Submitted:
20 June 2023
Posted:
21 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Cell-free DNAs (cfDNAs)
2.1. NETs
2.2. Vesicle-bound DNA
3. EccDNA
3.1. Biogenesis of eccDNA in tissue
4. CfDNA and eccDNA in inflammation
5. The role of computational biology in profiling of eccDNA for personalized medicine
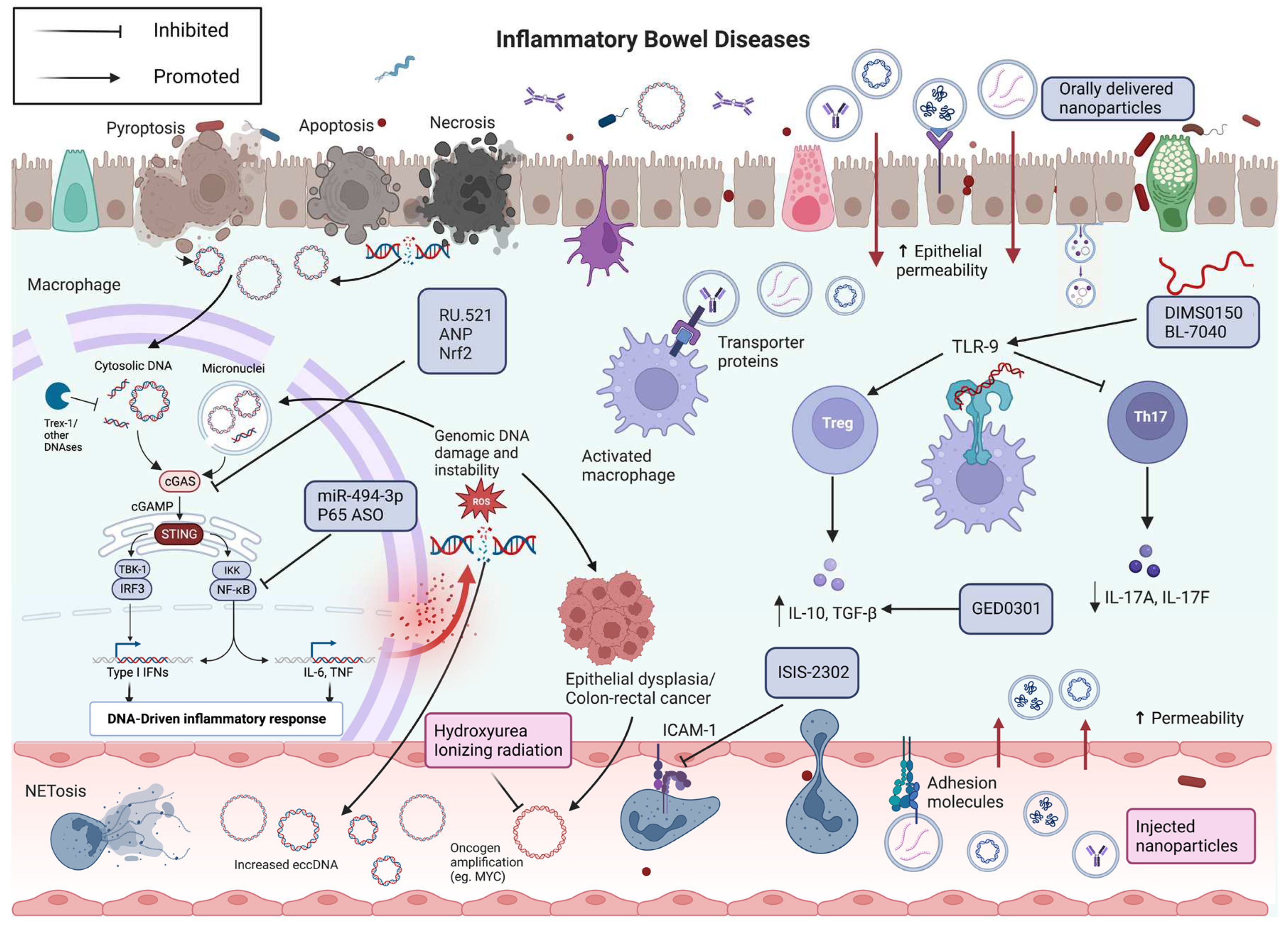
6. Towards molecular therapies for IBD
6.1. TLR9 therapeutics
6.2. cGAS-STING therapeutics
6.3. Extracellular vesicles therapeutics
6.4. Oligonucleotide therapeutics
6.5. eccDNA therapeutics
7. Concluding remarks and future perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordon, H.; Biancone, L.; Fiorino, G.; Katsanos, K.H.; Kopylov, U.; Sulais, E.A.; Axelrad, J.E.; Balendran, K.; Burisch, J.; de Ridder, L.; et al. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J Crohns Colitis 2022. [Google Scholar] [CrossRef]
- Kim, D.H.; Cheon, J.H. Pathogenesis of Inflammatory Bowel Disease and Recent Advances in Biologic Therapies. Immune Netw 2017, 17, 25–40. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef]
- Singh, S.; Fumery, M.; Sandborn, W.J.; Murad, M.H. Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe Crohn's disease. Aliment Pharmacol Ther 2018, 48, 394–409. [Google Scholar] [CrossRef]
- Singh, S.; Murad, M.H.; Fumery, M.; Dulai, P.S.; Sandborn, W.J. First- and Second-Line Pharmacotherapies for Patients With Moderate to Severely Active Ulcerative Colitis: An Updated Network Meta-Analysis. Clin Gastroenterol Hepatol 2020, 18, 2179–2191.e2176. [Google Scholar] [CrossRef]
- Moustafa, A.; Li, W.; Anderson, E.L.; Wong, E.H.M.; Dulai, P.S.; Sandborn, W.J.; Biggs, W.; Yooseph, S.; Jones, M.B.; Venter, J.C.; et al. Genetic risk, dysbiosis, and treatment stratification using host genome and gut microbiome in inflammatory bowel disease. Clin Transl Gastroenterol 2018, 9, e132. [Google Scholar] [CrossRef]
- Mandel, P.; Metais, P. [Nuclear Acids In Human Blood Plasma]. C R Seances Soc Biol Fil 1948, 142, 241–243. [Google Scholar]
- Annala, M.; Vandekerkhove, G.; Khalaf, D.; Taavitsainen, S.; Beja, K.; Warner, E.W.; Sunderland, K.; Kollmannsberger, C.; Eigl, B.J.; Finch, D.; et al. Circulating Tumor DNA Genomics Correlate with Resistance to Abiraterone and Enzalutamide in Prostate Cancer. Cancer Discov 2018, 8, 444–457. [Google Scholar] [CrossRef]
- Lo, Y.M.; Corbetta, N.; Chamberlain, P.F.; Rai, V.; Sargent, I.L.; Redman, C.W.; Wainscoat, J.S. Presence of fetal DNA in maternal plasma and serum. Lancet 1997, 350, 485–487. [Google Scholar] [CrossRef]
- Sin, S.T.K.; Jiang, P.; Deng, J.; Ji, L.; Cheng, S.H.; Dutta, A.; Leung, T.Y.; Chan, K.C.A.; Chiu, R.W.K.; Lo, Y.M.D. Identification and characterization of extrachromosomal circular DNA in maternal plasma. Proc Natl Acad Sci U S A 2020, 117, 1658–1665. [Google Scholar] [CrossRef]
- Kumar, P.; Dillon, L.W.; Shibata, Y.; Jazaeri, A.A.; Jones, D.R.; Dutta, A. Normal and Cancerous Tissues Release Extrachromosomal Circular DNA (eccDNA) into the Circulation. Mol Cancer Res 2017, 15, 1197–1205. [Google Scholar] [CrossRef]
- Møller, H.D.; Parsons, L.; Jørgensen, T.S.; Botstein, D.; Regenberg, B. Extrachromosomal circular DNA is common in yeast. Proc Natl Acad Sci U S A 2015, 112, E3114–3122. [Google Scholar] [CrossRef]
- Tie, J.; Cohen, J.D.; Lahouel, K.; Lo, S.N.; Wang, Y.; Kosmider, S.; Wong, R.; Shapiro, J.; Lee, M.; Harris, S.; et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N Engl J Med 2022, 386, 2261–2272. [Google Scholar] [CrossRef]
- Turner, K.M.; Deshpande, V.; Beyter, D.; Koga, T.; Rusert, J.; Lee, C.; Li, B.; Arden, K.; Ren, B.; Nathanson, D.A.; et al. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nature 2017, 543, 122–125. [Google Scholar] [CrossRef]
- Van Niel, G.; Mallegol, J.; Bevilacqua, C.; Candalh, C.; Brugière, S.; Tomaskovic-Crook, E.; Heath, J.K.; Cerf-Bensussan, N.; Heyman, M. Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut 2003, 52, 1690–1697. [Google Scholar] [CrossRef]
- Burger, D.; Schock, S.; Thompson, C.S.; Montezano, A.C.; Hakim, A.M.; Touyz, R.M. Microparticles: biomarkers and beyond. Clin Sci (Lond) 2013, 124, 423–441. [Google Scholar] [CrossRef]
- Dignat-George, F.; Boulanger, C.M. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol 2011, 31, 27–33. [Google Scholar] [CrossRef]
- Morel, O.; Jesel, L.; Freyssinet, J.M.; Toti, F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol 2011, 31, 15–26. [Google Scholar] [CrossRef]
- Lamarre, Y.; Nader, E.; Connes, P.; Romana, M.; Garnier, Y. Extracellular Vesicles in Sickle Cell Disease: A Promising Tool. Bioengineering (Basel) 2022, 9. [Google Scholar] [CrossRef]
- Pisetsky, D.S.; Ullal, A.J.; Gauley, J.; Ning, T.C. Microparticles as mediators and biomarkers of rheumatic disease. Rheumatology (Oxford) 2012, 51, 1737–1746. [Google Scholar] [CrossRef]
- Tesse, A.; Meziani, F.; David, E.; Carusio, N.; Kremer, H.; Schneider, F.; Andriantsitohaina, R. Microparticles from preeclamptic women induce vascular hyporeactivity in vessels from pregnant mice through an overproduction of NO. Am J Physiol Heart Circ Physiol 2007, 293, H520–525. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Li, C.; Yu, Y.; Yi, Y.; Wang, J.; Chen, D. Exosome-Induced Regulation in Inflammatory Bowel Disease. Front Immunol 2019, 10, 1464. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, A.V.; Konkova, M.S.; Kostyuk, S.V.; Izevskaya, V.L.; Baranova, A.; Veiko, N.N. Oxidized extracellular DNA as a stress signal in human cells. Oxid Med Cell Longev 2013, 2013, 649747. [Google Scholar] [CrossRef]
- Glebova, K.; Veiko, N.; Kostyuk, S.; Izhevskaya, V.; Baranova, A. Oxidized extracellular DNA as a stress signal that may modify response to anticancer therapy. Cancer Lett 2015, 356, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, S.V.; Tabakov, V.J.; Chestkov, V.V.; Konkova, M.S.; Glebova, K.V.; Baydakova, G.V.; Ershova, E.S.; Izhevskaya, V.L.; Baranova, A.; Veiko, N.N. Oxidized DNA induces an adaptive response in human fibroblasts. Mutat Res 2013, 747-748, 6–18. [Google Scholar] [CrossRef]
- Drury, B.; Hardisty, G.; Gray, R.D.; Ho, G.T. Neutrophil Extracellular Traps in Inflammatory Bowel Disease: Pathogenic Mechanisms and Clinical Translation. Cell Mol Gastroenterol Hepatol 2021, 12, 321–333. [Google Scholar] [CrossRef]
