1. Introduction
Gastric cancer (GC), one of the most common digestive cancers is the leading cause of cancer-related death worldwide [
1]. The clinical outcomes of patients with GC, particularly overall survival (OS), has gradually improved following the introduction of chemotherapy and immune checkpoint inhibitors (ICIs). In fact, ATTRACTION-2, the first phase 3 study on patients with GC receiving two or more chemotherapy regimens, showed that the anti-PD-1 antibody nivolumab improved overall survival (OS) [
2]. Furthermore, the CheckMate 649 study recommended nivolumab plus chemotherapy as the primary treatment for GC [
3]. However, patients with GC have shown unsatisfactory response rates to ICIs. Concerns regarding the impact of expensive ICIs on the health care economy have also emerged. As such, a robust biomarker is needed to more effectively assess the positive clinical therapeutic effects of nivolumab in patients with GC.
Nejman et al. showed that intratumor bacteria were present in cancer cells and were mostly intracellular. They also showed that bacterial lipopolysaccharides (LPS) were present in tumor cells and that intratumor bacteria could affect responses to immunotherapy [
4]. The cell wall of Gram-negative bacteria is composed of LPS. Reports have shown that LPS were associated with the development of GC by regulating cell proliferation, autophagy, and epithelial–mesenchymal transition (EMT) [
5,
6,
7]. In particular, Helicobacter pylori LPS have been found to activated both NFkB and STAT3 signaling pathways [
8,
9,
10]. NFkB induces PD-L1 expression and EMT by activating transforming growth factor β (TGF-β) signaling [
11].
TGF-β plays a critical role in EMT, which causes therapeutic resistance to ICIs [
12,
13]. In particular, TGF-β activates a pathway mediated by small mother against decapentaplegic (SMAD) proteins that induces the downstream activation of EMT, including N-cadherin and vimentin, via SNAIL, TWIST, and ZEB1 [
14,
15]. However, the appropriate method for evaluating TGF-β signaling in patients with cancer remains elusive given the genetic variations in TGF-β pathway genes across several cancers [
16,
17]. Our previous report showed that high stromal transforming growth factor-beta-induced protein (TGFBI) in lung cancer was associated with poor prognosis and therapeutic resistance to ICIs [
