Submitted:
13 June 2023
Posted:
13 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Raw Material, Supplies and Location of the Research
2.2. Obtaining Surimi from Bullfrog MDM
2.3. Production of Surimi Gel Emulsions
2.4. Proximate Composition of Surimi Gel Emulsions
2.5. Physical and Technological Quality Parameters of Surimi Gel Emulsions
2.6. Texture Profile Analysis of Surimi Gel Emulsions
2.7. Evaluation of Lipid Oxidation of Surimi Gel Emulsions and Determination of Antioxidant Activity
2.8. Statistical Treatment of Data
3. Results
3.1. Proximate Chemical Composition
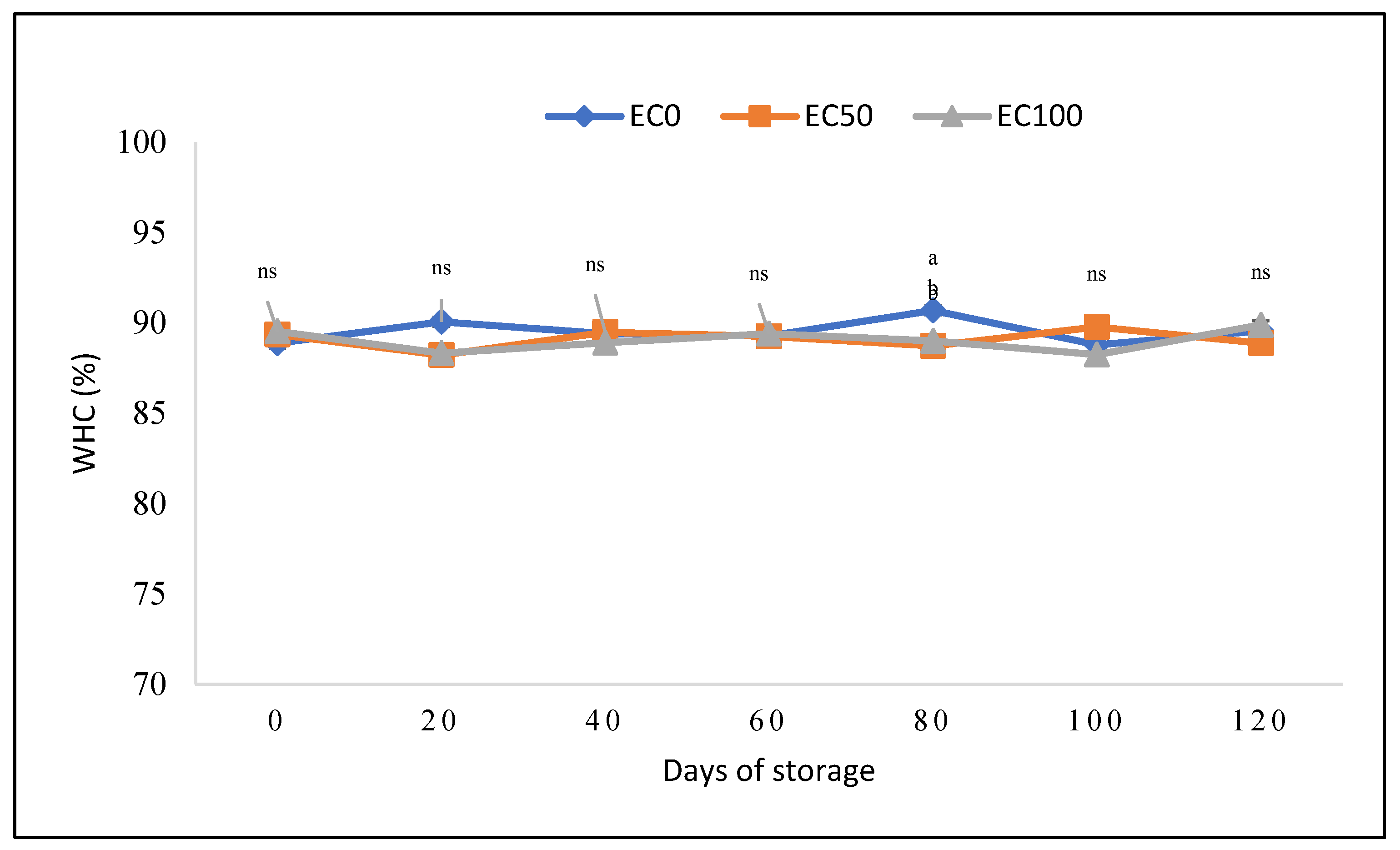
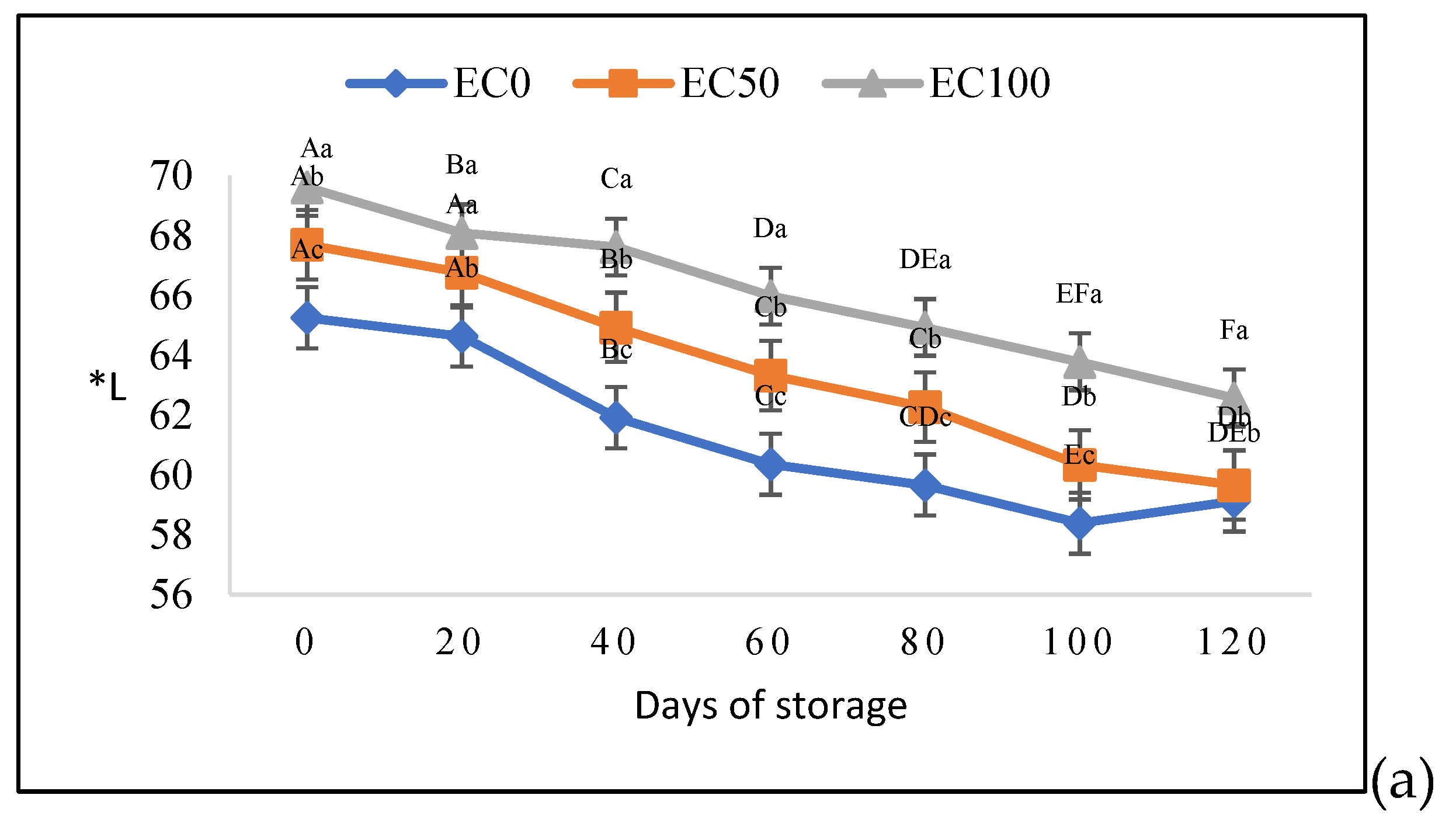