- Liu, H.; Tian, Y.; Xue, C.; Niu, Q.; Chen, C.; Yan, X. Analysis of extracellular vesicle DNA at the single-vesicle level by nano-flow cytometry. J Extracell Vesicles 2022, 11, e12206. [Google Scholar] [CrossRef]
- Maronek, M.; Gromova, B.; Liptak, R.; Konecna, B.; Pastorek, M.; Cechova, B.; Harsanyova, M.; Budis, J.; Smolak, D.; Radvanszky, J.; et al. Extracellular DNA Correlates with Intestinal Inflammation in Chemically Induced Colitis in Mice. Cells 2021, 10. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Djekidel, M.N.; Chen, H.; Liu, D.; Alt, F.W.; Zhang, Y. eccDNAs are apoptotic products with high innate immunostimulatory activity. Nature 2021, 599, 308–314. [Google Scholar] [CrossRef]
- Beccard, I.J.; Hofmann, L.; Schroeder, J.C.; Ludwig, S.; Laban, S.; Brunner, C.; Lotfi, R.; Hoffmann, T.K.; Jackson, E.K.; Schuler, P.J.; et al. Immune Suppressive Effects of Plasma-Derived Exosome Populations in Head and Neck Cancer. Cancers (Basel) 2020, 12. [Google Scholar] [CrossRef]
- Mitsuhashi, S.; Feldbrügge, L.; Csizmadia, E.; Mitsuhashi, M.; Robson, S.C.; Moss, A.C. Luminal Extracellular Vesicles (EVs) in Inflammatory Bowel Disease (IBD) Exhibit Proinflammatory Effects on Epithelial Cells and Macrophages. Inflamm Bowel Dis 2016, 22, 1587–1595. [Google Scholar] [CrossRef]
- Zhang, M.; Johnson-Stephenson, T.K.; Wang, W.; Wang, Y.; Li, J.; Li, L.; Zen, K.; Chen, X.; Zhu, D. Mesenchymal stem cell-derived exosome-educated macrophages alleviate systemic lupus erythematosus by promoting efferocytosis and recruitment of IL-17(+) regulatory T cell. Stem Cell Res Ther 2022, 13, 484. [Google Scholar] [CrossRef]
- Jahr, S.; Hentze, H.; Englisch, S.; Hardt, D.; Fackelmayer, F.O.; Hesch, R.D.; Knippers, R. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res 2001, 61, 1659–1665. [Google Scholar]
- Lui, Y.Y.; Chik, K.W.; Chiu, R.W.; Ho, C.Y.; Lam, C.W.; Lo, Y.M. Predominant hematopoietic origin of cell-free DNA in plasma and serum after sex-mismatched bone marrow transplantation. Clin Chem 2002, 48, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014, 6, 224ra224. [Google Scholar] [CrossRef]
- Leon, S.A.; Shapiro, B.; Sklaroff, D.M.; Yaros, M.J. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res 1977, 37, 646–650. [Google Scholar]
- Kustanovich, A.; Schwartz, R.; Peretz, T.; Grinshpun, A. Life and death of circulating cell-free DNA. Cancer Biol Ther 2019, 20, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Chan, C.W.; Chan, K.C.; Cheng, S.H.; Wong, J.; Wong, V.W.; Wong, G.L.; Chan, S.L.; Mok, T.S.; Chan, H.L.; et al. Lengthening and shortening of plasma DNA in hepatocellular carcinoma patients. Proc Natl Acad Sci U S A 2015, 112, E1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Malíčková, K.; Duricová, D.; Bortlík, M.; Hrušková, Z.; Svobodová, B.; Machková, N.; Komárek, V.; Fučíková, T.; Janatková, I.; Zima, T.; et al. Impaired deoxyribonuclease I activity in patients with inflammatory bowel diseases. Autoimmune Dis 2011, 2011, 945861. [Google Scholar] [CrossRef]
- Bonaventura, A.; Liberale, L.; Carbone, F.; Vecchié, A.; Diaz-Cañestro, C.; Camici, G.G.; Montecucco, F.; Dallegri, F. The Pathophysiological Role of Neutrophil Extracellular Traps in Inflammatory Diseases. Thromb Haemost 2018, 118, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Takei, H.; Araki, A.; Watanabe, H.; Ichinose, A.; Sendo, F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J Leukoc Biol 1996, 59, 229–240. [Google Scholar] [CrossRef]
- D'Haens, G.; Ferrante, M.; Vermeire, S.; Baert, F.; Noman, M.; Moortgat, L.; Geens, P.; Iwens, D.; Aerden, I.; Van Assche, G.; et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012, 18, 2218–2224. [Google Scholar] [CrossRef]
- Hirschfeld, J.; Chicca, I.J.; Moonen, C.G.J.; White, P.C.; Ling, M.R.; Wright, H.J.; Cooper, P.R.; Milward, M.R.; Chapple, I.L.C. Characterization, Quantification, and Visualization of Neutrophil Extracellular Traps. Methods Mol Biol 2023, 2588, 451–472. [Google Scholar] [CrossRef]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med 2016, 22, 146–153. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Kremer Hovinga, J.A.; Schatzberg, D.; Wagner, D.D.; Lämmle, B. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood 2012, 120, 1157–1164. [Google Scholar] [CrossRef]
- Li, T.; Wang, C.; Liu, Y.; Li, B.; Zhang, W.; Wang, L.; Yu, M.; Zhao, X.; Du, J.; Zhang, J.; et al. Neutrophil Extracellular Traps Induce Intestinal Damage and Thrombotic Tendency in Inflammatory Bowel Disease. J Crohns Colitis 2020, 14, 240–253. [Google Scholar] [CrossRef]
- Clancy, D.M.; Sullivan, G.P.; Moran, H.B.T.; Henry, C.M.; Reeves, E.P.; McElvaney, N.G.; Lavelle, E.C.; Martin, S.J. Extracellular Neutrophil Proteases Are Efficient Regulators of IL-1, IL-33, and IL-36 Cytokine Activity but Poor Effectors of Microbial Killing. Cell Rep 2018, 22, 2937–2950. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, Y.; Li, J.; Huang, J.; Zhang, L.; Feng, J.; Li, J.; Xia, Q.; Zhao, Q.; Huang, L.; et al. Eosinophil extracellular traps drive asthma progression through neuro-immune signals. Nat Cell Biol 2021, 23, 1060–1072. [Google Scholar] [CrossRef]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef]
- Yamamoto, S.; Azuma, E.; Muramatsu, M.; Hamashima, T.; Ishii, Y.; Sasahara, M. Significance of Extracellular Vesicles: Pathobiological Roles in Disease. Cell Struct Funct 2016, 41, 137–143. [Google Scholar] [CrossRef]
- Gelderman, M.P.; Simak, J. Flow cytometric analysis of cell membrane microparticles. Methods Mol Biol 2008, 484, 79–93. [Google Scholar] [CrossRef]
- Díaz-Garrido, N.; Badia, J.; Baldomà, L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J Extracell Vesicles 2021, 10, e12161. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, F.; Zhang, Q.; Liu, Y.; You, P.; Sun, S.; Lin, J.; Chen, N. Salivary exosomal PSMA7: a promising biomarker of inflammatory bowel disease. Protein Cell 2017, 8, 686–695. [Google Scholar] [CrossRef]
- Barrachina, F.; Battistone, M.A.; Castillo, J.; Mallofré, C.; Jodar, M.; Breton, S.; Oliva, R. Sperm acquire epididymis-derived proteins through epididymosomes. Hum Reprod 2022, 37, 651–668. [Google Scholar] [CrossRef]
