Submitted:
13 June 2023
Posted:
14 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cohorts
2.2. Methods
2.2.1. Strategy
2.2.2. Measurement of Serum Tumor Markers.
2.2.3. Radiological Evaluation of PNEN Disease Extent.
2.2.4. Histological Diagnosis
2.2.5. Statistical Analysis
3. Results
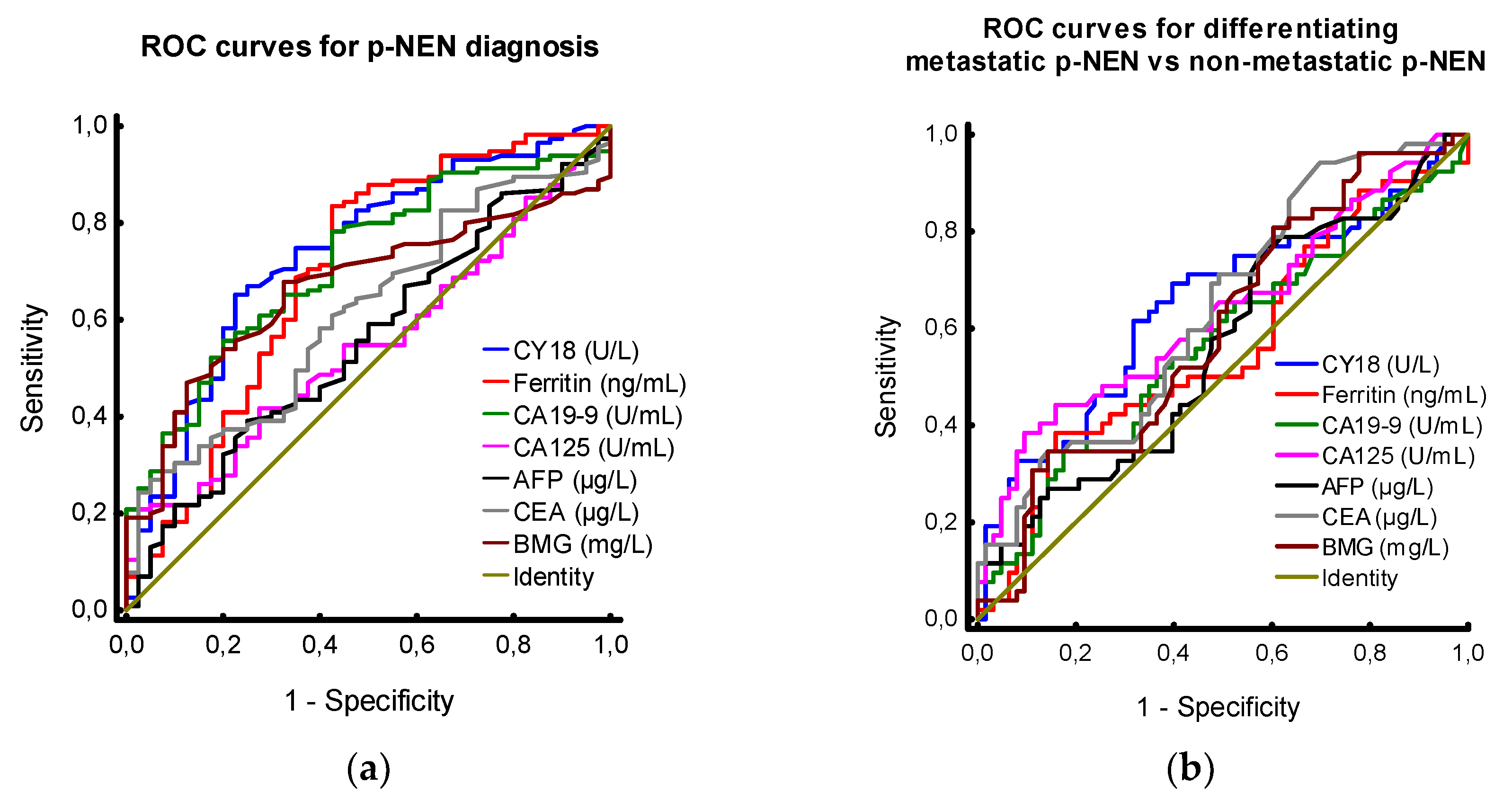
3.1. Tumour Markers in Patients with PNEN vs. Controls
3.2. Tumour Markers in Patients with PNEN - Analysis by Absence and Presence of Metastases
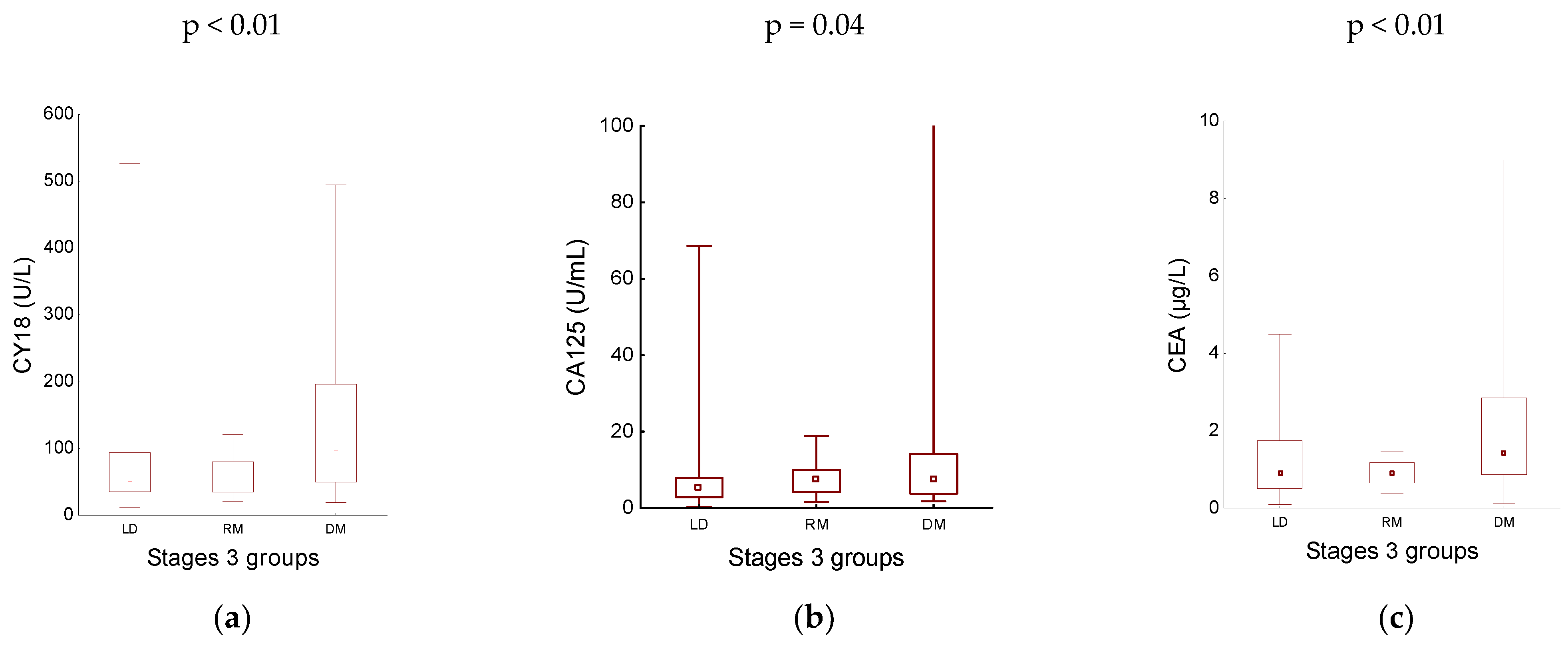
3.3. Tumour Markers in Patients with PNEN - Analysis by Disease Stage
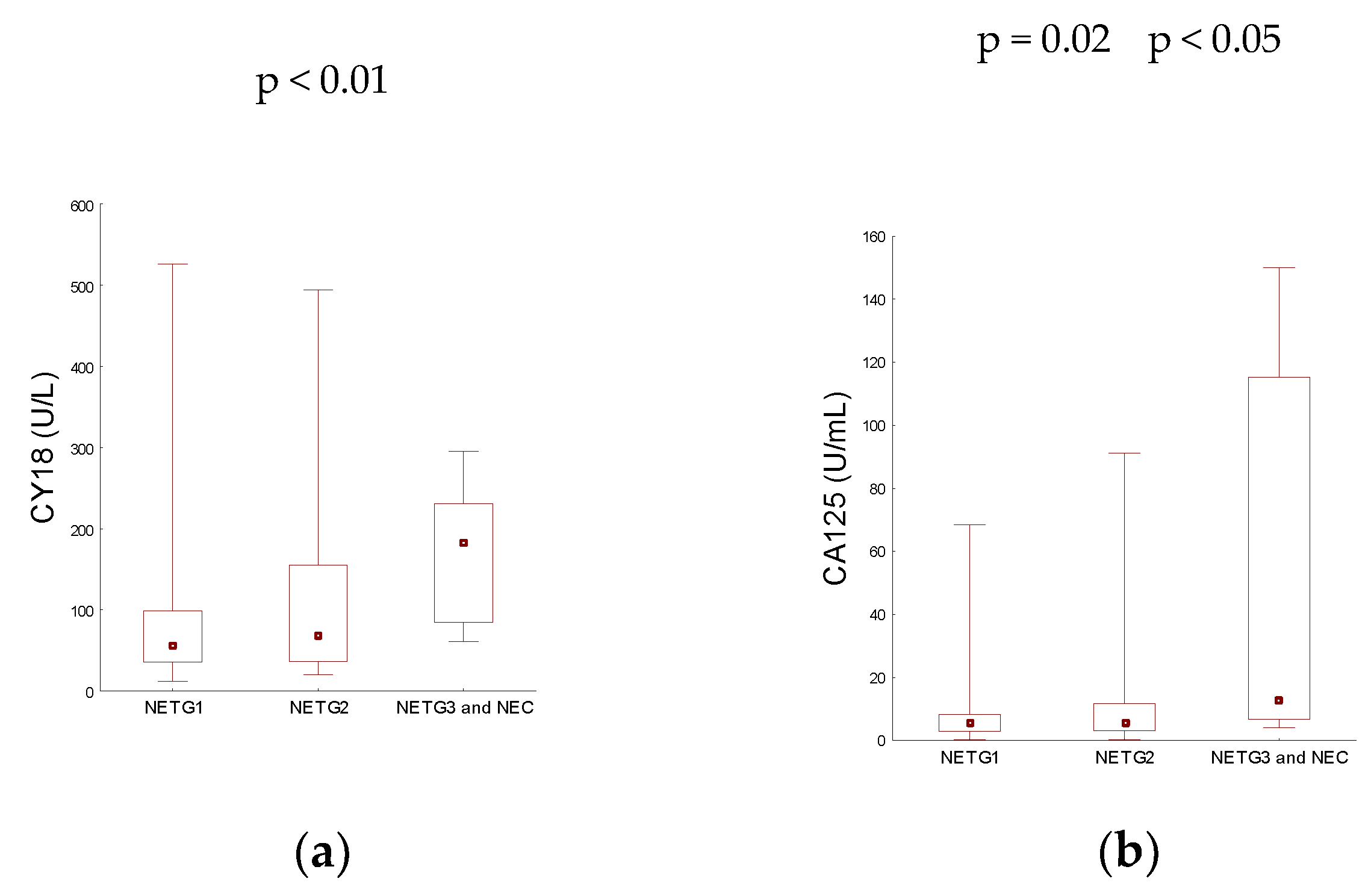
3.3. Analysis by Disease Grade
3.4. R Spearman’s Correlation
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kos-Kudła, B.; Rosiek, V.; Borowska, M.; Bednarczuk, T.; Bolanowski, M.; Chmielik, E.; Ćwikła, J.B.; Foltyn, W.; Gisterek, I.; Handkiewicz-Junak, D.; et al. Pancreatic neuroendocrine neoplasms - update of the diagnostic and therapeutic guidelines (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol Pol. 2022, 73, 491–548. [Google Scholar] [CrossRef]
- Kos-Kudła, B.; Foltyn, W.; Malczewska, A.; Bednarczuk, T.; Bolanowski, M.; Borowska, M.; Chmielik, E.; Ćwikła, J.B.; Gisterek, I.; Handkiewicz-Junak, D.; et al. Update of the diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours) [Aktualizacja zaleceń ogólnych dotyczących postępowania diagnostyczno-terapeutycznego w nowotworach neuroendokrynnych układu pokarmowego (rekomendowane przez Polską Sieć Guzów Neuroendokrynnych)]. Endokrynol Pol 2022, 73, 387–454. [Google Scholar] [CrossRef]