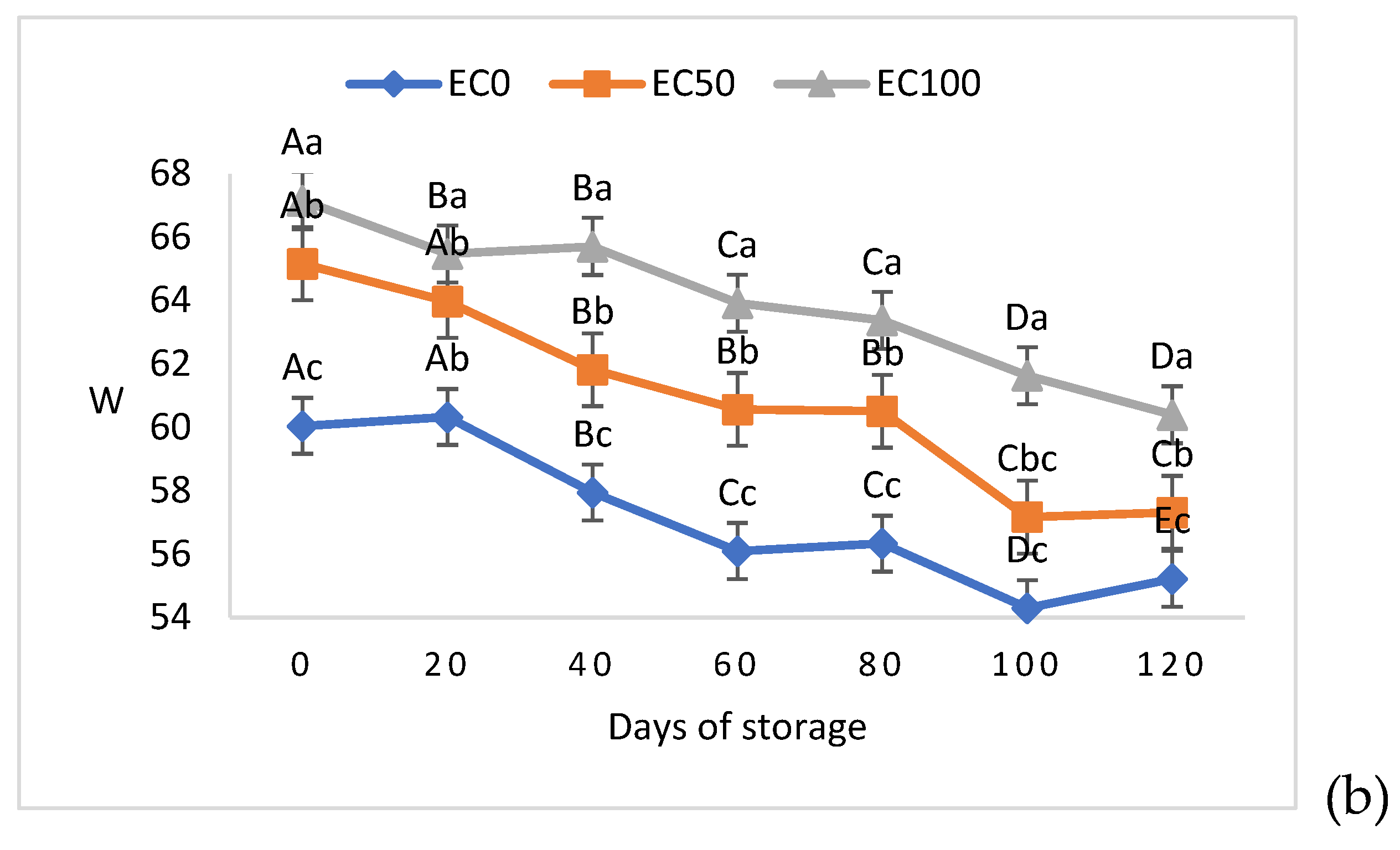
3.2. Physical and Technological Quality Parameters of Surimi Gel Emulsions
3.3. Texture Profile Analysis of Surimi Gel Emulsions
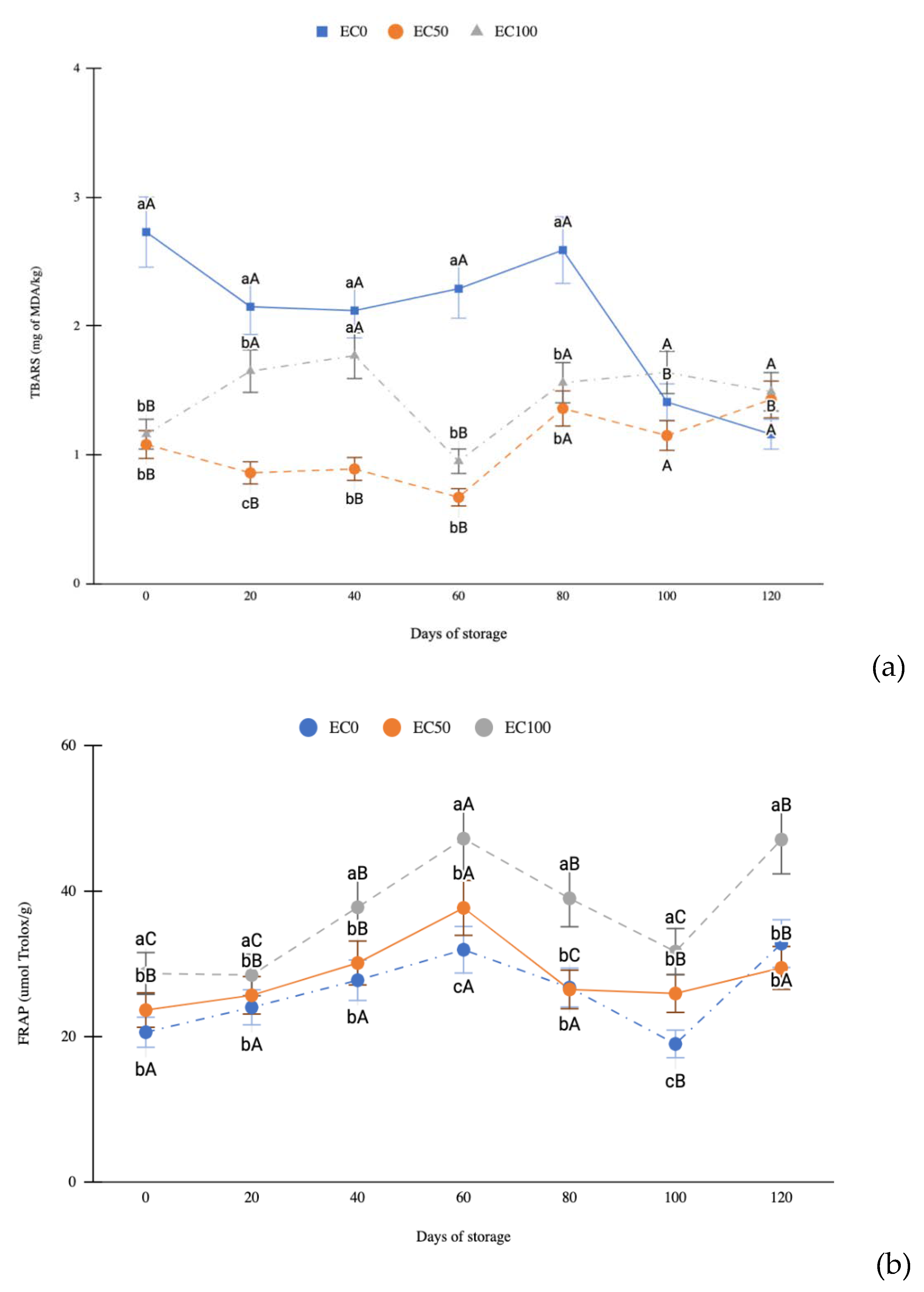
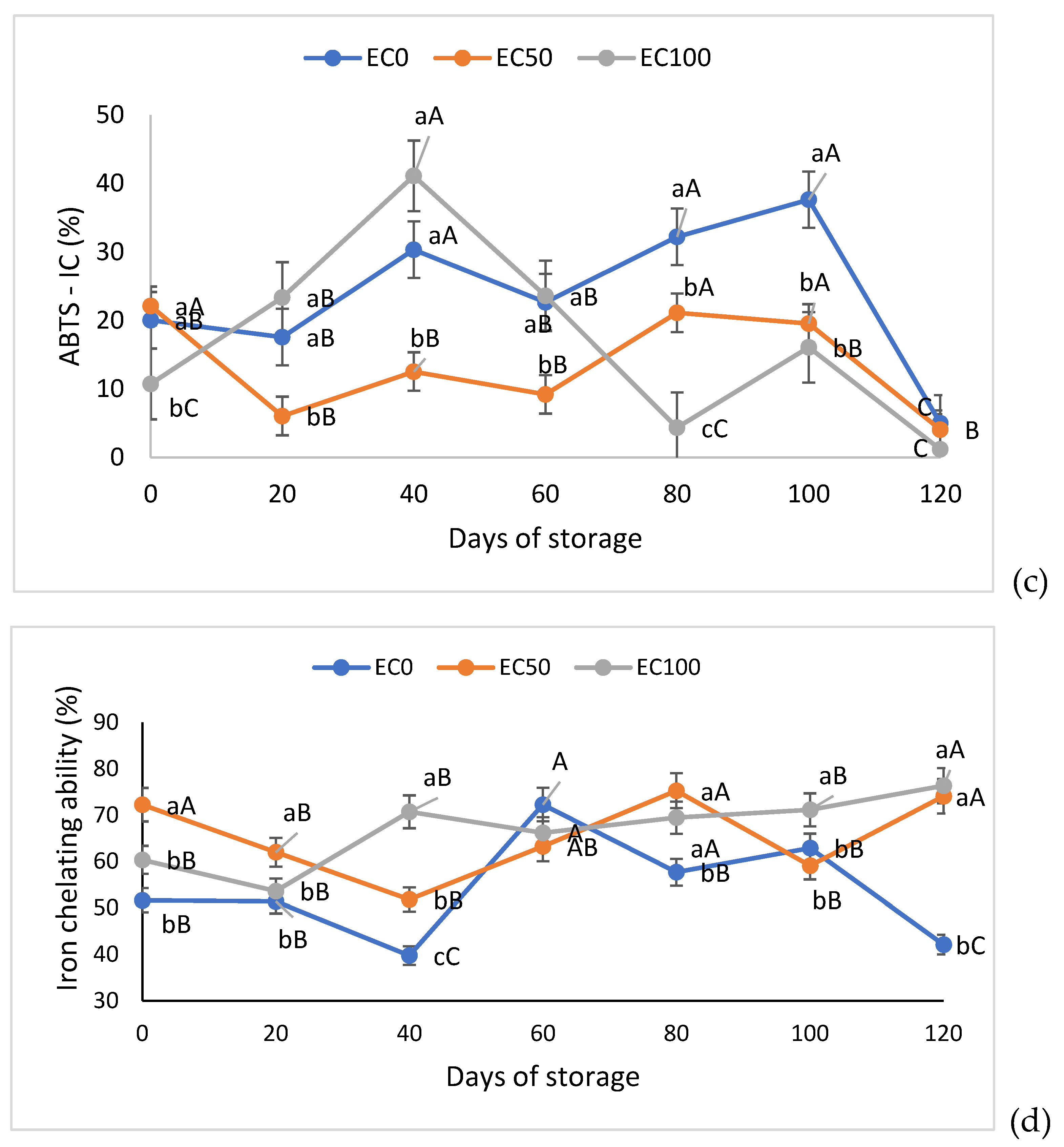
3.4. Evaluation of Lipid Oxidation of Surimi Gel Emulsions and Determination of Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Ma, D.; Wang, Y.; Qin, W. A comparative study of the properties and selfaggregation behavior of collagens from the scales and skin of grass carp (Ctenopharyngodon idella). Int. J. Biol. Macromol 2018, 106, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Verma, A.K.; Patel, R. Collagen extraction and recent biological activities of collagen peptides derived from sea-food waste: A review. Sustain. Chem. Pharm. 