1. Introduction
We have entered the Anthropocene - an era where humans are a dominant geological force - and at the same time we have entered an urban era. More than half of humanity now lives in cities, and by 2030 this proportion will reach 60% [
1]. In other words, in just over two decades, from 2010 to 2030, an additional 1.5 billion people will be added to the urban population. Creating healthy, livable urban living spaces for so many additional people will be one of the major challenges of our time. The quality of the urban environment-both its built and natural components-will determine the quality of life for an estimated total of five billion people, existing and future by 2030 [
2]. In the same vein, scientists have begun by questioning the place of the urban environment and its spaces with a natural character (known as urban vegetated spaces) in the protection, enhancement of biodiversity and optimization of ecosystem services [
3].
In Benin, a West African country, the urbanization rate increased from 11% in 1960 to 40% in 1990, and then from 42% in 2005 to 44% in 2015 [
4]. Moreover, in future projections, more than half of Benin's population will live in cities by 2025, with an estimated urban population rate of 56.2 %. Urban anthropogenic activities have enormous impacts on biodiversity and ecosystem services [
5,
6]. While balancing the need for urban growth with biological conservation at a reasonable threshold remains a concern in an approach to perpetually improving the quality of the living environment in high concentration areas [
7,
8,
9]. In urban areas with current environment issues like climate change and biodiversity loss, trees contribute to air purification through carbon dioxide sequestration, microclimate creation, natural balance, species restoration, and land attractiveness [
10,
11]. These benefits attribute social, economic, and environmental functions to trees [
12,
13], the importance of which is reflected in ecosystem services. Although urban floral diversity is essential for providing ecosystem services and enhancing human well-being, it remains threatened by the anthropogenic and environmental consequences of urbanization [
14,
15,
33].
According to [
9,
16], it is evident that urban nature planning, management, and development can be combined with biodiversity conservation and preservation objectives for the perpetual improvement of the living environment in a coherent urban territory. The literature reveals that little research has been conducted on the composition and structure of urban forests in urban centers in West Africa, particularly in cities with high population growth [
17]. In Benin, the work of [
18] focused on the study of species diversity in green spaces in the city of Cotonou, that of [
19] addressed the effect of the avenue trees of the Boulevard of Missèbo-Zongo on the local microclimate of the city of Cotonou while [
20] assessed the diversity and structure of woody vegetation in the city of Malanville in North Benin. Although research has been conducted based on species diversity in the city [
21,
42], the extent of distribution and prioritization of ecologically important species in urban land use units is lacking. [
22] highlighted the lack of urban biodiversity data in tropical cities and the immediate need to undertake research within the current frontiers of urban ecology. The ecological information deficit could be one of the major hindrances to the design of national urban forestry and environmental improvement program, and it could also hinder the optimization of ecosystem services needed to circumvent and adapt to the effects of climate change in a sustainable urban development context. Therefore, the present work aims to fill these knowledge gaps on the diversity of the different land use units in plant species and those with high ecological importance to guide the green development policies of future African and Beninese cities.
2. Methodology
2.1. Study Area
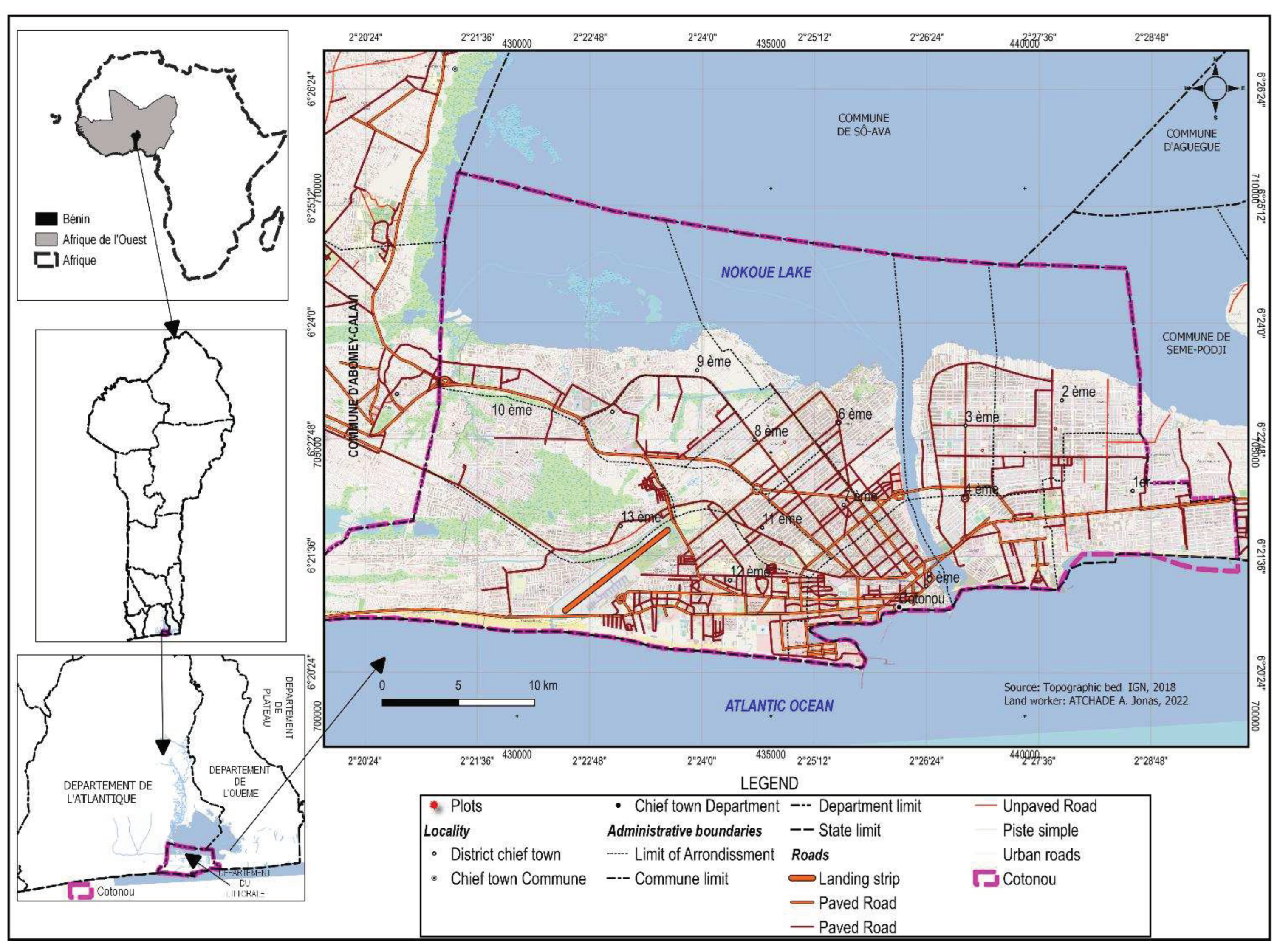
The city of Cotonou is in the South of the Republic of Benin between 6°20' and 6°23' North latitude and 2°22' and 2°30' East longitude. It is bordered to the north by Lake Nokoué, to the west by the Commune of Abomey-Calavi, to the east by the Commune of Sèmè-Kpodji and to the south by the Atlantic Ocean (
Figure 1). The city covers an area of 79 km
2 [
23].
