Submitted:
26 June 2023
Posted:
29 June 2023
You are already at the latest version
Abstract
Keywords:
Introduction and Methods
Results and Discussion
| *Rattan explains the concept of ‘Homeodynamic space’ as encompassing “three characteristics: stress response, damage control, and constant remodeling, which provide
measurable biomarkers reflecting the survival ability, robustness, and resilience of a biological system. A biological definition of health thus involves measures of functionality, tolerance and adaptation” [21]. * The homeodynamic space refers to the range of physiological states and processes that an organism can tolerate and maintain without suffering harm or death. This range is determined by the organism's capacity to adapt to and cope with stress [22]. * The concept of homeodynamic space is related to the concept of homeostasis. However, while homeostasis refers to a narrow range of physiological states that are optimal for survival, homeodynamic space refers to a much broader range of physiological states and processes that an organism can tolerate. It highlights the dynamic nature of physiological systems and the importance of resilience and adaptability in maintaining health and well-being. |
| Hormetin supplements | Description | Reference |
| Rhodiola | Adaptogen, antioxidant | [37,38] |
| Schisandra | Adaptogen, liver conditions, tonic | [39,40,41,42] |
| Spermidine | Biological modulator, Longevity(See section on spermidine) | [43,44,45] |
| Caffeine | Cognitive enhancer | [46] |
| Ginger | General health | [47,48] |
| Turmeric (Curcumin) | Anti-inflammation, antioxidant | [49,50,51] |
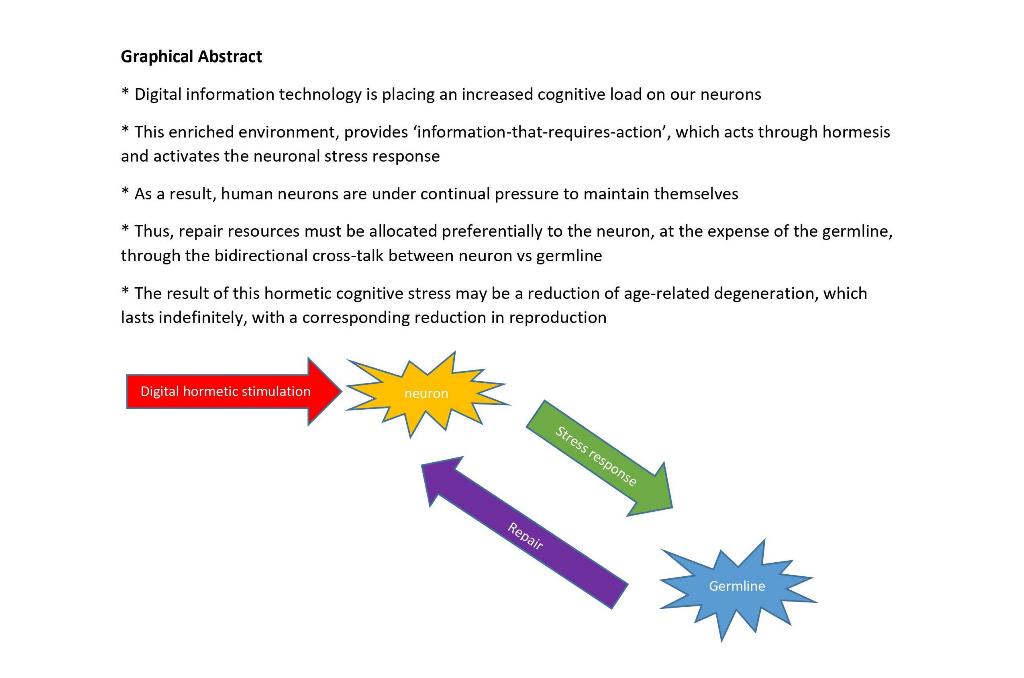
| * DNA damage in germ cells increases resilience in somatic cells * Neuronal stress causes an increased demand (by neurons) for repair resources, which are diverted from the germline * Germline cells have the capacity to become neurons. Neural precursors from the germline are able to mature and integrate within the existing neural network * A direct communication pathway between germline cells and the soma depends on the endoplasmic reticulum stress factor inositol requiring enzyme-1 (IRE-1) * Ectopic germline cells can be found in the brain, and could contribute to altered neuronal development, resulting in neurodevelopmental disorders * Progesterone, which modulates sperm function, acts by interacting with a membrane receptor which resembles the neuronal GABA(A) receptor in the brain * Eradication of germ cells in Drosophila, has a positive impact on its lifespan, possibly through modulation of the nutrient sensing insulin/insulin-like (IIS) growth factor signaling * The repressor element 1-silencing transcription factor (REST) which modulates multipotent stem cells, is present in both neonatal and adult testes, and regulates target genes in neurons * There is a conserved mechanism of modulation of neural development regulated by REST which is present in spermatogonial cells * The germline may influence the function of distant somatic cells, including neurons. For instance, germline stem cells influence proteostasis and thus control abnormal protein accumulation in neurons * We mention the example of the hormetin spermidine, which modulates autophagy both in the germline and in the neuron |
Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kyriazis, M. Aging as "Time-Related Dysfunction": A Perspective. Front Med (Lausanne). 2020, 7, 371. [Google Scholar] [CrossRef]
- Kyriazis, M. Clinical Effects of a 'Human-Computer' Interaction (June 21, 2016). Available at SSRN. Available online: https://ssrn.com/abstract=2798529 and http://dx.doi.org/10.2139/ssrn.2798529, (accessed on 20 June 2023).