18]. TGFBI had initially been described as a protein strongly induced by TGF-β and one of the representative downstream genes in TGF-β signaling [
19]. However, few studies have addressed the relationship between LPS and TGFBI, as well as the therapeutic effects of the anti-PD-1 antibody nivolumab in GC.
The current study therefore aimed to clarify the significance of LPS in patients with GC. Moreover, we investigated whether LPS could be a biomarker for predicting treatment resistance in patients receiving nivolumab for GC.
2. Results
2.1. Presence of LPS in GC surgical tissues
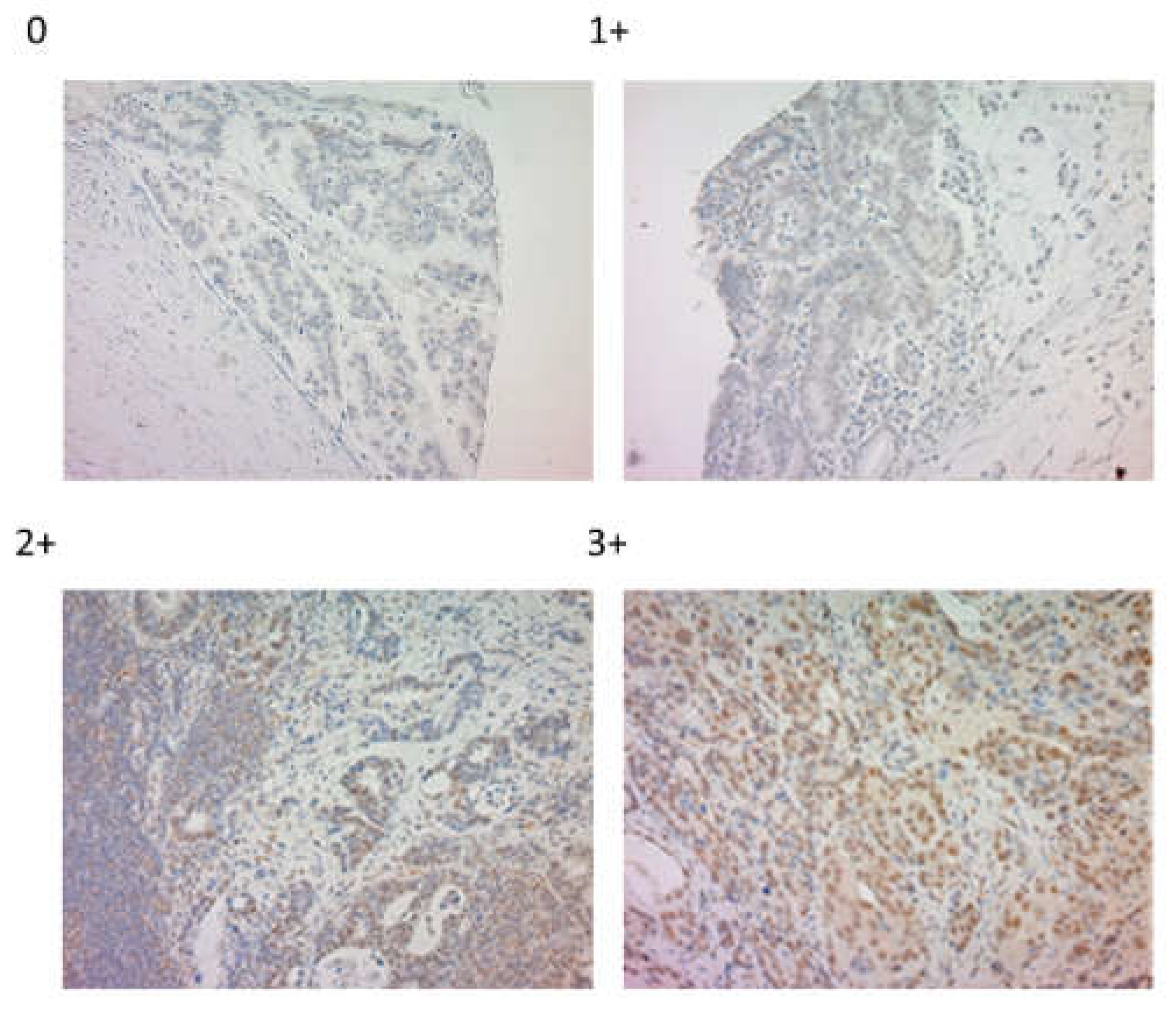
LPS were stained in the nucleus of cancer cells (
Figure 1), with our results showing 150 (75.8%) and 48 (24.2%) negative and positive cases, respectively.
2.2. Relationship between LPS and clinicopathological factors of patients with GC
The results for the relationship between LPS and clinicopathological factors are summarized in
Table 1. Accordingly, no significant difference in age, sex, tumor size, depth, differentiation, presence of lymph node metastasis, and stage were observed between positive and negative cases. The LPS-positive group showed significantly greater cancer stromal TGFBI expression (p < 0.0001), more HER2-negative cases (p = 0.012), greater PD-L1 expression (p = 0.0029), increased wnt3a signaling (p = 0.0028) and lower E-cadherin expression (p = 0.0055) (EMT marker) than did the LPS-positive group.
2.3. Kaplan–Meier curve for overall survival according to the presence of LPS in surgical cases with GC
Figure 2. presents the Kaplan–Meier curve for overall survival according to the presence of LPS in surgical cases with GC. Accordingly, the presence of LPS was not significantly associated with overall survival (p = 0.71).
2.4. Kaplan–Meier curve for overall survival with TGFBI expression and prognosis according to the presence or absence of LPS in surgical cases with GC
Figure 3 also presents the Kaplan–Meier curve for overall survival with TGFBI expression and prognosis according to the presence or absence of LPS in surgical cases with GC. High TGFBI expression in the presence of LPS was associated with a worse prognosis than that in the absence of LPS (p = 0.049).
2.5. Relationship between LPS and nivolumab sensitivity
Table 2 outlines the results for the relationship between LPS and nivolumab sensitivity. Notably, the LPS-negative and -positive groups showed a disease control rate of 66.7% and 11.8%, respectively (p = 0.049).
