz, m) (2H, K2He, m+n), 3.11-2.84 (m, 9Н, F6Hb(a)+X7Hg+X7Ha+X3He(mn)+F6Hb(b)+F5Hb(a)), 2.70-2.60 (m, 1Н, F5Hb(b)), 2.40-2.10 (m, 8H, X3Ham+X4Hbmn+E1Hg+X4Hamn+X3Han), 1.92-1.80 (m, 1Н, E1Hb(a)), 1.72-1.62 (m, 1Н, E1Hb(b)), 1.62-1.55 (m, 1Н, K2Hb(a)), 1.54-1.10 (m, 13Н, X7Hb+X3Hb+K2Hb(b)+X3Hd +K2Hd+K2Hg +X3Hg, m+n), 1.40-1.34 (m, 27H, tBu).
13C NMR (100 MHz, DMSO-d6, δ): 172.79 (X4Cg(n)), 172.76 (X4Cg(m)), 172.24 (K2C(n)), 172.20 (K2C(m)), 172.15 (X3C(n)), 172.13 (X3C(m)), 171.92 (E1C), 171.57 (X4C(mn)), 171.45 (E1Cd), 171.11 (F5C), 170.67 (F6C) 157.15 (U(mn)), 156.12 (C(O)Fmoc), 143.94 (FmocCg), 141.16 (X8Cb(m)), 140.77 (X8Cb(n)+FmocCte), 138.12 (F6Cg), 138.02 (F5Cg), 133.44 (X8Ck(n)), 133.09 (X8Ck(m)), 130.59 (X8Cd(n)), 130.23 (X8Cd(m)), 129.04 (F6Cd+F5Cd), 128.16 (F6Ce), 128.06 (F5Ce), 127.63 (FmocCk), 127.21 (X8Ct(m)), 127.15 (X8Ce(n)), 127.09 (FmocCt), 126.86 (X8Ce(m)), 126.29 (F6Ck+X8Ct(n)), 126.24 (F5Ck), 126.05 (X8Cg(m)), 125.17 (FmocCe), 124.95 (X8Cg(n)), 120.13 (FmocCd), 80.59 (E1tBu), 80.42 (K2tBu(m)), 80.33 (K2tBu(n)), 79.77 (E1dtBu), 65.32 (FmocCa), 54.94 (F5Ca), 54.40 (F6Ca), 53.01 (K2Ca(n)), 52.87 (K2Ca(m)), 52.20 (E1Ca), 49.62 (X8Ca(n)), 47.11 (X8Ca(m)), 46.80 (FmocCb+K2Ce(m)), 45.19 (K2Ce(n)), 38.65 (X3Ce(m)), 38.60 (X3Ce(n)), 37.90 (X7Cg), 37.11 (F5Cb), 36.82 (F6Cb), 36.39 (X7Ca), 32.32 (X3Ca(n)), 31.95 (X3Ca(m)), 31.82 (K2Cb), 30.93 (E1Cg), 30.69 (X4Ca), 30.58 (X4Cb), 29.20 (X7Cb), 29.05 (X3Cd(m)), 28.96 (X3Cd(n)), 27.74 (tBuE1), 27.65 (tBuK2+K2Cd(m)), 27.62 (tBuE1d+E1Cb), 26.69 (K2Cd(n)), 26.31 (X3Cg(m)), 26.22 (X3Cg(n)), 24.73 (X3Cb(m)), 24.60 (X3Cb(n)), 22.43 (K2Cg(n)) 22.25 (K2Cg(m)).
ESI-MS C77H101ClN8O14: m/z calcd. for [M+H+]+: 1397.72, found: 1397.65
HRMS (m/z, ESI): calcd. for C77H101ClN8O14 - [M+H]+ 1397.7199, found: 1397.7210
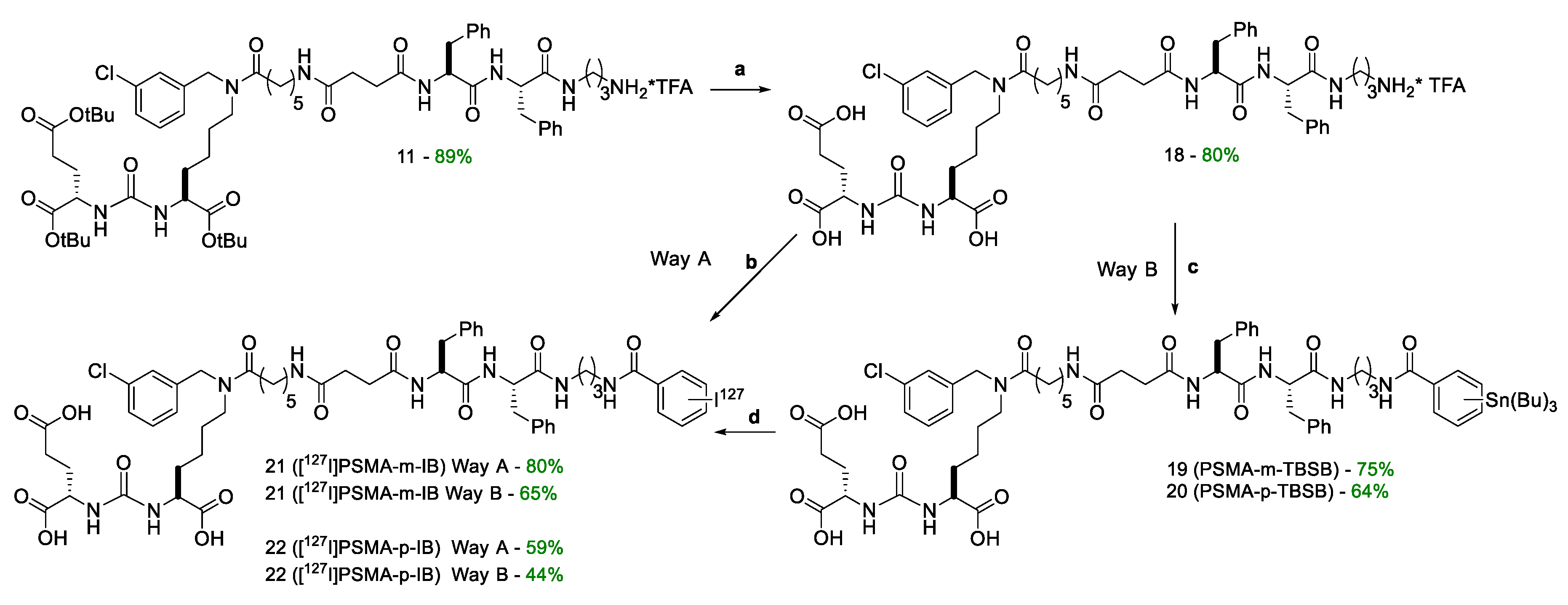
Compound 11. Compound 10 (1 eq; 200 mg; 0.143 mmol) was dissolved in Et2NH/DMF (20 eq Et2NH, 10 ml DMF) mixture and stirred for 20 minutes, then the solvent was removed under reduced pressure and the residue was reevaporated with DCM three times. The product was precipitated with Et2O and washed twice with Et2O (2 ml). The residue was purified by reverse phase column chromatography (Puriflash PF-15C18AQ-F0025 (15μ 35g): H2O*TFA(0.1%) (80%)/MeCN(20%) => H2O*TFA(0.1%) (0 %)/MeCN(100%) for 30 minutes after MeCN (100%) for 5 minutes. Compound 11 was obtained as a *TFA salt as a white amorphous solid (160 mg, 89% yield).