- Digital Watch, https://dig.watch/trends/digital-and-environment, (accessed on 20 June 2023).
- Germline: National Human Genome Research institute. Available online: https://www.genome.gov/genetics-glossary/germ-line, (accessed on 20 June 2023).
- Gaddy, M.A.; Kuang, S.; Alfhili, M.A.; Lee, M.H. The soma-germline communication: implications for somatic and reproductive aging. BMB Rep. 2021, 54, 253–259. [Google Scholar] [CrossRef]
- Sharma, A. Transgenerational epigenetics: Integrating soma to germline communication with gametic inheritance. Mechanisms of Ageing and Development. 2017, 163, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Xu, F.; Heimbucher, T.; Baumeister, R. Protection of germline immortality by the soma via a secreted endoribonuclease. BioEssays. 2021, 43, 2100195. [Google Scholar] [CrossRef]
- Conine, C.C.; Rando, O.J. Soma-to-germline RNA communication. Nat Rev Genet. 2022, 23, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Levi-Ferber, M.; Shalash, R.; Le-Thomas, A.; Salzberg, Y.; Shurgi, M.; Benichou, J.I.; Ashkenazi, A.; Henis-Korenblit, S. Neuronal regulated ire-1-dependent mRNA decay controls germline differentiation in Caenorhabditis elegans. Elife. 2021, 10, e65644. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, M. Biological ageing and clinical consequences of modern technology. Biogerontology. 2017, 18, 711–715. [Google Scholar] [CrossRef]
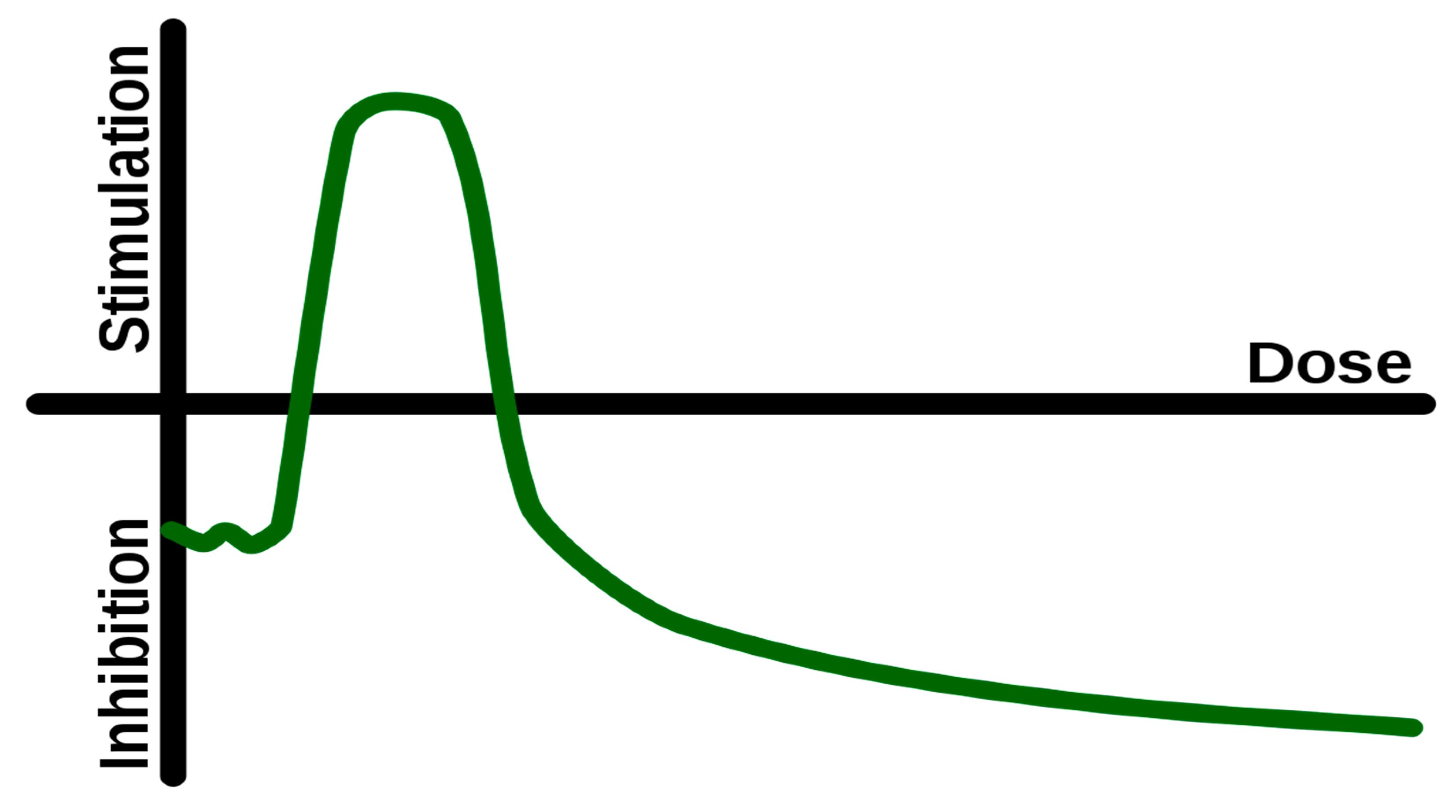
- Calabrese, E.J.; Baldwin, L.A. Defining hormesis. Hum. Exp. Toxicol. 2002, 21, 91–97. [Google Scholar] [CrossRef]
- Rattan, S. Hormesis in aging. Ageing Res Rev. 2008, 7, 63–78. [Google Scholar] [CrossRef]
- Santoro, A.; Martucci, M.; Conte, M.; Capri, M.; Franceschi, C.; Salvioli, S. Inflammaging, hormesis and the rationale for anti-aging strategies. Ageing Res Rev. 2020, 64, 101142. [Google Scholar] [CrossRef]
- Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C.; Traverso, N. Hormesis and Oxidative Distress: Pathophysiology of Reactive Oxygen Species and the Open Question of Antioxidant Modulation and Supplementation. Antioxidants (Basel). 2022, 11, 1613. [Google Scholar] [CrossRef]
- Li, X.; Yang, T.; Sun, Z. Hormesis in Health and Chronic Diseases. Trends Endocrinol Metab. 2019, 30, 944–958. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis defined. Ageing Res Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: Highly Generalizable and Beyond Laboratory. Trends Plant Sci. 2020, 25, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Pande, S.; Raisuddin, S. The Underexplored Dimensions of Nutritional Hormesis. Curr Nutr Rep. 2022, 11, 386–394. [Google Scholar] [CrossRef]
- Demirovic, D.; Rattan, S.I. Establishing cellular stress response profiles as biomarkers of homeodynamics, health and hormesis. Exp Gerontol. 2013, 48, 94–8. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? Aging Mech Dis 2017, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I.S. Biological Health and Homeodynamic Space. In Explaining Health Across the Sciences. Healthy Ageing and Longevity; Sholl, J., Rattan, S.I., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Rattan, SI. Aging is not a disease: implications for intervention. Aging Dis. 2014, 5, 196–202. [Google Scholar] [CrossRef]
