1. Introduction
The use of enzymes to catalyze biotransformations represents a desirable alternative within the context of more environmentally friendly conditions. However, in their native form, they are not always the best option compared to chemical catalysts. The use of enzymes can increase the cost of industrial process, thus reusable forms are gaining relevance and are being employed more frequently [
1,
2]. In this way, immobilization is a desirable alternative since it is usually accompanied by greater operational stability of the enzyme. In many cases, the immobilized enzyme significantly improves the pH and temperature range in which it can be used. This, coupled with the possibility of reusing the catalyst, increase the economic viability for the use of biocatalysts and make it a desirable option in the transition of many industrial processes towards green chemistry [
3].
Protein immobilization plays an important role in several areas. In life sciences and medicine, it forms the basis of many applications, such as biosensors, biomedical implants, recyclable biocatalysts, and protein arrays for drug screening, involving protein-protein or protein-ligand interaction [
4]. In the chemical and food industry, it also plays a fundamental role, where the main reason for immobilization and more recently co-immobilization, is reuse or continuous use within process reactors [
5,
6].
Methods for enzyme immobilization can be chemical and physicochemical [
7] and their choice will depend on the nature of the support, the enzyme, and the application and use of the enzyme [
8]. Enzymes can be immobilized on natural or synthetic supports through chemical means (binding them to supports by covalent bonds) or physical means (electrostatic, ionic forces, membranes). They can be adsorbed and also trapped/encapsulated in some material through the addition of agents that form a protective film around the immobilized enzyme, allowing the selective passage of reagents and small-sized products [
8].
The adsorption method, which is mainly based on physical adsorption or ionic binding, is a simple and reversible approach [
9]. However, it can be challenging to find the conditions under which the enzyme maintains the bond strength as well as its activity. On the other hand, entrapping enzymes within microporous gels, which are formed in the presence of the enzyme, prevents the enzyme from escaping the microporous structure while still allowing the entry and exit of substrates and products. This approach has been successfully tested in the pharmaceutical industry and other chemical processes and is known to enhance the mechanical stability of the biocatalyst [
6,
10]. In the case of covalent immobilization, the reaction occurs between amino acids on the enzyme surface and reactive groups placed on the support surface [
11]. Immobilization of enzymes by covalent bonds using mesoporous silica and chitosan supports has been reported, resulting in improvements in the lifetime and thermal stability of the enzyme [
10].
Chitosan, a poly-
N-acetylglucosamine, is a modified oligosaccharide obtained by deacetylation of chitin, the second most abundant biopolymer after cellulose. Chitosan is insoluble in water, but in most organic acids, the amino group in the molecular chain is converted to ammonium ion, allowing solubility in the form of a gel that precipitates when the pH is increased, forming water-insoluble complexes. In this way, the gel forms spheres, membranes, capsules, and fibers, among others, which can be modified with treatments that improve stability and durability. Moreover, chitosan has the advantages of being biocompatible, biodegradable, non-toxic, non-antigenic, easy to obtain, and low cost, among others, and at the same time has unique characteristics of molecular structure, chemical, and biological properties [
12,
13].
Enzymes with potential applications in biomass utilization processes become relevant in the promotion of the use of clean and renewable energy sources [
14,
15]. Such is the case of β-xylosidase from
Geobacillus stearothermophillus (XynB2) which has been successfully overexpressed by recombinant technology and purified [
16,
17] and previously subjected to thorough biochemical [
18,
19,
20] and biophysical characterization [
17]. It has also been demonstrated that XynB2 can be transformed into a glycosynthase through site-directed mutagenesis of the catalytic nucleophile [
21]. Additionally, the introduction of a new exo-xylanase activity into XynB2 was achieved by replacing tyrosine 509 with glutamic acid [
22]. Notably, this mutant enzyme variant retained its xylosidase activity. These characteristics of XynB2 make it a highly promising candidate for industrial applications [
23]. In this sense, the Y509E mutant of XynB2 (XynB2
Y509E) has been immobilized in Cross-Linked Enzyme Aggregates (CLEAs) with remarkable improvements in its operational stability [
24].
This present work addresses the importance of selection of optimal kind of immobilization with respect to the chitosan spheres which were prepared using the mutant XynB2Y509E. Two protocols were implemented to immobilize the purified β-xylosidase mutant: by entrapment into chitosan beads and by the reinforcement of chitosan beads with glutaraldehyde. The second protocol was expected to promote covalent links with primary amino groups of XynB2Y509E. In this context, both protocols were evaluated trough the immobilization conditions and kinetic properties of the free and immobilized mutant enzyme, intending to choose the more efficient catalyst. In addition, the storage stability and reusability of the catalysts were also determined.
2. Materials and Methods
2.1. Materials
Materials used to make the spheres and to measure the enzyme activity like low molecular mass chitosan, acetic acid, glutaraldehyde (GTA), p-nitrophenyl-β-d-xylopyranoside (pNPX), and birchwood xylan were from Sigma (St. Louis, MO, USA). Acrylamide, bromophenol blue and Coomassie R-250 brilliant blue used were from Fisher Scientific (Hampton, NH, USA). Bis-acrylamide, sodium dodecyl sulfate (SDS), glycerol, isopropyl β-d-1-thiogalactopyranoside (IPTG), molecular mass marker V849, and tris-base were from Promega (Madison, WI, USA). All other chemicals used were analytical grade reagents.
2.2. Mutagenesis, Overexpression, and Partial Purification of XynB2Y509E
Site-directed mutagenesis of the
xynB2 gene was developed using the QuikChange II kit (Stratagene, La Jolla, CA, USA), the plasmid with the mutation was named pJAVI100.
E. coli C43 was transformed with pJAVI100 plasmid, overexpressed, and partially purified as described previously by Romero
et al. [
24].
2.3. Preparation of chitosan spheres and immobilization
The spheres were prepared by the neutralization method [
25], which consists of dripping the chitosan gel solution into an alkaline solution. Since chitosan is insoluble at basic pH, the drop solidifies because of polymer precipitation. Immobilization in chitosan was tested in two different ways:
By entrapment: 0.2 g of low molecular mass chitosan were dissolved in 10 mL of acetic acid (0.1 M), under continuous stirring at 120 rpm and with heating (60 °C). Four mL of the latest solution were added to 2 mL of the partially purified protein; the resulting suspension was added by dripping with a 27 G syringe into 200 mL of 0.5 M citrate - phosphate - glycine (CFG) buffer, pH 8.5 and 20% ethanol, forming spheres of approximately 2 mm in diameter. For hardening, spheres were stirred gently for 1 h in this solution. Then, the beads were extracted on a sieve, and washed with CFG buffer pH 6.5, reserving the buffer and wash water to verify the activity of the protein that was not incorporated into the spheres.
By formation of covalent bonds on chitosan activated with glutaraldehyde: spheres were prepared with 2% chitosan in acetic acid (0.1 M) and dripped into a solution of CFG buffer pH 8.5 (0.5 M) and 20% ethanol, allowing maturation for 1 h (described above). Then, the spheres were washed with distilled water and immersed in 1% glutaraldehyde solution for activation for 4 h. After that, spheres were washed sufficiently to remove excess glutaraldehyde. For covalent protein binding, 0.3 g of wet spheres were incubated with 0.5 mL of protein partially purified by heating and 0.5 mL of CFG buffer pH 7.5 (0.1 M), under agitation of 200 rpm at 25 °C for 4 h. Then, the derivatives were filtered and washed with distilled water. After washing, the derivatives were stored in CFG buffer pH 7.5 (0.1 M), at 4 °C until further use.
The yield of the XynB2Y509E immobilization procedure was estimated by the difference between the protein measured from the starting amount (0.35 mg/mL) and the protein measured in the buffer solution used as means for immobilization plus the protein measured in the water of the 3 consecutive washes, according to equation:
In immobilization by entrapment, P0 is the initial protein amount; P is the sum of protein released during precipitation process and the protein released in the washes.
In immobilization on glutaraldehyde-activated chitosan spheres, P0 is the initial protein amount and P is the protein in the supernatant after immobilization.
To calculate the expression yield of XynB2Y509E after immobilization on chitosan by both methods, the following equation was used:
The term total activity of expressed enzyme refers to the total enzyme activity multiplied by the protein immobilization yield.
