1. Introduction
Heart failure is an increasing public health problem, mainly in the elderly, and is one of the important sources of death and hospitalization in elderly patients. The cardiotonic drug pimobendan (C
19H
18N
4O
2) in
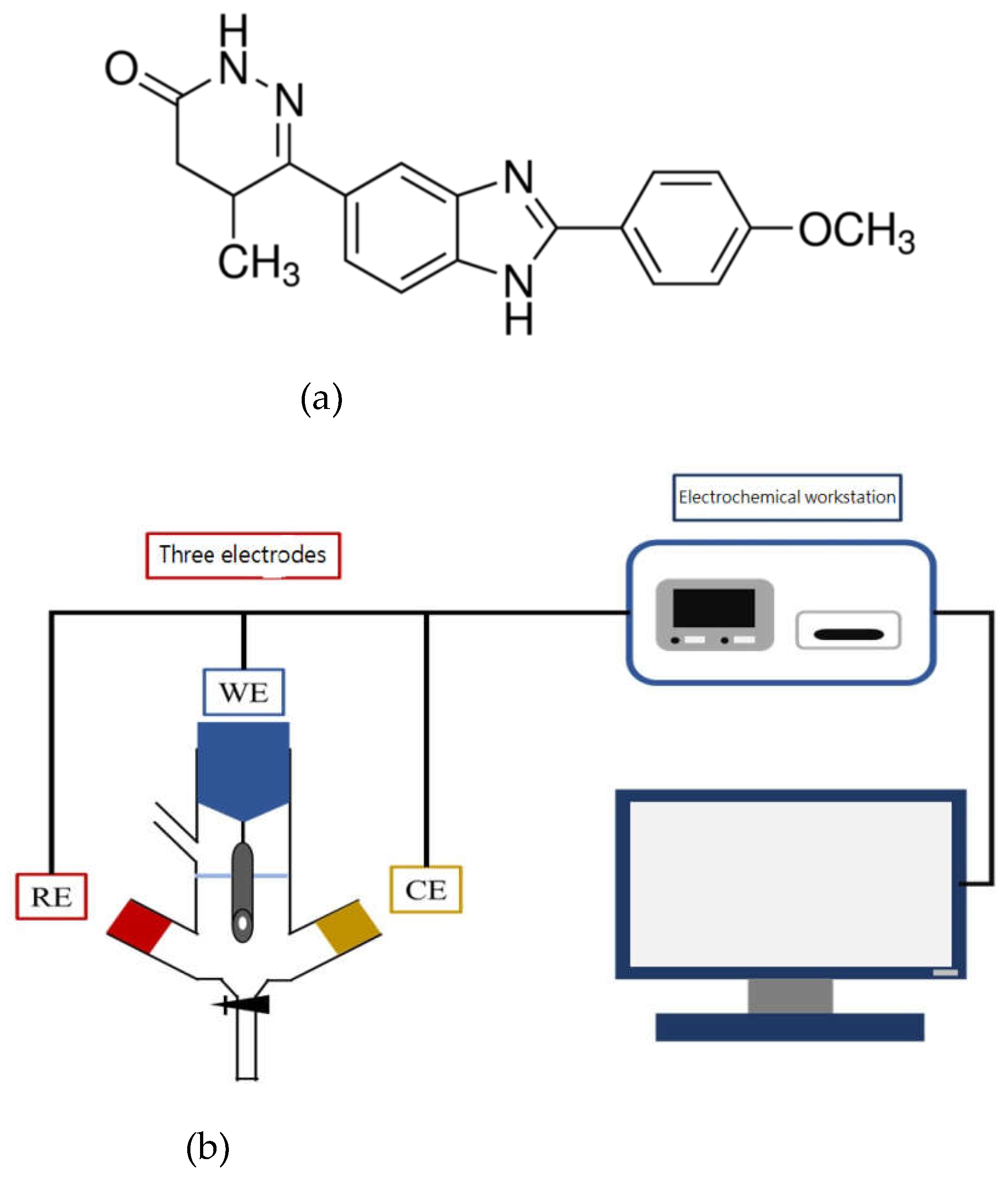
Figure 1(a) is a calcium sensitizer and selective inhibitor of phosphodiesterase III, studies have shown that as a canine cardiotonic therapy can increase lifespan [
1]. Pimobendan is a drug licensed for veterinary use indicated for the treatment of heart failure in dogs and it is also used in Japan in human patients [
1]. Oral pimobendan is used for treating congestive heart failure (CHF) in dogs and cats [
2]. Pimobendan is current in patients with chronic heart failure but is less effective in patients with old myocardial infarction (OMI) than in patients with dilated cardiomyopathy (DCM) or other heart diseases [
3,
4].
Currently used for clinical analysis of pimobendan is by high performance liquid chromatography (HPLC) [
5] and Liquid Chromatography Tandem Mass Spectrometry (LC/MS) [
6]. It is time-consuming (more than a few minutes), expensive equipment, and the coexistence of many complex sample preparations that interfere with the actual sample, that is not suitable for tests that require a large number of samples in a short analysis time.
To our knowledge, the fast detections of pimobendan by electrochemical method have not been studied. Therefore, it is hoped to develop a cardiotonic drug measurement with high sensitivity, high selectivity and high long-term stability by cyclic voltammetry and differential pulse voltammetry in electrochemical systems. This study is expected to gain a deeper understanding of the mechanisms of adsorption and surface redox reactions during cardiotonic drug sensing. Electrochemical detection technique has been considered as an outstanding candidate for the determination of pimobendan n owing to its advantages such as excellent sensitivity, high selectivity, fast response, simple operation and cost-effective.
Graphite carbonitride (g-C
3N
4) is a planar two-dimensional sheet structure similar to graphene, which is a typical polymer semiconductor. Due to its special structure and excellent properties, g-C
3N
4 has become a popular material for research of some electrochemical reactions [
7,
8,
9]. Since g-C
3N
4 has attracted consideration in various fields due to its excellent optical properties, high thermal stability in basic/acidic media, earth abundance and environmentally friendly nature [
10]. However low conductivity of g-C
3N
4 limits its application in electrochemical electrode, to overcome this deficiency to dope the metal is a way to improve its conductivity [
11].
Among nanomaterials, gold nanoparticles is functional material that has received increased attention [
12]. Gold has been used in promotion of some oxidation reactions and electrochemical reaction system [
12]. Until now, the Au-based nanomaterials have been observed as dynamic nanostructured materials and expected to be important element for electrode device [
12]. Therefore, the main objective of the present work was to establish a simplified cyclic voltammetry method for determination of pimobendan using electrochemical sensors based on Au/g-C
3N
4/glassy carbon electrode. The morphology, crystal phase, chemical component and microstructure of the as-prepared electrochemical sensors were characterized using various techniques like XRD (X-ray diffraction) and TEM (transmission electron microscopy). Moreover, the electrochemical behavior of the as-synthesized electrochemical sensors was investigated by cyclic voltammetry.
2. Experimental section
2.1. Chemicals and reagent
Melamine (99%), Methyl alcohol (99%) and tetrachloroauric acid trihydrate (99%) were purchased from Alfa Aesar Co., Ltd. Chitosan (>75%), Sodium phosphate dibasic (99%), Potassium phosphate monobasic (99%), Potassium chloride (99%) and Sodium chloride (99%) were obtained from SIGMA ALDRICH Company. The reagents used in the experiment were analytically pure without further purification. The laboratory-grade distilled and deionized water (Millpore, Milli-Q Water Purification System) was used throughout the experiment.
2.2. Electrochemical sensors preparation
A facile method for the fabrication of electrochemical sensors and the construction process is as behind. Take melamine for gradient calcining to obtain g-C3N4 of light yellow powder. Then take g-C3N4, add appropriate amount of tetrachloroauric acid (HAuCl4) solution and methanol (Methyl alcohol), heat and stir until powdery. Then the powers were dried, and carried out gradient calcination to obtain a light gray powder of various percentages of Au/g-C3N4 products. To shake and mix Au/g-C3N4 and chitosan solution evenly, and then was coating on the surface of the glassy carbon electrode. After drying to form a film, the preparation of the working electrode was completed.
2.3. Characterization apparatus
The crystal structures of the preparation samples were characterized by X-ray diffraction (XRD) using a Bruker D8 focus diffractometer operating at 35 kV and 35 mA with a scan step of 2º/min between 10º and 80º (2θ), using Cu Kα radiation (Cu Kα1, 1.5404Å). The microstructure and morphology of the samples were performed using JEM-2010 (JEOL, Japan, 200 kV) transmission electron microscope and scanning electron microscope FESEM/ Energy-dispersive X-ray spectroscopy (EDS) (JEOL JSM-7500F, JSM-6500F, Japan, 15 kV). Fourier transform infrared (FTIR) spectroscopy was by using an Agilent Cary 630 ATR-FTIR spectrophotometer (USA).
2.4. Electrode preparation and electrochemical measurements
Prior to use, the surface of the bare electrode (glasses carbon electrode, GCE) was successively polished with aluminum oxide powder on a polishing cloth to a mirror finish and rinsed with water. As shown in
Figure 1(b), the electrochemical sensing system includes three electrodes and an electrochemical workstation. Pt and Ag/AgCl were used as the counter and reference electrodes, respectively. G-C
3N
4 and Au/g-C
3N
4 electrodes were used as the working electrode. All electrochemical measurements were performed out on a ZIVE SP1 compact type electrochemical workstation (Won A Tech/ZIVE LAB). The scanning range was from -1.0 to 1.0 V at a rate from 10 to 200 mVs
-1 by using cyclic voltammetry (CV) method. The detection limit (DL) is measured as DL = (3×SD)/m, where SD is the standard deviation of the blank sample signal and the m is revealed the slope of the response curve versus pimobendan concentration (range from 0.0 to 55 μM).