- Tong, L.; Hao, H.; Zhang, Z.; Lv, Y.; Liang, X.; Liu, Q.; Liu, T.; Gong, P.; Zhang, L.; Cao, F.; et al. Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics 2021, 11, 8570–8586. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006, Chapter 3, Unit 3.22. [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Zhou, H.; Pisitkun, T.; Aponte, A.; Yuen, P.S.; Hoffert, J.D.; Yasuda, H.; Hu, X.; Chawla, L.; Shen, R.F.; Knepper, M.A.; et al. Exosomal Fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney Int 2006, 70, 1847–1857. [Google Scholar] [CrossRef]
- Borges, F.T.; Reis, L.A.; Schor, N. Extracellular vesicles: structure, function, and potential clinical uses in renal diseases. Braz J Med Biol Res 2013, 46, 824–830. [Google Scholar] [CrossRef]
- Vrablicova, Z.; Tomova, K.; Tothova, L.; Babickova, J.; Gromova, B.; Konecna, B.; Liptak, R.; Hlavaty, T.; Gardlik, R. Nuclear and Mitochondrial Circulating Cell-Free DNA Is Increased in Patients With Inflammatory Bowel Disease in Clinical Remission. Front Med (Lausanne) 2020, 7, 593316. [Google Scholar] [CrossRef]
- Valter, M.; Verstockt, S.; Finalet Ferreiro, J.A.; Cleynen, I. Extracellular Vesicles in Inflammatory Bowel Disease: Small Particles, Big Players. J Crohns Colitis 2021, 15, 499–510. [Google Scholar] [CrossRef]
- Asea, A.; Rehli, M.; Kabingu, E.; Boch, J.A.; Bare, O.; Auron, P.E.; Stevenson, M.A.; Calderwood, S.K. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem 2002, 277, 15028–15034. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, J.; Tang, X.; Rui, K.; Tian, X.; Ma, J.; Ma, B.; Xu, H.; Lu, L.; Wang, S. Exosomes released by granulocytic myeloid-derived suppressor cells attenuate DSS-induced colitis in mice. Oncotarget 2016, 7, 15356–15368. [Google Scholar] [CrossRef]
- Leoni, G.; Neumann, P.A.; Kamaly, N.; Quiros, M.; Nishio, H.; Jones, H.R.; Sumagin, R.; Hilgarth, R.S.; Alam, A.; Fredman, G.; et al. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest 2015, 125, 1215–1227. [Google Scholar] [CrossRef]
- Zhang, X.; Deeke, S.A.; Ning, Z.; Starr, A.E.; Butcher, J.; Li, J.; Mayne, J.; Cheng, K.; Liao, B.; Li, L.; et al. Metaproteomics reveals associations between microbiome and intestinal extracellular vesicle proteins in pediatric inflammatory bowel disease. Nat Commun 2018, 9, 2873. [Google Scholar] [CrossRef]
- Cohen, S.; Houben, A.; Segal, D. Extrachromosomal circular DNA derived from tandemly repeated genomic sequences in plants. Plant J 2008, 53, 1027–1034. [Google Scholar] [CrossRef]
- Cohen, S.; Yacobi, K.; Segal, D. Extrachromosomal circular DNA of tandemly repeated genomic sequences in Drosophila. Genome Res 2003, 13, 1133–1145. [Google Scholar] [CrossRef]
- Møller, H.D.; Ramos-Madrigal, J.; Prada-Luengo, I.; Gilbert, M.T.P.; Regenberg, B. Near-Random Distribution of Chromosome-Derived Circular DNA in the Condensed Genome of Pigeons and the Larger, More Repeat-Rich Human Genome. Genome Biol Evol 2020, 12, 3762–3777. [Google Scholar] [CrossRef]
- Kumar, P.; Dillon, L.W.; Shibata, Y.; Jazaeri, A.A.; Jones, D.R.; Dutta, A. Normal and Cancerous Tissues Release Extrachromosomal Circular DNA (eccDNA) into the CirculationHuman and Mouse microDNA in Circulation. Molecular Cancer Research 2017, 15, 1197–1205. [Google Scholar] [CrossRef]
- Dillon, L.W.; Kumar, P.; Shibata, Y.; Wang, Y.H.; Willcox, S.; Griffith, J.D.; Pommier, Y.; Takeda, S.; Dutta, A. Production of Extrachromosomal MicroDNAs Is Linked to Mismatch Repair Pathways and Transcriptional Activity. Cell Rep 2015, 11, 1749–1759. [Google Scholar] [CrossRef]
- Meng, X.; Qi, X.; Guo, H.; Cai, M.; Li, C.; Zhu, J.; Chen, F.; Guo, H.; Li, J.; Zhao, Y.; et al. Novel role for non-homologous end joining in the formation of double minutes in methotrexate-resistant colon cancer cells. J Med Genet 2015, 52, 135–144. [Google Scholar] [CrossRef]
- Møller, H.D.; Lin, L.; Xiang, X.; Petersen, T.S.; Huang, J.; Yang, L.; Kjeldsen, E.; Jensen, U.B.; Zhang, X.; Liu, X.; et al. CRISPR-C: circularization of genes and chromosome by CRISPR in human cells. Nucleic Acids Res 2018, 46, e131. [Google Scholar] [CrossRef]
- Shoshani, O.; Brunner, S.F.; Yaeger, R.; Ly, P.; Nechemia-Arbely, Y.; Kim, D.H.; Fang, R.; Castillon, G.A.; Yu, M.; Li, J.S.Z.; et al. Chromothripsis drives the evolution of gene amplification in cancer. Nature 2021, 591, 137–141. [Google Scholar] [CrossRef]
- Noer, J.B.; Hørsdal, O.K.; Xiang, X.; Luo, Y.; Regenberg, B. Extrachromosomal circular DNA in cancer: history, current knowledge, and methods. Trends in Genetics 2022, 38, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Arrey, G.; Keating, S.T.; Regenberg, B. A unifying model for extrachromosomal circular DNA load in eukaryotic cells. Semin Cell Dev Biol 2022, 128, 40–50. [Google Scholar] [CrossRef]
- Noer, J.B.; Hørsdal, O.K.; Xiang, X.; Luo, Y.; Regenberg, B. Extrachromosomal circular DNA in cancer: history, current knowledge, and methods. Trends Genet 2022, 38, 766–781. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr. Pillars article: approaching the asymptote? Evolution and revolution in immunology. Cold spring harb symp quant biol. 1989. 54: 1-13. J Immunol 2013, 191, 4475–4487. [Google Scholar]
- Matzinger, P. Tolerance, danger, and the extended family. Annu Rev Immunol 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Cao, X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat Rev Immunol 2016, 16, 35–50. [Google Scholar] [CrossRef]
- Kindrachuk, J.; Potter, J.E.; Brownlie, R.; Ficzycz, A.D.; Griebel, P.J.; Mookherjee, N.; Mutwiri, G.K.; Babiuk, L.A.; Napper, S. Nucleic acids exert a sequence-independent cooperative effect on sequence-dependent activation of Toll-like receptor 9. J Biol Chem 2007, 282, 13944–13953. [Google Scholar] [CrossRef]
- Abrams, S.T.; Zhang, N.; Manson, J.; Liu, T.; Dart, C.; Baluwa, F.; Wang, S.S.; Brohi, K.; Kipar, A.; Yu, W.; et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med 2013, 187, 160–169. [Google Scholar] [CrossRef]
- Li, Y.; Berke, I.C.; Modis, Y. DNA binding to proteolytically activated TLR9 is sequence-independent and enhanced by DNA curvature. Embo j 2012, 31, 919–931. [Google Scholar] [CrossRef]
- Ligi, D.; Lo Sasso, B.; Giglio, R.V.; Maniscalco, R.; DellaFranca, C.; Agnello, L.; Ciaccio, M.; Mannello, F. Circulating histones contribute to monocyte and MDW alterations as common mediators in classical and COVID-19 sepsis. Crit Care 2022, 26, 260. [Google Scholar] [CrossRef] [PubMed]