In order to evaluate more precisely the immobilization process, the percentage of activity recovery was calculated using the following equation:
2.4. Enzymatic Assays
β-xylosidase activity: β-xylosidase activity of free and immobilized enzyme was determined by measurement of
p-nitrophenol (pNP) released from the substrate
p-nitrophenyl β-
d-xylopyranoside (pNPX) (Sigma). The reaction of immobilized XynB2
Y509E was initiated by the addition of 18 mg of beads with immobilized enzyme, to 50 μL 2.2 mM substrate and 150 mM citrate-phosphate-glycine buffer at 50 °C. After 5 min the reaction was stopped and color developed through the addition of 600 μL of 1M Na
2CO
3. Color intensity was read at 410 nm by using the extinction coefficient ∆ε = 18 mM
−1 cm
−1 [
24], in an SP-830 plus spectrophotometer (Metertech). One enzyme unit (IU) was defined as the amount of enzyme required to produce 1 μmol of pNP per minute and specific activity was defined as units per mg weight of protein-containing spheres. All assays were performed in triplicate and used as control spheres prepared according to the described technique without added protein. Protein determination was assayed by the Bradford method [
26].
Xylanase activity: this activity was determined by measuring the amount of reducing sugars released from birchwood xylan employing the 3,5-dinitrosalicylic acid (DNS) method [
27]. The reaction mixture consisted of 60 mg of spheres with immobilized enzyme and 0.9 mL of birchwood xylan at different concentrations in 0.1 M CFG buffer at pH 6.5. The enzymatic reaction was incubated for 1 h at 50 °C. After that, the reaction was stopped by adding 1 mL of DNS reagent, and tubes were incubated at 100 °C for 15 min. Subsequently, 2 mL of distilled water was added to the reaction tubes and mixed thoroughly. The absorbance of the liberated xylose was measured at 540 nm and the concentration was calculated by using the extinction coefficient ∆ε = 0.082 mM−1 cm−1 [
24]. One unit of enzymatic activity was defined as the amount of enzyme required to release 1 µmol of reducing sugar xylose equivalents per minute. All assays were performed in triplicate and used as control spheres prepared according to the described technique without added protein. Protein determination was assayed by the Bradford method [
26].
2.5. Optimization of immobilization processes
In order to establish the optimal conditions during the immobilization by entrapment process, the following parameters were investigated: chitosan concentration (1.0-5.0% w/v), type of acid and its concentration for chitosan solubility, precipitant agents (sodium hydroxide, potassium hydroxide, and CFG buffer) at different final concentrations (0.1-1 M), and maturation time (1-4 h).
To determine the ideal conditions for the immobilization covalent process, the following parameters were investigated: enzyme concentration (0.753 and 0.389 mg/mL), glutaraldehyde concentration (1-5% v/v), crosslinking time (1-24 h), and immobilization time (1-24 h).
All experiments were conducted in triplicate. The β-xylosidase activity under optimal conditions was defined as 100%.
2.6. Biochemical characterization of immobilized XynB2Y509E
Effect of pH on the activity and stability of free and immobilized XynB2Y509E. The optimum pH of free and immobilized enzymes was determined by incubating with CFG buffer ranging from pH 4.3 – 11 and keeping the same ionic strength (100 mM). To determine the pH stability, free and immobilized enzyme were incubated in CFG buffers at pH 4.3 – 11 for 1 h at 4 °C. The residual activity was measured using the standard chromogenic assay described above. Experiments for optimum pH and pH stability were performed by triplicates.
Effect of temperature on the activity and stability of free and immobilized XynB2Y509E. The optimum temperature for both free and immobilized enzymes were determined by assaying enzymatic activity in 0.1 M CFG buffer (pH 6.5) at temperatures ranging from 40 to 80 °C for 5 min on p-NPX 2.2 mM as substrate. To investigate the thermal stability, the free and immobilized β-xylosidase were incubated in 0.1 M CFG buffer (pH 6.5) at different temperatures (30–90 °C) for 60 min. After that, the residual enzymatic activity was measured by the method described above. Experiments for optimum temperature and thermostability were performed in triplicate.
Kinetic parameters of free and immobilized XynB2Y509E. The kinetic constants (Km and Vmax) for the free and immobilized enzymes were determined by measuring the enzymatic activity in a 200 mM CFG buffer (pH 6.5) at 50 °C with different substrate concentrations (0.22–2.00 mM). Km and Vmax were calculated from the hyperbolic adjustment using the Origin 8.0 program (Originlab Corporation, Inc., Northhampton, MA, USA). All assays were carried out by triplicates.
Operational stability. The retention of the immobilized enzyme activity was assayed under standard conditions for β-xylosidase activity. After developing each reaction, the chitosan spheres with the immobilized enzyme were removed and washed with CFG buffer (pH 6.5) to remove any excess of substrate on the spheres. Then the biocatalysts were re-used with freshly buffer and substrate in a new consecutive cycle. The β-xylosidase activity in the first cycle was considered as 100%.
Storage stability. The enzyme immobilized on chitosan spheres by entrapment and formation of covalent bonds was stored in CFG buffer at 4 °C for 2 months. At different times, free enzyme and biocatalysts were assayed for β-xylosidase activity. The relative β-xylosidase activity (%) was measured as a percentage of initial β-xylosidase activity (100%) considered as control.
3. Results and Discussion
Within the general framework of obtaining more efficient biocatalysts, β-xylosidase XynB2Y509E was immobilized onto chitosan spheres using two different methods. The effectiveness of both immobilization methods was evaluated by comparing the activity and stability of β-xylosidase when either trapped in, or covalently attached to chitosan. In this regard, the chitosan spheres employed in this research were synthesized through the neutralization method.
XynB2
Y509E possesses dual activity, including its natural xylosidase activity, as well as a newly discovered exo-xylanase activity [
22,
24]. The introduction of a new catalytic function into the active site of XynB2 makes it an attractive candidate for studying various immobilization approaches. For industrial use, the enzyme must have high reusability, ease of recovery, and high operational stability. Enzyme immobilization is a promising alternative to improve the enzyme efficacy and performance in the industrial field. In general, immobilization results in an increase of operational stability, which ultimately results in reduction of process costs [
28,
29].
Chitosan is a frequently used material for the preparation of supports [
12,
28,
30]. An advantage of its use is its versatility in obtaining support preparations in various sizes and forms [
13,
25]. Moreover, there have been reports of enhancements in the usefulness and practical applications of chitosan after modifications with compounds such thiols [
31], alginate [
32], carrageenan, gelatin [
33] and glutaraldehyde [
34].
3.1. Conditions for the immobilization of XynB2Y509E in chitosan spheres by entrapment
In the entrapment method, a solid or gel is formed or reformed in the presence of the enzyme, whereby the enzyme is trapped within the matrix of the material [
35]. Ideally, in this type of immobilization, the enzyme retains the native structure as no strong modifications occur within the gel. Nonetheless, the primary drawback of this method is associated with the constraints on mass transfer that occur during an enzymatic cycle. Therefore, to achieve a successful entrapment, it is crucial to create a suitable environment within a porous material that enables unimpeded diffusion of the substrate and product, while limiting the mobility of the enzyme.
The efficiency of immobilizing XynB2
Y509E by entrapment, calculated according to equation 1, was 68%. However, only 7.9% of the protein activity is expressed. In addition, we also calculated the global enzyme activity yield, which is another parameter really pertinent to define the immobilization process [
1]. According to equation 3, the enzyme activity yield for the entrapment immobilization was estimated in 38%. Several factors determine the optimal conditions for the preparation of chitosan spheres as a support for enzyme immobilization. In the case of immobilization by entrapment, one of the crucial factors is the concentration of chitosan used to prepare the spheres. Therefore, different concentrations of chitosan ranging from 1.0 to 5.0% (w/v) were studied in order to produce beads with better mechanical strength.