Administratively, the city of Cotonou comprises, 13 arrondissements subdivided into 144 neighborhoods. Its population is 679,012 inhabitants according to the general population and housing census [
4]. The climate is humid subequatorial, with two dry seasons (mid-November to mid-March and mid-July to August) and two rainy seasons (mid-March to mid-July and September to mid-November). The average annual rainfall is 1,200 mm, with 700-800 mm in the long rainy season and 400-500 mm in the short rainy season [
24]. The average temperature in the coastal zone is 26.8°C with extremes of 36.6°C and 16.5°C. The average relative humidity in Cotonou is 84%. The hydrographic network consists of Lake Nokoué and the Atlantic Ocean. The types of soil encountered include sandy soils, ferruginous soils, and hydromorphic soils [
18]. All these characteristics favor plant development. The current urban matrix of the city offers a wide range of types of artificial and natural environments and vegetation ranging from totally unvegetated environments in the city centers to wooded private parks in residential areas to spontaneous vegetation in abandoned estates in the neighborhoods to fallows, plantations, ponds, marshes, and swamps in the peripheral areas of the city [
25].
2.2. Data Collection and Analysis
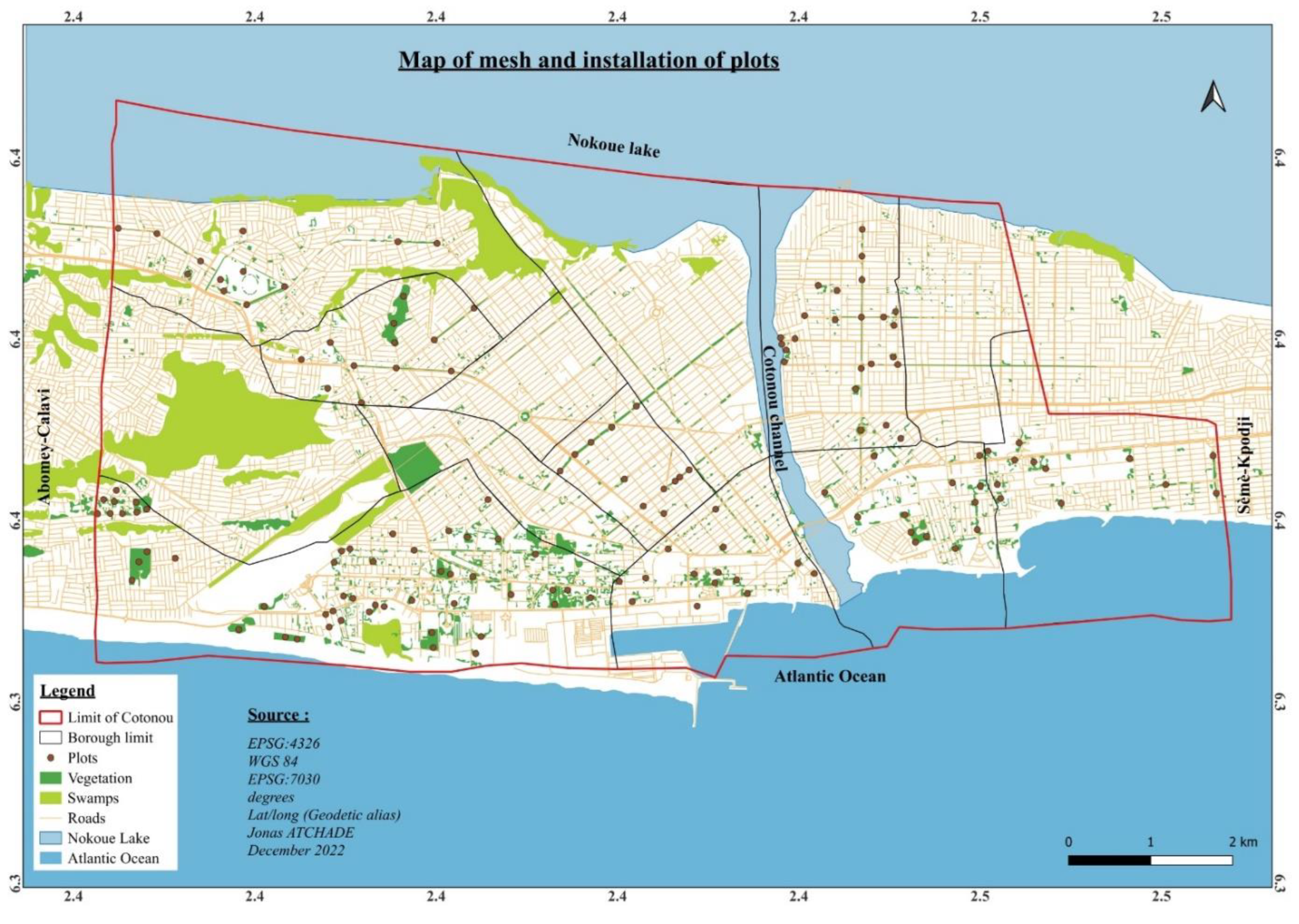
After having mapped the city of Cotonou, we randomly selected 8 of the 13 districts in the city to cover all the localities (central and peripheral boroughs) as recommended by [
21,
31].