2020, 18, 100315. [Google Scholar] [CrossRef]
- Gorlov, I.F.; Titov, E.I.; Semenov, G.V.; Slozhenkina, M.I.; Sokolov, A. Y.; Omarov, R.S.; Goncharov, A.I.; Zlobina, E.Y.; Litvinova, E.V.; Karpenko, E.V. Collagen from porcine skin: a method of extraction and structural properties. Int. J. Food Prop. 2018, 21, 1031–1042. [Google Scholar] [CrossRef]
- Noorzai, S.; Verbeek, C.J.R.; Lay, M.C.; Swan, J. Collagen Extraction from Various Waste Bovine Hide Sources. Waste Biomass 2020, 11, 5687–5698. [Google Scholar] [CrossRef]
- Techatanawat, S.; Surarit, R.; Suddhasthira, T.; Khovidhunkit, S. Type I collagen extracted from rat-tail and bovine Achilles tendon for dental application: a comparative study. Asian Biomed. 2017, 6, 787–798. [Google Scholar] [CrossRef]
- Hashim, P.; Mohd Ridzwan, M.S.; Bakar, J. Isolation and Characterization of Collagen from Chicken Feet. Int. J. Biol. Life Sci. 2014, 8, 250–254. [Google Scholar]
- Araújo, Í.B.S.; Bezerra, T.K.A.; do Nascimento, E.S.; de A. Gadelha, C.A.; Santi-Gadelha, T.; Madruga, M.S. Optimal conditions for obtaining collagen from chicken feet and its characterization. Food Sci. Technol 2018, 38, 167–173. [Google Scholar] [CrossRef]
- Espinales, C.; Romero-Pena, M.; Calderon, G.; Vergara, K.; Caceres, P.J.; Castillo, P. Collagen, protein hydrolysates and chitin from by-products of fish and shellfish: An overview. Heliyon 2023, 9, 14937. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef]
- Indriani, S.; Benjakul, S.; Kishimura, H.; Karnjanapratu, S.; Nalinanon, S. Impact of extraction condition on the yield and molecular characteristics of collagen from Asian bullfrog (Rana tigerina) skin. LWT-Food Sci. Technol. 2022, 162, 113439. [Google Scholar] [CrossRef]
- GlobeNewswire by notifiend. Available online: https://www.globenewswire.com/news-release/2023/05/24/2675606/0/en/Global-Collagen-Market-Size-To-Exceed-USD-8-2-Billion-By-2032-CAGR-5-9.html (accessed on 10 June 2023).