- Mehdi, M.M.; Solanki, P.; Singh, P. Oxidative stress, antioxidants, hormesis and calorie restriction: The current perspective in the biology of aging. Arch Gerontol Geriatr. 2021, 95, 104413. [Google Scholar] [CrossRef]
- Toussaint, O.; Remacle, J.; Dierick, J.F.; Pascal, T.; Frippiat, C.; Royer, V.; Chainiaux, F. Approach of evolutionary theories of ageing, stress, senescence-like phenotypes, calorie restriction and hormesis from the view point of far-from-equilibrium thermodynamics. Mech Ageing Dev. 2002, 123, 937–46. [Google Scholar] [CrossRef]
- Testa, G.; Biasi, F.; Poli, G.; Chiarpotto, E. Calorie restriction and dietary restriction mimetics: a strategy for improving healthy aging and longevity. Curr Pharm Des. 2014, 20, 2950–77. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, M. Challenging Aging: The Anti-Senescence Effects of Hormesis, Environmental Enrichment, and Information Exposure. Series Title: Frontiers in Aging Sciences. Bentham Science Publishers, UAE, Vol 1, ISSN: 2468-5933.
- Kyriazis, M. Systems neuroscience in focus: from the human brain to the global brain? Front Syst Neurosci. 2015, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.C.; Grosso, R.A.; Fader, C.M. Hallmarks of Aging: An Autophagic Perspective. Frontiers in Endocrinology 2019, 9, 790. [Google Scholar] [CrossRef]
- He, C.; Sumpter, R.; Levine, B. Exercise induces autophagy in peripheral tissues and in the brain. Autophagy. 2012, 8, 1548–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jiang, M.; Bi, Y.; Zhu, H.; Zhou, Z. Autophagy and Apoptosis act as partners to induce germ cell death after heat stress in mice. PLOS ONE 2012, 7, e41412. [Google Scholar] [CrossRef]
- Penke, B.; Bogár, F.; Crul, T.; Sántha, M.; Tóth, M.E.; Vígh, L. Heat Shock Proteins and Autophagy Pathways in Neuroprotection: from Molecular Bases to Pharmacological Interventions. Int J Mol Sci. 2018, 19, 325. [Google Scholar] [CrossRef]
- Kumsta, C.; Chang, J.; Schmalz, J.; et al. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat Commun. 2017, 8, 14337. [Google Scholar] [CrossRef]
- Alirezaei, M.; Kemball, C.C.; Flynn, C.T.; Wood, M.R.; Whitton, J.; Kiosses, W.B. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010, 6, 702–10. [Google Scholar] [CrossRef]
- Pietrocola, F.; Pol, J.; Vacchelli, E.; Rao, S.; Enot, D.P.; Baracco, E.E.; Levesque, S.; et al. Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell. 2016, 30, 147–160. [Google Scholar] [CrossRef]
- Kim, I.; Lemasters, JJ. Mitochondrial degradation by autophagy (mitophagy) in GFP-LC3 transgenic hepatocytes during nutrient deprivation. Am J Physiol Cell Physiol. 2011, 300, C308–17. [Google Scholar] [CrossRef]
- Rattan, SIS. Hormetins as drugs for healthy aging. In Anti-aging drugs: from basic research to clinical practice; Vaiserman, M., Ed.; The Royal Society of Chemistry: 2017; pp. 170-180.
