Submitted:
29 June 2023
Posted:
03 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Gastric Cancer and Preoperative Therapies
3. Gastric Cancer Staging and Tumor Response Grade
4. Tumor Response Grading Systems

5. Tumor Response Grade Prognostication
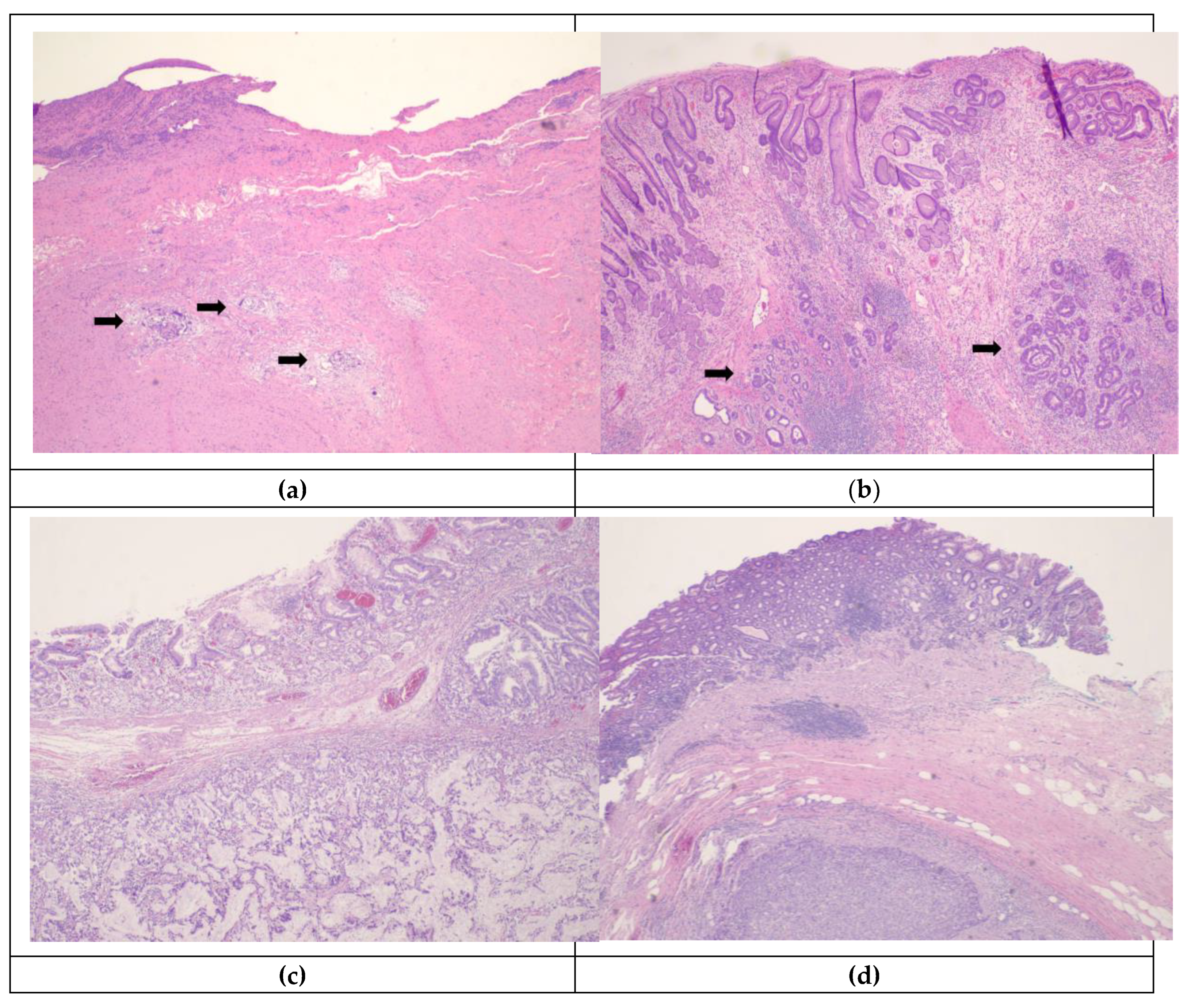
6. Radiographic and Endoscopic Response to Chemotherapy and Prognostication
7. Future Directions
8. Conclusions
Conflicts of Interest
References
- Jacques Ferlay, M.C., Isabelle Soerjomataram, Donald M. Parkin, Marion Piñeros, Ariana Znaor, Freddie Bray. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer. 2020 [cited 2023 February 23]; Available from: https://gco.iarc.fr/today/home.
- Ramos-Santillan, V., et al., The order of surgery and chemotherapy matters: Multimodality therapy and stage-specific differences in survival in gastric cancer. J Surg Oncol, 2023. 127(1): p. 56-65. [CrossRef]
- National Comprehensive Cancer Network.. Gastric Cancer (Version 1.2023). 2023 April 11, 2023]; Available from: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf.
- Petrillo, A. and E.C. Smyth, Multimodality treatment for localized gastric cancer: state of the art and new insights. Curr Opin Oncol, 2020. 32(4): p. 347-355. [CrossRef]
- Cunningham, D., et al., Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med, 2006. 355(1): p. 11-20. [CrossRef]
- Ychou, M., et al., Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol, 2011. 29(13): p. 1715-21. [CrossRef]
- Al-Batran, S.E., et al., Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet, 2019. 393(10184): p. 1948-1957. [CrossRef]
- Ajani JA, In H., Sano T, Gaspar LE, Erasmus JJ, Tang LH, Washington MK, Gerdes H, Wittekind CW, Mansfield PF, Rimmer C, Hofetetter WL, Kelson D. Stomach. In: Amin, M.B., Edge, S.B., Greene, F.L., et al. (Eds.) AJCC Cancer Staging Manual. 8th Ed. New York: Springer; 2017: 203-220.
