Submitted:
30 June 2023
Posted:
30 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
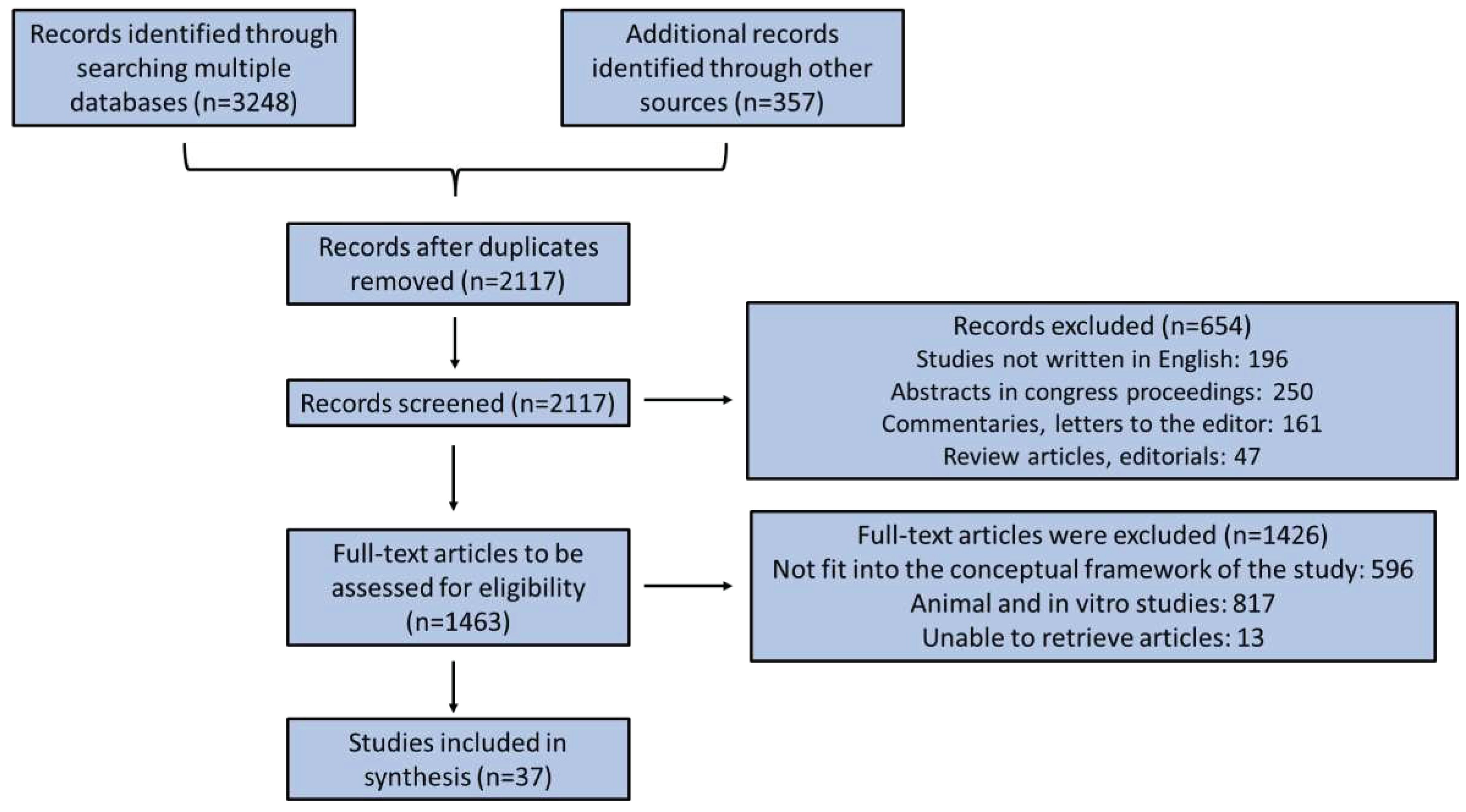
2. Methods
3. Results and Discussion
3.1. Oesophageal cancer
3.2. Pharyngeal cancer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer; 2020. 20 December. Available online: https://gco.iarc.fr/today (accessed on December 2022).