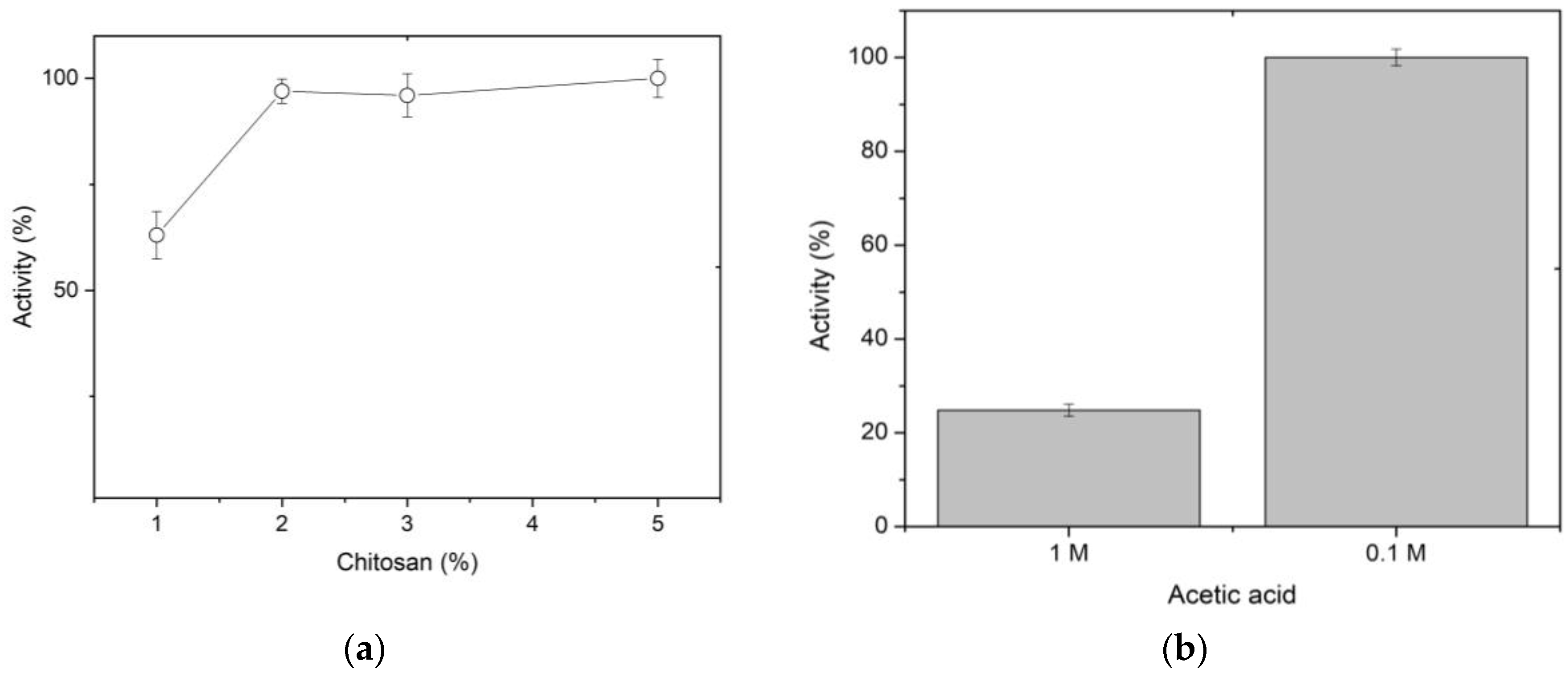
Figure 1a shows that, at a very low concentration, the immobilization efficiency is lower due to enzyme leakage, which could be explained by a larger pore size in the spheres formed at a lower chitosan concentration. As the chitosan concentration increases, there is an improvement in immobilization efficiency which may be due to the greater amount of groups available to form the porous crosslinking. Although high chitosan concentrations favor entrapment, it was observed that there were mechanical difficulties in producing regular spheres from the 3% chitosan solution. Therefore, 2% was selected as the optimum chitosan concentration.
Another important factor to consider during entrapment is the molecular mass of the chitosan used in the fabrication of the spheres. Sun & Zhang (2009) [
36] have suggested that increasing the mass of chitosan, e.g. a polymer with longer molecular chains would promote stronger spheres due to increased interaction between them. Higher sphere strength is not always the ideal situation, as it has been observed that in high molecular mass chitosan spheres, the surface pore size is smaller. The smaller size prevents or restricts the passage of the substrate to the trapped enzyme, thus decreasing the efficiency of the biocatalyst. Based on the aforementioned, the chitosan utilized in this study had a low molecular mass (< 190,000 Da).
During the optimization of the immobilization by entrapment, other factors were also evaluated, such as the type of acid and its concentration for a good chitosan solubility; the precipitating agent, and the maturation time of the spheres. Chitosan is soluble in solutions of different acids, thus in this work, this polysaccharide was dissolved in two acids, a weak one such as organic acetic acid [
13,
25], and a strong one such as inorganic hydrochloric acid, at two different concentrations. As expected, the enzyme entrapped within the spheres formed upon dissolving chitosan in hydrochloric acid exhibited no activity (results not shown). At 1 M acetic acid, the activity of the entrapped enzyme was recorded to be 25% (
Figure 1b). Significantly, when the concentration of acetic acid was decreased to 100 mM, the activity recovered from the spheres was 100%.
The wild-type form of β-xylosidase XynB2 from G. stearothermophilus has been thoroughly characterized [
16,
17,
18,
19,
20]. The β-xylosidase activity of Y509E mutant of XynB2 has been previously characterized biochemically [
22,
24] presenting no major differences compared to the wild type form, in relation to optimal conditions for enzyme activity and stability. From these works, it is known that, at the acid pH necessary to solubilize chitosan, the enzyme irreversibly decreases its activity over time. With this in mind, we can suggest that the absence of activity when chitosan is solubilized in hydrochloric acid is due to the inactivation of the enzyme by acid pH.
During beads preparation, solutions of sodium hydroxide, potassium hydroxide, and CFG buffer were evaluated to select the best precipitant agent. It was found that the enzyme trapped in the spheres precipitated in strong alkalis did not exhibit activity (results not shown). This could be because XynB2
Y509E loses its whole activity at a pH higher than 10, which is related to the ionic forms of the residues involved in the formation of the catalytic pocket [
22,
24]. Significantly, the enzyme entrapped in the spheres, which were precipitated using any of the four tested conditions of CFG buffer, remained active (
Figure 1c). In this regard, 100% of recoverd activity was obtained with the combination of CFG buffer and 20% ethanol.
Finally, the last parameter analyzed was the maturation time of the spheres (
Figure 1d). Results indicate that 1 h of maturation is the optimum time to recover 100% activity. Longer curing times, e.g. 2 or 4 h decrease the activity by 25% or 40%, respectively. In summary, the entrapment immobilization of XynB2
Y509E on chitosan spheres was achieved by mixing the enzyme with 2% chitosan in 0.1 M acetic acid, followed by precipitating the beads using CFG buffer with ethanol and maturing them for 1 h.
3.2. Conditions for the immobilization of XynB2Y509E on chitosan spheres by covalent bond formation
Since some enzymes can be inactivated by glutaraldehyde, a frequently used enzyme-support crosslinking agent, the concentration of this agent as well as the activation time were factors evaluated during the process of covalent immobilization of XynB2
Y509E on chitosan spheres. During the activation reaction of chitosan, glutaraldehyde facilitates the cross-linking of the polymeric chains, thereby improving the mechanical strength of the support and preventing its solubilization in an acidic medium due to its cationic nature [
37]. Activation reaction also generates aldehyde groups on the chitosan surface that can react mainly with amino groups of the enzyme, although they may eventually react with other functional groups such as thiols, imidazoles or phenols [
38]. Even though the mechanism of the reaction is not fully elucidated, it has been proposed that the formation of Schiff bases and nucleophilic substitutions may be involved [
38,
39].