- Rekvig, O.P.; Hannestad, K. Human autoantibodies that react with both cell nuclei and plasma membranes display specificity for the octamer of histones H2A, H2B, H3, and H4 in high salt. J Exp Med 1980, 152, 1720–1733. [Google Scholar] [CrossRef] [PubMed]
- Cavalier, E.; Guiot, J.; Lechner, K.; Dutsch, A.; Eccleston, M.; Herzog, M.; Bygott, T.; Schomburg, A.; Kelly, T.; Holdenrieder, S. Circulating Nucleosomes as Potential Markers to Monitor COVID-19 Disease Progression. Front Mol Biosci 2021, 8, 600881. [Google Scholar] [CrossRef]
- Marsman, G.; Zeerleder, S.; Luken, B.M. Extracellular histones, cell-free DNA, or nucleosomes: differences in immunostimulation. Cell Death Dis 2016, 7, e2518. [Google Scholar] [CrossRef]
- Tsung, A.; Sahai, R.; Tanaka, H.; Nakao, A.; Fink, M.P.; Lotze, M.T.; Yang, H.; Li, J.; Tracey, K.J.; Geller, D.A.; et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med 2005, 201, 1135–1143. [Google Scholar] [CrossRef]
- Deng, J.; Pan, W.; Ji, N.; Liu, N.; Chen, Q.; Chen, J.; Sun, Y.; Xie, L.; Chen, Q. Cell-Free DNA Promotes Inflammation in Patients With Oral Lichen Planus via the STING Pathway. Front Immunol 2022, 13, 838109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, G.; Luo, R.; Lei, J.; Song, Y.; Wang, B.; Ma, L.; Liao, Z.; Ke, W.; Liu, H.; et al. Cytosolic escape of mitochondrial DNA triggers cGAS-STING-NLRP3 axis-dependent nucleus pulposus cell pyroptosis. Exp Mol Med 2022, 54, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.R.; El Messaoudi, S.; Gahan, P.B.; Anker, P.; Stroun, M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev 2016, 35, 347–376. [Google Scholar] [CrossRef]
- Li, J.; Huynh, L.; Cornwell, W.D.; Tang, M.S.; Simborio, H.; Huang, J.; Kosmider, B.; Rogers, T.J.; Zhao, H.; Steinberg, M.B.; et al. Electronic Cigarettes Induce Mitochondrial DNA Damage and Trigger TLR9 (Toll-Like Receptor 9)-Mediated Atherosclerosis. Arterioscler Thromb Vasc Biol 2021, 41, 839–853. [Google Scholar] [CrossRef]
- Tumburu, L.; Ghosh-Choudhary, S.; Seifuddin, F.T.; Barbu, E.A.; Yang, S.; Ahmad, M.M.; Wilkins, L.H.W.; Tunc, I.; Sivakumar, I.; Nichols, J.S.; et al. Circulating mitochondrial DNA is a proinflammatory DAMP in sickle cell disease. Blood 2021, 137, 3116–3126. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Ueki, S.; Kamide, Y.; Miyabe, Y.; Fukuchi, M.; Yokoyama, Y.; Furukawa, T.; Azuma, N.; Oka, N.; Takeuchi, H.; et al. Increased Circulating Cell-Free DNA in Eosinophilic Granulomatosis With Polyangiitis: Implications for Eosinophil Extracellular Traps and Immunothrombosis. Front Immunol 2021, 12, 801897. [Google Scholar] [CrossRef]
- Johnson, P.; Zhou, Q.; Dao, D.Y.; Lo, Y.M.D. Circulating biomarkers in the diagnosis and management of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2022, 19, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Huang, X.; Xu, M.; Qin, Z.; Zhang, F.; Hua, F.; Jiang, X.; Wang, Y. Value of circulating miRNA-21 in the diagnosis of subclinical diabetic cardiomyopathy. Mol Cell Endocrinol 2020, 518, 110944. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, F.; Dunger, N.; Deml, L.; Herfarth, H.; Schölmerich, J.; Falk, W. CpG motifs of bacterial DNA exacerbate colitis of dextran sulfate sodium-treated mice. Eur J Immunol 2002, 32, 2084–2092. [Google Scholar] [CrossRef]
- Hajizadeh, S.; DeGroot, J.; TeKoppele, J.M.; Tarkowski, A.; Collins, L.V. Extracellular mitochondrial DNA and oxidatively damaged DNA in synovial fluid of patients with rheumatoid arthritis. Arthritis Res Ther 2003, 5, R234–240. [Google Scholar] [CrossRef]
- Rachmilewitz, D.; Katakura, K.; Karmeli, F.; Hayashi, T.; Reinus, C.; Rudensky, B.; Akira, S.; Takeda, K.; Lee, J.; Takabayashi, K.; et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 2004, 126, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Műzes, G.; Sipos, F.; Fűri, I.; Constantinovits, M.; Spisák, S.; Wichmann, B.; Valcz, G.; Tulassay, Z.; Molnár, B. Preconditioning with intravenous colitic cell-free DNA prevents DSS-colitis by altering TLR9-associated gene expression profile. Dig Dis Sci 2014, 59, 2935–2946. [Google Scholar] [CrossRef]
- Delgado, M.A.; Elmaoued, R.A.; Davis, A.S.; Kyei, G.; Deretic, V. Toll-like receptors control autophagy. Embo j 2008, 27, 1110–1121. [Google Scholar] [CrossRef]
- Műzes, G.; Kiss, A.L.; Tulassay, Z.; Sipos, F. Cell-free DNA-induced alteration of autophagy response and TLR9-signaling: Their relation to amelioration of DSS-colitis. Comp Immunol Microbiol Infect Dis 2017, 52, 48–57. [Google Scholar] [CrossRef]
- Zhao, F.; Zheng, T.; Gong, W.; Wu, J.; Xie, H.; Li, W.; Zhang, R.; Liu, P.; Liu, J.; Wu, X.; et al. Extracellular vesicles package dsDNA to aggravate Crohn's disease by activating the STING pathway. Cell Death Dis 2021, 12, 815. [Google Scholar] [CrossRef]
- Schiller, M.; Bekeredjian-Ding, I.; Heyder, P.; Blank, N.; Ho, A.D.; Lorenz, H.M. Autoantigens are translocated into small apoptotic bodies during early stages of apoptosis. Cell Death Differ 2008, 15, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Navratil, J.S.; Sabatine, J.M.; Ahearn, J.M. Apoptosis and immune responses to self. Rheum Dis Clin North Am 2004, 30, 193–212. [Google Scholar] [CrossRef]
- Zhou, Z.; Ménard, H.A. Autoantigenic posttranslational modifications of proteins: does it apply to rheumatoid arthritis? Curr Opin Rheumatol 2002, 14, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Rondas, D.; Crèvecoeur, I.; D'Hertog, W.; Ferreira, G.B.; Staes, A.; Garg, A.D.; Eizirik, D.L.; Agostinis, P.; Gevaert, K.; Overbergh, L.; et al. Citrullinated glucose-regulated protein 78 is an autoantigen in type 1 diabetes. Diabetes 2015, 64, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 2013, 5, 178ra140. [Google Scholar] [CrossRef]
- Makrygiannakis, D.; af Klint, E.; Lundberg, I.E.; Löfberg, R.; Ulfgren, A.K.; Klareskog, L.; Catrina, A.I. Citrullination is an inflammation-dependent process. Ann Rheum Dis 2006, 65, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xu, H.; Bashir, M.; Sharma, M.; Williams, K.; Anyanwu, C.; Okawa, J.; Werth, V. Proinflammatory microvesicles in patients with cutaneous lupus erythematosus. In Proceedings of the Journal of Investigative Dermatology, 2014; pp. S13-S13.
