1. Introduction
Triplin is a channel-forming structure found in
E. coli. It forms a set of three pores whose effective size and conductance resemble OmpC, whereas its weak ion selectivity resembles OmpF [
1]. Triplin differs dramatically for all porins described to date e.g. [
2,
3,
4,
5] in that it displays steep voltage dependence [
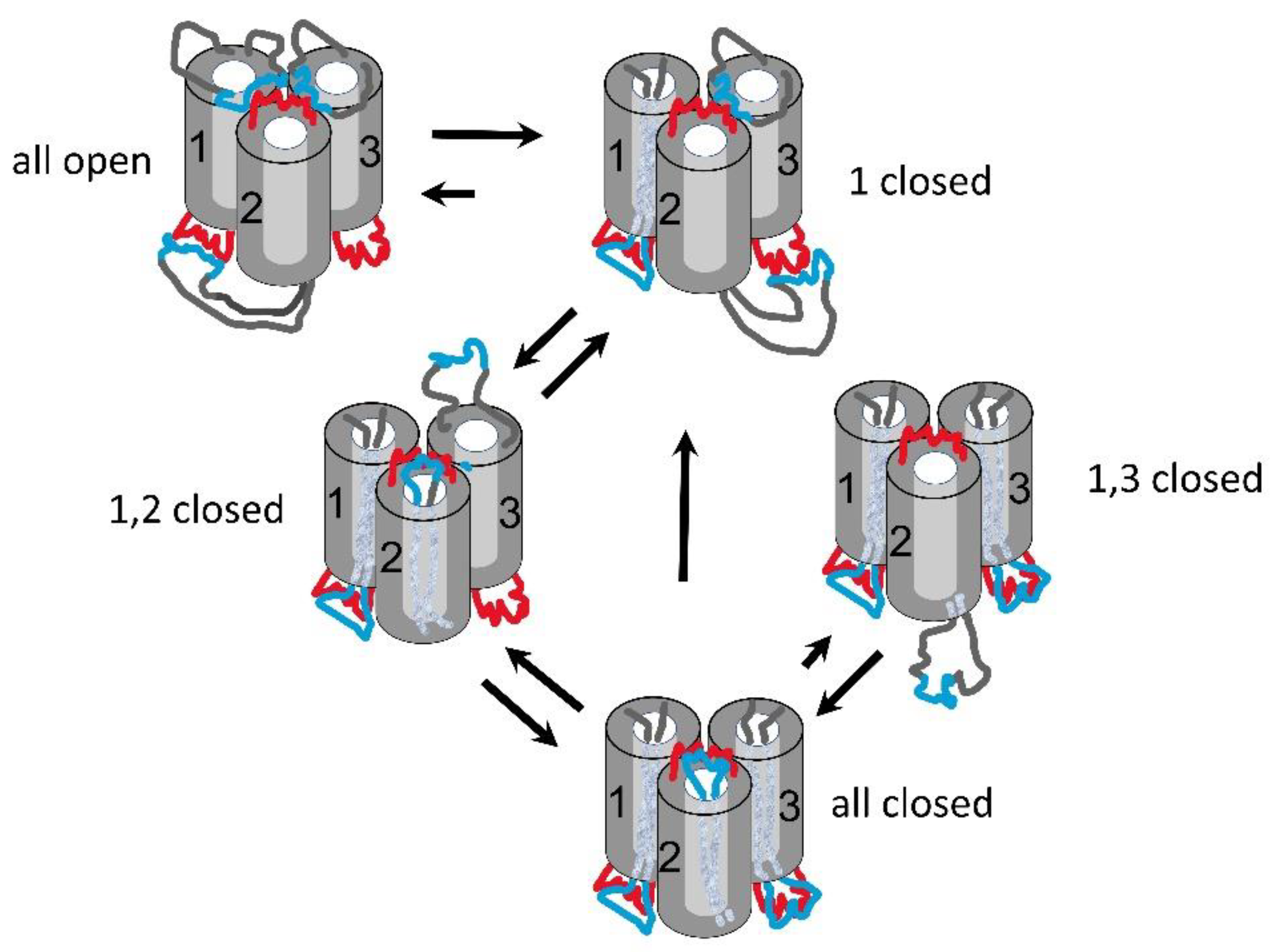
6] comparable to that of the voltage-gated channels responsible for the electrical excitability of the mammalian nervous system. A unique property of Triplin that distinguishes it from all membrane channels and pores described to date is the remarkable interpore cooperative behavior in that pore 1 must close first, its closure then allows pore 2 to close and, in turn, pore 2 closure allows pore 3 to close [
6]. This is illustrated in
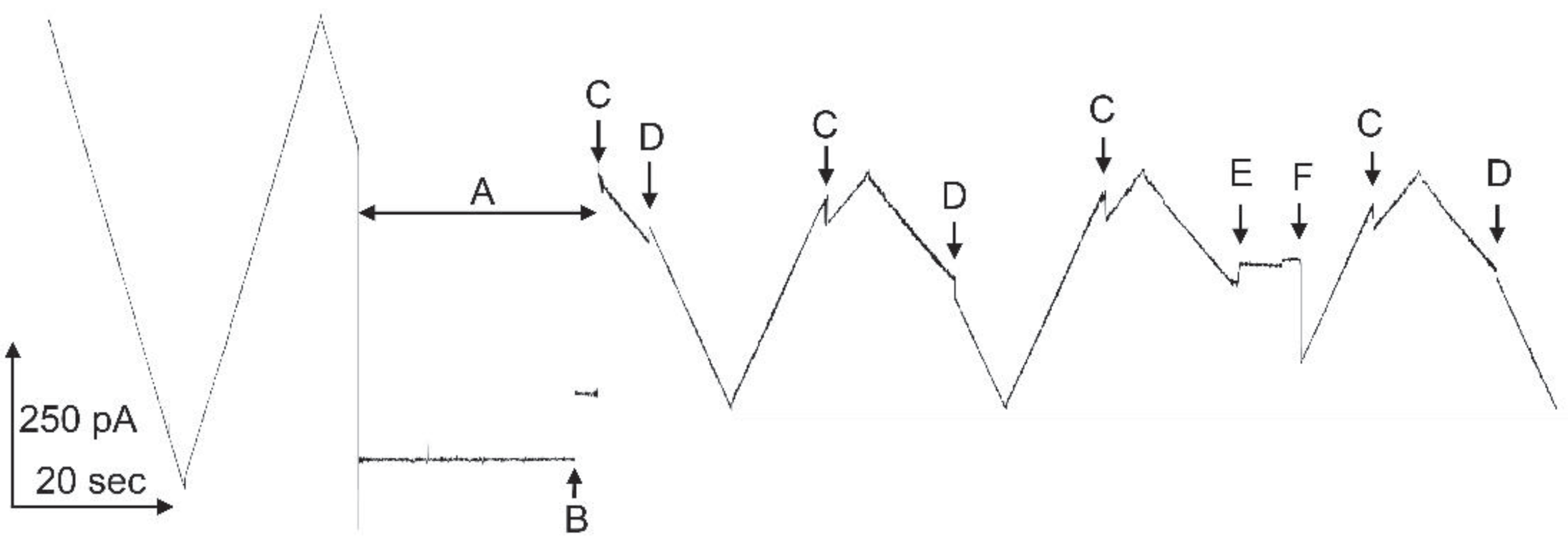
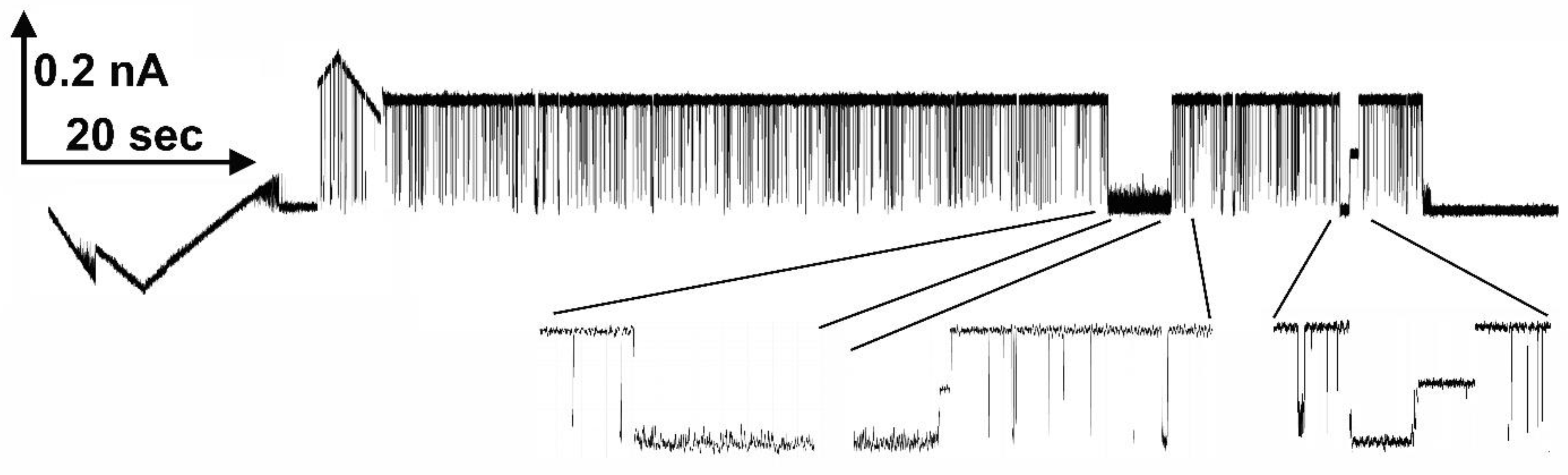
Figure 1. At left a single Triplin shows no voltage-dependent gating. The application of a high positive potential during time interval “A”, closes pore 1 (at point “B”) and it remains closed. That closure allows pore 2 to close at negative voltages (at points “C”). The reopening of pore 2 (at points “D”) prevents the closing of pore 3 (at points “E”). A delayed reopening of pore 2 allows pore 3 to close at positive voltages.