- Bhuimbar, M.V.; Bhagwat, P.K.; Dandge, P.B. Extraction and characterization of acid soluble collagen from fish waste: Development of collagen-chitosan blend as food packaging film. J. Environ. Chem. Eng. 2019, 7, 102983. [Google Scholar] [CrossRef]
- Dierickx, K.; Presslee, S.; Harvey, V.L. Rapid collagen peptide mass fingerprinting as a tool to authenticate Pleuronectiformes in the food industry. Food Control 2023, 148, 109680. [Google Scholar] [CrossRef]
- Santana, J.C.C.; Gardim, R.B.; Almeida, P.F.; Borini, G.B.; Quispe, A.P.B.; Llanos, S.A.V.; Heredia, J.A.; Zamuner, S.; Gamarra, F.M.C.; Farias, T.M.B.; Ho, L.L.; Berssaneti, F.T. Valorization of Chicken Feet By-Product of the Poultry Industry: High Qualities of Gelatin and Biofilm from Extraction of Collagen. Polymers 2020, 12, 529. [Google Scholar] [CrossRef]
- Ju, H.; Liu, X.; Zhang, G.; Liu, D.; Yang, Y. Comparison of the Structural Characteristics of Native Collagen Fibrils Derived from Bovine Tendons Using Two Different Methods: Modified Acid-Solubilized and Pepsin-Aided Extraction. Materials 2020, 13, 358. [Google Scholar] [CrossRef]
- Naomi, R.; Bahari, H.; Ridzuan, P.M.; Othman, F. Natural-Based Biomaterial for Skin Wound Healing (Gelatin vs. Collagen): Expert Review. Polymers 2021, 13, 2319. [Google Scholar] [CrossRef]
- Matinong, A.M.E.; Chisti, Y.; Pickering, K.L.; Haverkamp, R.G. Collagen Extraction from Animal Skin. Biology 2022, 11, 905. [Google Scholar] [CrossRef]
- Sultana, S.; Eaqub Ali, Md.; Ahamad, M.N.U. Gelatine, collagen, and single cell proteins as a natural and newly emerging food ingredients. Preparation and Processing of Religious and Cultural Foods 2018, 215–239. [Google Scholar] [CrossRef]
- Araújo, Í.B.S.; Lima, D.A.S.; Pereira, S.F.; Madruga, M.S. Quality of low-fat chicken sausages with added chicken feet collagen. Poult. Sci. 2019, 98, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Araújo, Í.B.S.; Lima, D.A.S.; Pereira, S.F.; Paseto, R.P.; Madruga, M.S. Effect of storage time on the quality of chicken sausages produced with fat replacement by collagen gel extracted from chicken feet. Poult. Sci. 2021, 100, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.C.; Fragoso, S.P.; Penna, C.R.A.; Arcanjo, N.M.O.; Silva, F.A.P.; Ferreira, V.C.S.; Barreto, M.D.S.; Araújo, Í.B.S. Quality parameters of frankfurter-type sausages with partial replacement of fat by hydrolyzed collagen. LWT-Food Sci. Technol. 2017, 76, 320–325. [Google Scholar] [CrossRef]
- Guimarães, J.L.B.; Calixto, F.A.A.; Keller, L.A.M.; Torrezan, R.; Furtado, A.A.L. Mesquita, E.F.M. Quality of mechanically separated meat (MSM) and surimi obtained from low commercial value fish. Bol. Inst. Pesca 2018, 44, e243. [Google Scholar] [CrossRef]
- Fragoso, S.P.; Nascimento, E.S.; Gadelha, C.A.A. Electrophoretic profile of proteins present in the rinse water of surimi produced with bullfrog (Lithobates catesbeianus) meat. 60th International Congress of Meat Science and Technology, 2014. 2014. Available online: https://www.academia.edu/65517741/ELECTROPHORETIC_PROFILE_OF_PROTEINS_PRESENT_IN_THE_RINSE_WATER_OF_SURIMI_PRODUCED_WITH_BULLFROG_Lithobates_catesbeianus_MEAT?from_sitemaps=true&version=2 (accessed on 10 June 2023).