- Dewys, W.D.; Begg, C.; Lavin, P.T.; Band, P.R.; Bennett, J.M.; Bertino, J.R.; Cohen, M.H.; Douglass, H.O.Jr.; Engstrom, P.F.; Ezdinli, E.Z.; et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am. J. Med. 1980, 69, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M. Cancer-associated malnutrition. Eur. J. Oncol. Nurs. 2005, 9, S39–S50. [Google Scholar] [CrossRef] [PubMed]
- Laviano, A. , Preziosa, I.; Fanelli, F.R. Cancer and Nutritional Status. In Nutrition and Cancer, C. Shaw (Ed.): Nutrition and cancer. Wiley-Blackwell Publishing Ltd, Oxford 2011. [Google Scholar] [CrossRef]
- Santarpia, L., Contaldo; Pasanisi, F. Nutritional screening and early treatment of malnutrition in cancer patients. J. Cachexia Sarcopenia Muscle 2011, 2, 27–35. [Google Scholar] [CrossRef]
- Cristofaro, M.G.; Barca, I.; Ferragina, F.; Novembre, D.; Ferro, Y.; Pujia, R.; Montalcini, T. The health risks of dysphagia for patients with head and neck cancer: a multicentre prospective observational study. J. Transl. Med. 2021, 19, 472. [Google Scholar] [CrossRef] [PubMed]
- Martin-Harris, B.; McFarland, D.; Hill, E.G.; Strange, C.B.; Focht, K.L.; Wan, Z.; Blair, J.; McGrattan, K. Respiratory-swallow training in patients with head and neck cancer. Arch. Phys. Med. Rehabil. 2015, 96, 885–893. [Google Scholar] [CrossRef]
- Bressan, V.; Stevanin, S.; Bianchi, M.; Aleo, G.; Bagnasco, A.; Sasso, L. The effects of swallowing disorders, dysgeusia, oral mucositis and xerostomia on nutritional status, oral intake and weight loss in head and neck cancer patients: A systematic review. Cancer Treat. Rev. 2016, 45, 105–119. [Google Scholar] [CrossRef]
- Jordan, T.; Mastnak, D.M.; Palamar, N.; Kozjek, N.R. Nutritional Therapy for Patients with Esophageal Cancer. Nutr. Cancer 2018, 70, 23–29. [Google Scholar] [CrossRef]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef]
- Bossi, P.; De Luca, R.; Ciani, O.; D'Angelo, E.; Caccialanza, R. Malnutrition management in oncology: An expert view on controversial issues and future perspectives. Front. Oncol. 2022, 12, 910770. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; Krznaric, Z.; Laird, B, Larsson, M. ; Laviano, A.; Mühlebach, S.; Muscaritoli, M.; Oldervoll, L.; Ravasco, P.; Solheim, T.; Strasser, F.; de van der Schueren, M.; Preiser, J.C. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Mantzorou, M.; Koutelidakis, A.; Theocharis, S.; Giaginis, C. Clinical Value of Nutritional Status in Cancer: What is its Impact and how it Affects Disease Progression and Prognosis? Nutr. Cancer 2017, 69, 1151–1176. [Google Scholar] [CrossRef] [PubMed]
- Attar, A.; Malka, D.; Sabaté, J.M.; Bonnetain, F.; Lecomte, T.; Aparicio, T.; Locher, C.; Laharie, D.; Ezenfis, J.; Taieb, J. Malnutrition is high and underestimated during chemotherapy in gastrointestinal cancer: an AGEO prospective cross-sectional multicenter study. Nutr. Cancer 2012, 64, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Cushen, S.J.; Orsso, C.E.; Ryan, A.M. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc. Nutr. Soc. 2016, 75, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Ozorio, G.A.; Barão, K.; Forones, N.M. Cachexia Stage, Patient-Generated Subjective Global Assessment, Phase Angle, and Handgrip Strength in Patients with Gastrointestinal Cancer. Nutr. Cancer 2017, 69, 772–779. [Google Scholar] [CrossRef]
- Anandavadivelan, P. , Lagergren, P. Cachexia in patients with oesophageal cancer. Nat. Rev. Clin. Oncol. 2006, 13, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A. M.; Rowley, S.P.; Healy, L.A.; Flood, P.M.; Ravi, N.; Reynolds, J.V. Post-oesophagectomy early enteral nutrition via a needle catheter jejunostomy: 8-year experience at a specialist unit. Clin. Nutr. 2006, 25, 386–393. [Google Scholar] [CrossRef]
- Grotenhuis, B.A.; Wijnhoven, B.P.; Grüne, F.; van Bommel, J.; Tilanus, H.W.; van Lanschot, J.J. Preoperative risk assessment and prevention of complications in patients with esophageal cancer. J. Surg. Oncol. 2010, 101, 270–278. [Google Scholar] [CrossRef]
- Cox, S.; Powell, C.; Carter, B.; Hurt, C.; Mukherjee, S.; Crosby, T.D. Role of nutritional status and intervention in oesophageal cancer treated with definitive chemoradiotherapy: outcomes from SCOPE1. Br. J. Cancer 2016, 115, 172–177. [Google Scholar] [CrossRef]
- Wang, P.Y.; Chen, X.K.; Liu, Q.; Xu, L.; Zhang, R.X.; Liu, X.B.; Li, Y. Application of four nutritional risk indexes in perioperative management for esophageal cancer patients. J. Cancer Res. Clin. Oncol. 2021, 147, 3099–3111. [Google Scholar] [CrossRef]
- Chen, M.F.; Hsieh, C.C.; Chen, P.T.; Lu, M.S. Role of Nutritional Status in the Treatment Outcome for Esophageal Squamous Cell Carcinoma. Nutrients 2021, 13, 2997. [Google Scholar] [CrossRef]