- Piette, E.W.; Foering, K.P.; Chang, A.Y.; Okawa, J.; Ten Have, T.R.; Feng, R.; Werth, V.P. Impact of smoking in cutaneous lupus erythematosus. Arch Dermatol 2012, 148, 317–322. [Google Scholar] [CrossRef]
- Gerovska, D.; Araúzo-Bravo, M.J. Skeletal Muscles of Sedentary and Physically Active Aged People Have Distinctive Genic Extrachromosomal Circular DNA Profiles. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Gerovska, D.; Araúzo-Bravo, M.J. Systemic Lupus Erythematosus Patients with DNASE1L3·Deficiency Have a Distinctive and Specific Genic Circular DNA Profile in Plasma. Cells 2023, 12. [Google Scholar] [CrossRef]
- Prada-Luengo, I.; Møller, H.D.; Henriksen, R.A.; Gao, Q.; Larsen, Camilla E.; Alizadeh, S.; Maretty, L.; Houseley, J.; Regenberg, B. Replicative aging is associated with loss of genetic heterogeneity from extrachromosomal circular DNA in Saccharomyces cerevisiae. Nucleic Acids Research 2020, 48, 7883-7898. [CrossRef]
- Infante, A.; Gener, B.; Vázquez, M.; Olivares, N.; Arrieta, A.; Grau, G.; Llano, I.; Madero, L.; Bueno, A.M.; Sagastizabal, B.; et al. Reiterative infusions of MSCs improve pediatric osteogenesis imperfecta eliciting a pro-osteogenic paracrine response: TERCELOI clinical trial. Clin Transl Med 2021, 11, e265. [Google Scholar] [CrossRef] [PubMed]
- Araúzo-Bravo, M.J.; Erichsen, L.; Ott, P.; Beermann, A.; Sheikh, J.; Gerovska, D.; Thimm, C.; Bendhack, M.L.; Santourlidis, S. Consistent DNA Hypomethylations in Prostate Cancer. Int J Mol Sci 2022, 24. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, E.C.; Murphy, C.; O'Neill, L.A.; Creagh, E.M. The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis. Mucosal Immunol 2010, 3, 17–28. [Google Scholar] [CrossRef]
- Cario, E.; Gerken, G.; Podolsky, D. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 2007, 132, 1359–1374. [Google Scholar] [CrossRef]
- Lee, J.; Mo, J.H.; Katakura, K.; Alkalay, I.; Rucker, A.N.; Liu, Y.T.; Lee, H.K.; Shen, C.; Cojocaru, G.; Shenouda, S.; et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol 2006, 8, 1327–1336. [Google Scholar] [CrossRef]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef]
- Bleich, A.; Janus, L.M.; Smoczek, A.; Westendorf, A.M.; Strauch, U.; Mähler, M.; Hedrich, H.J.; Fichtner-Feigl, S.; Schölmerich, J.; Falk, W.; et al. CpG motifs of bacterial DNA exert protective effects in mouse models of IBD by antigen-independent tolerance induction. Gastroenterology 2009, 136, 278–287. [Google Scholar] [CrossRef]
- Obermeier, F.; Strauch, U.G.; Dunger, N.; Grunwald, N.; Rath, H.C.; Herfarth, H.; Schölmerich, J.; Falk, W. In vivo CpG DNA/toll-like receptor 9 interaction induces regulatory properties in CD4+CD62L+ T cells which prevent intestinal inflammation in the SCID transfer model of colitis. Gut 2005, 54, 1428–1436. [Google Scholar] [CrossRef]
- Rachmilewitz, D.; Karmeli, F.; Takabayashi, K.; Hayashi, T.; Leider-Trejo, L.; Lee, J.; Leoni, L.M.; Raz, E. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology 2002, 122, 1428–1441. [Google Scholar] [CrossRef]
- Lee, J.; Rachmilewitz, D.; Raz, E. Homeostatic effects of TLR9 signaling in experimental colitis. Ann N Y Acad Sci 2006, 1072, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Rachmilewitz, D.; Karmeli, F.; Shteingart, S.; Lee, J.; Takabayashi, K.; Raz, E. Immunostimulatory oligonucleotides inhibit colonic proinflammatory cytokine production in ulcerative colitis. Inflamm Bowel Dis 2006, 12, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Fuse, K.; Katakura, K.; Sakamoto, N.; Ohira, H. Toll-like receptor 9 gene mutations and polymorphisms in Japanese ulcerative colitis patients. World J Gastroenterol 2010, 16, 5815–5821. [Google Scholar] [CrossRef]
- Török, H.P.; Glas, J.; Tonenchi, L.; Bruennler, G.; Folwaczny, M.; Folwaczny, C. Crohn's disease is associated with a toll-like receptor-9 polymorphism. Gastroenterology 2004, 127, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.C.; Liang, C.M.; Lai, C.Y.; Liang, S.M. Involvement of heat shock protein (Hsp)90 beta but not Hsp90 alpha in antiapoptotic effect of CpG-B oligodeoxynucleotide. J Immunol 2007, 178, 6100–6108. [Google Scholar] [CrossRef]
- Kuo, C.C.; Liang, S.M.; Liang, C.M. CpG-B oligodeoxynucleotide promotes cell survival via up-regulation of Hsp70 to increase Bcl-xL and to decrease apoptosis-inducing factor translocation. J Biol Chem 2006, 281, 38200–38207. [Google Scholar] [CrossRef] [PubMed]
- Malago, J.J.; Koninkx, J.F.; van Dijk, J.E. The heat shock response and cytoprotection of the intestinal epithelium. Cell Stress Chaperones 2002, 7, 191–199. [Google Scholar] [CrossRef]
- O'Hara, J.R.; Feener, T.D.; Fischer, C.D.; Buret, A.G. Campylobacter jejuni disrupts protective Toll-like receptor 9 signaling in colonic epithelial cells and increases the severity of dextran sulfate sodium-induced colitis in mice. Infect Immun 2012, 80, 1563–1571. [Google Scholar] [CrossRef]
- Abreu, M.T. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 2010, 10, 131–144. [Google Scholar] [CrossRef]
- Creed, T.J.; Lee, R.W.; Newcomb, P.V.; di Mambro, A.J.; Raju, M.; Dayan, C.M. The effects of cytokines on suppression of lymphocyte proliferation by dexamethasone. J Immunol 2009, 183, 164–171. [Google Scholar] [CrossRef]
- Kuznetsov, N.V.; Zargari, A.; Gielen, A.W.; von Stein, O.D.; Musch, E.; Befrits, R.; Lofberg, R.; von Stein, P. Biomarkers can predict potential clinical responders to DIMS0150 a toll-like receptor 9 agonist in ulcerative colitis patients. BMC Gastroenterol 2014, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Musch, E.; Lutfi, T.; von Stein, P.; Zargari, A.; Admyre, C.; Malek, M.; Löfberg, R.; von Stein, O.D. Topical treatment with the Toll-like receptor agonist DIMS0150 has potential for lasting relief of symptoms in patients with chronic active ulcerative colitis by restoring glucocorticoid sensitivity. Inflamm Bowel Dis 2013, 19, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Bloom, S.; Scaldaferri, F.; Gerardi, V.; Admyre, C.; Karlsson, Å.; Knittel, T.; Kowalski, J.; Lukas, M.; Löfberg, R.; et al. Clinical Effects of a Topically Applied Toll-like Receptor 9 Agonist in Active Moderate-to-Severe Ulcerative Colitis. J Crohns Colitis 2016, 10, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Reinisch, W.; Peyrin-Biroulet, L.; Scaldaferri, F.; Admyre, C.; Knittel, T.; Kowalski, J.; Neurath, M.F.; Hawkey, C. Clinical efficacy of the Toll-like receptor 9 agonist cobitolimod using patient-reported-outcomes defined clinical endpoints in patients with ulcerative colitis. Dig Liver Dis 2018, 50, 1019–1029. [Google Scholar] [CrossRef]
- Atreya, R.; Peyrin-Biroulet, L.; Klymenko, A.; Augustyn, M.; Bakulin, I.; Slankamenac, D.; Miheller, P.; Gasbarrini, A.; Hébuterne, X.; Arnesson, K.; et al. Cobitolimod for moderate-to-severe, left-sided ulcerative colitis (CONDUCT): a phase 2b randomised, double-blind, placebo-controlled, dose-ranging induction trial. Lancet Gastroenterol Hepatol 2020, 5, 1063–1075. [Google Scholar] [CrossRef]
- Dotan, I.; Levy-Nissenbaum, E.; Chowers, Y.; Fich, A.; Israeli, E.; Adar, T.; Shteingart, S.; Soreq, H.; Goldin, E. Ameliorating Active Ulcerative Colitis via an Orally Available Toll-Like Receptor-9 Modifier: A Prospective Open-Label, Multicenter Phase II Trial. Dig Dis Sci 2016, 61, 3246–3254. [Google Scholar] [CrossRef]
- Burman, C.; Ktistakis, N.T. Autophagosome formation in mammalian cells. Semin Immunopathol 2010, 32, 397–413. [Google Scholar] [CrossRef]
- Wu, J.; Yan, N. No Longer A One-Trick Pony: STING Signaling Activity Beyond Interferon. J Mol Biol 2022, 434, 167257. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, R.; Tang, D. The STING1 network regulates autophagy and cell death. Signal Transduct Target Ther 2021, 6, 208. [Google Scholar] [CrossRef]
- Gui, X.; Yang, H.; Li, T.; Tan, X.; Shi, P.; Li, M.; Du, F.; Chen, Z.J. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature 2019, 567, 262–266. [Google Scholar] [CrossRef]