Figure 2a shows that higher concentrations of glutaraldehyde resulted in a decreased activity. It has been reported that in aqueous solution, glutaraldehyde can exist in its simplest form, a monomeric dialdehyde, but also as a dimer, trimer, and polymer [
39]. As a result, the efficacy of glutaraldehyde to activate support could be rationalized with the multiplicity of structures. In this sense, Betancor
et al. (2006) suggest that a polymerizing effect is also generated on the glutaraldehyde in solution, promoted by the increased reactivity of the molecule after the first amino-glutaraldehyde reaction [
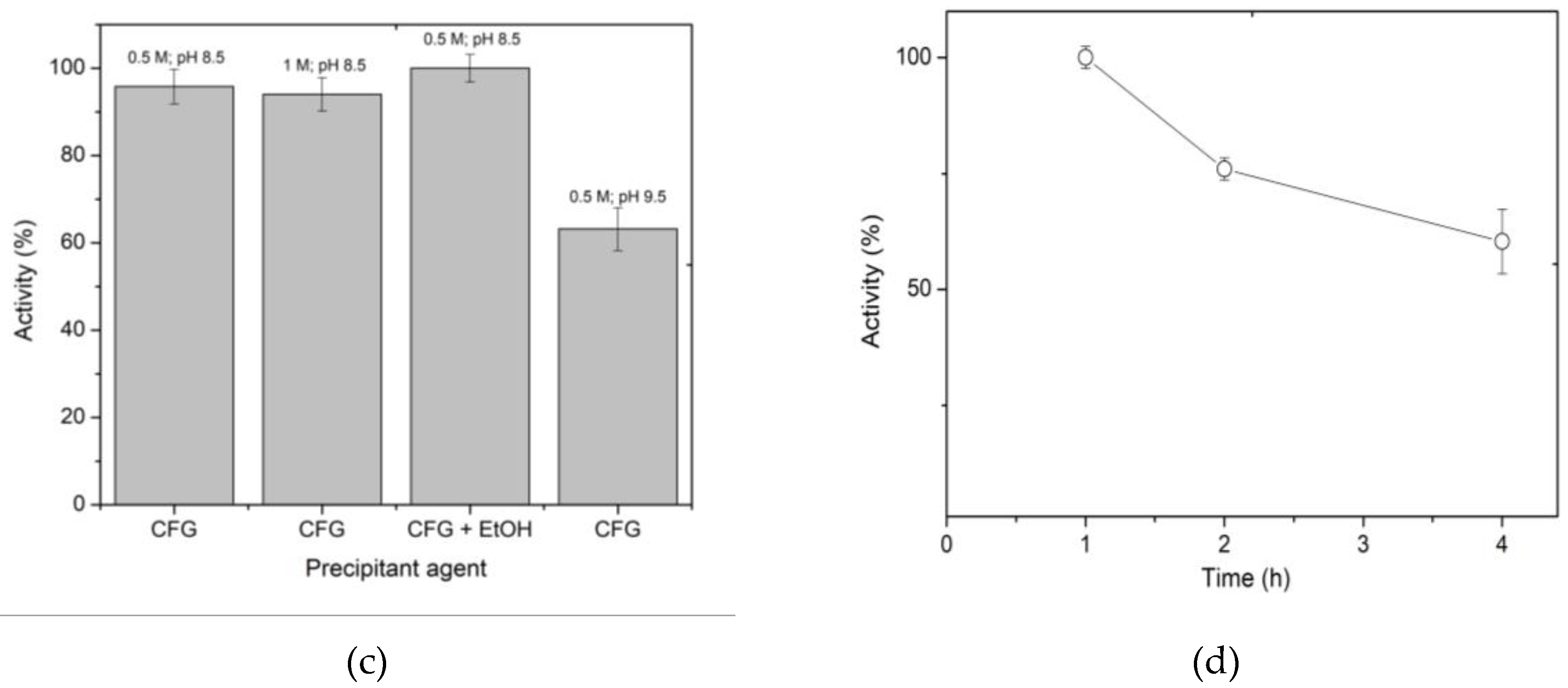
40]. Since 1% glutaraldehyde allows the recovery of 100% of the activity, this concentration was considered optimal. In relation to the activation time of the spheres with 1% glutaraldehyde, it was found that between 4 and 6 h preserve 100% of the activity. The results of
Figure 2a,b allow us to conclude that the conditions of activation of the spheres for an optimum immobilization are 1.0% glutaraldehyde for 4 h.
Another factor evaluated during glutaraldehyde reaction with chitosan was the pH of the solution. The assay was run at two pH values (6.5 and 8), since it is known that at acidic pH values glutaraldehyde is in its monomeric form, which produces shorter bonds, while at more alkaline pH, larger polymers are formed, with the possibility of broader bonds [
39]. Given that the XynB2
Y509E enzyme variant has dual activity of β-xylosidase and xylanase, it was relevant to consider both pH conditions.
Figure 2c shows that the activation of spheres at pH 6.5 and 8 preserves β-xylosidase activity, but there is a significant reduction in xylanase activity when sphere activation and enzyme binding occur at pH 6.5. This finding could be related to the difficulty of the substrate (xylan in the case of xylanase activity) to access the active site, as has been previously reported when XynB2
Y509E was immobilized as cross-linked enzyme aggregates [
24].
After determining the optimal conditions for immobilizing XynB2
Y509E on activated chitosan spheres, the immobilization yield was evaluated according to equation 1.
Figure 2d shows the yields obtained for the four immobilization reaction times that were studied. The results reveal that the immobilization yield increases with longer reaction times, although the maximum yield obtained was 77%. In order to increase the immobilization yield, the amount of protein to be immobilized was reduced by half to 0.389 mg/mL, resulting in a 90% yield. The reason for the decreased yield when a higher enzyme concentration was employed can be attributed to the saturation of the support. The degree of deacetylation which influences the saturation degree, ranges from 60 to 100% [
41] in commercial applications and is ≥ 75% according to the manufacturer [
42]. In this way, the enzyme concentration should be considered a crucial factor when using porous supports to immobilize enzymes [
1,
43].
Another parameter often used to determine the success of enzyme immobilization is the percentage of expression yield which was calculated according to equation 2. The values obtained, namely 30% for immobilization by covalent bonds and 7.9% for immobilization by trapping, can be attributed to alterations in the microenvironment of the catalytic pocket, enzyme distortion, or limitations in diffusion caused by the immobilization process itself. The findings of this research indicate that the expressed activity of immobilized XynB2Y509E is notably enhanced by activating the spheres using glutaraldehyde.
Finally, the recovery of the enzyme activity in the chitosan spheres activated with glutaraldehyde was evaluated by means of equation 3. For the two protein concentrations examined, the activity recovery was 70%. The loss of activity has also been found in other reports of enzyme immobilizations by the covalent bond formation and it would be related to conformational changes in the structure as a result of covalent coupling [
44].
3.3. Effect of temperature and thermostability.
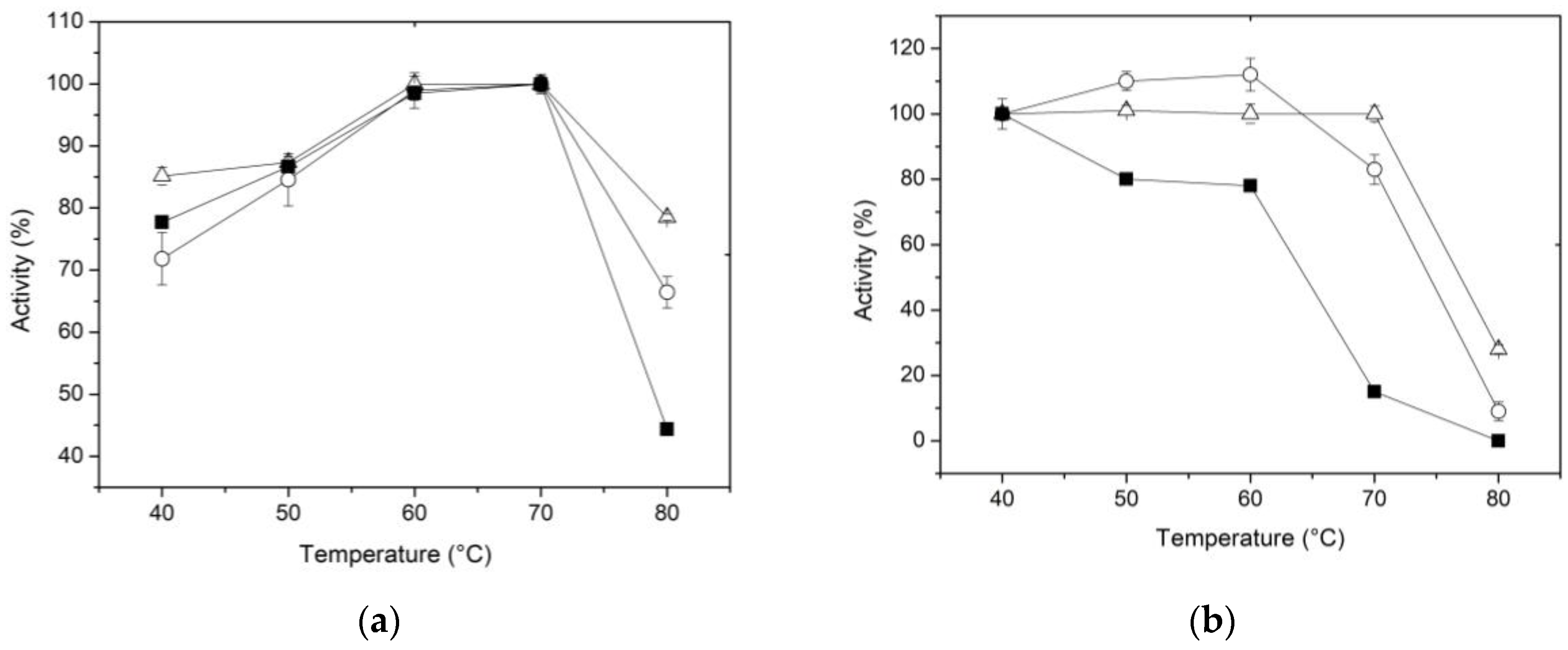
The temperature effect on the β-xylosidase activity of the free and immobilized enzyme using the two proposed methods was determined in 0.1 M CFG buffer within the range of 40 to 80 °C. The results presented in
Figure 3a show an increase in activity as the temperature rises, followed by a subsequent decrease after reaching a peak at maximum activity. Although this trend is consistent in both the free and immobilized forms; it is worth noting that at 80 °C, the enzyme -regardless of the immobilized method- exhibits a higher percentage of activity compared to the free enzyme (
Figure 3a). These results suggest that the conformational flexibility of XynB2
Y509E was influenced by both immobilization methods. According to Munjal and Sawhney (2002), the use of a matrix to entrap enzymes can potentially cause hydrophobic and other secondary interactions, resulting in changes to the enzyme´s conformational flexibility. As a result, higher temperatures may be necessary to attain the correct conformation and maintain its reactivity [
45]. Furthermore, Asgher
et al. (2017a) proposed that the chitosan support can also protect the enclosed enzyme from the surrounding environment, effectively preventing denaturation that could potentially happen in the soluble form of the enzyme [
46].
