1. Introduction
Prosthetic joint infection (PJI) is a complication that occurs approximately in 1%-2% patients undergoing prosthetic surgery and it is related to important morbidity and mortality rates, as well as sanitary costs [
1,
2,
3].
As the main causative factor associated with PJI is microorganisms’ ability to form a biofilm on the prosthesis surface, it makes difficult its eradication [
4,
5,
6]. The conventional management is based on radical debridement, one or two stage or even resection arthroplasty, combined with different antibiotic protocols [
7,
8,
9,
10].
Regarding the use of antibiotics loaded in bone cement when two stage replacement is approached, polymethylmethacrylate (PMMA) is the most commonly used due to the wide experience in its use, as well as its high safety profile [
11,
12]. Even few antibiotics are recommended to be used loaded in bone cement, we recently described, for the first time, that dalbavancin was a suitable alternative to be used in bone cement based on its good elution capacity during a 14-day
in vitro study period [
13]. However, it was needed to assess both whether elution is maintained for a long period and the capacity of eluted dalbavancin to reduce Staphylococcal biofilm.
Therefore, we aimed to assess the elution capacity and the anti-biofilm activity of dalbavancin and compare it with that of vancomycin both at 2.5% and 5% concentrations up to a 3-month period.
2. Results
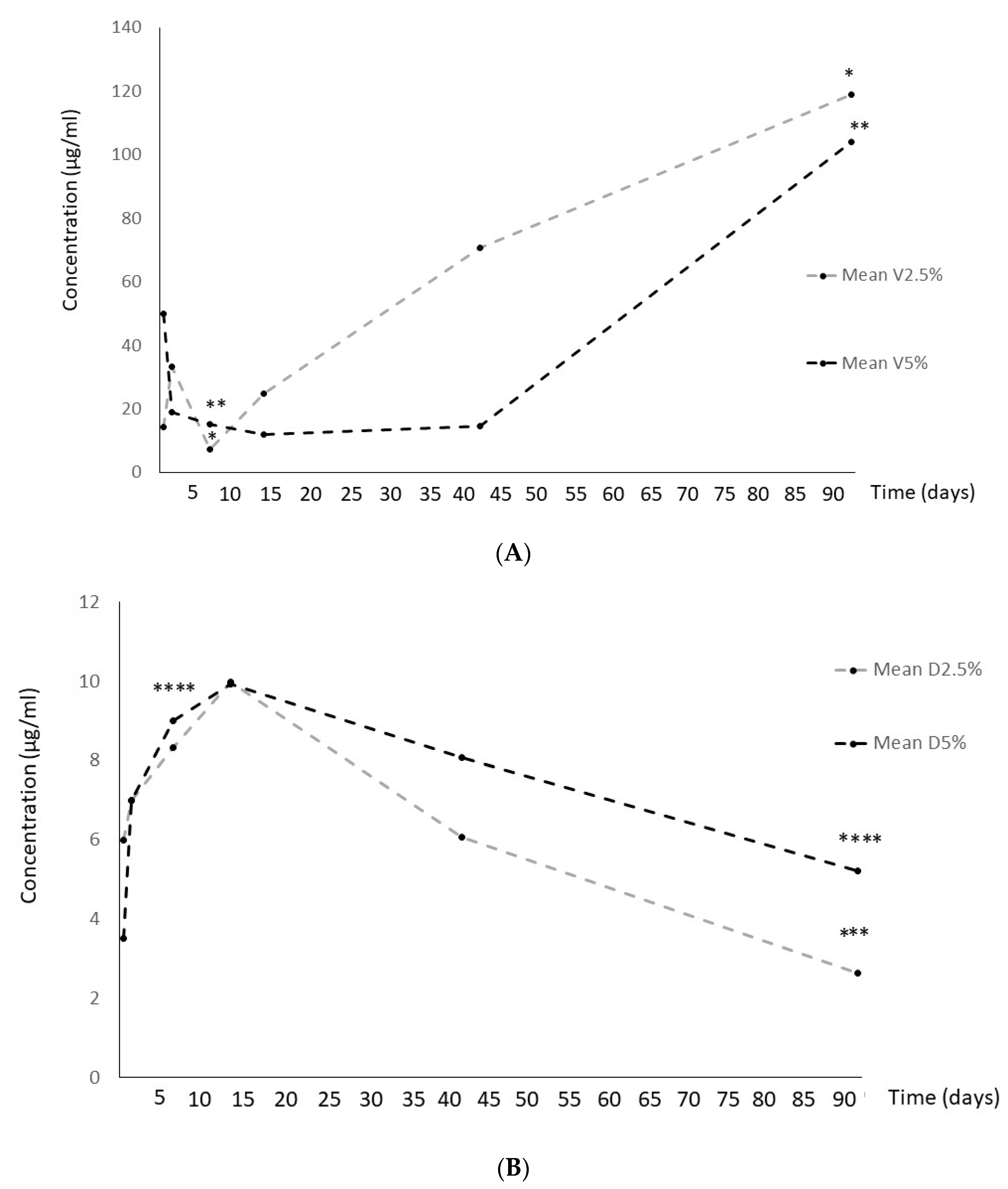
2.1. Antibiotic concentrations
Despite vancomycin showed a significant concentration decrase at 2 weeks, then it gradually increased significantly both at 2.5% and 5% formulations (
Figure 1A).