Insights into the molecular basis for this complex were obtained by probing the nature and dynamics of the voltage sensors of Triplin [
1]. This led the authors to propose the following model.
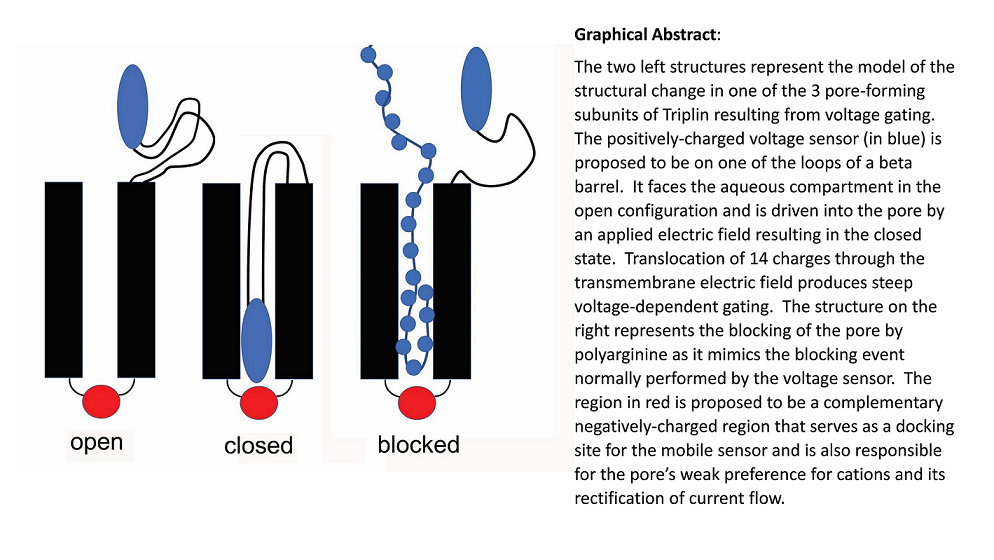
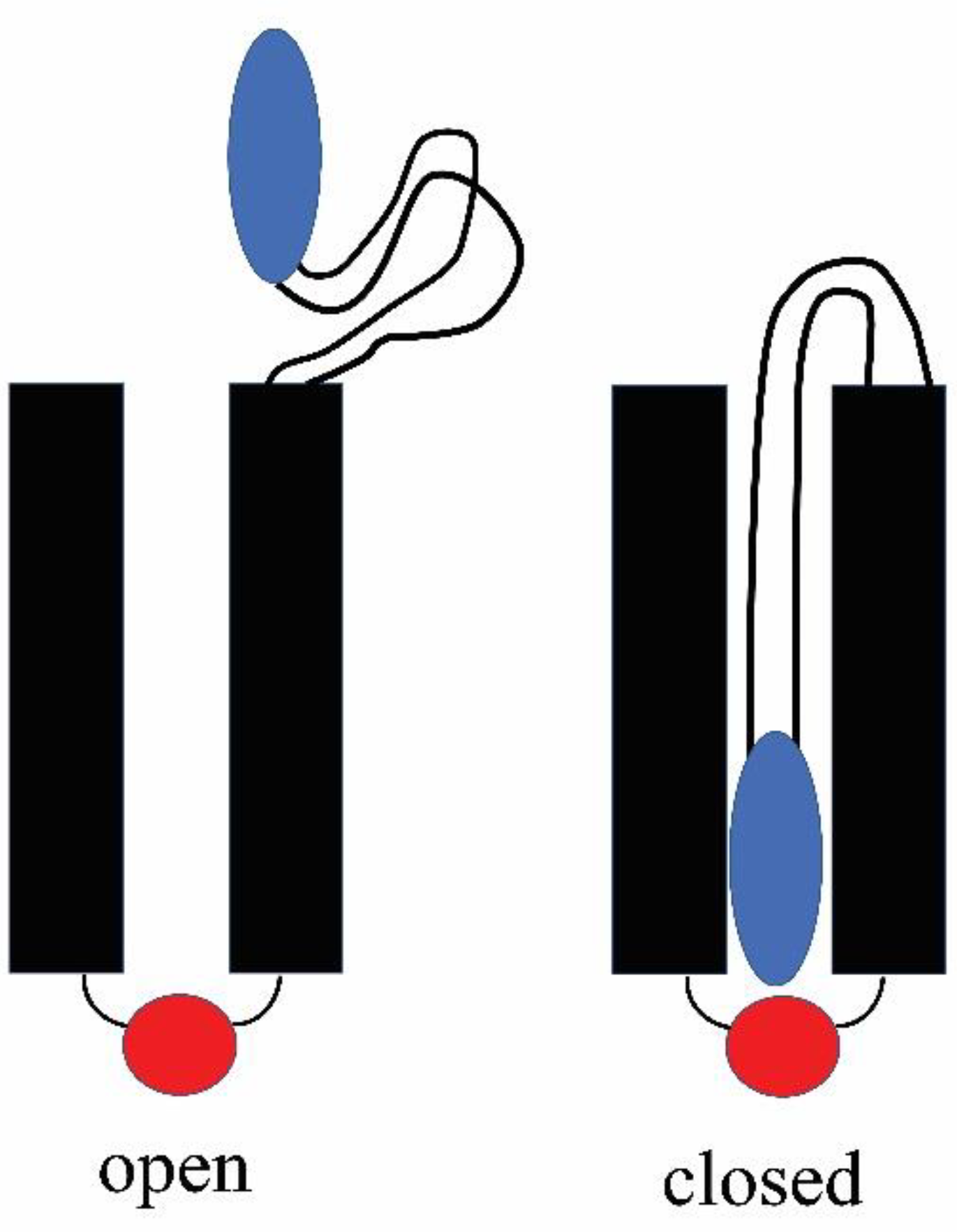
Figure 2.