The increased rigidity of the enzyme resulting from covalent immobilization has also been reported previously. Roy
et al. (1989) observed an improvement in the heat stability of β-glucosidase from
Myceliophthora thermophile when it was immobilized on CNBr-activated sepharose [
47]. Additionally, Abdel-Naby (1993) reported that the broader heat tolerance exhibited by covalently bound β-xylosidase and a xylanase to chitosan could be due to an increase in enzyme rigidity [
48]. Furthermore, Figueira
et al. (2011) reported that the immobilization of a fungal β-glucosidase in Sol-Gel results in an augmented heat tolerance [
49]. More recently, a similar result was observed when the β-glucosidase from
Aspergillus niger was immobilized trough covalent binding [
44]. A possible explanation of the better heat tolerance capacity of the XynB2
Y509E immobilized by covalent method might be attributed to the formation of stronger bonds between the enzyme and the chitosan network.
Thermostability was assessed by incubating the enzyme, whether free or immobilized, at temperatures ranging from 40 to 80 °C for 1 h in the absence of substrate and then the activity of the enzyme was determined at the optimal reaction temperature. The data regarding relative activities are displayed in
Figure 3b. Between 50 and 60 °C, a slight increase in activity was observed in the enzyme immobilized by entrapment compared to the form immobilized on activated chitosan. This could be attributed to a phenomenon where the pores of the chitosan sphere expand with rising temperature, facilitating better access of the substrate to the active site. At higher temperatures, however, the immobilized enzyme by covalent binding shows better stability. For example, at 70 °C, the free enzyme was quickly denatured, whereas the immobilized XynB2
Y509E, whether by entrapment or covalent binding, retained approximately 80% and 100% of their activity, respectively. Furthermore, the relative activity of XynB2
Y509E immobilized on activated chitosan remains above 30% even after 1 h at 80 °C. Taken together, the results suggest that immobilization by entrapment or covalent binding leads to an increase in the thermal stability of XynB2
Y509E, which makes these forms of the enzyme industrially more valuable than the free enzyme. To sum up, our findings are consistent with the notion that chitosan microspheres may offer protection to the enzyme against environmental factors that would otherwise impair its performance [
46,
50].
3.4. Effect of pH on activity and stability
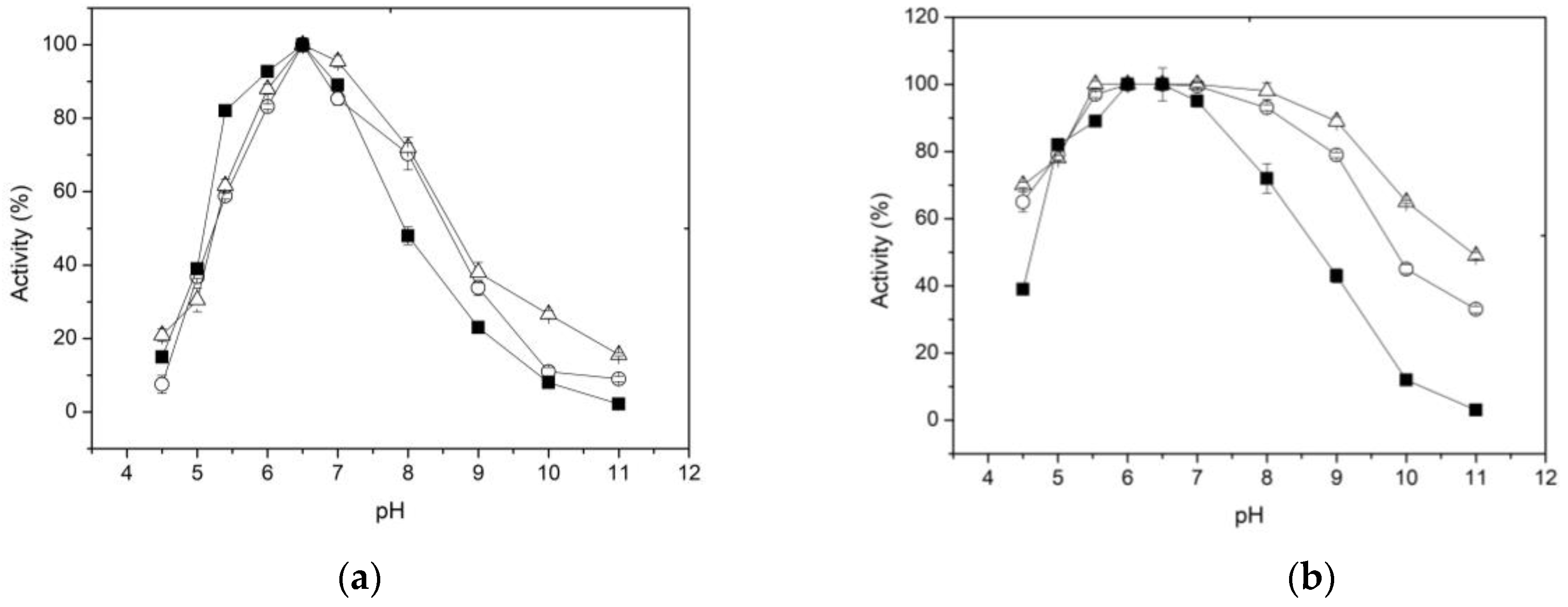
It is well known that variations in pH values can affect the formation and/or dissociation of the enzyme-substrate complex, hindering the formation of the catalytic pocket or active center of the enzyme and its stability. The effect of pH on enzyme activity, free and immobilized, is shown in
Figure 4a. It is observed that the relative activity of the free and immobilized enzyme increases gradually in the pH range of 5.5 to 6.5, while the pH optima were almost the same (pH 6.5). At pH 7, the decrease in activity is more drastic in the free enzyme than in the two forms of immobilized enzyme. Furthermore, at pH 8, while the free enzyme retains 45% of activity, the immobilized forms retained 75% of their initial activity. Additionally, the pH-activity profiles indicated that XynB2
Y509E, when entrapped within chitosan or covalent bounded to chitosan, were less susceptible to pH changes compared to the free enzyme. This effect can be attributed to the buffering properties of chitosan, which bears a reported p
Ka value of 6.2–6.6 and acts as a buffer within the pH range of 5.5 to 7.4 [
51,
52].
Regarding the effect of pH on the stability of the free and immobilized enzyme, it was found that immobilization results in greater chemical stability of the enzyme at pH values from 7 to 11 (
Figure 4b). For example, after incubation at pH 9 for 1 h, the free enzyme retains only 40% of its activity. In contrast, the enzyme immobilized forms by entrapment or covalent bonding retain 80 and 90% of their original activity, respectively. The improved alkaline tolerance capacity of XynB2
Y509E immobilized either by entrapment or covalent binding, could be potentially attributed to the electrostatic interactions formed between the enzyme molecule and the chitosan matrix or network. These interactions could facilitate the maintenance of the biologically active conformation of the enzyme, thereby resulting in improved resistance to environmental changes [
53]. The broad pH tolerance exhibited by XynB2
Y509E after chitosan immobilization makes it highly valuable for industrial applications.