In contrast, dalbavancin reached its highest concentration at 2 weeks and then it gradually decreased at both formulations used (
Figure 1B).
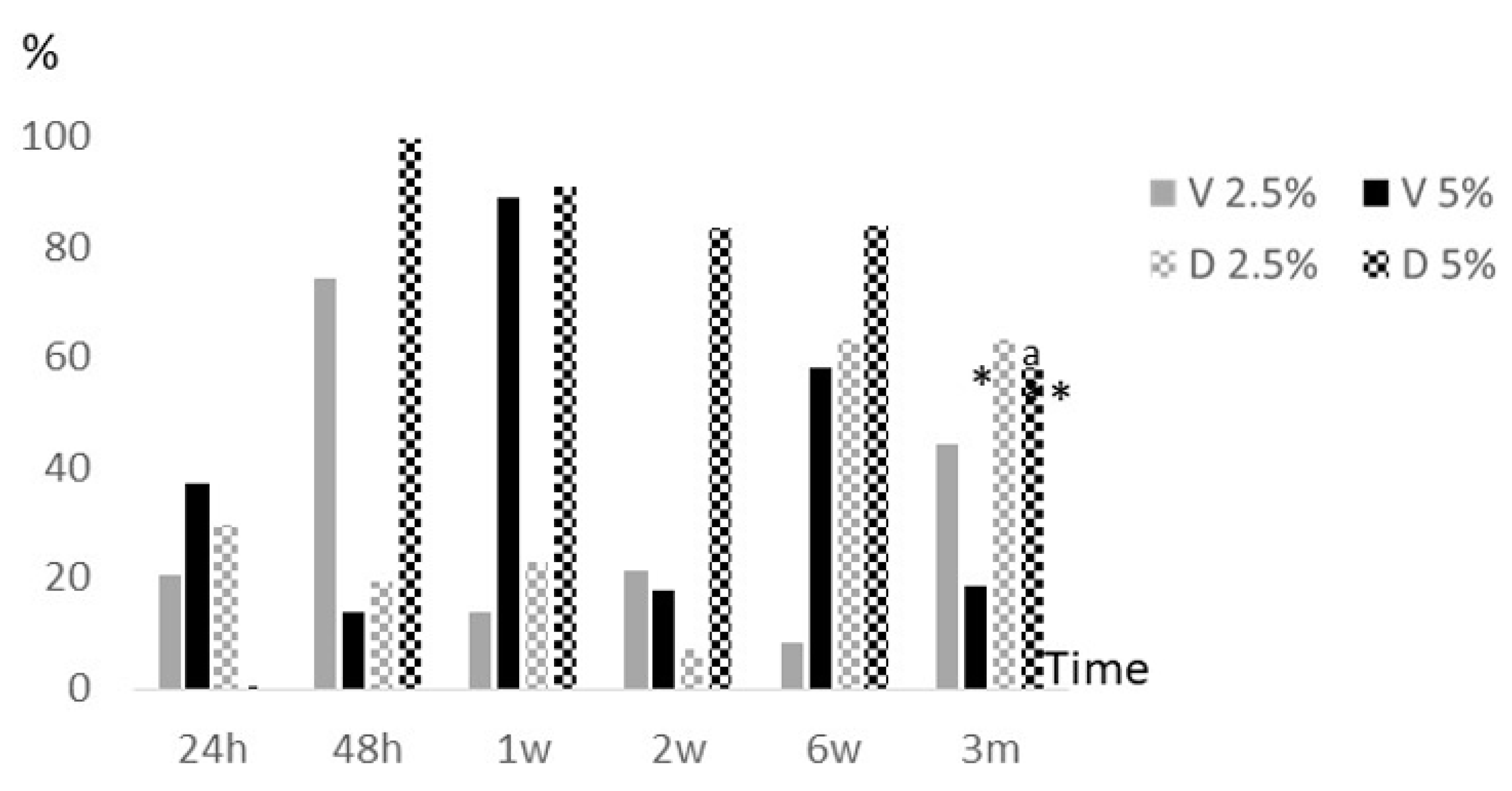
2.2. Anti-biofilm activity of eluted antibiotics
Overall, we observed a significant decrease of median (IQR) percentage reduction of metabolic activity at the final study period (3 months) with respect to 24 hours and 1 week in 5% vancomycin (8.7% [0.0%-47.5%] vs. 42.6% [20.2%-49.1%], p=0.0044; and 90.5% [83.6%-93.0%], p<0.001; respectively). In addition, it was also observed a significant reduction of median (IQR) percentage reduction of metabolic activity at 3 months with respect 1 week in 5% dalbavancin (43.9% [35.4%-95.3%] vs. 89.3% [84.1%-100%], p=0.044) (
Figure 2).