- Takagi, K.; Buettner, S.; Ijzermans, J.N.M.; Wijnhoven, B.P.L. Systematic Review on the Controlling Nutritional Status (CONUT) Score in Patients Undergoing Esophagectomy for Esophageal Cancer. Anticancer Res. 2020, 40, 5343–5349. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Cheng, Q.; Ma, Y.; Wu, C.; Zhang, X.; Ma, Q.; He, L.; Li, Q.; Tao, J. Prognostic Effect of the Controlling Nutritional Status Score in Patients With Esophageal Cancer Treated With Immune Checkpoint Inhibitor. J. Immunother. 2022, 45, 415–422. [Google Scholar] [CrossRef]
- Hirahara, N.; Matsubara, T.; Hayashi, H.; Takai, K.; Nakada, S.; Tajima, Y. Prognostic Importance of Controlling Nutritional Status in Patients Undergoing Curative Thoracoscopic Esophagectomy for Esophageal Cancer. Am. J. Ther. 2018, 25, e524–e532. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Baba, Y.; Shigaki, H.; Harada, K.; Iwatsuki, M.; Kurashige, J.; Sakamoto, Y.; Miyamoto, Y.; Ishimoto, T.; Kosumi, K.; et al. Preoperative Nutritional Assessment by Controlling Nutritional Status (CONUT) is Useful to estimate Postoperative Morbidity After Esophagectomy for Esophageal Cancer. World J. Surg. 2016, 40, 1910–1917. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Harada, K.; Baba, Y.; Kosumi, K.; Iwatsuki, M.; Kinoshita, K.; Nakamura, K.; Sakamoto, Y.; Miyamoto, Y.; Karashima, R.; et al. Preoperative controlling nutritional status (CONUT) is useful to estimate the prognosis after esophagectomy for esophageal cancer. Langenbecks Arch. Surg. 2017, 402, 333–341. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Yang, X.; Chen, Q.; Cheng, X. The usefulness of pretreatment controlling nutritional status score for predicting recurrence in patients with esophageal squamous cell carcinoma undergoing neoadjuvant immunochemotherapy: A real-world study. Front. Immunol. 2022, 13, 1015365. [Google Scholar] [CrossRef]
- Okadome, K.; Baba, Y.; Yagi, T.; Kiyozumi, Y.; Ishimoto, T.; Iwatsuki, M.; Miyamoto, Y.; Yoshida, N.; Watanabe, M.; Baba, H. Prognostic Nutritional Index, Tumor-infiltrating Lymphocytes, and Prognosis in Patients with Esophageal Cancer. Ann. Surg. 2020, 271, 693–700. [Google Scholar] [CrossRef]
- Qi, Q.; Song, Q.; Cheng, Y.; Wang, N. Prognostic Significance of Preoperative Prognostic Nutritional Index for Overall Survival and Postoperative Complications in Esophageal Cancer Patients. Cancer Manag. Res. 2021, 13, 8585–8597. [Google Scholar] [CrossRef]
- Sakai, M.; Sohda, M. ; Miyazaki, T.; Yoshida, T.; Kumakura, Y.; Honjo, H.; Hara, K.; Ozawa, D.; Suzuki, S.; Tanaka, N.; Yokobori, T.; Kuwano, H. Association of Preoperative Nutritional Status with Prognosis in Patients with Esophageal Cancer Undergoing Salvage Esophagectomy. Anticancer Res. 2018, 38, 933–938. [Google Scholar] [CrossRef]
- Nakatani, M.; Migita, K.; Matsumoto, S.; Wakatsuki, K.; Ito, M.; Nakade, H.; Kunishige, T.; Kitano, M.; Kanehiro, H. Prognostic significance of the prognostic nutritional index in esophageal cancer patients undergoing neoadjuvant chemotherapy. Dis. Esophagus. 2017, 30, 1–7. [Google Scholar] [CrossRef]
- Wang, R.; Cai, H.; Li, Y.; Chen, C.; Cui, Y. Impact Exerted by Nutritional Risk Screening on Clinical Outcome of Patients with Esophageal Cancer. Biomed. Res. Int. 2018, 2018, 7894084. [Google Scholar] [CrossRef] [PubMed]
- Movahed, S.; Norouzy, A.; Ghanbari-Motlagh, A.; Eslami, S.; Khadem-Rezaiyan, M.; Emadzadeh, M.; Nematy, M.; Ghayour-Mobarhan, M.; Varshoee Tabrizi, F.; Bozzetti, F.; Seilanian Toussi, M. Nutritional Status in Patients with Esophageal Cancer Receiving Chemoradiation and Assessing the Efficacy of Usual Care for Nutritional Managements. Asian Pac. J. Cancer Prev. 2020, 21, 2315–2323. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.B.; Liu, D.T.; Chen, Y.P. The Impact of Preoperative Nutritional Status on the Survival of Patients With Esophageal Squamous Cell Carcinoma. Front. Surg. 2021, 8, 752792. [Google Scholar] [CrossRef]
- Cao, Y.; Han, D.; Zhou, X.; Han, Y.; Zhang, Y.; Li, H. Effects of preoperative nutrition on postoperative outcomes in esophageal cancer: a systematic review and meta-analysis. Dis. Esophagus. 2022, 35, doab028. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Wan, Q.; Yu, W. ; Li, J.; Lu, S.; Xie, C.; Wang, H.; Fang, M. Pretreatment nutritional risk scores and performance status are prognostic factors in esophageal cancer patients treated with definitive chemoradiotherapy. Oncotarget 2017, 8, 98974–98984. [Google Scholar] [CrossRef]
- Noh, J.H.; Na, H.K.; Kim, Y.H.; Song, H.J.; Kim, H.R.; Choi, K.D.; Lee, G.H.; Jung, H.Y. Influence of Preoperative Nutritional Status on Patients Who Undergo Upfront Surgery for Esophageal Squamous Cell Carcinoma. Nutr. Cancer 2022, 74, 2910–2919. [Google Scholar] [CrossRef]
- Wang, J.; Yu, B.; Ye, Y.; Shen, J.; Ding, N.; Tang, H.; Xu, Y.; Song, L.; Zhu, Z.; Chen, Y.; Xie, S.; Chen, M. Predictive Value of Nutritional Risk Screening 2002 and Prognostic Nutritional Index for Esophageal Cancer Patients Undergoing Definitive Radiochemotherapy. Nutr. Cancer 2018, 70, 879–885. [Google Scholar] [CrossRef]