Based on [
26] definition of urban forest, the study area was stratified into six land use types that correspond to urban forests in Cotonou: (1) commercial areas including markets, stores, boutiques, restaurants, and vehicle repair shops; (2) roads covering main streets and boulevards; (3) residential areas covering houses, mosques, and churches; (4) schools covering private and public training and learning institutions such as elementary school, secondary schools, universities, schools and vocational training centers; (5) administrative areas such as public and private utilities; (6) urban green spaces areas consisting of urban agricultural plots, agroforestry systems and wetlands, irrigated agricultural land and botanical gardens. In view of the type of land use, rectangular and square plots of 25 m x 100 m and 50 m x 50 m were set up (
Figure 2). In each of the plots, a systematic inventory of the trees was made on the one hand. On the other hand, dendrometric parameters such as diameter at breast height (Dbh) and height, as well as the number of stems of each tree were evaluated. Note that only trees with Dhp ≥ 10 cm were visited for the data collection.
2.3. Data Processing and Analysis
Taxonomic diversity
Species whose scientific names were not known were identified using the flora of Benin [
27]. Diversity parameters such as species richness (S), Shannon diversity index (H, Equation (1)) and Pielou diversity index (S, Equation (2)) were globally calculated for each type of land use. In addition, the number of families and genera were counted. The relative frequencies of occurrence of species were calculated to highlight the most represented families and genera.
Specific richness (R): This is the number of species present in each area of the city of Cotonou. In this study, it is represented on each land use unit in the city.
Shannon diversity index (H)
The Shannon diversity index varies both in terms of the number of species present and in terms of the relative proportion of individuals of various species. It is expressed in bits. It is all the higher that a great number of species participates in the recovery. That is to say, the higher its value, the greater the diversity. It is calculated according to the formula.
Pi (between 0 and 1): relative proportion of the number of individuals of a species i in the set of individuals of all species involved; Pi = ni /∑ ni; with ni: number of individuals of species i and ∑ ni: set of individuals of all species.
Pielou's equitability (E)
Pielou's equitability index (E) is the measure of the degree of diversity achieved by a plant grouping. It reflects the way individuals are distributed across plant groups. It varies between 0 and 1. The Pielou equitability index E < 0.5, when there is a phenomenon of dominance (the individuals belong to a single species). Pielou's equitability index E ≥ 0.5, when the distribution of individuals is homogeneous and results in an equitable distribution. Species are evenly distributed, and individuals have the same recovery.
Equitability reflects the degree of diversity achieved by a stand, and corresponds to the ratio between the effective diversity (H) and the theoretical maximum diversity (Hmax)
In Equation (2), Hmax is the theoretical maximum Shannon value in each occupancy type. This value is maximum when species are identically abundant in each occupancy type and minimum when one species or a small group of species dominates in each occupancy type. The ''vegan'' package [
28] was used to calculate the diversity indices in R software.
Study of species with high ecological importance in the city of Cotonou
The Importance Value Index (IVI) of each land use type is calculated (Equation (3)). This index is used to determine the dominant species of each type of land use using (i) relative frequency (ii) relative density; and (iii) relative dominance of basal area, (
Table 1). The importance index value is between 0 and 300. Species with an IVI value greater than or equal to 10 were considered ecologically important species in the city of study. Frequency is calculated as the number of plots where a species is observed divided by the total number of plots surveyed. Relative frequency is calculated as frequency divided by the sum of the frequencies of all species, multiplied by 100 (to obtain a percentage). Density is calculated as the total number of individuals of a species. Relative density is calculated as density divided by the sum of the densities of all species, multiplied by 100 (to obtain a percentage). Dominance is calculated as the total basal area of a species. Relative dominance is calculated by dividing dominance by the sum of the dominances of all species, multiplied by 100 (to obtain a percentage) (
Table 1). For a species α, the IVI is calculated as [
29] :
In Equation (3), RDα is the relative density of species α, RFα is the relative frequency of species α, and DoRα is the relative dominance of species α. These parameters are presented in
Table 3 with value calculated from
Table 1.
Statistical software
The data collected was first processed and formatted using Microsoft 365's Excel spreadsheet. To carry out the statistical analyses, the biodiveristy R package was used to calculate the various taxonomic diversity indices (Specific richness, Shannon diversity index, Pielou's equitability index). And then, statistical analyses were performed using the statistical software R (version 4.0.0, R Foundation for Statistical Computing, Vienna, Austria) [
30].
3. Results
3.1. Floristic Diversity in the Land Use Units in the City of Cotonou
A total of 1536 trees were visited and surveyed in 149 plots. Overall, 62 plant species of Dhp ≥ 10 cm belonging to 55 genera and 27 families were counted. The total number of species inventoried and the floristic diversity in the city of Cotonou varies according to the types of land use in the city (
Table 2). Analysis of Table II shows that in the city of Cotonou, residential areas recorded the highest number of plant species (S=39) from 37 genera and 20 families, while the lowest number of species is recorded in commercial areas (S=7) from 5 genera and 4 families. After residential areas (S=39); administrative areas (S=23) present the highest species richness followed by establishments (S=14), roads (S=11), green areas (S=8) and commercial areas (S=8). The values of the Shannon index and Piélou equitability varying from one land use unit to another and justify the floristic diversity and the distribution of individuals by land use units.
Overall, Shannon's diversity index (H=2.86 bits) and Piélou's equitability (Eq=0.69) indicate a significant floristic diversity throughout the city. Observation of the results of the analysis of variance of the diversity parameters reveals significant differences (
Table 2) between the city's land-use units in terms of Shannon's diversity index (P <0.001), in contrast to Piélou's equitability which remains statistically similar (P >0.5). From the comparative analysis, it appears that the highest Shannon diversity index value (H) is observed at the residential level (H=3.15 bits) while the lowest value is recorded at the green space level (H=1.58 bits) with some statistical significance (P<0.001) at the 5% threshold. However, there is no statistically significant difference between the Shannon diversity index values of the land use units such as residences (H=3.15 bits) and administrations (H=2.68bits). The Shannon index is statistically similar for commercial area (H=1.59bits), green space (H=1.58bits), roads (H=1.75bits), and establishment areas (H=2.07bits) and reflects low floristic diversity of these land use units compared to administrative (H=2.68bits) and residential areas (H=3.15bits) (
Table 2).