Model of the gating of Triplin. The top of the structure is the
cis side of the membrane, the side from which Triplin inserted. The bottom of the structure is the
trans side and that is the side maintained at virtual ground by the amplifier. All indicated voltages refer to the
cis side. For simplicity, the closed state of the pore is illustrated as the result of blockage by a single loop of the beta barrel but, of course, multiple loops may be involved. Blue regions are positively charged whereas red are negatively charged. From Figure 14 ref [
1].
Figure 2.
Model of the gating of Triplin. The top of the structure is the
cis side of the membrane, the side from which Triplin inserted. The bottom of the structure is the
trans side and that is the side maintained at virtual ground by the amplifier. All indicated voltages refer to the
cis side. For simplicity, the closed state of the pore is illustrated as the result of blockage by a single loop of the beta barrel but, of course, multiple loops may be involved. Blue regions are positively charged whereas red are negatively charged. From Figure 14 ref [
1].
Triplin is proposed to form three beta barrel pores, similar to those of other 3-pore porins but one pore-forming structure is oriented opposite to the others [
1]. In the model, each pore-forming subunit has a positively-charged voltage sensor (blue region) located in external loops on one end of the pore. Pore closure is proposed to result from the entry of the sensor into the pore, thus blocking ion flow. When the sensor is in the pore it is proposed to interact with a negative domain located at the other end of the pore, that negative domain also being responsible for the pore’s weak cation selectivity and rectification [
1]. No evidence was presented to support this closing mechanism and this paper addresses that issue.
2. Results:
2.1. Kinetic measurements
2.1.1. Kinetic measurements can distinguish between viable gating mechanisms
The molecular mechanism for the voltage gating of the pore structures formed by Triplin assumes that these are formed by beta barrels. The similarity of the conductance and selectivity of the Triplin pores to those of porins like OmpF and OmpC whose structure is well established, makes the assumption of a beta barrel very likely to be correct. The steep voltage-dependence of the Triplin pores, requiring the translocation of 14 charges across the membrane [
6], only leaves 2 mechanisms: translocation of charges through the lipid bilayer or translocation through the hole formed by the pore. Other models for the gating of porins such as the movement of a loop located withing the pore itself, are not tenable. Whereas the movement of such a loop could easily obstruct the flow of ions, it would not be able to translocate sufficient charge to account for the steep voltage dependence. As to the 2 viable models, they make different predictions on the kinetics of the gating process. Translocation of charges through the lipid bilayer should result in the same voltage dependence of the rate constant for pore opening and closure because the energy barrier for both processes is the energy required to insert a highly charged domain into the hydrophobic portion of the membrane and thus the barrier height should be the same. If the sensor enters the pore and binds to the proposed negative domain at the other end of the pore, then it may travel through a large portion of the electric field before reaching the energy barrier. Thus, kinetic studies could distinguish between the two processes.
2.1.2. Measurement of the kinetics of the gating of pore 2
The only pore-forming subunit amenable to kinetic measurements was pore 2. The kinetics of pore 1 are too slow. Pore 3 gating depends on pore 2 being closed and performing kinetic measurements in the positive voltage region leads to pore 2 opening. Another limitation is the necessity to perform these measurements on single Triplins. In a multi-Triplin experiment, one cannot be certain that the gating of the pores of other Triplins would not influence the data collected. The data on the reopening of pore 2 was influenced by the closing of pore 3 at the higher positive voltages (section 2.1.3) but that can be corrected.
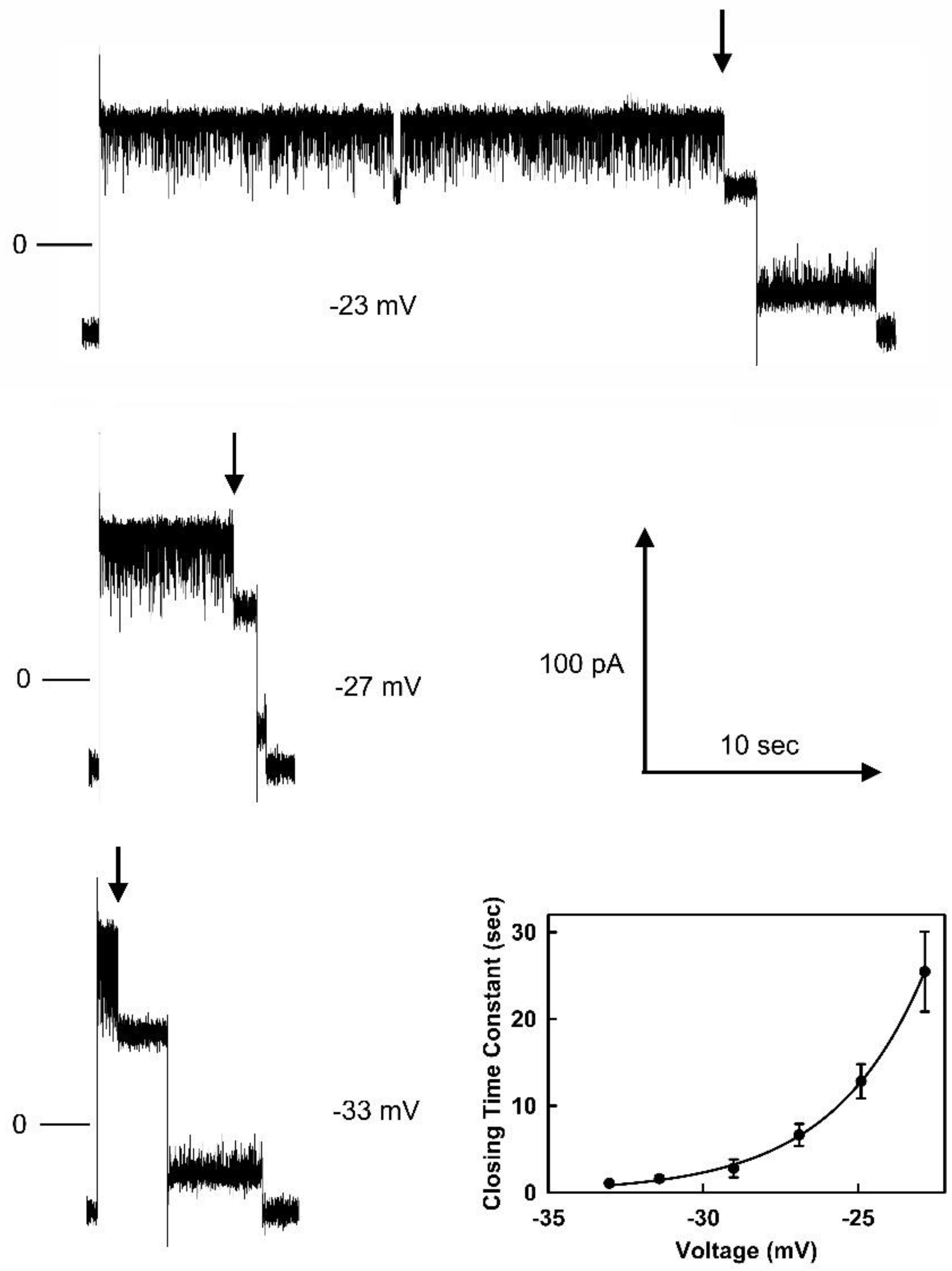
Figure 3 shows examples of single records of the time for pore 2 closure at three negative voltage values. Note that the pore is flickering to an unstable closed state and the closing time measured was the time to reach a stable closed state indicated by the arrow and the current remaining stable at the level at which only pore 3 is conducting. The times for closure varied stochastically as expected for single-molecule experiments. The mean time of typically 15 recordings was used to obtain a reliable value for the closing time constant, τ. The inset shows how τ varied with the applied voltage for one complete experiment.
