Submitted:
03 July 2023
Posted:
04 July 2023
You are already at the latest version
Abstract
Keywords:
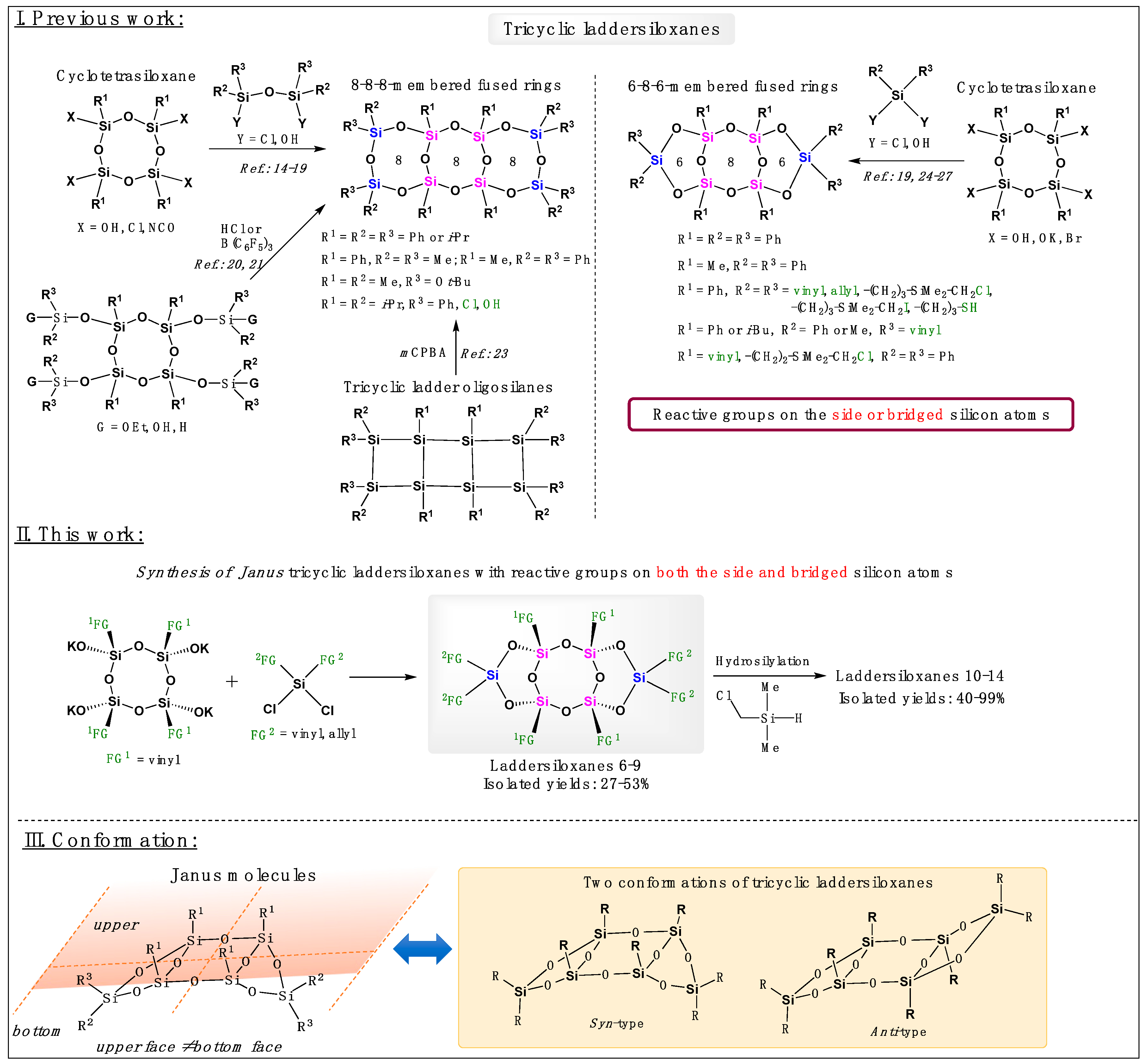
1. Introduction
2. Results and Discussion
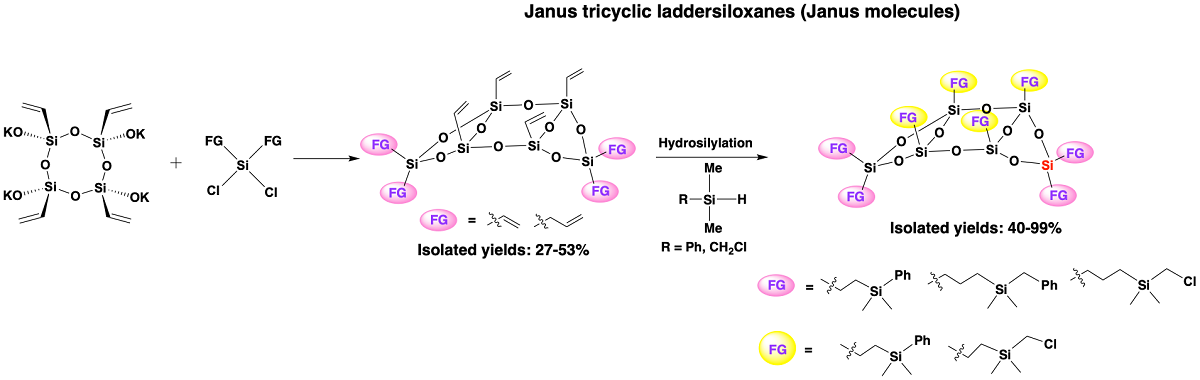
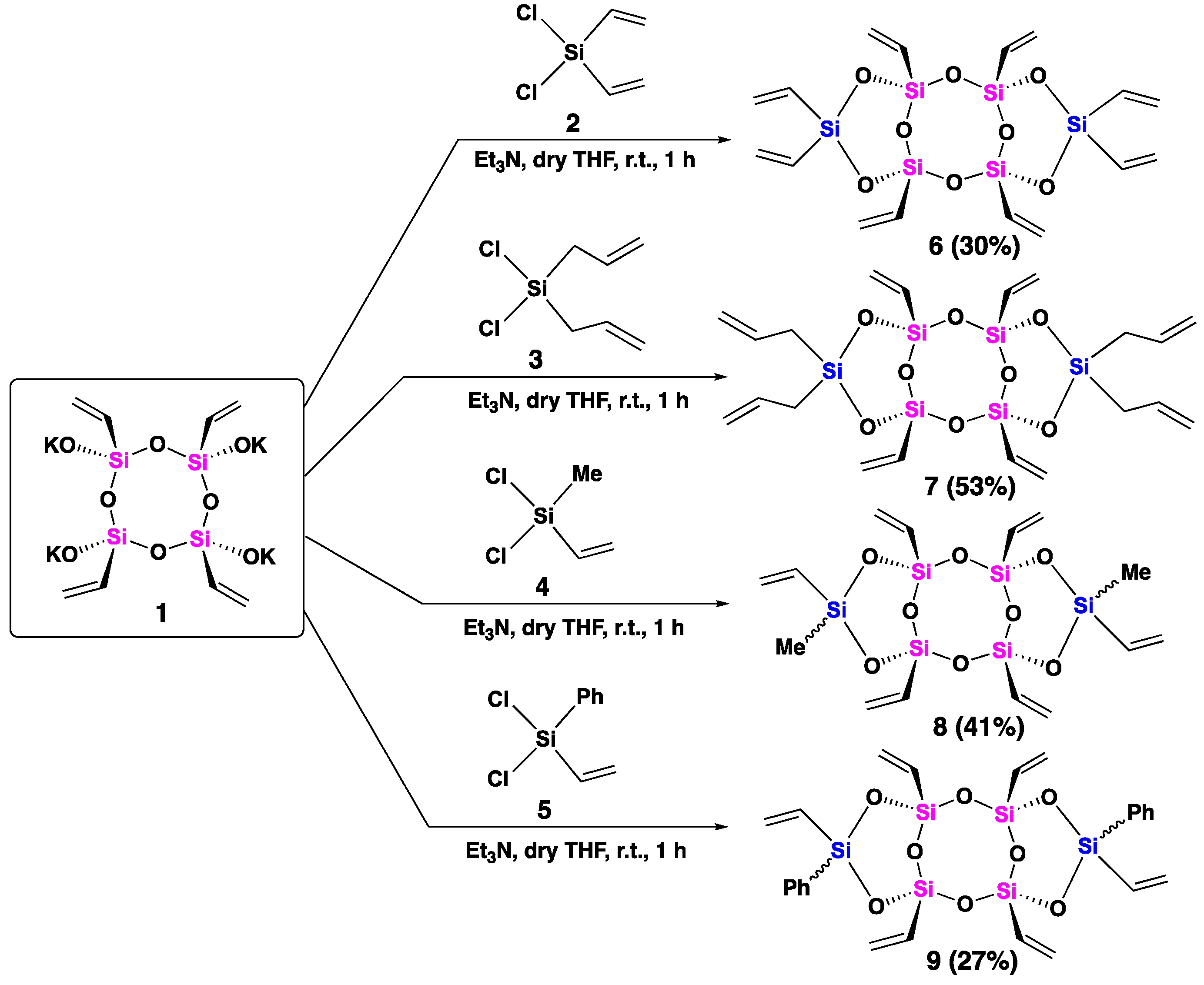
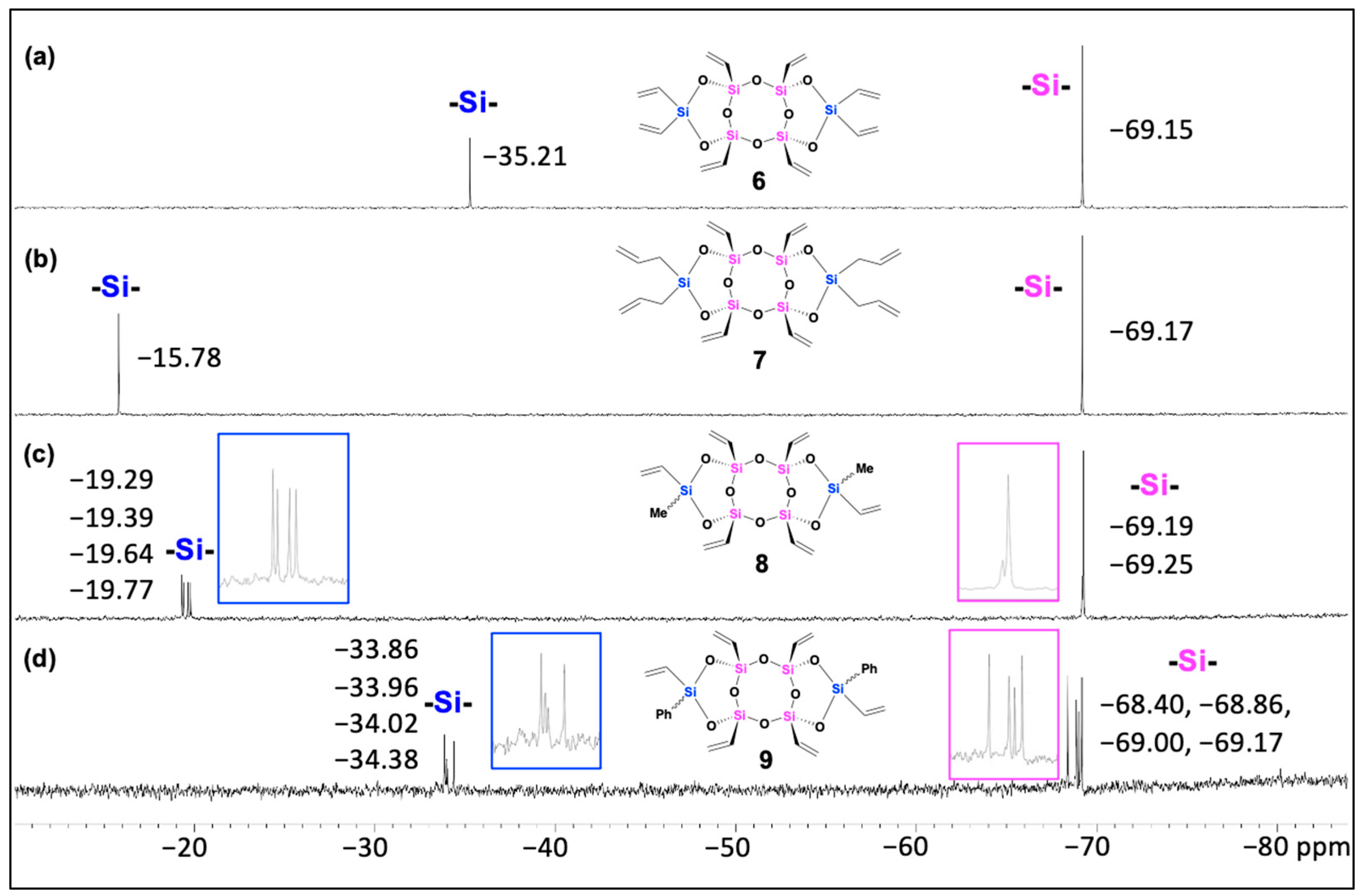
2.1. Preparation of Janus tricyclic laddersiloxanes bearing eight or six alkenyl groups
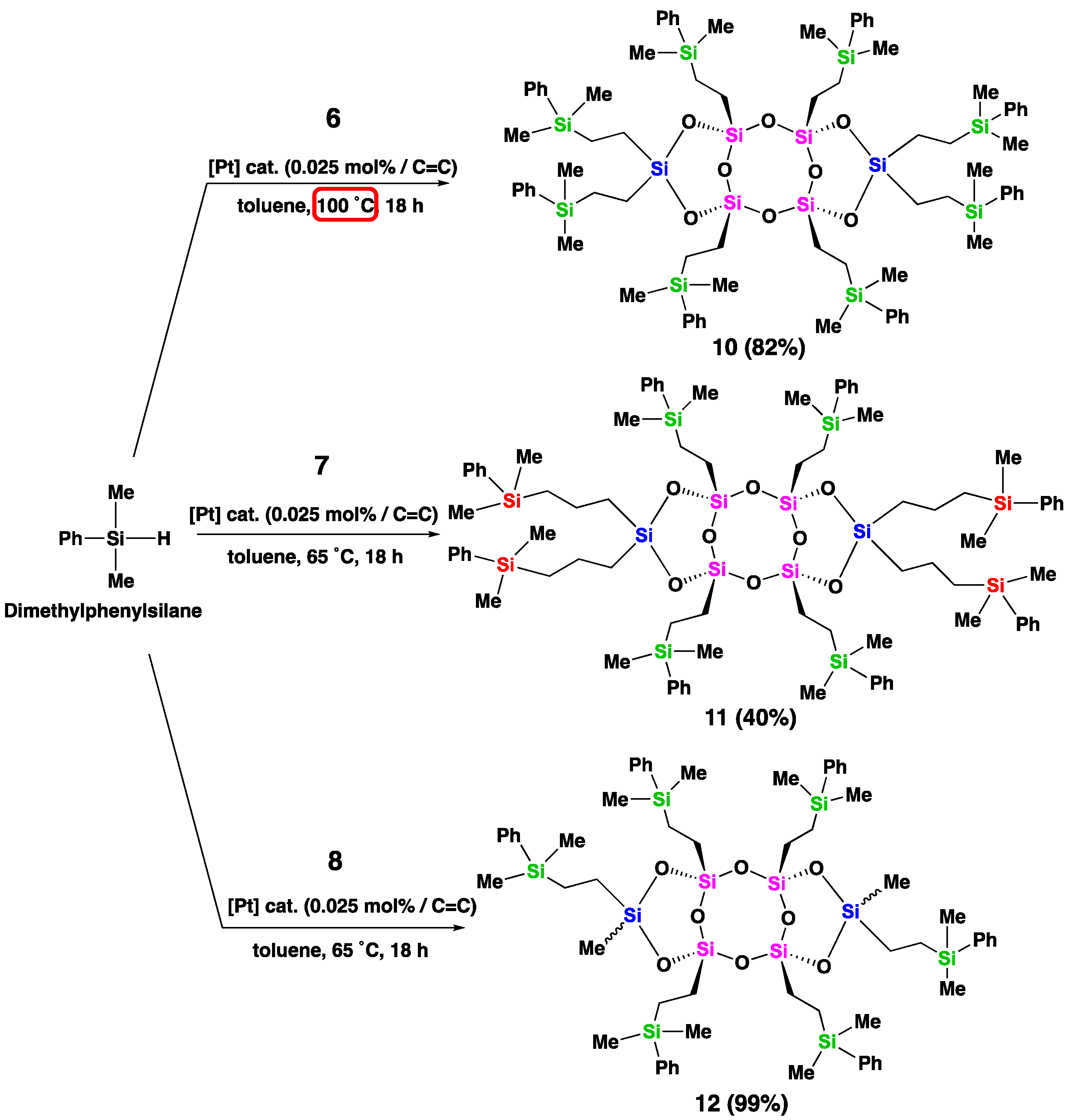
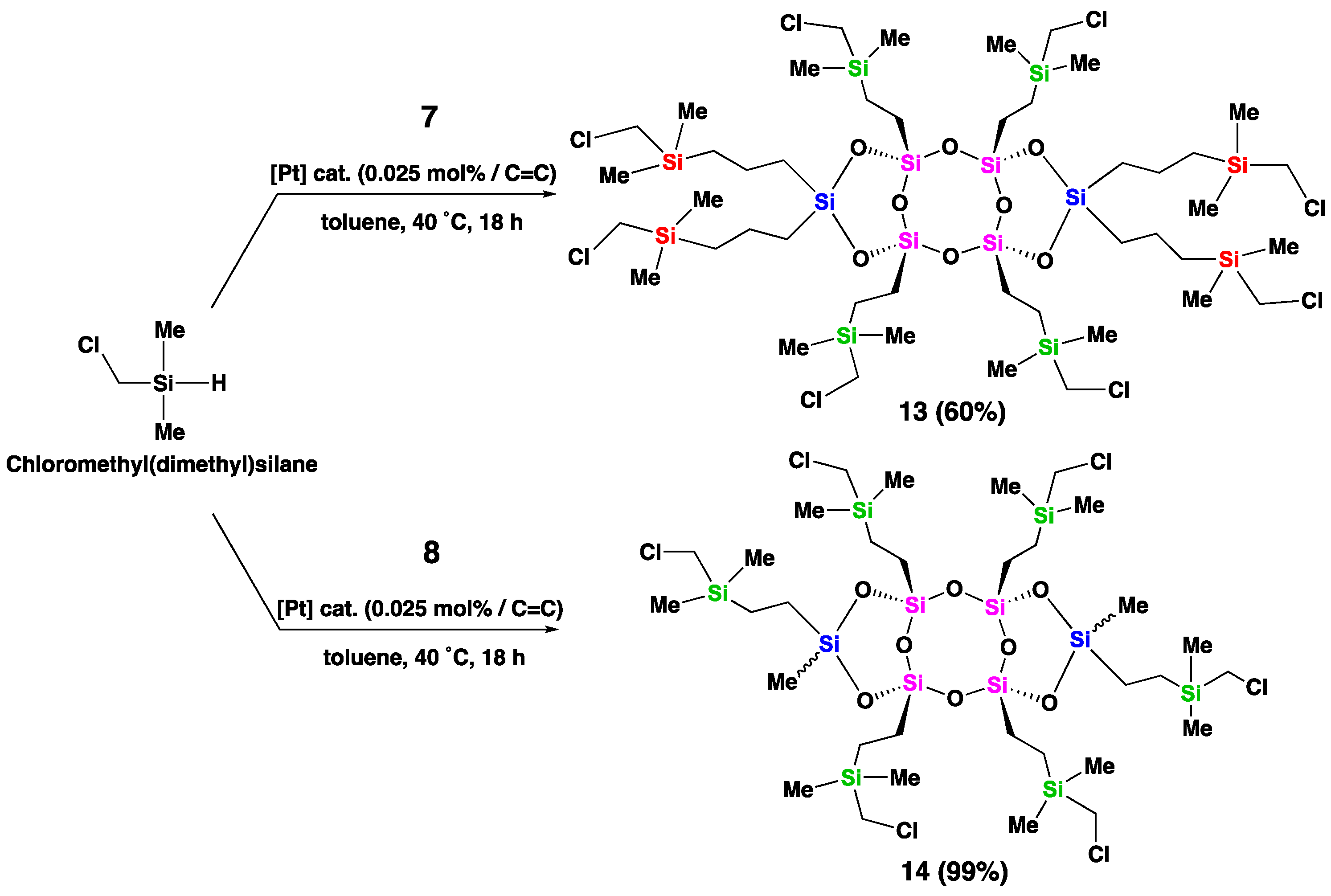