1HNMR (400 MHz, DMSO-d6, δ): 8.37 (d, J=7.3 Hz, 1Н, F5NHmn), 8.23-8.15 (br.d, 1Н, F6NHmn), 8.00 (t, J=5.4 Hz, m) & 7.97 (t, J=5.4 Hz, n) ) (1H, X3NHk, m+n), 7.82 (br.s, 3H, X7NHd), 7.77-7.69 (m, 1H, X7NH), 7.42-7.09 (m, 14H, X8Hdn+X8Hen +X8Hdm+X8Hem+F6He+F6Hd +X8Htmn+F5He+F6Hk+F5Hk+F5Hd+X8Hgmn), 6.39-6.23 (m, 2Н, K2NHm+ K2NHn+E1NHm +E1NHn), 4.55 (s, n) & 4.47 (s, m) (2H, X8Ha, m+n), 4.43-4.33 (m, 1H, F6Ha), 4.33-4.23 (m, 1H, F5Ha), 4.08-3.99 (m, 1H, E1Ha), 3.99-3.90 (m, 1H, K2Ham+K2Han), 3.26-3.11 (m, 3H, K2Hemn+X7Hg(a)), 3.11-2.85 (m, 6Н, F6Hb(a)+X7Hg(b)+X3He(mn)+F6Hb(b)+F5Hb(a)), 2.78-2.61 (m, 3Н, X7Ha+F5Hb(b)), 2.40-2.10 (m, 8H, X3Ham+X4Hbmn+E1Hg +X4Hamn+X3Han), 1.92-1.80 (m, 1Н, E1Hb(a)), 1.73-1.61 (m, 3Н, X7Hb+E1Hb(b)), 1.62-1.55 (m, 1Н, K2Hb(a)), 1.54-1.10 (m, 11Н, X3Hb+K2Hb(b)+X3Hd+K2Hd +K2Hg+X3Hg, m+n), 1.40-1.34 (m, 27H, tBu).
13C NMR (100 MHz, DMSO-d6, δ): 172.91 (X4Cg(n)), 172.88 (X4Cg(m)), 172.24 (K2C(n)), 172.20 (K2C(m)), 172.15 (X3C(n)), 172.13 (X3C(m)), 171.92 (E1C), 171.61 (X4C(mn)), 171.46 (E1Cd), 171.23 (F5C), 171.09 (F6C), 157.18 (U(m)), 157.16 (U(n)), 141.18 (X8Cb(m)), 140.78 (X8Cb(n)), 138.08 (F6Cg), 138.02 (F5Cg), 133.41 (X8Ck(n)), 133.07 (X8Ck(m)), 130.61 (X8Cd(n)), 130.25 (X8Cd(m)), 129.05 (F6Cd+F5Cd), 128.20 (F6Ce), 128.07 (F5Ce), 127.20 (X8Ct(m)), 127.15 (X8Ce(n)), 126.86 (X8Ce(m)), 126.34 (F6Ck), 126.31 (X8Ct(n)), 126.25 (F5Ck), 126.06 (X8Cg(m)), 124.97 (X8Cg(n)), 80.55 (E1tBu), 80.38 (K2tBu(m)), 80.30 (K2tBu(n)), 79.77 (E1dtBu), 55.10 (F5Ca), 54.53 (F6Ca), 53.01 (K2Ca(n)), 52.88 (K2Ca(m)), 52.19 (E1Ca), 49.62 (X8Ca(n)), 47.10 (X8Ca(m)), 46.81 (K2Ce(m)), 45.22 (K2Ce(n)), 38.64 (X3Ce(m)), 38.58 (X3Ce(n)), 36.92 (F5Cb), 36.79 (F6Cb), 36.58 (X7Cg), 35.81 (X7Ca), 32.33 (X3Ca(n)), 31.95 (X3Ca(m)), 31.79 (K2Cb), 30.92 (E1Cg), 30.66 (X4Ca), 30.57 (X4Cb), 29.05 (X3Cd(m)), 28.97 (X3Cd(n)), 27.75 (tBuE1), 27.66 (tBuK2+K2Cd(m)), 27.63 (tBuE1d), 27.57 (E1Cb), 27.09 (X7Cb), 26.70 (K2Cd(n)), 26.32 (X3Cg(m)), 26.23 (X3Cg(n)), 24.74 (X3Cb(m)), 24.60 (X3Cb(n)), 22.45 (K2Cg(n)) 22.26 (K2Cg(m)).
ESI-MS C62H91ClN8O12: m/z calcd. for [M+H+]+: 1175.65, found: 1175.6
HRMS (m/z, ESI): calcd. for C62H91ClN8O12 - [M+H]+ 1175,6518, found: 1175.6520
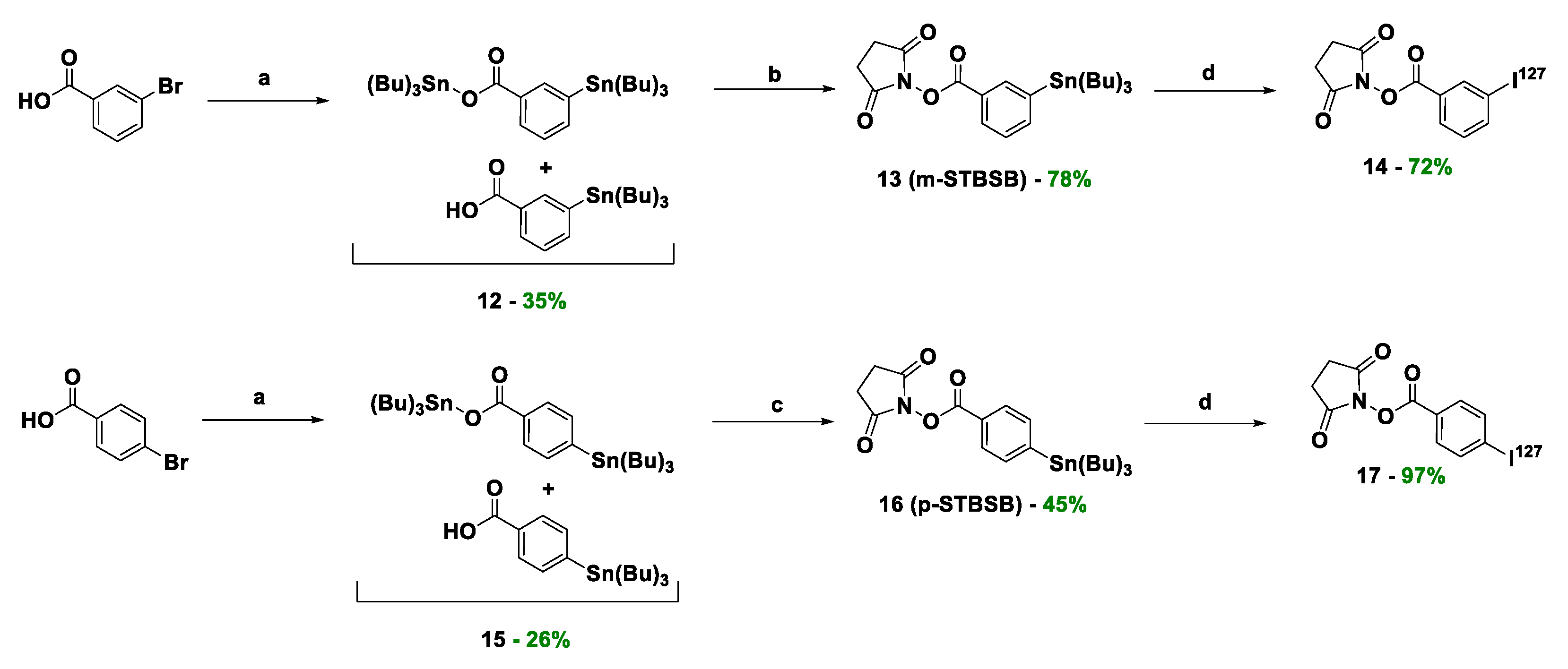
Compound 13. Compound 12 (1 eq; 1800 mg; 4.376 mmol, calculated assuming that 12 is only a mono-stanylated derivative) was dissolved in 40 ml dry DCM. Then NHS (1.2 eq; 604 mg; 5.25 mmol), DMAP (0.1 eq; 53 mg; 0.438 mmol) and EDC*HCl (1.1 eq; 924 mg; 4.82 mmol) in DMF (4 ml) were added dropwise. The mixture was stirred overnight. After the reaction proceeded, the solvent was removed on a rotary evaporator, the residue was dissolved in DCM (100 ml) and transferred to a separating funnel, washed twice with H2O and then with saturated NaCl solution. The organic layer was dried over Na2SO4. The solvent was removed on a rotary evaporator and the residue was purified using a column chromatography (Puriflash on a column (40μ-60 μ 120g)); P.E. (97%) / E.A. (3%) for 7 minutes, then P.E. (97 %)/E.A.(3%) => P.E.(60%)/E.A.(40%) for 40 minutes, then P.E.(60%)/E.A.( 40%) => P.E.(0%)/E.A.(100%) for 5 minutes, then E.A.(100%) for 10 minutes. As a result, a fraction was isolated, which is a pale-yellow transparent oily substance (m = 1729 mg, 78%).