On the other hand,
Table 2 shows that Pielou's equitability remains statistically similar (P>0.5) between all land-use units. This shows that the distribution of species is regular in all land-use units in the city of Cotonou.
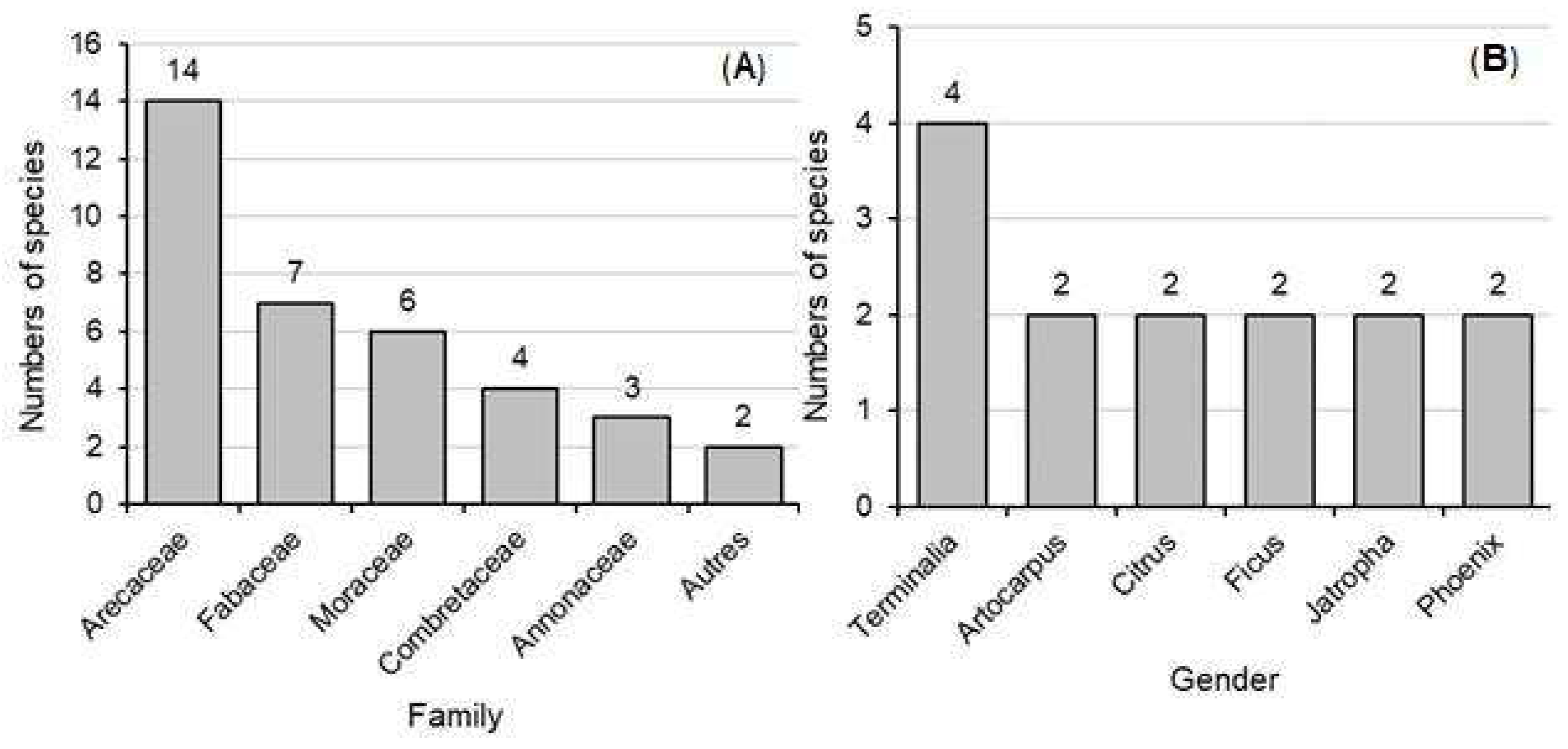
3.2. Most Represented Families and Genera in the Cotonou City
Figure 3 illustrates the species richness by family (A) and by genus (B) in the city of Cotonou. From the analysis of this figure, it appears that the Arecaceae are in the lead with 14 species, followed respectively by the Fabaceae with 7 species, the Moraceae with 6 species, the Combretaceae with 4 species and the Annonaceae with 3 species (
Figure 3A). As for the genera, those most represented are respectively the Terminalia with 4 species, followed by the Artocarpus, Citrus, Ficus, Jatropha, and Phoenix. These last genera have two species each (
Figure 3B).
For family, other includes the families of Anacardiaceae Euphorbiaceae Malvaceae Meliaceae Myrtaceae Rutaceae Sapotaceae.
