1. Introduction
Colorectal cancer (CRC) is a heterogenous disease, which can be stratified by genomic and gene expression profiles into several distinct molecular subtypes with major differences in prognosis and therapeutic response. Approximately 15% of CRCs arise from microsatellite instability (MSI) pathway caused by deficient mismatch repair (dMMR) system, leading to hypermutation and increased cancer susceptibility. The MMR system can be compromised by epigenetic changes, usually by acquired
MLH1 promoter hypermethylation, or by genetic inactivation of
MLH1, MSH2, MSH6, or
PMS2 genes characteristic to Lynch syndrome (LS) [
1,
2]. Among all CRC tumors, stage II-III MSI CRCs have a better prognosis than corresponding microsatellite stable (MSS) tumors, whereas stage IV MSI CRCs have a worse prognosis than corresponding MSS CRCs [
1,
2]. Further,
BRAFV600E mutation has been associated to aggressive behavior in MSS tumors but not in MSI tumors suggesting that the MSI phenotype may override the negative prognostic potential of
BRAFV600E mutation [
3,
4,
5,
6]. Immuno-oncological treatments with immune checkpoint inhibitors have shown excellent responses in advanced dMMR/MSI CRC patients [
7,
8] but only limited responses when treating MSS CRC patients [
9,
10]. Despite the clinical success, nearly half of metastatic dMMR/MSI CRCs fail to respond to immunotherapy [
11,
12]. The molecular mechanisms behind these differing responses are unclear.
All human cells have a complex glycan coating (the glycocalyx), which is involved in many essential cellular functions such as cell signaling, adhesion, differentiation, and proliferation [
13,
14]. Altered glycosylation, most often increased sialylation, fucosylation, and branching of N-linked glycans, has been observed in many types of cancer cells [
13,
14]. Aberrantly expressed glycans can modulate essential events of cancer development and progression, including angiogenesis, invasion and metastasis [
13,
14,
15]. Further, the surface glycans of cancer cells play a role in evasion of immune response [
16]. Glycan alterations may thus provide valuable novel molecular targets for cancer diagnostics and treatment.
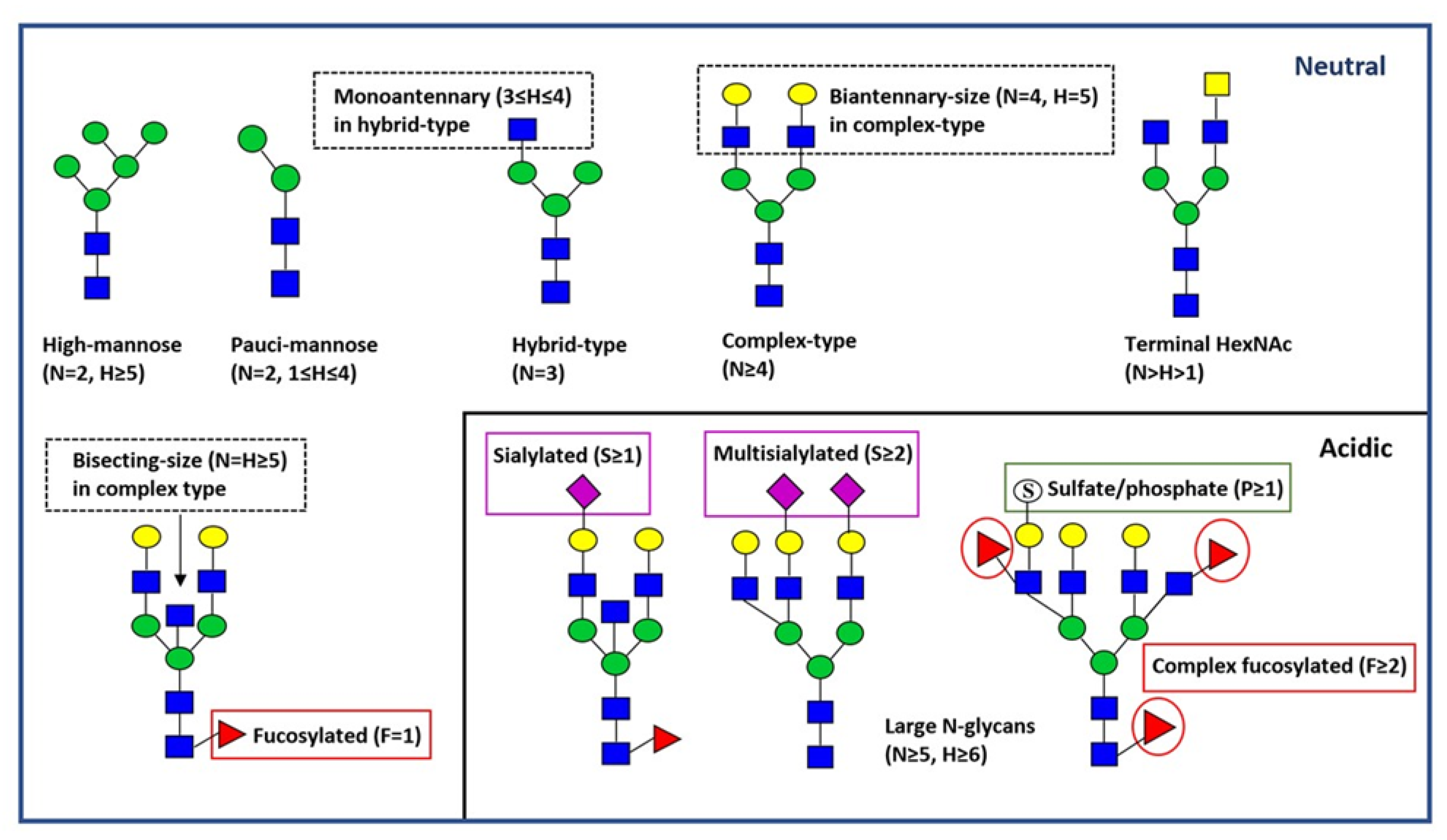