1HNMR (400 MHz, DMSO-d6, δ): 8.18-8.06 (m., 1Н, 2), 8.05-7.97 (m, 1H, 4), 7.96-7.82 (m, 1H, 6), 7.65-7.55 (m, 1H, 5), 2.89 (s, 4H, 9), 1.63-1.39 (m, 6H, 11), 1.35-1.21 (m, 6H, 12), 1.20-1.00 (m, 6H, 10), 0.84 (t, J = 7.3 Hz, 9H, 13)
13C NMR (100 MHz, DMSO-d6, δ): 170.33 (8), 162.14 (7), 143.46 (6), 143.24 (1), 137.05 (2), 129.64 (3), 128.84 (4), 124.07 (5), 28.52 (11), 26.67 (12), 25.57 (9), 13.48 (10), 9.36 (13).
ESI-MS C23H35NO4118Sn: m/z calcd. for [M+Na+]+: 530,15,found: 530.10
HRMS (m/z, ESI): calcd for C23H35NO4120Sn - [M+Na+]+ 532,1480, found: 532,1487
Compound 14. I2 (1 eq; 97.5 mg; 0.384 mmol) was dissolved in 0.1N NaOH (2300 µl = V1), AcOH (3%) in CHCl3 (2300 µl = V2) was added (V1 = V2), then tert-butyl hydroperoxide (TBHP) in CHCl3 (the solution is prepared in advance by adding 1800 µl of 70% TBHP in water to 11209 µl of CHCl3, after which Na2SO4 is added to the prepared mixture to bind water) (11513 µl), then 13 (1 eq; 195 mg; 0.383 mmol) in CHCl3 (3900 µl) were added. The mixture was stirred for 30 minutes, the solvent was then removed under reduced pressure. The product was precipitated with Н2O and washed twice with Н2O (2 ml) and twice with P.E. (2 ml). The residue was purified by reverse phase column chromatography (Puriflash PF-15C18AQ-F0025 (15μ 40g): H2O(90%)/MeCN(10%) => H2O(0 %)/MeCN(100%) for 30 minutes after MeCN (100%) for 15 minutes. As a result, a fraction was isolated, which is the target substance in the form of a white powder (m = 95 mg, 72%).
1H NMR (400 MHz, DMSO-d6, δ): 8.34 (t, J = 1.7 Hz, 1H, 2), 8.21 (ddd, J = 7.9, 1.7, 1.0 Hz, 1H, 6), 8.10 (ddd, J = 7.9, 1.7, 1.0 Hz, 1H, 4), 7.45 (t, J = 7.9 Hz, 1H, 5), 2.90 (s, 4H, 9).
13C NMR (100 MHz, DMSO-d6, δ): 170.22 (8), 160.60 (7), 144.11 (6), 137.87 (2), 131.62 (3), 129.30 (5), 126.41 (4), 95.53 (1), 25.57 (9).
Compound 16. Compound 15 (1 eq; 1380 mg; 3.35 mmol, calculated assuming that 15 is only a mono-stanylated derivative) was dissolved in 15 ml dry THF. Then NHS (1.2 eq; 463 mg; 4.02 mmol), and DCC (1 eq; 691 mg; 3.35 mmol) in THF (15 mL) were added dropwise. The mixture was stirred overnight. The precipitated dicyclohexylurea was removed by filtration through a fritted funnel. The precipitate was washed with 2×6 ml of THF, then the solvent was removed on a rotary evaporator. The residue was purified using a column chromatography (Puriflash on a column (40μ-60 μ 120g)); P.E. (97%) / E.A. (3%) for 7 minutes, then P.E. (97 %)/E.A.(3%) => P.E.(60%)/E.A.(40%) for 40 minutes, then P.E.(60%)/E.A.( 40%) => P.E.(0%)/E.A.(100%) for 5 minutes, then E.A.(100%) for 10 minutes. As a result, a fraction was isolated, which is a pale-yellow transparent oily substance (m = 772 mg, 45%).
1HNMR (400 MHz, DMSO-d6, δ): 8.05-7.93 (m., 1Н, 3), 7.82-7.66 (m, 1H, 4), 2.89 (s, 4H, 7), 1.65-1.39 (m, 6H, 9), 1.36-1.21 (m, 6H, 10), 1.20-1.00 (m, 6H, 8), 0.84 (t, J = 7.3 Hz, 9H, 11)
13C NMR (100 MHz, DMSO-d6, δ): 170.33 (6), 162.10 (5), 153.06 (1), 137.16 (2), 128.53 (3), 124.01 (4), 28.52 (9), 26.67 (10), 25.56 (7), 13.51(8), 9.37 (11).