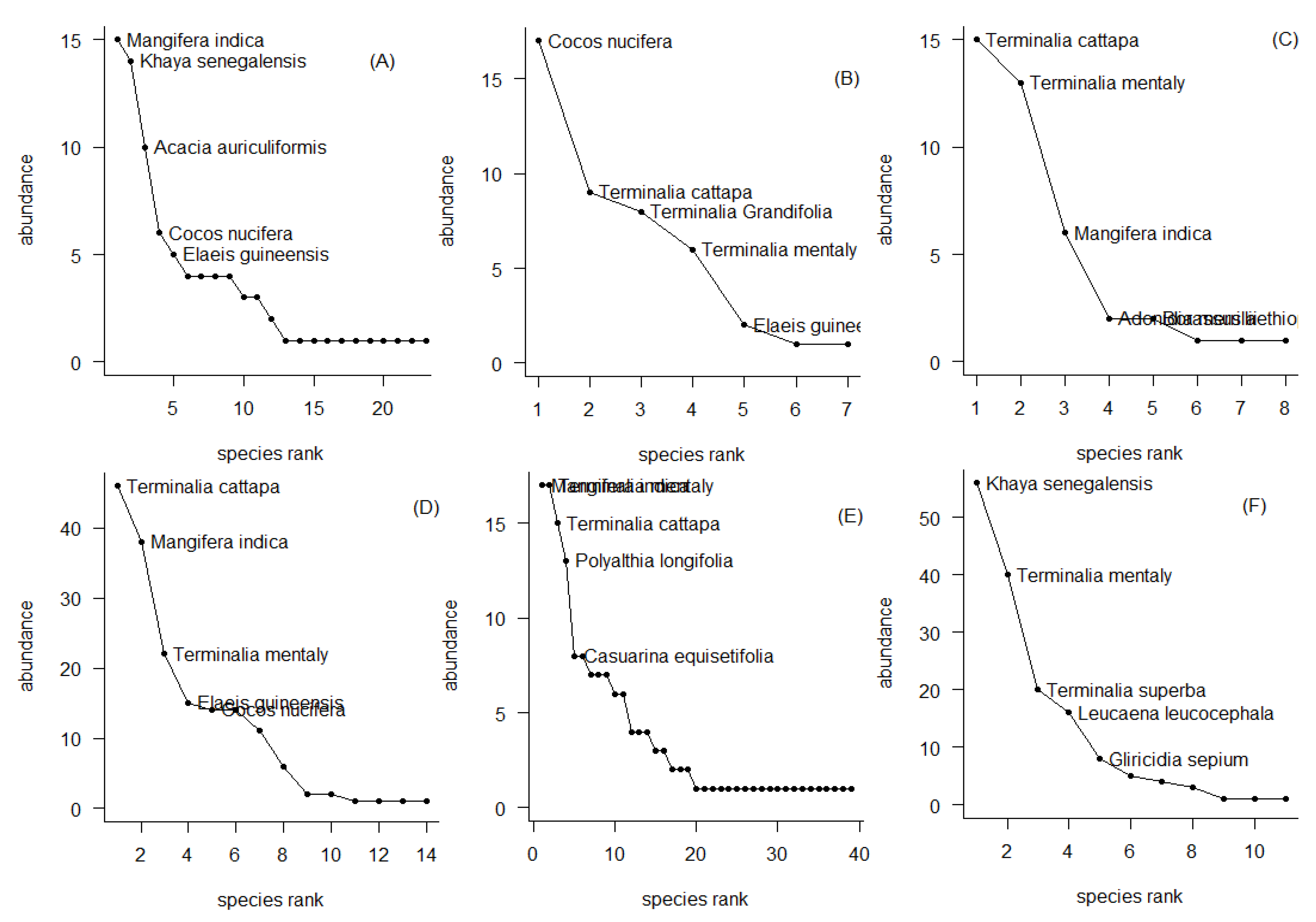
3.3. Top 5 Most Abundant Species in Land Use Units in the Cotonou City
The graphs in
Figure 4 highlight the top 5 species by land use unit. These graphs illustrate that the vegetation of all land use units in the city of Cotonou is strongly represented by a high frequency of exotic species.
The top 5 most abundant species in the administrative zones (A) of the city are Mangifera indica (15), Khaya senegalensis (about 14), Acacia auriculiformis (10), Cocos nucifera (6) and Elaeis guineensis (5). This compartment of the city represented by the public and private administration is taken over by species that are mostly edible and exotic. Khaya senegalensis (about 14) is the only resistant native species with a strong representation in front of three out of five species. At the level of the commercial centers (B) of the study city, we note the strong presence of species such as Cocos nucifera (15), Terminalia catappa (9), Terminalia grandifolia (8), Terminalia mentaly (6) and Elaeis guineensis (3). The abundant presence of these species justifies the ecosystem services provided in the city. The green spaces (C), which are represented by the woodlands and urban forests, botanical gardens, interstices and cemeteries and the peripheral zone, are mainly full of Terminalia Cattapa (15), Terminalia mentally (13), Mangifera indica (6), Adonidia merrillii (2) and Borassus aethiopum. The establishments (D), which are mainly training and apprenticeship centers, are peaked by the majority species such as Terminalia catappa (48), Mangifera indica (38), Terminalia mentaly (22), Elaeis guineensis (15) and Cocos nucifera (13). The first three most abundant species define the potential regulating services offered in this land use unit of the city. The residential compartment (E) is mainly and respectively full of species such as Terminalia mentaly (19), Mangifera indica (19), Terminalia catappa (15), Polyalthia longifolia (13) and Casuarina equisetifolia (8).
At the level of the road (F), the abundance of species reveals that the species Khaya senegalensis (59) is the most abundant followed by Terminalia mentaly (40), Terminalia superba (20), Leucaena leucocephala (18) and Gliricidia sepium (8).
3.4. Ecological Importance of Species in the City of Cotonou
The Importance Value Index (IVI) characterizes the importance within a stand of a species relative to the total of all species present in the vegetation under consideration.
Table 3 shows the ecologically important species (IVI ≥ 10%) by land use unit. The analysis of
Table 3 shows the five (5) species with the highest importance values (IVI) in the land use units of the city of Cotonou. From this analysis, it appears that
Mangifera indica (IVI = 64.06%) is the most ecologically important plant species in the administrative zones while
Terminalia catappa (121.47%) occupies this position in the commercial zones of the city. About of green areas and etablishments,
Terminalia catappa remains the most ecologically important plant species with 113.15% and 86.16% as the values of ecological importance index respectively. In relation to the land use unit’s residential areas and roads,
Mangifera indica (52.06%),
Khaya senegalensis (151.16%) are respectively the most ecologically important plant species. Not only did
Terminalia catappa, an exotic species, rank among the top 5 species of high ecological importance in all land use units except for the administrative zones, but it should also be noted that the species occupies the first place in three of the six land use units studied in the city of Cotonou. On the other hand,
Khaya senegalensis, even though the native species is present in the top five ecologically important species in all land use units except for the commercial areas, it only has the highest IVI value in one land use unit (road). As for
Mangifera indica, the exotic species occupies the first place in two land use units and is present in the top five ecologically important species in five of the six land use units. All of this shows that exotic plant species have taken over and predominated in the city's land use units, and that this is guiding the ecological development policies of Benin's cities.