3. Results and Discussion
3.1. Characterized the sensing materials
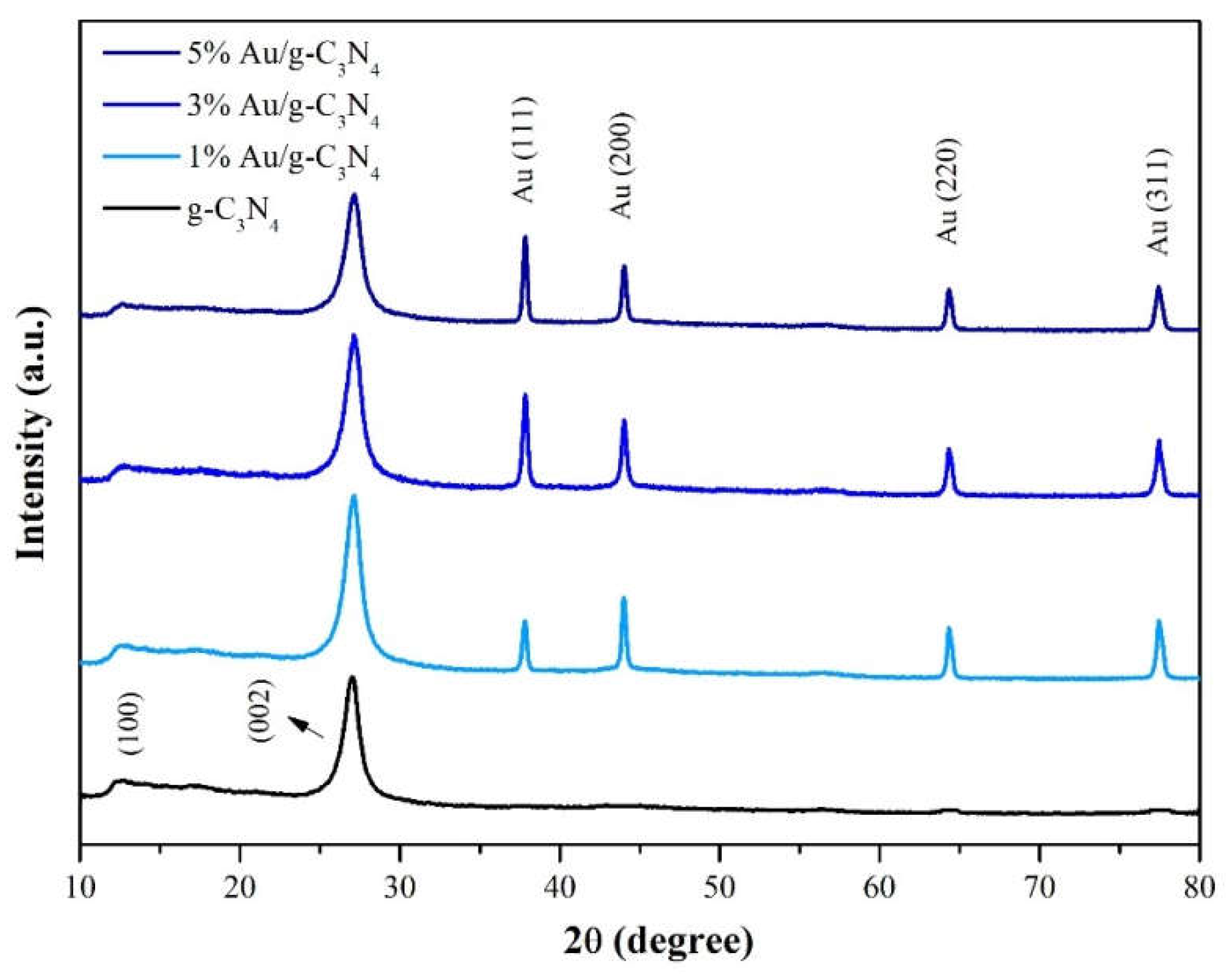
To investigate the structure of g-C
3N
4 and Au/g-C
3N
4, the XRD patterns of g-C
3N
4 and Au/g-C
3N
4 with different Au contents (1%, 3% and 5%) were studied. As can be seen in
Figure 2, g-C
3N
4 XRD has clear peaks (100) and (002) at 2θ = 13.1° and 27.3°, respectively. These represent the accumulation of conjugated π-π bonds in the aromatics of graphite carbonitrides [
7]. Furthermore in
Figure 2, the characteristic peaks at 37.7°, 44.0°, 64.3°, and 77.4° correspond to the crystalline planes of Au(111), Au(200), Au(220) and Au(311), respectively [
8]. From this, it can be seen that the characteristic peak of Au (111) increases with the increase of the proportion of Au doping, so it is known that Au has been successfully doped onto the g-C
3N
4 flakes. By using Scherer’s formula, the calculated average crystalline sizes of Au (111) of 3% Au/C
3N
4 was found to be approximately 18.6 nm.
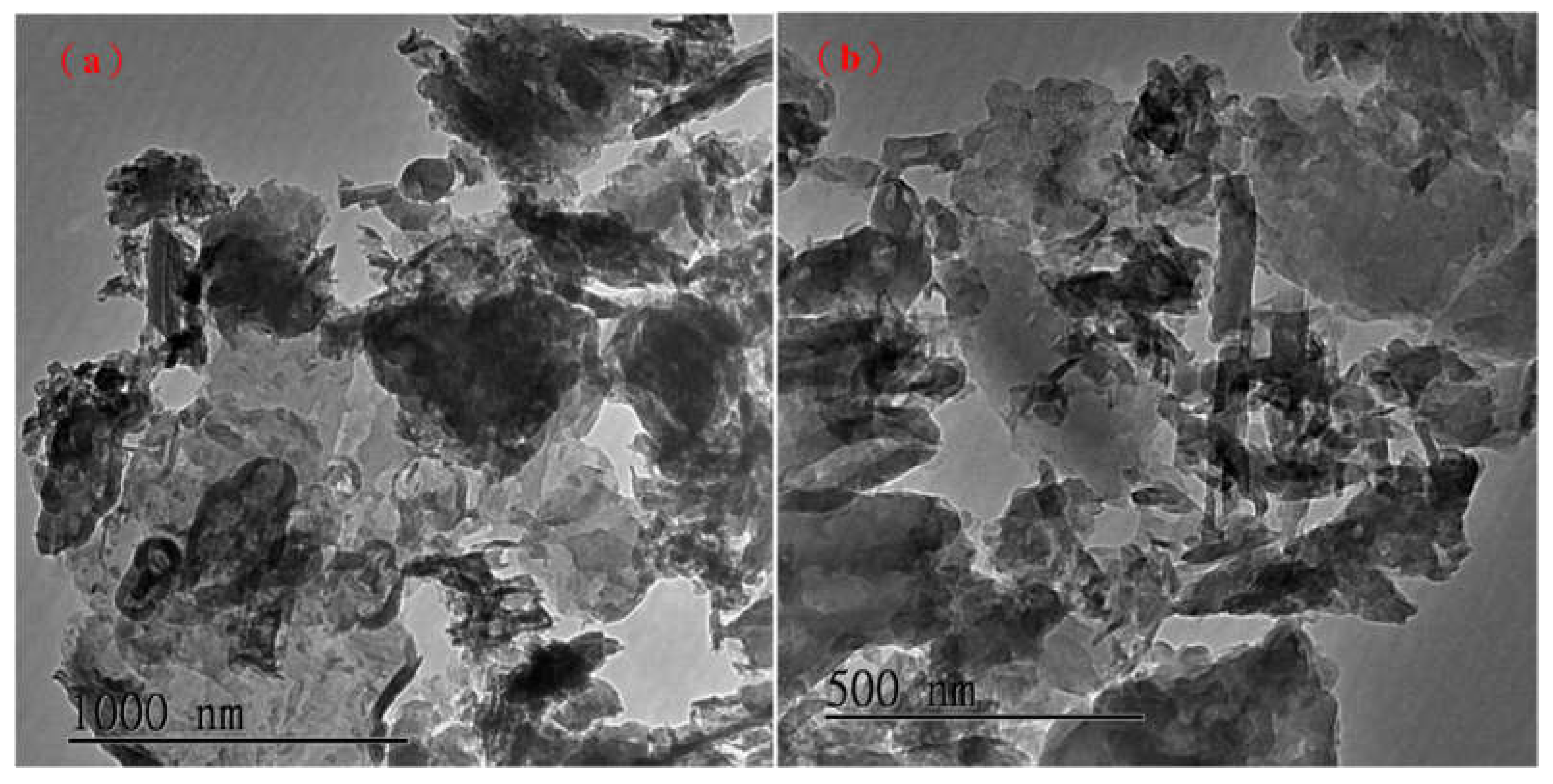
Figure 3(a) presents a g-C
3N
4 TEM with a magnification and scale bar 1000 nm, and (b) shows a g-C
3N
4 TEM with a magnification and the scale bar 500 nm. It can be seen from the figure that g-C
3N
4 presents a multilayer sheet structure.
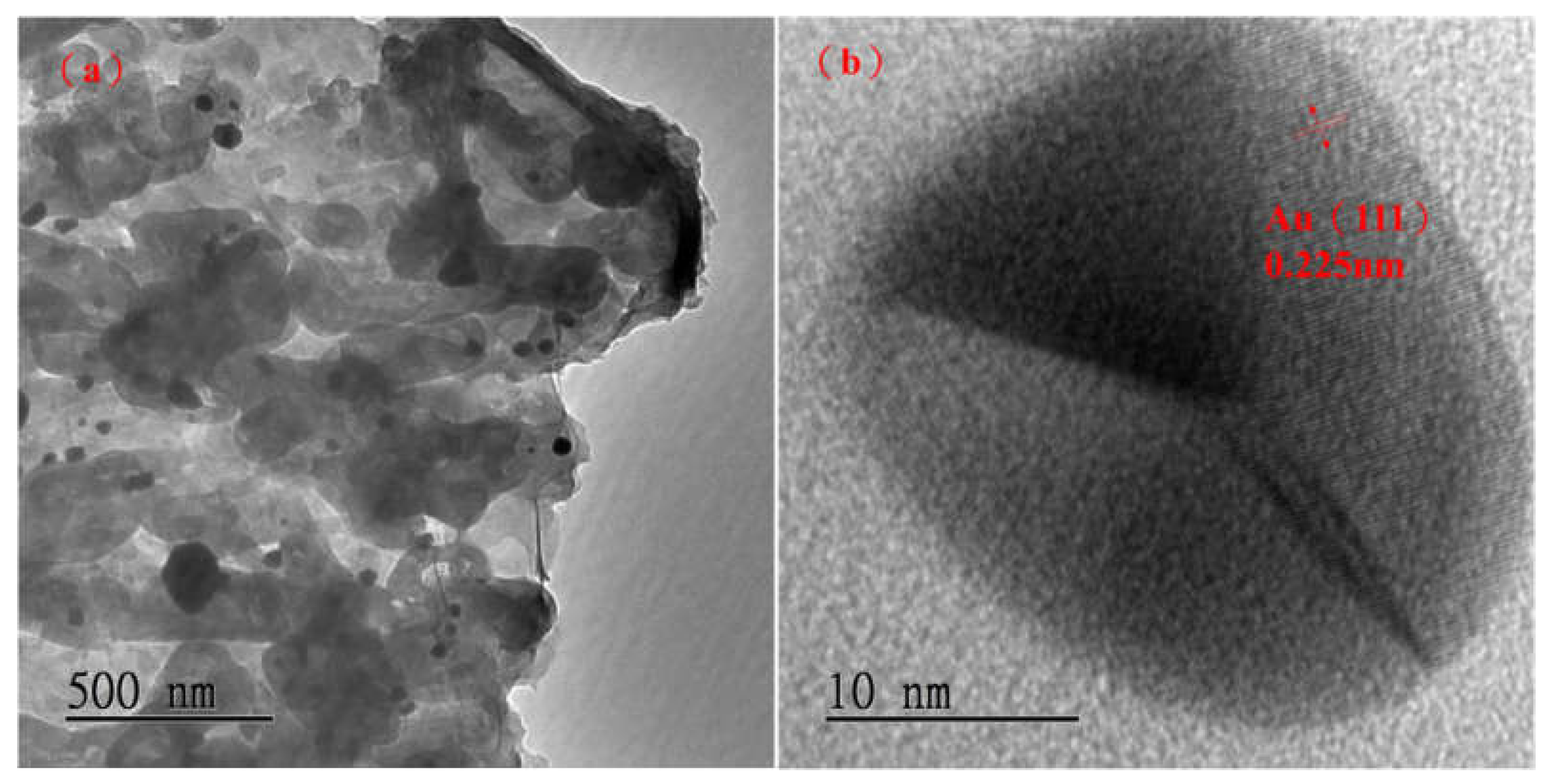
Figure 4(a) is a g-C
3N
4 TEM with a magnification and scale bar 500 nm, and (b) is a g-C
3N
4 TEM with a magnification and scale bar 10 nm. We can see from
Figure 4(b) where Au is doped on the surface of flake g-C
3N
4. The lattice line is used to calculate the lattice spacing of Au and compared with the literature. It is found that the Au (111) crystal plane has a lattice spacing of d = 0.225 nm [
9]. It can be seen that the spherical Au is successfully doped on the flake g-C
3N
4, the average crystalline size of Au (111) is calculated as 19.2 nm. It reveals similar result of XRD spectra in
Figure 2.
The microstructure and distribution of elements on samples can be characterized by SEM and EDS instruments.
Figure 5a showed a g-C
3N
4 SEM image with a scale bar of 50 μm, and 5b is the g-C
3N
4 SEM image of a scale bar of 20 μm. The surface of g-C
3N
4 exhibited an aggregation from unregular like smaller particles structure in
Figure 5a and b. To study the element distribution and composition of as-prepared samples, energy-dispersive X-ray spectroscopy (EDS) was employed. It is revealed a g-C
3N
4 EDS and element mapping images in
Figure 6.