3. Discussion
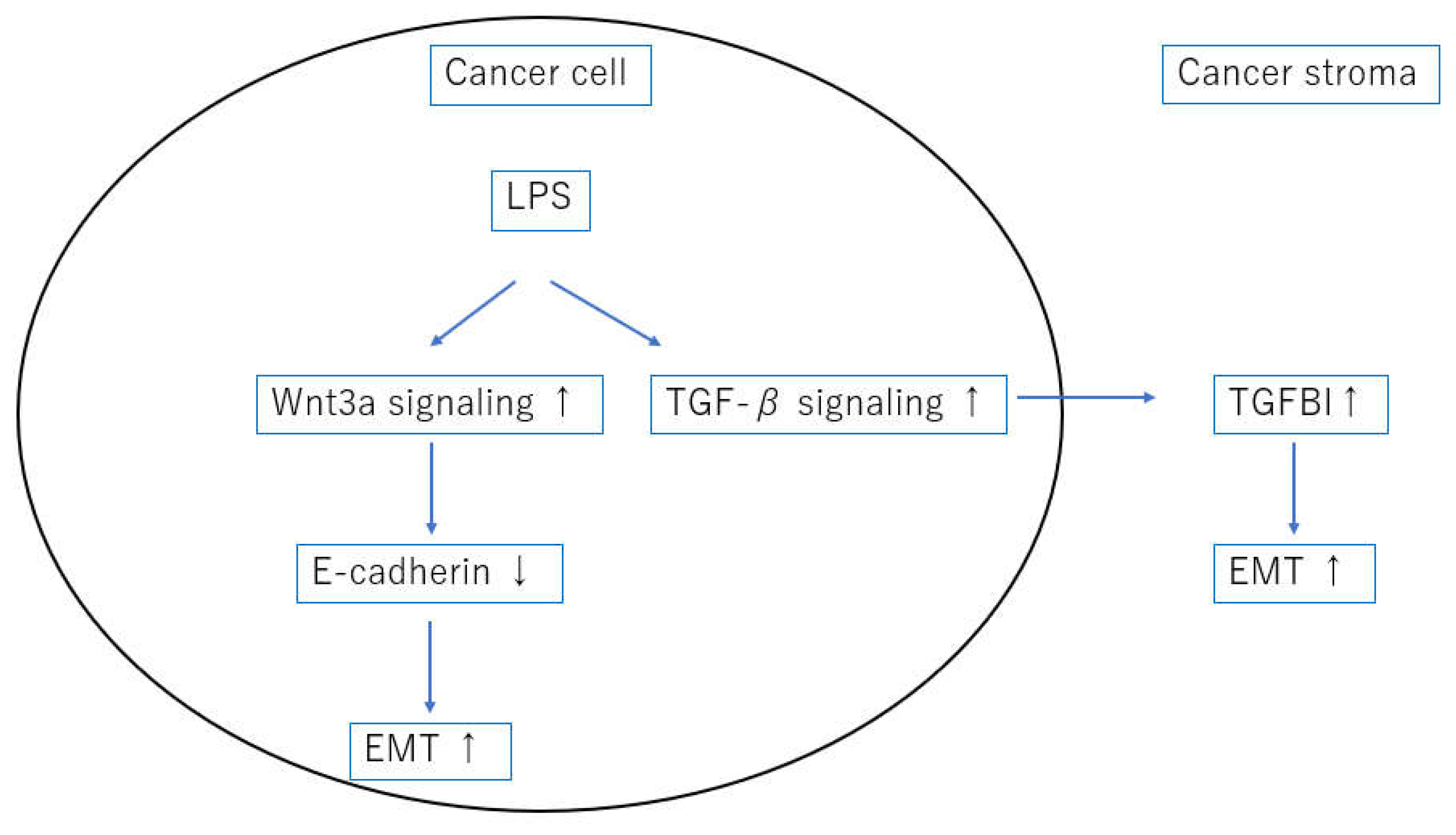
The current study showed that LPS in GC cells were associated with enhanced TGF-β signaling, wnt3a signaling, PD-L1 expression, and EMT. Although the presence of LPS did not contribute to the prognosis of GC, it did contribute to therapeutic resistance in patients receiving nivolumab for unresectable advanced recurrent GC (p = 0.049). Furthermore, high TGFBI expression in the presence of LPS was associated with a worse prognosis than that in the absence of LPS.
A previous report revealed that intratumor bacteria were present in both cancer and immune cells and were mostly intracellular. Evidence has also shown that intratumor bacteria or their predicted functions were correlated with tumor types and subtypes, patients' smoking status, and responses to immunotherapy [
4]. Moreover, recent studies have revealed that LPS contributes to the immune response of the host by functioning as a cancer antigen [
20]. In line with this, our study showed that LPS contributed to therapeutic resistance in patients receiving nivolumab for GC.