4. Discussion
4.1. Floristic Composition and Specific Diversity of the Urban Vegetation
Over all the plots, 62 species divided into 55 genera and 27 families were inventoried. Thus, the number of species recorded in Cotonou is close to that obtained by [
31] in the of the city of Porto-Novo (73 species). The results of [
9] revealed 67 species divided into 54 genera and 28 families in the city of Atakpamé (Togo). Our results reinforce those of these author’s work. Contrary to [
18,
31] found 22 plant species in their scientific work on the city of Cotonou. This discrepancy between the number of plant species in their work and ours could be explained by the fact that [
18] only considered public and private green spaces, while our investigation considered all the land use units of the city. On the other hand, in research entitled "Structure, diversity and carbon stocks of trees in the city of Kumasi in Ghana", [
33] obtained 176 species and 42 families in the different types of urban trees in the city of Kumasi. In the city of Lomé in Togo, high values to ours were also recorded by [
15] in the city of Lomé with 93 species and 47 families. These results show a high diversity of plant species in the city of Kumasi and Lomé in contrast to the city of Cotonou, which records more trees, but less diversified. The low diversity values of urban vegetation in the city of Cotonou could be explained not only by the climatic and soil conditions that are not very favorable to the survival of several plant species, but also by the city's ecological development policies as mentioned by the work of [
33]. That’s why [
14] found that species diversity among different cities are affected by climate and topography as well as human factors. This could be explained by the fact that in the city of Cotonou, we did not involve the horticultural species as [
15,
42] did in the species plots.
4.2. Specific Diversity of Vegetation, Abundance of Exotic Species, Ecological Importance in the Land Use Units
Observation of the results of the analysis of variance of the diversity parameters reveals significant differences (Table II) between the land use units of the city in terms of the Shannon diversity index (P <0.001), in contrast to the Piélou equitability which remains statistically similar (P >0.5). In the city of Cotonou, residential (H=3.15 bits), administrative (H=2.68bits) and settlement areas are the occupancy units that recorded the highest values of the Shannon index and testifies to the plant diversity of these occupancy units. The work of [
20] in the city of Malanville revealed that residential areas hold the highest Shannon index value. The results of [
17] on the city of Kumasi in Ghana expose administrative areas as among the most floristically diverse occupancy units. The work of [
9] in Togo prioritized settlements among the most floristically diverse places in terms of urban flora. The species richness and Shannon index varying from one occupancy unit to another, express the floristic diversity of the city's occupancy units. This was highlighted by [
35] in their study on analysis on the effects of ecological conservation redline policies in the Pearl River Delta area, China. They found that the Shannon’s diversity index in the inside of city is lower than in the outside area, which indicates a high degree and rapid landscape fragmentation in the outside area The diversity and richness of flora in residential areas could be justified by the arrangements that city dwellers make to introduce specific species into their homes based on preferences for the ecosystem services these species provide. Urban biodiversity is often attributed to the inherent and preferential location of cities in biodiversity hotspots, socio-ecological factors, and human actions through species introduction and landscape heterogeneity [
17]. Residential areas and administrative complexes reflect the ecological values, variety of interests, and socioeconomic status of the owners and users of these spaces. The high species diversity in residential areas is a manifestation of their multifunctional and structural complexity [
34] underpinned by a variety of socioecological constraints [
36]. In contrast to home gardens, which are predominantly private, vegetation in institutional compounds is primarily maintained by government administrative authorities for its qualities of shade and ornament, boundary demarcation, windbreak, and environmental protection, to cut wind, and sometimes for food reasons.
The study shows that exotic species such as
Terminalia Catappa, Terminalia mentaly and
Mangifera indica are very abundant in four out of six land use units in the city of Cotonou and outnumber the other species in terms of abundance. While
Khaya senegalensis, a native species, is present and abundant in only two of the six land-use units visited in Cotonou. These results corroborate those of [
9] who concluded that the vegetation in the city of Atakpamé is represented by a high frequency of exotic species. The abundance of widely distributed species (pantropical and introduced species) indicates that the study area belongs to the highly anthropized domain disturbed by phenomena catalyzed by the urbanization gradient [
18,
37] Only one dominant endogenous species (
Khaya senegalensis) of high ecological importance tops a single occupancy unit of the six cities. This result agrees with [
15,
38]; according to whom exotic species predominate over urban vegetation. According to [
41], populations to settle, fragment and degrade or destroy natural areas, thus disappearing or losing local or endogenous biodiversity and introduce exotic species for their comfort. The city's ecological development policies have catalyzed the storming of all land use units by exotic plant species. The endogenous species that are resistant and that are found in the top 5 plant species of high ecological importance are hosted by a single land use unit (the roads). However, the presence of certain exotic plant species in certain land-use units amply justifies certain ecosystem services offered by floral diversity in the city of Cotonou. According to [
38], the preponderance of these species has been favored by their promotion as species adapted to the urban environment and used during reforestation and regreening campaigns for the past three decades. Better, the choice of species in the occupation units would be explained by the fact that most of the species are better adapted to the climatic and edaphic conditions of the city [
33,
39]. In addition, these species are evergreen trees that can provide certain ecosystem services such as shade in all seasons [
20,
40]. This is, moreover, what could explain the high proportion of these species in urban areas. Others are found there for their beautifying flowers. The development of endogenous plant species in the city would facilitate the conservation of local plant diversity in urban ecosystems. The ecological implication of the Ecological Importance Value Index (IVI) lies in its potential to guide and redirect policy decisions in ecological land use planning.
5. Conclusions
The study provided a range of knowledge on the potential of the urban and peri-urban flora of the city of Cotonou. It allowed us to identify 62 tree species divided into 55 genera and 27 families. This study reveals that residential areas, administrations and establishments are the occupation units that have recorded the highest values of the Shannon index and are the most diverse in terms of urban flora. This flora is characterized by a strong preponderance of exotic species such as Terminalia Catappa, Terminalia mentaly and Mangifera indica. An indigenous species strongly resilient to the extreme weather resulting from the climate change and urbanization of the urban soils is Khaya senegalensis. These species are those of high ecological importance with high IVI values in the occupancy units and offer diversified ecosystem services to the city's residents. All this significant ecological potential should be developed to make a strong contribution to nature-based solutions in urban areas in the face of the adverse effects of climate change. The data can be used to formulate guidelines, policies, or strategies so that the selective development of this urban vegetation can improve the resilience of urban life to climate risks through the provision of urban ecosystem services, possible foundations of ecological infrastructures and urban nature-based solutions In view of this opportunity, it would be wise for all stakeholders in urban management to join forces to promote ecologically sustainable infrastructure to facilitate the achievement of the Sustainable Development Goals (SDGs), in this case SDG 11, in African cities. This multi-stakeholder approach could enable the country to honor its international commitments to the climate and the planet.
