1. Introduction
Light availability is of paramount importance for phytoplankton, especially in turbid ecosystems [
1,
2,
3,
4,
5], but it has not yet received the same attention as nutrients as an environmental driver of phytoplankton dynamics. Indeed, studies dealing with the effects of nutrients on phytoplankton are abundant (e.g., [
6,
7]), whilst papers focused on the effects of light are rather scarce (e.g., [
8,
9]). The main reason behind this discrepancy is probably methodological. Dissolved inorganic macronutrients can be easily and directly analysed in the lab, using simple colorimetric methods (e.g., [
10]). In addition, water samples can be collected and preserved for a considerable amount of time before the actual nutrient analysis is performed.
Measurements of underwater light are not as straightforward. Solar radiation reaching the Earth’s surface is composed by a large spectrum of radiation with different energies and wavelengths, including ultraviolet, infrared, and visible radiation. Photoautotrophic organisms, such as plants and algae, can only use a fraction of the total solar radiation for the process of photosynthesis; this photosynthetically active radiation (PAR) corresponds roughly to the visible light of the electromagnetic spectrum, with wavelengths between 400 and 700 nm. PAR constitutes approximately 50% of the total solar radiation that reaches the Earth’s surface, considering both direct and diffuse sources [
11]. The amount of light that penetrates the water surface depends on several factors, such as solar elevation and its daily and annual variation [
12], but a reflectance of 6.6% for a flat surface and irradiance coming equally from all directions is generally assumed [
11]. The radiation that penetrates the water is scattered and eventually all photons are absorbed by water molecules and by dissolved and particulate matter, resulting in an exponential decrease of light intensity with depth, according to the Beer-Lambert law. The rate at which light disappears in the water column with depth can be expressed by the light extinction coefficient or diffuse attenuation coefficient (K
d). K
d is classified as an apparent optical property of water, depending on the composition of the medium and on the directional structure of the ambient light field [
11].
The diffuse attenuation coefficient is typically estimated using the depth of disappearance of the Secchi disc (SD), as
k/SD, where
k is a constant;
k values of 1.7 [
13] and 1.44 [
14], for non-turbid and turbid (euphotic depths <5 m) waters, respectively, are commonly used. However, the constant
k can vary widely, between 1.27 and 2 [
15], and this uncertainty may lead to errors in the estimation of light availability for phytoplankton. In addition, this equation does not account for all of K
d variability, which is affected by any optically active component and represents the sum of water, phytoplankton, seston and chromophoric dissolved organic matter (CDOM) variability [
16]. Indeed, the relationship between SD and K
d may vary seven-fold in waters with high turbidity and CDOM variability [
17].
The best estimate of K
d (m
-1) is given by an exponential fit of light measurements in the water column as a function of depth, according to equation 1:
where I
z (µmol photons m
-2 s
-1) is the light intensity at depth level Z (m) and I
0 is the light intensity at the water surface. K
d may also be expressed as (equation 2):
where I
1 and I
2 are the light intensities at depth levels Z
1 and Z
2, respectively. Z
1 is the depth immediately below water surface. When light intensity in the water column is not available, K
d is generally estimated using empirical coefficients. Yet, a hyperbolic fit of Secchi disk measurements and K
d (obtained from vertical light attenuation measurements) data will result in a better function to estimate K
d from SD in a given ecosystem, than these constants (e.g., [
16]).
The use of K
d is deeply rooted in phytoplankton research and many efforts have been made to develop better empirical relationships between SD and K
d (e.g. [
18,
19,
20]). However, the diffuse attenuation coefficient
per se does not provide any information on the quantity of PAR available for phytoplankton and, therefore, does not allow the evaluation of the underwater light environment and its role as a limiting factor of phytoplankton growth. To determine potential light limitation, light intensity values are needed, just like nutrient concentrations are required to assess nutrient limitation [
21,
22]. Thus, the mean light intensity in the mixed layer (I
m) is a more relevant metric to evaluate the underwater light environment.