- Clavier, J.B.; Antoni, D.; Atlani, D.; Ben Abdelghani, M.; Schumacher, C.; Dufour, P.; Kurtz, J.E.; Noel, G. Baseline nutritional status is prognostic factor after definitive radiochemotherapy for esophageal cancer. Dis. Esophagus 2014, 27, 560–567. [Google Scholar] [CrossRef]
- Fan H, Ma W, Fu Y, Yi T, Tian J. Association of Geriatric Nutritional Risk Index with Survival Outcomes in Patients with Esophageal Squamous Cell Carcinoma: A Meta-Analysis. Nutr. Cancer 2022, 74, 2796–2802. [Google Scholar] [CrossRef]
- Zemanova, M.; Novak, F.; Vitek, P.; Pazdro, A.; Smejkal, M.; Pazdrova, G.; Petruzelka, L. Outcomes of patients with oesophageal cancer treated with preoperative chemoradiotherapy, followed by tumor resection: influence of nutritional factors. J. BUON 2012, 17, 310–316. [Google Scholar]
- Hamai, Y.; Hihara, J.; Emi, M.; Taomoto, J.; Aoki, Y.; Kishimoto, I.; Ibuki, Y.; Okada, M. Treatment outcomes and prognostic factors for thoracic esophageal cancer with clinical evidence of adjacent organ invasion. Anticancer Res. 2013, 33, 3495–3502. [Google Scholar] [PubMed]
- Di Fiore, F.; Lecleire, S.; Pop, D.; Rigal, O.; Hamidou, H.; Paillot, B.; Ducrotté, P.; Lerebours, E.; Michel, P. Baseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancer. Am. J. Gastroenterol. 2007, 102, 2557–2563. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Ida, S.; Baba, Y.; Ishimoto, T.; Kosumi, K.; Tokunaga, R.; Izumi, D.; Ohuchi, M.; Nakamura, K.; Kiyozumi, Y.; Imamura, Y.; Iwatsuki, M.; Iwagami, S.; Miyamoto, Y.; Sakamoto, Y.; Yoshida, N.; Watanabe, M.; Baba, H. Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis. Esophagus 2016, 29, 627–633. [Google Scholar] [CrossRef]
- Zemanová, M.; Staňková, B.; Ušiakova, Z.; Tvrzická, E.; Pazdro, A.; Petruželka, L.; Zeman, M. Serum adiponectin relates to shortened overall survival in men with squamous cell esophageal cancer treated with preoperative concurrent chemoradiotherapy: a pilot study. Med. Sci. Monit. 2014, 20, 2351–2357. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhang, W.; Zhang, T.; Chen, X.; Zhao, J.; Zeng, Y.; Chen, Y.; Wei, X.; Lei, T.; Wang, P.; et al. Baseline nutritional status could be a predictor for radiation esophagitis in esophageal cancer patients undergoing radiotherapy. Ann. Transl. Med. 2020, 8, 1148. [Google Scholar] [CrossRef] [PubMed]
- Lidoriki, I.; Mylonas, K.S.; Syllaios, A.; Vergadis, C.; Stratigopoulou, P.; Marinos, G.; Mastoraki, A.; Karavokyros, I.; Schizas, D. The Impact of Nutritional and Functional Status on Postoperative Outcomes following Esophageal Cancer Surgery. Nutr. Cancer 2022, 74, 2846–2858. [Google Scholar] [CrossRef]
- Hikage, M.; Taniyama, Y.; Sakurai, T.; Sato, C.; Takaya, K.; Okamoto, H.; Konno, T.; Ujiie, N.; Naitoh, T.; Unno, M.; Kamei, T. The Influence of the Perioperative Nutritional Status on the Survival Outcomes for Esophageal Cancer Patients with Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2019, 26, 4744–4753. [Google Scholar] [CrossRef]
- Horinouchi, T.; Yoshida, N.; Harada, K.; Eto, K.; Sawayama, H.; Iwatsuki, M.; Iwagami, S.; Baba, Y.; Miyamoto, Y.; Baba, H. A retrospective study of preoperative malnutrition based on the Controlling Nutritional Status score as an associated marker for short-term outcomes after open and minimally invasive esophagectomy for esophageal cancer. Langenbecks Arch. Surg. 2022, 407, 3367–3375. [Google Scholar] [CrossRef]
- Wu, C.Y.; Lin, Y.H.; Lo, W.C.; Cheng, P.C.; Hsu, W.L.; Chen, Y.C.; Shueng, P.W.; Hsieh, C.H.; Liao, L.J. Nutritional status at diagnosis is prognostic for pharyngeal cancer patients: a retrospective study. Eur. Arch. Otorhinolaryngol. 2022, 279, 3671–3678. [Google Scholar] [CrossRef]
- Tu, X.; Ren, J.; Zhao, Y. Prognostic value of prognostic nutritional index in nasopharyngeal carcinoma: A meta-analysis containing 4511 patients. Oral Oncol. 2020, 110, 104991. [Google Scholar] [CrossRef]
- Li, P.; Wang, X.; Lai, Y.; Zhou, K.; Tang, Y.; Che, G. The prognostic value of pre-treatment prognostic nutritional index in esophageal squamous cell carcinoma: A meta-analysis. Medicine (Baltimore) 2019, 98, e15280. [Google Scholar] [CrossRef] [PubMed]
- Topkan, E.; Yucel Ekici, N.; Ozdemir, Y.; Besen, A.A.; Mertsoylu, H.; Sezer, A.; Selek, U. Baseline Low Prognostic Nutritional Index Predicts Poor Survival in Locally Advanced Nasopharyngeal Carcinomas Treated With Radical Concurrent Chemoradiotherapy. Ear. Nose Throat J. 2021, 100, NP69–NP76. [Google Scholar] [CrossRef]