Regarding differences between both antibiotics, it was at month 3 where statistical significant decrease of median (IQR) percentage reduction of metabolic activity was observed in 5% vancomycin vs. 5% dalbavancin (8.7% [0.0%-47.5%] and 43.9% [35.4%-95.3%], p=0.044; respectively) (
Figure 2).
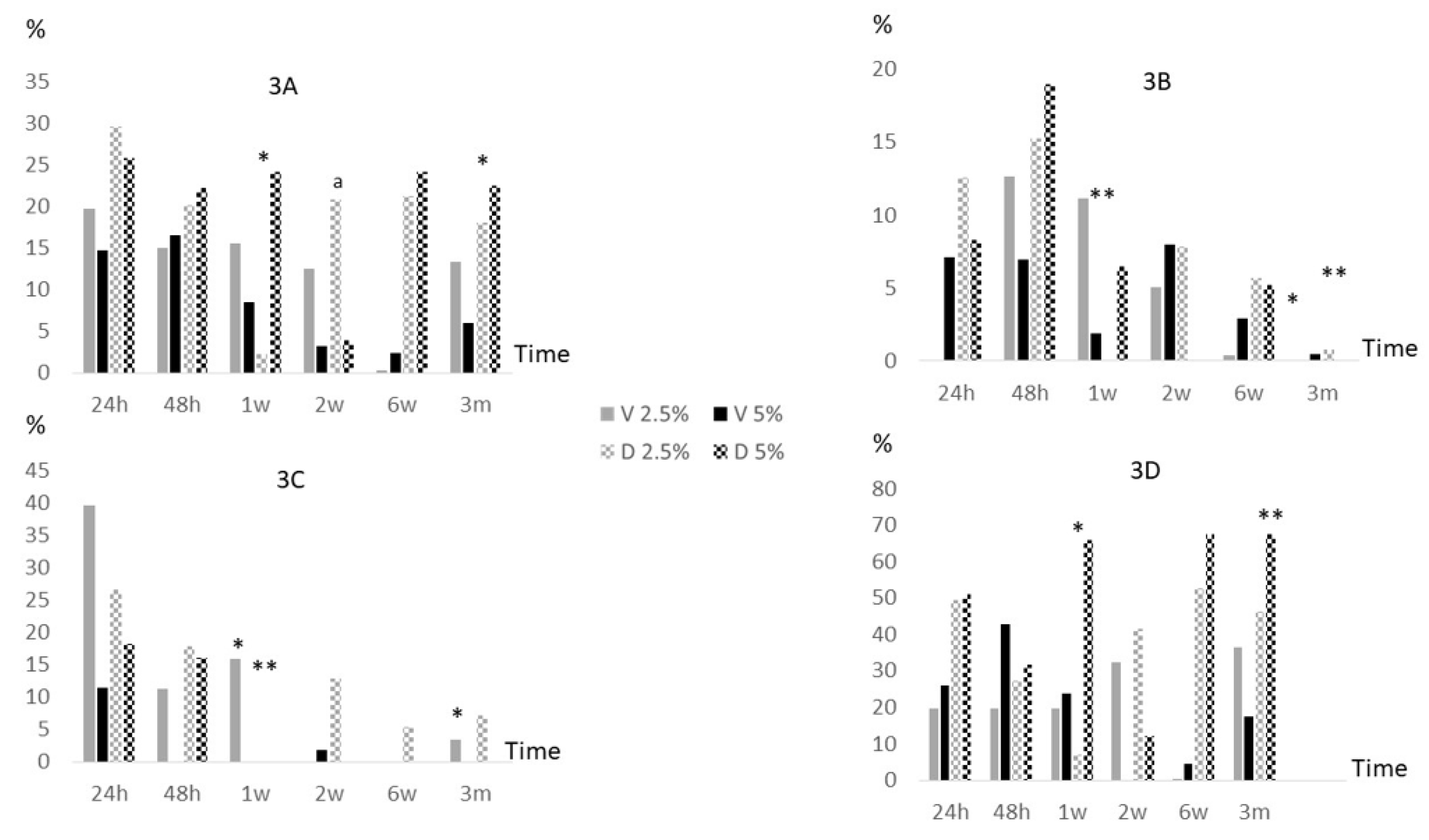
2.3. Anti-biofilm activity of antibiotics loaded in bone cements
Overall, median percentage reduction of log cfu/ml at the final study period (3 months) had no significant changes neither in vancomycin nor in dalbavancin at both concentrations used with respect to values at 24 hours. In between periods, it was only detected a slightly decrease of median (IQR) log cfu/ml percentage reduction between 24 hours and 1 week and between 1 week and 3 months in 2.5% dalbavancin (25.4% [14.8%-48.4%] vs. 0.0% [0.0%-0.6%], p=0.001; and 0.0% [0.0%-0.6%] vs. 2.9% [0.0%-35.7%], p=0.042 respectively) (
Figure 3A). In addition, when both antibiotics were compared, the median (IQR) percentage reduction of log cfu/ml at 2 weeks were significantly higher in 2.5% dalbavancin vs. 2.5% vancomycin (4.5% [0.0%-23.0%] vs. 11.2% [8.1%-28.6%], p=0.037).