N-glycan profiles of CRC tissues have previously been mainly compared with those of non-neoplastic colon tissues, and different stages of CRC have been compared without considering the MSI status. Increased glycosylation features in CRC include pauci-mannosidic, β1,6-branched, sulfated and sialylated N-glycans, especially α2,6-sialylated glycans, as well as glycans containing sialylated Lewis type epitopes [
17,
18]. The decreased features in turn include structures with a bisecting N-acetylglucosamine [
17,
18]. In our previous study, we also demonstrated differences in the levels of sialylated and sulfated glycans between stage II and III right-sided CRC samples, with stage III tumors showing predominantly sulfated and stage II sialylated N-glycans [
19]. Also, a study by Kaprio
et al. showed pauci-mannose and small high-mannose N-glycan structures, as well as sialylated structures to be relatively more abundant in rectal carcinomas than adenomas [
20].
The aim of this study was to analyze the N-glycan profiles of MSI CRC tumors to identify possible reasons for their differing behavior. We studied the neutral and acidic N-linked glycan profiles of MSI CRC samples (n=40) and pools of paired non-neoplastic colon controls (n=4) using MALDI-TOF mass spectrometry. The MSI CRC samples were further divided into subgroups according to the stage (II or IV) and BRAFV600E mutation status (wt or mut) (n=10 in each group). In addition, the N-glycan profiles of stage II BRAFwt MSI tumors were compared to stage II BRAFV600E wt MSS tumors (n= 9).
4. Discussion
In this study, we investigated the N-linked glycan profiles of MSI CRC tissue specimens subdivided into subgroups according to stage (II or IV) and BRAFV600E mutation status (wt or mut) using MALDI-TOF mass spectrometry. Further, we compared these glycan profiles to those of both paired non-neoplastic colon tissue samples and MSS CRC specimens. We found multiple differences between MSI CRC samples and the control samples, and between MSI stage II and MSS stage II CRC samples. When MSI CRC subgroups were compared to each other, only minor differences were found in neutral N-glycan profiles, whereas a clear association between tumor stage and BRAF mutation status was seen in acidic N-glycan profiles. Most interestingly, no acid ester-modified (sulfated/phosphorylated) N-glycans were identified in any of the stage II MSI tumors with BRAFV600E mutation.