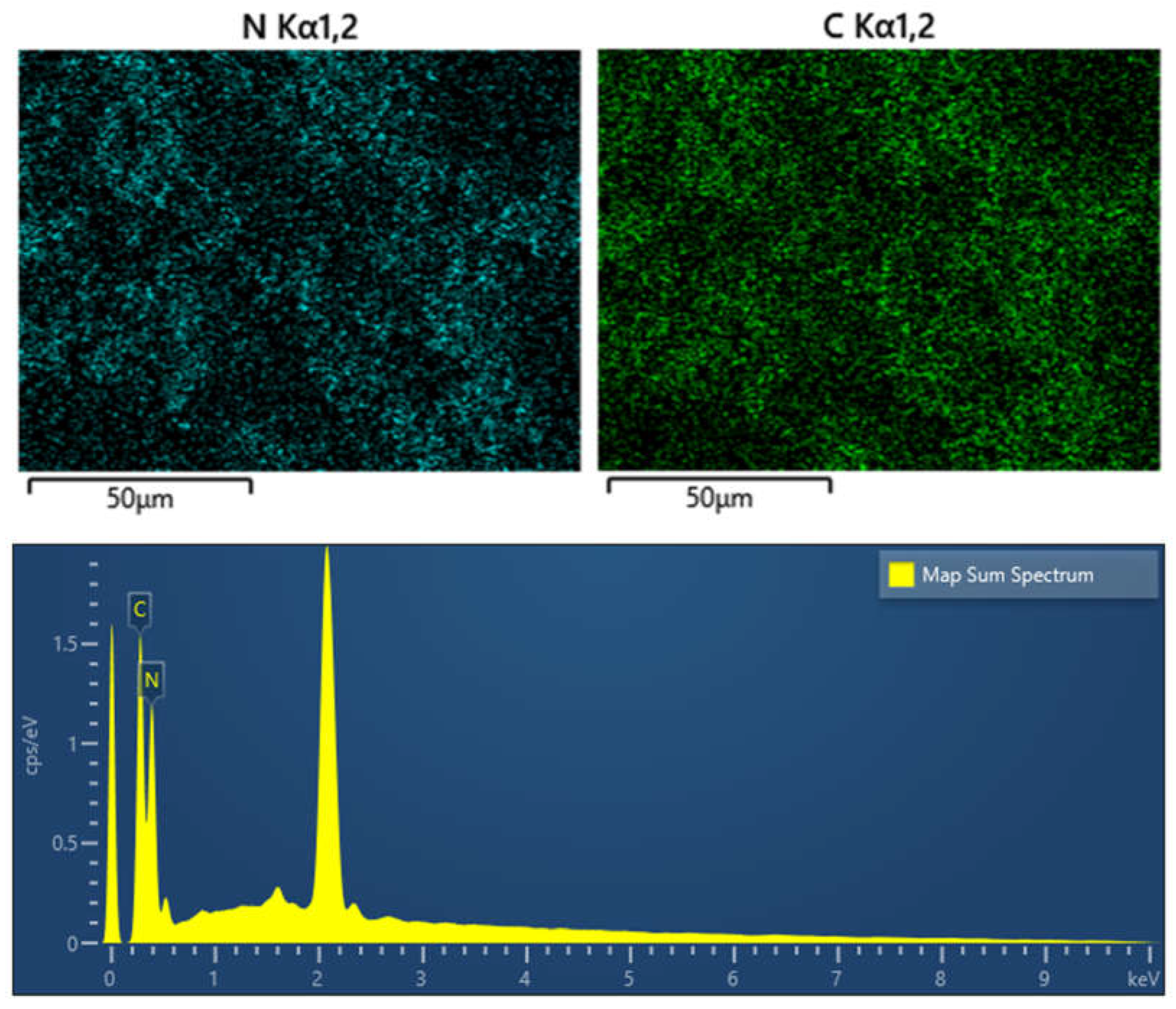
Figure 6 presents the carbon and nitrogen elements are coexisted in the g-C
3N
4, and the two elements are homogeneously distributed on the surface of the g-C
3N
4.
Figure 7a is a 3% Au/g-C
3N
4 SEM image with a scale bar of 50 μm, and 7b is a 3% Au/g-C
3N
4 SEM image with scale bar 20 μm. The 3% Au/g-C
3N
4 showed an aggregation structure from smaller particles in
Figure 7a and 7b.
Figure 8 shows 3% Au/g-C
3N
4 EDS and mapping images.
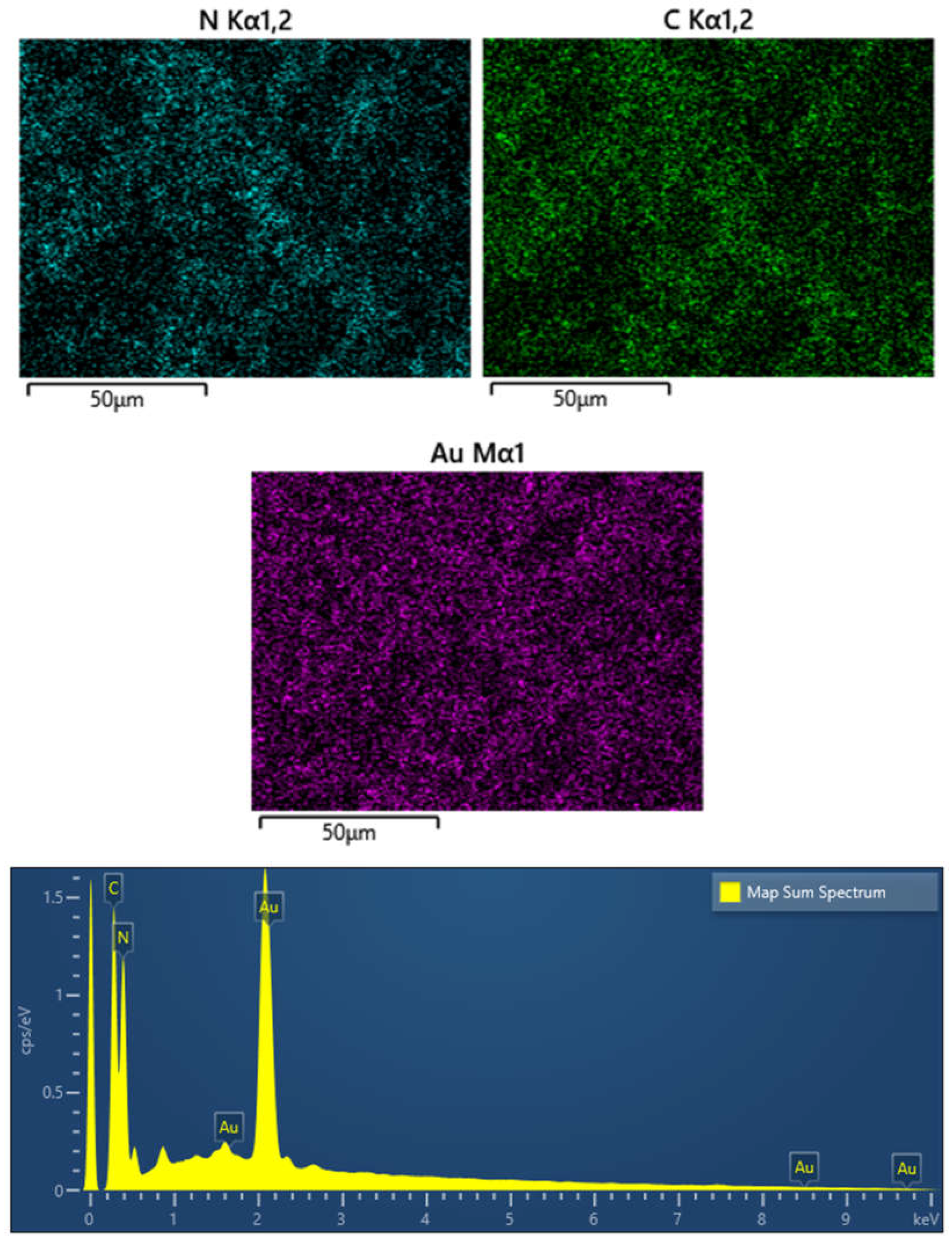
Figure 8 reveals the carbon, nitrogen and gold elements are coexisted on the 3% Au/g-C
3N
4, and the three elements are homogeneously distributed on the surface of the 3% Au/g-C
3N
4.
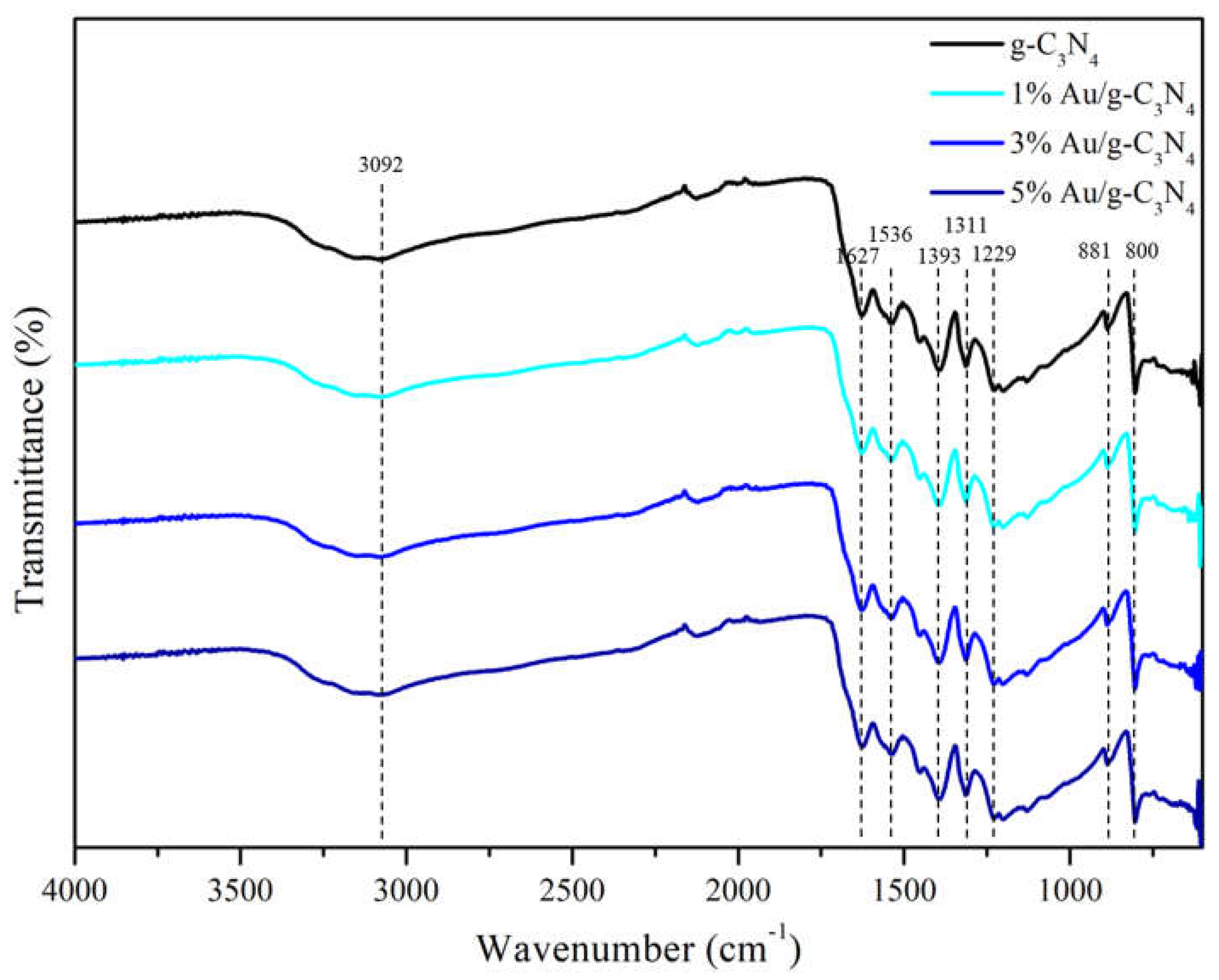
Figure 9 revealed the FTIR spectra of g-C
3N
4, 1% Au/g-C
3N
4, 3% Au/g-C
3N
4 and 5% Au/g-C
3N
4. Where the broadened peaks between 3000 and 3500 cm
−1 (highest at 3092 cm
−1) were correlated to the stretching vibration [
13] of remaining free N-H in the bridging C-NH-C units and O-H initiated from physically adsorbed water species on g-C
3N
4 surface, respectively. As shown, the main characteristic group peaks observed in the region from 900 to 1700 cm−1 were usually assigned to stretching vibration signals of aromatic heptazine-derived repeating units, including the typical sp
2 C=N stretching modes at 1627 cm
-1 and out-of-plane bending vibrations of the sp
3 C-N bonds at 1536, 1393, 1311 and 1229 cm
-1[
14]. The sharp absorption peak located at about 800 cm
−1 was attributed to the characteristic ring mode of tri-s-triazine cycles [
15], and the absorption band at 881 cm
−1 was assigned as the deformation mode of N-H in amino groups [
13,
14,
15], respectively. In
Figure 9, no obvious peak was observed by addition of gold on g-C
3N
4.