Particularly, in MSSA, we only observed a statistically significant decrease of median (IQR) log cfu/ml percentage reduction between 24 hours and 3 months in 2.5% dalbavancin (12.2% [7.0%-NA], 0.0% [0.0%-NA], p=0.046). In between periods, 2.5% vancomycin at 1 week vs. 3 months and 2.5% dalbavancin at 24 hours vs. 1 week also showed a significant decrease (10.9% [9.3%-NA] vs. 0.0% [0.0%-NA], p=0.037; and 12.2% [7.0%-NA] vs. 0.0% [0.0%-NA], p=0.037) (
Figure 3B). In MRSA, only 2.5% vancomycin showed a significant decrease of median (IQR) log cfu/ml percentage reduction between 24 hours and 3 months (38.6% [32.3%-NA] vs. 0.0% [0.0%-NA], p=0.046). In between periods, also 2.5% vancomycin at 24 hours vs. 1 week and 1 week vs. 3 months showed a significant decrease (38.6% [32.3%-NA] vs. 16.6% [13.1%-NA], p=0.05; and 16.6% [13.1%-NA] vs. 0.0% [0.0·-NA], p=0.046). Moreover, 2.5% dalbavancin showed a significant decrease of median (IQR) log cfu/ml percentage reduction between 24 hours and 1 week (25.4% [17.3%-NA] vs. 0.0% [0.0%-NA], p=0.037) (
Figure 3C). In S
. epidermidis, only 2.5% dalbavancin showed a significant decrease of median (IQR) log cfu/ml percentage reduction between 24 hours and 1 week (52.0% [44.8%-NA] vs. 1.2% [0.0%-NA], p=0.046). In addition, median (IQR) log cfu/ml percentage reduction significantly decreased in 5% vancomycin compared to 5% dalbavancin at 3 months (17.4% [15.5%-NA] vs. 67.5% [67.5%-67.5%], p=0.037) (
Figure 3D).
3. Discussion
Despite dalbavancin is approved for the treatment of skin and soft tissue infections, successful results in the outcome of patients with PJI have been widely demonstrated [
14,
15], as well as being cost-effective [
16].
This therapeutic success of dalbavancin in the treatment of PJI is mainly related to the eradication of bacterial biofilms, to which dalbavancin is highly active [
17]. Particularly, in the study of Pfaller et al., 800
S. aureus strains from United States and European hospitals isolated between 2011 and 2016 were tested against several antimicrobials, and minimum inhibitory concentration (MIC) for dalbavancin was at least 8-fold lower than others [
18]. Regarding minimal biofilm eradication concentration (MBEC
90) values for dalbavancin, Sivori et al. showed that they were significantly lower than those of linezolid and vancomycin in 32 MRSA tested strains. In addition, they demonstrated that its activity was affected by an increased concentration of extracellular DNA in the biofilm matrix [
19].
Moreover, in a time-kill kinetics in vitro model, dalbavancin was tested against
S. aureus and
S. epidermidis biofilms up to 7 days and it showed a higher activity than vancomycin [
20].