HRMS (m/z, ESI): calcd for C23H35NO4120Sn - [M+Na+]+ 532,1480, found: 532,1485
Compound 17. I2 (1 eq; 75 mg; 0.295 mmol) was dissolved in 0.1N NaOH (1770 µl = V1), AcOH (3%) in CHCl3 (1770 µl = V2) was added (V1 = V2), then tert-butyl hydroperoxide (TBHP) in CHCl3 (the solution is prepared in advance by adding 1264 µl of 70% TBHP in water to 7872 µl of CHCl3, after which Na2SO4 is added to the prepared mixture to bind water) (8856 µl), then 16 (1 eq; 150 mg; 0.295 mmol) in CHCl3 (3000 µl) were added. The mixture was stirred for 30 minutes, the solvent was removed under reduced pressure. The product was precipitated with Н2O and washed twice with Н2O (2 ml) and twice with P.E./E.A. (80/20) (2 ml). As a result, a fraction was isolated, which is the target substance in the form of a white powder (m = 98.5 mg, 97%).
1HNMR (400 MHz, DMSO-d6, δ): 8.06 (d, J = 8.5 Hz, 2H, 3), 7.83 (d, J = 8.5 Hz, 1H, 2), 2.89 (s, 4H, 7).
13C NMR (100 MHz, DMSO-d6, δ): 170.28 (6), 161.69 (5), 138.65 (2), 131.39 (3), 123.88 (4), 105.01 (1), 25.58 (7).
Compound 18. Compound 11 (1 eq.; 228.5 mg; 177.1 mmol) was dissolved in the mixture of DCM/TFA/TIPS/H2O (46.25%/46.25%/2.5%/5%; V/V respectively, 8 mL). The mixture was stirred for 3 h, then the solvent was removed under reduced pressure and the residue was re-evaporated with DCM three times. The product was precipitated with Et2O and washed twice with Et2O (1 mL). After the compound was purified by column chromatography (Puriflash on the column PF-15C18HP-F0012(15μ 20g), eluent: H2O(90%)/MeCN (10%) => H2O(0%)/MeCN (100%) for 30 min, after MeCN (100%) for 5 min. The individual compound 18 was obtained as white amorphous solid (159 mg, yield 80%).
1HNMR (400 MHz, DMSO-d6, δ): 8.36 (d, J=7.3 Hz, 1Н, F5NHmn), 8.24-8.16 (m, 1Н, F6NHm+F6NHn), 7.96 (t, J=5.4 Hz, m) & 7.93 (t, J=5.4 Hz, n) (1H, X3NHk, m+n), 7.75-7.58 (m, 4H, X7NH+X7NH3+d), ), 7.42-7.09 (m, 14H, X8Hdn+X8Hen+X8Hdm+ X8Hem+F6He+F6Hd+X8Htmn+F5He+F6Hk+F5Hk+F5Hd+X8Hgmn), 6.39-6.25 (m, 2Н, K2NHm+K2NHn +E1NHm+E1NHn), 4.55 (s, n) & 4.47 (s, m) (2H, X8Ha, m+n), 4.42-4.33 (m, 1H, F6Ha), 4.33-4.23 (m, 1H, F5Ha), 4.14-3.98 (m, 2H, E1Ha+K2Ham+K2Han), 3.25-3.10 (m, 3H, K2Hemn+X7Hg(a)), 3.10-2.85 (m, 6Н, F6Hb(a) +X7Hg(b)+X3He(mn)+F6Hb(b)+F5Hb(a)), 2.78-2.58 (m, 3Н, X7Ha+F5Hb(b)), 2.40-2.10 (m, 8H, X3Ham+X4Hbmn +E1Hg +X4Hamn+X3Han), 1.97-1.85 (m, 1Н, E1Hb(a)), 1.76-1.56 (m, 4Н, E1Hb(b)+X7Hb +K2Hb(a)), 1.56-1.11 (m, 11Н, X3Hb+K2Hb(b)+X3Hd+ K2Hd+K2Hg+X3Hg, m+n).
13C NMR (100 MHz, DMSO-d6, δ): 174.61 (K2C(n)), 174.57 (K2C(m)), 174.27 (E1C(mn)), 173.84 (E1Cd), 173.06 (X4Cg(n)), 173.03 (X4Cg(m)), 172.23 (X3C), 171.70 (X4C(mn)), 171.33 (F5C), 171.24 (F6C), 159.03 (C(O)TFA), 158.66 (C(O)TFA), 158.30 (C(O)TFA), 157.92 (C(O)TFA), 157.36 (U), 141.27 (X8Cb(m)), 140.87 (X8Cb(n)), 138.09 (F6Cg), 138.03 (F5Cg), 133.46 (X8Ck(n)), 133.11 (X8Ck(m)), 130.66 (X8Cd(n)), 130.30 (X8Cd(m)), 129.07 (F6Cd+F5Cd), 128.28 (F6Ce), 128.16 (F5Ce), 127.24 (X8Ct(m)), 127.19 (X8Ce(n)), 126.90 (X8Ce(m)), 126.44 (F6Ck), 126.34 (X8Ct(n))+F5Ck), 126.14 (X8Cg(m)), 125.02 (X8Cg(n)), 117.00 (CF3), 114.17 (CF3), 55.15 (F5Ca), 54.58 (F6Ca), 52.33 (K2Ca(n)), 52.20 (K2Ca(m)), 51.72 (E1Ca), 49.67 (X8Ca(n)), 47.21 (X8Ca(m)), 46.93 (K2Ce(m)), 45.39 (K2Ce(n)), 38.72 (X3Ce(m)), 38.65 (X3Ce(n)), 36.94 (F5Cb), 36.82 (F6Cb), 36.72 (X7Cg), 35.79 (X7Ca), 32.34 (X3Ca(n)), 31.93 (X3Ca(m)), 31.85 (K2Cb), 30.72 (X4Ca), 30.59 (X4Cb), 29.97 (E1Cg), 29.11 (X3Cd(m)), 29.01 (X3Cd(n)), 27.85 (K2Cd(m)), 27.59 (E1Cb), 27.23 (X7Cb), 26.80 (K2Cd(n)), 26.34 (X3Cg(m)), 26.26 (X3Cg(n)), 24.77 (X3Cb(m)), 24.63 (X3Cb(n)), 22.56 (K2Cg(n)) 22.40 (K2Cg(m)).