3.2. Sensing properties
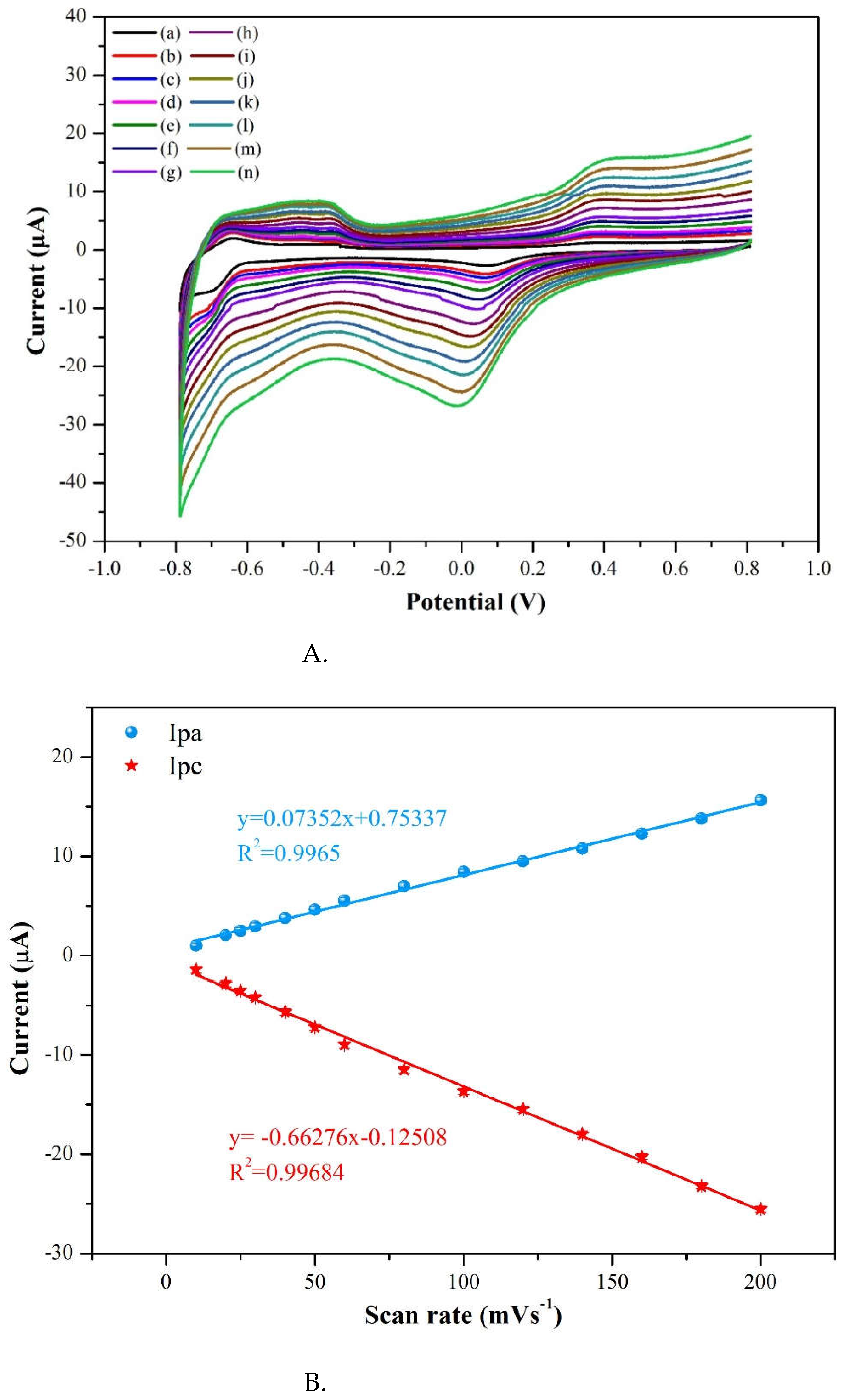
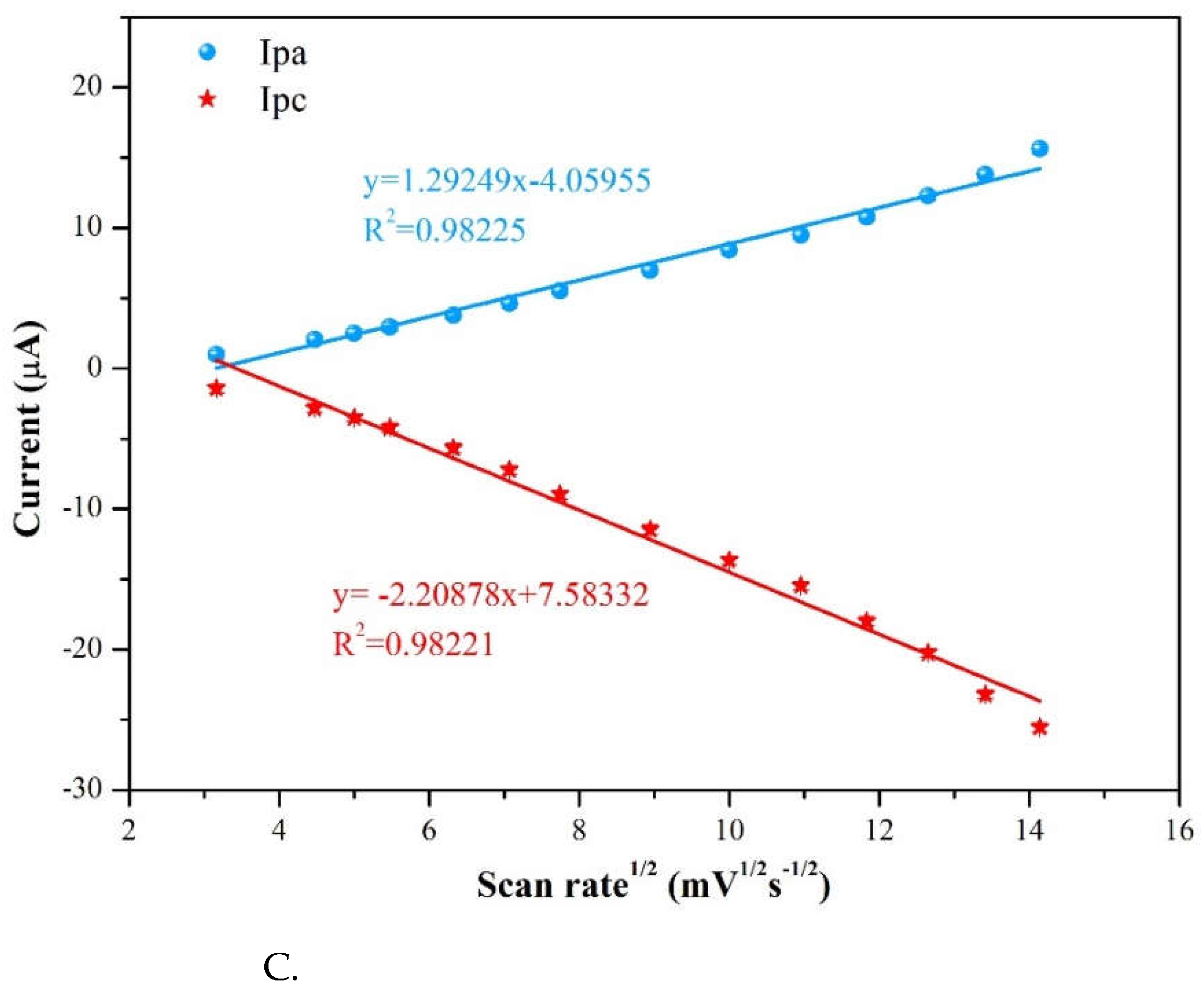
3.2.1. Scan rate effect
To conclude whether the redox process is controlled by a diffusion or adsorption, this study considered the relationship between oxidation current and scan rate. If it appears a linear relationship of peak current with sweep rate, then it usually means an adsorbed pimobendan molecule is controlled [
16]. At pH =7.0 the CV method was used of scan rates ranging from 10 to 200 mVs
-1 on oxidation current intensity at a pimobendan concentration at 55 μM. In
Figure 10A, the oxidative current peaks were located at around -0.40 V and 0.40 V and reduction current was found near 0.05 V of 3 %Au/g–C
3N
4 increased with the various scan rates. To determine the relationship between pimobendan current and scan rate, plots of the scan rate versus current intensity in
Figure 10B. The scan rate was presented a linear relationship with the anodic peak current (Ipa) at 0.4 V and cathodic peak current (Ipc) at 0.05 V, and it showed the R
2 were obtained as 0.9965 and 0.9968, respectively. In
Figure 10C the square root of scan rate exhibited a linear relationship with the anodic peak current (Ipa) and cathodic peak current (Ipc), it presented R
2 = 0.9823 and 0.9822, respectively. Above results showed the redox reaction of pimobendan on the modified electrode was an adsorption-controlled process, indicating that pimobendan was directly adsorption on the electrode surface [
16]. We choose the condition of scan rate at 50 mVs
-1 for it is easily for the peaks separations in
Figure 10A.