Therefore, based on these data and on the demonstration that it had excellent bone distribution, the potential use of dalbavancin loaded in bone cement is an attractive approach for the treatment of PJI [
21]. As we demonstrated in a previous study of our group, dalbavancin showed a good elution capacity when loaded in bone cement remaining almost to 14 days [
13], so we now have confirmed that eluted dalbavancin both from 2.5% and 5% formulations maintained concentrations above 2 µg/ml up to 3 months. In addition, despite specific fluctuations were observed in between periods, dalbavancin had efficacy enough to reduce 24h-Staphylococcal biofilms, as median percentage reduction of log cfu/ml at 3 months had no significant changes neither in vancomycin nor in dalbavancin at both concentrations used with respect to values at 24 hours. Particularly, it was mainly in S
. epidermidis where significant differences were observed between median (IQR) log cfu/ml percentage reduction of 5% vancomycin and 5% dalbavancin at 3 months (17.4% [15.5%-NA] vs. 67.5% [67.5%-67.5%], p=0.037). It was also important to highlight that median (IQR) percentage reduction of metabolic activity of Staphylococcal biofilms exposed to eluted antibiotics was significantly lower in 5% vancomycin compared to 5% dalbavancin
(8.7% [0.0%-47.5%] vs. 43.9% [35.4%-95.3%], p=0.044; respectively).
Regarding the significant differences found in the release profiles between the two antibiotics, it may be explained because of the high solubility and smaller size of the vancomycin molecule with respect to dalbavancin. It seems that vancomycin suffered an initial degradation at the beginning of the study period reflected in the reduction of concentration and, then, due to its high elution capacity, concentration became to accumulate. In contrast, dalbavancin reached its highest concentration at 2 weeks and it became to degrade, as there is no more elution. These data differ from our previous study because we there analyzed cumulative concentration [
13]. Either way, although the concentration of dalbavancin recovered during elution is lower than that of vancomycin, it is higher than the MIC against staphylococci.
Despite our findings are promising, complete biofilm eradication was never achieved. This is in concordance with those results obtained by Silva et. al, in which they reported in an
in vivo mouse model that, although administering intraperitoneal dalbavancin for 14 days decreased cfu/gr and cfu/ml both in the tibia and the implant, there were still signs of biofilm-induced infection [
22]. Therefore, new approaches are needed to improve dalbavancin activity in bone cements, such as combining it with rifampicin, as it has been previously reported [
23,
24,
25].
One of the main limitations of our study is that we used an in vitro static biofilm model which may not represent the real clinical scenario. Future in vivo or dynamic models mimicking PJI are needed to corroborate our findings. Moreover, performing cytotoxicity experiments using dalbavancin loaded in bone cements are also essential.
4. Materials and Methods
The study was carried out in a tertiary teaching hospital in Madrid, Spain.
4.1. Preparation of the Antibiotic-Loaded Bone Cements
Palacos
® R bone cements (Heraeus Medical GmbH, Wehrheim, Germany) were manually mixed with either vancomycin or dalbavancin powder in proportions of 2.5% (1 g of antibiotic per 40 g of cement) and 5% (2 g of antibiotic per 40 g of cement). Discs of 3x6 mm were prepared using a mould (
Figure S1). Discs were incubated in phosphate-buffered saline (PBS) at 37 °C under shaking (150 rpm) for 24 hours, 48 hours, 1 week, 2 weeks, 6 weeks, and 3 months. All experiments were performed in triplicate and negative controls were also included. At each time, 1 mL aliquots of the eluate were obtained and frozen for the following analysis: high-performance liquid chromatography (vancomycin) or mass cytometry (dalbavancin) and anti-biofilm activity against 24h-biofilms in well plates. Likewise, discs were rinsed with PBS for the anti-biofilm experiments in steel implants at each study period (
Figure S1).
4.2. Vancomycin Analysis
Samples were analyzed using an HPLC with a Varian Prostar 230 Solvent Delivery Module, Varian Prostar autosampler 410, and Varian Prostar 310 UV–visible detector (Varian, Palo Alto, CA, USA). Peak integration was analysed using the Galaxie software (version 1.9). Vancomycin was analyzed using a Nucleosil C18 column (250 mm × 4.6 mm, 5 μm). The mobile phase consisted of 50 mM ammonium phosphate: acetonitrile (92:8, v:v), with a final pH of 2.2, which was pumped at a flow rate of 0.7 mL/min and the injection volume of the sample was 40 µL. The column temperature was maintained at 25 °C and the detector was set at 205 nm.
4.3. Dalbavancin Analysis
Samples were analyzed using LC-ESI-QQQ-MS (LC-8030 Shimadzu, Manchester, U.K.). Dalbavancin was analyzed using a Phenomenex Gemini C18 columns (110 A 150 mm × 2 mm, 5 μm). The gradient mode consisted of 5% Phase B—7 min 95% Phase B—8 min 95% Phase B—8.5 min 5% Phase B. Phase A consisted of H2O + 0.1% formic acid and Phase B of CAN. It was pumped at a flow rate of 0.4 mL/min and the injection volume of the sample was 40 µL. The run time was 10 min and the MRM transitions for D were: Quantifier (m/z): 909.1 > 340.0 CE: -36; Qualifier (m/z): 909.1 > 730.5 CE: -26.
The elution tests were performed in triplicate.
