Submitted:
28 June 2023
Posted:
28 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
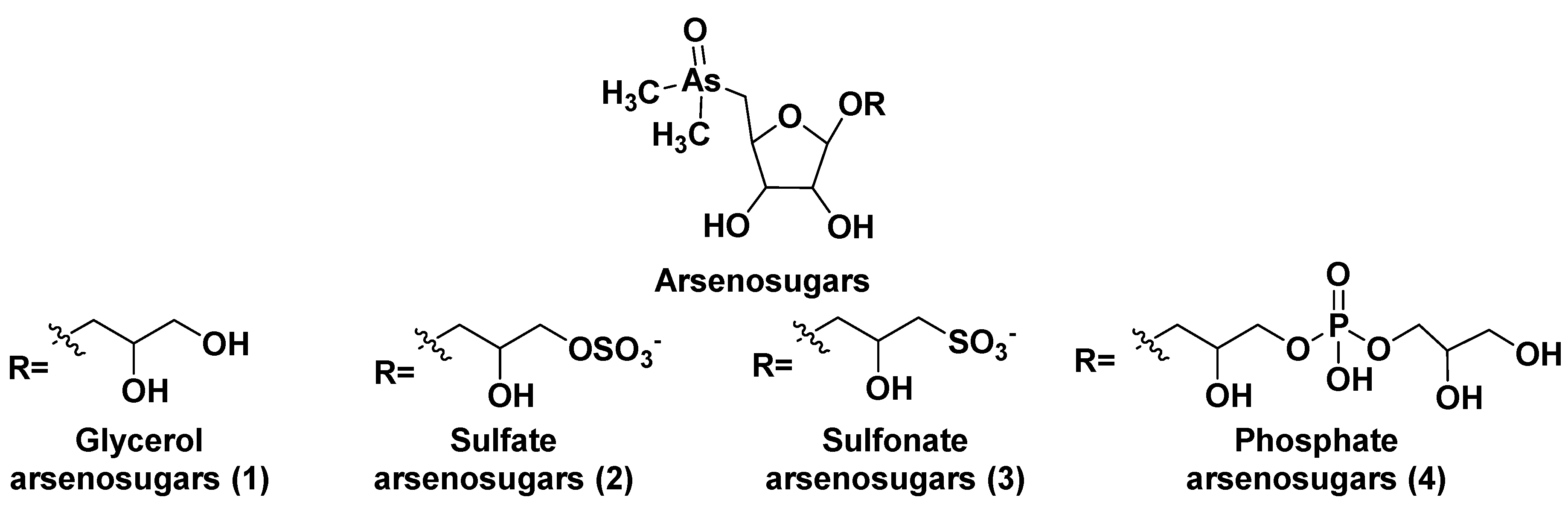
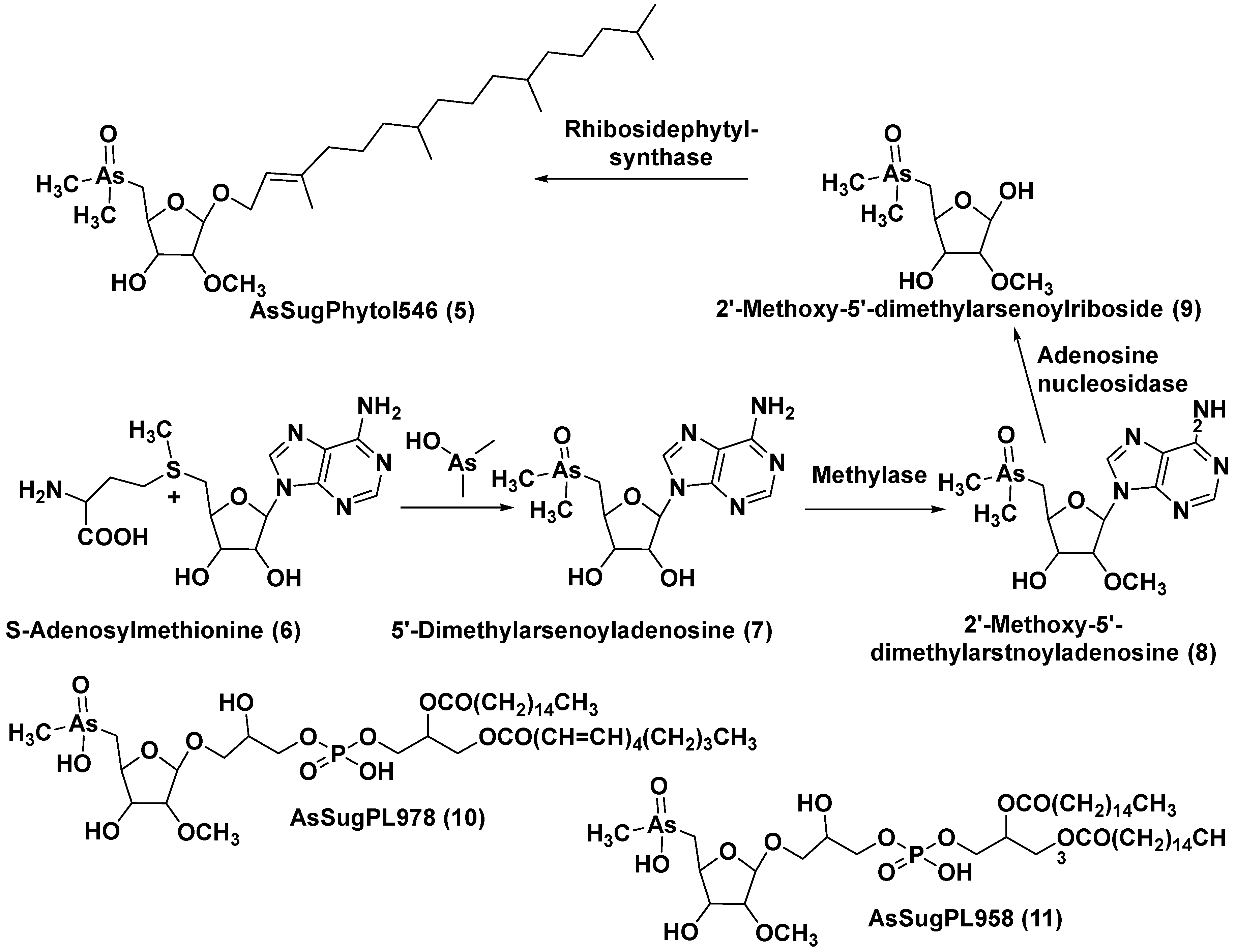
2. Glycosylated arsenicals
3. Glycolipids
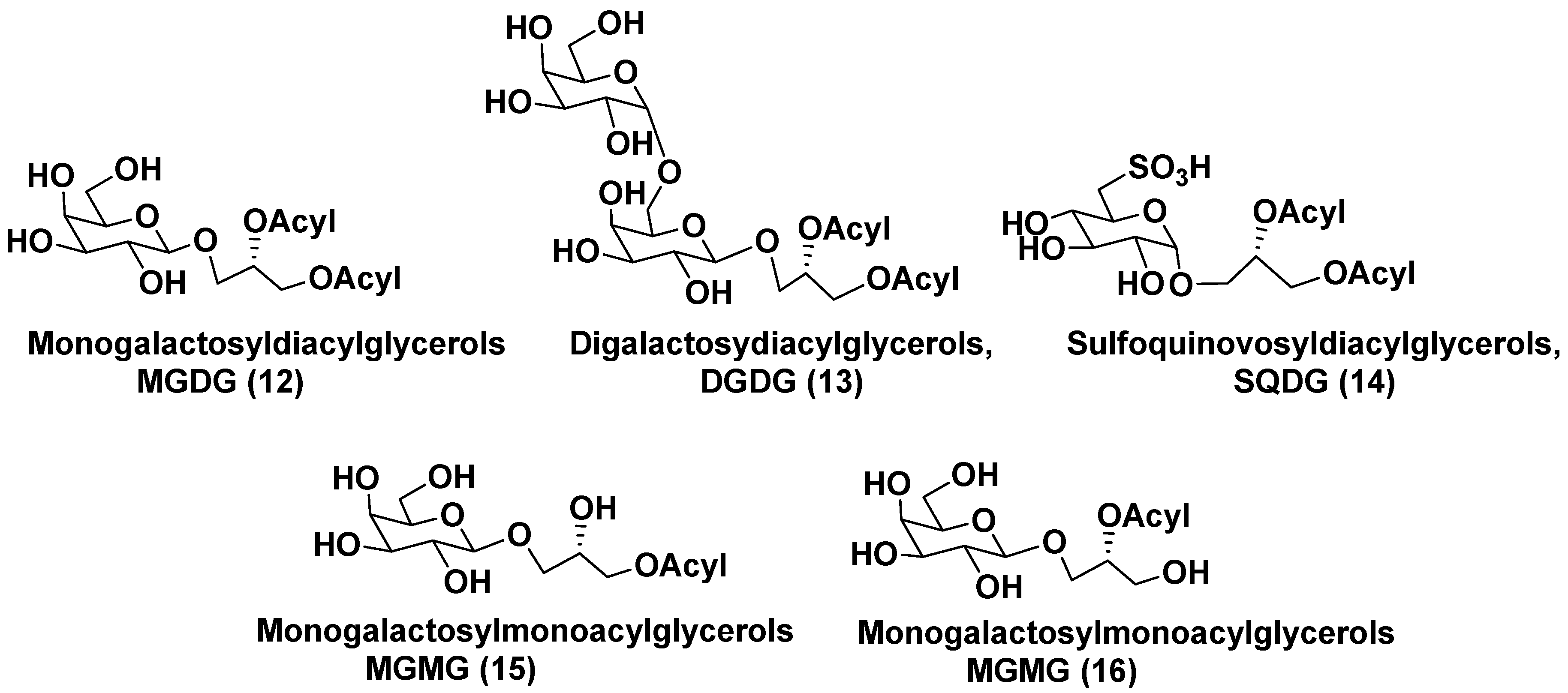
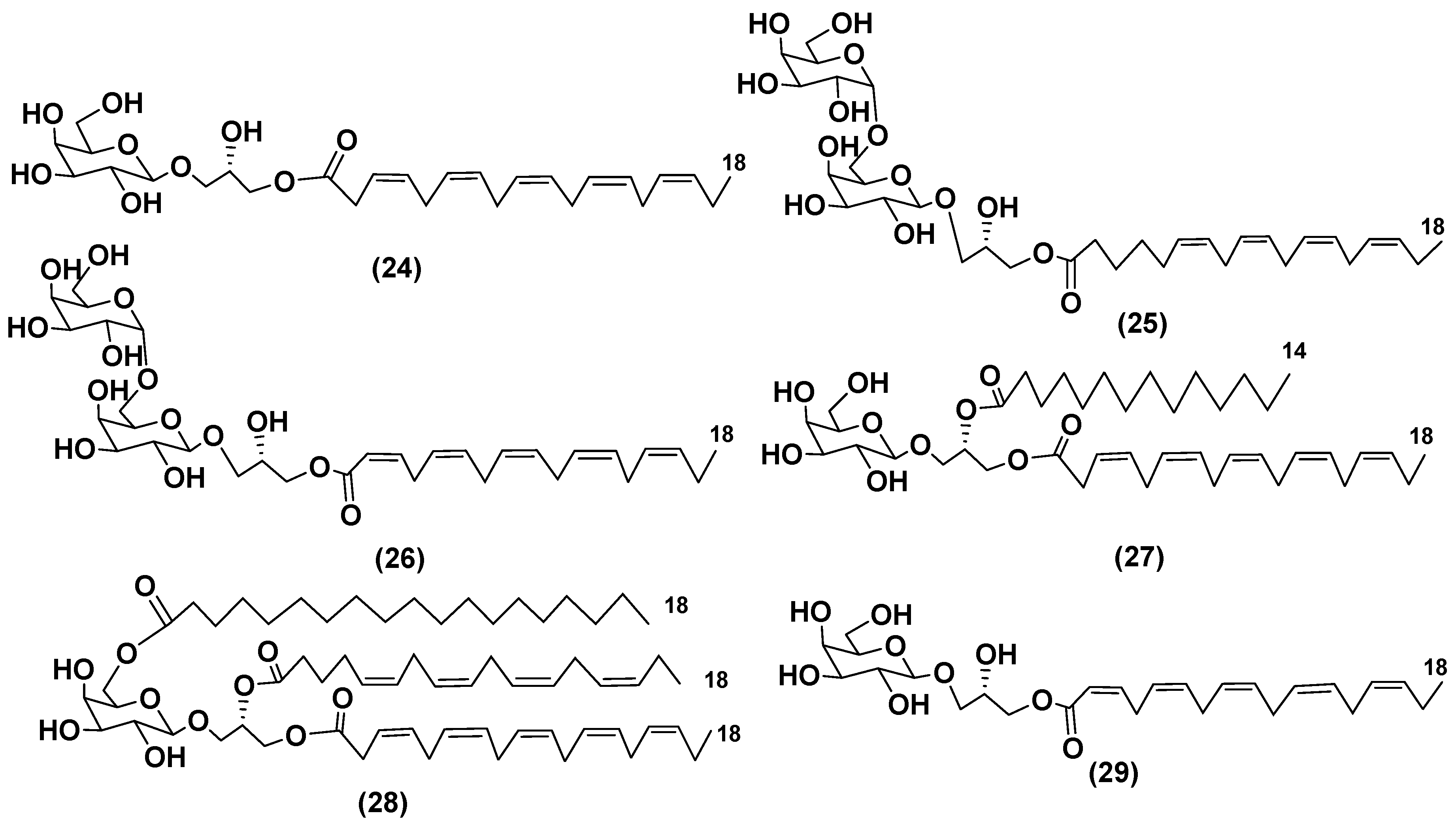
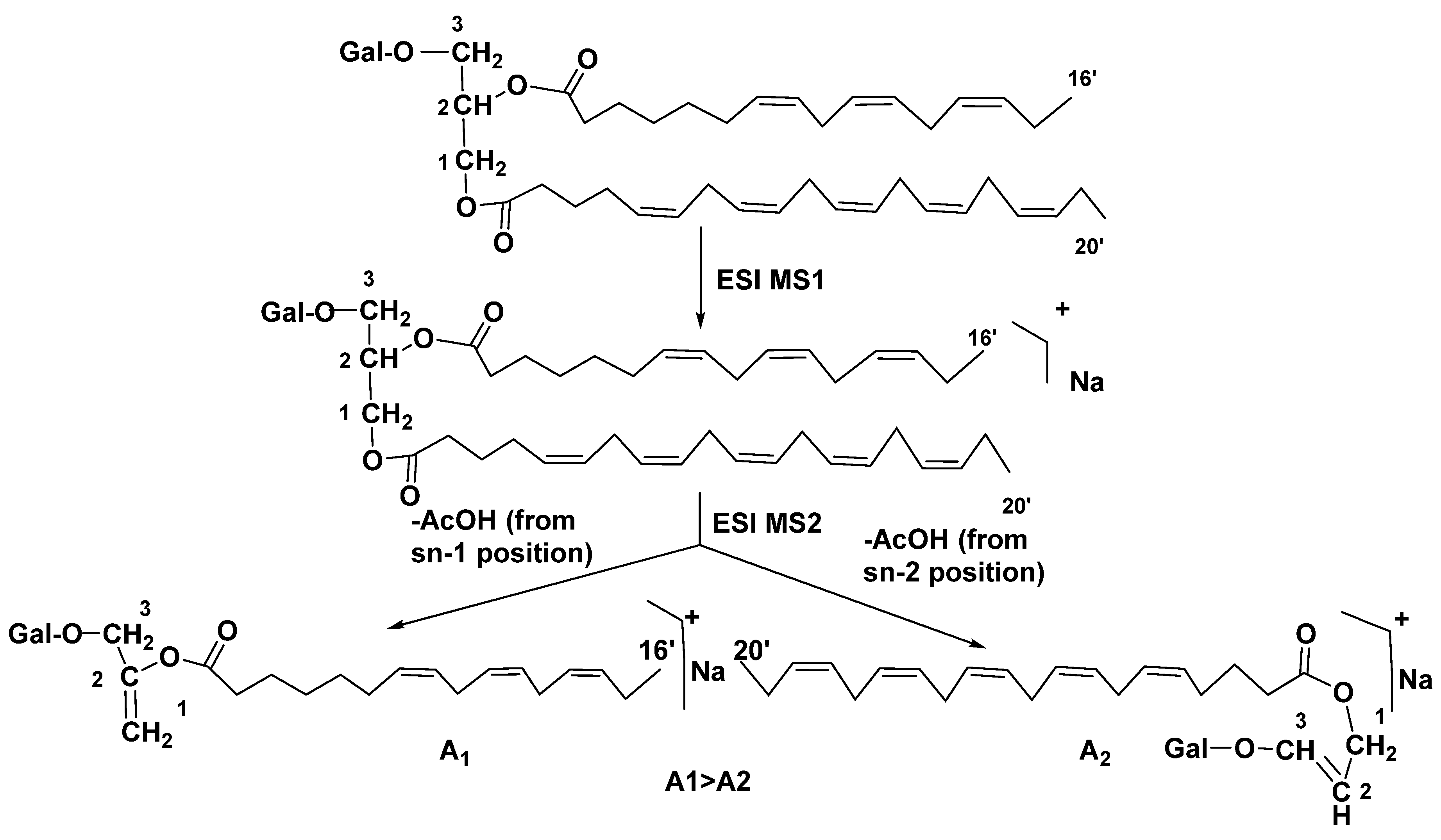
3.1. Galactolipids of microalgae
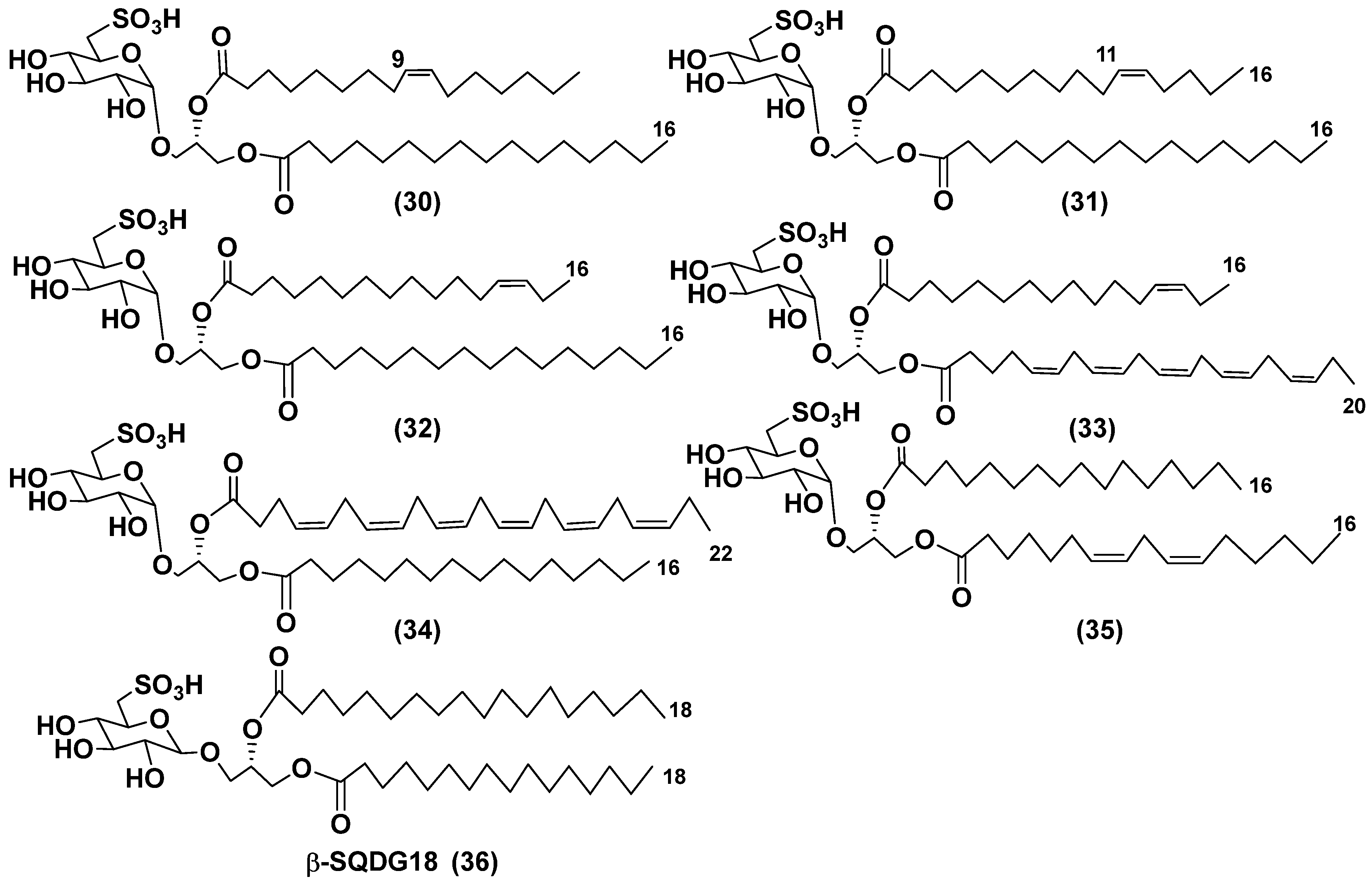
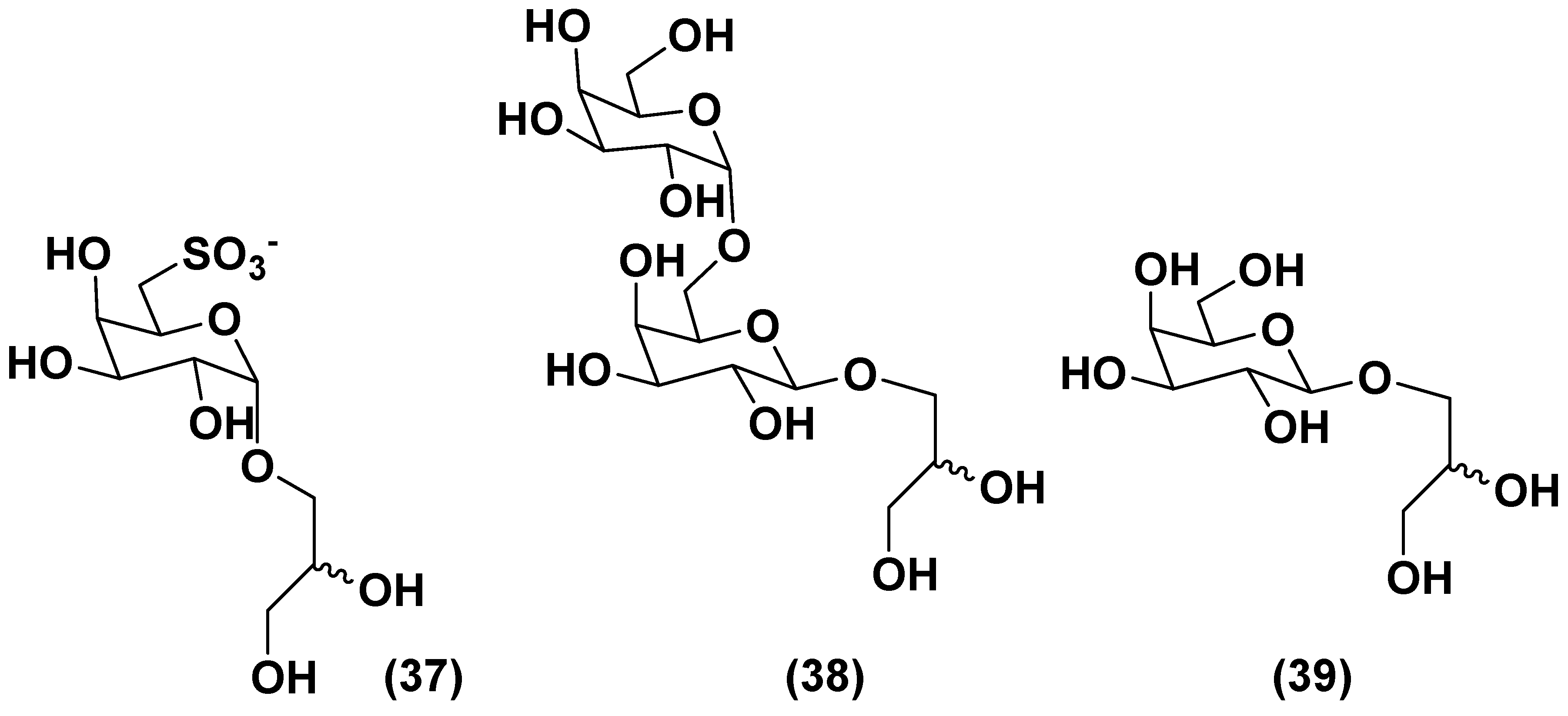
3.2. Sulfoquinovosyl-containing glycolipids
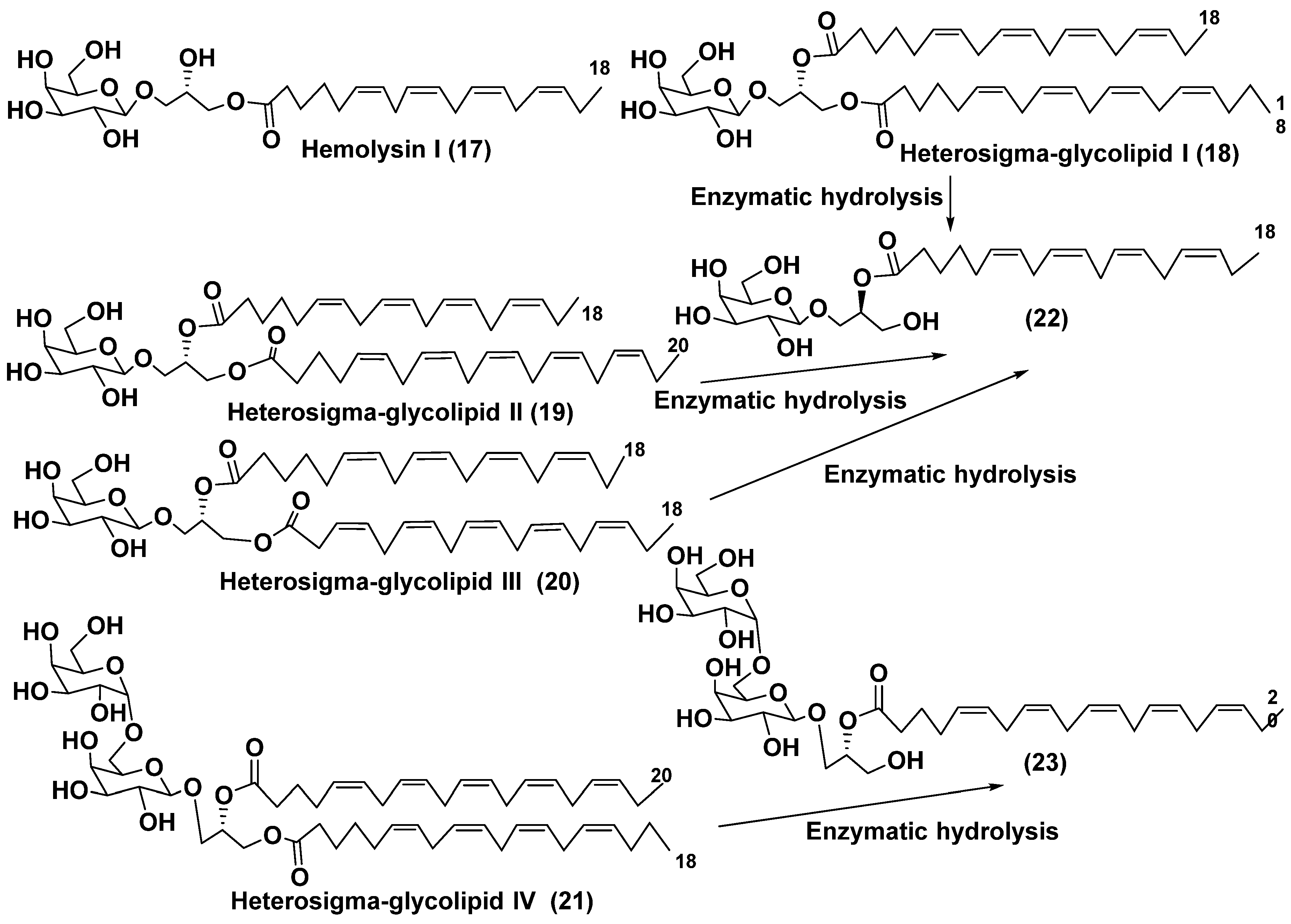
3.3. Deacylated glycolipids
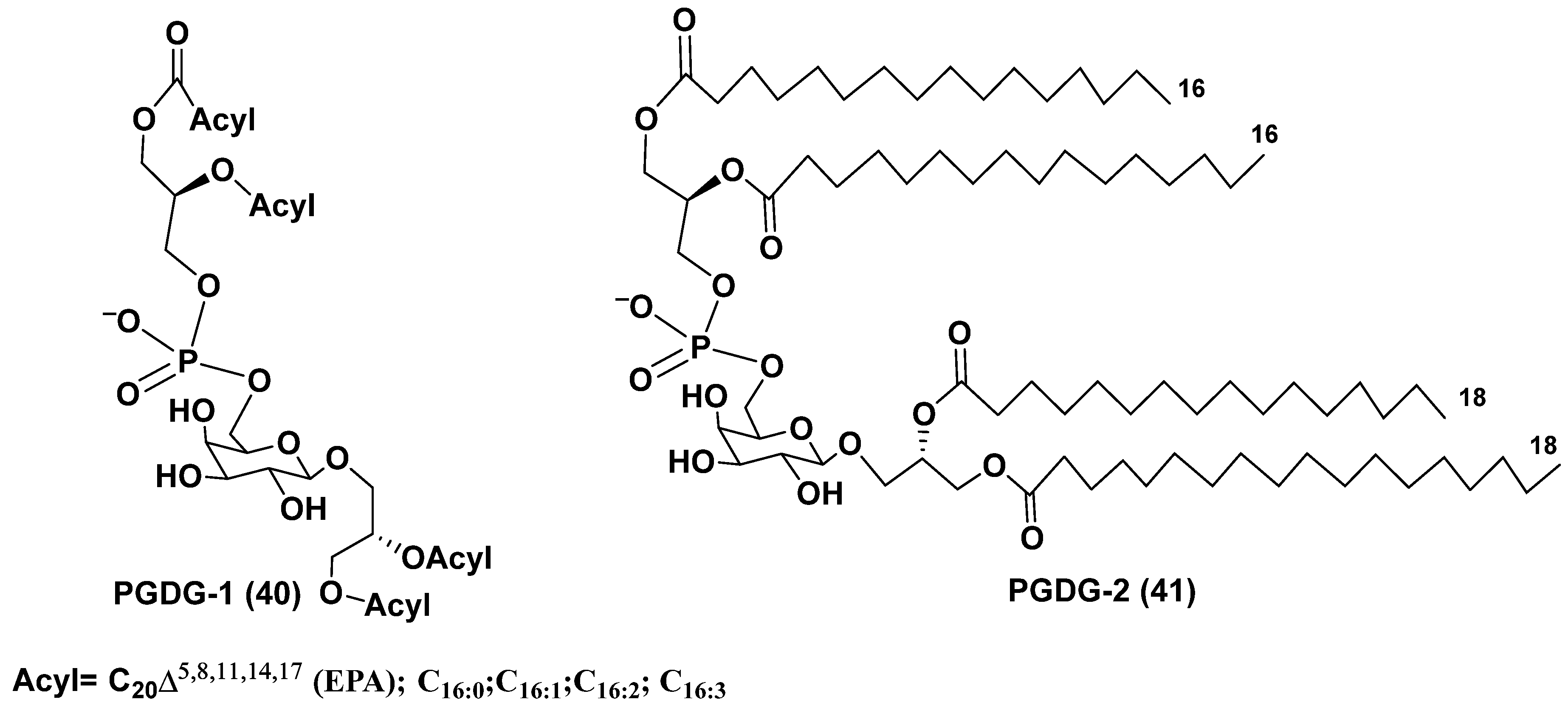
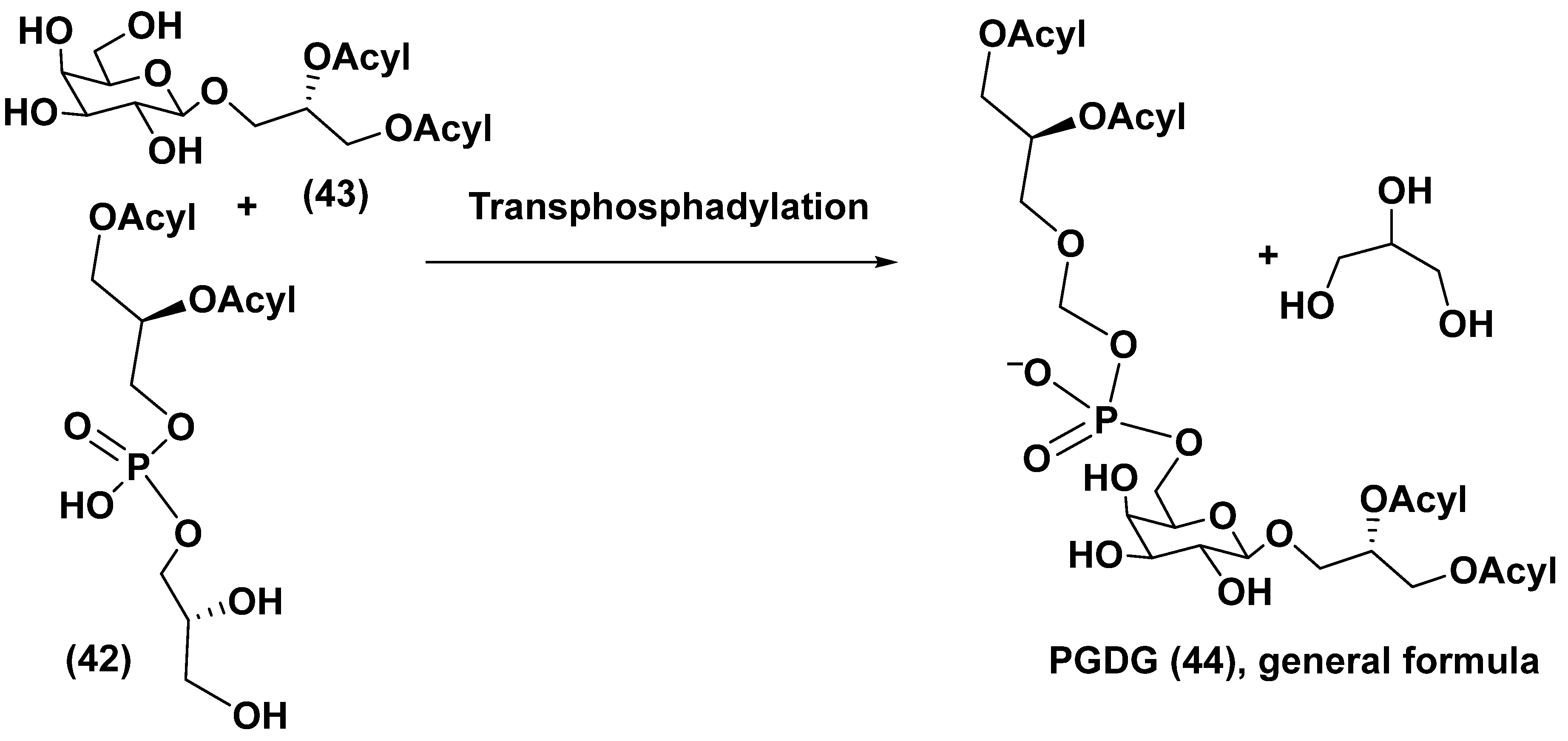
4. Phosphoglycolipids
5. Glycosides from microalgae
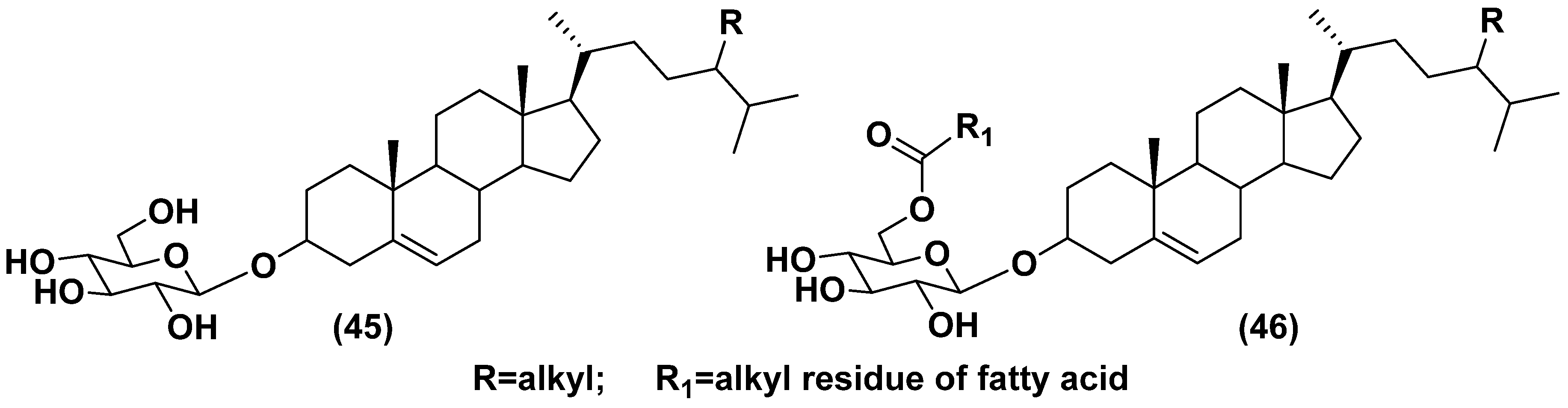
5.1. Steryl glycosides and other glycosylated derivatives from microalgae
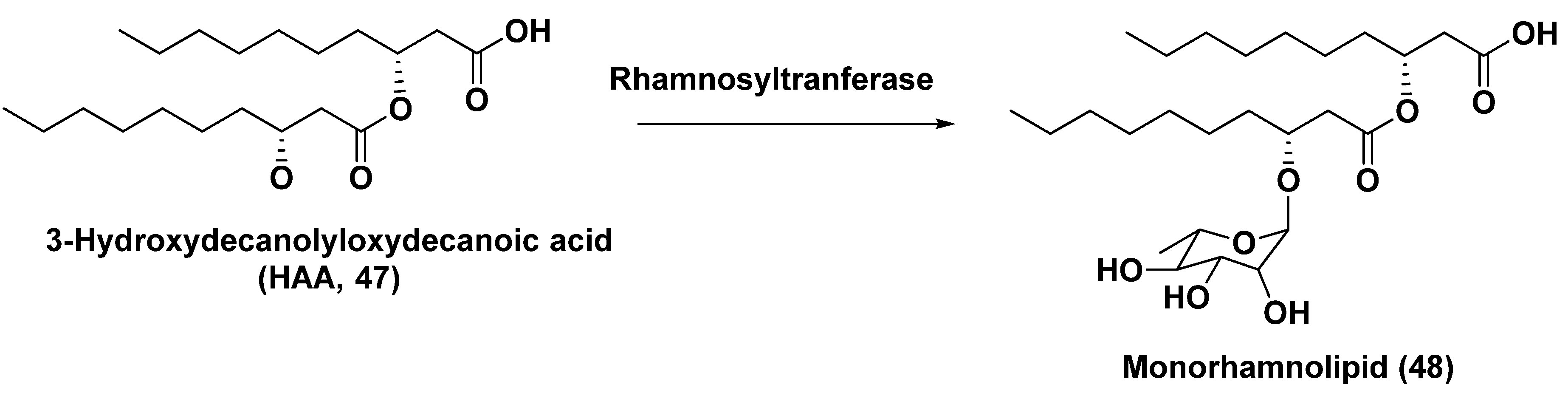
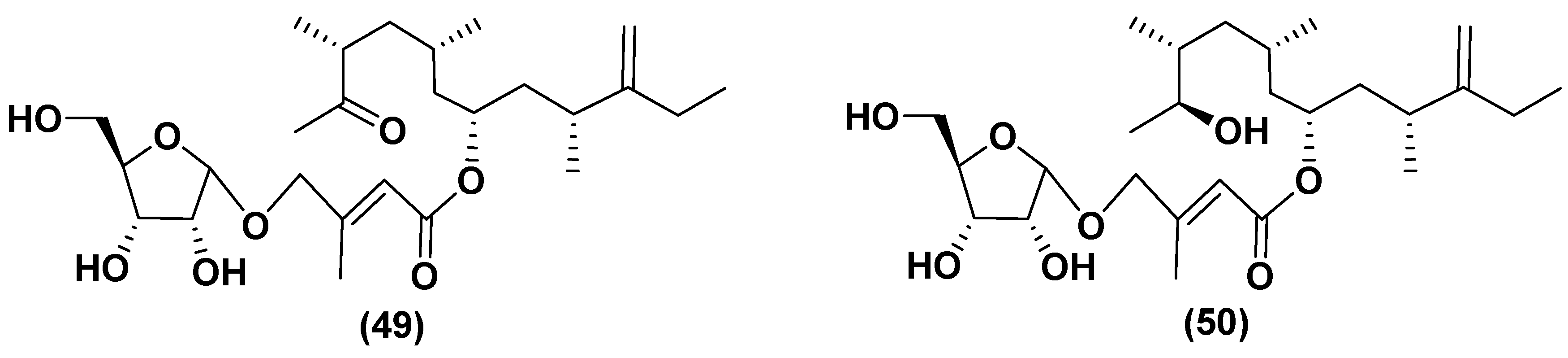
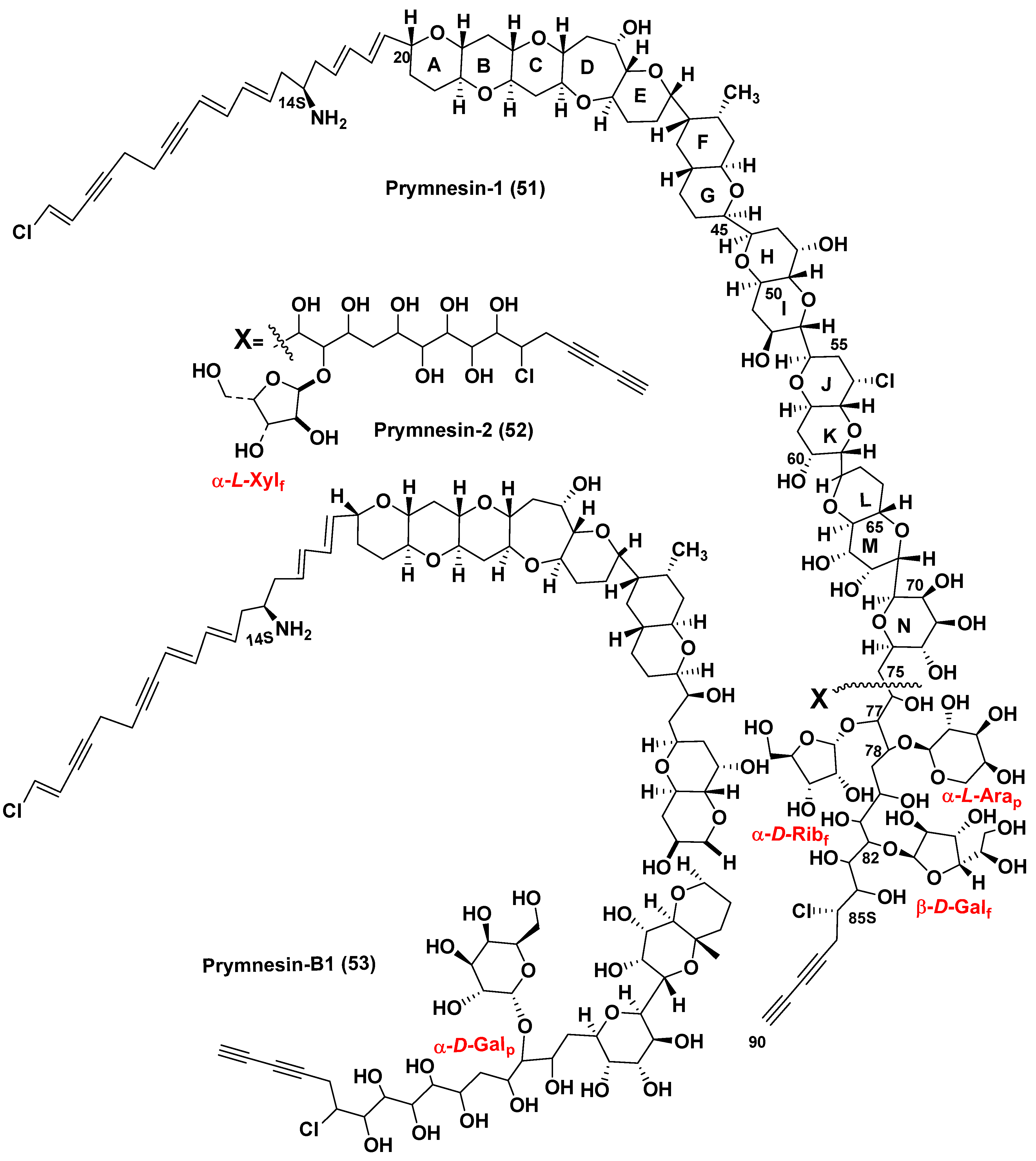
5.2. Glycosylated long chain polyketide derivatives
6. Viral regulation of microalgal blooms and glycoconjugates
6. Carbohydrate-containing metabolites of microalgae and deep-sea life.
7. Conclusive remarks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Downing, J.A. Marine nitrogen: Phosphorus stoichiometry and the global N:P cycle. Biogeochem. 1997, 37, 237–252. [Google Scholar] [CrossRef]
- Stonik, V.; Stonik, I. Low-molecular weight metabolites from diatoms: structures, biological roles and biosynthesis. Mar. Drugs 2015, 13, 3672–3709. [Google Scholar] [CrossRef]
- Stonik, V.A.; Stonik, I.V. Sterol and sphingoid glycoconjugates from microalgae. Mar. Drugs 2018, 16, 514. [Google Scholar] [CrossRef] [PubMed]
- Sadoli, E. Investigation into the occurrence of arsenic in the organism of fish. Biochem. Z. 1928, 201, 323–331. [Google Scholar]
- Wang, Y.; Wang, S.; Xu, P.; Liu, C.; Wang, Y.; Zhang, C.; Ge, Y. Review of arsenic specification, toxicity and metabolism in microalgae. Rev. Environ. Sci. Biotechnol. 2015, 14, 427–451. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Djordjevic, A.B.; Crisponi, G.; Alexander, J.; Bjørklund, G.; Aaseth, J. Arsenic toxicity: molecular targets and therapeutic agents. Biomolecules 2020, 10, 235. [Google Scholar] [CrossRef]
- Zaffiri, L.; Gardner, J.; Toledo-Pereyra, L.H. History of antibiotics. From salvarsan to cephalosporins. J. Investig. Surg. 2012, 25, 67–77. [Google Scholar] [CrossRef]
- Emadi, A.; Gore, S.D. Arsenic trioxide—an old drug rediscovered. Blood Rev. 2010, 24, 191–199. [Google Scholar] [CrossRef]
- Duncan, E.G.; Maher, W.A.; Foster, S.D.; Krikowa, F. Influence of culture regime on arsenic cycling by the marine phytoplankton Dunaliella tertiolecta and Thallassiosira pseudonana. Environ. Chem. 2013, 10, 99–101. [Google Scholar] [CrossRef]
- Foster, S.D.; Thomson, D.; Maher, W.A. Uptake and metabolism of arsenate by anexic cultures of the microalgae Dunaliella tertiolecta and Phaeodactylum tricornutum. Mar. Chem. 2008, 108, 172–183. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Sun, G.X.; Yin, X.X.; Rensing, C.; Zhu, Y.-G. Biomethylation and volatilization of arsenic by the marine microalgae Ostreococcus tauri. Chemosphere 2013, 93, 47–53. [Google Scholar] [CrossRef]