I
m represents the mean PAR to which cells are exposed throughout most of their life cycle, while being continuously mixed in the mixed layer. I
m is therefore a useful indicator of the underwater light environment, and it can be used to evaluate the occurrence of potential light limitation of phytoplankton growth. I
m determination (equation 3) considers the incident light at the water surface (I
0), the diffuse attenuation coefficient in the water column (K
d), and the depth of the mixed layer (Z
m):
However, given the easiness of Secchi depth determination, the high cost of quanta sensors [
20], and the common lack of daily integrated I
0 measurements, necessary for a realistic determination of I
m, K
d is frequently the only light-related variable determined in phytoplankton studies. In our opinion, K
d is used in a rather excessive and forced fashion to draw askew conclusions about the amount of light available for phytoplankton photosynthesis. It is commonly assumed that the relationship between K
d and light availability is inversely proportional, but light availability for phototrophs depends on the depth of the mixed layer, whereas K
d is unrelated with depth. For instance, a turbid (higher K
d) and shallow ecosystem (e.g., a coastal lagoon) may present higher light availability than a non-turbid system (lower K
d) with a deeper mixing layer (e.g., coastal zone, continental shelf, oceanic zone).
The assessment of underwater light availability is essential to understand phytoplankton dynamics and thus to assess the impacts of natural and human-induced perturbations to ecosystems. In this article we aim to expose constraints that phytoplankton researchers are faced with when characterizing the underwater light environment and to clarify the light-related variables of interest that, in our opinion, should be used in phytoplankton research and environmental monitoring. To accomplish this goal, we present underwater light data collected in distinct ecosystems in southern Portugal and analyse relationships between the diffuse attenuation coefficient and mean light intensity in the mixed layer.