- In, H., et al., The newly proposed clinical and post-neoadjuvant treatment staging classifications for gastric adenocarcinoma for the American Joint Committee on Cancer (AJCC) staging. Gastric Cancer, 2018. 21(1): p. 1-9. [CrossRef]
- Kim, G., et al., Providing Reliable Prognosis to Patients with Gastric Cancer in the Era of Neoadjuvant Therapies: Comparison of AJCC Staging Schemata. J Gastric Cancer, 2020. 20(4): p. 385-394. [CrossRef]
- Japanese Gastric Cancer, A., Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer, 2023. 26(1): p. 1-25.
- Eisenhauer, E.A., et al., New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer, 2009. 45(2): p. 228-47. [CrossRef]
- Chung, Y.R., et al., Prognostic implications of regression of metastatic axillary lymph nodes after neoadjuvant chemotherapy in patients with breast cancer. Sci Rep, 2021. 11(1): p. 12128. [CrossRef]
- Ahn, S., et al., Four-Tier Pathologic Tumor Regression Grading System Predicts the Clinical Outcome in Patients Who Undergo Surgical Resection for Locally Advanced Pancreatic Cancer after Neoadjuvant Chemotherapy. Gut Liver, 2022. 16(1): p. 129-137. [CrossRef]
- Peng, Y.F., et al., Tumor regression grades: potential outcome predictor of locally advanced rectal adenocarcinoma after preoperative radiotherapy. World J Gastroenterol, 2015. 21(6): p. 1851-6. [CrossRef]
- Li, J.Y., et al., Survival landscape of different tumor regression grades and pathologic complete response in rectal cancer after neoadjuvant therapy based on reconstructed individual patient data. BMC Cancer, 2021. 21(1): p. 1214. [CrossRef]
- Amin MB, E.S., Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, et al. (Eds.), AJCC Cancer Staging Manual. 8th ed. 2017: Springer International Publishing: American Joint Commission on Cancer.
- Becker, K., et al., Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer, 2003. 98(7): p. 1521-30. [CrossRef]
- Mandard, A.M., et al., Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer, 1994. 73(11): p. 2680-6. [CrossRef]
- Ryan, R., et al., Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology, 2005. 47(2): p. 141-6. [CrossRef]
- Japanese Gastric Cancer, A., Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer, 2011. 14(2): p. 101-12. [CrossRef]
- Lawrence J. Burgart, M.W.V.C., MD*; Dhanpat Jain, MD Protocol for the Examination of Specimens From Patients With Carcinoma of the Stomach. 2022.
- Mansour, J.C., et al., Does graded histologic response after neoadjuvant chemotherapy predict survival for completely resected gastric cancer? Ann Surg Oncol, 2007. 14(12): p. 3412-8. [CrossRef]
- West, C.M., A. Naveed A. Hayes S. Formela L. Welch I. West C. M. Pritchard S., Assessment of Histopathological Response in Gastric and Gastro-Oesophageal Junction Adenocarcinoma following Neoadjuvant Chemotherapy: Which Scoring System to Use? Pathology. Pathology, 2012.
- Lowy, A.M., et al., Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg, 1999. 229(3): p. 303-8. [CrossRef]
- Becker, K., et al., Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg, 2011. 253(5): p. 934-9. [CrossRef]
- Xie, J.W., et al., Prognostic Value of Tumor Regression Grading in Patients Treated With Neoadjuvant Chemotherapy Plus Surgery for Gastric Cancer. Front Oncol, 2021. 11: p. 587856. [CrossRef]
- Sinnamon, A.J., et al., Tumor Regression Grade and Overall Survival following Gastrectomy with Preoperative Therapy for Gastric Cancer. Ann Surg Oncol, 2023. 30(6): p. 3580-3589. [CrossRef]
- Smyth, E.C., et al., Effect of Pathologic Tumor Response and Nodal Status on Survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J Clin Oncol, 2016. 34(23): p. 2721-7. [CrossRef]
- Achilli, P., et al., Tumor response evaluation after neoadjuvant chemotherapy in locally advanced gastric adenocarcinoma: a prospective, multi-center cohort study. J Gastrointest Oncol, 2017. 8(6): p. 1018-1025. [CrossRef]
- Ott, K., et al., Neoadjuvant chemotherapy with cisplatin, 5-FU, and leucovorin (PLF) in locally advanced gastric cancer: a prospective phase II study. Gastric Cancer, 2003. 6(3): p. 159-67. [CrossRef]
- Kelsen, D., et al., Neoadjuvant therapy of high-risk gastric cancer: a phase II trial of preoperative FAMTX and postoperative intraperitoneal fluorouracil-cisplatin plus intravenous fluorouracil. J Clin Oncol, 1996. 14(6): p. 1818-28. [CrossRef]
- Ajani, J.A., et al., Enhanced staging and all chemotherapy preoperatively in patients with potentially resectable gastric carcinoma. J Clin Oncol, 1999. 17(8): p. 2403-11. [CrossRef]
- Park, S.R., et al., Endoscopic ultrasound and computed tomography in restaging and predicting prognosis after neoadjuvant chemotherapy in patients with locally advanced gastric cancer. Cancer, 2008. 112(11): p. 2368-76. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