- Duncan, E.G.; Maher, W.A.; Foster, S.D.; Krikowa, F. The influence of arsenate and phosphate exposure on arsenic uptake, metabolism and species formation in the marine phytoplankton Dunaliella tertiolecta. Mar. Chem. 2013, 157, 78–85. [Google Scholar] [CrossRef]
- Duncan, E.G.; Mather, W.A.; Foster, S.D. Contribution of arsenic species in unicellular algae to cycling of arsenic in marine ecosystem. Environ. Sci. Technol. 2015, 49, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Levitsky, D.O. Arsenolipids. Progress Lipid Res. 2004, 43, 403–448. [Google Scholar] [CrossRef]
- Xue, X.M.; Xiong, C.; Yoshinaga, M.; Rosen, B.; Zhu, Y.G. The enigma of environmental organoarsenicals: Insights and implications. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3835–3862. [Google Scholar] [CrossRef]
- Glabonjat, R.A.; Raber, G.; Jensen, K.B.; Guttenberger, N.; Zangger, K.; Francesconi, K.A. A 2-O-methylriboside unknown outside the RNAs world contains arsenic. Angew. Chem., Int. Ed. 2017, 56, 11963–11965. [Google Scholar] [CrossRef]
- Glabonjat, R.A.; Raber, G.; Jensen, K.B.; Guttenberg, N.; Zangger, K.; Francesconi, K.A. Arsenolipid biosynthesis by the unicellular alga Dunalliella tertiolecta is influenced by As/P ratio in culture experiments. Metallomics 2018, 10, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Glabonjat, R.A.; Raber, G.; Jensen, K.B.; Ehgartner, J.; Francesconi, K.A. Quantification of arsenolipids in the certified reference material NMIJ 7405-a (Hijiki) using HPLC/mass spectrometry after chemical derivatization. Anal. Chem. 2018, 6, 10282–10287. [Google Scholar] [CrossRef]
- Arora, N.; Gulati, K.; Patel, A.; Pruthi, P.A.; Poluri, K.M.; Pruthi, V. A hybrid approach integrating arsenic detoxification with biodiesel production using oleaginous microalgae. Algal Res. 2017, 24, 29–39. [Google Scholar] [CrossRef]
- Hölzl, G.; Witt, S.; Kelly, A.A.; Heinz, E. Functional differences between galactolipids and glucolipids revealed in photosynthesis of higher plants. PNAS 2006, 103, 7512–7517. [Google Scholar] [CrossRef] [PubMed]
- Gounaris, K.; Barber, J. Monogalactosyldiacylglycerol: the most abundant polar lipid in nature. Trends Biochem. Sci. 1983, 8, 378–381. [Google Scholar] [CrossRef]
- Da Costa, E.; Silva, J.; Mendonça, S.H.; Abreu, M.H.; Domingues, M.R. Lipidomic approaches towards deciphering glycolipids from microalgae as a reservoir of bioactive lipids. Mar. Drugs 2016, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Awai, K. Diversity of biosynthetic pathways of galactolipids in the light of endosymbiotic origin of chloroplasts. Front. Plant Sci., Sec. Plant Metabolism Chemodiv. 2016, 7, 117. [Google Scholar] [CrossRef]
- Petroutsos, D.; Amiar, S.; Abida, H.; Dolch, L.J.; Bastien, O.; Rébeillé, F.; Jouhet, J.; Falconet, D.; Block, M.A.; McFadden, G.I.; Bowler, C.; Botte, C.; Maréchal, E. Evolution of galactoglycerolipid biosynthetic pathways - From cyanobacteria to primary plastids and from primary to secondary plastids. Progr. Lipid Res. 2014, 54, 68–85. [Google Scholar] [CrossRef]
- Nuzzo, G.; Gallo, C.; D’Ippolito, G.; Cutignano, A.; Sardo, A.; Fontana, A. Composition and quantification of microalgal lipids by ERITIC 1H NMR spectroscopy. Mar. Drugs 2013, 11, 3742–3753. [Google Scholar] [CrossRef]