- Konno, H.; Konno, K.; Barber, G.N. Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell 2013, 155, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, H.; Wang, C.; Li, Y.; Tian, H.; Siraj, S.; Sehgal, S.A.; Wang, X.; Wang, J.; Shang, Y.; et al. STING directly activates autophagy to tune the innate immune response. Cell Death Differ 2019, 26, 1735–1749. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, T.; Bodda, C.; Krapp, C.; Zhang, B.C.; Christensen, M.H.; Sun, C.; Reinert, L.; Cai, Y.; Jensen, S.B.; Skouboe, M.K.; et al. Attenuation of cGAS-STING signaling is mediated by a p62/SQSTM1-dependent autophagy pathway activated by TBK1. Embo j 2018, 37. [Google Scholar] [CrossRef]
- Saitoh, T.; Fujita, N.; Hayashi, T.; Takahara, K.; Satoh, T.; Lee, H.; Matsunaga, K.; Kageyama, S.; Omori, H.; Noda, T.; et al. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc Natl Acad Sci U S A 2009, 106, 20842–20846. [Google Scholar] [CrossRef]
- Gonugunta, V.K.; Sakai, T.; Pokatayev, V.; Yang, K.; Wu, J.; Dobbs, N.; Yan, N. Trafficking-Mediated STING Degradation Requires Sorting to Acidified Endolysosomes and Can Be Targeted to Enhance Anti-tumor Response. Cell Rep 2017, 21, 3234–3242. [Google Scholar] [CrossRef]
- Martin, G.R.; Blomquist, C.M.; Henare, K.L.; Jirik, F.R. Stimulator of interferon genes (STING) activation exacerbates experimental colitis in mice. Sci Rep 2019, 9, 14281. [Google Scholar] [CrossRef]
- Howell, K.J.; Kraiczy, J.; Nayak, K.M.; Gasparetto, M.; Ross, A.; Lee, C.; Mak, T.N.; Koo, B.K.; Kumar, N.; Lawley, T.; et al. DNA Methylation and Transcription Patterns in Intestinal Epithelial Cells From Pediatric Patients With Inflammatory Bowel Diseases Differentiate Disease Subtypes and Associate With Outcome. Gastroenterology 2018, 154, 585–598. [Google Scholar] [CrossRef]
- An, X.; Zhu, Y.; Zheng, T.; Wang, G.; Zhang, M.; Li, J.; Ji, H.; Li, S.; Yang, S.; Xu, D.; et al. An Analysis of the Expression and Association with Immune Cell Infiltration of the cGAS/STING Pathway in Pan-Cancer. Mol Ther Nucleic Acids 2019, 14, 80–89. [Google Scholar] [CrossRef]
- Canesso, M.C.C.; Lemos, L.; Neves, T.C.; Marim, F.M.; Castro, T.B.R.; Veloso É, S.; Queiroz, C.P.; Ahn, J.; Santiago, H.C.; Martins, F.S.; et al. The cytosolic sensor STING is required for intestinal homeostasis and control of inflammation. Mucosal Immunol 2018, 11, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Aden, K.; Tran, F.; Ito, G.; Sheibani-Tezerji, R.; Lipinski, S.; Kuiper, J.W.; Tschurtschenthaler, M.; Saveljeva, S.; Bhattacharyya, J.; Häsler, R.; et al. ATG16L1 orchestrates interleukin-22 signaling in the intestinal epithelium via cGAS-STING. J Exp Med 2018, 215, 2868–2886. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yan, W.; Zhang, Y.; Zhao, X.; Tao, M.; Feng, Q.; Fu, Y. ANP attenuates intestinal inflammation by regulating STING pathway. Available at SSRN 3756807 2021. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Tao, M.; Zhao, X.; Feng, Q.; Fei, X.; Fu, Y. Atrial Natriuretic Peptide Attenuates Colitis via Inhibition of the cGAS-STING Pathway in Colonic Epithelial Cells. Int J Biol Sci 2022, 18, 1737–1754. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yang, D.; Wang, B.; Wu, C.; Wu, Y.; Li, S.; Liu, X.; Lassen, K.; Dai, L.; Yang, S. Gasdermin D in macrophages restrains colitis by controlling cGAS-mediated inflammation. Sci Adv 2020, 6, eaaz6717. [Google Scholar] [CrossRef]
- Erttmann, S.F.; Swacha, P.; Aung, K.M.; Brindefalk, B.; Jiang, H.; Härtlova, A.; Uhlin, B.E.; Wai, S.N.; Gekara, N.O. The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity 2022, 55, 847–861.e810. [Google Scholar] [CrossRef]
- Ahn, J.; Son, S.; Oliveira, S.C.; Barber, G.N. STING-Dependent Signaling Underlies IL-10 Controlled Inflammatory Colitis. Cell Rep 2017, 21, 3873–3884. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.; Adura, C.; Gao, P.; Luz, A.; Lama, L.; Asano, Y.; Okamoto, R.; Imaeda, T.; Aida, J.; Rothamel, K.; et al. Small molecule inhibition of cGAS reduces interferon expression in primary macrophages from autoimmune mice. Nat Commun 2017, 8, 750. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, Y.; Zhou, X.; Zhou, J. Inhibitory targeting cGAS-STING-TBK1 axis: Emerging strategies for autoimmune diseases therapy. Front Immunol 2022, 13, 954129. [Google Scholar] [CrossRef]
- Haag, S.M.; Gulen, M.F.; Reymond, L.; Gibelin, A.; Abrami, L.; Decout, A.; Heymann, M.; van der Goot, F.G.; Turcatti, G.; Behrendt, R.; et al. Targeting STING with covalent small-molecule inhibitors. Nature 2018, 559, 269–273. [Google Scholar] [CrossRef]
- Gantier, M.; Ullah, T.; Johansen, M.; Balka, K.; Ambrose, R.; Gearing, L.; Wenholz, D.; Zeng, J.; Miemczyk, S.; Nguyen, D. Pharmacological inhibition of TBK1/IKKε blunts COVID-19 immunopathology. 2022.
- Hong, Z.; Mei, J.; Li, C.; Bai, G.; Maimaiti, M.; Hu, H.; Yu, W.; Sun, L.; Zhang, L.; Cheng, D.; et al. STING inhibitors target the cyclic dinucleotide binding pocket. Proc Natl Acad Sci U S A 2021, 118. [Google Scholar] [CrossRef]
- Olagnier, D.; Brandtoft, A.M.; Gunderstofte, C.; Villadsen, N.L.; Krapp, C.; Thielke, A.L.; Laustsen, A.; Peri, S.; Hansen, A.L.; Bonefeld, L.; et al. Nrf2 negatively regulates STING indicating a link between antiviral sensing and metabolic reprogramming. Nat Commun 2018, 9, 3506. [Google Scholar] [CrossRef]
- Lamprecht, A.; Schäfer, U.; Lehr, C.M. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm Res 2001, 18, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, M.; Yang, H. Recent Progress in the Diagnosis and Precise Nanocarrier-Mediated Therapy of Inflammatory Bowel Disease. J Inflamm Res 2021, 14, 1701–1716. [Google Scholar] [CrossRef] [PubMed]
- Coco, R.; Plapied, L.; Pourcelle, V.; Jérôme, C.; Brayden, D.J.; Schneider, Y.J.; Préat, V. Drug delivery to inflamed colon by nanoparticles: comparison of different strategies. Int J Pharm 2013, 440, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Narula, N.; Peyrin-Biroulet, L. Management Strategies to Improve Outcomes of Patients With Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 351–361.e355. [Google Scholar] [CrossRef]
- Cuvelier, C.A.; Quatacker, J.; Mielants, H.; De Vos, M.; Veys, E.; Roels, H.J. M-cells are damaged and increased in number in inflamed human ileal mucosa. Histopathology 1994, 24, 417–426. [Google Scholar] [CrossRef]
- Mohan, L.J.; Daly, J.S.; Ryan, B.M.; Ramtoola, Z. The future of nanomedicine in optimising the treatment of inflammatory bowel disease. Scand J Gastroenterol 2019, 54, 18–26. [Google Scholar] [CrossRef]
- Pichai, M.V.; Ferguson, L.R. Potential prospects of nanomedicine for targeted therapeutics in inflammatory bowel diseases. World J Gastroenterol 2012, 18, 2895–2901. [Google Scholar] [CrossRef]
- Song, W.; Shen, L.; Wang, Y.; Liu, Q.; Goodwin, T.J.; Li, J.; Dorosheva, O.; Liu, T.; Liu, R.; Huang, L. Synergistic and low adverse effect cancer immunotherapy by immunogenic chemotherapy and locally expressed PD-L1 trap. Nat Commun 2018, 9, 2237. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, J.; Pan, Y.; Gao, Y.; Huang, L.; Luan, X.; Lin, Z.; Zhu, W.; Li, Y.; Song, Y. Mesopore to Macropore Transformation of Metal-Organic Framework for Drug Delivery in Inflammatory Bowel Disease. Adv Healthc Mater 2021, 10, e2000973. [Google Scholar] [CrossRef]
- Youshia, J.; Lamprecht, A. Size-dependent nanoparticulate drug delivery in inflammatory bowel diseases. Expert Opin Drug Deliv 2016, 13, 281–294. [Google Scholar] [CrossRef]
- Xiao, B.; Xu, Z.; Viennois, E.; Zhang, Y.; Zhang, Z.; Zhang, M.; Han, M.K.; Kang, Y.; Merlin, D. Orally Targeted Delivery of Tripeptide KPV via Hyaluronic Acid-Functionalized Nanoparticles Efficiently Alleviates Ulcerative Colitis. Mol Ther 2017, 25, 1628–1640. [Google Scholar] [CrossRef] [PubMed]