Funding
This research was funded by the Regional Centre of Excellence on Sustainable Cities in Africa (CERViDA_DOUNEDON), Association of African Universities (AUA) and the World Bank Group.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We are grateful to the Regional Centre of Excellence on Sustainable Cities in Africa (CERViDA_DOUNEDON), Association of African Universities (AUA) and the World Bank for providing the necessary funding that facilitated our research work leading to these results. We would also like to express our gratitude to Cyprien AHOLOU, Kossiwa ZINSOU Epse KLASSOU and Yanik AKIN, for their all support in this scientific journey.
References
- United Nations. Revision of World Urbanization Prospects. 2018. Available online: https://www.un.org/development/desa/pd/news/world-urbanization-prospects-2018 (accessed on 18 May 2023).
- Elmqvist, T.; Fragkias, Michail, J. G.; Burak G. P. J. M.; McDonald, I. S. P.; Sendstad, M. S. M.; Wilkinson, K. C. S. C. Urbanization, Biodiversity and Ecosystem Services: Challenges and Opportunities. A Global Assessment, 2013. Springer, Dordrecht. ISBN 978-94-007-7088-1. [CrossRef]
- Selmi Wissal and Christiane Weber. Évaluation des services écosystémiques urbains : de la rhétorique à la pratique. L’apport de l’approche par habitat. Environnement Urbain / Urban Environment. 2017, Volume 11.
- Institut National de la Statistique et de l’Analyse Economique (INSAE). 2015. Rgph4: Que Retenir Des Effectifs De Population En 2013 ?
- Semeraro, T.; Scarano, A.; Pandey, R. Ecosystem Services Analysis and Design through Nature-Based Solutions in Urban Planning at a Neighbourhood Scale. Urban Sci. 2022, 6, 23. [CrossRef]
- McDonald, R. I.; Marcotullio, P. J. & Güneralp, B. Urbanization and global trends in biodiversity and ecosystem services, in: Urbanization, Biodiversity and Ecosystem Services: Challenges and Opportunitie. Springer, Dordrecht. 2013, pp. 31–52.
- D. Doucoure & A. Fleury. La place de l’agriculture urbaine dans les dispositifs institutionnels et la planification. In Developpement Durable de L’agriculture urbaine en Afrique francophone : Enjeux, concepts et methodes (C. CIRAD, ed), CIRAD, CRDI. 2004, pp 45 78.
- Reghezza-Zitt, M.& Sanseverino-Godfrin,V. Aménagement durable des territoires soumis à de fortes contraintes : enjeux et perspectives à travers l’examen des outils juridiques. L’exemple de la basse vallée du Var (06). Ann. Georgr. 2012, 685 (3), 242. [CrossRef]
- Folega Fousséni1 ; Atakpama Wouyo ; Kanda Madjouma ; Konate Djibril ; Gmadjom Kissao ; Wala Kperkouma1 & Akpagana Koffi. Flore des espaces verts urbains de la ville d’Atakpamé au Togo Rev. Sci. Technol., Synthèse. 2019, Vol 25, numéro 2: 25-39 (2019).
- Pendall, E.; Hewitt, A.; Boer, M.M.; Carrillo, Y.; Glenn, N.F.; Griebel, A.; Middleton, J.H.; Mumford, P.J.; Ridgeway, P.; Rymer, P.D.; et al. Remarkable Resilience of Forest Structure and Biodiversity Following Fire in the Peri-Urban Bushland of Sydney, Australia. Climate. 2022, 10, 86. [Google Scholar] [CrossRef]
- Seastedt, T.R.; Oldfather, M.F. Climate Change, Ecosystem Processes and Biological Diversity Responses in High Elevation Communities. Climate 2021, 9, 87. [Google Scholar] [CrossRef]
- Adhikari, S.; Struwig, M.; Siebert, S.J. Identifying Common Trees and Herbaceous Plants to Mitigate Particulate Matter Pollution in a Semi-Arid Mining Region of South Africa. Climate 2023, 11, 9. [Google Scholar] [CrossRef]
- Gómez-Baggethun Erik and Barton N. David. Classifying and valuing ecosystem services for urban planning. Ecological Economics. 2013, 86 235–245.
- Li, K.; Zhang, G. Species Diversity and Distribution Pattern of Heritage Trees in the Rapidly-Urbanizing Province of Jiangsu, China. Forests 2021, 12, 1543. [Google Scholar] [CrossRef]
- Polorigni B. and Radji R.,Kokou K. Perceptions, tendances et préférences en foresterie urbaine: cas de la ville de Lomé au Togo. European Scientific Journal. 2014, 10:261-277.
- Alpaidze, L.; Pace, R. Ecosystem Services Provided by Urban Forests in the Southern Caucasus Region: A Modeling Study in Tbilisi, Georgia. Climate 2021, 9, 157. [Google Scholar] [CrossRef]
- Nero, Bertrand F., Benjamin B. Campion, Nelson Agbo, Daniel Callo-Concha , Manfred Denich. Tree and trait diversity, species coexistence, and diversityfunctional relations of green spaces in Kumasi, Ghana. Procedia Engineering, 2017, 99 – 115.
- Sehoun, L. C.; Lougbegnon, T.O; Codjia, J. C. T. Connaissances Et Perceptions Des Services Écosystémiques Des Espaces Verts Des Villes De Cotonou, Abomey-Calavi Et Allada Du Sud Bénin : Implications Pour La Gestion Durable Des Forêts Urbaines Et Péri-Urbaines. European Scientific Journal. 2020, ESJ ISSN: 1857-7881 (Print) e -Vol.16, No.36.