- Küçükarda, A.; Erdoğan, B.; Gökyer, A.; Sayın, S.; Gökmen, İ.; Özcan, E.; Hacıoğlu, M.B.; Uzunoğlu, S.; Çiçin, İ. Prognostic nutritional index and its dynamics after curative treatment are independent prognostic factors on survival in non-metastatic nasopharyngeal carcinoma. Support Care Cancer 2022, 30, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.H.; Hsieh, J.C.; Yeh, K.Y.; Chen, E.Y.; Yang, S.W.; Huang, J.S.; Lai, C.H.; Wu, T.H.; Huang, Y.M.; Chang, Y.S.; Chou, W.C.; Wang, C.H. Prognostic nutritional index relevance in chemoradiotherapy for advanced oral cavity, oropharyngeal and hypopharyngeal cancer. Asia Pac. J. Clin. Nutr. 2018, 27, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.L.; Oei, R.W.; Kong, F.F.; Du, C.R.; Zhai, R.P.; Ji, Q.H.; Hu, C.S.; Ying, H.M. The prognostic value of preoperative prognostic nutritional index in patients with hypopharyngeal squamous cell carcinoma: a retrospective study. J. Transl. Med. 2018, 16, 12. [Google Scholar] [CrossRef]
- Deng, J.; He, Y.; Sun, X.S.; Li, J.M.; Xin, M.Z.; Li, W.Q.; Li, Z.X.; Nie, S.; Wang, C.; Li, Y.Z.; et al. Mai, H.Q. Construction of a comprehensive nutritional index and its correlation with quality of life and survival in patients with nasopharyngeal carcinoma undergoing IMRT: A prospective study. Oral Oncol. 2019, 98, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.Y.; Deng, J.; Su, D.F.; Li, W.Q.; Han, Y.; Li, Z.X.; Huan, X.Z.; Zhu, S.H.; Yang, Q.L.; Hu, W.; Xin, M.Z.; Tang, L.Q.; Mai, H.Q.; Fan, Y.Y.; He, Y. Construction of a comprehensive nutritional index and comparison of its prognostic performance with the PNI and NRI for survival in older patients with nasopharyngeal carcinoma: a retrospective study. Support Care Cancer 2021, 29, 5371–5381. [Google Scholar] [CrossRef]
- Su, L.; Lin, Q.; Li, R.; Hua, Y.; Zhang, H.; Song, X.; Ye, J.; Zhang, W.; Hong, J. Prognostic value of nutritional impairment on treatment-related toxicity and survival in patients with nasopharyngeal carcinoma taking normal nutrition before radiotherapy. Head Neck 2020, 42, 3580–3589. [Google Scholar] [CrossRef]
- Song, X.; Su, L.; Lin, Q.; Liu, S.; Zhang, W.; Hong, J. Effect of nutritional status before radiotherapy on radiation-induced acute toxicities in patients with nasopharyngeal carcinoma. Head Neck 2023, 45, 620–628. [Google Scholar] [CrossRef]
- Hong, J.S.; Hua, Y.J.; Su, L.; Zhang, H.R.; Lv, W.L.; Chen, X.Y.; Tian, J.; Zhang, W.J. Modified-Nutrition Index is a Significant Prognostic Factor for the Overall Survival of the Nasopharyngeal Carcinoma Patients who Undergo Intensity-modulated Radiotherapy. Nutr. Cancer 2017, 69, 1011–1018. [Google Scholar] [CrossRef]
- Peng, H, Chen, B. B.; Tang, L.L.; Chen, L.; Li, W.F.; Zhang, Y.; Mao, Y,P,; Sun, Y.; Liu, L.Z.; Tian, L.; Guo, Y.; Ma, J. Prognostic value of nutritional risk screening 2002 scale in nasopharyngeal carcinoma: A large-scale cohort study. Cancer Sci. 2018, 109, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Ikeguchi, M. Glasgow prognostic score and neutrophil-lymphocyte ratio are good prognostic indicators after radical neck dissection for advanced squamous cell carcinoma in the hypopharynx. Langenbecks Arch. Surg. 2016, 401, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Gao, J.; Liu, Z.G.; Tao, Y.L.; Xu, B.Q.; Tu, Z.W.; Zhang, X.P.; Zeng, M.S.; Xia, Y.F. Influence of pretreatment ideal body weight percentile and albumin on prognosis of nasopharyngeal carcinoma: Long-term outcomes of 512 patients from a single institution. Head Neck 2014, 36, 660–666. [Google Scholar] [CrossRef]
- Bozec, A.; Benezery, K.; Chamorey, E, Ettaiche, M. ; Vandersteen, C.; Dassonville, O.; Poissonnet, G.; Riss, J.C.; Hannoun-Lévi, J.M.; Chand, M.E.; Leysalle, A.; Saada, E.; Sudaka, A.; Haudebourg, J.; Hebert, C.; Falewee, M.N.; Demard, F.; Santini, J.; Peyrade F. Nutritional status and feeding-tube placement in patients with locally advanced hypopharyngeal cancer included in an induction chemotherapy-based larynx preservation program. Eur. Arch. Otorhinolaryngol. 2016, 273, 2681–2687. [Google Scholar] [CrossRef] [PubMed]
- Jager-Wittenaar H, Dijkstra PU, Vissink A, van Oort RP, van der Laan BF, Roodenburg JL. Malnutrition in patients treated for oral or oropharyngeal cancer--prevalence and relationship with oral symptoms: an explorative study. Support Care Cancer 2011, 19, 1675–1683. [Google Scholar] [CrossRef]
- Jogiat, U.M.; Sasewich, H.; Turner, S.R.; Baracos, V.; Eurich, D.T.; Filafilo, H.; Bédard, E.L.R. Sarcopenia Determined by Skeletal Muscle Index Predicts Overall Survival, Disease-free Survival, and Postoperative Complications in Resectable Esophageal Cancer: A Systematic Review and Meta-analysis. Ann. Surg. 2022, 276, e311–e318. [Google Scholar] [CrossRef]
- Leoncini, E.; Vukovic, V.; Cadoni, G.; Giraldi, L.; Pastorino, R.; Arzani, D.; Petrelli, L.; Wünsch-Filho, V.; Toporcov, T.N.; Moyses, R.A.; et al. Tumour stage and gender predict recurrence and second primary malignancies in head and neck cancer: a multicentre study within the INHANCE consortium. Eur. J. Epidemiol. 2018, 33, 1205–1218. [Google Scholar] [CrossRef]
- Ravasco, P.; Monteiro Grillo, I.; Camilo, M. Cancer wasting and quality of life react to early individualized nutritional counselling! Clin Nutr. 2007, 26, 7–15. [Google Scholar] [CrossRef]
- Ravasco, P. Nutrition in Cancer Patients. J. Clin. Med. 2019, 8, 1211. [Google Scholar] [CrossRef]