- Charania, M.A.; Laroui, H.; Liu, H.; Viennois, E.; Ayyadurai, S.; Xiao, B.; Ingersoll, S.A.; Kalman, D.; Merlin, D. Intestinal epithelial CD98 directly modulates the innate host response to enteric bacterial pathogens. Infect Immun 2013, 81, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Liu, S.; Xie, X.N.; Tan, Z.R. Regulation profile of the intestinal peptide transporter 1 (PepT1). Drug Des Devel Ther 2017, 11, 3511–3517. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Y.; Li, P.; Weng, Y.; Kamada, N.; Jiang, H.; Smith, D.E. Expression and regulation of proton-coupled oligopeptide transporters in colonic tissue and immune cells of mice. Biochem Pharmacol 2018, 148, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Laroui, H.; Viennois, E.; Ayyadurai, S.; Charania, M.A.; Zhang, Y.; Zhang, Z.; Baker, M.T.; Zhang, B.; Gewirtz, A.T.; et al. Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice. Gastroenterology 2014, 146, 1289–1300.e1281-1219. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, M.; Torkvist, L.; Bresso, F.; Halfvarson, J.; Hellquist, A.; Anedda, F.; Assadi, G.; Lindgren, G.B.; Svanfeldt, M.; Janson, M.; et al. PepT1 oligopeptide transporter (SLC15A1) gene polymorphism in inflammatory bowel disease. Inflamm Bowel Dis 2009, 15, 1562–1569. [Google Scholar] [CrossRef]
- Farzi, B.; Young, D.; Scrimgeour, J.; Cetinkaya, C. Mechanical properties of P-selectin PSGL-1 bonds. Colloids Surf B Biointerfaces 2019, 173, 529–538. [Google Scholar] [CrossRef]
- Sakhalkar, H.S.; Dalal, M.K.; Salem, A.K.; Ansari, R.; Fu, J.; Kiani, M.F.; Kurjiaka, D.T.; Hanes, J.; Shakesheff, K.M.; Goetz, D.J. Leukocyte-inspired biodegradable particles that selectively and avidly adhere to inflamed endothelium in vitro and in vivo. Proc Natl Acad Sci U S A 2003, 100, 15895–15900. [Google Scholar] [CrossRef]
- Chung, C.H.; Jung, W.; Keum, H.; Kim, T.W.; Jon, S. Nanoparticles Derived from the Natural Antioxidant Rosmarinic Acid Ameliorate Acute Inflammatory Bowel Disease. ACS Nano 2020, 14, 6887–6896. [Google Scholar] [CrossRef]
- Sun, T.; Kwong, C.H.T.; Gao, C.; Wei, J.; Yue, L.; Zhang, J.; Ye, R.D.; Wang, R. Amelioration of ulcerative colitis via inflammatory regulation by macrophage-biomimetic nanomedicine. Theranostics 2020, 10, 10106–10119. [Google Scholar] [CrossRef]
- Xiao, B.; Merlin, D. Oral colon-specific therapeutic approaches toward treatment of inflammatory bowel disease. Expert Opin Drug Deliv 2012, 9, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, J.; Nejati, V.; Mahmoodi, M.; Ahmadi, M. Mesenchymal stem cells derived extracellular vesicles: A promising nanomedicine for drug delivery system. Biochem Pharmacol 2022, 203, 115167. [Google Scholar] [CrossRef] [PubMed]
- Keener, A.B. How extracellular vesicles can enhance drug delivery. Nature 2020, 582, S14–S14. [Google Scholar] [CrossRef]
- Reynolds, A.; Leake, D.; Boese, Q.; Scaringe, S.; Marshall, W.S.; Khvorova, A. Rational siRNA design for RNA interference. Nat Biotechnol 2004, 22, 326–330. [Google Scholar] [CrossRef]
- González, V.M.; Martín, M.E.; Fernández, G.; García-Sacristán, A. Use of Aptamers as Diagnostics Tools and Antiviral Agents for Human Viruses. Pharmaceuticals (Basel) 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.I.; Herrera, A.; Rossi, J.J.; Zhou, J. Current Advances in Aptamers for Cancer Diagnosis and Therapy. Cancers (Basel) 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Khvorova, A.; Watts, J.K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat Biotechnol 2017, 35, 238–248. [Google Scholar] [CrossRef]
- Gewirtz, A.T.; Sitaraman, S. Alicaforsen. Isis Pharmaceuticals. Curr Opin Investig Drugs 2001, 2, 1401–1406. [Google Scholar]
- Vainer, B.; Nielsen, O.H. Changed colonic profile of P-selectin, platelet-endothelial cell adhesion molecule-1 (PECAM-1), intercellular adhesion molecule-1 (ICAM-1), ICAM-2, and ICAM-3 in inflammatory bowel disease. Clin Exp Immunol 2000, 121, 242–247. [Google Scholar] [CrossRef]
- Schreiber, S.; Nikolaus, S.; Malchow, H.; Kruis, W.; Lochs, H.; Raedler, A.; Hahn, E.G.; Krummenerl, T.; Steinmann, G. Absence of efficacy of subcutaneous antisense ICAM-1 treatment of chronic active Crohn's disease. Gastroenterology 2001, 120, 1339–1346. [Google Scholar] [CrossRef]
- Yacyshyn, B.; Chey, W.Y.; Wedel, M.K.; Yu, R.Z.; Paul, D.; Chuang, E. A randomized, double-masked, placebo-controlled study of alicaforsen, an antisense inhibitor of intercellular adhesion molecule 1, for the treatment of subjects with active Crohn's disease. Clin Gastroenterol Hepatol 2007, 5, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Yacyshyn, B.R.; Bowen-Yacyshyn, M.B.; Jewell, L.; Tami, J.A.; Bennett, C.F.; Kisner, D.L.; Shanahan, W.R., Jr. A placebo-controlled trial of ICAM-1 antisense oligonucleotide in the treatment of Crohn's disease. Gastroenterology 1998, 114, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Yacyshyn, B.R.; Chey, W.Y.; Goff, J.; Salzberg, B.; Baerg, R.; Buchman, A.L.; Tami, J.; Yu, R.; Gibiansky, E.; Shanahan, W.R. Double blind, placebo controlled trial of the remission inducing and steroid sparing properties of an ICAM-1 antisense oligodeoxynucleotide, alicaforsen (ISIS 2302), in active steroid dependent Crohn's disease. Gut 2002, 51, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Greuter, T.; Biedermann, L.; Rogler, G.; Sauter, B.; Seibold, F. Alicaforsen, an antisense inhibitor of ICAM-1, as treatment for chronic refractory pouchitis after proctocolectomy: A case series. United European Gastroenterol J 2016, 4, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Greuter, T.; Vavricka, S.R.; Biedermann, L.; Pilz, J.; Borovicka, J.; Seibold, F.; Sauter, B.; Rogler, G. Alicaforsen, an Antisense Inhibitor of Intercellular Adhesion Molecule-1, in the Treatment for Left-Sided Ulcerative Colitis and Ulcerative Proctitis. Dig Dis 2018, 36, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Fantini, M.C.; Onali, S.; Zorzi, F.; Sancesario, G.; Bernardini, S.; Calabrese, E.; Viti, F.; Monteleone, I.; Biancone, L.; et al. Phase I clinical trial of Smad7 knockdown using antisense oligonucleotide in patients with active Crohn's disease. Mol Ther 2012, 20, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Kumberova, A.; Croft, N.M.; McKenzie, C.; Steer, H.W.; MacDonald, T.T. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest 2001, 108, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Neurath, M.F.; Ardizzone, S.; Di Sabatino, A.; Fantini, M.C.; Castiglione, F.; Scribano, M.L.; Armuzzi, A.; Caprioli, F.; Sturniolo, G.C.; et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn's disease. N Engl J Med 2015, 372, 1104–1113. [Google Scholar] [CrossRef]
- Suzuki, K.; Arumugam, S.; Yokoyama, J.; Kawauchi, Y.; Honda, Y.; Sato, H.; Aoyagi, Y.; Terai, S.; Okazaki, K.; Suzuki, Y.; et al. Pivotal Role of Carbohydrate Sulfotransferase 15 in Fibrosis and Mucosal Healing in Mouse Colitis. PLoS One 2016, 11, e0158967. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yokoyama, J.; Kawauchi, Y.; Honda, Y.; Sato, H.; Aoyagi, Y.; Terai, S.; Okazaki, K.; Suzuki, Y.; Sameshima, Y.; et al. Phase 1 Clinical Study of siRNA Targeting Carbohydrate Sulphotransferase 15 in Crohn's Disease Patients with Active Mucosal Lesions. J Crohns Colitis 2017, 11, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, X.; Chen, Y.; Zhang, J.; Wu, B.; Zheng, L.; Haruehanroengra, P.; Wang, R.; Li, S.; Lin, J.; et al. Crystal structure of an RNA-cleaving DNAzyme. Nat Commun 2017, 8, 2006. [Google Scholar] [CrossRef] [PubMed]
- Popp, V.; Gerlach, K.; Mott, S.; Turowska, A.; Garn, H.; Atreya, R.; Lehr, H.A.; Ho, I.C.; Renz, H.; Weigmann, B.; et al. Rectal Delivery of a DNAzyme That Specifically Blocks the Transcription Factor GATA3 and Reduces Colitis in Mice. Gastroenterology 2017, 152, 176–192.e175. [Google Scholar] [CrossRef] [PubMed]