ESI-MS C50H67СlN8O12: m/z calcd. for [M+H+]+: 1007.46,found: 1007.55
HRMS (m/z, ESI): calcd. for C50H67СlN8O12 - [M+H]+ 1007.4640, found: 1007.4622
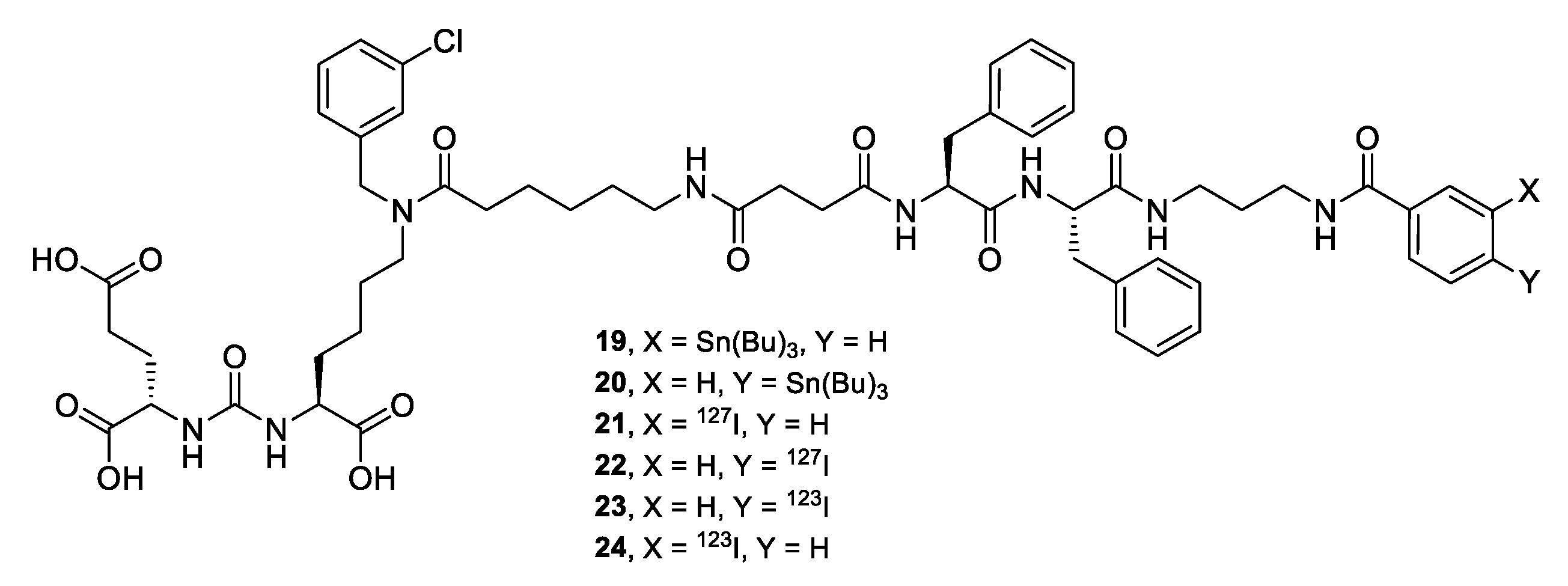
Compound 19. Compound 18 (1 eq.; 30 mg; 26.75 μmol) and DIPEA (6 eq.; 28 μl; 160.5 μmol) were dissolved in DMF (2 mL). Compound 13 (1 eq.; 14 mg; 26.75 μmol) was added to the obtained mixture and the system was purged with argon. The mixture was stirred for 6 h and the solvent was evaporated under reduced pressure. The residue was then purified by column chromatography (Puriflash on a column of PF-15C18HP-F0012(15μ 20g), eluent: H2O(90%)/MeCN(10%) => H2O(0%)/MeCN(100%) for 20 min after MeCN (100%) for 5 min. Compound 19 was obtained as white powder (28 mg, yield 75%).
1HNMR (400 MHz, DMSO-d6, δ): 13.30-11.30 (br.s, 3H, COOH), 8.45-8.36 (m, 1H, X7NHδ), 8.33-8.25 (m, 1Н, F5NHmn), 8.24-8.14 (m, 1Н, F6NHm+F6NHn), 7.97-7.83 (m, 2H, X3NHζmn+2), 7.78-7.70 (m, 1H, 6), 7.69-7.59 (m, 1H, X7NH), 7.59-7.50 (m, 1H, 4), 7.45-7.08 (m, 15H, 5+X8Hδn+X8Hεn+X8Hδm+X8Hεm+F6Hε+F6Hδ+X8Hηmn+F5Hε+F6Hζ+F5Hζ+F5Hδ +X8Hγmn), 6.38-6.24 (m, 2Н, K2NHm+K2NHn +E1NHm+E1NHn), 4.54 (s, n) & 4.47 (s, m) (2H, X8Hα, m+n), 4.44-4.37 (m, 1H, F6Hα), 4.37-4.27 (m, 1H, F5Hα), 4.14-3.98 (m, 2H, E1Hα+K2Hαm+K2Hαn), 3.26-2.84 (m, 11H, K2Hεmn+X7Hγ(a))+F6Hβ(a) +X7Hγ(b) +X3Hε(mn) +F6Hβ(b)+X7Hα+F5Hβ(a)), 2.70-2.60 (m, 1Н, F5Hβ(b)), 2.40-2.10 (m, 8H, X3Hαm+X4Hβmn+E1Hγ +X4Hαmn+X3Hαn), 1.97-1.85 (m, 1Н, E1Hβ(a)), 1.76-1.67 (m, 1Н, E1Hβ(b)), 1.67-1.13 (m, 26Н, X7Hβ+K2Hβ(a)+8+X3Hβ +K2Hβ(b)+X3Hδ+9+ K2Hδ+K2Hγ+X3Hγ, m+n), 1.13-0.95 (m, 6H, 10), 0.83 (t, J = 7.1 Hz, 9H, 11).
13C NMR (100 MHz, DMSO-d6, δ): 174.54 (K2C(n)), 174.51 (K2C(m)), 174.22 (E1C(mn)), 173.80 (E1Cδ), 172.74 (X4Cγ(n)), 172.68 (X4Cγ(m)), 172.12 (X3C), 171.50 (X4C(mn)), 171.12 (F5C), 170.78 (F6C), 166.60 (7), 157.26 (U), 141.50 (6), 141.22 (X8Cβ(m)), 140.80 (X8Cβ(n)), 138.90 (1), 138.10 (F6Cγ), 138.00 (F5Cγ), 135.53 (2), 134.73 (3), 133.92 (5), 133.40 (X8Cζ(n)), 133.04 (X8Cζ(m)), 130.59 (X8Cδ(n)), 130.23 (X8Cδ(m)), 129.04 (F6Cδ+F5Cδ), 128.16 (F6Cε), 128.04 (F5Cε), 127.75 (4), 127.20 (X8Cη(m)), 127.12 (X8Cε(n)), 126.83 (X8Cε(m)), 126.29 (F6Cζ+X8Cη(n)), 126.22 (F5Cζ), 126.08 (X8Cγ(m)), 124.95 (X8Cγ(n)), 54.86 (F5Cα), 54.41 (F6Cα), 52.25 (K2Cα(n)), 52.14 (K2Cα(m)), 51.68 (E1Cα), 49.61 (X8Cα(n)), 47.13 (X8Cα(m)), 46.85 (K2Cε(m)), 45.31 (K2Cε(n)), 38.64 (X3Cε(m)), 38.57 (X3Cε(n)), 37.09 (F5Cβ), 36.86 (F6Cβ), 36.69 (X7Cγ), 35.48 (X7Cα), 32.28 (X3Cα(n)), 31.86 (X3Cα(m)), 31.81 (K2Cβ), 30.67 (X4Cα), 30.56 (X4Cβ), 29.97 (E1Cγ), 29.07 (X3Cδ(m)+X7Cβ), 28.95 (X3Cδ(n)), 28.60 (9), 27.80 (K2Cδ(m)), 27.60 (E1Cβ), 26.70 (K2Cδ(n)+10), 26.28 (X3Cγ(m)), 26.21 (X3Cγ(n)), 24.72 (X3Cβ(m)), 24.57 (X3Cβ(n)), 22.53 (K2Cγ(n)) 22.34 (K2Cγ(m)), 13.58 (8), 9.20 (11).