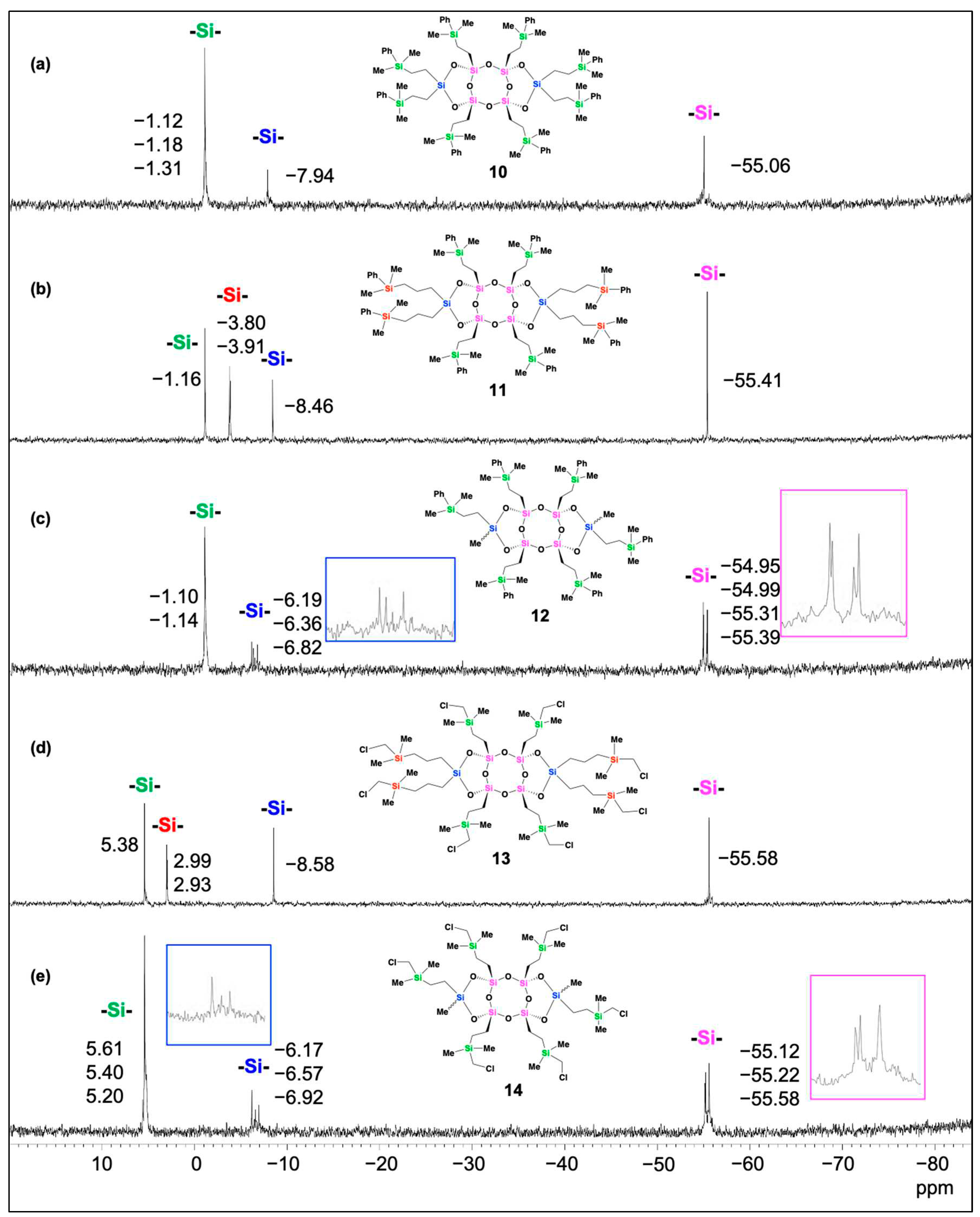
2.2. Full functionalization of Janus tricyclic laddersiloxanes bearing eight or six alkenyl groups
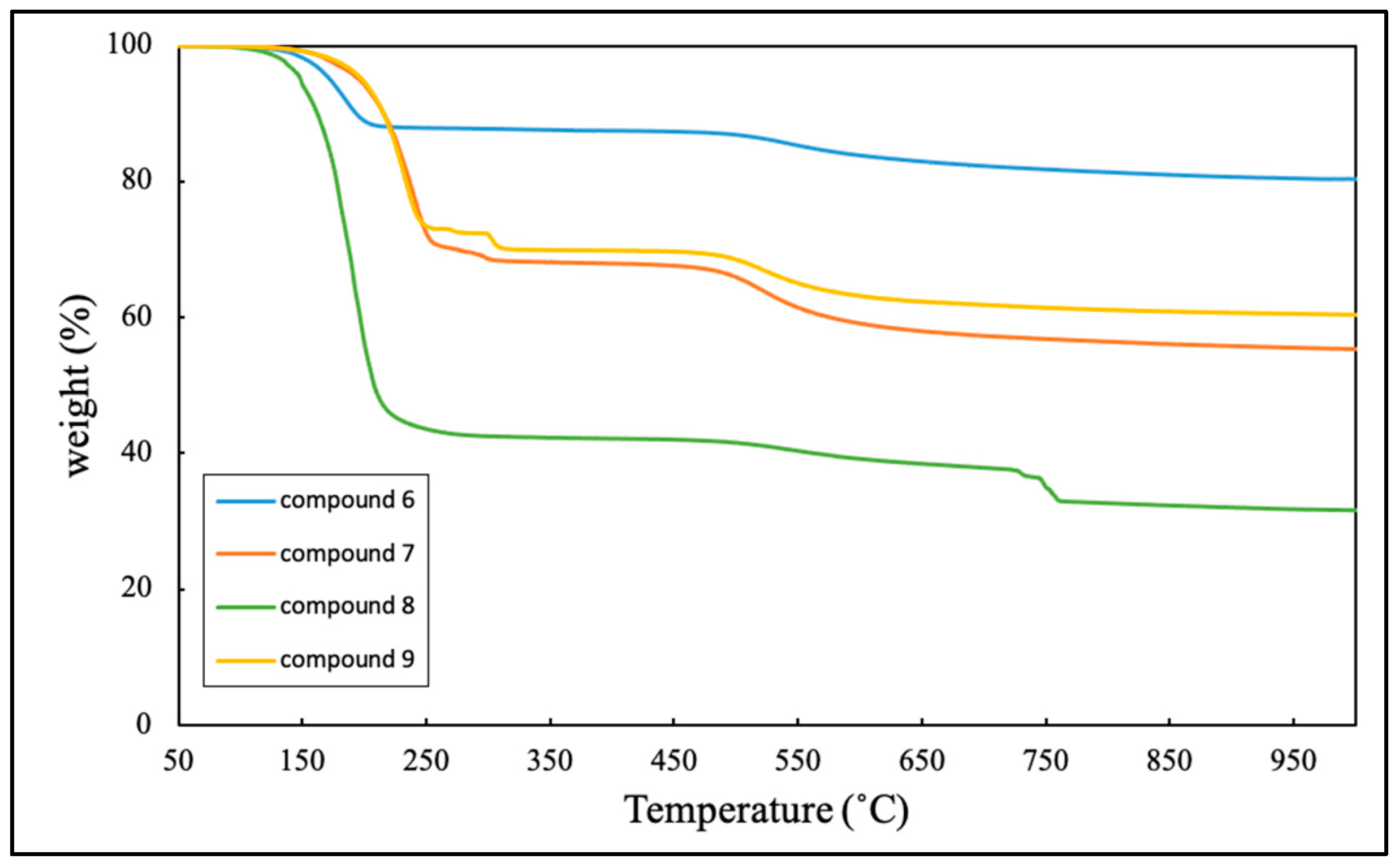
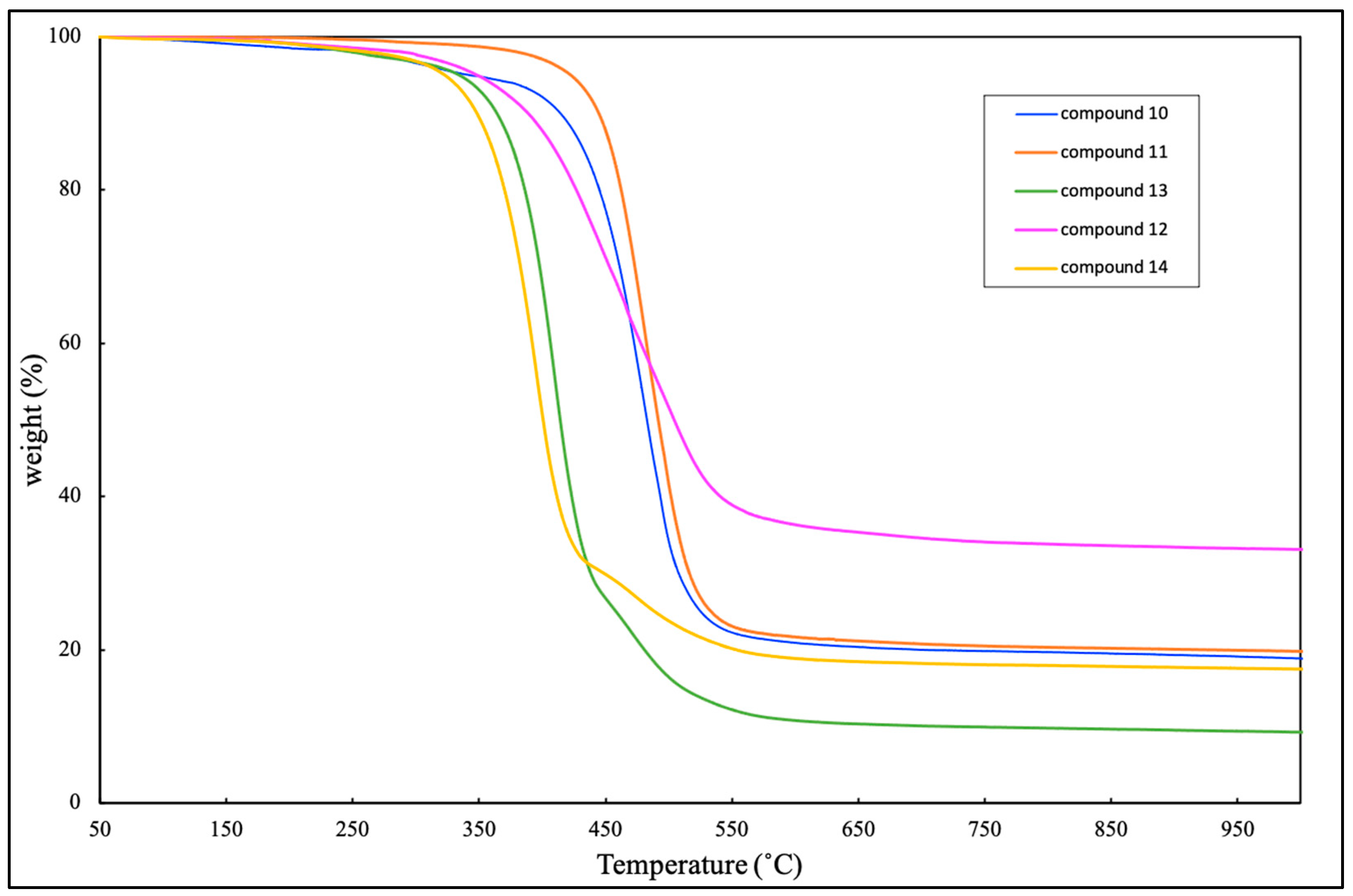
2.3. Thermal properties of laddersiloxanes 6-14
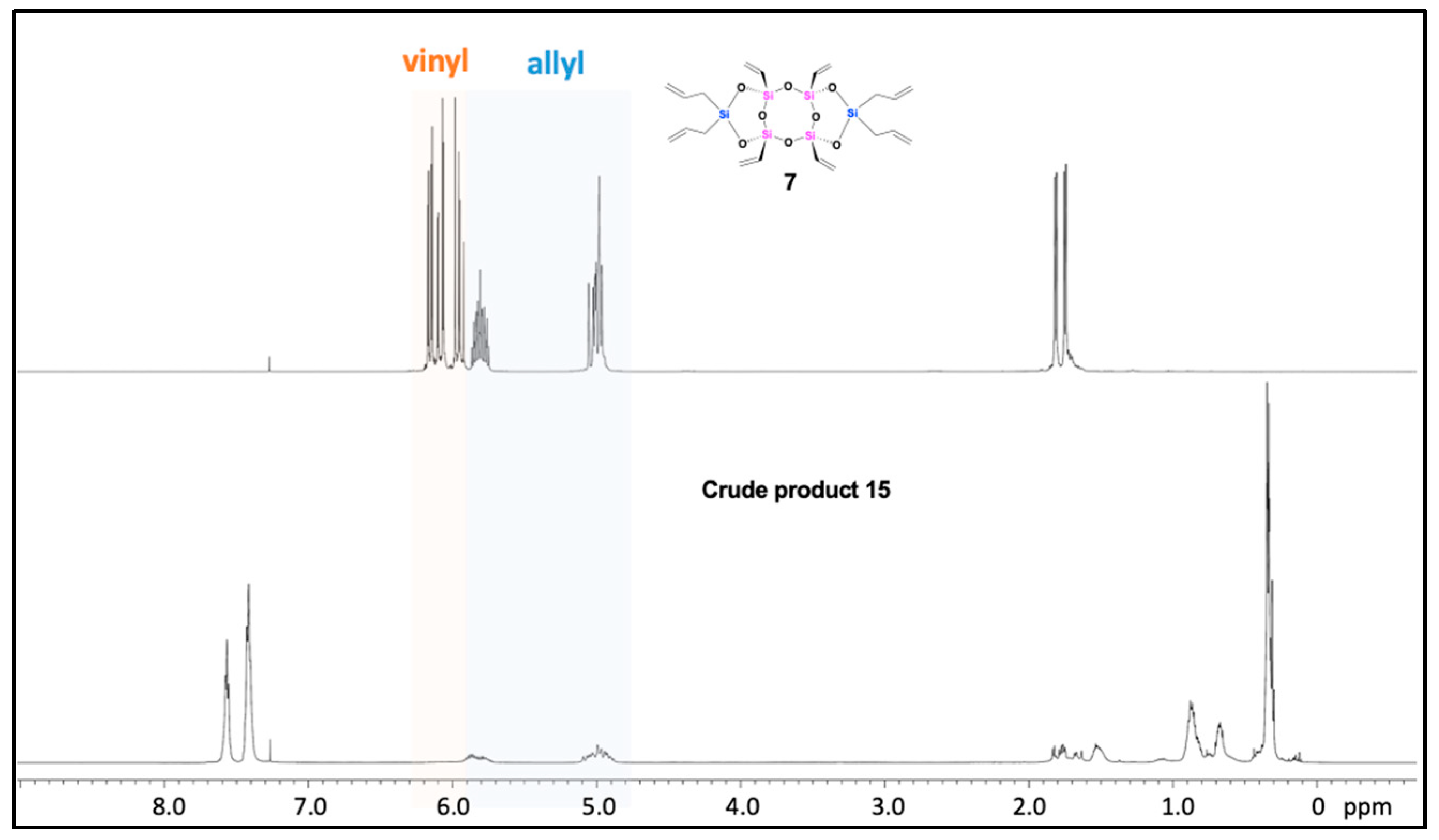
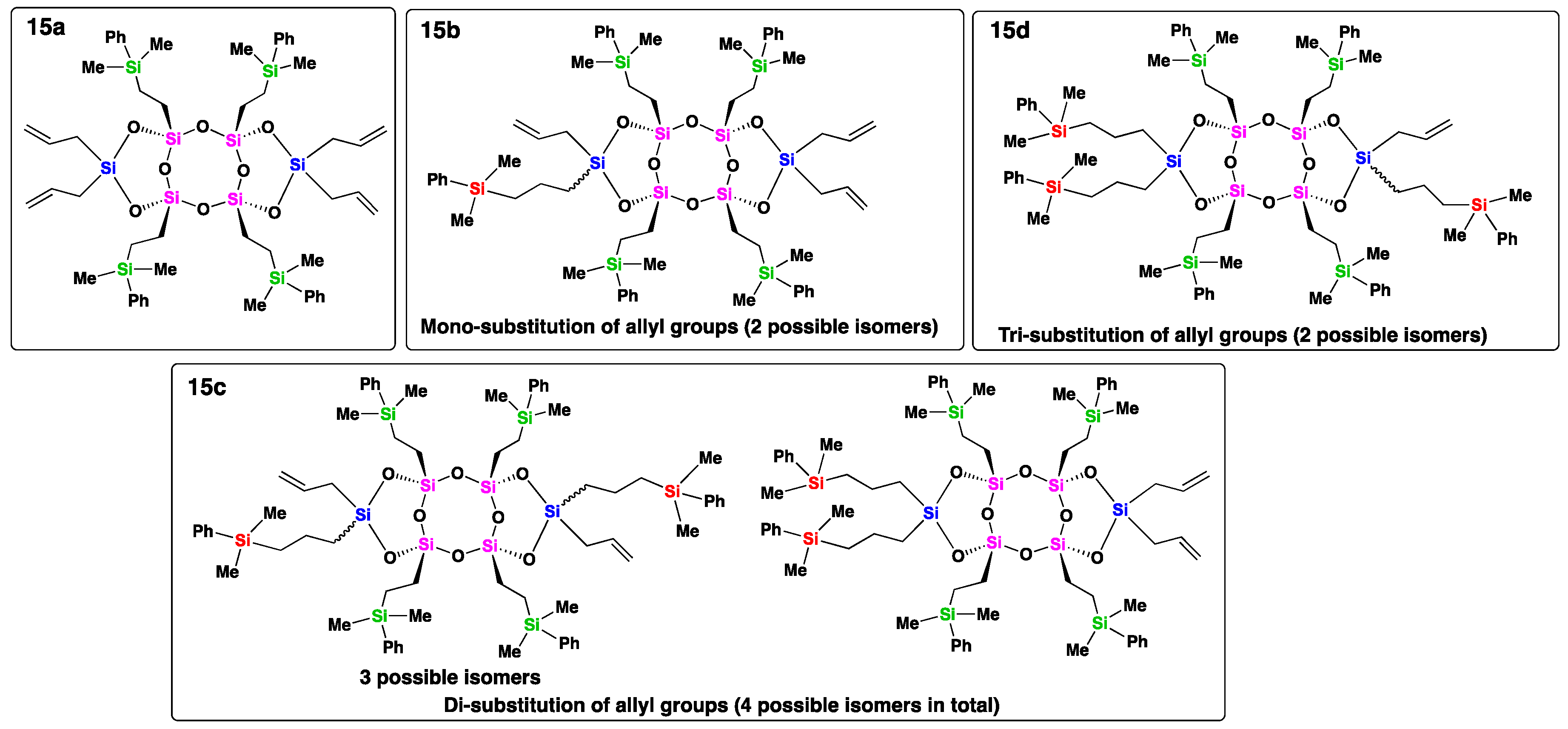
2.4. Trials of selective functionalization of Janus tricyclic laddersiloxane 7
3. Materials and Methods
3.1. General considerations
3.2. Synthetic procedures of compounds 6-15