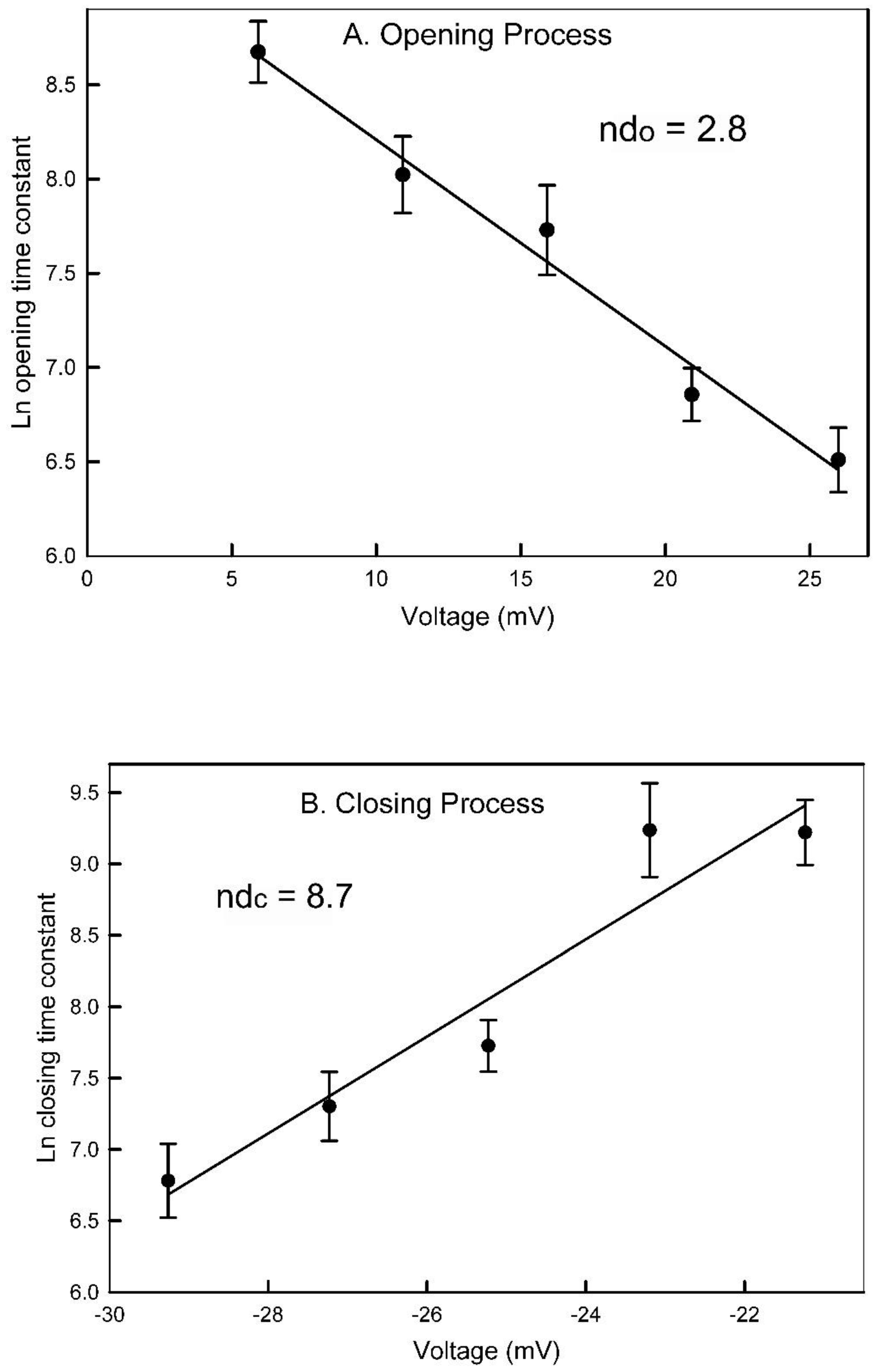
A log transform of τ was then used to obtain an estimate of the fraction of the electric field, “d”, traversed by the sensor when going either from the closed state, d
c, or from the open state, d
o, to the peak of the energy barrier (
Figure 4). The slope of the fit lines when multiplied by RT/F yields the product of the number of gating charges times the fraction of the field traversed, nd
c and nd
o. The summation yields the effective number of gating charges which for this experiment was 11.5. The average of 6 independent experiments resulted in nd
c = 8.7± 0.7 and nd
o = 2.5±1.2 (mean ± SD). That yields a value for n of 11.2. Thus, the d
c = 0.78 and d
o= 0.22.
The blockage of the ion flow through the pore will change the shape of the transmembrane electric field and so hard conclusions can only be made if the whole process were understood in detail. Regardless, the sensor is moving through a major portion of the electric field before reaching the peak energy barrier and this is consistent with the sensor moving into the pore eventually obstructing the pore. The charged region is leading the way and thus translocating though a large portion of the electric field.
2.1.3. Kinetics insights into interpore control
The opening process for pore 2 takes place at a manageable rate at positive voltages (see
Figure 4). However, the closing process for pore 3 also takes place at positive voltages.
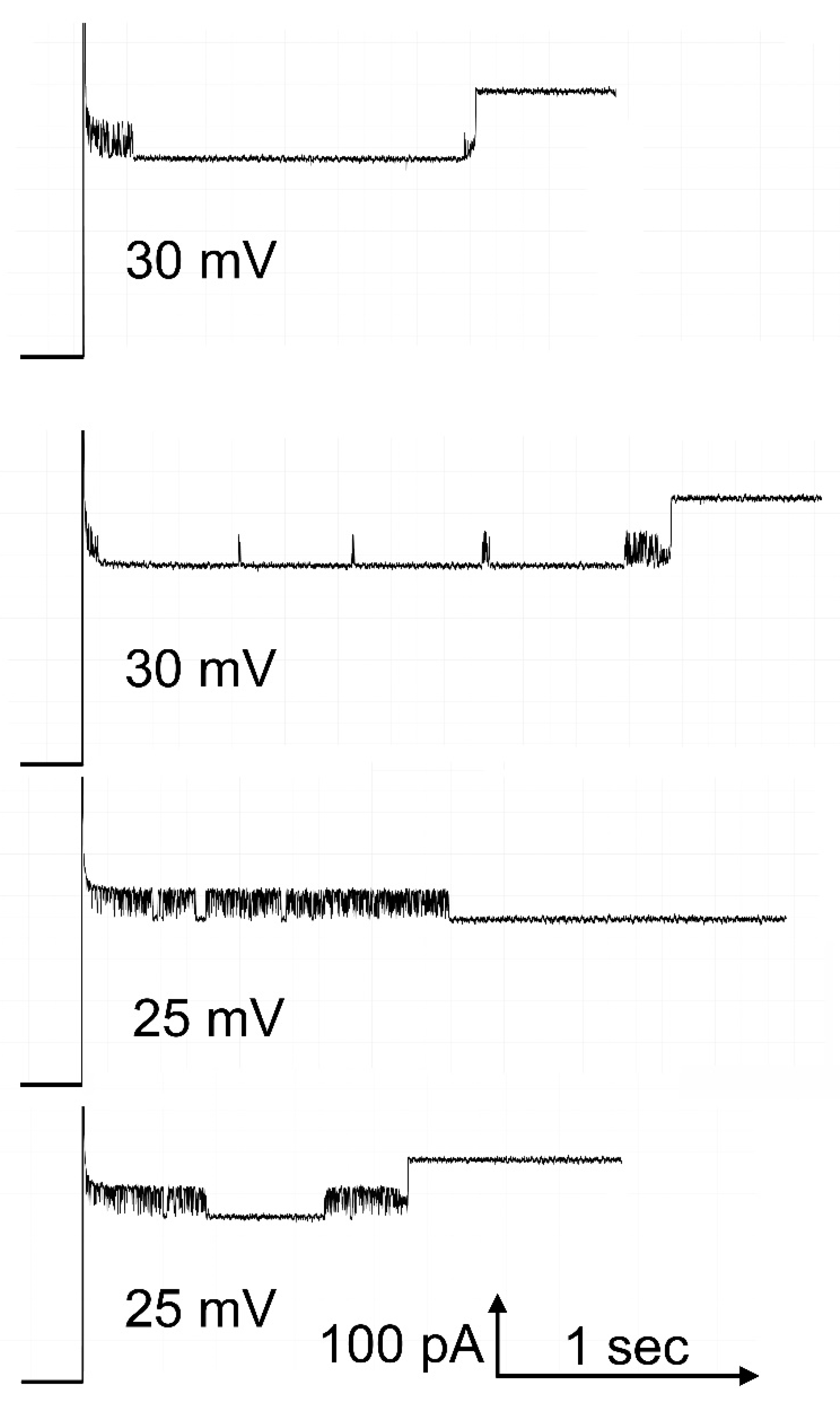
Figure 5 shows examples of the opening of pore 2 at 25 mV and 30 mV. Under both conditions, while waiting for pore 2 to open, pore 3 is either rapidly accessing the closed state or resting in the closed state for some time. If the pores were functioning independently, then the probability of pore 2 opening and that of pore 3 closing should take place regardless of the state of the other pore. That is not the case. When pore 2 opens, pore 3 does not close (see lower trace and ref [