- Cao, J.; Xu, H.; Li, W.; Guo, Z.; Lin, Y.; Shi, Y.; Hu, W.; Ba, Y.; Li, S.; Li, Z.; et al. Investigation on Nutrition Status and Clinical Outcome of Common Cancers (INSCOC) Group, Chinese Society of Nutritional Oncology. Nutritional assessment and risk factors associated to malnutrition in patients with esophageal cancer. Curr. Probl. Cancer 2021, 45, 100638. [Google Scholar] [CrossRef]
| Number of patients and cancer type | Assessment tool | Results | Reference |
|---|---|---|---|
| 258 patients randomly assigned to definitive chemoradiotherapy (dCRT) +/- cetuximab | NRI | Baseline NRI<100 predicted worse overall survival. | Cox 2016 [20] |
| Retrospective study on 69 advanced oesophageal carcinoma patients, aged 18-80 years, treated with Immune Checkpoint Inhibitor (ICI) | CONUT | CONUT score (cut-off point =1) was an independent prognosticator for overall survival and progression-free survival in patients undergoing ICI. | Chang 2022 [24] |
| Retrospective study of 148 patients with oesophageal squamous cell carcinoma who underwent potentially curative esophagectomy (complete resection) | CONUT | CONUT score was an independent prognosticator for cancer-specific survival in patients aged < 70 years old. | Hirahara 2018 [25] |
| Retrospective study of 352 patients who underwent elective oesophagectomy with lymphadenectomy for oesophageal carcinoma | CONUT | Malnourished patients had a substantial greater prevalence of any morbidity, serious morbidities, and surgical site infection. Hospitalization of malnourished patients was considerably longer. Moderate or severe malnutrition was an independent risk factor for any morbidity and serious morbidities. | Yoshida 2016 [26] |
| Retrospective study of 373 patients who underwent three-incision oesophagectomy with 2- or 3-field lymphadenectomy for oesophageal carcinoma | CONUT | CONUT score was able to predict malnutrition and acted as a prognosticator of overall and disease-specific survival in patients undergone surgery. | Yoshida 2017 [27] |
| 216 patients with oesophageal squamous cell carcinoma, receiving neoadjuvant immunochemotherapy | CONUT | ↑CONUT score (cut-off point=3): ↑risk of relapse. CONUT score: independent prognosticator for disease-free survival at one year. |
Feng 2022 [28] |
| Database of 337 curatively resected oesophageal cancers | PNI | ↓PNI led to considerably poorer overall survival in both univariate and multivariate analysis. | Okadome 2020 [29] |
| Retrospective study of 407 oesophageal carcinoma patients who underwent esophagectomy | PNI | Multivariable analysis identified PNI as an independent prognosticator for overall survival and post-operative complications. | Qi 2021 [30] |
| retrospective study with 32 patients with oesophageal squamous cell carcinoma who underwent salvage oesophagectomy | PNI | PNI (cut-off point=45) was an independent prognostic preoperative factor for overall survival after adjustment for age, clinical response, and preoperative PNI. PNI was not a prognosticator for disease-specific survival. |
Sakai 2018 [31] |
| 66 squamous cell oesophageal carcinoma patients undergoing neoadjuvant chemotherapy | PNI | The mean pre-treatment and preoperative PNI were 50.2 ± 5.7 and 48.1 ± 4.7, respectively. PNI decreased following chemotherapy in 66.7% patients. Pre-chemotherapy PNI and preoperative PNI were considerably correlated with the overall survival and relapse-free survival rates. In multivariate analysis, only preoperative PNI was an independent prognosticator for worse overall and relapse-free survival. |
Nakatani 2017 [33] |
| 97 esophageal carcinoma patients earlier cured with definitive chemo-radiotherapy (CRT) | PNI | ↑Baseline PNI or PNI at the end of CRT (≥45) was related with better 2-year overall survival. PNI was a prognosticator for overall, but not for progression-free survival, organ metastasis-free survival, or local regional recurrence-free survival post CRT. | Wang 2018 [33] |
| 71 newly diagnosed patients followed for 1 year | PG-SGA | 1 year mortality was considerably associated with reduced BMI after CRT, primary PG-SGA score, weight loss, BMI<18.5, MUAC, physical performance, living in rural or urban areas, and addiction. | Movahed 2020 [34] |
| Retrospective study on 340 esophageal-squamous cell carcinoma patients who completed curative treatment | PG-SGA | Well-nourished patients benefited from surgery. Malnutrition was associated with worse prognosis, regarding metastases and survival. |
Chen 2021 [35] |
| 202 patients with unresectable locally advanced oesophageal carcinoma (stages 3 and 4) who were treated with definitive concurrent chemoradiotherapy | NRS-2002 | NRS-2002 score (cut-off point ≥3) (was an independent prognosticator for the response to chemoradiotherapy, overall survival and progression-free survival. | Song 2017 [37] |