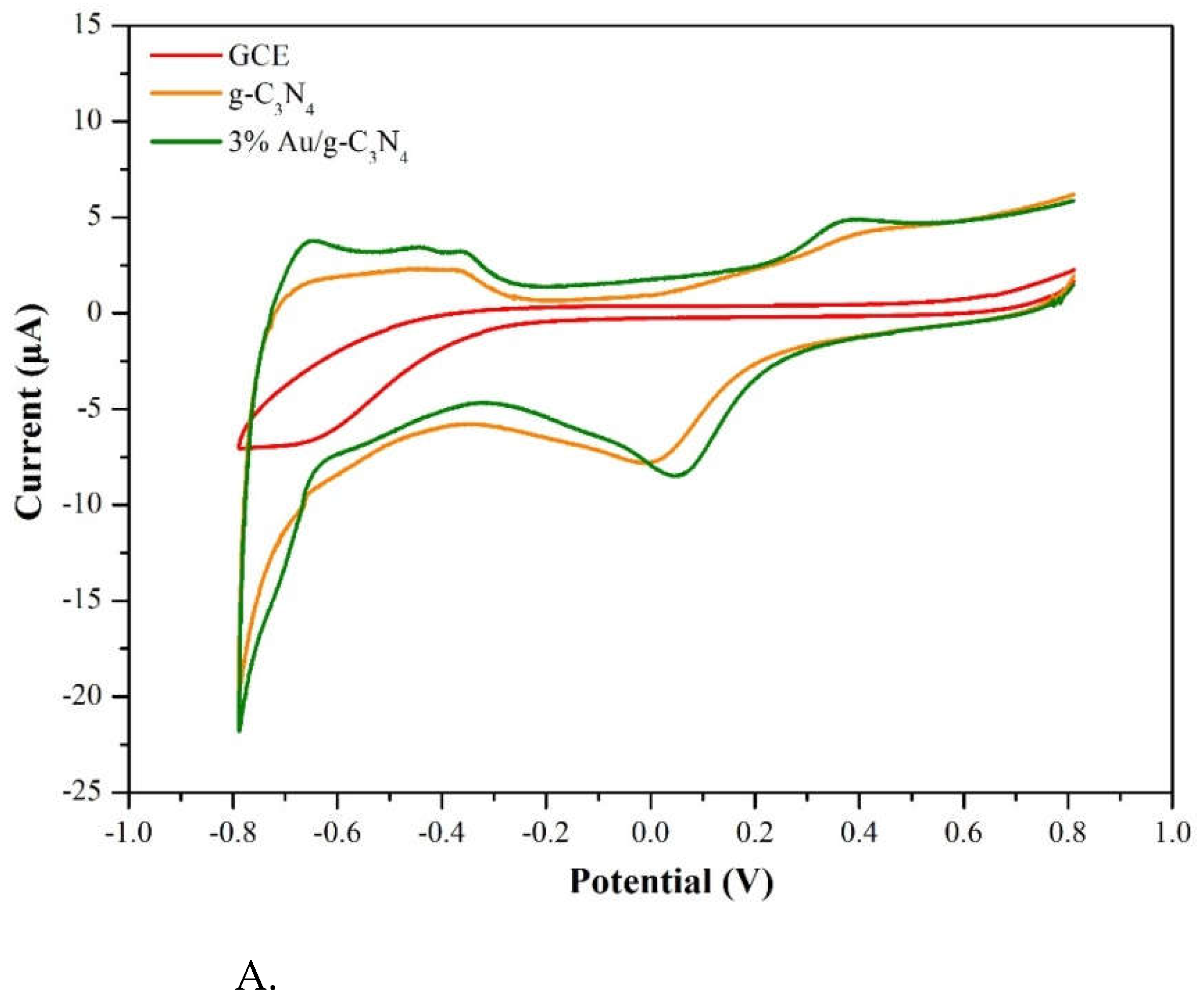
3.2.2. Various kind materials of sensing properties
Figure 11A presents CV plots which indicating the current signals of the GCE, g-C
3N
4 electrode and 3%Au/g–C
3N
4 electrode at a scan rate of 50 mV/s and pimobendan concentration of 55 μM at pH=7.0. No significant CV current signal was observed on the GCE, a reduction peak was observed at 0.0 V on g–C
3N
4 electrode in
Figure 11A. The 3%Au/g-C
3N
4 composite had the highest oxidation peak current at approximately 0.4 V and reduction peak at 0.05 V.
In
Figure 11B, the oxidation peaks were placed at around -0.40 V and 0.40 V which were identified as the oxidative products from pimobendan in
Figure 11B (a) and (b) [
17]. And reduction peak was found at 0.10 V which was identified as the pimobendan from the oxidative products [
17].
3.2.3. CV current versus concentration
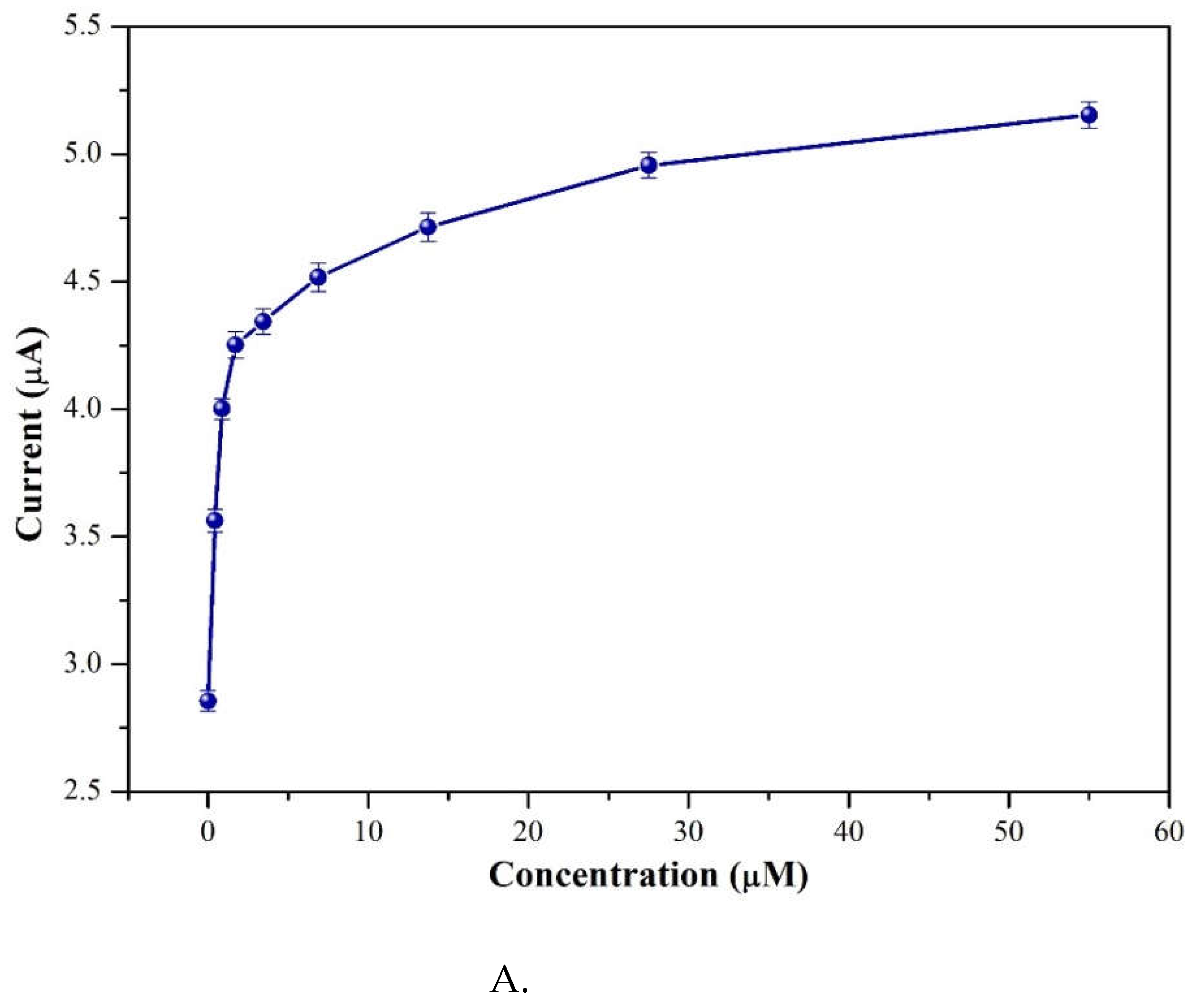
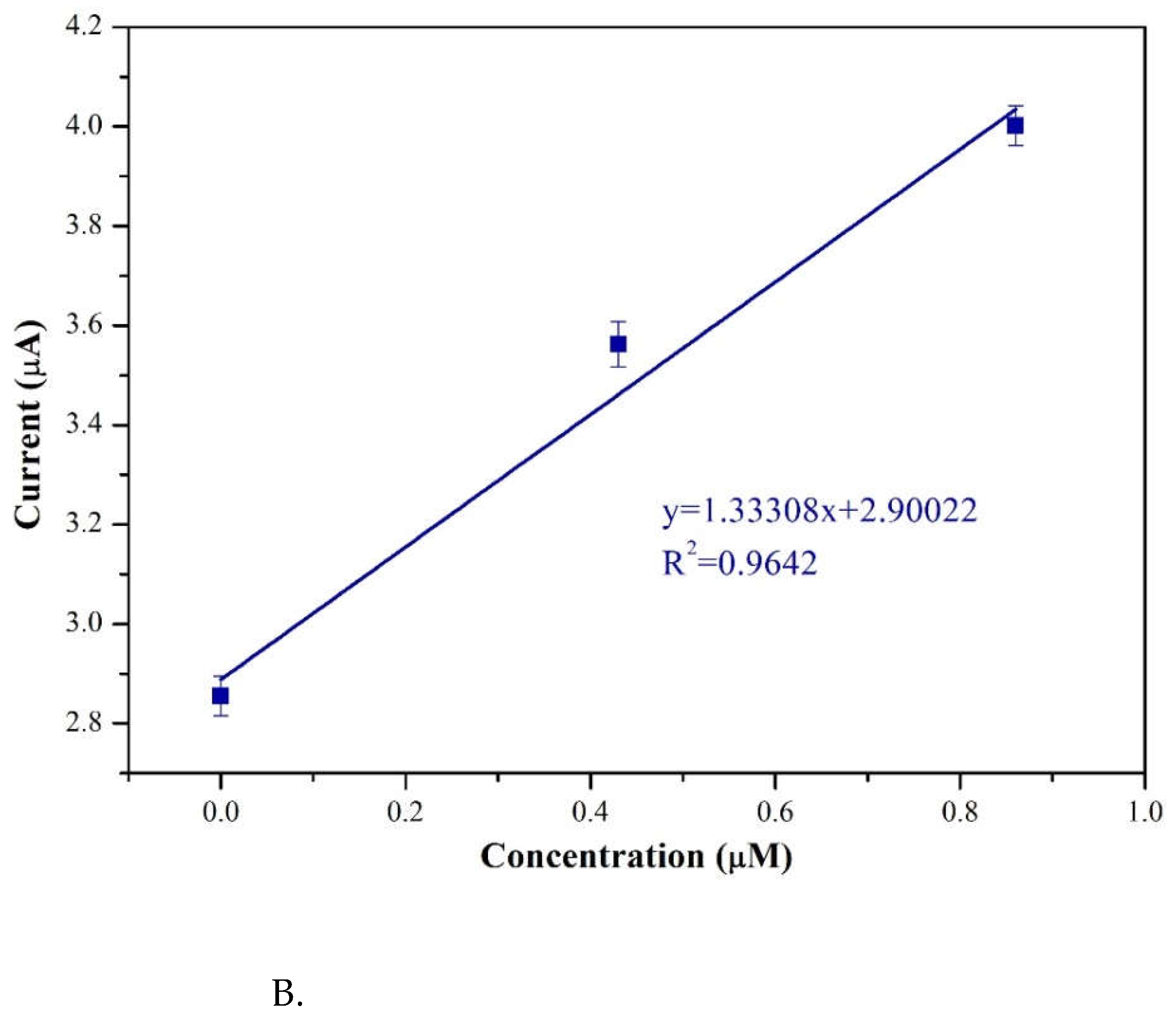
Figure 12A revealed the CV currents versus pimobendan concentrations (ranging from 0.0 to 55 μM). It showed the 3 %Au/g-C
3N
4 at higher concentrations (
Figure 12A) the reduction peak current (cathodic peak current) exhibited a higher current value. From low concentration of pimobendan (0.0–0.84 μM) indicating a linear relationship between the cathodic peak current and the pimobendan concentrations in Fig. 12B. It was obtained the R
2 as 0.9642, and the detection limit was calculated to 0.28 μM.