HRMS (m/z, ESI): calcd. for C69H97ClN8O13Sn - [M+H]+ 1401,5958, found: 1401,5990.
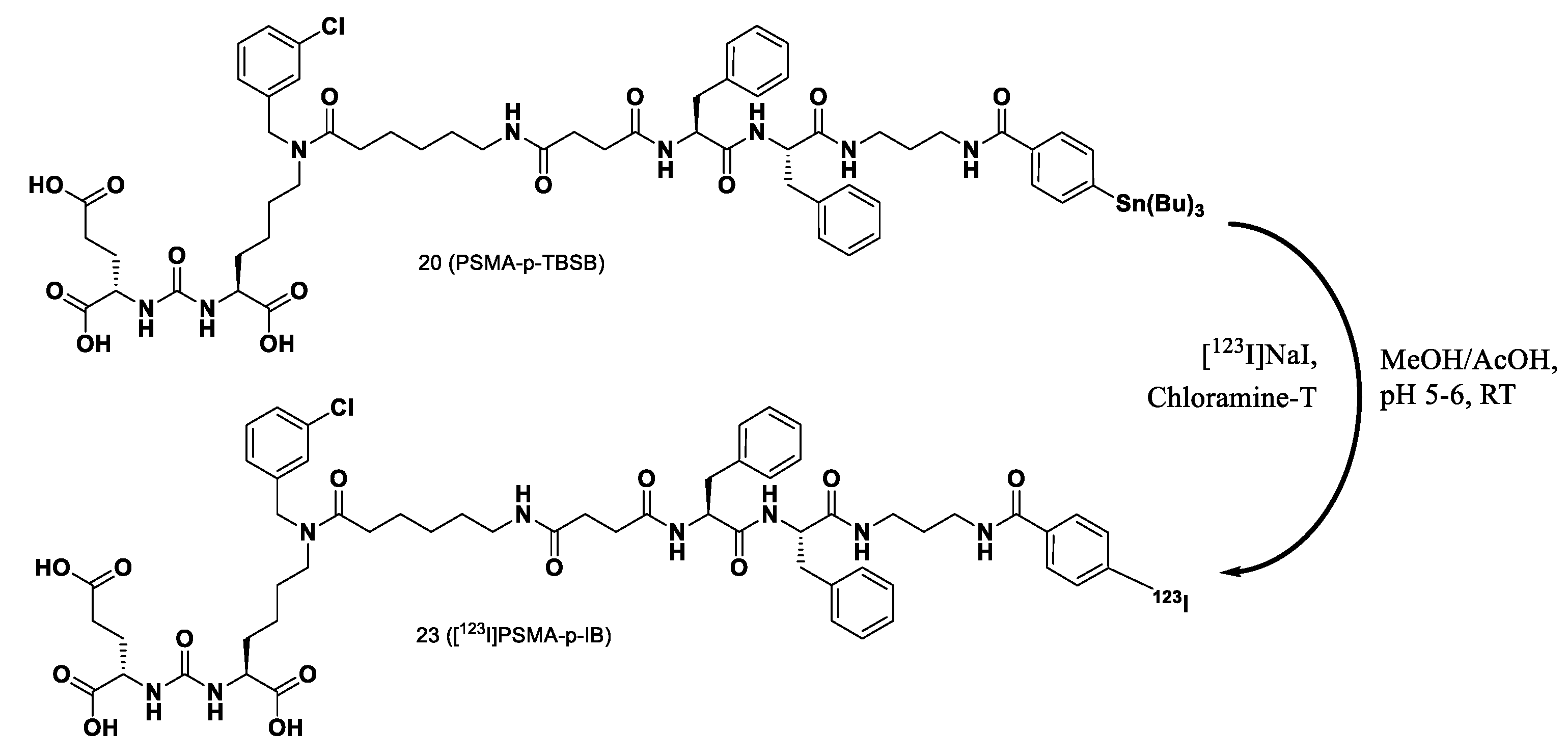
Compound 20. Compound 18 (1 eq.; 38 mg; 34 μmol) and DIPEA (6 eq.; 30 μl; 174.36 μmol) were dissolved in DMF (2 mL). Compound 16 (1 eq.; 17.3 mg; 34 μmol) was added to the obtained mixture, the system was purged with argon and the mixture was stirred for 6 h. The solvent was evaporated under reduced pressure and the residue was then purified by column chromatography (Puriflash on a column of PF-15C18HP-F0012(15μ 20g), eluent: H2O(90%)/MeCN(10%) => H2O(0%)/MeCN(100%) for 20 min after MeCN (100%) for 5 min. Compound 19 was obtained as white powder (30.5 mg, yield 64%).
1HNMR (400 MHz, DMSO-d6, δ): 12.95-11.50 (br.s, 3H, COOH), 8.44-8.34 (m, 1H, X7NHδ), 8.34-8.26 (m, 1Н, F5NHmn), 8.25-8.15 (m, 1Н, F6NHm+F6NHn), 7.92 (t, J=5.4 Hz, m) & 7.89 (t, J=5.4 Hz, n) (1H, X3NHζ, m+n), 7.75 (d, J=7.5 Hz, 2H, 3), 7.70-7.60 (m, 1H, X7NH), 7.51 (d, J=7.5 Hz, 2H, 2), 7.41-7.07 (m, 14H, X8Hδn+X8Hεn+X8Hδm+X8Hεm+F6Hε+F6Hδ+X8Hηmn +F5Hε+F6Hζ+F5Hζ +F5Hδ+X8Hγmn), 6.39-6.22 (m, 2Н, K2NHm+K2NHn +E1NHm+E1NHn), 4.54 (s, n) & 4.46 (s, m) (2H, X8Hα, m+n), 4.44-4.37 (m, 1H, F6Hα), 4.37-4.27 (m, 1H, F5Hα), 4.14-3.98 (m, 2H, E1Hα +K2Hαm+K2Hαn), 3.25-2.84 (m, 11H, K2Hεmn+X7Hγ(a))+F6Hβ(a) +X7Hγ(b) +X3Hε(mn) +F6Hβ(b)+X7Hα+ F5Hβ(a)), 2.70-2.60 (m, 1Н, F5Hβ(b)), 2.40-2.10 (m, 8H, X3Hαm+X4Hβmn+E1Hγ +X4Hαmn+X3Hαn), 1.96-1.84 (m, 1Н, E1Hβ(a)), 1.77-1.67 (m, 1Н, E1Hβ(b)), 1.67-1.13 (m, 26Н, X7Hβ+K2Hβ(a)+6+X3Hβ +K2Hβ(b)+X3Hδ+7+ K2Hδ+K2Hγ+X3Hγ, m+n), 1.13-0.95 (m, 6H, 8), 0.83 (t, J = 7.1 Hz, 9H, 9).