- Arabit, J.G.J.; Elhaj, R.; Schriner, S.E.; Sevrioukov, E.A.; Jafari, M. Rhodiola rosea Improves Lifespan, Locomotion, and Neurodegeneration in a Drosophila melanogaster Model of Huntington's Disease. Biomed Res Int. 2018, 6726874. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Simoneau, A.R.; Jafari, M.; Zi, X. Rhodiola rosea extracts and salidroside decrease the growth of bladder cancer cell lines via inhibition of the mTOR pathway and induction of autophagy. Mol Carcinog. 2012, 51, 257–67. [Google Scholar] [CrossRef]
- Yu, C.; Yu, C.; Jing, S.; Li, H.; Jiang, E.; Ju, W.; Chen, J. Effects of Schisandra total lignin on autophagy and apoptosis of mouse brain aging induced by D-galactose. Journal of Jilin University (Medicine Edition) 2014, 40, 1210–1215. [Google Scholar]
- Zhao, X.; Liu, C.; Xu, M.; Li, X.; Bi, K.; Jia, Y. Total Lignans of Schisandra chinensis Ameliorates Aβ1-42-Induced Neurodegeneration with Cognitive Impairment in Mice and Primary Mouse Neuronal Cells. PLOS ONE 2016, 11, e0152772. [Google Scholar] [CrossRef]
- Kim, J.S.; Yi, H.K. Schisandrin C enhances mitochondrial biogenesis and autophagy in C2C12 skeletal muscle cells: potential involvement of anti-oxidative mechanisms. Naunyn Schmiedebergs Arch Pharmacol. 2018, 391, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jing, S.; Jiang, R.; Wang, C.; Zhang, C.; Chen, J.; Li, H. Metabolomics study of the therapeutic mechanism of Schisandra chinensis lignans on aging rats induced by d-galactose. Clin Interv Aging. 2018, 13, 829–841. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. ; Spermidine in health and disease. Science, 2018, 359, Issue 6374. [Google Scholar] [CrossRef]
- Sigrist, S.J.; Carmona-Gutierrez, D.; Gupta, V.K.; Bhukel, A.; Mertel, S.; Eisenberg, T.; Madeo, F. Spermidine-triggered autophagy ameliorates memory during aging. Autophagy. 2014, 10, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, S.; Zhang, Y.; Lin, X.; Song, Y.; Xue, Z.; Qian, H.; Wang, S.; Wan, G.; Zheng, X.; Zhang, L. Induction of autophagy by spermidine is neuroprotective via inhibition of caspase 3-mediated Beclin 1 cleavage. Cell Death Dis. 2017, 8, e2738. [Google Scholar] [CrossRef]
- Luan, Y.; Ren, X.; Zheng, W.; Zeng, Z.; Guo, Y.; Hou, Z.; Guo, W.; Chen, X.; Li, F.; Chen, J.F. Chronic Caffeine Treatment Protects Against α-Synucleinopathy by Reestablishing Autophagy Activity in the Mouse Striatum. Front Neurosci. 2018, 12, 301. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.Y.; Hsu, Y.L.; Li, C.; Ko, Y.C.; Ni, W.C.; Huang, M.S.; Kuo, P.L. 6-Shogaol, an active constituent of dietary ginger, induces autophagy by inhibiting the AKT/mTOR pathway in human non-small cell lung cancer A549 cells. J Agric Food Chem. 2009, 57, 9809–16. [Google Scholar] [CrossRef]
- Li, T.Y.; Chiang, B.H. 6-shogaol induces autophagic cell death then triggered apoptosis in colorectal adenocarcinoma HT-29 cells. Biomed Pharmacother. 2017, 93, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Jiang, J.; Guan, C.; Dong, C.; Wang, G.; Bai, L.; Sun, J.; Hu, C.; Bai, C. Curcumin induces autophagy via activating the AMPK signaling pathway in lung adenocarcinoma cells. J Pharmacol Sci. 2013, 123, 102–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Li, X.H.; Zeng, X.C.; Li, J.; Zhou, J.; Xiao, B.; Hu, K. Curcumin protects neuronal cells against status-epilepticus-induced hippocampal damage through induction of autophagy and inhibition of necroptosis. Can J Physiol Pharmacol. 2017, 95, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Brimson, J.M.; Prasanth, M.I.; Malar, D.S.; Thitilertdecha, P.; Kabra, A.; Tencomnao, T.; Prasansuklab, A. Plant Polyphenols for Aging Health: Implication from Their Autophagy Modulating Properties in Age-Associated Diseases. Pharmaceuticals (Basel) 2021, 14, 982. [Google Scholar] [CrossRef]
- Stavoe, A.K.H.; Holzbaur, E.L.F. Autophagy in Neurons. Annu Rev Cell Dev Biol. 2019, 35, 477–500. [Google Scholar] [CrossRef]
- Ji, S.; Xiong, M.; Chen, H.; Liu, Y.; Zhou, L.; Hong, Y.; Wang, M.; Wang, C.; Fu, X.; Sun, X. Cellular rejuvenation: molecular mechanisms and potential therapeutic interventions for diseases. Signal Transduct Target Ther. 2023, 8, 116. [Google Scholar] [CrossRef]
- Martinelli, S.; Anderzhanova, E.A.; Bajaj, T.; Wiechmann, S.; Dethloff, F.; Weckmann, K.; Heinz, D.; Ebert, T.; Hartmann, J.; Geiger, T.M.; et al. Stress-primed secretory autophagy promotes extracellular BDNF maturation by enhancing MMP9 secretion. Nat Commun. 2021, 12, 4643. [Google Scholar] [CrossRef]