- Teka Oscar; Codjo E. Togbe; Rosos Djikpo; Romeo Chabi; Bruno Djossa. Effects of Urban Forestry on the Local Climate in Cotonou, Benin Republic. Agriculture, Forestry and Fisheries. 2017, Vol. 6, No. 4, 2017, pp. 123-129. [CrossRef]
- Orou W. Baké; Soufouyane Zakari; Mama Djaouga; Ismaïla T. Imorou ; Ibouraïma Yabi ; Brice A. H. Tenté et Julien G. Djego. Diversité et structure de la végétation ligneuse dans la ville de Malanville au Nord-Bénin. Int. J. Biol. Chem. Sci. 2021, 15(1): 129-143, February 2021 ISSN 1997-342X (Online), ISSN 1991-8631 (Print).
- Moussa Soulé ; Boateng Kyereh; Shem Kuyah; Abasse Tougiani and Mahamane Saadou. Composition Floristique et Structure des Forêts Urbaines des Villes Sahéliennes : Cas de Niamey et Maradi, Niger. Science de la vie, de la terre et agronomie. rev. ramres – 2020, vol.07 num.00. 2019 issn 2424-7235.
- Aronson, M.F.J.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, S.G.; Cilliers, S.; Clarkson, B., Dobbs; C., Dolan, R.; Hedblom, M.; Klotz, S.; Kooijmans, J.L.; Kuhn, I.; MacGregorFors, L.; McDonnell, M.; Mortberg, U.; Pyšek, P.; Siebert, S.; Sushinsky, J.; Werner, P.; Winter, M. A global analysis of the impacts of urbanization on bird and plant diversiy reveals key anthropogenic drivers. Proc. R. Soc. 2014, 281, 20133330.
- Agbani, B. S.; Vissoh, S. A.; Kadjegbin, T. R. G.; Bio bigou, L. B. Gestion Des Espaces Verts Dans La Ville De Cotonou Au Sud Du Benin : Etat Des Lieux Et Perspectives. International Journal of Progressive Sciences and Technologies (IJPSAT), 2021.ISSN: 2509-0119.
- Boko, M. Climats et communautés rurales du Bénin : Rythmes climatiques et rythmes de développement. Thèse, Dijon. 1988.
- Lougbegnon, O. Toussaint and Codjia, T. Jean-Claude. Avifaune urbaine de Cotonou et sa distribution en relation avec les facteurs de l’habitat : implications pour l’aménagement écologique de la ville. Afrique SCIENCE. 2011, 07(1) (2011) 116 – 136.
- FAO. Guidelines on urban and peri-urban forestry FAO. Forestry Paper. FAO, Rome, Italia. 2016, (No. 178).
- Akoegninou A.; Van der Burg WJ., Van der Maesen LJG. Flore Analytique du Bénin. Backhuys Publishers: Wageningen. 2006.
- Oksanen Jar; Guillaume Blanchet; Michael Friendly; Roeland Kindt; Pierre Legendre; Dan McGlinn; Peter R. Minchin,R. B. O'Hara; Gavin L. Simpson; Peter Solymos; Henry H. Stevens; Eduard Szoecs and Helene Wagner.vegan: Community Ecology Package. R package version 2.5-7. https://CRAN.R-project.org/package=vegan, 2020.
- Curtis JT; McIntosh RP. An upland forest continuum in the prairie-forest border region of Wisconsin. Ecology. 1951, 32: 476–496.
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. 2021.
- Amontcha AAM. Typologie, utilités et contraintes à l’aménagement des espaces verts dans les villes du grand Nokoué (sud-Bénin). PhD thèse, Université d’Abomey-Calavi, Abomey-Calavi, 243 p. 2018.
- Nero BF; Callo-Concha D; Denich M. Structure, Diversity, and Carbon Stocks of the Tree, Community of Kumasi, Ghana. Forests. 2018, 9(519): 17. [CrossRef]
- Peters, D.P.C.; Savoy, H.M.; Stillman, S.; Huang, H.; Hudson, A.R.; Sala, O.E.; Vivoni, E.R. Plant Species Richness in Multiyear Wet and Dry Periods in the Chihuahuan Desert. Climate 2021, 9, 130. [Google Scholar] [CrossRef]
- Agbogidi E. B. and Adolor O.M. &, “Home gardens in the maintenance of biological diversity,” Appl. Sci. 2013, vol. 1, no. 1, pp. 19–25.
- Xin, W.; Zhe, Z.; Haiguang, H.; Chao, Z.; Ding, W. Analysis on the effects of ecological conservation redline policies in the Pearl River Delta area, China. Front. Ecol. Evol. Sec. Urban Ecology. 2023, Volume 11 - 2023. 2023. [Google Scholar] [CrossRef]
- Eichemberg, M. T., M. Christina, D. M. Amorozo, and C. De Moura, “Species composition and plant use in old urban homegardens in Rio Claro, Southeast of Brazil,” Acta Bot. Brasilica. 2009, vol. 23, no. 4, pp. 1057– 1075.
- Sinsin B.,Oumorou M. Étude de la diversité spécifique du groupement à Cochlospermum tinctorium A. Rich, des savanes arbustives du nord-Bénin. Acta botanica gallica. 2000,147: 345-360.
- Osseni A., Toko M., Tohozin B.,Sinsin B. SIG et gestion des espaces verts dans la ville de PortoNovo au Bénin. Tropicultura. 2015, 33: 146-156.
- Dardour M., Daroui E.A., Boukroute A., Kouddane N.- E., Berrichi A. Inventaire et état sanitaire des arbres d’alignement de la ville de Saïdia (Maroc oriental). Nature Technology. 2014, 10: 2.
- Vroh B.T.A. Évaluation de la diversité et estimation de la biomasse aérienne des arbres du jardin botanique de Bingerville (District d’Abidjan, Côte d’Ivoire). European Scientific Journal. 2016, 12:185-201.
- Logbo J, Yedomonhan P, Tente Akoegninou A. Distribution et habitats de Newbouldia laevis (P.Beauv.) Seemann ex Bureau et de Dracaena arborea (Willd.) Link dans les zones bioclimatiques du Bénin. Int. J. Biol. Chem. Sci. 2020, 14(8): 2903-2927. [CrossRef]
- Radji R, Kokou K, Akpagana K. Etude diagnostique de la flore ornementale togolaise. Int. J. Biol. Chem. Sci. 2010, 4(2): 491-508. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).