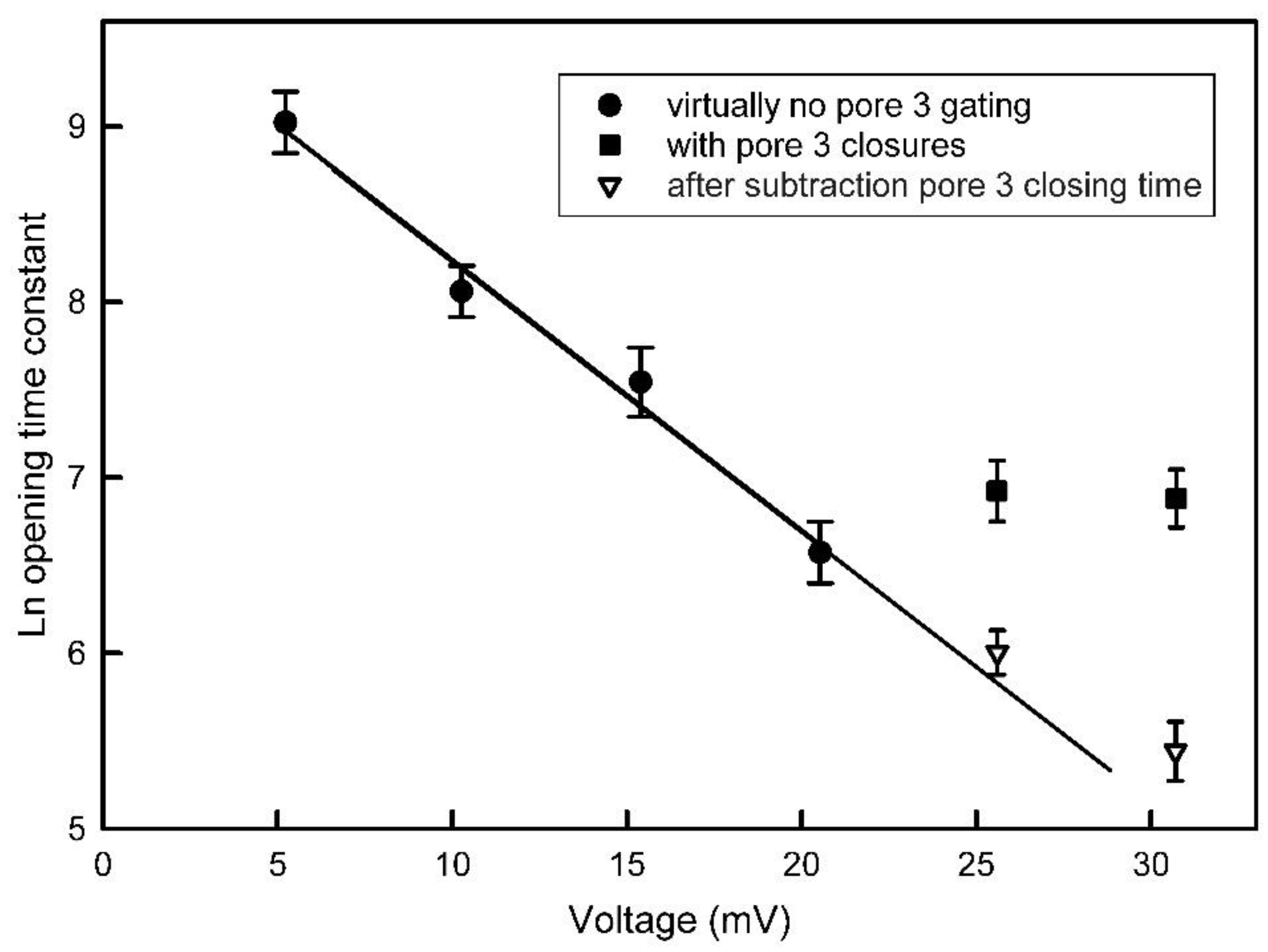
1]). In addition, pore 2 opening does not take place when pore 3 is closed. The rate of pore 2 opening is greatly slowed at the higher positive voltages (
Figure 6). Without correcting for the time that pore 3 is closed the data for 25 and 30 mV deviates markedly from the linear relationship of the log of the time constant as a function of voltage. However, by subtracting the time pore 3 is closed, the data follows the expected relationship. Thus, the kinetic measurements also demonstrate the interdependence of the gating of Triplin’s pores.
2.2. Pore blockage by polyarginine
Evidence indicates that the sensors responsible for the closure of the Triplin pores must contain a minimum of 14 positive charges and most of those are likely to be arginines [
1]. If, as proposed, a sensor were to physically block a pore by inserting into the pore, it is reasonable to conclude that evolutionary adaptation of the process would result in a binding surface that complements the physical nature of the sensor. Thus, polyarginine might be sufficiently similar to the sensor to mimic its ability to block the pores. Experiments using polyarginine, with an average molecular mass of 10,000, show that blockage can begin to be measured at a concentration in the solution next to the membrane of 40nM. This signifies strong binding. The same experiments using polylysine with an average molecular mass of 15,000 resulted in no blockage even with a final concentration of 23µM. 800µM spermine (a polyamine containing 4 amines) also had no blocking effect. Thus, it appears that there is a surface complementary to the sensor and it is specific for polyarginine.