3.2.4. Interference effect
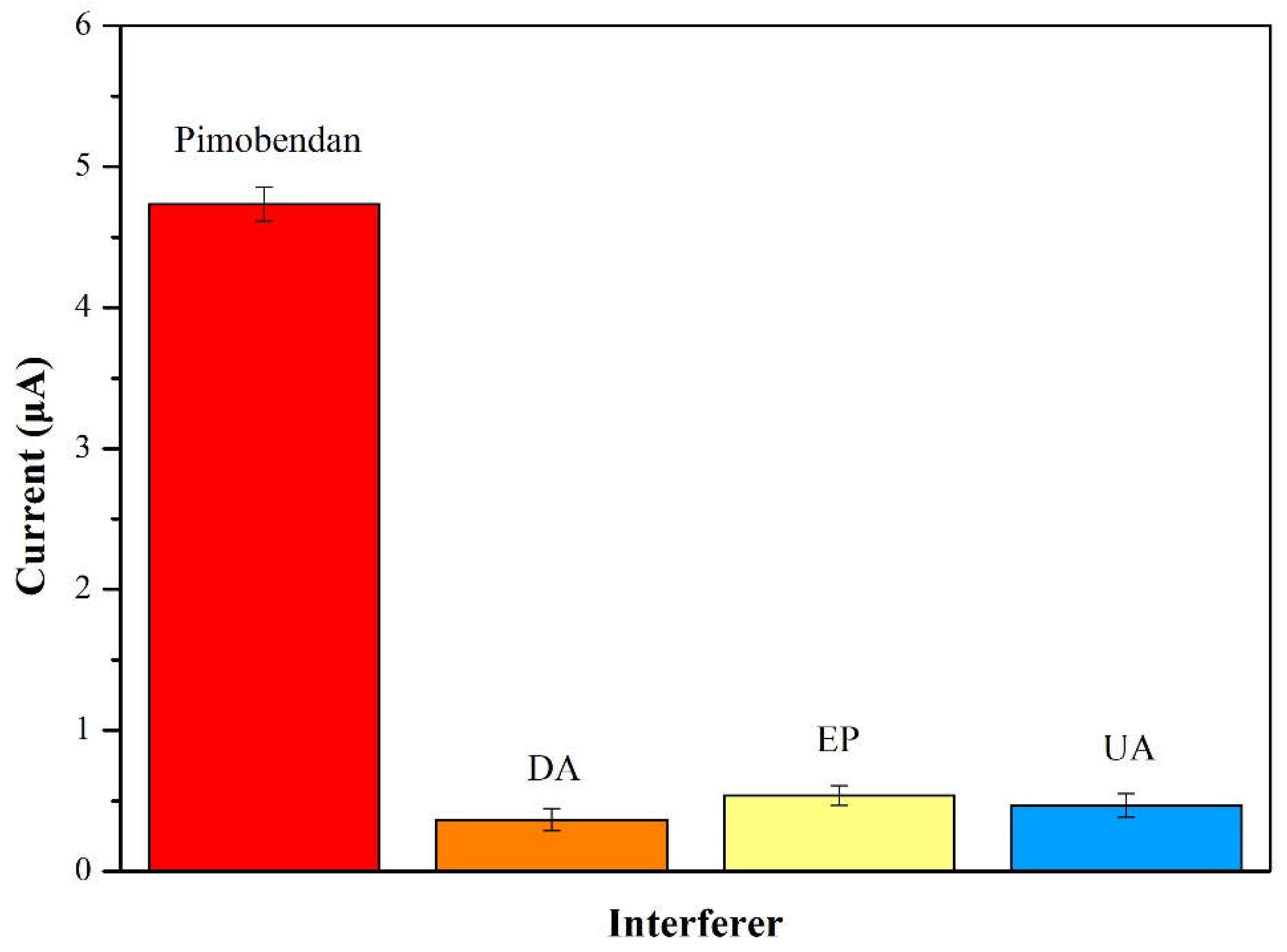
From the literature, the interferences of 55 μM dopamine (DA), 55 μM epinephrine (EP) and 55 μM uric acid (UA) are naturally coexist in physiological samples. Accordingly, the interference tests by plotting the CV reduction current versus some interferences materials of DA, EP and UA was displayed in
Figure 13. The CV current of pimobendan (4.7 µA) was higher than those of DA (0.38 µA), EP (0.51 µA) and UA (0.49 µA) at the same concentration in
Figure 13. It presented the sensing material of pimobendan detection was high selectivity and little interferences with DA, EP and UA.
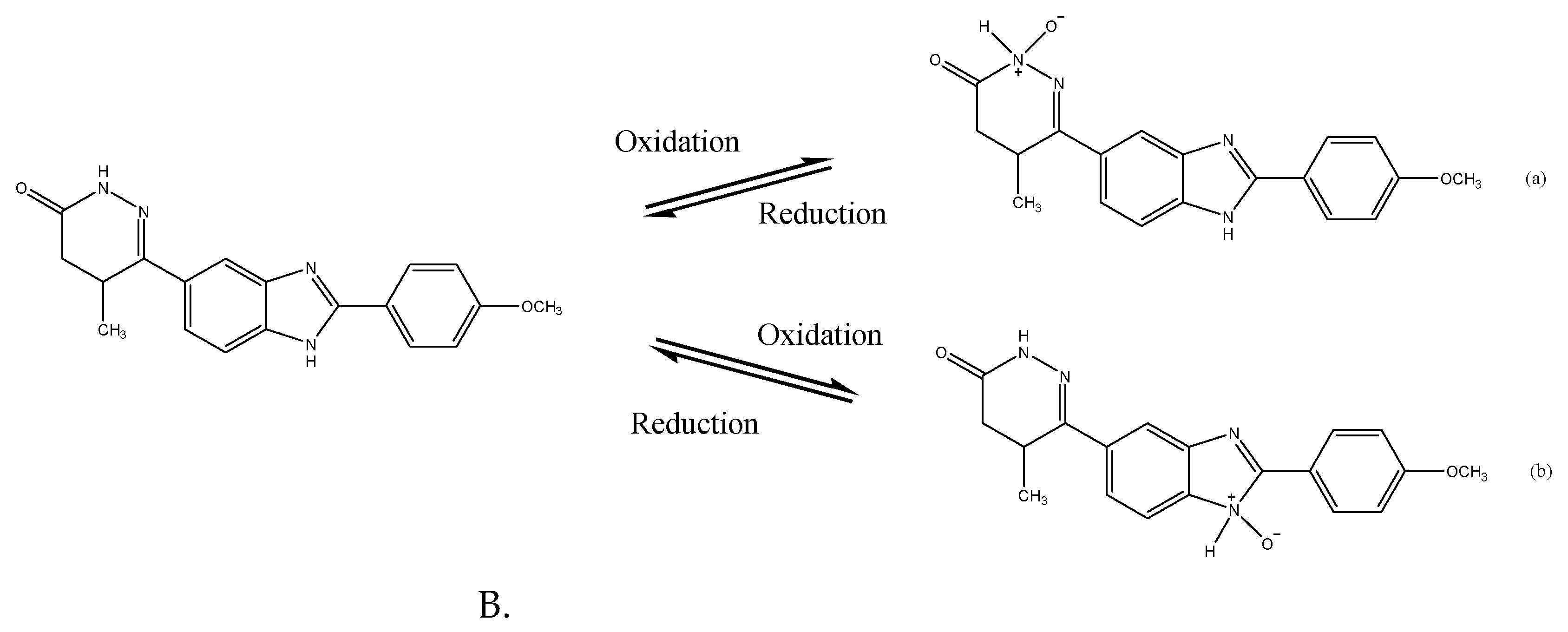
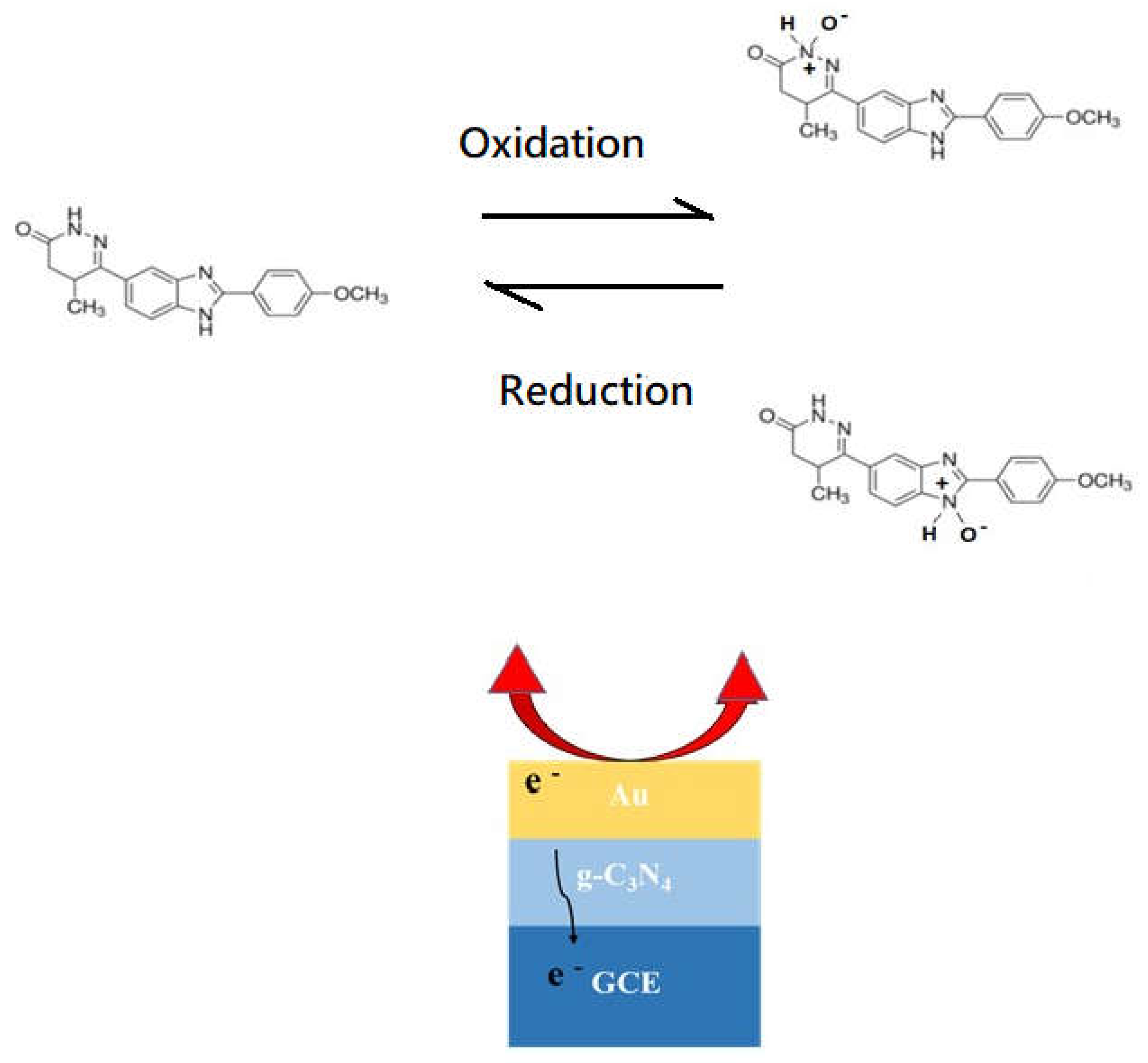
3.3. Sensing mechanism
The 3 %Au/g-C
3N
4 composite had the highest oxidation peak current at approximately 0.4 V and reduction peak at 0.05 V in
Figure 11A. The redox reaction mechanisms of the pimobendan sensing under the amide oxidation and amide oxide reduction reactions on the Au/g-C
3N
4 electrode are illustrated in
Figure 14 [
17,
18,
19,
20,
21]. The C
3N
4 showed a flake-like morphology of TEM images in
Figure 3 and
Figure 4. Graphitic carbon nitride (g-C
3N
4) is a two-dimensional organic semiconductor with a high specific surface area, additionally g-C
3N
4 owns high electrical conductivity for its sole delocalized conjugated structure [
22,
23,
24,
25,
26]. Au is applied in promotion of some oxidation-reduction reactions and electrochemical reaction system [
12,
16,
21]. The binding of pimobendan molecules on nano Au increased the electron transfer, yielding the modification in response current. The presence of Au, therefore, enhanced the detection of oxidation and reduction current sensitivity [
26,
27,
28,
29,
30]. The high sensing circuit of the electrode was feasibly (
Figure 11) because the Au nanoparticles on the surfaces of C
3N
4 efficiently collected the pimobendan oxidation–induced electrons during the electrocatalytic reaction and transferred them to the C
3N
4 composite and then to the surface of the GCE [
18]. Two possible products of pimobendan (amide) oxidation on the Au catalyst are presented in
Figure 14 [
17,
19,
20].