3.5. Effect of immobilization on kinetic parameters
The kinetic parameters of the reaction of the free enzyme and both immobilized forms are compiled in
Table 1. The K
m value for free XynB2
Y509E was found to be 0.9 mM, which aligns with findings from previous studies [
22,
24]. The XynB2
Y509E immobilized through entrapment on chitosan beads displayed an apparent K
m value of 0.7 mM, whereas the immobilized enzyme via the covalent method exhibited the same K
m value as the free enzyme (K
m=0.9 mM). Since the K
m values were similar in magnitude, we can infer that the catalytic function of XynB2
Y509E was not significantly compromised by either of the two immobilization methods. On the other hand, enzyme immobilization caused a moderate decrease in V
max, which might be caused by diffusion limitation of substrate and product when XynB2
Y509E was entrapped within the chitosan sphere or to interaction of the enzyme with the functional groups on the surface of the chitosan beads in the covalent immobilization [
53]. Other researchers have reported similar observations of a lower V
max following the immobilization of fungal xylanolytic enzymes either by covalent binding on chitosan spheres or by adsorption on aluminum hydroxide particles [
54,
55]. More recently, it has been reported that covalent bonding immobilization of acetylcholinesterase on chitosan spheres also resulted in a decrease in V
max. This decrease was attributed to the restricted mobility of the enzyme, which could potentially induce alterations in its three-dimensional structure [
56].
Catalytic turnover and catalytic efficiency (
Table 1), calculated from
Km and
Vmax parameters reflect the changes after immobilization. In general, the enzyme´s
kcat and catalytic efficiency (
kcat/
Km) decreased after immobilization. A significant decrease in turnover number (
kcat) was observed for the two immobilized enzyme forms. Furthermore, the catalytic efficiency,
kcat/
Km, of the two immobilized enzyme forms was significantly lower than the value achieved by the free enzyme. The decrease in
kcat/
Km was 420-fold for the enzyme immobilized by entrapment and 42-fold for the enzyme immobilized by covalent bonding. In other words, immobilization by covalent bonding showed a 10-fold improvement compared to immobilization by entrapment. Taken together, these results suggest a lower catalytic activity of the XynB2
Y509 when it was immobilized. Several factor may contribute to the observed reduction in enzymatic activity, specifically, the diffusion of the substrate to the active site of the enzyme, the restricted mobility of the immobilized enzyme and the partial denaturation of the enzyme during immobilization [
53,
54,
55,
56,
57]. Although the catalytic efficiency of XynB2
Y509E decline with immobilization, the two immobilization methods have significantly improved the thermal and chemical stability of the enzyme.
3.6. Operational Stability of XynB2Y509E Immobilized on Chitosan Spheres
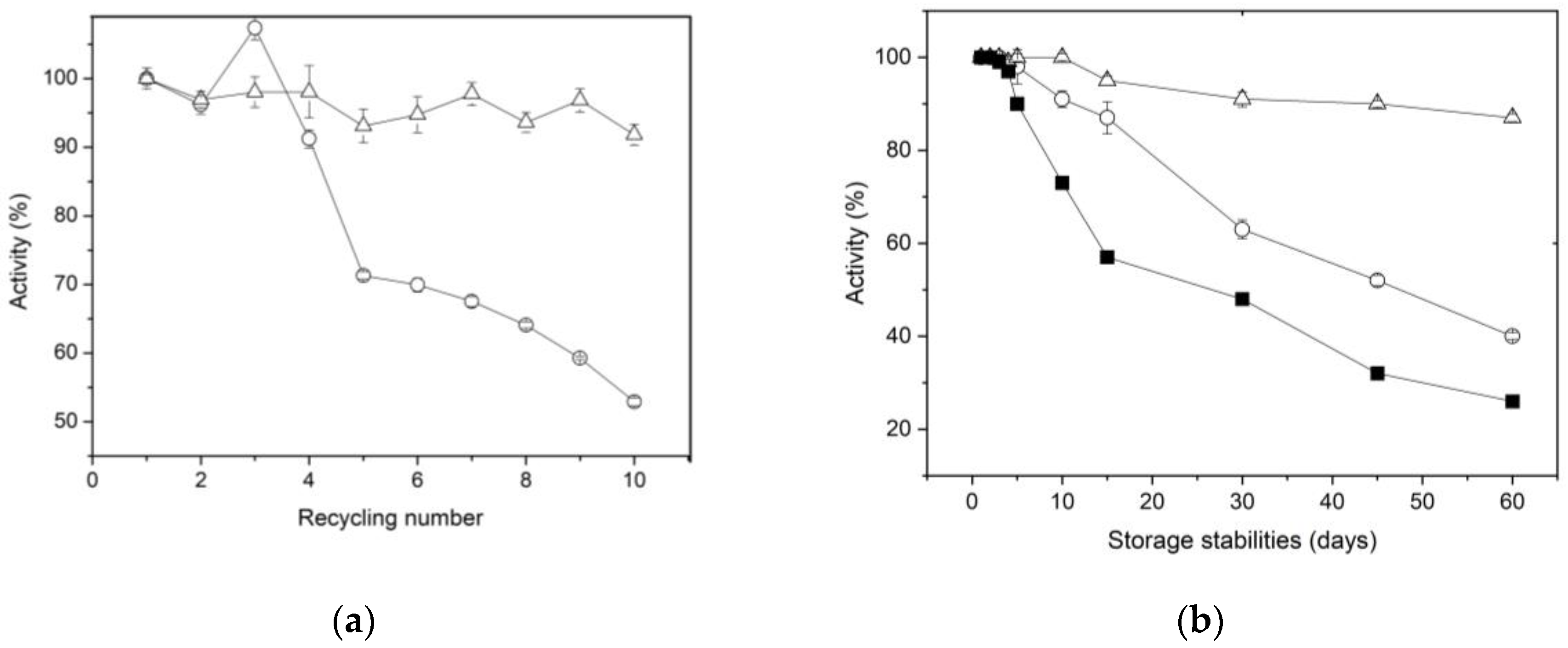
A key factor that reduces the overall cost of enzymes during industrial applications is the ability of immobilized enzymes to be regenerated and used continuously across multiple cycles [
55]. The reusability of XynB2
Y509E immobilized by entrapment and covalent bonding on chitosan spheres was assesed by conducting a series of 10 cycles pNPX substrate hydrolysis under the standard conditions described in the methodology section (
Figure 5a). It was observed that the immobilized XynB2
Y509E by entrapment preserves up to 65% of its activity after 5 cycles of reuse and more than 50% by the end of 10 reuse cycles. The observed decrease in activity for XynB2
Y509E entrapped in chitosan may be attributed to the enzyme leaking from the beads, as reported for other immobilized enzyme using entrapment methods [
46]. This phenomenon was also supported by the determination of β-xylosidase activity in the washing water. The XynB2
Y509E immobilized through covalent linkages to chitosan retained 90% of its original activity after 10 cycles of reuse, indicating high and stable pNPX degradation performance in the repeated batches. This lower loss in activity can be attributed to the robust covalent attachment of XynB2
Y509E to chitosan spheres, which reduces the leaching of enzyme during catalysis. A similar result was observed by Pal & Khanum (2011) for immobilized xylanase on glutaraldehyde activated alginate beads [
58]. Although our results suggest that the immobilization of XynB2
Y509E on chitosan beads, whether through entrapment or covalent binding, exhibited favorable reusability, the biocatalyst immobilized on glutaraldehyde activated chitosan beads emerged as a superior choice due to its higher reusability efficiency.