4.4. Anti-biofilm activity of eluted antibiotics against 24h-biofilms in well plates according to metabolic activity reduction at each study period
At each study period, 24h-biofilms of each specie were formed on 96-well plates by adding 100 µl of a 10
8 cfu/ml (0.5 McFarland) bacterial suspension prepared in broth media (TSB for S. aureus and TSB+1% glucose for S. epidermidis) previously incubated at 37ºC during 24h under agitation. Plates were then incubated for 24h at 37ºC and 3 gently washes with PBS were performed. Once the biofilms on the wells were dried, 100 µl of eluted antibiotics or PBS (in the positive control wells) from each study period were added and plates were incubated for 24h at 37ºC. Then, 3 gently washes with PBS were performed and, once the wells were dried, 100 µl of XTT (with menadione at 1:1000) were added and plates were incubated for 2h at 37ºC at darkness. After incubation, the content of the wells were transferred to a new plate and absorbance was measured in an spectrophotometer at 490nm (
Figure S1). We calculated percentage reduction of metabolic activity as follows:
[1-(abs treated well/abs positive control)] x 100, were abs is the absorbance value at 490nm.
were abs is the absorbance value at 490nm.
Experiments were only performed once.
4.5. Anti-biofilm activity of antibiotics loaded in bone cements against 24h-biofilms in steel implants according to log cfu/ml reduction at each study period
At each study period, 24h-biofilms of each specie were formed on steel implant discs of 6x3 mm (previously sterilized with ethanol followed by autoclave) by adding in a glass tube 1 ml of a 10
8 cfu/ml (0.5 McFarland) bacterial suspension prepared in broth media (TSB for S. aureus and TSB+1% glucose for S. epidermidis) previously incubated at 37ºC during 24h under agitation. Tubes were then incubated for 24h at 37ºC under agitation and 3 gently washes with PBS were performed. Once the biofilms on the implant were dried, both steel disc and antibiotic loaded cement bone disc (or without antibiotics for the positive control) of each study period were put togheter in a glass tube with 1 ml of PBS. Tubes were incubated for 24h at 37ºC under agitation. Then, 3 gently washes with PBS were performed and steel implant discs were then sonicated for 10 min at 40 kHz in 1 ml of PBS. Serial dilutions of the sonicate were done and 100 were cultured onto blood agar plates (
Figure S1). Colony forming units were counted and data were expresed as log cfu/ml. We calculated percentage reduction of log cfu/ml as follows:
[1-(log cfu ml-1 antibiotic loaded cement/log cfu ml-1 non-antibiotic loaded cement)] x 100.
Experiments were performed in triplicate.
This procedure (until sonication step) was performed twice at month 3 in oder to analyze implant biofilm structure by scanning electron microscopy (SEM).
4.6. Scanning electron microscopy analysis of implant discs at month 3
Once implant discs have faced 3-month incubated bone cement discs and 3 gently washes with PBS were performed, they were transferred to a new tube with 10% glutaraldehide to be further analyzed by SEM. Prior to the visualization by SEM, the samples were subjected to a dehydration process using increasing concentrations of ethanol.
4.7. Statistical Analysis
For the comparison of quantitative variable among groups we used Student’s t-test.
Statistical significance was set at p < 0.05 for all the tests. The statistical analysis was performed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp, Armonk, NY, USA).
We compared median concentration for each antibiotic between the following periods: 24 hours vs. 1 week, 24 hours vs. 3 months, 1 week vs. 3 months, and 2 weeks vs. 3 months. We compared median percentage reduction of log cfu/ml and median percentage reduction of metabolic activity for each antibiotic between the two extremes of study time (24 hours vs. 3 months) and, in addition, between the following intermediate periods: 24 hours vs. 1 week and 1 week vs. 3 months. Comparisons between vancomycin and dalbavancin were tested only at 2 weeks and 3 months for each concentration used. The analysis was performed for all microorganisms together and for each specie (MSSA, MRSA and S. epidermidis), except for metabolic activity analysis, in which no triplicates were performed, so comparison of median percentage reduction was only assessed at all microorganisms together, but not for each specie.
The statistical significance comparisons between groups are detailed in the figure legends, showing only those in which a significant reduction was observed.
5. Conclusions
Overall, dalbavancin showed a significant concentration decrease from 2 weeks of incubation but maintain its anti-biofilm activity up to 3 months (>50% reduction of metabolic activity), whereas, despite vancomycin concentration showed a significant decrease at 1w and then it gradually increased, its anti-biofilm activity was significantly lower.
Dalbavancin loaded in bone cements seems a promising alternative approach for the treatment of PJI. It has showed better results than vancomycin both at 2.5% and 5% concentrations.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Laboratory procedure.