4. Conclusions
The composite 3% Au/g-C3N4 sensing electrode was fabricated for detection pimobendan. The Sensing materials characterization by XRD and TEM. The cyclic voltammetry was applied to detect the concentration and redox properties of pimobendan. It can be seen that when the voltage value is 0.05 V, and a reduction peak was presented and the pimobendan concentration from 0 to 55 μM has a relationship with the reductive currents. At low concentrations of pimobendan concentration from 0.0 to 0.8 μM, the linearity R2 = 0.9642 with a detection limit of 0.28 μM. Little interferences of DA, EP and UA were detected with pimobendan. A possible pimobendan sensing mechanism on 3% Au/g-C3N4 was proposed.
Author Contributions
Conceptualization, H.-N.L. and R.-J.W.; methodology, R.-J.W.; software, X.-J.C.; validation, X.-J.C.; formal analysis, X.-J.C.and H.-J.Z; investigation, R.-J.W.; data curation, X.-J.C.; writing—original draft preparation, R.-J.W.; writing—review and editing, H-N.L. and R.-J.W.; supervision, R.-J.W.; project administration, R.-J.W.; funding acquisition, R.-J.W. All authors have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science and Technology council (Grant No.: NSTC 111-2113-M-126-003), Taiwan, R.O.C, for funding this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors are thankful to the National Science and Technology council (Grant No.: NSTC 111-2113-M-126-003), Taiwan, R.O.C, for funding this study. We are also thankful Prof. Hong-jian Zhao for her kind help for this research work.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| XRD |
X-ray diffraction analysis |
| TEM |
Transmission electron Microscope |
| CV |
cyclic voltammetry |
References
- Mathieu Magnin, Jeanne Marie Bonnet-Garin, Chiara Laurenza, Caroline Didier, Morgane Gavet, Alexandra Nectoux, Bernard Allaouchiche, St´ephane Junot, Evaluation of pimobendan effect on sublingual microcirculation in an experimental pharmacology induced hypotension porcine model, Research in Veterinary Science 148 (2022) 7–14. [CrossRef]
- L.V. Kost, T.M. L.V. Kost, T.M. Glaus, A. Diana, M. Baron Toaldo, Effect of a single dose of pimobendan on right ventricular and right atrial function in 11 healthy cats, Journal of Veterinary Cardiology 37 (2021) 52-61. [CrossRef]
- N. Takeda, Y Hayashi, T Arino, A Takeda, K Noma, Effect of pimobendan in patients with chronic heart failure, Exp Clin Cardiol. 2001 Winter; 6(4): 195–199. PMCID: PMC2858999.
- Hiroaki Kawano, Shuji Arakawa, Osami Satoh, Yuji Matsumoto, Motonobu Hayano, Daisuke Nakatomi, Toshihiko Yamasa, Koji Maemura, Effect of pimobendan in addition to standard therapy for heart failure on prevention of readmission in elderly patients with severe chronic heart failure, Geriatr Gerontol Int 2014;14:109–114. [CrossRef]
- Jiwoong Her, Kendon W. Kuo, Randolph L. Winter, Crisanta Cruz-Espindola, Lenore M. Bacek, Dawn M. Boothe, Pharmacokinetics of Pimobendan and Its Metabolite O-Desmethyl-Pimobendan Following Rectal Administration to Healthy Dogs, Front Vet Sci. 7 (2020) 423. [CrossRef]
- Nakkawee Saengklub, Tussapon Boonyarattanasoonthorn, Anusak Kijtawornrat, Doungdaw Chantasart, Preliminary Bioequivalence of an Oral Pimobendan Solution Formulation with Reference Solution Formulation in Beagle Dogs. Vet. Sci. 2022, 9, 141. [CrossRef] [PubMed]
- Jitong Yan, Yanyan Liu, Runan Wang, Meirong Xia, Jing Wang, Faming Gao, Yongfu Tang, Bamboo-like carbonitride nanotubes with multi-type active sites for oxygen reduction reaction in both alkaline and acid mediums, International Journal of Hydrogen Energy 47 (2022) 7949-7960. [CrossRef]
- Dongyan Tian, Jie Wang, Qiandong Zhuang, Songmei Wu, Yu Yu, Kejian Ding, An electrochemiluminescence biosensor based on Graphitic carbon nitride luminescence quenching for detection of AFB1, Food Chemistry 404 (2023) 134183. [CrossRef]
- Fangmu Qua, Magdalena Graczyk-Zajac, Dragoljub Vrankovic, Nan Chai, Zhaoju Yu, Ralf Riedel, Effect of morphology of C-rich silicon carbonitride ceramic on electrochemical properties of sulfur cathode for Li-S battery, Electrochimica Acta 384 (2021) 138265. [CrossRef]
- Javad Safaei, Nurul Aida Mohamed, Mohamad Firdaus Mohamad Noh, Mohd Fairuz Soh, Norasikin Ahmad Ludin, Mohd Adib Ibrahim, Wan Nor Roslam Wan Isahak, Mohd Asri Mat Teridi, Graphitic carbon nitride (g-C3N4) electrodes for energy conversion and storage: a review on photoelectrochemical water splitting, solar cells and supercapacitors. J. Mater. Chem. A, 2018, 6, 22346–22380. [CrossRef]
- Haoye Wang, Aijuan Xie, Shuji Li, Jiajun Wang, Kaixuan Chen, Zilong Su, Ningning Song, Shiping Luo, Three-dimensional g-C3N4/MWNTs/GO hybrid electrode as electrochemical sensor for simultaneous determination of ascorbic acid, dopamine and uric acid, Analytica Chimica Acta 1211 (2022) 339907. [CrossRef]
- Samarjeet Siwal, Nishu Devi, Venkata K. Perla, Sarit K. Ghosh, Kaushik Mallick, Promotional role of gold in electrochemical methanol oxidation, Catalysis, Structure & Reactivity 5 (2019) 1-9. [CrossRef]
- Wee-Jun Ong, Lling-Lling Tan, Yun Hau Ng, Siek-Ting Yong, Siang-Piao Chai, Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability, Chem Rev. 116 (2016) 7159–7329. https://pubs.acs.org/doi/10.1021/acs.chemrev.6b00075.
- Federica Fina, Samantha K. Callear, George M. Carins, John T. S. Irvine, Structural Investigation of Graphitic Carbon Nitride via XRD and Neutron Diffraction, Chem. Mater. 27 (2015) 2612–2618. https://pubs.acs.org/doi/10.1021/acs.chemmater.5b00411.
- PanelJiuqing Wen, Jun Xie, Xiaobo Chen, Xin Li, A review on g-C3N4-based photocatalysts, Applied Surface Science 391 (2017) 72-123. [CrossRef]
- Zhen Zhu, Hsiang-Ning Luk, Yu-Shih Liu, Ren-Jang Wu, Ming-Hung Chung, Xu-Jia Chang, Preparation of Bimetallic Au-Pd/MWCNTs Electrode for Detection of Dopamine, Minerals 12 (2022) 1145. [CrossRef]
- Zhen Zhu, Hsiang-Ning Luk, Yi-Min Huang, Yu-Cheng Zhang, Xu-Jia Chang, Ren-Jang Wu, Silver/graphene–polypyrrole composite for levosimendan detection, Journal of Chinese Chemical Society 70 (2023) 928-937. [CrossRef]
- Ye-Cheng Li, Xiao-Song Li, Bin Zhu, Ai-Min Zhu, Boosting low-temperature water gas shift reaction over Au/TiO2 nanocatalyst activated by oxygen plasma, Chemical Engineering Journal 430 (2021) 133013. [CrossRef]
- R. Garg, S. Mondal, L. Sahoo, C. P. Vinod, U. K. Gautam, Nanocrystalline Ag3PO4 for Sunlight- and Ambient Air-Driven Oxidation of Amines: High Photocatalytic Efficiency and a Facile Catalyst Regeneration Strategy. ACS Appl. Mater. Interfaces 2020, 12, 29324. [CrossRef]
- D. Bernier, U. K. D. Bernier, U. K. Wefelscheid, S. Woodward, Organic Preparations and Procedures International 41 (2009) 175. [CrossRef]
- Ting Xiao, Jianshe Huang, Dewen Wang, Tian Meng, Xiurong Yang, Au and Au-Based nanomaterials: Synthesis and recent progress in electrochemical sensor applications, Talanta 206 (2020) 120210. [CrossRef]
- Atefeh Nasri, Babak Jaleh, Milad Daneshnazar, Rajender S. Varma, Sensing Properties of g-C3N4/Au Nanocomposite for Organic Vapor Detection, Biosensors 13 (2023) 315. [CrossRef]
- Furong Chen, Layue Bao, Ying Zhang, Ruili Wang, Jinghai Liu, Wenfeng Hai, Yushuang Liu, NiCoP/g-C3N4 Nanocomposites-Based Electrochemical Immunosensor for Sensitive Detection of Procalcitonin, Sensors 23 (2023), 4348. [CrossRef]
- Mehrab Pourmadadi, Maryam Rajabzadeh-Khosroshahi, Fatemeh Saeidi Tabar, Narges Ajalli, Amirmasoud Samadi, Mahsa Yazdani, Fatemeh Yazdian, Abbas Rahdar, Ana M. Díez-Pascual, Two-Dimensional Graphitic Carbon Nitride (g-C3N4) Nanosheets and Their Derivatives for Diagnosis and Detection Applications, J. Funct. Biomater. 13 (2022) 204. [CrossRef]
- Shaolin Zhang, Nguyen Thuy Hang, Zhijun Zhang, Hongyan Yue, Woochul Yang, Preparation of g-C3N4/Graphene Composite for Detecting NO2 at Room Temperature, Nanomaterials 7 (2017) 12. [CrossRef]
- Sanjida Yeasmin, Bo Wu, Ye Liu, Ahasan Ullah, Li-Jing Cheng, Nano gold-doped molecularly imprinted electrochemical sensor for rapid and ultrasensitive cortisol detection, Biosensors and Bioelectronics 206 (2022) 114142. [CrossRef]
- Saied Jafari, Mohammad Dehghani, Navid Nasirizadeh, Mostafa Azimzadeh, An azithromycin electrochemical sensor based on an aniline MIP film electropolymerized on a gold nano urchins/graphene oxide modified glassy carbon electrode, Journal of Electroanalytical Chemistry 829 (2018) 27-34. [CrossRef]
- Mari Elancheziyan, Sellappan Senthilkumar, Redox-active gold nanoparticle-encapsulated poly(amidoamine) dendrimer for electrochemical sensing of 4-aminophenol, Journal of Molecular Liquids 325 (2021) 115131. [CrossRef]
- Yongying Zhou, Jin Zhao, Shenghua Li, Minjie Guo, Zhi Fan, An electrochemical sensor for the detection of p-nitrophenol based on a cyclodextrin-decorated gold nanoparticle–mesoporous carbon hybrid, Analyst 144 (2019) 4400-4406. [CrossRef]
- Jinmei Luo, Shuhuai Li, Yuwei Wu, Chaohai Pang, Xionghui Ma, Mingyue Wang, Chenghui Zhang, Xu Zhi, Bei Li, Electrochemical sensor for imidacloprid detection based on graphene oxide/gold nano/β-cyclodextrin multiple amplification strategy, Microchemical Journal 183 (2022) 107979. [CrossRef]
Figure 1.