13C ЯМР (100 MHz, DMSO-d6, δ): 174.56 (K2C(mn)), 174.26 (E1C(mn)), 173.85 (E1Cδ), 172.77 (X4Cγ(n)), 172.72 (X4Cγ(m)), 172.16 (X3C), 171.53 (X4C(mn)), 171.18 (F5C), 170.81 (F6C), 166.50 (5), 157.29 (U), 145.70 (4), 141.23 (X8Cβ(m)), 140.83 (X8Cβ(n)), 138.12 (F6Cγ), 138.02 (F5Cγ), 136.10 (3), 134.29 (1), 133.42 (X8Cζ(n)), 133.06 (X8Cζ(m)), 130.60 (X8Cδ(n)), 130.25 (X8Cδ(m)), 129.06 (F6Cδ+F5Cδ), 128.19 (F6Cε), 128.06 (F5Cε), 127.21 (X8Cη(m)), 127.13 (X8Cε(n)), 126.84 (X8Cε(m)), 126.36 (2), 126.31 (F6Cζ+X8Cη(n)), 126.23 (F5Cζ), 126.10 (X8Cγ(m)), 124.97 (X8Cγ(n)), 54.89 (F5Cα), 54.47 (F6Cα), 52.29 (K2Cα(n)), 52.20 (K2Cα(m)), 51.76 (E1Cα), 49.65 (X8Cα(n)), 47.16 (X8Cα(m)), 46.88 (K2Cε(m)), 45.33 (K2Cε(n)), 38.65 (X3Cε(m)), 38.59 (X3Cε(n)), 37.09 (F5Cβ), 36.87 (F6Cβ), 36.71 (X7Cγ), 35.48 (X7Cα), 32.30 (X3Cα(n)), 31.89 (X3Cα(m)), 31.83 (K2Cβ), 30.69 (X4Cα), 30.58 (X4Cβ), 30.08 (E1Cγ), 29.07 (X3Cδ(m)), 29.01 (X7Cβ), 28.97 (X3Cδ(n)), 28.62 (7), 27.82 (K2Cδ(m)), 27.73 (E1Cβ), 26.71 (K2Cδ(n)+8), 26.29 (X3Cγ(m)), 26.22 (X3Cγ(n)), 24.74 (X3Cβ(m)), 24.59 (X3Cβ(n)), 22.55 (K2Cγ(n)) 22.36 (K2Cγ(m)), 13.59 (6), 9.23 (9).
HRMS (m/z, ESI): calcd. for C69H97ClN8O13Sn - [M+H]+ 1401,5958, found: 1401,5978.
Compound 21. Way A. Compound 18 (1 eq.; 32.5 mg; 29 μmol) and DIPEA (6 eq.; 30 μl; 174.36 μmol) were dissolved in DMF (4 mL). Compound 14 (1 eq.; 10 mg; 29 μmol) was added to the odtained mixture and the system was purged with argon. The mixture was stirred for 6 h. The solvent was evaporated under reduced pressure and the residue was then purified by column chromatography (Puriflash on a column of PF-15C18HP-F0012(15μ 20g), eluent: H2O(90%)/MeCN(10%) => H2O(0%)/MeCN(100%) for 20 min after MeCN (100%) for 5 min. Compound 19 was obtained as white powder (28.6 mg, yield 80%).
Way B. I2 (1 eq; 3.4 mg; 13.136 μmol) was dissolved in 0.1N NaOH (79 µl = V1), AcOH (3%) in CHCl3 (79 µl = V2) was added (V1 = V2), then tert-butyl hydroperoxide (TBHP) in CHCl3 (the solution is prepared in advance by adding 1800 µl of 70% TBHP in water to 11209 µl of CHCl3, after which Na2SO4 is added to the prepared mixture to bind water) (394 µl) and then 19 (1 eq; 18.4 mg; 13.136 μmol l) in DMF (131 µl) were added. The mixture was stirred for 30 minutes. Next, the solvent was removed under reduced pressure and the residue was purified by reverse phase column chromatography (Puriflash PF-15C18AQ-F0012 (15μ 20g): H2O(90%)/MeCN(10%) => H2O(0 %)/MeCN(100%) for 30 minutes after MeCN (100%) for 5 minutes. Compound 21 was obtained as white powder (10.5 mg, yield 65%).
1HNMR (400 MHz, DMSO-d6, δ): 12.74-11.94 (br.s, 3H, COOH), 8.55-8.47 (m, 1H, X7NHδ), 8.29 (d, J = 7.4 Hz, 1Н, F5NH), 8.22-8.13 (m, 1Н, F6NHmn+2), 7.93-7.80 (m, 3H, X3NHζmn+4+6), 7.67-7.58 (m, 1H, X7NH), 7.42-7.08 (m, 15H, X8Hδn+5+X8Hεn+ X8Hδm+X8Hεm+F6Hε+F6Hδ +X8Hηmn+F5Hε+F6Hζ+F5Hζ+F5Hδ+X8Hγmn), 6.37-6.23 (m, 2Н, K2NHm +K2NHn +E1NHm+E1NHn), 4.55 (s, n) & 4.47 (s, m) (2H, X8Hα, m+n), 4.44-4.37 (m, 1H, F6Hα), 4.37-4.28 (m, 1H, F5Hα), 4.14-3.98 (m, 2H, E1Hα+K2Hαm+K2Hαn), 3.26-2.84 (m, 11H, K2Hεmn+X7Hγ(a)) +F6Hβ(a) +X7Hγ(b)+X3Hε(mn)+F6Hβ(b)+X7Hα+F5Hβ(a)), 2.70-2.60 (m, 1Н, F5Hβ(b)), 2.40-2.10 (m, 8H, X3Hαm+X4Hβmn+E1Hγ +X4Hαmn+X3Hαn), 1.97-1.85 (m, 1Н, E1Hβ(a)), 1.76-1.67 (m, 1Н, E1Hβ(b)), 1.67-1.11 (m, 14Н, X7Hβ+K2Hβ(a)+X3Hβ+K2Hβ(b)+X3Hδ+ K2Hδ+K2Hγ+X3Hγ, m+n).
13C NMR (100 MHz, DMSO-d6, δ): 174.56 (K2C(n)), 174.53 (K2C(m)), 174.25 (E1C(mn)), 173.81 (E1Cδ), 172.82 (X4Cγ(n)), 172.77 (X4Cγ(m)), 172.16 (X3C), 171.55 (X4C(mn)), 171.16 (F5C), 170.75 (F6C), 164.71 (7), 157.30 (U), 141.24 (X8Cβ(m)), 140.82 (X8Cβ(n)), 139.68 (6), 138.13 (F6Cγ), 138.02 (F5Cγ), 136.59 (3), 135.63 (2), 133.42 (X8Cζ(n)), 133.07 (X8Cζ(m)), 130.61 (X8Cδ(n)), 130.54 (5), 130.25 (X8Cδ(m)), 129.06 (F6Cδ+F5Cδ), 128.19 (F6Cε), 128.08 (F5Cε), 127.21 (X8Cη(m)), 127.14 (X8Cε(n)), 126.85 (X8Cε(m)), 126.64 (4), 126.31 (F6Cζ+X8Cη(n)), 126.25 (F5Cζ), 126.09 (X8Cγ(m)), 124.97 (X8Cγ(n)), 94.74 (1), 54.96 (F5Cα), 54.45 (F6Cα), 52.29 (K2Cα(n)), 52.16 (K2Cα(m)), 51.68 (E1Cα), 49.65 (X8Cα(n)), 47.17 (X8Cα(m)), 46.89 (K2Cε(m)), 45.33 (K2Cε(n)), 38.67 (X3Cε(m)), 38.60 (X3Cε(n)), 37.09 (F5Cβ), 36.97 (X7Cγ), 36.86 (F6Cβ), 36.55 (X7Cα), 32.28 (X3Cα(n)), 31.86 (X3Cα(m)), 31.81 (K2Cβ), 30.67 (X4Cα), 30.56 (X4Cβ), 29.97 (E1Cγ), 29.07 (X3Cδ(m)), 28.95 (X3Cδ(n)), 28.87 (X7Cβ), 27.80 (K2Cδ(m)), 27.60 (E1Cβ), 26.70 (K2Cδ(n), 26.28 (X3Cγ(m)), 26.21 (X3Cγ(n)), 24.72 (X3Cβ(m)), 24.57 (X3Cβ(n)), 22.53 (K2Cγ(n)) 22.34 (K2Cγ(m)).