- Peker, N.; Gozuacik, D. Autophagy as a Cellular Stress Response Mechanism in the Nervous System. J Mol Biol. 2020, 432, 2560–2588. [Google Scholar] [CrossRef]
- Cappucci, U.; Noro, F.; Casale, A.; Pimpinell, S. The Hsp70 chaperone is a major player in stress-induced transposable element activation. Proceedings of the National Academy of Sciences 2019, 116, 17943–17950. [Google Scholar] [CrossRef]
- Leon, M.; Woo, C. Environmental Enrichment and Successful Aging. Front Behav Neurosci. 2018, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Colavitta, M.F.; Grasso, L.; Barrantes, F.J. Environmental Enrichment in Murine Models and Its Translation to Human Factors Improving Conditions in Alzheimer Disease. J Prev Alzheimers Dis. 2023, 10, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Birch, A.M.; Kelly, Á.M. Lifelong environmental enrichment in the absence of exercise protects the brain from age-related cognitive decline. Neuropharmacology. 2019, 145 Pt A, 59–74. [Google Scholar] [CrossRef]
- Balietti, M.; Conti, F. Environmental enrichment and the aging brain: is it time for standardization? Neurosci Biobehav Rev. 2022, 139, 104728. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Haase, M.; Best, L.; Groth, M.; Lindner, J.; Witte, O.W.; Kaleta, C.; Frahm, C. Restoring Age-Related Cognitive Decline through Environmental Enrichment: A Transcriptomic Approach. Cells. 2022, 11, 3864. [Google Scholar] [CrossRef]
- Barone, I.; Novelli, E.; Strettoi, E. Long-term preservation of cone photoreceptors and visual acuity in rd10 mutant mice exposed to continuous environmental enrichment. Mol Vis 2014, 20, 1545–1556. [Google Scholar]
- Levine, J.N.; Chen, H.; Gu, Y.; Cang, J. Environmental Enrichment Rescues Binocular Matching of Orientation Preference in the Mouse Visual Cortex. J Neurosci. 2017, 37, 5822–5833. [Google Scholar] [CrossRef]
- Gurfein, B.T.; Davidenko, O.; Premenko-Lanier, M.; Milush, J.M.; Acree, M.; Dallman, M.F.; Touma, C.; Palme, R.; York, V.A.; Fromentin, G.; Darcel, N.; Nixon, D.F.; Hecht, F.M. Environmental enrichment alters splenic immune cell and enhances secondary influenza vaccine responses in mice. Mol Med 2014, 20, 179–190. [Google Scholar] [CrossRef]
- Vitalo, A.G.; Gorantla, S.; Fricchione, J.G.; Scichilone, J.M.; Camacho, J.; Niemi, S.M.; Denninger, J.W.; Benson, H.; Yarmush, M.L.; Levine, J.B. Environmental enrichment with nesting material accelerates wound healing in isolation-reared rats. Behav Brain Res 2012, 226, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Bice, B.D.; Stephens, M.R.; Georges, S.J.; Venancio, A.R.; Bermant, P.C.; Warncke, A.V.; Affolter, K.E.; Hidalgo, J.R.; Angus-Hill, M.L. Environmental Enrichment Induces Pericyte and IgA-Dependent Wound Repair and Lifespan Extension in a Colon Tumor Model. Cell Rep. 2017, 19, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, D.; Aranda, M.L.; González Fleitas, M.F.; Chianelli, M.S.; Fernandez, D.C.; Sande, P.; Rosenstein, R.E. Environmental enrichment protects the retina from early diabetic damage in adult rats. PLoS ONE 2014, 9, e101829. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Mahato, N.K.; Nakazawa, M.; Law, T.D.; Thomas, J.S. ; The power of the mind: the cortex as a critical determinant of muscle strength/weakness. J Neurophysiol 2014, 112, 3219–3226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, K.; Shen, X. Environmental enrichment attenuated sevoflurane-induced neurotoxicity through the PPAR-γ signaling pathway. Biomed Res Int 2015, 107149. [Google Scholar]
- Scarola, S.; Bardi, M. Environmental enrichment modulates inflammation during development in long-evans rats (Rattus norvegicus). Dev Psychobiol. 2021, 63, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Matur, E.; Akyaz, İ.; Eraslan, E.; Ergul Ekiz, E.; Eseceli, H.; Keten, M.; Metiner, K.; Aktaran Bala, D. The effects of environmental enrichment and transport stress on the weights of lymphoid organs, cell-mediated immune response, heterophil functions and antibody production in laying hens. Anim Sci J 2016, 87, 284–292. [Google Scholar] [CrossRef]
- Wolinsky, F.; Unverzagt, F.W.; Smith, D.M.; Jones, R.; Wright, E.; Tennstedt, S.L. The effects of the ACTIVE cognitive training trial on clinically relevant declines in health-related quality of life. J Gerontol B 2006, 61, S281–S287. [Google Scholar] [CrossRef]
- Rebok, G.W.; Ball, K.; Guey, L.T.; Jones, R.N.; Kim, H.Y.; King, J.W.; Marsiske, M.; Morris, J.N.; Tennstedt, S.L.; Unverzagt, F.W.; Willis, S.L. ACTIVE Study Group. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc 2014, 62, 16–24. [Google Scholar] [CrossRef]