- Miller, H.C.; Drymousis, P.; Flora, R.; Goldin, R.; Spalding, D.; Frilling, A. Role of Ki-67 proliferation index in the assessment of patients with neuroendocrine neoplasias regarding the stage of disease. World J. Surg. 2014, 38, 1353–1361. [Google Scholar] [CrossRef]
- Bałdys-Waligórska, A.; Nowak, A. Neuroendocrine neoplasms of the digestive system—Current classification and terminology. Nowotwory 2021, 71, 26–37. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree. I.A. WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Kos-Kudła, B.; Rosiek, V.; Borowska, M.; Bałdys-Waligórska, A.; Bednarczuk, T.; Blicharz-Dorniak, J.; Bolanowski, M.; Boratyn-Nowicka, A.; Cichocki, A.; Ćwikła, J.B.; et al. Pancreatic neuroendocrine neoplasms — management guidelines (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol Pol. 2017, 68, 169–197. [Google Scholar] [CrossRef] [PubMed]
- Foulfoin, M.; Graillot, E.; Adham, M.; Rousset, P.; Forestier, J.; Hervieu, V.; Robinson, P.; Scoazec, J.Y.; Lombard-Bohas, C.; Walter, T. Treatment of metastatic pancreatic neuroendocrine tumors: relevance of ENETS 2016 guidelines. Endocr. Relat. Cancer. 2017, 24, 71–81. [Google Scholar] [CrossRef]
- O'Grady, H.L.; Conlon, K.C. Pancreatic neuroendocrine tumours. Eur. J. Surg. Oncol. 2008, 34, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Lombard-Bohas, C.; Baudin, E.; Kvols, L.K.; Rougier, P.; Ruszniewski, P.; Hoosen, S.; St Peter, J.; Haas, T.; Lebwohl, D.; et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J. Clin. Oncol. 