- Zhu, S.; Chen, X.; Zheng, J.; Fan, W.; Ding, Y.; Zhou, X. Emulsion surimi gel with tunable gel properties and improved thermal stability by modulating oil types and emulsification degree. Foods 2022, 11, 179. [Google Scholar] [CrossRef]
- Jiménez Muñoz, L.M.; Sotelo Díaz, I.; Salgado Rohner, C.; Cáez Ramirez, G.; Filomena Ambrosio, A. Effectiveness of High Power Ultrasound for Surimi-based Preparation of Lionfish (Pterois Volitans) Patties by Textural, Sensory and Shape Preference. J. Culin. Sci. Technol. 2019, 17, 89–102. [Google Scholar] [CrossRef]
- Muñoz, L.M.J.; Díaz, I.S.; Rohner, C.S.; Ramirez, G.C.; Ambrosio, A.F. Effectiveness of High Power Ultrasound for Surimi-Based Preparation of Lionfish (Pterois volitans) Patties by Textural, Sensory and Shape Preference. J. Culin. Sci. Technol. 2019, 17, 89–102. [Google Scholar] [CrossRef]
- Schilling, M.W. Emulsifier applications in meat products. Food emulsifiers and their applications 2019, 347–77. [Google Scholar] [CrossRef]
- Câmara, A.K.F.I.; Vidal, V.A.S.; Santos, M.; Bernardinelli, O.D.; Sabadini, E.; Pollonio, M.A.R. Reducing phosphate in emulsified meat products by adding chia (Salvia hispanica L.) mucilage in powder or gel format: A clean label technological strategy. Meat Sci. 2020, 163, 108085. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jiang, S.; Zhao, D.; Zhang, J.; Gu, S.; Pan, Z.; Ding, Y. Changes in physicochemical properties and protein structure of surimi enhanced with camellia tea oil. LWT-Food Sci. Technol. 2017, 84, 562–571. [Google Scholar] [CrossRef]
- Yan, B.; Jiao, X.; Zhu, H.; Wang, Q.; Huang, J.; Zhao, H.; Cao, H.; Zhou, W.; Zhang, W.; Ye, W.; Zhang, H.; Fan, D. Chemical interactions involved in microwave heat-induced surimi gel fortified with fish oil and its formation mechanism. Food Hydrocoll. 2020, 105, 105779. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists. Official methods of analysis the of AOAC International. 17th ed. Washington, USA, 2000.
- Folch, J.; Lees, M.; Stanley, S.G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol.Chem. 1957, 26, 497–509. [Google Scholar] [CrossRef]
- Horita, C.N.; Morgano, M.A.; Celeguini, R.M.S.; Pollonio, M.A.R. Physicochemical and sensory properties of reduced-fat mortadella prepared with blends of calcium, magnesium and potassium chloride as partial substitutes for sodium chloride. Meat Sci. 2011, 89, 426–433. [Google Scholar] [CrossRef]
- Jiao, X.; Cao, H.; Fan, D.; Huang, J.; Zhao, J. Effects of fish oil incorporation on the gelling properties of silver carp surimi gel subjected to microwave heating combined with conduction heating treatment. Food Hydrocoll. 2019, 94, 164–173. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S. M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Seighalani, F.Z.B.; Jamilah, B.; Saari, N. Physico-chemical properties of red tilapia (Oreochromis spp.) during surimi and kamaboko gel preparation. Int. Food Res. J. 2017, 24, 1248–1254. [Google Scholar]
- Rosmini, M.R.; Perlo, F.; Pérez-Alvarez, J.A.; Pagán-Moreno, M.J.; Gago-Gago, A.; López-Santoveña, F.; Aranda-Catalá, V. TBA teste by na extractive method appliend to pate. Meat Sci. 1996, 42, 103–110. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.P.; Munekata, P.E.S.; Lorenzo, J.M.; De Melo, M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Technol. 2015, 53, 1–10. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-JiménesÉREZ-JIMÉNEZ, J.; SAURA-CALIXTO, F.D. Metodologia científica: Determinação da atividade antioxidante total em frutas pela captura do radical livre DPPH. Comunicado Técnico Embrapa 2007, 1679–6535. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.M.M. Guia del inspector veterinário titular: 1- bromotologia sanitária. Barcelona: Biblioteca Veterinária Aedos 1997, 1975, 190–260. [Google Scholar]