Author Contributions
Conceptualization, M.S.-S., P.S.-R. and M.G.; methodology, M.D.-N and A.B.; software, M.D.-N.; validation, J.M., J.V. and P.M.; formal analysis, M.D.-N.; investigation, M.S.-S.; resources, M.G.; data curation, M.D-N.; writing—original draft preparation, M.S.-S-; writing—review and editing, A.B.; visualization, M.G.; supervision, M.G. and P.S.-R.; project administration, M.S.-S.; funding acquisition, M.G., M.S.-S., M.D.-N. and P.S.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundación Mutua Madrileña, grant number FMM21/01 and by the ISCIII and the European Regional Development Fund (FEDER) “A way of making Europe”, grant number PI21/00344. M. Guembe is supported by the Miguel Servet Program (ISCIII-MICINN, MSII18/00008) from the Health Research Fund (FIS) of the Carlos III Health Institute (ISCIII), Madrid, Spain. M. Díaz-Navarro is supported by the ISCIII (FI22/00022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Thomas O’Boyle for his help in the preparation of the manuscript. We thank Mª Dolores Serrano, from the Galenic Department, Faculty of Pharmacy, Complutense University of Madrid for her help in the HPLC analysis. We thank Estefanía García Calvo and Cristina Gutierrez from the Faculty of Chemical Science, Complutense University of Madrid, for their help in the MS analysis. We thank Juan Carlos del Real Romero from the Pontificia Comillas University of Madrid for his help in the preparation of the steel discs and the mould for the elaboration of cement discs. We thank the laboratory and all the installations of Surgery and Experimental Medicine Unit of HGUGM for the technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gundtoft, P.H.; Pedersen, A.B.; Varnum, C.; Overgaard, S. Increased Mortality After Prosthetic Joint Infection in Primary THA. Clin. Orthop. Relat. Res. 2017, 475, 2623–2631. [Google Scholar] [CrossRef]
- Wildeman, P.; Rolfson, O.; Söderquist, B.; Wretenberg, P.; Lindgren, V. What Are the Long-term Outcomes of Mortality, Quality of Life, and Hip Function after Prosthetic Joint Infection of the Hip? A 10-year Follow-up from Sweden. Clin. Orthop. Relat. Res. 2021, 479, 2203–2213. [Google Scholar] [CrossRef]
- Blom, A.W.; Lenguerrand, E.; Strange, S.; Noble, S.M.; Beswick, A.D.; Burston, A.; Garfield, K.; Gooberman-Hill, R.; Harris, S.R.S.; Kunutsor, S.K.; et al. Clinical and cost effectiveness of single stage compared with two stage revision for hip prosthetic joint infection (INFORM): pragmatic, parallel group, open label, randomised controlled trial. BMJ 2022, 379, e071281. [Google Scholar] [CrossRef] [PubMed]
- Malchau, K.S.; Tillander, J.; Zaborowska, M.; Hoffman, M.; Lasa, I.; Thomsen, P.; Malchau, H.; Rolfson, O.; Trobos, M. Biofilm properties in relation to treatment outcome in patients with first-time periprosthetic hip or knee joint infection. J. Orthop. Transl. 2021, 30, 31–40. [Google Scholar] [CrossRef]
- Davidson, D.J.; Spratt, D.; Liddle, A.D. Implant materials and prosthetic joint infection: the battle with the biofilm. EFORT Open Rev. 2019, 4, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Dhillon MS, Hooda A, Moriarty TF, Sharma S. Biofilms-What Should the Orthopedic Surgeon know? 2023;57(1):44-51.
- Moore, A.J.; Wylde, V.; Whitehouse, M.R.; Beswick, A.D.; Walsh, N.E.; Jameson, C.; Blom, A.W. Development of evidence-based guidelines for the treatment and management of periprosthetic hip infection. Bone Jt. Open 2023, 4, 226–233. [Google Scholar] [CrossRef]
- Ometti, M.; Delmastro, E.; Salini, V. Management of prosthetic joint infections: a guidelines comparison. Musculoskelet. Surg. 2022, 106, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Minassian, A.M.; Osmon, D.R.; Berendt, A.R. Clinical guidelines in the management of prosthetic joint infection. J. Antimicrob. Chemother. 2014, 69, i29–i35. [Google Scholar] [CrossRef]
- Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25.