Epithelial cells can acquire a mesenchymal phenotype through a process called EMT. More specifically, EMT can be described as a process of cell plasticity in which epithelial cells acquire mesenchymal characteristics, such as fibroblast-like morphology, increased motility, and increased expression of mesenchymal markers such as vimentin, while simultaneously decreasing cell-to-cell contact, E-cadherin and claudin expression, and other epithelial characteristics [
21]. Cytotoxin-associated gene A (CagA) is the major virulence factor and oncoprotein of H. pylori [
22]. Previous reports have shown that CagA promoted EMT by reducing GSK-3 activity [
23], and downregulating programmed cell death protein 4 [
24]. The current study showed that LPS, a constituent molecule of Gram-negative bacteria, were present in cancer cells, suggesting a new possibility for inducing EMT. We also showed that the activation of wnt3a signaling may downregulate E-cadherin expression and induce EMT during these processes [
Figure 4].
TGF-β signaling is considerably complex in addition to being time- and context-dependent, with different signaling processes occurring in cancerous and noncancerous areas [
25]. Hence, evaluating TGF-β signaling is quite challenging. TGFBI is a representative downstream gene of TGF-β signaling and a protein whose expression is induced downstream of SMAD [
19]. In other words, TGFBI expression can be a good indicator of TGF-β signaling activation. Furthermore, one study showed that TGFBI expression is highly induced in the process of EMT [
26]. LPS induced IL-6 and TGF-β1 secretion in esophageal cancer cell lines, and E-cadherin was significantly decreased after LPS treatment, promoting EMT [
27]. Interestingly, the current study showed that cancer stromal TGFBI expression was strongly correlated with the presence of LPS in cancer cells. This indicates that bacterial-derived LPS activate TGF-β signaling, suggesting that LPS regulation may be useful for regulating TGF-β signaling and, consequently, EMT [
Figure 4].
Our previous study reported that cancer stromal TGFBI expression was significantly associated with therapeutic resistance in patients receiving nivolumab for lung cancer [
18]. Similarly, the present study found that the presence of LPS was significantly correlated with cancer stromal TGFBI expression, which may have contributed to therapeutic resistance in patients with GC using nivolumab. Furthermore, reports of increased EMT and resistance to ICI treatment have been observed in other cancers [
28,
29]. Although the presence of LPS was positively correlated with PD-L1 expression in cancer cells in present study, but the aforementioned EMT findings suggest that the presence of LPS may have caused treatment resistance in patients receiving nivolumab for GC.
V Gopalakrishnan et al., who studied the oral and gut microbiota of patients with melanoma receiving anti-PD-1 immunotherapy, reported significant differences in the diversity and composition of patient gut bacteria between responders and non-responders. Immune profiling also suggested enhanced systemic and antitumor immunity in reactive patients with favorable gut microbiota and in sterile mice transplanted with feces from reactive patients [
30]. Given that H. pylori infections have been suggested to cause GC, we would like to examine the history of H. pylori eradication and sensitivity to ICIs in the future.
Our study has several limitations worth noting. First, this is a single-center, retrospective study with small sample size. In particular, the GC samples treated by nivolumab might not be enough to indicate strong evidence of LPS as a promising biomarker for ICIs treatment. Further prospective studies are needed to clarify the importance of LPS evaluation as an ICI sensitivity marker for GC patients in the clinic. Second, no in vitro or in vivo studies were conducted herein. Third, this study did not examine the association between LPS and H. pylori. In the future, we would like to use surgical specimens to investigate the relationship between 16srDNA and LPS at cancer sites. Finally, lymphocytes were also stained for LPS in immunostaining. The significance of this staining has not been examined.
In conclusion, the current study found that LPS was associated with wnt3a, TGF-β signaling and EMT and that its expression affected therapeutic resistance to nivolumab in patients with GC.