- Joëls, M. , Karst, H., Sarabdjitsingh, R.A. The stressed brain of humans and rodents. Acta Physiol (Oxf). 2018, 223, e13066. [Google Scholar] [CrossRef]
- Korneeva, N.L. ; Integrated Stress Response in Neuronal Pathology and in Health. Biochemistry (Mosc). 2022, 87 (Suppl 1), S111-S127), S111–S127). [Google Scholar] [CrossRef]
- Farley, M.M. , Watkins, T.A. Intrinsic Neuronal Stress Response Pathways in Injury and Disease. Annu Rev Pathol. 2018, 13, 93–116. [Google Scholar] [CrossRef]
- Kim, K.W.; Jin, Y. Neuronal responses to stress and injury in C. elegans. FEBS Lett. 2015, 589, 1644–52. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Sekine, Y.; Oyeyemi, M.J.; Abrams, A.J.; Basavaraju, M.; Han, S.M.; Groth, M.; Morrison, H.; Strittmatter, S.M.; Hammarlund, M. The stress-responsive gene GDPGP1/mcp-1 regulates neuronal glycogen metabolism and survival. J Cell Biol. 2020, 219, e201807127. [Google Scholar] [CrossRef] [PubMed]
- Freeland, K.; Boxer, L.M.; Latchman, D.S. The cyclic AMP response element in the Bcl-2 promoter confers inducibility by hypoxia in neuronal cells. Brain Res Mol Brain Res. 2001, 92, 98–106. [Google Scholar] [CrossRef]
- Korte, M. The impact of the digital revolution on human brain and behavior: where do we stand? Dialogues Clin Neurosci. 2020, 22, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.K.; Kanai, R. How Has the Internet Reshaped Human Cognition? Neuroscientist. 2016, 22, 506–20. [Google Scholar] [CrossRef] [PubMed]
- Menghini, F.; van Rijsbergen, N.; Treves, A. Modelling adaptation aftereffects in associative memory. Neurocomputing, 2007, 70, 2000–2004. [Google Scholar] [CrossRef]
- Small, G.W.; Lee, J.; Kaufman, A.; Jalil, J.; Siddarth, P.; Gaddipati, H.; Moody, T.D.; Bookheimer, S.Y. Brain health consequences of digital technology use . Dialogues Clin Neurosci. 2020, 22, 179–187. [Google Scholar] [CrossRef]
- Advanced Cognitive Training in Vital Elderly – ACTIVE – study. IU School of Medicine Nov 16, 2017. Available online: https://medicine.iu.edu/news/2017/11/brain-exercise-dementia-prevention (accessed on 2 June 2023).
- Abd-Alrazaq, A.; Abuelezz, I.; AlSaad, R.; Al-Jafar, E.; Ahmed, A.; Aziz, S.; Nashwan, A.; Sheikh, J. Serious Games for Learning Among Older Adults With Cognitive Impairment: Systematic Review and Meta-analysis. J Med Internet Res. 2023, 25, e43607. [Google Scholar] [CrossRef]
- Abd-Alrazaq, A.; Alhuwail, D.; Ahmed, A.; Househ, M. Effectiveness of Serious Games for Improving Executive Functions Among Older Adults With Cognitive Impairment: Systematic Review and Meta-analysis. JMIR Serious Games. 2022, 10, e36123. [Google Scholar] [CrossRef]
- Yang, C.; Han, X.; Jin, M.; Xu, J.; Wang, Y.; Zhang, Y.; Xu, C.; Zhang, Y.; Jin, E.; Piao, C. The Effect of Video Game-Based Interventions on Performance and Cognitive Function in Older Adults: Bayesian Network Meta-analysis. JMIR Serious Games. 2021, 9, e27058. [Google Scholar] [CrossRef]
- Clemenson, G.D.; Stark, S.M.; Rutledge, S.M.; Stark, C.E.L. Enriching hippocampal memory function in older adults through video games. Behav Brain Res. 2020, 390, 112667. [Google Scholar] [CrossRef] [PubMed]
- Ramnath, U.; Rauch, L.; Lambert, E.V.; Kolbe-Alexander, T. Efficacy of interactive video gaming in older adults with memory complaints: A cluster-randomized exercise intervention. PLoS One. 2021, 16, e0252016. [Google Scholar] [CrossRef]
- Chen, H.Y.; Jolly, C.; Bublys, K.; Immler, S. Trade-off between somatic and germline repair in a vertebrate supports the expensive germ line hypothesis. PNAS 2020, 117, 8973–8979. [Google Scholar] [CrossRef] [PubMed]
- Ermolaeva, M.A.; Segref, A.; Dakhovnik, A. DNA damage in germ cells induces an innate immune response that triggers systemic stress resistance. Nature 2013, 501, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, N.; Shai, N.; Ben-Zvi, A. Germline stem cell arrest inhibits the collapse of somatic proteostasis early in Caenorhabditis elegans adulthood. Aging Cell 2013, 12, 814–822. [Google Scholar] [CrossRef]
- Ermolaeva, M.; Schumacher, B. The innate immune system as mediator of systemic DNA damage responses. Commun Integr Biol 2013, 6, e26926. [Google Scholar] [CrossRef]
- Khodakarami, A.; Saez, I.; Mels, J.; Vilchez, D. ; Mediation of organismal aging and somatic proteostasis by the germline. Front Mol Biosci. 2015, 2, 3. [Google Scholar] [CrossRef]
- Monaghan, P.; Metcalfe, N.B. The deteriorating soma and the indispensable germline: gamete senescence and offspring fitness. Proc Biol Sci. 2019, 286, 20192187. [Google Scholar] [CrossRef]
- Avise, J.C. The evolutionary biology of aging, sexual reproduction and DNA repair. Evolution 1993, 47, 1293–1301. [Google Scholar] [CrossRef]