In line with previous glycomic profiling reports of CRC tissues [
17,
18,
19], MSI CRC tumors showed a higher relative abundance of neutral pauci-mannose N-glycans, especially the fucosylated glycan H2N2F1, and decreased relative abundances of the putative terminal HexNAc (e.g. H3N5, H3N5F1, and H4N5F1) containing glycans, as well as the bisecting-size structure H5N5F1 as compared to the control tissues. In contrast to previous CRC reports, which have not specifically taken into account the MSI/MSS status [
29,
30,
31,
32], MSI tumors had a lower relative abundance of high-mannose type N-glycans than the non-neoplastic control tissues. Boyaval
et al. [
32] have demonstrated even higher levels of high-mannose type N-glycans in dysplastic regions of pre-cursor lesions than in early-stage CRC. Notably, in a recent study, overexpression of high-mannose N-glycans was demonstrated specifically in MSS CRC tumor tissue [
33]. The acidic N-glycan profiles of MSI tumors were relatively simple, and, in contrast to previous reports, MSI tumors showed decreased relative abundances of some sulfated/phosphorylated and complex fucosylated structures e.g. H5N4F3P1 and H5N6F4P1 as compared to the control tissues [
17,
19]. Fucosylated neutral pauci-mannose and hybrid-type glycans were detected to increase but fucosylated acidic complex-type glycans to decrease in MSI CRC samples compared to non-neoplastic controls. Similarly, a higher abundance of fucosylated neutral N-glycans has been reported by Holm
et al. [
19] and a lower abundance of fucosylated complex-type N-glycans by Boyaval
et al. [
31] in CRC compared to adjacent normal colon epithelium. Further, we did not find any statistically significant difference in the abundances of sialylated N-glycans between MSI CRC samples and control tissues, whereas previous reports have reported increased levels of sialylation in CRC [
17,
29,
31,
34], and increased sialylation has also been attributed to metastatic potential and therapeutic resistance in CRC [
35,
36].
When comparing BRAFwt stage II MSI and MSS tumors, multiple significant differences were seen, both in neutral and acidic N-glycan profiles. MSI tumors showed distinctively higher relative abundances of neutral complex-type and monoantennary hybrid-type glycans, as well as fucosylation, especially in pauci-mannose glycans. On the contrary, clearly lower abundances of 2 HexNAc and high-mannose type glycans, as well as putative terminal HexNAc complex-type structures were observed in MSI than in MSS stage II tumors. In the acidic N-glycan profiles, MSI stage II BRAFwt tumors showed increased relative abundances of biantennary-size complex-type N-glycans and 4 HexNAc complex-type glycans than corresponding MSS samples. Yet, sulfated/phosphorylated hybrid-type glycans, large glycans and putative terminal HexNAc containing complex-type glycans, as well as fucosylated, especially fucosylated/complex fucosylated complex-type glycans were significantly less abundant in MSI stage II BRAFwt tumors. These neutral and acidic N-glycan types with differing abundances in MSI stage II CRC compared to corresponding MSS tumors, may be linked to the MSI pathway of CRC. To our knowledge this is the first study to report significant N-glycosylation differences between MSI and MSS CRC tissue samples.