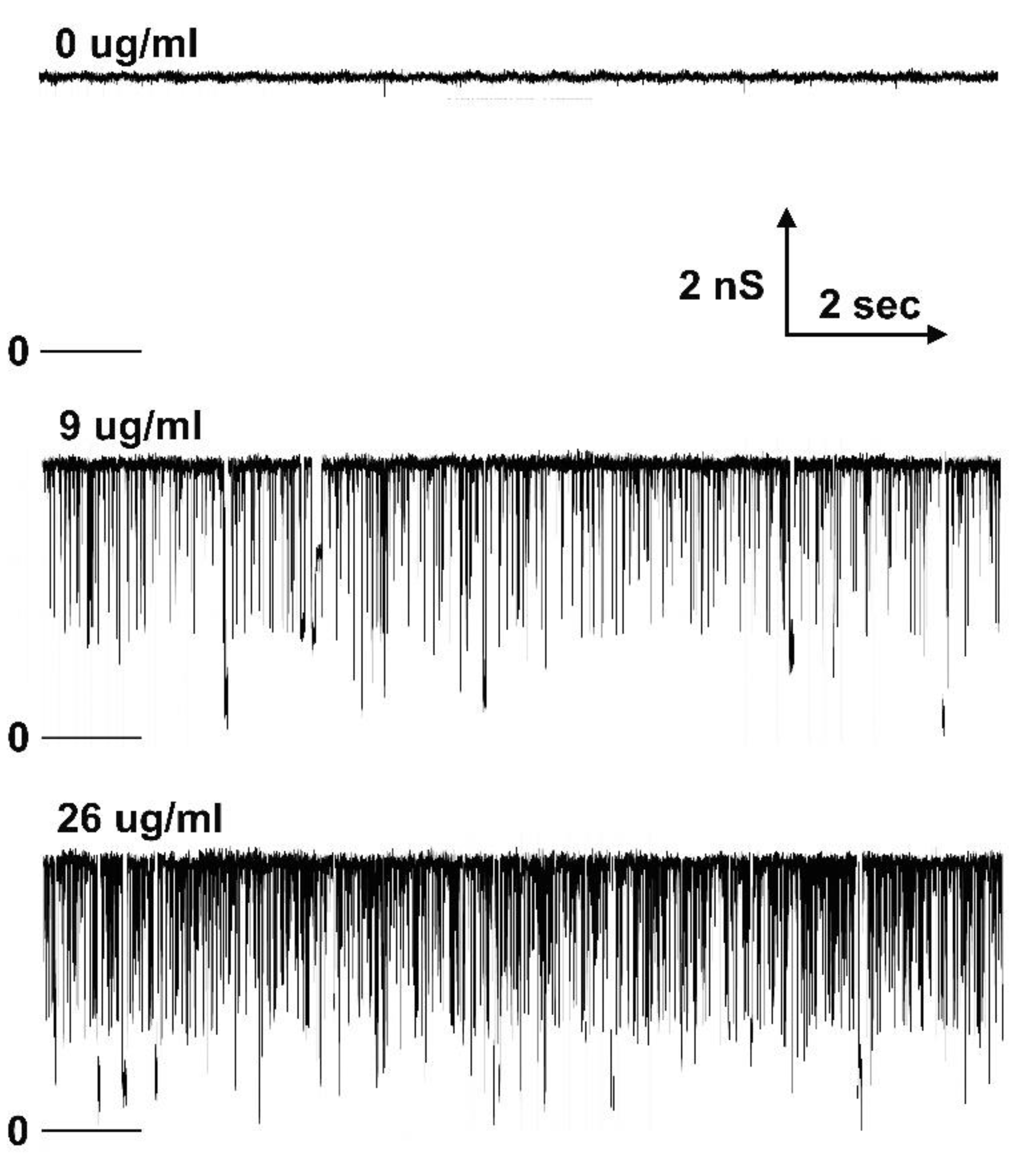
Figure 7 shows the transient blockages observed when +40mV was applied to the cis compartment of a membrane containing a single Triplin. Records were taken prior to and after sequential additions of polyarginine. Transient blockages were observed only at positive potentials as expected since a positive potential would drive polyarginine into the pores. In this record all three pores were open and were blocked, sometimes individually, but often two at a time or all at once. This is seen more clearly in
Figure 8. In the illustrated portion of this experiment, pore 1 was closed and pores 2 and 3 gated normally when a triangular voltage wave was applied (far left). The low dose of polyarginine used in this experiment did not induce pore 1 opening. The application of a constant +50 mV resulted in frequent fast blockage but also 2 periods of prolonged blockage. The simultaneous blockage of both pores was often followed by separate pore unblockage. On the right side of the figure, the blockage was persistent, and a negative potential was necessary to unblock the pores and repeat the blocking experiment. The simultaneous blockage of 2 and 3 pores is easily explained by considering that the long polyarginine chain (average of about 65 residues) can straddled two and three pores at the same time.
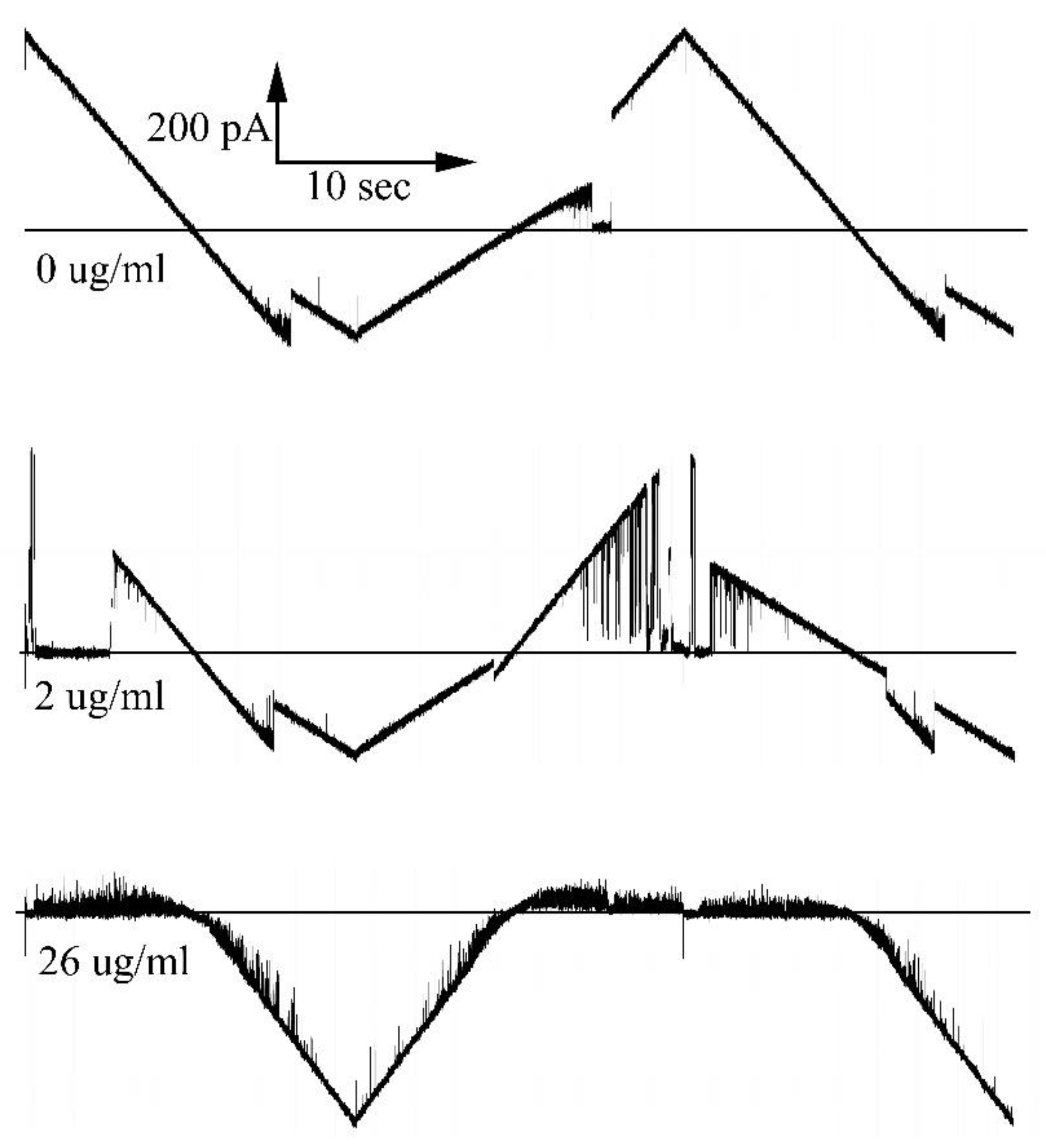
Figure 9 shows current recordings as triangular voltage waves were applied. Blocking occurred only at positive voltages on the side of the membrane to which polyarginine was added. Negative voltages unblock the pore including long-lived blocking events that are more common at high concentrations of polyarginine.