- Vasconcelos, E.L.Q.; Andrade, E.G.; Rocha, M.P.S.; Taveira, I. S.; Inhamuns, A.J.; Oloveira, P.R.; Uchôa, N.M.; Souza, A.L. Crioprotetores na estabilidade do surimi de matrinxã (Brycon amazonicus Spix and Agassiz 1819) sob congelamento. PUBVET 2016, 10, 352–355. Available online: https://www.researchgate.net/profile/Euclides-Luis/publication/308274599_Crioprotetores_na_estabilidade_do_surimi_de_matrinxa_Brycon_amazonicus_Spix_e_Agassiz_1819_sob_congelamento/links/5b3e8b10a6fdcc8506face2b/Crioprotetores-na-estabilidade-do-surimi-de-matrinxa-Brycon-amazonicus-Spix-e-Agassiz-1819-sob-congelamento.pdf (accessed on 10 June 2023). [CrossRef]
- Shi, L.; Yin, T.; Huang, Q.; You, J.; Hu, Y.; Jia, D.; Xiong, S. Effects of filleting methods on composition, gelling properties and aroma profile of grass carp surimi. Food Science and Human Wellness 2021, 10, 308–315. [Google Scholar] [CrossRef]
- Sousa, T.C.A.; Silva, E.L.L.; Ferreira, V.C.S.; Madruga, M.S. Silva, F.A.P. Oxidative stability of green weakfish (Cynoscion virescens) by-product surimi and surimi gel enhanced with a Spondias mombin L. waste phenolic-rich extract during cold storage. Food Bioscience 2022, 10, 102021. [Google Scholar] [CrossRef]
- Marín-Peñalver, D.; Alemán, A.; Montero, M.P.; Gómez-Guillén, M.C. Entrapment of natural compounds in spray-dried and heat-dried iota-carrageenan matrices as functional ingredients in surimi gels. Food Funct. 2021, 12, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
| Paramaters (g/100g) | Treatments | |||
| EC0 | EC50 | EC100 | ||
| Moisture | 61.39±0,62a | 59.66± 0.80ab | 57.64±2.21b | |
| Ash | 4.45±0,29a | 4.02± 0.39a | 4.01±0.14a | |
| Protein | 18.71±0,60b | 21.03± 0.78a | 20.82±1.16a | |
| Lipids | 9.28±0,45a | 8.85± 0.20ab | 8.15±0.20b1 | |
| Parameters | Treatments | ||
| EC0 | EC50 | EC100 | |
| ES (%) | 75.45±0.41a | 71.51±0.54b | 71.20±1.26b |
| Total fluid released (%) | 24.55±0.41b | 28.49±0.54b | 28.80±1.26a2 |
| pH | |||
|---|---|---|---|
| Days of storage | Treaments | ||
| EC0 | EC50 | EC100 | |
| 0 | 7.55±0.03aB | 7.41±0.02bB | 7.40±0.01bB |
| 20 | 7.88±0.01aA | 7.83±0.01bA | 7.83±0.01bA |
| 40 | 6.98±0.02aCD | 6.94±0.02bCD | 6.91±0.01bCD |
| 60 | 6.85±0.03aE | 6.87±0.06aD | 6.82±0.02aE |
| 80 | 7.01±0.05aC | 7.04±0.09aC | 6.93±0.02aC |
| 100 | 7.01±0.03aCD | 6.98±0.03abCD | 6.91±0.03bCD |
| 120 | 6.92±0.05aDE | 6.96±0.05aCD | 6.87±0.01aD3 |
| Parameters | Treatments | Storage (days) | ||
| 0 | 60 | 120 | ||
| Hardness (N) | EC0 | 8.58 ± 0.19cB | 9.05 ± 0.38bAB | 9,46 ± 0,23bA |
| EC50 | 10.29 ± 0.88bB | 12.09 ± 0.22aB | 16,84 ± 1,13aA | |
| EC100 | 17.56 ± 0.13aA | 12.52 ± 1.35aB | 16,84 ± 0,58aA | |
| Gumminess (N) | EC0 | 1.67 ± 0.34aA | 1.59 ± 0.01bA | 1.61 ± 0.44bA |
| EC50 | 2.19 ± 0.29aA | 2.48 ± 0.38aA | 3.14 ± 0.57aA | |
| EC100 | 2.17 ± 0.22aAB | 1.98 ± 0.26abB | 2.76 ± 0.29aA | |
| Resilience | EC0 | 0.07 ± 0.02aA | 0.07 ± 0.01bA | 0.07 ± 0.01bA |
| EC50 | 0.09 ± 0.03aA | 0.11 ± 0.01aA | 0.11 ± 0.02aA | |
| EC100 | 0.07 ± 0.01aB | 0.11 ± 0.01aA | 0.11 ± 0.01aA | |
| Cohesiviness | EC0 | 0.19 ± 0.03aA | 0.19 ± 0.02aA | 0.16 ± 0.03bA |
| EC50 | 0.23 ± 0.06aA | 0.19 ± 0.01aA | 0.21 ± 0.01aA | |
| EC100 | 0.15 ± 0.02aA | 0.16 ± 0.01aA | 0.17 ± 0.02bA | |
| Elasticity (mm) | EC0 | 4.46 ± 0.76aA | 4.61 ± 0.42bA | 5.11 ± 0.36bA |
| EC50 | 5.35 ±0.77aB | 6.90 ± 0.30aA | 6.21 ± 0.66aAB | |
| EC100 | 5.50 ± 0.14aB | 6.16 ± 0.52aAB | 6.80 ± 0.11aA | |
| Chewiness (mJ) | EC0 | 6.35 ± 0.75cB | 6.95 ± 0.35cAB | 9.24 ± 1.70bA |
| EC50 | 11.63 ± 0.55bC | 14.65 ± 0.15aB | 23.23 ± 1.33aA | |
| EC100 | 13.40 ± 0.20aB | 12.64 ± 1.30bB | 20.60 ± 0.87aA4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