2010, 28, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, K.; Ohtsuka, T.; Mori, Y.; Fujino, M.; Yasui, T.; Aishima, S.; Takahata, S.; Nakamura, M.; Ito, T.; Tanaka, M. Analysis of lymph node metastasis in pancreatic neuroendocrine tumors (PNETs) based on the tumor size and hormonal production. J. Gastroenterol. 2012, 47, 678–685. [Google Scholar] [CrossRef]
- Van Loon, K.; Zhang, L.; Keiser, J.; Carrasco, C.; Glass, K.; Ramirez, M.T.; Bobiak, S.; Nakakura, E.K.; Venook, A.P.; Shah, M.H.; et al. Bone metastases and skeletal-related events from neuroendocrine tumors. Endocr. Connect. 2015, 4, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Falconi, M.; Bartsch, D.K.; Eriksson, B.; Klöppel, G.; Lopes, J.M.; O'Connor, J.M.; Salazar, R.; Taal, B.G.; Vullierme, M.P.; O'Toole, D. Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology. 2012, 95, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; O'Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology. 2016, 103, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W.E.; Gonzalez, R.S.; Cates, J.M.M.; Liu, E.; Shi, C. Hepatic micrometastases are associated with poor prognosis in patients with liver metastases from neuroendocrine tumors of the digestive tract. Hum Pathol. 2018, 79, 109–115. [Google Scholar] [CrossRef]
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef]
- Perri, G.; Prakash, L.R.; Katz, M.H.G. Pancreatic neuroendocrine tumors. Current opinion in gastroenterology. 2019, 35, 468–477. [Google Scholar] [CrossRef]