3. Results and Discussion
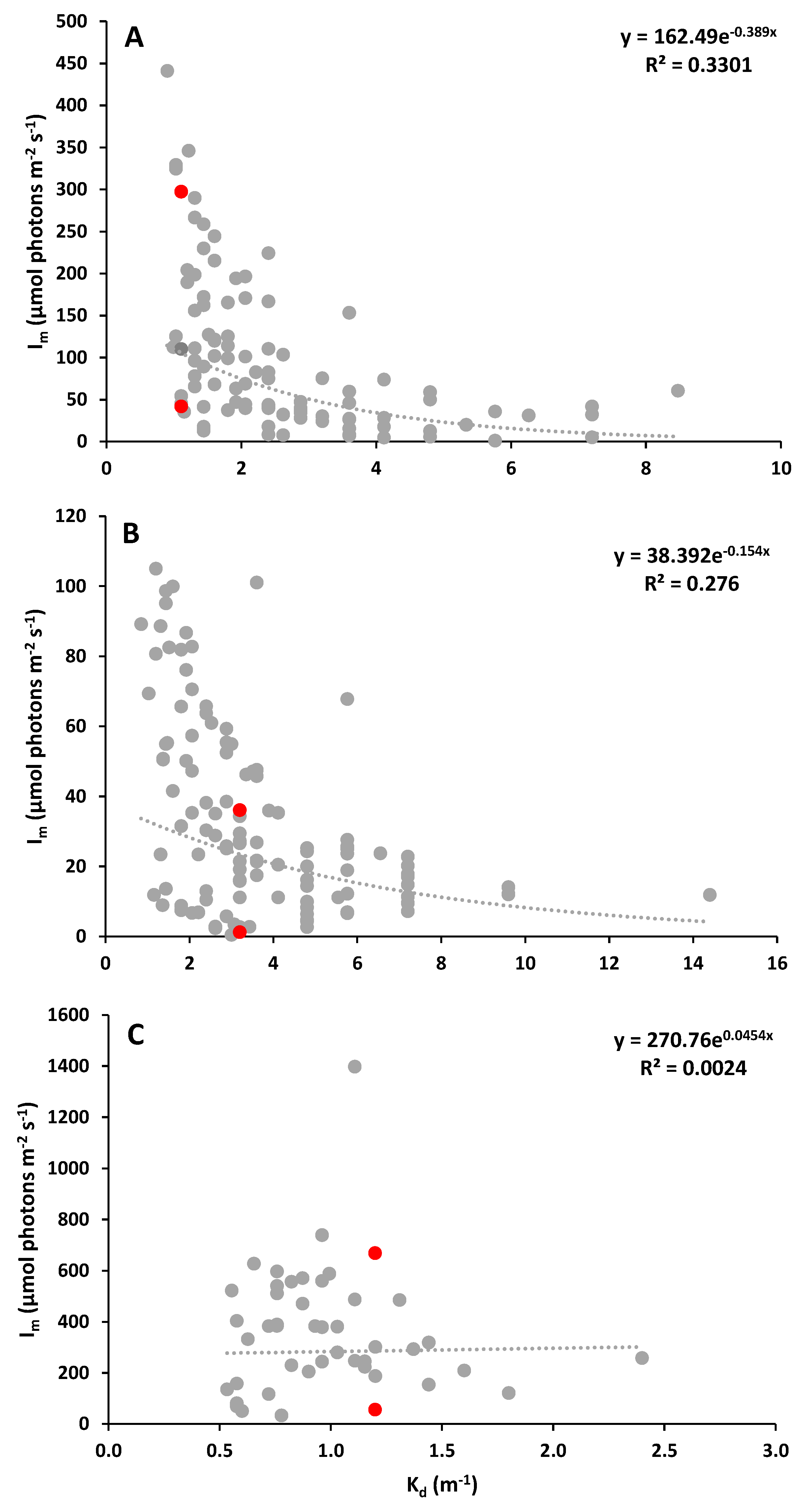
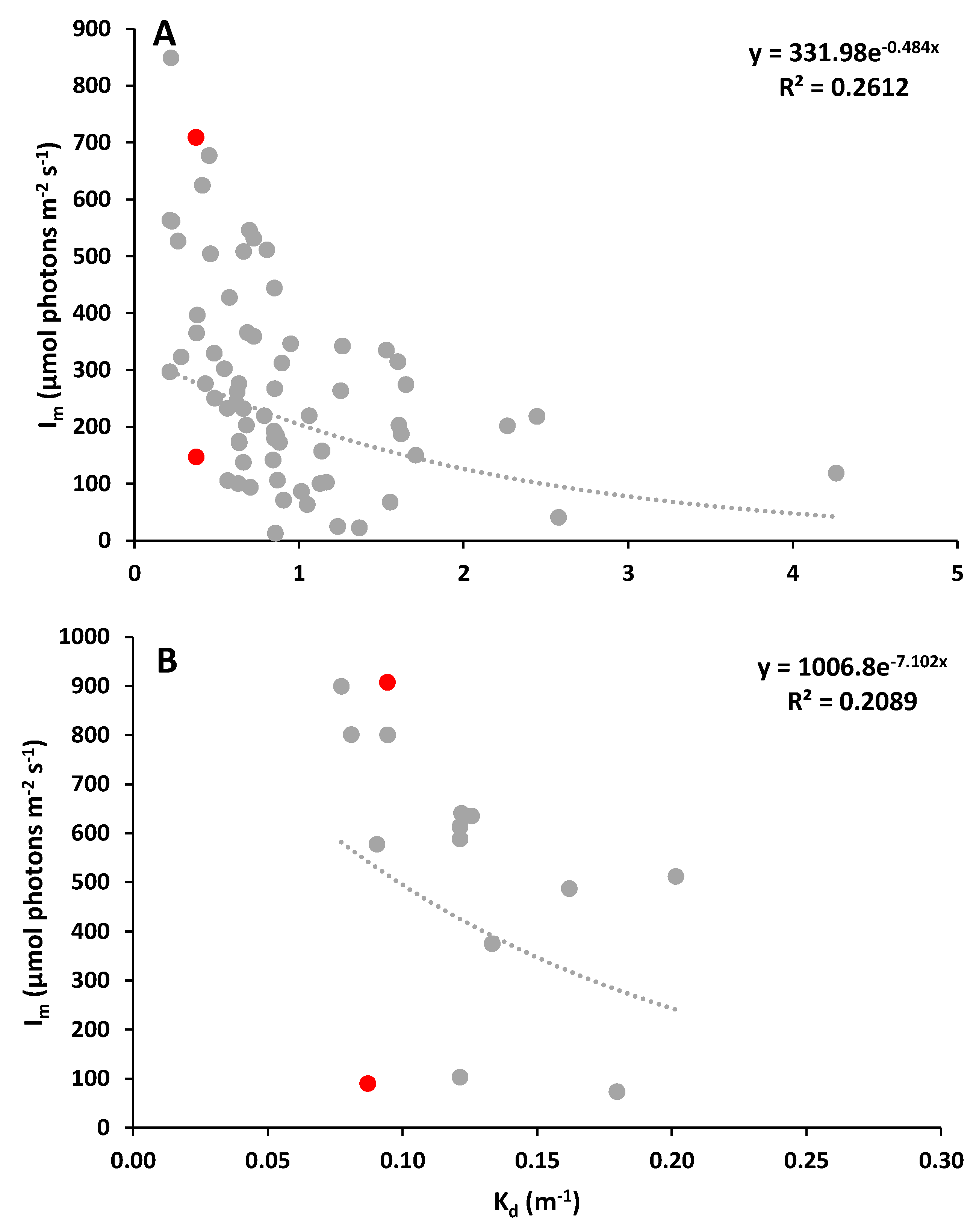
The relationship between the diffuse attenuation coefficient and the amount of light available for photosynthesis, expressed as the mean light intensity in the mixed layer, is typically inversely proportional, but not always significant. The highest K
d and lower I
m values were found in the freshwater (K
d = 0.90 - 8.47 m
-1, I
m = 0.99 – 440.86 µmol photons m
-2 s
-1) and brackish (K
d = 0.85 - 14.40 m
-1; I
m = 0.42 – 104.97 µmol photons m
-2 s
-1) estuarine zones of the Guadiana estuary (
Figure 1), whereas for coastal locations, K
d values were lower and I
m was higher (K
d = 0.08 – 4.26 m
-1, I
m = 12.82 – 1397.64 µmol photons m
-2 s
-1) (
Figure 2). The strength of the relationship between the two variables in the different coastal ecosystems was always weak, with determination coefficients ranging between 0.00 to 0.33.
It was also clear that similar K
d values were associated with different I
m (
Table 1), reflecting the importance of considering the depth of the mixed layer to properly evaluate PAR availability for photosynthesis. For instance, a K
d of 0.6 m
-1 corresponds to I
m values ranging between 100 and 427 µmol photons m
-2 s
-1 in the Ria Formosa coastal lagoon, and between 50 and 332 µmol photons m
-2 s
-1 in the Guadiana estuary (See
Figure 1 and
Figure 2). For each type of ecosystem, each Kd value presents a wide range of corresponding Im values; for instance, in coastal zone, a K
d of 0.09 m
-1 was associated with I
m values of 89 and 907 µmol photons m
-2 s
-1 (
Table 1). These I