- Steadman, W.; Chapman, P.R.; Schuetz, M.; Schmutz, B.; Trampuz, A.; Tetsworth, K. Local Antibiotic Delivery Options in Prosthetic Joint Infection. Antibiotics 2023, 12, 752. [Google Scholar] [CrossRef] [PubMed]
- Fraval, A.; Wang, J.; Tarabichi, S.; Parvizi, J. Optimal timing for reimplantation in the setting of two stage revision for prosthetic joint infection. 2023, 67, 246–252. [CrossRef]
- Sánchez-Somolinos, M.; Díaz-Navarro, M.; Benjumea, A.; Tormo, M.; Matas, J.; Vaquero, J.; Muñoz, P.; Sanz-Ruíz, P.; Guembe, M. Determination of the Elution Capacity of Dalbavancin in Bone Cements: New Alternative for the Treatment of Biofilm-Related Peri-Prosthetic Joint Infections Based on an In Vitro Study. Antibiotics 2022, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- De Vito, A.; Fiore, V.; Colpani, A.; Zauli, B.; Fanelli, C.; Tiseo, G.; Occhineri, S.; Babudieri, S.; Falcone, M.; Madeddu, G. The current and future off-label uses of dalbavancin: a narrative review. . 2023, 27, 1222–1238. [Google Scholar] [CrossRef] [PubMed]
- Buzón Martín L, Mora Fernández M, Perales Ruiz JM, Ortega Lafont M, Álvarez Paredes L, Morán Rodríguez MA, et al. Dalbavancin for treating prosthetic joint infections caused by Gram-positive bacteria: A proposal for a low dose strategy. A retrospective cohort study. Rev Esp Quimioter. 2019;32(6):532-8.
- Bouza, E.; Valerio, M.; Soriano, A.; Morata, L.; Carus, E.G.; Rodríguez-González, M.C.; Hidalgo-Tenorio, C.; Plata, A.; Muñoz, P.; Vena, A.; et al. Dalbavancin in the treatment of different gram-positive infections: a real-life experience. Int. J. Antimicrob. Agents 2018, 51, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Stefani, S.; Venditti, M.; Di Domenico, E.G. Biofilm-Related Infections in Gram-Positive Bacteria and the Potential Role of the Long-Acting Agent Dalbavancin. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Flamm, R.K.; Castanheira, M.; Sader, H.S.; Mendes, R.E. Dalbavancin in-vitro activity obtained against Gram-positive clinical isolates causing bone and joint infections in US and European hospitals (2011–2016). Int. J. Antimicrob. Agents 2018, 51, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Sivori, F.; Cavallo, I.; Kovacs, D.; Guembe, M.; Sperduti, I.; Truglio, M.; Pasqua, M.; Prignano, G.; Mastrofrancesco, A.; Toma, L.; et al. Role of Extracellular DNA in Dalbavancin Activity against Methicillin-Resistant Staphylococcus aureus (MRSA) Biofilms in Patients with Skin and Soft Tissue Infections. Microbiol. Spectr. 2022, 10, e0035122. [Google Scholar] [CrossRef] [PubMed]
- Di Pilato, V.; Ceccherini, F.; Sennati, S.; D'Agostino, F.; Arena, F.; D'Atanasio, N.; Di Giorgio, F.P.; Tongiani, S.; Pallecchi, L.; Rossolini, G.M. In vitro time-kill kinetics of dalbavancin against Staphylococcus spp. biofilms over prolonged exposure times. Diagn. Microbiol. Infect. Dis. 2019, 96, 114901. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.W.; Puttagunta, S.; Sprenger, C.R.; Rubino, C.; Van Wart, S.; Baldassarre, J. Extended-Duration Dosing and Distribution of Dalbavancin into Bone and Articular Tissue. Antimicrob. Agents Chemother. 2015, 59, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Antão, H.S.; Guimarães, J.; Prada, J.; Pires, I.; Martins, Â.; Maltez, L.; E Pereira, J.; Capelo, J.L.; Igrejas, G.; et al. Efficacy of dalbavancin against MRSA biofilms in a rat model of orthopaedic implant-associated infection. J. Antimicrob. Chemother. 2020, 75, 2182–2187. [Google Scholar] [CrossRef] [PubMed]
- Jacob, B.; Makarewicz, O.; Hartung, A.; Brodt, S.; Roehner, E.; Matziolis, G. In vitro additive effects of dalbavancin and rifampicin against biofilm of Staphylococcus aureus. Sci. Rep. 2021, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- El Haj, C.; Benavent, E.; Sierra, Y.; Soldevila, L.; Rigo-Bonnin, R.; Torrejón, B.; Gomez-Junyent, J.; Rosselló, I.; Murillo, O. Comparative efficacy of dalbavancin alone and with rifampicin against in vitro biofilms in a pharmacodynamic model with methicillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 2022, 60, 106664. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Ruiz P, Matas-Diez JA, Villanueva-Martínez M, Santos-Vaquinha Blanco AD, Vaquero J. Is Dual Antibiotic-Loaded Bone Cement More Effective and Cost-Efficient Than a Single Antibiotic-Loaded Bone Cement to Reduce the Risk of Prosthetic Joint Infection in Aseptic Revision Knee Arthroplasty? J Arthroplasty. 2020;35(12):3724-3729.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).