The long-term storage of industrial enzymes without any decline in biological activity has garnered significant attention in recent years. To study the impact of immobilization on the storage stability of XynB2
Y509E, the free and immobilized forms of the enzyme mutant were stored at 4 °C for a duration of 60 days. The residual activity of the enzymes was assessed daily for the initial 5 days and subsequently at 15-day intervals (
Figure 5b). The results show that the storage stability of the two forms of immobilized enzyme is substantially higher compared to the free enzyme. During the first 5 days, residual activity for all three forms was 100%. From the 5th day on, a noticeable decline in activity occurs in the free enzyme, reaching 50% after 15 days of storage at 4 °C. In contrast to the free enzyme, XynB2
Y509E immobilized by entrapment retained 85% of its initial activity, while the enzyme immobilized by covalent bonding retained 94% of its original activity. After a storage period of two months, it was observed that while the enzyme immobilized by entrapment gradually loses its catalytic stability, the enzyme covalently linked to glutaraldehyde-activated chitosan maintains its residual activity above 90%. This remarkable difference in storage stability suggests that best immobilization for XynB2
Y509E is covalent binding. This finding is a desirable feature for the possible exploitation of this enzyme in industrial sectors that demand high storage stability.
4. Conclusions
When separated from their natural biocatalytic environment, enzymes tend to lose their activity or stability. This situation further underscores the importance of exploring new immobilization methods. In the present work, successful immobilization of a recombinant mutant of the family 52 glycoside hydrolase onto chitosan spheres was achieved using two different methods. The entrapment method resulted in an immobilization yield of 68%, whereas the covalent binding method achieved a yield of 90%. XynB2Y509E immobilization on chitosan improved stability properties to various parameters, such as pH, temperature, storage and reuse, making it industrially useful. Altogether, our results suggest that the immobilization of xylanolytic enzymes on chitosan spheres is a valid and efficient approach. Significantly, this type of immobilization fulfills the requirement of being an environmentally friendly technique and, moreover, it enhances the long-term stability of both forms of the biocatalyst produced. Furthermore, the findings presented in this work contribute to the growing list of successful immobilizations using activated chitosan to immobilize enzymes. Finally, our findings also offer valuable insights into the optimization of supports and/or enzymes to enhance biocatalyst performance.
Author Contributions
Conceptualization, G.R., C.A., and L.M.C.; methodology, G.R., C.A., L.M.C and J.M.C.-J.; software, G.R.; validation, G.R. and C.A.; formal analysis, G.R., L.M.C., J.W., and F.J.L.H.-V.; investigation, G.R., C.A., and L.M.C.; resources, C.A., J.W., F.J.L.H.-V., and F.R.-V.; data curation, G.R., L.M.C., and J.M.C.-J.; writing—original draft preparation, G.R. and L.M.C.; writing—review and editing, L.M.C., J.W., J.M.C.-J., F.R.-V., and F.J.L.H.-V.; visualization, G.R., C.A., and L.M.C.; supervision, J.W., F.R.-V., and F.J.L.H.-V.; project administration, J.M.C.-J., F.R.-V., and F.J.L.H.-V.; funding acquisition, L.M.C., C.A., J.M.C.-J., F.R.-V., and F.J.L.H.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by research grants from the European Regional Development Fund Andalucía 2014-2020 (grant UAL18-CTS-B032-A, FRV), the Own Research and Transfer Plan 2020 of the University of Almeria (grant PPUENTE2020/006, FJLHV), the Council for Scientific and Humanistic Development of the University of Carabobo (grant 345-2012, LMC) and University of Carabobo (grant CD-004-534-2016, GR.). GR thanks support from Universidad Católica de la Santísima Concepción for her research stay.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters necessary to define an immobilized enzyme preparation. Process Biochem. 2020, 90, 66–80. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Sheldon, R.A. Engineering a more sustainable world through catalysis and green chemistry. J. R. Soc. Interface. 2016, 13, 20160087. [Google Scholar] [CrossRef]
- Meldal, M.; Schoffelen, S. Recent advances in covalent, site-specific protein immobilization. F1000Research. 2016, 5. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Carballares, D.; Morellon-Sterlling, R.; Berenguer-Murcia, Á.; Alcántara, A.R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Enzyme co-immobilization: Always the biocatalyst designers’ choice…or not? Biotechnol. Adv. 2020, 107584. [Google Scholar] [CrossRef]
- Guisan, J.M. New opportunities for immobilization of enzymes. Methods Mol. Biol. 2013, 1051, 1–13. [Google Scholar] [CrossRef]
- Sheldon, R.; van Pelt, S. Enzyme immobilization in biocatalysis: why, what and how. Chem. Soc. Rev. 2013, 42, 6223. [Google Scholar] [CrossRef]
- Rehn, F.B.; Chen, S.; Rehn, B.H. Enzyme engineering for in situ immobilization. Molecules 2016, 21, 1370. [Google Scholar] [CrossRef]
- Brena, B.; González-Pombo, P.; Batista-Viera, F. Immobilization of enzymes: a literature survey. Methods Mol. Biol. 2013, 1051, 15–31. [Google Scholar] [CrossRef]
- Ali, S.; Zafar, W.; Shafiq, S.; Manzoor, M. Enzymes Immobilization: An overview of techniques, support materials and its applications. Int. J. Sci. Res. 2017, 6, 4–72. [Google Scholar]
- Graebin, N.; Schöffer, J.; de Andrades, D.; Hertz, P.; Ayub, M.; Rodrigues, R. Immobilization of glycoside hydrolase familie GH1, GH13, and GH70: state of the art and perspectives. Molecules. 2016, 21, 1074. [Google Scholar] [CrossRef] [PubMed]
- Nunes, Y.L.; de Menezes, F.L.; de Sousa, I.G.; Cavalcante, A.L.G.; Cavalcante, F.T.T.; da Silva Moreira, K.; … & dos Santos, J.C.S.; … & dos Santos, J. C.S. Chemical and physical Chitosan modification for designing enzymatic industrial biocatalysts: How to choose the best strategy? Int. J. Biol. Macromol. 2021, 181, 1124–1170. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial – a review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Corradini, F.A.; Milessi, T.S.; Gonçalves, V.M.; Ruller, R.; Sargo, C.R.; Lopes, L.A.; Zangirolami, T.C.; Tardioli, P.W.; Giordano, R.C.; Giordano, R.L. High stabilization and hyperactivation of a Recombinant β-xylosidase through Immobilization Strategies. Enzyme Microb. Technol. 2021, 145, 109725. [Google Scholar] [CrossRef]
- Canio, D.; Bari, D.; Patrizi, R. Latest Frontiers in the Biotechnologies for Ethanol Production from Lignocellulosic Biomass [Internet]. Biofuel Production-Recent Developments and Prospects. InTech. 2011. [Google Scholar] [CrossRef]
- Bravman, T.; Zolotnitsky, G.; Shulami, S.; Belakhov, V.; Solomon, D.; Baasov, T.; Shoham, G.; Shoham, Y. Stereochemistry of family 52 glycosyl hydrolases: a beta-xylosidase from Bacillus stearothermophilus T-6 is a retaining enzyme. FEBS Lett. 2001, 495, 39–43. [Google Scholar] [CrossRef]
- Contreras, L.M.; Gómez, J.; Prieto, J.; Clemente-Jiménez, J.M.; Las Heras-Vázquez, F.J.; Rodríguez-Vico, F.; Blanco, F.J.; Neira, J.L. The family 52 β-xylosidase from Geobacillus stearothermophilus is a dimer: Structural and biophysical characterization of a glycoside hydrolase. Biochim. Biophys. Acta - Proteins Proteom. 2008, 1784, 1924–1934. [Google Scholar] [CrossRef]
- Bravman, T.; Belakhov, V.; Solomon, D.; Shoham, G.; Henrissat, B.; Baasov, T.; Shoham, Y. Identification of the catalytic residues in family 52 glycoside hydrolase, a beta-xylosidase from Geobacillus stearothermophilus T-6. J. Biol. Chem. 2003, 278, 26742–26749. [Google Scholar] [CrossRef]
- Bravman, T.; Zolotnitsky, G.; Belakhov, V.; Shoham, G.; Henrissat, B.; Baasov, T.; Shoham, Y. Detailed kinetic analysis of a family 52 glycoside hydrolase: a β-xylosidase from Geobacillus stearothermophilus. Biochemistry 2003, 42, 10528–10536. [Google Scholar] [CrossRef]