| 274 patients (stages 1 to 3, median age 63 years) undergone direct surgery for oesophageal squamous cell carcinoma, with a median follow-up of 55 months | NRS-2002 | Overall survival rates were shorter in the NRS 2002 group with elevated scores. A high NRS 2002 score was related with more frequent postoperative complications. | Noh 2022 [38] |
| 97 oesophageal carcinoma patients treated with CRT | NRS-2002 | NRS-2002 score 3 at baseline was associated with better 2-year overall survival than NRS-2002 score ≥4. NRS-2002 score at baseline was an independent risk factor for prognosis. |
Wang 2018 [39] |
| Retrospective study with 143 patients with oesophageal squamous cell carcinoma and adenocarcinoma followed for 20.8 months | NRI | Overall Survival: NRI > 97.5 and PS = 0 were independent predictive factors. Disease-free survival: NRI > 97.5 and PS = 0 were independent predictive factors. |
Clavier 2014 [40] |
| Meta-analysis of 8 retrospective studies with 1460 oesophageal squamous cell carcinoma patients | GNRI | Low GNRI was correlated with shorter overall and cancer-specific survival. | Fan 2022 [41] |
| Retrospective study on 107 esophageal carcinoma patients cured with neoadjuvant CRT and surgery | Weight status, Performance statusAlbumin | Low PS, serious dysphagia, above-average pre-treatment weight decline, weight decrease >5% throughout CRT, and serum albumin ≤ 35 g/l prior to or next to CRT implied shorter survival times. Serum albumin concentrations, nasogastric tube insertion and pretreatment body weight decline were independent prognosticators for overall survival. Serum albumin concentrations, along with nasogastric tube insertion after CRT was prognostic factor for time to progression. |
Zemanova 2012 [42] |
| Retrospective study on 74 patients with locally advanced esophageal carcinoma with adjacent organ invasion | AlbuminandHemoglobin | Younger age (<60 years) and hemoglobin ≥13 g/dl were independent predictors of favorable treatment outcome.Pre-treatment higher serum albumin (≥3.5 g/dl) was an independent favorable predictor of patients’ survival. | Hamai 2013 [43] |
| Retrospective study on 105 non-metastatic patients with a locally advanced oesophageal carcinoma cured with definitive CRT | Albumin | Serum albumin > 35 g/L was the only independent predictive factor of complete therapy response. Independent prognostic factors of survival were BMI > 18 Kg/m2, dysphagia Atkinson score <2, dose of RT > 50 Grays and CR to CRT were independent prognosticators of favorable patients’ survival. |
Di Fiore 2007 [44] |
| Retrospective study on 325 oesophageal squamous cell carcinoma patients (256 surgical and 69 dCRT cases) | Sarcopenia | Sarcopenia substantially lowered the overall survival of patients without lymph node metastasis, but not in patients presenting lymph involvement. | Harada 2015 [45] |
| Retrospective study on 42 patients, treated with a multimodal regimen of simultaneous neoadjuvant CRT, followed by surgery. | AdiponectinSerum albumin, and Cholesterol | In univariate analysis, elevated serum adiponectin was linked with poorer overall survival, while elevated serum albumin, and cholesterol were associated with favorable overall survival. In multivariate analysis, only a tendency for negative serum adiponectin relationship with the overall survival was noted. | Zemanova 2014 [46] |
| Retrospective study on 100 patients with oesophageal carcinoma cured with definitive chemoradiotherapy, preoperative chemoradiation and definitive radiotherapy | PG-SGABMI%Weight loss in 3 months Albumin Hemoglobin CRP GPS | PG-SGA score ≥9 was recognized as an independent predictor of radiation oesophagitis. | Dong 2020 [47] |
| 70 patients with oesophageal and gastroesophageal junction carcinoma who underwent oesophagectomy | GNRIAlbuminMuscle mass%weight loss | Albumin and GNRI were decreased in patients developing severe complications compared to patients without postoperative complications. Major complications were related with ↑%weight loss and with ↓ handgrip strength. Albumin and poor muscle mass were considerably correlated with anastomotic leakage occurrence. |
Lidoriki 2022 [48] |
| Retrospective study on 141 oesophageal carcinoma patients undergone neoadjuvant chemotherapy after radical oesophagectomy | CONUTPNI | In multivariate analysis, malnutrition 14 days after surgery according to CONUT and ↓PNI before surgery were identified as independent prognosticators of overall patients’ survival. | Hikage 2019 [49] |
| c674 patients who underwent three-incision oesophagectomy for oesophageal carcinoma | CONUT | Malnutrition according to CONUT was an independent risk factor for severe, respiratory, and cardiovascular morbidities after surgical operation. | Horinouchi 2022 [50] |
| Number of patients and cancer type | Assessment tool | Results | Author, date |
|---|---|---|---|