- Gracida, X.; Eckmann, C.R. Fertility and germline stem cell maintenance under different diets requires nhr-114/HNF4 in C. elegans. Curr Biol 2013, 23, 607–613. [Google Scholar] [CrossRef]
- Heininger, K. Aging is a deprivation syndrome driven by a germ-soma conflict. Ageing Res Rev. 2002, 1, 481–536. [Google Scholar] [CrossRef] [PubMed]
- Douglas, P.M.; Dillin, A. The disposable soma theory of aging in reverse. Cell Res 2014, 24, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Ng, C.L.; Schulz, C. CSN maintains the germline cellular microenvironment and controls the level of stem cell genes via distinct CRLs in testes of Drosophila melanogaster. Dev Biol 2015, 398, 68–79. [Google Scholar] [CrossRef]
- Sharma, U. Paternal contributions to offspring health: role of sperm small RNAs in intergenerational transmission of epigenetic information. Front. Cell Dev. Biol. 2019, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S.A.; Jayasooriah, N.; Buckland, M.E.; Martin, D.I.K.; Cropley, J.E.; Suter, C.M. Roll over Weismann: extracellular vesicles in the transgenerational transmission of environmental effects. Epigenomics 2015, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Jolly, C.; Bublys, K.; Marcu, D.; Immler, S. Trade-off between somatic and germline repair in a vertebrate supports the expensive germ line hypothesis. Proc Natl Acad Sci U S A. 2020, 117, 8973–8979. [Google Scholar] [CrossRef]
- Marchal, I.; Tursun, B. Induced Neurons From Germ Cells in Caenorhabditis elegans. Front Neurosci. 2021, 15, 771687. [Google Scholar] [CrossRef]
- Glaser, T.; Opitz. T.; Kischlat, T. Adult germ line stem cells as a source of functional neurons and glia. Stem Cells. 2008, 26, 2434–43. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, Y.A.; Kim, K.J. Effects of paracrine factors on CD24 expression and neural differentiation of male germline stem cells. Int J Mol Med. 2015, 36, 255–62. [Google Scholar] [CrossRef]
- Streckfuss-Bömeke, K.; Vlasov, A.; Hülsmann, S. Generation of functional neurons and glia from multipotent adult mouse germ-line stem cells. Stem Cell Res. 2009, 2, 139–54. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Hai, Y. Efficient Conversion of Spermatogonial Stem Cells to Phenotypic and Functional Dopaminergic Neurons via the PI3K/Akt and P21/Smurf2/Nolz1 Pathway. Mol Neurobiol. 2015, 52, 1654–69. [Google Scholar] [CrossRef]
- Wang, X.; Chen, T.; Zhang, Y.; Li, B.; Xu, Q.; Song, C. Isolation and Culture of Pig Spermatogonial Stem Cells and Their in Vitro Differentiation into Neuron-Like Cells and Adipocytes. Int J Mol Sc. 2015, 16, 26333–46. [Google Scholar] [CrossRef] [PubMed]
- Teichert, A.M.; Pereira, S.; Coles, B. The neural stem cell lineage reveals novel relationships among spermatogonial germ stem cells and other pluripotent stem cells. Stem Cells Dev 2014, 23, 767–78. [Google Scholar] [CrossRef] [PubMed]
- Levi-Ferber, M.; Shalash, R.; Le-Thomas, A. Neuronal regulated ire- 1-dependent mRNA decay controls germline differentiation in Caenorhabditis elegans. Elife. 2021, 10, e65644. [Google Scholar] [CrossRef] [PubMed]
- Bonefas, K.M.; Iwase, S. Soma-to-germline transformation in chromatin-linked neurodevelopmental disorders? FEBS J. 2022, 289, 2301–2317. [Google Scholar] [CrossRef]
- Calogero, A.E.; Burrello, N.; Barone, N.; Palermo, I.; Grasso, U.; D'Agata, R. Effects of progesterone on sperm function: mechanisms of action. Hum Reprod. 2000, 15 (Suppl 1), 28–45. [Google Scholar] [CrossRef]
- Flatt, T.; Min, K.J.; D'Alterio, C.; Villa-Cuesta, E.; Cumbers, J.; Lehmann, R.; Jones, D.L.; Tatar, M. Drosophila germ-line modulation of insulin signaling and lifespan. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 6368–6373. [Google Scholar] [CrossRef]
- Kimura, R.; Inoue, Y.U.; Kikkawa, T.; Tatehana, M.; Morimoto, Y.; Inada, H.; Oki, S.; Inoue, T.; Osumi, N. Detection of REST expression in the testis using epitope-tag knock-in mice generated by genome editing. Dev Dyn. 2022, 251, 525–535. [Google Scholar] [CrossRef]
- Cheng, Y.; Yin, Y.; Zhang, A.; Bernstein, A.M.; Kawaguchi, R.; Gao, K.; Potter, K.; Gilbert, H.Y.; Ao, Y.; Ou, J.; Fricano-Kugler, C.J.; Goldberg, J.L.; He, Z.; Woolf, C.J.; Sofroniew, M.V.; Benowitz, L.I.; Geschwind, D.H. Transcription factor network analysis identifies REST/NRSF as an intrinsic regulator of CNS regeneration in mice. Nat Commun. 2022, 13, 4418. [Google Scholar] [CrossRef]