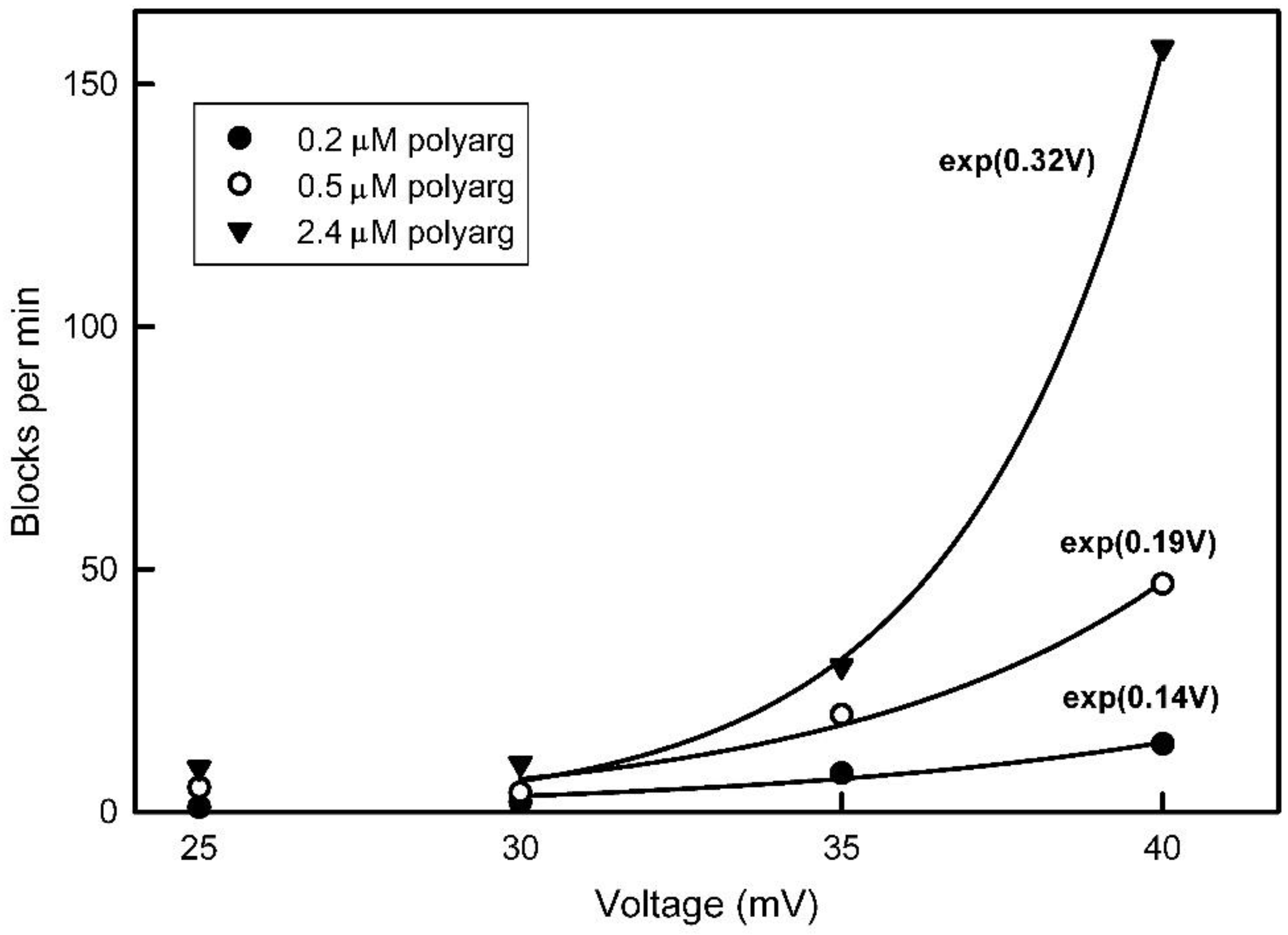
Transient blockages increase weakly with voltage whereas long-lived blockages are far more voltage dependent. The frequency of such blockage increases exponentially with applied voltage (
Figure 10). Increasing voltages also increases the occurrence of blockages that require a negative voltage to unblock.
3. Discussion
Triplin is a 3-pore complex, each pore possessing a positively-charged voltage sensor consisting of 14 net charges [
1,
6]. Each sensor domain translocates across the membrane in a steeply voltage-dependent manner and this translocation is coupled to the closer of each pore [
1]. The proposed molecular mechanism by which sensor translocation results in pore closure is for the sensor to enter the pore and obstruct the flow of ions [
1]. Other mechanisms are possible [
7] but the experimental evidence presented here supports the proposed mechanism.
Kinetic measurements can not only provide insight into the molecular mechanism by which voltage-gating takes place but, more importantly, can provide compelling evidence against the proposed mechanism. The kinetics of a well-studied beta barrel channel-former, VDAC, provide useful contrasting kinetic properties to Triplin. For Triplin, the rates of pore closure for pores 2 and 3 are fast whereas the rates of reopening are slow [
6]. For VDAC, pore closure is slow (τ≈400sec.) and voltage-dependent whereas reopening is fast (τ≈2.5 msec.) and voltage independent [
8]. The voltage dependent effective closure of VDAC (actually a reduction in pore size and selectivity inversion that results in no flux of ATP) is achieved by the translocation of portions of the beta barrel being moved to the membrane surface [
8]. The slow closure rate of VDAC can be readily understood by the need to break at least two sets of hydrogen bonds that anchor the mobile domain to the rest of the transmembrane beta barrel before they can translocate to the membrane surface. Reopening involves moving this mobile region from the membrane surface and reinserting it into the beta barrel. Half as many hydrogen bonds in the beta barrel would need to be broken for the reinsertion of the mobile domain to take place.
For Triplin, the proposed mechanism would reverse the energetics. Closure of the pores in Triplin is proposed to result from the entry of a surface loop-like domain into the pore led by the positively-charged region (blue;
Figure 11). Once in the pore, this mobile sensor domain is proposed to bind to a negatively-charged domain (red) on the other end of the pore. The breaking of that electrostatic interaction then would be responsible for the slow reopening kinetics.
The measured voltage dependence of pore closing and opening time constants provide independent support for the pore-blocking closure mechanism. The pore closing process is 4 times more voltage dependent than the opening process indicating that the energy barrier for the process is deep within the pore. Therefore, the closing process would require the sensor to move through most of the electric field before reaching the peak of the energy barrier and the reverse would be the case for the reopening process. An important contributor to the energy barrier is the necessity to remove the water of hydration from both the sensor and the proposed complementary binding region, perhaps the proposed negative domain on the other end of the pore. The dehydration energy is critical to binding selectivity as demonstrated by George Eisenman [
9]. The existence of a binding region complementary to the sensor domain is strongly supported by the polyarginine blocking results.
The differential ability to block the pore by polyarginine as opposed to polylysine is greater than 500 fold since no detectable blocking by polylysine was observed. This specificity of the block to polyarginine over polylysine is not related to any propensity for secondary structure formation as both have an extended conformation under the experimental conditions used [
10,
11]. Thus, the very strong preference for polyarginine indicates a specific interacting surface in Triplin.
Is the blockage of ion flow through the Triplin pores really mimicking the blockage produced by the sensor domain? Whereas the long-lived blockages must be physical blockages, the transient ones might be the result of polyarginine translocating through the pore as it moves from one side of the membrane to the other. However, calculations indicate that an extended polyarginine chain driven through the pore with an electric field should travel at a rate 104 times faster than that observed from the typical duration of the fast current blocking (flickering) events. Thus, it is more likely that the flickering is the result of transient blocking of the pore. That conclusion is supported by the observation that frequently all the conductance is blocked simultaneously and thus all open pores of a Triplin are blocked simultaneously. That would be a very unlikely event if the conductance drops were due to polyarginine translocating through the pores.
An observation that would seem to indicate that the polyarginine is translocating through the pores rather than blocking is the finding that the flickering rate increases with an increase in the aqueous concentration of polyarginine. However, the interaction between polyarginine and Triplin may be quite labile, undergoing a dynamic equilibrium with dissolved polymer. Thus, increasing the concentration of polyarginine would increase the blocking frequency.