- Synthesis of 6-8-6 tricyclic laddersiloxane (6)
- Synthesis of 6-8-6 tricyclic laddersiloxane (7)
- Synthesis of 6-8-6 tricyclic laddersiloxane (8)
- Synthesis of 6-8-6 tricyclic laddersiloxane (9)
- Synthesis of 6-8-6 tricyclic laddersiloxane (10):
- Synthesis of 6-8-6 tricyclic laddersiloxane (11):
- Synthesis of 6-8-6 tricyclic laddersiloxane (12):
- Synthesis of 6-8-6 tricyclic laddersiloxane (13):
- Synthesis of 6-8-6 tricyclic laddersiloxane (14):
- Synthesis of 6-8-6 tricyclic laddersiloxane (15):
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Baney, R.H.; Itoh, M.; Sakakibara, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409-1430.
- Kickelbick, G. Silsesquioxanes in Functional Molecular Silicon Compounds I; Structure and Bonding; Scheschkewitz, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; 155, pp. 1-28.
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081-2173. [CrossRef]
- Laine, R.M.; Roll, M.F. Polyhedral Phenylsilsesquioxanes. Macromolecules 2011, 44, 1073-1109. [CrossRef]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications. Mater. Chem. Front. 2017, 1, 212-230. [CrossRef]
- Chen, F.; Lin, F.; Zhang, Q.; Cai, R.; Wu, Y.; Ma, X. Polyhedral Oligomeric Silsesquioxane Hybrid Polymers: Well-Defined Architectural Design and Potential Functional Applications. Macromol. Rapid Commun. 2019, 40, 1900101. [CrossRef]
- Du, Y.; Liu, H. Cage-like silsesquioxanes-based hybrid materials. Dalton Trans. 2020, 49, 5396-5405. [CrossRef]