- Akoka, S.; Barantin, L.; Trierweiler, M. Concentration measurement by proton NMR using the ERETIC method. Anal. Chem. 1999, 71, 2554–2557. [Google Scholar] [CrossRef]
- Kozaki, H.; Oshima, Y.; Yasumoto, T. lsolation and structural elucidation of Hemolysin I from the phytoflagellate Prymnesium parvum. Agric. Biol. Chem. 1982, 46, 233–236. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hayashi, K.; Kawazoe, K.; Kitagawa, I. Marine Natural Products. XXIX. Heterosigma-glycolipids I, II, III, and IV, four diacylglyceroglycolipids possessing ω3-polyunsaturated fatty acid residues, from the Raphidopycean dinoflagellate Heterosigma akashiwo. Chem. Pharm. Bull. 1992, 40, 1404–1410. [Google Scholar] [CrossRef]
- Hiraga, Y.; Shikano, T.; Widianti, T.; Ohkata, K. Three new glycolipids with cytolytic activity from cultured marine dinoflagellate Heterocapsa circularisquama. Nat. Prod. Res. 2008, 22, 649–657. [Google Scholar] [CrossRef]
- Daranas, A.H.; Fernández, J.J.; Norte, M. New monogalactosyl triacylglycerol from a cultured marine dinoflagellate Amphidinium sp. Nat. Prod. Lett. 1999, 14, 107–114. [Google Scholar] [CrossRef]
- Oshima, Y.; Yamada, S.-H.; Moriya, T.; Ohimuzu, Y. A monogalactosyl diacylglycerol from a culture marine dinoflagellate Scripsiella trocholidea. J. Nat. Prod., 1994, 57, 534–536. [Google Scholar] [CrossRef]
- Leutou, A.S.; McCall, J.R.; York, R.; Govindapur, R.R.; Bourdelais, A.J. Anti-inflammatory activity of glycolipids and a polyunsaturated fatty acid methyl ester isolated from the marine dinoflagellate Karenia mikimotoi. Mar. Drugs 2020, 18, 138. [Google Scholar] [CrossRef] [PubMed]
- Parrish, C.C.; Bodennec, G.; Gentien, P. Haemolytic glycoglycerolipids from Gymnodinium species. Phytochemistry 1998, 47, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Monsour, M.P.; Volkmam, J.K.; Jackson, A.E.; Blackburn, S.I. Very-long-chain (C28) highly unsaturated fatty acids in marine dinoflagellates. Phytochemistry 1999, 50, 541–548. [Google Scholar] [CrossRef]
- Volkman, J.K.; Barrett, S.M.; Blackburn, S.I.; Mansour, M.P.; Sikes, E.L.; Gelin, F. Microalgal biomarkers: a review of recent research developments. Org. Geochem. 1998, 29, 1168–1179. [Google Scholar] [CrossRef]
- Leblond, J.D.; Chapman, P.J. Lipid class distribution of highly unsaturated long chain fatty acids in marine dinoflagellates. J. Phycol. 2000, 36, 1103–1198. [Google Scholar] [CrossRef]
- Renaud, S.M.; Parry, D.L.; Thinh, L.V.; Kuo, C.; Padovan, A.; Sammy, N. Effect of light intensity on the proximate biochemical and fatty acid composition of Isochrysis sp. and Nannochloropsis oculata for use in tropical aquaculture. J. Appl. Phycol. 1991, 3, 43–53. [Google Scholar] [CrossRef]
- Okyama, H.; Morita, N.; Kogame, K. Occurrence of octadecapentaenoic acid in lipids of a cold stenotermic alga prymnesiophyte strain B. J. Phycol. 1992, 25, 465–472. [Google Scholar] [CrossRef]
- Nichols, P.D.; Volkman, J.K.; Hallegraeff, G.M.; Blackburn, S.I. Sterols and fatty acids of the red tide flagellates Heterosigma akashiwo and Chattonella antiqua (Raphidophyceae). Phytochemistry 1987, 26, 2537–2541. [Google Scholar] [CrossRef]