- Zullo, J.M.; Drake, D.; Aron, L.; O'Hern, P.; Dhamne, S.C.; Davidsohn, N.; Mao, C.A; Klein, W.H.; Rotenberg, A.; Bennett, D.A.; Church, G.M.; Colaiácovo, M.P.; Yankner, B.A. Regulation of lifespan by neural excitation and REST. Nature. 2019, 574, 359–364. [Google Scholar] [CrossRef]
- Calculli, G.; Lee, H.J.; Shen, K.; Pham, U.; Herholz, M.; Trifunovic, A.; Dillin, A.; Vilchez, D. Systemic regulation of mitochondria by germline proteostasis prevents protein aggregation in the soma of C. elegans. Sci Adv. 2021, 7, eabg3012. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science. 2018, 359, eaan2788. [Google Scholar] [CrossRef]
- Jing, Y.H.; Yan, J.L.; Wang, Q.J.; Chen, H.C.; Ma, X.Z.; Yin, J.; Gao, L.P. Spermidine ameliorates the neuronal aging by improving the mitochondrial function in vitro. Exp Gerontol. 2018, 108, 77–86. [Google Scholar] [CrossRef]
- Ghosh, I.; Sankhe, R.; Mudgal, J.; Arora, D.; Nampoothiri, M. Spermidine, an autophagy inducer, as a therapeutic strategy in neurological disorders. Neuropeptides 2020, 83, 102083. [Google Scholar] [CrossRef]
- Yang, N.; Liu, X.; Niu, X.; Wang, X.; Jiang, R.; Yuan, N.; Wang, J.; Zhang, C.; Lim, K.L.; Lu, L. Activation of Autophagy Ameliorates Age-Related Neurogenesis Decline and Neurodysfunction in Adult Mice. Stem Cell Rev Rep. 2022, 18, 626–641. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Tian, G.G.; Pei, X.; Hu, X.; Wu, J. Spermidine induces cytoprotective autophagy of female germline stem cells in vitro and ameliorates aging caused by oxidative stress through upregulated sequestosome-1/p62 expression. Cell Biosci 2021, 11, 107. [Google Scholar] [CrossRef]
- Bhukel, A.; Madeo, F.; Sigrist, S. Spermidine boosts autophagy to protect against synapse ageing. Autophagy. 2017, 13, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, P.; Deshmukh, R. Neuroprotective potential of spermidine against rotenone induced Parkinson's disease in rats. Neurochem Int. 2018, 116, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, S.; Hofer, S.J.; Zimmermann, A.; Pechlaner, R.; Dammbrueck, C.; Pendl, T.; Marcello, G.M.; et al. Dietary spermidine improves cognitive function. Cell Rep 2021, 35, 108985. [Google Scholar] [CrossRef]
- Babajanyan, S.G.; Koonin, E.V.; Allahverdyan, A.E. Thermodynamic selection: mechanisms and scenarios. New J Phys. 2022, 24, 053006. [Google Scholar] [CrossRef]
- Teulière, J.; Bhattacharya, D.; Bapteste, E. Ancestral germen/soma distinction in microbes: Expanding the disposable soma theory of aging to all unicellular lineages. Ageing Res Rev. 2020, 60, 101064. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, A.; Stamato, T.; Sell, C. The disposable soma theory revisited: time as a resource in the theories of aging. Cell Cycle. 2011, 10, 3853–6. [Google Scholar] [CrossRef] [PubMed]
- Drenos, F.; Kirkwood, T.B. Modelling the disposable soma theory of ageing. Mech Ageing Dev. 2005, 126, 99–103. [Google Scholar] [CrossRef]
- Kyriazis, M. Neurons vs. Germline: A War of Hormetic Tradeoffs. Curr Aging Sci, 2017, 10, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, M. Reversal of informational entropy and the acquisition of germ-like immortality by somatic cells. Curr Aging Sci, 2014, 7, 9–16. [Google Scholar] [CrossRef]
- Aiello, L.C.; Wheeler, P. The expensive-tissue hypothesis. Curr Anthropol. 1995, 36, 199–221. [Google Scholar] [CrossRef]
- Roff, D.A.; Mostowy, S.; Fairbairn, D.J. ; The evolution of trade-offs: testing predictions on response to selection and environmental variation. Evolution. 2002, c56, 84–95. [Google Scholar]
- Lesch, R.; Kotrschal, K.; Kitchener, A.C.; Fitch, W.T.; Kotrschal, A. The expensive-tissue hypothesis may help explain brain-size reduction during domestication. Commun Integr Biol. 2022, 15, 190–192. [Google Scholar] [CrossRef]
- Huang, C.H.; Yu, X.; Liao, W.B. The Expensive-Tissue Hypothesis in Vertebrates: Gut Microbiota Effect, a Review. Int J Mol Sci. 2018, 19, 1792. [Google Scholar] [CrossRef]
- Nengovhela, A.; Ivy, C.M.; Scott, G.; Denys, C.; Taylor, P.J. Counter-gradient variation and the expensive tissue hypothesis explain parallel brain size reductions at high elevation in cricetid and murid rodents. Sci Rep. 2023, 13, 5617. [Google Scholar] [CrossRef]
- Maklakov, A.A.; Immler, S. The Expensive Germline and the Evolution of Ageing. Curr Biol. 2016, 26, R577–R586. [Google Scholar] [CrossRef] [PubMed]
- Teplyuk, N.M. Near-to-perfect homeostasis: examples of universal aging rule which germline evades. J Cell Biochem. 2012, 113, 388–96. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, M. The Indispensable Soma Hypothesis. Available online: www.indispensablesoma.info (accessed on 13 June 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