- Xiao, Z.; Xu, H.; Strosberg, J.R.; Lu, R.; Zhu, X.; Deng, S.; Ding, L.; Ni, Q.; Warshaw, A.L.; Yu, X.; et al. EGFR is a potential therapeutic target for highly glycosylated and aggressive pancreatic neuroendocrine neoplasms. Int J Cancer. 2023, 153, 164–172. [Google Scholar] [CrossRef]
- Gu, Y.L.; Lan, C.; Pei, H.; Yang, S.N.; Liu, Y.F.; Xiao, L.L. Applicative Value of Serum CA19-9, CEA, CA125 and CA242 in Diagnosis and Prognosis for Patients with Pancreatic Cancer Treated by Concurrent Chemoradiotherapy. Asian Pac J Cancer Prev. 2015, 16, 6569–6573. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Chen, M.; Chen, J. [Prognostic value of carcinoembryonic antigen, alpha fetoprotein, carbohydrate antigen 125 and carbohydrate antigen 19-9 in gastroenteropancreatic neuroendocrine neoplasms]. Zhonghua Wei Chang Wai Ke Za Zhi. 2017, 20, 1002–1008. [Google Scholar]
- Zhuge, X.; Guo, C.; Chen, Y.; Feng, L.; Jia, R.; Zhao, Y.; Sun, K.; Wang, Z.; Chen, X. The Levels of Tumor Markers in Pancreatic Neuroendocrine Carcinoma and Their Values in Differentiation Between Pancreatic Neuroendocrine Carcinoma and Pancreatic Ductal Adenocarcinoma. Pancreas. 2018, 47, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, Y.; Zhang, P.; Zhang, X.; Li, J.; Zhou, J.; Wang, X.; Peng, Z.; Sun, Y.; Li, J.; et al. Serum Biomarker Status with a Distinctive Pattern in Prognosis of Gastroenteropancreatic Neuroendocrine Carcinoma. Neuroendocrinology. 2022, 112, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Kanda, S.; Fukushima, T.; Kobayashi, T.; Kondo, R.; Koizumi, T. Increased carbohydrate antigen 19-9 expression in a thymic neuroendocrine tumor. Thorac Cancer. 2021, 12, 2949–2952. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Fan, Z.; Yang, J.; Shi, M.; Li, Y.; Zhan, H. Diagnostic role and prognostic value of tumor markers in high-grade gastro-enteropancreatic neuroendocrine neoplasms. Pancreatology. 2023, 23, 204–212. [Google Scholar] [CrossRef]
- Webb, A.; Scott-Mackie, P.; Cunningham, D.; Norman, A.; Andreyev, J.; O'Brien, M.; Bensted, J. The prognostic value of CEA, beta HCG, AFP, CA125, CA19-9 and C-erb B-2, beta HCG immunohistochemistry in advanced colorectal cancer. Ann Oncol. 1995, 6, 581–587. [Google Scholar] [CrossRef]
- McShane, L.M.; Hayes, D.F. Publication of tumor marker research results: the necessity for complete and transparent reporting. J Clin Oncol. 2012, 30, 4223–4232. [Google Scholar] [CrossRef]
| Variables | PNEN n = 115 |
Controls n = 40 |
|---|---|---|
| Number | data | data |
| Gender: | ||
| Males | 49 (42.61 %) | 19 (47.50 %) |
| Females | 66 (57.39 %) | 21 (52.50 %) |
| Age [years] | ||
| Mean (range) | 52.88 (19 – 79) | 48.53 (22 - 76) |
| Functionality status: | N/A | |
| Non-functional | 108 (93.91 %) | |
| Functional | 7 (6.09 %) | |
| Grade: | N/A | |
| G1 NET | 60 (52.17 %) | |
| G2 NET | 47 (40.87 %) | |
| G3 NET and G3 NEC | 8 (6.96 %) | |
| TNM stage: | N/A | |
| Stage I | 31 (26.96 %) | |
| Stage IIA | 20 (17.39 %) | |
| Stage IIB | 6 (5.22 %) | |
| Stage IIIA | 6 (5.22 %) | |
| Stage IIIB | 8 (6.95 %) | |
| Stage IV | 44 (38.26 %) | |
| Disease extend: | N/A | |
| Localized disease | 63 (54.78 %) | |
| Regional metastatic | 8 (6.96 %) | |
| Distant metastatic | 44 (38.26 %) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