- Mostaert, A.S.; Karsten, U.; Hara, Y.; Watanabe, M.M. Pigments and fatty acids of marine raphidophytes: a chemotaxonomic re-evaluation. Phycol. Res. 1998, 46, 215–220. [Google Scholar] [CrossRef]
- Lynch, D.V.; Gundersen, R.E.; Thompson, G.A. Jr. Separation of galactolipids molecular species by high-performance liquid chromatography. Plant Physiol. 1983, 72, 903–905. [Google Scholar] [CrossRef]
- Yongmanitchai, W.; Ward, O.P. Positional distribution of fatty acids, and molecular species of polar lipids, in the diatom Phaeodactylum tricornutum. Microbiology, 1993, 139, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.-H.; Hong, J.; Choe, J.C.; Kim, Y.-H. Analysis of fatty acyl groups of diacyl galactolipid molecular species by HPLC/ESI-MS with in-source fragmentation. Bull. Korean Chem. Soc. 2003, 24, 1163–1168. [Google Scholar] [CrossRef]
- Guella, G.; Frasssantio, R.; Mancini, I. A new solution for an old problem: the regiochemical distribution of the acyl chains in galactolipids can be established by electrospray ionization tandem mass spectrometry. Rapid Comm. Mass Spectrom. 2003, 17, 982–1984. [Google Scholar] [CrossRef]
- Gray, C.G.; Lasiter, A.D.; Li, C.; Leblond, J.D. Mono- and digalactosyldiacylglycerol composition of dinoflagellates. I. Peridinin-containing taxa. Eur. J. Phycol. 2009, 44, 191–197. [Google Scholar] [CrossRef]
- Yoon, H.S.; Hackett, J.D.; Van Dolah, F.M.; Nosenko, T.; Lidie, K.L.; Bhattacharya, D. Tertiary endosymbiosis driven genome evolution in dinoflagellate algae. Mol. Biol. Evol. 2005, 22, 1299–1308. [Google Scholar] [CrossRef]
- Dorrell, R.G.; Howe, C.J. Integration of plastids with their hosts: Lessons learned from dinoflagellates. Proc. Natl Acad. Sci. USA 2015, 112, 10247–10254. [Google Scholar] [CrossRef]
- Leblond, J.D.; Andrew, D.; Lasiter, A.D. Mono- and digalactosyldiacylglycerol composition of dinoflagellates. II. Lepidodinium chlorophorum, Karenia brevis, and Kryptoperidinium foliaceum, three dinoflagellates with aberrant plastids. Eur. J. Phycol. 2009, 44, 199–205. [Google Scholar] [CrossRef]
- Leblond, J.D.; Elkins, F.C.; Graeff, J.E.; Sabir, K. Galactolipids of the genus Amphidinium (Dinophyceae): an hypothesis that they are basal to those of other peridinin-containing dinoflagellates. Eur. J. Phycol. 2022, 58, 190–198. [Google Scholar] [CrossRef]
- Awai, K.; Matsuoka, R.; Shioi, Y. Lipid and fatty acid compositions of Symbiodinium strains. In Proceedings of the 12th international coral reef symposium. 2012, 9–12. [Google Scholar]
- Imbs, A.B.; Yakovleva, I.M.; Dautova, T.N.; Bui, L.H.; Jones, P. Diversity of fatty acid composition of symbiotic dinoflagellates in corals: evidence for the transfer of host PUFAs to the symbionts. Phytochemistry 2014, 101, 76–82. [Google Scholar] [CrossRef]
- Imbs, A.B.; Rybina, V.G.; Kharlamenko, V.I.; Dang, L.P.T.; Nguyen, N.T.; Pham, K.M.; Pham, L.Q. Polyunsaturated molecular species of galactolipids: Markers of zooxanthellae in a symbiotic association of the soft coral Capnella sp. (Anthozoa: Alcyonacea). Russ. J. Mar Biol. 2015, 41, 461–467. [Google Scholar] [CrossRef]
- Imbs, A.B.; Yakovleva, I.M.; Pham, L.Q. Distribution of lipids and fatty acids in the zooxanthellae and host of the soft coral Sinularia sp. Fish. Sci. 2010, 76, 375–380. [Google Scholar] [CrossRef]