| 319 pharyngeal cancer patients with nasopharyngeal carcinoma, oropharyngeal carcinoma and hypopharyngeal carcinoma | BMI, hemoglobin, albumin, PNI, NRI, HALP | HALP score was an independent factor for overall survival after adjusting for sex, age, tumor site, clinical stage, and BMI. PNI was the most important independent factor for overall and cancer-specific survival. |
Wu 2022 [51] |
| Meta-analysis of 10 studies with 4511 patients with nasopharyngeal carcinoma | PNI | Patients with decreased PNI exhibited a worse overall, distant metastasis-free, progression-free and locoregional recurrence-free survival. Subgroup analysis: ↓ PNI was a significant prognostic factor for overall and distant metastasis-free survival. |
Tu 2020 [52] |
| 154 patients presenting locoregionally advanced nasopharyngeal cancer treated with concurrent chemoradiotherapy | PNI | ↓ baseline PNI (cut-off point=51) is an independent prognosticator for overall, cancer-specific, locoregional progression-free, organ metastasis-free, and progression-free survival. | Topkan 2021 [54] |
| 107 non-metastatic nasopharyngeal carcinoma patients | PNI | Pre- and post-treatment PNI were independent predictors for overall survival. | Küçükarda 2022 [55] |
| 143 patients presenting stage III, IVA, and IVB pharyngeal cancers who were treated with concurrent chemoradiotherapy | PNI | Patients with ↓ PNI had higher likelihoods of grade 3/4 hematological toxicities, sepsis and toxic death. Patients with ↓ PNI were less probable to tolerate concurrent chemoradiotherapy, even when they treated with a considerably lower dosage of cisplatin, showing a decreased completion rate of planned radiotherapy, or a longer overall radiotherapy treatment time. |
Chang 2018 [56] |
| 123 hypopharyngeal squamous cell carcinoma patients treated with radical surgery | PNI | ↑ preoperative PNI was an independent prognosticator for better overall, progression-free, locoregional recurrence-free and organ metastasis-free survival. | Ye 2018 [57] |
| 359 newly diagnosed nasopharyngeal cancer patients undergoing intensity-modulated radiation therapy | CNI | CNI decreased after therapy. CNI was an independent prognosticator of overall survival. |
Deng 2019 [58] |
| Retrospective study with 309 older nasopharyngeal carcinoma patients | CNI | CNI was an independent prognosticator of overall and disease-free survival. Kaplan-Meier analysis indicated that reduced CNI was correlated with unfavorable overall and disease-free survival. |
Duan 2021 [59] |
| 187 nasopharyngeal cancer patients who had a normal nutrition before treatment | modified Nutrition Index (m-NI) | Severe nutritional impairment, assessed as a reduction in m-NI score ≥50%, was an independent prognosticator for overall survival and an independent risk factor for grade ≥2 oral mucositis. | Su 2020 [60] |
| 228 nasopharyngeal cancer patients with NPC treated with intensity-modulated radiotherapy | modified Nutrition Index |
m-NI ≤6 was a risk factor for xerostomia, oral mucositis, dysgeusia and dysphagia. Total score of radiation-induced acute toxicities of malnourished patients was considerably greater compared to that of patients with normal nutrition. |
Song 2023 [61] |
| 323 patients with nasopharyngeal carcinoma undergoing intensity-modulated radiotherapy | modified Nutrition Index |
The 1-, 3-, and 5-year overall survival rates between malnutrition and normal nutrition groups assessed by m-NI were 93.0% vs. 96.9%, 76.4% vs. 82.8%, and 61.8% vs. 77.1%, respectively. Regression analysis indicated that m-NI was an independent prognosticator for overall survival. |
Hong 2017 [62] |
| 3232 nasopharyngeal carcinoma patients from big-data database | NRS-2002 | NRS2002 ≤3 vs >3 had significantly different 5-year disease-free, overall, distant metastasis-free and locoregional relapse-free survival. | Peng 2018 [63] |
| 59 patients presenting clinical stage III and IV hypopharyngeal squamous cell carcinoma who underwent pharyngo-laryngo-cervical esophagectomy with definitive tracheostomy followed by free jejunal graft reconstruction. | GPSNLR | ↑ GPS (1 or 2) and ↑NLR (≥5) were independent unfavorable prognosticators for 5-year overall survival. | Ikeguchi 2016 [64] |
| Prospective survey on 512 patients with nasopharyngeal carcinoma undergone radical RT | Weight status (ideal body weight, IBW)Albumin | Prior to radiotherapy, IBW% <90% was associated with shorter overall and organ metastasis-free survival. Albumin ≤43.0 g/L was related to shorter overall and metastasis-free survival. |
Li 2014 [65] |
| 53 patients presenting locally advanced hypopharyngeal carcinoma (stage 3 and 4) assigned to an induction chemotherapy (ICT)-based larynx preservation program without prophylactic feeding-tube placement | Weight loss | Maximum weight loss was considerably correlated with a greater probability of enteral tube feeding during treatment and a higher likelihood of complications during radiotherapy. | Bozec 2016 [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