- Calabrese, C.; Aprile, C.; Gruttadauria, M.; Giacalone, F. POSS nanostructures in catalysis. Catal. Sci. Technol. 2020, 10, 7415-7447. [CrossRef]
- Dudziec, B.; Marciniec, B. Double-decker Silsesquioxanes: Current Chemistry and Applications. Curr. Org. Chem. 2017, 21, 2794-2813. [CrossRef]
- Unno, M.; Suto, A.; Matsumoto, T. Laddersiloxanes−silsesquioxanes with defined ladder structure. Russ. Chem. Rev. 2013, 82, 289-302. [CrossRef]
- Kim, M. J.; Heo, Y. M.; Cho, J. H. Ladder-type silsesquioxane copolymer gate dielectrics for gating solution-processed IGZO field-effect transistors. Org. Electron. 2017, 43, 41-46. [CrossRef]
- Endo, H.; Takeda, N.; Takanashi, M.; Imai, T.; Unno, M. Refractive Indices of Silsesquioxanes with Various Structures. Silicon 2015, 7, 127-132. [CrossRef]
- Brown Jr., J.F.; Vogt Jr., L.H.; Katchman, A.; Eustance, J.W.; Kiser, K.M.; Krantz, K.W. Double Chain Polymers of Phenylsilsesquioxane. J. Am. Chem. Soc. 1960, 82, 6194-6195.