- Gray, C.G.; Lasiter, A.D.; Leblond, J.D. Mono- and digalactosyldiacylglycerol composition of dinoflagellates. III. Four cold-adapted, peridinin-containing taxa and the presence of trigalactosyldiacylglycerol as an additional glycolipid. Eur. J. Phycol. 2009, 44, 439–445. [Google Scholar] [CrossRef]
- Graeff, J.E.; Elkins, L.C.; Leblond, J.D.; Graeff, J.E.; Elkins, L.C.; Leblond, J.D. Plastid-associated galactolipid composition in eyespot-containing dinoflagellates: a review. Algae 2021, 36, 73–90. [Google Scholar] [CrossRef]
- Yongmanitchai, W.; Ward, O.P. Positional distribution of fatty acids, and molecular species of polar lipids, in the diatom Phaeodactylum tricornutum. J. Gen. Microbiol. 1993, 139, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, G.A.; Volkman, J.K.; Barrett, S.M.; Leroi, J.M.; Jeffrey, S.W. Essential polyunsaturated fatty acids from 14 species of diatom (Bacillariophyceae). Phytochemistry 1994, 35, 155–161. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Aizdaicher, N.A. Fatty acid composition of 15 species of marine microalgae. Phytochemistry 1995, 39, 351–356. [Google Scholar] [CrossRef]
- Yan, X.; Chen, D.; Xu, J.; Zhou, C. Profiles of photosynthetic glycerolipids in three strains of Skeletonema determined by UPLC-Q-TOF-MS. J. Appl. Phycol. 2011, 23, 271–282. [Google Scholar] [CrossRef]
- Dodson, J.; Dahmen, J.L.; Mouget, J.L.; Leblond, J.D. Mono-and digalactosyldiacylglycerol composition of the marennine-producing diatom, Haslea ostrearia: Comparison to a selection of pennate and centric diatoms. Phycol. Res. 2013, 61, 199–207. [Google Scholar] [CrossRef]
- Roche, S.A.; Leblond, J.D. Mono- and digalactosyldiacylglycerols compositions of rhaphidophytes (Raphidophyceae): a modern interpretation using positive-ion/ mass-spectrometry/mass-spectrometry. Journal of phycology 2011, 47, 106–111. [Google Scholar] [CrossRef]
- Sato, N.; Moriyama, T. Genomic and biochemical analysis of lipid biosynthesis in the unicellular rhodophyte Cyanidioschyzon merolae: lack of a plastidic desaturation pathway results in the coupled pathway of galactolipid synthesis. Eukaryotic cell 2007, 6, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Benning, C. Biosynthesis and functions of the sulfolipid sulfoquinosyl diacylglycerol. Annual Rev. Plant Physiol. Plant Molecul. Biol. 1998, 49, 53–75. [Google Scholar] [CrossRef]
- Keusgen, M.; Curtis, J.M.; Thibault, P.; Walter, J.A.; Windust, A.; Ayer, S.W. Sulfoquinovosyl diacylglycerols from the alga Heterosigma carterae. Lipids 1997, 32, 1102–1112. [Google Scholar] [CrossRef]
- Gustafson, K.R.; Cardellina, J.H., II; Fuller, R.W.; Weislow, O.S.; Kiser, R.F.; Snader, K.M.; Patterson, G.M.L.; Boyd, M.R. AIDS-Antiviral Sulfolipids from Cyanobacteria (Blue-Green Algae). J. Natl. Cancer Inst. 1989, 81, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.Y.; Yang, A.R.; Park, J.; Moon, S.J.; Jeong, E.J.; Rho, J.-R. Characterization of a new trioxilin and a sulfoquinovosyl diacylglycerol with anti-inflammatory properties from the dinoflagellate Oxyrrhis marina. Mar. Drugs, 2017, 15, 57. [Google Scholar] [CrossRef]