ESI-MS C57H70СlIN8O13: m/z calcd. for [M+H+]+: 1237.38, found: 1237.55
HRMS (m/z, ESI): calcd. for C57H70СlIN8O13 - [M+H]+ 1237.3868, found: 1237.3873
Compound 22. Way A. Compound 18 (1 eq.; 20 mg; 18 μmol) and DIPEA (6 eq.; 19 μl; 107 μmol) were dissolved in DMF (4 mL). Compound 17 (1 eq.; 6.1 mg; 18 μmol) was added to the obtained mixture and the system was purged with argon. The mixture was stirred for 6 h, the solvent was then evaporated under reduced pressure. The residue was purified by column chromatography (Puriflash on a column of PF-15C18HP-F0012(15μ 20g), eluent: H2O(90%)/MeCN(10%) => H2O(0%)/MeCN(100%) for 20 min after MeCN (100%) for 5 min. Compound 19 was obtained as white powder (13 mg, yield 59%).
Way B. I2 (1 eq; 5.4 mg; 21.42 μmol) was dissolved in 0.1N NaOH (129 µl = V1), AcOH (3%) in CHCl3 (129 µl = V2) was added (V1 = V2), then tert-butyl hydroperoxide (TBHP) in CHCl3 (the solution is prepared in advance by adding 1800 µl of 70% TBHP in water to 11209 µl of CHCl3, after which Na2SO4 is added to the prepared mixture to bind water) (643 µl), then 20 (1 eq; 30 mg; 21.42 μmol l) in DMF (214 µl) were added. The mixture was stirred for 30 minutes and the solvent was then removed under reduced pressure. The residue was purified by reverse phase column chromatography (Puriflash PF-15C18AQ-F0012 (15μ 20g): H2O(90%)/MeCN(10%) => H2O(0 %)/MeCN(100%) for 30 minutes after MeCN (100%) for 5 minutes. Compound 21 was obtained as white powder (11.7 mg, yield 44%).
1HNMR (400 MHz, DMSO-d6, δ): 12.74-11.94 (br.s, 3H, COOH), 8.54-8.45 (m, 1H, X7NHδ), 8.38-8.28 (m, 1Н, F5NH), 8.26-8.15 (m, 1Н, F6NHmn+2), 7.93 (t, J=5.4 Hz, m) & 7.90 (t, J=5.4 Hz, n) (1H, X3NHζ, m+n), 7.86-7.81 (m, 2H, 3), 7.68-7.58 (m, 3H, X7NH+2), 7.41-7.09 (m, 14H, X8Hδn+X8Hεn+X8Hδm+X8Hεm+F6Hε+F6Hδ+X8Hηmn+F5Hε+F6Hζ+F5Hζ +F5Hδ+X8Hγmn), 6.37-6.23 (m, 2Н, K2NHm+K2NHn +E1NHm+E1NHn), 4.54 (s, n) & 4.47 (s, m) (2H, X8Hα, m+n), 4.44-4.35 (m, 1H, F6Hα), 4.35-4.27 (m, 1H, F5Hα), 4.14-3.98 (m, 2H, E1Hα+K2Hαm+K2Hαn), 3.26-2.84 (m, 11H, K2Hεmn+X7Hγ(a)) +F6Hβ(a) +X7Hγ(b) +X3Hε(mn)+F6Hβ(b)+X7Hα+F5Hβ(a)), 2.70-2.60 (m, 1Н, F5Hβ(b)), 2.40-2.10 (m, 8H, X3Hαm+X4Hβmn+E1Hγ +X4Hαmn+X3Hαn), 1.97-1.85 (m, 1Н, E1Hβ(a)), 1.76-1.11 (m, 15Н, E1Hβ(b)+X7Hβ+K2Hβ(a)+X3Hβ+K2Hβ(b) +X3Hδ+K2Hδ+K2Hγ+X3Hγ, m+n).
13C NMR (100 MHz, DMSO-d6, δ): 174.57 (K2C(n)), 174.54 (K2C(m)), 174.25 (E1C(mn)), 173.82 (E1Cδ), 172.80 (X4Cγ(n)), 172.75 (X4Cγ(m)), 172.16 (X3C), 171.53 (X4C(mn)), 171.17 (F5C), 170.76 (F6C), 165.51 (5), 157.28 (U), 141.23 (X8Cβ(m)), 140.83 (X8Cβ(n)), 139.68 (6), 138.11 (F6Cγ), 138.01 (F5Cγ), 137.19 (3), 133.97 (2), 133.42 (X8Cζ(n)), 133.07 (X8Cζ(m)), 130.61 (X8Cδ(n)), 130.25 (X8Cδ(m)), 129.06 (F6Cδ+F5Cδ), 128.19 (F6Cε), 128.08 (F5Cε), 127.21 (X8Cη(m)), 127.14 (X8Cε(n)), 126.85 (X8Cε(m)), 126.31 (F6Cζ+X8Cη(n)), 126.25 (F5Cζ), 126.09 (X8Cγ(m)), 124.97 (X8Cγ(n)), 98.73 (1), 54.96 (F5Cα), 54.45 (F6Cα), 52.29 (K2Cα(n)), 52.16 (K2Cα(m)), 51.68 (E1Cα), 49.65 (X8Cα(n)), 47.17 (X8Cα(m)), 46.89 (K2Cε(m)), 45.33 (K2Cε(n)), 38.67 (X3Cε(m)), 38.60 (X3Cε(n)), 37.09 (F5Cβ), 36.88 (X7Cγ+F6Cβ), 36.55 (X7Cα), 32.28 (X3Cα(n)), 31.86 (X3Cα(m)), 31.81 (K2Cβ), 30.67 (X4Cα), 30.56 (X4Cβ), 30.01 (E1Cγ), 29.07 (X3Cδ(m)), 28.95 (X3Cδ(n)), 28.87 (X7Cβ), 27.80 (K2Cδ(m)), 27.60 (E1Cβ), 26.70 (K2Cδ(n), 26.28 (X3Cγ(m)), 26.21 (X3Cγ(n)), 24.72 (X3Cβ(m)), 24.57 (X3Cβ(n)), 22.53 (K2Cγ(n)) 22.34 (K2Cγ(m)).
ESI-MS C57H70СlIN8O13: m/z calcd. for [M+H+]+: 1237.38, found: 1237.55
HRMS (m/z, ESI): calcd. for C57H70СlIN8O13 - [M+H]+ 1237.3868, found: 1237.3890