- Kurz, L.; García, V.; Wilkesman, J.; Contreras, L.M. Enzymatic characterization of the recombinant beta-xylosidase XynB2. JMBSR. 2014, 1, 14–19. [Google Scholar]
- Ben-David, A.; Bravman, T.; Balazs, Y.S.; Czjzek, M.; Schomburg, D.; Shoham, G.; Shoham, Y. Glycosynthase activity of Geobacillus stearothermophilus GH52 β-xylosidase: efficient synthesis of xylooligosaccharides from a D-xylopyranosyl fluoride through a conjugated reaction. Chembiochem. 2007, 8, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, X.; Zhang, S.; Liu, Z. GH52 xylosidase from Geobacillus stearothermophilus: characterization and introduction of xylanase activity by site-directed mutagenesis of Tyr509. J. Ind. Microbiol. Biotechnol. 2014, 41, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Kyung, M.; Jo, I.; Kim, Y.R.; Ha, N.C. Structure-based protein engineering of bacterial β-xylosidase to increase the production yield of xylobiose from xylose. Biochem. Biophys. Res. Commun. 2018, 501, 703–710. [Google Scholar] [CrossRef]
- Romero, G.; Contreras, L.M.; Aguirre, C.; Wilkesman, J.; Clemente-Jiménez, J.M.; Rodríguez-Vico, F.; Las Heras-Vázquez, F.J. Characterization of Cross-Linked Enzyme Aggregates of the Y509E Mutant of a Glycoside Hydrolase Family 52 β-xylosidase from G. stearothermophilus. Molecules 2021, 26, 451. [Google Scholar] [CrossRef] [PubMed]
- Kamburov, M.; Lalov, I. Preparation of chitosan beads for trypsin immobilization. Biotechnol. Biotechnol. Equip. 2012, 26 (Suppl. 1), 156–163. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Verma, M.L.; Kumar, S.; Das, A.; Randhawa, J.; Chamundeeswar, M. Chitin and chitosan-based support materials for enzyme immobilization and biotechnological applications. Environ. Chem. Lett. 2020, 18, 315–323. [Google Scholar] [CrossRef]
- Moehlenbrock, M.J.; Minteer, S.D. Introduction to the field of enzyme immobilization and stabilization. Methods Mol. Biol. 2010, 679, 1–7. [Google Scholar] [CrossRef]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic modifications of chitin, chitosan, and chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, W.; Deng, W.; Xu, C.; Cai, Y.; Wang, X. Antibacterial and hemostatic thiol-modified chitosan-immobilized AgNPs composite sponges. ACS Appl. Mater Interfaces. 2020, 12, 20307–20320. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.A.; Arumainathan, S. Crosslinked Chitosan-Gelatin Biocompatible Nanocomposite as a Neuro Drug Carrier. ACS Omega. 2022, 7, 18732–18744. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Kumari, A.; Chauhan, G.S.; Chauhan, K. Modified chitosan microspheres in non-aggregated amylase immobilization. Int. J. Biol. Macromol. 2014, 66, 46–51. [Google Scholar] [CrossRef]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme entrapment, biocatalyst immobilization without covalent attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Sun, S.F.; Zhang, Y. A novel process to prepare chitosan macrospheres without shrinkage and its application to immobilize β-galactosidase. E-J CHEM. 2009, 6, 1211–1220. [Google Scholar] [CrossRef]
- Bilal, M.; Jing, Z.; Zhao, Y.; Iqbal, H.M.N. Immobilization of fungal laccase on glutaraldehyde cross-linked chitosan beads and its bio-catalytic potential to degrade bisphenol A. Biocatal. Agric. Biotechnol. 2019, 19, 101174. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: a useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Betancor, L.; López-Gallego, F.; Hidalgo, A.; Alonso-Morales, N.; Mateo, G.D.O.C.; Fernández-Lafuente, R.; Guisán, J.M. Different mechanisms of protein immobilization on glutaraldehyde activated supports: effect of support activation and immobilization conditions. Enzyme Microb. Technol. 2006, 39, 877–882. [Google Scholar] [CrossRef]
- Chitosan [Internet]. Matexcel, NY 11967. 2023. Available online: https://www.matexcel.com/category/products/natural-materials/naturally-extracted-materials/chitosan/#:~:text=Chitosan%20is%20produced%20commercially%20from,can%20be%20converted%20to%20chitosan (accessed on 19 June 2023).
- Chitosan-low molecular weight. Product specification. Sigma-Aldrich. Saint Louis, USA. 2023. Available online: https://www.sigmaaldrich.com/specification-sheets/462/398/448869-BULK_______ALDRICH__.pdf (accessed on 19 June 2023).
- Zaak, H.; Siar, E.H.; Kornecki, J.F.; Fernandez-Lopez, L.; Pedrero, S.G.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Effect of immobilization rate and enzyme crowding on enzyme stability under different conditions. The case of lipase from Thermomyces lanuginosus immobilized on octyl agarose beads. Process Biochem. 2017, 56, 117–123. [Google Scholar] [CrossRef]
- Karami, F.; Ghorbani, M.; Mahoonak, A.S.; Khodarahmi, R. Fast, inexpensive purification of β-glucosidase from Aspergillus niger and improved catalytic/physicochemical properties upon the enzyme immobilization: Possible broad prospects for industrial applications. Food. Sci. Technol. 2020, 118, 108770. [Google Scholar] [CrossRef]
- Munjal, N.; Sawhney, S.K. Stability and properties of mushroom tyrosinase entrapped in alginate, polyacrylamide and gelatin gels. Enzyme Microb. Technol. 2002, 30, 613–619. [Google Scholar] [CrossRef]
- Asgher, M.; Noreen, S.; Bilal, M. Enhancement of catalytic, reusability, and long-term stability features of Trametes versicolor IBL-04 laccase immobilized on different polymers. Int. J. Biol. Macromol. 2017, 95, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Raha, S.K.; Dey, S.K.; Chakrabarty, S.L. Immobilization of β-glucosidase from Myceliophthora thermophila D-14. Enzyme Microb. Technol. 1989, 11, 431–435. [Google Scholar] [CrossRef]
- Abdel-Naby, M.A. Immobilization of Aspergillus niger NRC 107 xylanase and b-xylosidase, and properties of the immobilized enzymes. Appl. Biochem. Biotechnol. 1993, 38, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Figueira, J.D.A.; Dias, F.F.G.; Sato, H.H.; Fernandes, P. Screening of Supports for the Immobilization of-Glucosidase. Enzyme Res. 2011, 642460. [Google Scholar] [CrossRef]
- Asgher, M.; Noreen, S.; Bilal, M. Enhancing catalytic functionality of Trametes versicolor IBL-04 laccase by immobilization on chitosan microspheres. Chem. Eng. Res. Des. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Aslam, S.; Asgher, M.; Khan, N.A.; Bilal, M. Immobilization of Pleurotus nebrodensis WC 850 laccase on glutaraldehyde cross-linked chitosan beads for enhanced biocatalytic degradation of textile dyes. J. Water. Proc. Eng. 2021, 40, 101971. [Google Scholar] [CrossRef]
- Blagodatskikh, I.V.; Kulikov, S.N.; Vyshivannaya, O.V.; Bezrodnykh, E.A.; Tikhonov, V.E. N-reacetylated oligochitosan: pH dependence of self-assembly properties and antibacterial activity. Biomacromolecules 2017, 18, 1491–1498. [Google Scholar] [CrossRef]
- Jaiswal, N.; Pandey, V.P.; Dwivedi, U.N. Immobilization of papaya laccase in chitosan led to improved multipronged stability and dye discoloration. Int. J. Biol. Macromol. 2016, 86, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, L.; Lv, S.; Liu, X.; Wang, M.; Song, A.; Jia, X. Immobilization of Aspergillus niger xylanase on chitosan using dialdehyde starch as a coupling agent. Appl. Biochem. Biotechnol. 2010, 162, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, Y.; Li, H. Immobilization of Thermomyces lanuginosus Xylanase on Aluminum Hydroxide Particles Through Adsorption: Characterization of Immobilized Enzyme. J. Microbiol. Biotechnol. 2015, 25, 2016–2023. [Google Scholar] [CrossRef] [PubMed]
- Işık, M. High Stability of Immobilized Acetylcholinesterase on Chitosan Beads. Chemistry Select. 2020, 5, 4623–4627. [Google Scholar] [CrossRef]
- Monier, M.; Ayad, D.M.; Wei, Y.; Sarhan, A.A. Immobilization of horseradish peroxidase on modified chitosan beads. Int. J. Biol. Macromol. 2010, 46, 324–330. [Google Scholar] [CrossRef]
- Pal, A.; Khanum, F. Covalent immobilization of xylanase on glutaraldehyde activated alginate beads using response surface methodology: characterization of the immobilized enzyme. Process Biochem. 2011, 46, 1315–1322. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).