- Zianni, R.; Bianco, G.; Lelario, F.; Losito, I.; Palmisano, F.; Cataldi, T.R. Fatty acid neutral losses observed in tandem mass spectrometry with collision-induced dissociation allows regiochemical assignment of sulfoquinovosyl-diacylglycerols. J. Mass Spectr. 2013, 48, 205–215. [Google Scholar] [CrossRef] [PubMed]
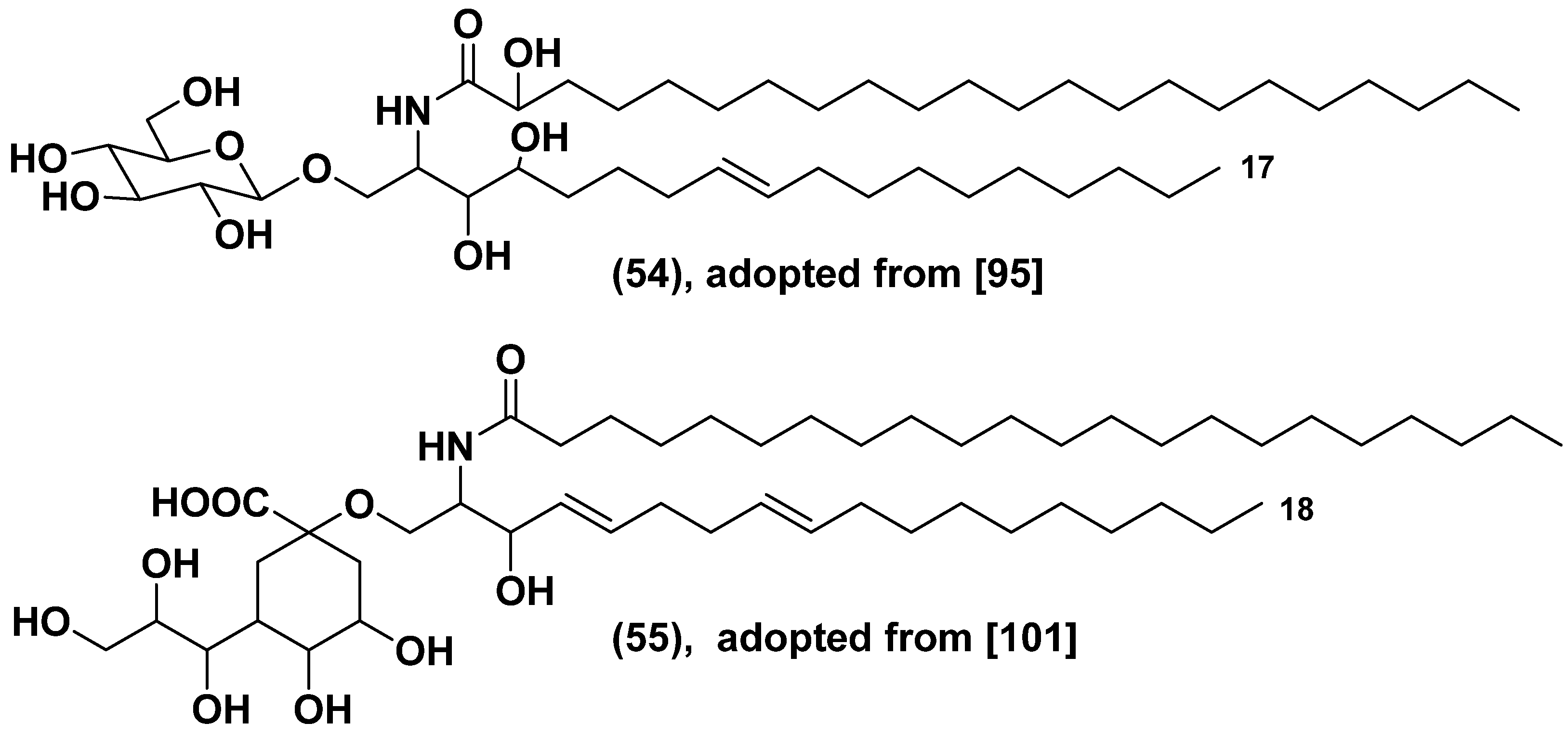
- Manzo, E.; Cutignano, A.; Pagano, D.; Gallo, C.; Barra, G.; Nuzzo, G.; Sansone, C.; Ianora, A.; Urbanek, K.; Fenoglio, D.; Ferrera, F.; Bernardi, C.; Parodi, A.; Pasqually, G.; Leonardi, A.; Filaci, G.; De Palma, R.; Fontana, A. A new marine-derived sulfoglycolipid triggers dendritic cell activation and immune adjuvant response. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Mazepa, E.; Malburg, B.V.; Mógor, G.; de Oliveira, A.C.; Amatussi, J.O.; Corrêa, D.O.; Lemos, J.S.; Diogo, R.B.; Ducatti, D.R.; Duarte, M.E.R.; Mogor, A.F.; Noseda, M.D. Plant growth biostimulant activity of the green microalga Desmodesmus subspicatus. Algal Res. 2021, 59, 102434. [Google Scholar] [CrossRef]
- Manzo, E.; Gallo, C.; Sartorius, R.; Nuzzo, G.; Sardo, A.; De Berardinis, P.; Fontana, A.; Cutignano, A. Immunostimulatory phosphatidylmonogalactosyldiacylglycerols (PGDG) from the Marine Diatom Thalassiosira weissflogii: Inspiration for a Novel Synthetic Toll-Like Receptor 4 Agonist. Mar. Drugs, 2019, 17, 103. [Google Scholar] [CrossRef] [PubMed]
- Gallo, C.; Barra, G.; Saponaro, M.; Manzo, E.; Fioretto, L.; Zico, M.; Nuzzo, G.; d’Ippolito, G.; De Palma, R.; Fontana, A. A new bioassay platform design for the discovery of small molecules with anticancer immunotherapeutic activity. Mar. drugs, 2020, 18, 604. [Google Scholar] [CrossRef]
- Stonik, V.A. Marine polar steroids. Russ. Chem. Rev., 2001, 70, 673–715. [Google Scholar] [CrossRef]
- Shimamura, M. Structure, metabolism and biological functions of steryl glycosides in mammals. Archiv. Immunolog. Therap. 2012, 60, 351–359. [Google Scholar] [CrossRef]
- Carlucci, R.; Jäger, S.N.; Labadie, G.R. Steryl glucosides recovered from biodiesel tank deposits are an excellent source of phytosterols. Industrial Crops and Prod. 2022, 187, 115307. [Google Scholar] [CrossRef]
- Mario-Vynvyals, B.; Artigues, M.; Wostrikoff, K.; Monte, E.; Bruto-Puig, G. Chloroplast engineering of the green microalgae Chlamydomonas reinhardtii for the production of HAA the lipid moiety of rhamnolipid biosurfactants. New biotechn. 2023, 76, 1–12. [Google Scholar]
- Kubota, T.; Iwai, T.; Sakai, K.; Gonoi, T.; Kobayashi, J. Amphidinins C−F, Amphidinolide Q Analogues from marine dinoflagellate Amphidinium sp. Org. Lett. 2014, 16, 5624–5627. [Google Scholar] [CrossRef]
- Liebert, F.; Deerns, W.M. Onderzoek naar de oorzak van een Vischsterfte in den Polder Workumer Nieuwland, nabij Workum. Verhandungen en Rapporten uitgegeven door Rijkinstituten voor Visscherijonderzoek, 1920, 1, 81–93. [Google Scholar]
- Wagstaff, B.A.; Hems, E.S.; Rejzek, M.; Pratscher, J.; Brooks, E.; Kuhaudomlarp, S.K.; O’Neill, E.C.; Matthew, I.; Donaldson, M.I.; Lane, S.; Currie, J.; Hindes, A.M.; Malin, G.; Murrell, J.C.; Field, R.A. Insights into toxic Prymnesium parvum blooms: the role of sugars and algal viruses. Biochem. Soc. Transactions, 2018, 46, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Satake, M.; Yasumoto, T. Prymnesin-2: a potent ichthyotoxic and hemolytic glycoside isolated from the red tide alga Prymnesium parvum. J. Am. Chem. Soc. 1996, 118, 479–480. [Google Scholar] [CrossRef]