- Unno, M.; Suto, A.; Takada, K.; Matsumoto, H. Synthesis of Ladder and Cage Silsesquioxanes from 1,2,3,4-Tetrahydroxycyclotetrasiloxane. Bull. Chem. Soc. Jpn. 2000, 73, 215-220. [CrossRef]
- Unno, M.; Suto, A.; Matsumoto, H. Pentacyclic Laddersiloxane. J. Am. Chem. Soc. 2002, 124, 1574-1575. [CrossRef]
- Suyama, K.; Gunji, T.; Arimitsu, K.; Abe, Y. Synthesis and Structure of Ladder Oligosilsesquioxanes: Tricyclic Ladder Oligomethylsilsesquioxanes. Organometallics 2006, 25, 5587-5593. [CrossRef]
- Unno, M.; Matsumoto, T.; Matsumoto, H. Synthesis of laddersiloxanes by novel stereocontrolled approach. J. Organomet. Chem. 2007, 692, 307-312. [CrossRef]
- Seki, H.; Abe, Y.; Gunji, T. Stereochemistry of the reaction of cis, trans, cis-2,4,6,8-tetraisocyanato-2,4,6,8-tetramethylcyclotetrasiloxane with triphenylsilanol and 1,1,3,3-tetraphenyldisiloxane-1,3-diol. J. Organomet. Chem. 2011, 696, 846-851. [CrossRef]
- Endo, H.; Takeda, N.; Unno, M. Synthesis and Properties of Phenylsilsesquioxanes with Ladder and Double-Decker Structures. Organometallics 2014, 33, 4148-4151. [CrossRef]
- Sugiyama, T.; Shiba, H.; Yoshikawa, M.; Wada, H.; Shimojima, A.; Kuroda, K. Synthesis of Polycyclic and Cage Siloxanes by Hydrolysis and Intramolecular Condensation of Alkoxysilylated Cyclosiloxanes. Chem. Eur. J. 2019, 25, 2764-2772. [CrossRef]
- Chaiprasert, T.; Liu, Y.; Takeda, N.; Unno, M. Janus ring siloxane: versatile precursor of extended Janus ring and tricyclic laddersiloxanes. Dalton Trans. 2020, 49, 13533-13537. [CrossRef]
- Chaiprasert, T.; Liu, Y.; Intaraprecha, P.; Kunthom, R.; Takeda, N.; Unno, M. Synthesis of Tricyclic Laddersiloxane with Various Ring Sizes (Bat Siloxane). Macromol. Rapid Commun. 2021, 42, 2000608.
- Unno, M.; Tanaka, R.; Tanaka, S.; Takeuchi, T.; Kyushin, S.; Matsumoto, H. Oligocyclic Ladder Polysiloxanes: Alternative Synthesis by Oxidation. Organometallics 2005, 24, 765-768. [CrossRef]
- Unno, M.; Chang, S.; Matsumoto, H. cis-trans-cis-Tetrabromotetramethylcyclotetrasiloxane: a Versatile Precursor of Ladder Silsesquioxanes. Bull. Chem. Soc. Jpn. 2005, 78, 1105-1109. [CrossRef]
- Liu, Y.; Onodera, K.; Takeda, N.; Ouali, A.; Unno, M. Synthesis and Characterizatioin of Functionalizable Silsesquioxanes with Ladder-type Structures. Organometallics 2019, 38, 4373-4376.
- Liu, Y.; Endo, A.; Zhang, P.; Takizawa, A.; Takeda, N.; Ouali, A.; Unno, M. Synthesis, Characterization, and Reaction of Divinyl-substituted Laddersiloxanes. Silicon 2022, 14, 2723-2730.
- Liu, Y.; Katano, M.; Yingsukkamol, P.; Takeda, N.; Unno, M.; Ouali, A. Tricyclic 6-8-6 laddersiloxanes derived from all-cis-tetravinylcyclotetrasiloxanolate: Synthesis, characterization and reactivity. J. Organomet. Chem. 2022, 959, 122213.
- Pang, X.; Wan, C.; Wang, M.; Lin, Z. Strictly Biphasic Soft and Hard Janus Structures: Synthesis, Properties, and Applications. Angew. Chem. Int. Ed. 2014, 53, 5524-5538. [CrossRef]
- Yi, Y.; Sanchez, L.; Gao, Y.; Yu, Y. Janus particles for biological imaging and sensing. Analyst 2016, 141, 3526-3539. [CrossRef]
- Ng, S.-W.; Noor, N.; Zheng, Z. Graphene-based two-dimensional Janus materials. NPG Asia Materials 2018, 10, 217-237. [CrossRef]
- Peng, Z.; Huang, J.; Guo, Z. Anisotropic Janus materials: from micro-/nanostructures to applications. Nanoscale 2021, 13, 18839-18864.
- Liu, Y.; Kigure, M.; Okawa, R.; Takeda, N.; Unno, M.; Ouali, A. Synthesis and characterization of tetrathiol-substituted double-decker or ladder silsesquioxane nano-cores. Dalton Trans. 2021, 50, 3473-3478. [CrossRef]
- Guan, J.; Sun, Z.; Ansari, R.; Liu, Y.; Endo, A.; Unno, M.; Ouali, A.; Mahbub, S.; Furgal, J.C.; Yodsin, N.; Jungsuttiwong, S.; Hashemi, D.; Kieffer, J.; Laine, R.M. Conjugated Copolymers That Shouldn’t Be. Angew. Chem. Int. Ed. 2021, 60, 11115-11119. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