- Murano, M.; Maemura, K.; Hirata, I.; Toshina, K.; Nishikawa, T.; Hamamoto, N.; Sasaki, S.; Saitoh, O.; Katsu, K. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol 2000, 120, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Fuss, I.; Schürmann, G.; Pettersson, S.; Arnold, K.; Müller-Lobeck, H.; Strober, W.; Herfarth, C.; Büschenfelde, K.H. Cytokine gene transcription by NF-kappa B family members in patients with inflammatory bowel disease. Ann N Y Acad Sci 1998, 859, 149–159. [Google Scholar] [CrossRef]
- Sands, B.E.; Feagan, B.G.; Sandborn, W.J.; Schreiber, S.; Peyrin-Biroulet, L.; Frédéric Colombel, J.; Rossiter, G.; Usiskin, K.; Ather, S.; Zhan, X.; et al. Mongersen (GED-0301) for Active Crohn's Disease: Results of a Phase 3 Study. Am J Gastroenterol 2020, 115, 738–745. [Google Scholar] [CrossRef]
- van Deventer, S.J.; Wedel, M.K.; Baker, B.F.; Xia, S.; Chuang, E.; Miner, P.B., Jr. A phase II dose ranging, double-blind, placebo-controlled study of alicaforsen enema in subjects with acute exacerbation of mild to moderate left-sided ulcerative colitis. Aliment Pharmacol Ther 2006, 23, 1415–1425. [Google Scholar] [CrossRef]
- Song, L.; Chang, R.; Sun, X.; Lu, L.; Gao, H.; Lu, H.; Lin, R.; Xu, X.; Liu, Z.; Zhan, L. Macrophage-derived EDA-A2 inhibits intestinal stem cells by targeting miR-494/EDA2R/β-catenin signaling in mice. Commun Biol 2021, 4, 213. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, Y.; Wang, Y.; Sun, W.; Wei, M.; Yuan, L.; Yang, G. Selective Encapsulation of Therapeutic mRNA in Engineered Extracellular Vesicles by DNA Aptamer. Nano Lett 2021, 21, 8563–8570. [Google Scholar] [CrossRef]
- Fay, N.C.; Muthusamy, B.P.; Nyugen, L.P.; Desai, R.C.; Taverner, A.; MacKay, J.; Seung, M.; Hunter, T.; Liu, K.; Chandalia, A.; et al. A Novel Fusion of IL-10 Engineered to Traffic across Intestinal Epithelium to Treat Colitis. J Immunol 2020, 205, 3191–3204. [Google Scholar] [CrossRef]
- Stephens, M.; Keane, K.; Roizes, S.; Liao, S.; Weid, P.V. Mincle-binding DNA aptamer demonstrates therapeutic potential in a model of inflammatory bowel disease. Mol Ther Nucleic Acids 2022, 28, 935–947. [Google Scholar] [CrossRef]
- Prochazka, P.; Hrabeta, J.; Vícha, A.; Eckschlager, T. Expulsion of amplified MYCN from homogenously staining chromosomal regions in neuroblastoma cell lines after cultivation with cisplatin, doxorubicin, hydroxyurea, and vincristine. Cancer Genet Cytogenet 2010, 196, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Valent, A.; Bénard, J.; Clausse, B.; Barrois, M.; Valteau-Couanet, D.; Terrier-Lacombe, M.J.; Spengler, B.; Bernheim, A. In vivo elimination of acentric double minutes containing amplified MYCN from neuroblastoma tumor cells through the formation of micronuclei. Am J Pathol 2001, 158, 1579–1584. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Waddelow, T.; Forseth, B.; Davidson, K.; Scott, J.; Wahl, G. Hydroxyurea accelerates loss of extrachromosomally amplified genes from tumor cells. Cancer Res 1991, 51, 6273–6279. [Google Scholar]
- Yu, L.; Zhao, Y.; Quan, C.; Ji, W.; Zhu, J.; Huang, Y.; Guan, R.; Sun, D.; Jin, Y.; Meng, X.; et al. Gemcitabine eliminates double minute chromosomes from human ovarian cancer cells. PLoS One 2013, 8, e71988. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Faivre, S.; Weiss, G.; McGill, J.; Davidson, K.; Izbicka, E.; Kuhn, J.G.; Allred, C.; Clark, G.M.; Von Hoff, D.D. Effects of hydroxyurea on extrachromosomal DNA in patients with advanced ovarian carcinomas. Clin Cancer Res 2001, 7, 1171–1180. [Google Scholar] [PubMed]
- Sanchez, A.M.; Barrett, J.T.; Schoenlein, P.V. Fractionated ionizing radiation accelerates loss of amplified MDR1 genes harbored by extrachromosomal DNA in tumor cells. Cancer Res 1998, 58, 3845–3854. [Google Scholar]
- Schoenlein, P.V.; Barrett, J.T.; Kulharya, A.; Dohn, M.R.; Sanchez, A.; Hou, D.Y.; McCoy, J. Radiation therapy depletes extrachromosomally amplified drug resistance genes and oncogenes from tumor cells via micronuclear capture of episomes and double minute chromosomes. Int J Radiat Oncol Biol Phys 2003, 55, 1051–1065. [Google Scholar] [CrossRef]
- Feng, W.; Arrey, G.; Zole, E.; Lv, W.; Liang, X.; Han, P.; Mohiyuddin, M.; Pilegaard, H.; Regenberg, B. Targeted removal of mitochondrial DNA from mouse and human extrachromosomal circular DNA with CRISPR-Cas9. Comput Struct Biotechnol J 2022, 20, 3059–3067. [Google Scholar] [CrossRef]
- Chen, D.; Luo, Y.; Cheng, G. Single cell and immunity: Better understanding immune cell heterogeneities with single cell sequencing. Clin Transl Med 2023, 13, e1159. [Google Scholar] [CrossRef]
| Molecule name | Compound | Target | Mechanism of action | Disease | Study design - References | Outcomes | Route of administration | Developmental stage |
|---|---|---|---|---|---|---|---|---|
| Mongersen | ASO | SMAD7 | Restoring TGF-β1 activity | CD | Randomized quadruple blind, clinical trial [208] | Not superior to placebo | Oral | Phase III |
| Alicaforsen | ASO | ICAM-1 | Leukocyte trafficking | CD | Randomized double blind clinical trial [NCT00048113] | Ongoing | Intravenous | phase III |
| UC | Double-blind, placebo controlled clinical trial [209] | Statistical benefit over placebo for prolonged reduction of DAI | Enema | Phase II | ||||
| Pouchitis | Double-blind randomized controlled clinical trial [NCT02525523] | Ongoing | Enema | Phase III | ||||
| STNM01 | ds-RNA | CHST15 | Inhibition of collagen fibril formation | CD | Randomized, double blind, placebo controlled clinical trial [203] | Amelioration of SES-CD and fibrosis | Intracolonic | phase I |
| UC | Randomized, multicenter, double-blind, placebo-controlled clinical trial | Higher rates of mucosal healing and clinical remission in left-sided refractory colits | Intracolonic | Phase IIa | ||||
| Hdg40/SB012 | DNAzyme | GATA3 | Inhibition of Th2-driven response | UC | Double blind randomized clinical trial [NCT02129439] | Clinical and endoscopic improvement of disease activity | Intrarectal | phase IIa |
| DIMS0150, Cobitolimod | ss-DNA | TLR9 | Induction of anti-inflammatory cytokines | UC | Randomized quadruple blind placebo controlled clinical trial [NCT01493960] | Higher clinical remission in moderate-to-severe UC | Intracolonic | phase III |
| BL-7040, Monarsen | Synthetic oligonucleotide | TLR9 | Induction of anti-inflammatory cytokines | UC | Single group assignment, open label clinical trial [138] | Higher clinical response and remission in moderate UC | Oral | phase II |
| P65 ASO | ASO | nF-kB | Reduction of pro-inflammatory cytokines | UC | murine models [206] | Downregulation of NF-kB and proinflammatory cytokines | Intrarectal | Pre-clinical studies |
| miR-494-3p | microRNA | IKKβ/NF-κB, EDA2R/EDA-A2 | inhibits M1 macrophage recruitment, suppresses colonic stemness and epithelial repair | DSS induced colitis in mice | murine models [210] | Ameliorated severity of colonic colitis | intraperitoneal injection | Pre-clinical studies |
| interleukin-10 (IL-10) mRNA | mRNA | anti-inflammatory cytokine | DSS induced colitis in mice | murine models [211] | Anti-inflammatory effect on intestinal mucosa | Pre-clinical studies | ||
| AMT-101 | Chx386–hIL-10 fusion protein | IL-10 receptor | exerting IL-10 anti-inflammatory activity | DSS induced colitis in mice | murine models [212] | Efficient transcytosis towards intestinal lamina propria and activation of IL-10R | Oral | Pre-clinical studies |
| AptMincleDRBL | Aptamer | Mincle | blocking Mincle (PRR) pathway | DSS induced colitis in mice | murine models [213] | Reduction of disease activity | intraperitoneal injection | Pre-clinical studies |
| ASO antisense oligonucleotide, CD Crohn’s disease, UC ulcerative colitis, DSS dextran sodium sulfate, IL Inteleukin, CHST15 carbohydrate sulfotransferase 15, ds double-stranded, ICAM intercellular adhesion molecule, NF-kB nuclear factor kappa-light-chain-enhancer of activated B cells, ss single-stranded, TGF transforming growth factor, mRNA Messenger ribonucleic acid, Th2 type 2 T helper, TLR Toll-like receptor, PRR pattern recognition receptors. | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