(a) Chemical structure of pimobendan (b) The electrochemical detection system.
Figure 1.
(a) Chemical structure of pimobendan (b) The electrochemical detection system.
Figure 2.
XRD patterns of g-C3N4, 1% Au/g-C3N4, 3% Au/g-C3N4 and 5% Au/g-C3N4.
Figure 2.
XRD patterns of g-C3N4, 1% Au/g-C3N4, 3% Au/g-C3N4 and 5% Au/g-C3N4.
Figure 3.
TEM images of (a) g-C3N4 scale bar 1000 nm; (b) g-C3N4 scale bar 500 nm.
Figure 3.
TEM images of (a) g-C3N4 scale bar 1000 nm; (b) g-C3N4 scale bar 500 nm.
Figure 4.
TEM images of (a) 3% Au/g-C3N4 scale bar 500 nm (b) 3% Au/g-C3N4 scale bar 10 nm.
Figure 4.
TEM images of (a) 3% Au/g-C3N4 scale bar 500 nm (b) 3% Au/g-C3N4 scale bar 10 nm.
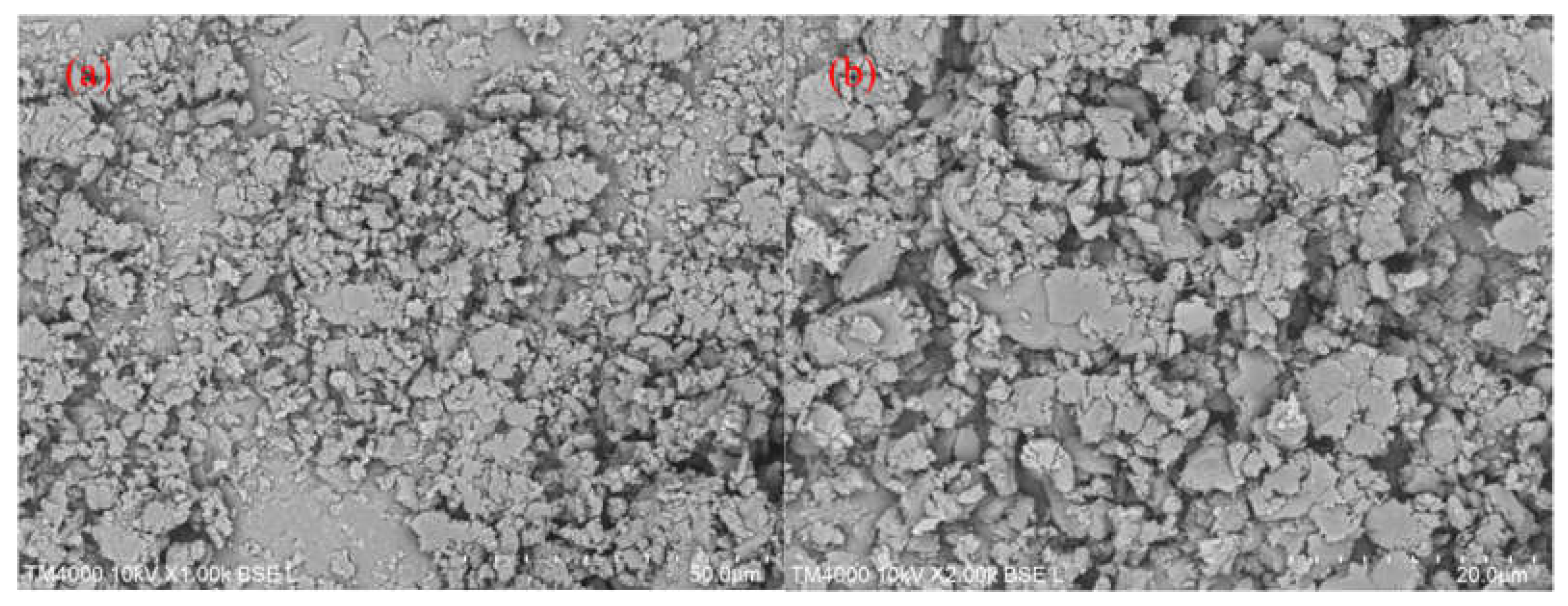
Figure 5.
SEM images of (a) g-C3N4 scale bar 50 μm (b) g-C3N4 scale bar 20 μm.
Figure 5.
SEM images of (a) g-C3N4 scale bar 50 μm (b) g-C3N4 scale bar 20 μm.
Figure 6.
EDS and elements mapping images of g-C3N4.
Figure 6.
EDS and elements mapping images of g-C3N4.
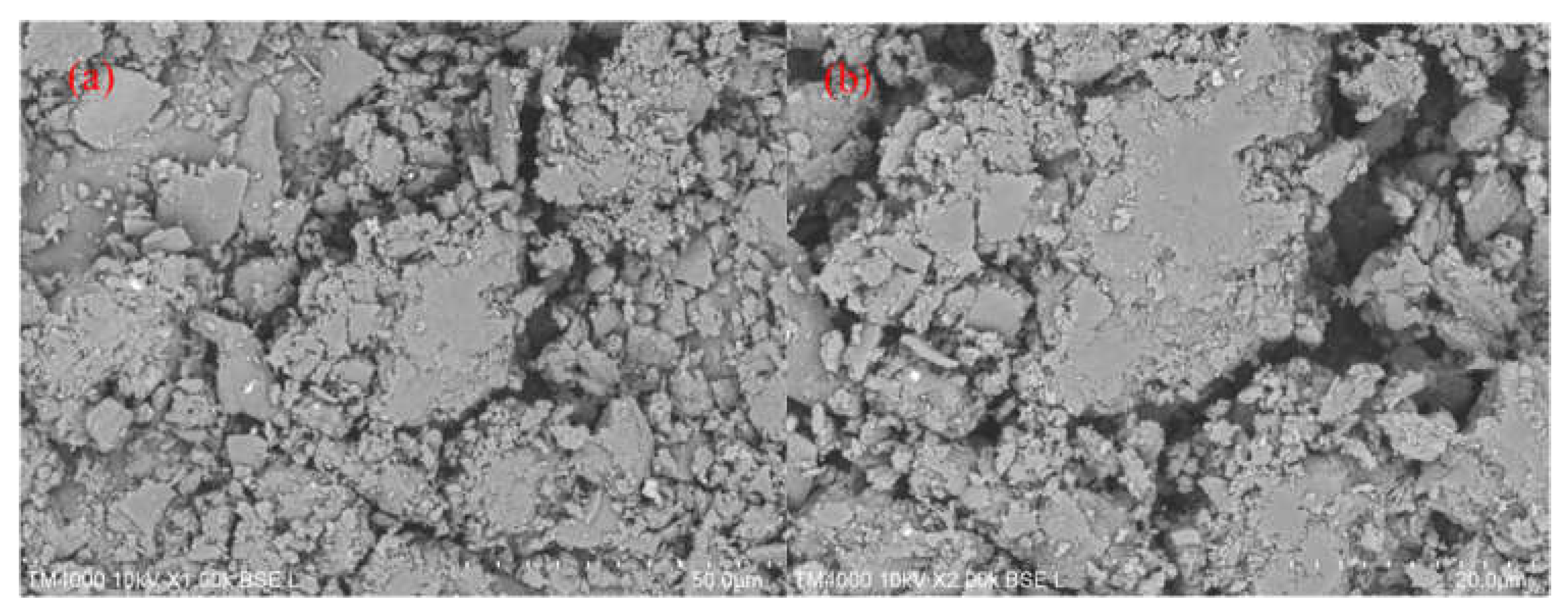
Figure 7.
SEM images of (a) 3% Au/g-C3N4 scale bar 50 μm (b) 3% Au/g-C3N4 scale bar 20 μm.
Figure 7.
SEM images of (a) 3% Au/g-C3N4 scale bar 50 μm (b) 3% Au/g-C3N4 scale bar 20 μm.
Figure 8.
EDS and elements mapping images of 3% Au/g-C3N4.
Figure 8.
EDS and elements mapping images of 3% Au/g-C3N4.
Figure 9.
FTIR spectra of g-C3N4, 1% Au/g-C3N4, 3% Au/g-C3N4 and 5% Au/g-C3N4.
Figure 9.
FTIR spectra of g-C3N4, 1% Au/g-C3N4, 3% Au/g-C3N4 and 5% Au/g-C3N4.
Figure 10.
A. Scan rate of 3%Au/g-C3N4 at (a) 10 (b) 20 (c) 25 (d) 30 (e) 40 (f) 50 (g) 60 (h) 80 (i) 100 (j) 120 (k) 140 (l) 160 (m) 180 (n) 200 mVs-1, B. The scan rate was presented a linear relationship with the Ipa at 0.4 V and Ipc at 0.05 V, C. The square root of scan rate exhibited a linear relationship with the Ipa at 0.4 V and Ipc at 0.05 V.
Figure 10.
A. Scan rate of 3%Au/g-C3N4 at (a) 10 (b) 20 (c) 25 (d) 30 (e) 40 (f) 50 (g) 60 (h) 80 (i) 100 (j) 120 (k) 140 (l) 160 (m) 180 (n) 200 mVs-1, B. The scan rate was presented a linear relationship with the Ipa at 0.4 V and Ipc at 0.05 V, C. The square root of scan rate exhibited a linear relationship with the Ipa at 0.4 V and Ipc at 0.05 V.
Figure 11.
A. CV plots of the GCE, g-C3N4 electrode and 3 %Au/g–C3N4 electrode at a scan rate of 50 mV/s and pimobendan concentration of 55 μM at pH=7.0. B. The possible oxidation and reduction reactions of pimobendan.
Figure 11.
A. CV plots of the GCE, g-C3N4 electrode and 3 %Au/g–C3N4 electrode at a scan rate of 50 mV/s and pimobendan concentration of 55 μM at pH=7.0. B. The possible oxidation and reduction reactions of pimobendan.
Figure 12.
A. CV currents versus pimobendan concentrations ranging from 0.0 to 55 μM on 3%Au/g-C3N4 at a scan rate of 50 mV/s and pH=7.0, B. A linear relationship between the cathodic peak current and the pimobendan concentrations.
Figure 12.
A. CV currents versus pimobendan concentrations ranging from 0.0 to 55 μM on 3%Au/g-C3N4 at a scan rate of 50 mV/s and pH=7.0, B. A linear relationship between the cathodic peak current and the pimobendan concentrations.
Figure 13.
The interference effect for 55 μM pimobendan of 55 μM dopamine (DA), 55 μM epinephrine (EP) and 55 μM uric acid (UA) at a scan rate of 50 mV/s and pH=7.0.
Figure 13.
The interference effect for 55 μM pimobendan of 55 μM dopamine (DA), 55 μM epinephrine (EP) and 55 μM uric acid (UA) at a scan rate of 50 mV/s and pH=7.0.
Figure 14.
Sensing mechanism of pimobendan on Au/g-C3N4 modified electrode.
Figure 14.
Sensing mechanism of pimobendan on Au/g-C3N4 modified electrode.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).