The properties of the polyarginine block are consistent with the model of the gating mechanism. The long, highly charged polypetide chain should easily flow through a simple cylindrical pore, especially when driven by an electric field. Published work indicates that polyarginine should have a primarily extended conformation under the conditions of neutral pH used in these experiments [
10]. Thus, the observation of blockage indicates some stabilizing interaction within the pore and/or interference to flow by the proposed negatively-charged domain at one end of each pore.
4. Conclusion
The gating process used by each of the 3 pores formed by Triplin requires the movement of a highly positively charged domain from one side of the membrane to the other. Kinetic studies show that the sensor moves through most of the transmembrane electric field prior to reaching the peak of the interaction energy between the sensor and the pore structure. This agrees with the model that the sensor enters the pore and travels to the opposite side interacting with a complementary domain on the other side of the membrane and thus blocking the flow of ions and producing the closed state. The complementary domain would likely be produced by negatively charged loop structures near the pore opening because, in the closed state, the sensor is cleaved by trypsin added to the opposite side. Polyarginine mimics the pore blocking mechanism used by the sensor including voltage dependence of the polyarginine block. The inability of polylysine to block the pores supports the conclusion that the complementary surface binds arginines specifically and is in harmony with previous findings that the charges on the sensor are mainly arginines. There is no alternative mechanism that is consistent with all the experimental observations.
5. Materials and Methods
5.1. Sources of materials used
All chemicals used were reagent grade. The phospholipids were obtained from Avanti Polar Lipids (Alabaster, AL). Cholesterol, polylysine, polyarginine and spermine were purchased from Sigma (St. Louis, MO).
5.2. Electrophysiological recordings
All experiments were performed on Triplin reconstituted into planar phospholipid membranes made from monolayers as described previously [
12,
13]. In brief, the membrane was formed across a 0.1 mm hole in a thin polyvinylidene chloride partition separating two aqueous compartments containing 5 ml of 1.0 M KCl, 1 mM MgCl
2, buffered with 10 mM HEPES, pH 7.8. The monolayers were formed from a solution of 0.5% (w/v) diphytanoylphosphatidylcholine, 0.5% (w/v) polar extract of soybean phospholipids, and 0.05% (w/v) cholesterol in hexane. The hexane was allowed to evaporate prior to membrane formation and thus no solvents were present in the membrane. This membrane is identical to a natural cell membrane but lacking proteins or carbohydrates. Samples containing Triplin were generated as previously described [
6], flash-frozen in 0.1 ml aliquots and stored at -80
oC. After thawing, β-octyl-glucoside was added to a final concentration of 1% (w/v) and kept on ice during the experiment. Typically, 10 µl of the sample was dispersed into one aqueous compartment (designated “
cis”) and, with time, Triplin would insert into the membrane. The membrane voltage was clamped and the current recorded using Clampex software. Calomel electrodes were used to interface the solution with the electronics. Voltages was applied to the
cis side, the
trans held at virtual ground. The signal was low-pass filtered at 500 Hz. The sample containing Triplin was always added to the
cis compartment.
5.3. Kinetic measurements
Kinetic measurements were only made on pore 2 because the conditions necessary for making those measurements of pore 3 often resulted in the spontaneous opening of pore 2 and thus interfering with the collection of sufficient data to achieve valid measurements. Recordings were made on membranes containing a single Triplin so that the meaning of any conductance change was clearly defined. For the closing kinetics, pore 2 was held in the open state at 10 mV and then switched to a negative voltage until pore 2 closed and thus the time required for closure was recorded. This process was repeated 15 to 20 times, depending on the experiment, for negative voltages ranging from -20 to -30 mV. For the opening process, pore 2 was closed by applying -40 mV and then the voltage was switched to a positive value until the pore reopened. Again, the process was repeated 15 to 20 times, depending on the experiment, for positive voltages ranging from 5 to 40 mV. At the higher voltages pore 3 would frequently close and reopen during the waiting period for pore 2 to reopen.
5.3.1. Theoretical basis for estimating the fraction of the electric field traversed prior to reaching the peak of the energy barrier
Using Eyring Rate Theory, one can obtain an estimate of the fraction of the transmembrane electric field that the sensor needed to traverse prior to reaching the peak of the energy barrier.
mean time to open pore =
mean time to close pore =
From Eyring rate theory: Where h is Plank’s constant, kB is Boltzmann’s constant, n is the number of charges on the voltage sensor, dc is the fraction of the electric field that the sensor must traverse to move from the closed state to the peak of the energy barrier whereas do is from the open state to the barrier peak. V is the transmembrane voltage and Vo is the voltage at which half the pores are open and half are closed. R,T, and F have their usual meanings.
After log transform and simplifying:
And
Author Contributions
Conceptualization, M.C. and P.L.; Methodology, M.C.; Formal Analysis, M.C., C.D., P.L.; Investigation, M.C., C.D., P.L.; Data Curation, M.C.; Writing Review Editing, M.C., C.D., P.L.; Project Administration, M.C.; Funding Acquisition, M.C.
Acknowledgments
We thank Emmy Hudak and Harley Mocker for performing some of the experiments reported in this manuscript.
Conflicts of interest
None.
References
- Colombini, M., Barnes, K., Chang, K-T, Younis, M.H. and Aguilella, V.M. Triplin: Functional Probing of Its Structure and the Dynamics of the Voltage-Gating Process. Int. J. Mol. Sci. (2022) 23,13765. [CrossRef]
- Schindler, H., Rosenbusch, J.P. Matrix protein in planar membranes: clusters of channels in a native environment and their functional reassembly. Proc. Natl. Acad. Sci. USA . (1981) 78: 2302-2306. [CrossRef]
- Van Gelder, P., Saint, N., Phale, P., Eppens, E.F., Prilipov, A., van, B.R., Rosenbusch, J.P., and Tommassen, J. Voltage sensing in the PhoE and OmpF outer membrane porins of Escherichia coli: role of charged residues. J. Mol. Biol. (1997) 269: 468–472. [CrossRef]
- Robertson, K.M. and Tieleman, D.P. Molecular basis of voltage gating of OmpF porin. Biochem. Cell Biol. (2002) 80: 517–523. [CrossRef]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. (2003) 67: 593-656. [CrossRef]
- Lin, S.H., Chang, K-T., Cherian, N., Wu, B., Phee, H., Cho, C. and Colombini, M. Cooperativity and steep voltage dependence in a bacterial channel. Int. J. Mol. Sci. (2019) 20: 4501. [CrossRef]
- Hille, B. Ionic Channels of Excitable Membranes, Second Ed., Publisher: Sinauer Associates Inc., USA, (1992) Chapter 18 p. 479.
- Colombini, M. VDAC structure, selectivity, and dynamics. Biochim. Biophys. Acta (2012) 1818: 1457-1465. [CrossRef]
- Eisenman, G. and Horn, R. Ionic selectivity revisited: The role of kinetic and equilibrium processes in ion permeation through channels. J. Membr. Biol. (1983) 76: 197–225. [CrossRef]
- Morga, M., Batys, P., Kosior, D., Bonarek,P. and Adamczyk Z. Poly-L-Arginine Molecule Properties in Simple Electrolytes: Molecular Dynamic Modeling and Experiments. Int J Environ Res Public Health (2022) 19(6):3588. [CrossRef]
- Adamczyk, Z., Morga, M., Kosior, D., and Batys, P. Conformations of poly-l-lysine molecules in electrolyte solutions: modeling and experimental measurements. J. Phys. Chem. C (2018) 122 (40):23180–23190. [CrossRef]
- Montal, M., Mueller, P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. U.S.A. (1972) 69: 3561–3566. [CrossRef]
- Colombini, M. Characterization of channels isolated from plant mitochondria. Method Enzymol. (1987) 148: 465-475. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).