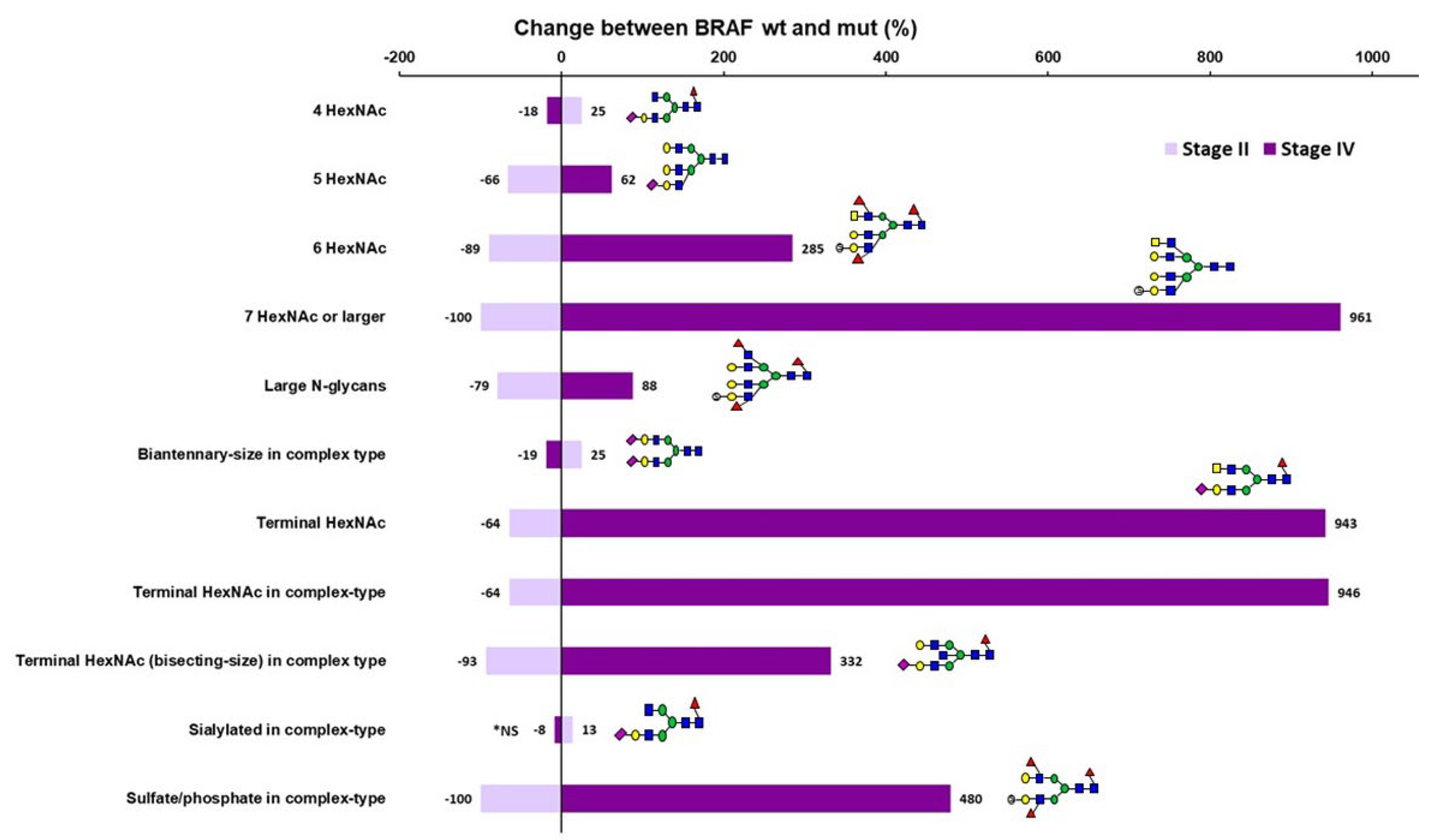
Between MSI CRC subgroups, only minor differences were found in neutral N-glycan profiles and the major differences were observed in the acidic N-glycan profiles. Most interestingly, no sulfated/phosphorylated N-glycans were identified in any of the stage II MSI tumors containing BRAF
V600E mutation. Between all MSI stage II and stage IV CRC, only acidic biantennary-size complex-type glycans showed a clear decrease in stage IV samples. When comparing MSI BRAFwt stage II and IV CRC samples to each other, biantennary-size complex type glycans were more abundant and large N-glycans less abundant in stage IV samples. Between BRAFmut stage II and stage IV samples, large N-glycans, putative terminal HexNAc containing and sulfated/phosphorylated complex-type glycans were significantly more abundant, and 4 HexNAc, biantennary-size complex-type and sialylated complex-type glycans less abundant in stage BRAFmut IV samples. Most interesting differences were observed when comparing MSI BRAFwt and BRAFmut samples within the stage. In these comparisons, the same glycan classes differed between BRAFwt and BRAFmut samples, but the direction of the change was totally opposite in stage II versus stage IV. In stage II BRAFmut samples, sulfated, large, and putative terminal HexNAc containing acidic N-glycans were decreased as compared to the corresponding BRAFwt samples, whereas in stage IV these same N-glycan features were increased in BRAFmut samples as compared to BRAFwt ones. On the other hand, increased levels of biantennary-size complex-type glycans, especially 4 HexNAc glycans were observed in stage II BRAFmut samples as compared to corresponding BRAFwt samples, whereas in stage IV samples these same glycan classes were decreased in BRAFmut samples relative to BRAFwt ones. BRAF
V600E mutation is known to have a negative prognostic value in MSS CRC, while MSI has been suggested to override this negative effect [
3,
4,
6]. Some studies have reported that BRAF mutation could be a positive prognostic marker in early stage MSI CRC [
37] and, on the other hand, a negative prognostic factor in advanced MSI CRC [
38,
39]. The oppositely behaving acidic N-glycan types, especially large, sulfated/phosphorylated, and putative terminal HexNAc containing glycans, showed a clear dependency on tumor stage and BRAF mutation status and may thus be associated with MSI CRC progression according to BRAF mutation.
Large N-glycans commonly contain β1,6-branching, and increase in β1,6-branched N-linked glycans has been related to the malignant transformation and metastatic potential in many cancers, including CRC [
13,
40,
41,
42]. Importantly, the modification of epithelial cadherin (E-cadherin) with branched glycans is known to interfere cellular adhesion and promote tumour invasiveness and metastasis [
13,
43]. Also, Kaprio
et al. [
20] has reported a significantly higher abundance of acidic N5 glycans in tissue samples of stage III CRC compared to stage I-II CRC samples. The enzyme catalyzing β1,6-branching of N-glycans is N-acetylglucosaminyltransferase V (GnT-V) encoded by the
MGAT5 gene.