4. Materials and Methods
4.1. Patients
This study included 198 patients who underwent potentially curative surgery for GC at the Department of General Surgical Science, Gunma University Hospital between 1996 and 2006. In this study, we performed tissue microarray analysis. Preoperatively treated cases and endoscopic mucosal resection cases were excluded, and multiple blocks containing preinvasive sites were collected and subjected to tissue microarray. Clinicopathological factors, such as age, sex, tumor size, depth, differentiation, presence of lymph node metastasis, stage, and HER2 status, were determined. Furthermore, we analyzed 20 patients receiving nivolumab for postoperative recurrent or unresectable advanced GC from 2017 to 2021. Nivolumab was used after third-line treatment in all cases. Among such cases, the response to nivolumab was evaluated using the Response Evaluation Criteria in Solid Tumors version 1.1 [
31]. The resected specimens and pretreatment biopsy specimens were included in nivolumab sensitivity consideration. Our study was approved by the institutional review board of Gunma University (approval no. HS2021-085).
4.2. Immunohistochemistry
All specimens were cut into 4-µm-thick sections and mounted on glass slides. Their sections were deparaffinized with xylene, hydrated, and incubated in 0.3% hydrogen peroxide for 30 min at room temperature to block endogenous peroxidase activity. Antigen retrieval was performed in ImmunoSaver (Nisshin EM, Tokyo, Japan) at 98°C for 45 min. Nonspecific binding sites were blocked by incubation with Protein Block Serum-Free (Dako, Carpinteria, CA) for 30 min at room temperature. The primary antibody for LPS (Hycult Biotech, HM6011, Mouse mAb, 1:100 dilution) was diluted with Dako REAL Antibody Diluent and incubated overnight at 4°C. The Histofine Simple Stain MAX-PO (Multi) Kit (Nichirei, Tokyo, Japan), which was used as the secondary antibody, was incubated for 30 min at room temperature. The stain 3,3-diaminobenzidine tetrahydrochloride was applied as a 0.02% solution in 50 mM of ammonium acetate-citrate acid buffer (pH 6.0) containing 0.005% hydrogen peroxide. Sections were lightly contrast-stained with hematoxylin and then mounted. We divided LPS into four groups: negative, weak, moderate, and strong, with moderate and strong indicating positivity. For TGFBI, CD8, and PD-L1, we employed the same staining and evaluation methods as previously report [
18]. And we also employed the same staining and evaluation method for wnt3a [
32]. We performed immunohistochemical staining using E-cadherin antibody (HECD-1, mouse monoclonal, 1:500 dilution), with sections boiled in 10 mM citrate buffer (pH 6.0) at 98°C for 30 min for E-cadherin activation. A positive case was defined as that in which ≥50% of the cancer cells had moderate staining intensity in E-cadherin.
4.3. Statistical analysis
Statistically significant differences were analyzed using the Mann–Whitney U test for continuous variables and the Chi squared test for categorical variables. Survival rates were calculated using the Kaplan–Meier method, with statistical significance being determined by the log rank test. All analyses were performed using JMP Pro 15.0 software (SAS Institute Inc., Cary, NC, USA), with p < 0.05 indicating statistical significance.
5. Conclusions
The current study showed that lipopolysaccharides positivity was associated with wnt3a, TGF-β signaling and epithelial–mesenchymal transition and was considered to promote therapeutic resistance to nivolumab.
Author Contributions
Conception and design: N. Nakazawa, and T.Yokobori; Acquisition of data: N. Nakazawa, K.Tateno, T.Watanabe, Y.Shimoda, and M.Ide; Analysis and interpretation: N. Nakazawaa, M. Sohda, A. Sano, M. Sakai, H. Ogawa, K. Shirabe, and H. Saeki: Writing, review, and/or revision of the manuscript: N. Nakazawa, M. Sohda, K. Shirabe, and H. Saeki: Study supervision: K. Shirabe, and H. Saeki. All Authors have read and approved the final manuscript.
Funding
The authors disclose no grant support.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Gunma University (approval No. HS2021-085) and was conducted according to the Declaration of Helsinki.