- Igarashi, T.; Satake, M.; Yasumoto, T. Structures and partial stereochemical assignments for prymnesin-1 and prymnesin-2: potent hemolytic and ichthyotoxic glycosides isolated from the red tide alga Prymnesium parvum. J. Am. Chem. Soc. 1999, 121, 37–8499. [Google Scholar] [CrossRef]
- Sasaki, M.; Shida, T.; Tachibana, K. Synthesis and stereochemical confirmation of the HI/JK ring system of prymnesins, potent hemolytic and ichthyotoxic glycoside toxins isolated from the red tide alga. Tetrahedron Lett. 2001, 42, 5725–5728. [Google Scholar] [CrossRef]
- Sasaki, M.; Ebine, M.; Takagi, H.; Takakura, H.; Shida, T.; Satake, M.; Oshima, Y.; Igarashi, T.; Yasumotao, T. Synthesis of the CDE/FG ring models of prymnesins: reassignment of the relative configuration of the E/F ring juncture. Org. Lett. 2004, 6, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Meier, S.; Andersen, N.G.; Blossom, H.E.; Duus, J.Q.; Nielsen, K.F.; Hansen, P.J.; Larsen, T.O. Chemodiversity of Ladder-Frame Prymnesin Polyethers in Prymnesium parvum. J. Nat. Prod. 2016, 79, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- Binzer, S.B.; Svenssen, D.K.; Daugbjerg, N.; Alves-de-Souza, C.; Pinto, E.; Hansen, P.J.; Varga, E. A-, B-and C-type prymnesins are clade specific compounds and markers in Prymnesium parvum. Harmful Algae, 2019, 81, 10–17. [Google Scholar] [CrossRef]
- Manning, S.R.; La Claire II, J.W. Prymnesins: Toxic Metabolites of the Golden Alga, Prymnesium parvum Carter (Haptophyta). Mar. Drugs 2010, 8, 678–704. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, U. Phagotrophy by a plastidic haptophyte, Prymnesium patelliferum. Aquat. Microb. Ecol. 1998, 14, 155–160. [Google Scholar] [CrossRef]
- Tillmann, U. Kill and eat your predator: A winning strategy of the planktonic flagellate Prymnesium parvum. Aquat. Microb. Ecol. 2003, 32, 73–84. [Google Scholar] [CrossRef]
- La Claire, J.W., II. Analysis of expressed sequence tags from the harmful alga, Prymnesium parvum (Prymnesiophyceae, Haptophyta). Mar. Biotech. 2006, 8, 534–546. [Google Scholar] [CrossRef]
- Anestis, K.; Kohli, G.; Wohlrab, S.S.; Varga, E.; Larsen, T.O.; Hansen, P.J.; Jon, U. Polyketide synthase genes and molecular trade-offs in the ichthyotoxic species Prymnesium parvum. Sci. Total Environ., 2021, 795, 148878. [Google Scholar] [CrossRef]
- Van Wagoner, R.M.; Satake, M.; Wright, J.L. Polyketide biosynthesis in dinoflagellates: what makes it different? Nat. Prod. Rep. 2014, 31, 1101–1137. [Google Scholar] [CrossRef]
- Freitag, M.; Beszteri, S.; Vogel, H.; John, U. Effects of physiological shock treatments on toxicity and polyketide synthase gene expression in Prymnesium parvum (Prymnesiophyceae). Eur. J. Phycol., 2011, 46, 193–201. [Google Scholar] [CrossRef]
- Nagasaki, K.; Masashi, A.; Itakura, S.; Imai, I.; Ishida, Y. Viral mortality in the final stage of Heterosigma akashiwo (Raphidophyceae) red tide. J. Plankt. Res. 1994, 16, 1595–1599. [Google Scholar] [CrossRef]