MGAT5 expression is regulated by the RAS–RAF–MAPK pathway, and mutations of this oncogenic pathway are known to up-regulate GnT-V expression and concomitant β1,6-branching [
13]. This is in line with our findings of increased levels of large (N≥5) acidic N-glycans in BRAFmut stage IV tumors as compared to corresponding BRAFwt ones but contradicts with BRAFmut stage II tumors showing lower levels of these glycans as compared to corresponding BRAFwt ones. Also, in our study, BRAFwt MSI stage II samples showed lower relative abundances of large acidic N-glycans than BRAFwt stage II MSS tumors thus potentially explaining for some degree the better prognosis of early stage MSI CRC as compared to corresponding MSS tumors.
Sulfated glycans have been shown to play an important role in many cell surface-related functions such as cellular adhesion and selectin-ligand interactions [
44]. Higher levels of glycan-sulfotransferase activities have been demonstrated in poorly differentiated gastric carcinomas than in moderately differentiated ones, thus being associated with gastric tumorigenesis [
45]. Also, sulfated Lewis X determinants form a predominant structural glycan motif in xenograft tumor mucin of LS174T-HM7 cells, a highly metastatic subline of the LS174T human CRC cell line [
46]. Sulfated glycans are preferably bound by galectin-1 and galectin-2 [
47], and upregulation of galectin-1 has been related to malignant progression in CRC [
48,
49]. In our study, sulfated/phosphorylated complex-type glycans were increased with the tumor stage in MSI BRAFmut samples, but not in MSI BRAFwt samples. Strikingly, sulfated/phosphorylated N-glycans were not found in any of the stage II MSI BRAFmut tumors whereas MSI BRAFwt stage II tumors displayed these glycans. Further, MSI stage II BRAFwt tumors showed a lower relative abundance of sulfated/phosphorylated hybrid-type glycans when compared to corresponding MSS samples.
Putative terminal HexNAc-containing N-glycans (N>H>1) have previously been identified in various CRC cell lines and increased abundances of terminal HexNAc residues have further been correlated with caudal-related homeobox protein 1 (CDX1) expressing CRC cells [
50,
51]. CDX1 is a transcription factor regulating normal development and differentiation of intestinal epithelium and associated with tumor suppressing potential in the colon [
52]. More specifically, increased terminal N-acetylglucosamine (GlcNAc) has been identified in various carcinomas [
22]. N-glycans containing bisecting GlcNAc have yet more been attributed to suppression of tumor progression and metastasis through regulation of cell surface glycoproteins such as stabilizing the E-cadherin mediated cell-cell adhesion [
13,
43]. N-glycans containing bisecting GlcNAc have also been reported to decrease in CRC tissue samples with more advanced tumor stages [
30,
34]. In our study, stage IV BRAFmut tumors showed in contrast higher relative abundances of acidic putative terminal HexNAc, especially in bisecting-size, containing complex-type glycans when compared to corresponding stage II samples. Although, a higher expression of bisecting N-glycans in a metastatic MSI CRC cell line (LIM1215) as compared with two non-metastatic MSI CRC cell lines (LIM1899 and LIM2405) has also controversially been demonstrated by Sethi
et al. [
53]. Further, the acidic putative terminal HexNAc and terminal HexNac in bisecting-size complex-type glycans showed significantly lower relative abundances in MSI stage II samples than in corresponding MSS samples.
A limitation of our study is that the mass spectrometric analyses were conducted using flakes from FFPE tissue blocks. It is thus not possible to specify from which cell type (e.g. cancer or stromal cells) the detached glycans originated. The tumor stroma is composed of various non-neoplastic cells e.g immune cells, fibroblasts and endothelial cells as well as extracellular matrix that forms a tumor microenvironment promoting cancer growth and spreading [
54]. Moreover, the cancer related N-glycan signature has been demonstrated to spread into the stroma at the invasive front of the tumor [
31]. We, however, used macrodissection to exclude the distant stroma and to achieve the highest percentage of epithelial cells in the carcinoma and non-neoplastic tissues. The analyzed tissues (tumor epithelium percentages 30-80%) although included varying amounts of tumor mucin, intra-tumor stroma and surrounding interface stroma which may contribute to the heterogeneity of the N-glycan signatures found in this study. Other limitation of this study is that we used pools of paired non-neoplastic colon samples from each MSI CRC patient set instead of individual paired non-neoplastic control samples. However, the main aim of this study was to compare the N-glycan profiles between MSI and MSS CRC samples and, especially, between different MSI CRC subgroups.