Informed Consent Statement
As this was a retrospective study, the requirement to obtain informed consent was waived by the IRB of the Gunma University Hospital. An opt-out method was used to obtain the participant’s consent.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
We thank Mariko Nakamura, Kao Abe, Yukiko Suto, Sayaka Okada, and Harumi Kanai for their assistance with this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J Clin 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Kang, Y.K.; Boku, N.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef]
- Janjigian, Y.Y; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Bragagnoli, A.C.; et al. Nivolumab plus chemotherapy versus chemotherapy as first-line treatment for advanced gastric cancer/gastroesophageal junction cancer/oesophageal adenocarcinoma (CheckMate 649): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Muhammad, J.S.; Nanjo, S.; Ando, T.; Yamashita, S.; Maekita, T.; Ushijima, T.; Tabuchi, Y.; Sugiyama, T. Autophagy impairment by Helicobacter pylori-induced methylation silencing of MAP1LC3Av1 promotes gastric carcinogenesis. Int J Cancer 2017, 140, 2272–2283. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Okabayashi, T.; Rehli, M.; Fujii, N.; Amano, K. Helicobacter pylori lipopolysaccharides upregulate toll-like receptor 4 expression and proliferation of gastric epithelial cells via the MEK1/2-ERK1/2 mitogen-activated protein kinase pathway. Infect Immun 2010, 78, 468–476. [Google Scholar] [CrossRef]
- Chen, G.; Tang, N.; Wang, C.; Xiao, L.; Yu, M.; Zhao, L.; Cai, H.; Han, L.; Xie, C.; Zhang, Y. TNF-α-inducing protein of Helicobacter pylori induces epithelial-mesenchymal transition (EMT) in gastric cancer cells through activation of IL-6/STAT3 signaling pathway. Biochem Biophys Res Commun 2017, 484, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xia, L.; Liu, Q.; Wang, H.; Lin, J.; Oyang, L.; Chen, X.; Luo, X.; Tan, S.; Tian, Y.; et al. Induction of pro-inflammatory response via activated macrophage-mediated NF-κB and STAT3 pathways in gastric cancer cells. Cell Physiol Biochem 2018, 47, 1399–1410. [Google Scholar] [CrossRef]
- Orlowski, R.Z.; Baldwin, A.S. NF-kappaB as a therapeutic target in cancer. Trends Mol Med 2002, 8, 385–389. [Google Scholar] [CrossRef]
- Chavarría-Velázquez, C.O; Torres-Martínez, A.C.; Montaño, L.F.; Rendón-Huerta, E.P. TLR2 activation induced by H. pylori LPS promotes the differential expression of claudin-4, -6, -7 and -9 via either STAT3 and ERK1/2 in AGS cells. Immunobiology 2018, 223, 38–48. [Google Scholar]
- Xiao, Z.; Su, Z.; Han, S.; Huang, J.; Lin, L.; Shuai, X. Dual pH-sensitive nanodrug blocks PD-1 immune checkpoint and uses T cells to deliver NF-κB inhibitor for antitumor immunotherapy. Sci Adv 2020, 6, eaay7785. [Google Scholar] [CrossRef]
- Lanitis, E.; Dangaj, D.; Irving, M.; Coukos, G. Mechanisms regulating T-cell infiltration and activity in solid tumors. Ann Oncol 2017, 28, xii18–xii32. [Google Scholar] [CrossRef] [PubMed]
- Cannito, S.; Novo, E.; Di Bonzo, L.V.; Busletta, C.; Colombatto, S.; Parola, M. Epithelial-mesenchymal transition: from molecular mechanisms, redox regulation to implications in human health and disease. Antioxid Redox Signal 2010, 12, 1383–1430. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, Z.; Dam, H.v.; Zhang, L.; Zhou, F. Regulation of TGF-β superfamily signaling by SMAD mono-ubiquitination. Cells 2014, 3, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Turini, S.; Bergandi, L.; Gazzano, E.; Prato, M.; Aldieri, E. Epithelial to mesenchymal transition in human mesothelial cells exposed to asbestos fibers: role of TGF-β as mediator of malignant mesothelioma development or metastasis via EMT event. Int J Mol Sci 2019, 20, 150. [Google Scholar] [CrossRef]