- Nagasaki, K.; Yamaguchi, M. Isolation of a virus infectious to the harmful bloom causing microalga Heterosigma akashiwo (Raphidophyceae). Aquat. Microb. Ecol. 1997, 13, 135–140. [Google Scholar] [CrossRef]
- Fuhrman, J.A. Marine viruses and their biogeochemical and ecological effects. Nature 1999, 399, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, D.C.; Oke, J.; Malin, G.; Wilson, W.H. Coccolithovirus (Phycodnaviridae): Characterization of a new large dsDNA algal virus that infects Emiliana huxleyi. Arch. Virol. 2002, 147, 1685–1698 doiorg103390/v9030052. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.H.; Tarran, G.A.; Schroeder, D.; Cox, M.; Oke, J.; Malin, G. Isolation of viruses responsible for the demise of an Emiliania huxleyi bloom in the English Channel. J. Mar. Biol. Assoc. UK, 2002, 82, 369–377. [Google Scholar] [CrossRef]
- Vardi, A.; Haramaty, L.; Van Mooy, B.A.S.; Fredricks, H.F.; Kimmance, S.; Larsen, A.; Bidle, K.D. Host-virus dynamics and subcellular controls of cell fate in a natural coccolithophore population. Proc. Natl. Acad. Sci. U S A, 2012, 109, 19327–19332. [Google Scholar] [CrossRef] [PubMed]
- Vardi, A.; Van Mooy, B.A.S.; Fredricks, H.F.; Popendorf, K.J.; Ossolinski, J.E.; Haramaty, L.; Bidle, K.D. Viral glycosphingolipids induce lytic infection and cell death in marine phytoplankton. Science 2009, 326, 861–865. [Google Scholar] [CrossRef]
- Pagarete, A.; Allen, M.J.; Wilson, W.H.; Kimmance, S.A.; de Vargas, C. Host–virus shift of the sphingolipid pathway along an Emiliania huxleyi bloom: survival of the fattest. Environ. Micobiol. 2009, 11, 2840–2848. [Google Scholar] [CrossRef]
- Wilson, W.H.; Schroeder, D.C.; Allen, M.J.; Holden, M.T.G.; Parkhill, J.; Barrell, B.G.; Ghazal, P. Complete genome sequence and lytic phase transcription profile of a Coccolithovirus. Science 2005, 309, 1090–1092. [Google Scholar] [CrossRef]
- Fulton, J.M.; Fredricks, H.F.; Bidle, K.D.; Bidle, K.D.; Vardi, A.; Kendrick, B.J.; DiTullio, G.R.; Van Mooy, B.A.S. Novel molecular determinants of viral susceptibility and resistance in the lipidome of Emiliania huxleyi. Environ. Microbiol. 2014, 16, 1137–1149. [Google Scholar] [CrossRef]
- Wagstaff, B.A.; Vladu, I.C.; Barclay, J.E.; Schroeder, D.C.; Malin, G.; Field, R.A. Isolation and Characterization of a Double Stranded DNA Megavirus Infecting the Toxin-Producing Haptophyte Prymnesium parvum. Viruses 2017, 9, 40. [Google Scholar] [CrossRef]
- Fogg, G.T.; Nalewajko, C.; Watt, W. Extracellular products of phytoplankton photosynthesis. Proc. Roy Soc London B 1965, 162, 517–534. [Google Scholar] [CrossRef]
- Fenchel, T. Marine phytoplankton food chains. Ann. Rev. Ecol. Syst. 1988, 19, 19–38. [Google Scholar] [CrossRef]
- Moore, J.C.; Berlow, E.L.; Coleman, D.C.; de Ruiter, P.C.; Dong, Q.; Hastings, A.; Johnson, N.C.; McCann, K.S.; Melville, K.; Morin, P.J.; Nadelhoffer, K.; Rosemond, A.D.; Post, D.M.; Sabo, J.L.; Scow, K.M.; Vanni, M.J.; Wall, D.H. Detritus, trophic dynamics and biodiversity. Ecol. letters 2004, 7, 584–600. [Google Scholar] [CrossRef]
- Sirohi, R.; Joun, J.; Choi, H.I.; Gaur, V.K.; Sim, S.J. Algal glycobiotechnology: omics approaches for strain improvement. Microbial Cell Fact 2021, 20, 163. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