- Lin, M.; Stewart, D.J.; Spitz, M.R.; Hildebrandt, M.A.T.; Lu, C.; Lin, J.; Gu, J.; Huang, M.; Lippman, S.M.; Wu, X. Genetic variations in the transforming growth factor-beta pathway as predictors of survival in advanced non-small cell lung cancer. Carcinogenesis 2011, 32, 1050–1056. [Google Scholar] [CrossRef]
- Javle, M.; Li, Y.; Tan, D.; Dong, X.; Chang, P.; Kar, S.; Li, D. Biomarkers of TGF-β signaling pathway and prognosis of pancreatic cancer. PLoS One 2014, 9, e85942. [Google Scholar] [CrossRef]
- Nakazawa, N.; Yokobori, T.; Kaira, K.; Turtoi, A.; Baatar, S.; Gombodorj, N.; Handa, T.; Tsukagoshi, M.; Ubukata, Y.; Kimura, A.; et al. High stromal TGFBI in lung cancer and intratumoral CD8-positive T cells were associated with poor prognosis and therapeutic resistance to immune checkpoint inhibitors. Ann Surg Oncol 2020, 27, 933–942. [Google Scholar] [CrossRef]
- Skonier, J.; Neubauer, M.; Madisen, L.; Bennett, K.; Plowman, G.D.; Purchio, A.F. cDNA cloning and sequence analysis of beta ig-h3, a novel gene induced in a human adenocarcinoma cell line after treatment with transforming growth factor-beta. DNA Cell Biol 1992, 11, 511–522. [Google Scholar] [CrossRef]
- Kalaora, S.; Nagler, A.; Nejman, D.; Alon, M.; Barbolin, C.; Barnea, E.; Ketelaars, S.L.C.; Cheng, K.; Vervier, K.; Shental, N.; et al. Identification of bacteria-derived HLA-bound peptides in melanoma. Nature 2021, 592, 138–143. [Google Scholar] [CrossRef]
- Gavert, N.; Ben-Ze'ev, A. Epithelial-mesenchymal transition and the invasive potential of tumors. Trends Mol Med 2008, 14, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Odenbreit, S.; Püls, J.; Sedlmaier, B.; Gerland, E.; Fischer, W.; Haas, R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 2000, 287, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Kim, H.S.; Lee, Y.S. ; Kim,S. ; Cha, S.Y.; Ota, I.; Kim, N.H.; Cha, Y.H.; Yang, D.H.; Lee, Y.; et al. Helicobacter pylori CagA promotes Snail-mediated epithelial-mesenchymal transition by reducing GSK-3 activity. Nat Commun 2014, 5, 4423. [Google Scholar]
- Yu, H.; Zeng, J.; Liang, X.; Wang, W.; Zhou, Y.; Sun, Y.; Liu, S.; Li, W.; Chen, C.; Jia, J. Helicobacter pylori promotes epithelial-mesenchymal transition in gastric cancer by downregulating programmed cell death protein 4 (PDCD4). PLoS One 2014, 9, e105306. [Google Scholar] [CrossRef]
- Costanza, B.; Umelo, I.A.; Bellier, J.; Castronovo, V.; Turtoi, A. Stromal modulators of TGF-β in cancer. J Clin Med 2017, 6, 7. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, M.; Du, S.; Feng, W.; Zhang, K.; Zhang, L.; Liu, H.; Jia, G.; Wu, L.; Hu, X.; et al. Competitive endogenous RNA is an intrinsic component of EMT regulatory circuits and modulates EMT. Nat Commun 2019, 10, 1637. [Google Scholar] [CrossRef]
- Peng, Z.; Wan, P.; Deng, Y.; Shen, W.; Liu, R. Lipopolysaccharide exacerbates to the migration, invasion, and epithelial-mesenchymal transition of esophageal cancer cells by TLR4/NF-κB axis. Environ Toxicol 2023, 38, 1090–1099. [Google Scholar] [CrossRef]
- Wang, L.; Saci, A.; Szabo, P.M.; Chasalow, S.D.; Castillo-Martin, M.; Domingo-Domenech, J.; Siefker-Radtke, A.; Sharma, P.; Sfakianos, J.P.; Gong, Y.; et al. EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. Nat Commun 2018, 9, 3503. [Google Scholar] [CrossRef]
- Chae, Y.K.; Chang, S.; Ko, T.; Anker, J.; Agte, S.; Iams, W.; Choi, W.M.; Lee, K.; Cruz, M. Epithelial-mesenchymal transition (EMT) signature is inversely associated with T-cell infiltration in non-small cell lung cancer (NSCLC). Sci Rep 2018, 8, 2918. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; Rubinstein, L.; et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1). Eur J Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, N.; Sohda, M.; Yokobori, T.; Gombodorj, N.; Sano, A.; Sakai, M.; Oyama, T.; Kuwano, H.; Shirabe, K.; Saeki, H. Cytoplasmic localization of connexin 26 suppresses transition of β-catenin into the nucleus in intestinal- and mix-type gastric cancer. Ann Gastroenterol Surg 2022, 6, 505–514. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).