m values may have different physiological effects on phytoplankton. For instance, light enrichment experiments conducted in the Guadiana estuary demonstrated that exposure of natural phytoplankton communities to I
m values of 50, 70, 120, and 225 µmol photons m
-2 s
-1 had significantly different effects, from an enhancement to a decline of phytoplankton growth, leading to changes in phytoplankton biomass and community structure (Domingues et al. 2011b). Therefore, a K
d value of 0.6 m
-1 may correspond not only to different mean light intensities in the mixed layer, but also to different degrees of light-limited growth and photoinhibition of phytoplankton photosynthesis. To avoid erroneous conclusions about the availability of underwater light for phytoplankton based solely on light attenuation coefficients, we urge phytoplankton researchers to include estimates of both K
d and I
m in phytoplankton studies and monitoring programs. But how can I
m me estimated in a timely and inexpensive manner?
Firstly, the depth of the mixed layer (Z
m) should be established. The mixed layer is the top, unstratified layer in the water column where no significant density gradients are found, and phytoplankton is thus continuously mixed. Empirically, the mixed layer is where temperature or density variability with depth is less than 0.5ºC or 0.125 sigma-t, respectively [
25]. In shallow, well mixed ecosystems, Z
mix usually corresponds to the whole water column depth [
26]. In an estuarine ecosystem, for instance, Z
mix will vary along the channel’s cross-section and with tidal phase. A mean mixing depth should therefore be established for coastal, shallow waters, taking into consideration bathymetry and tidal amplitude. For deeper waters, the mixing depth should be determined in each sampling campaign, using vertical profiles of T and S.
A common lapse in phytoplankton studies is the characterization of light availability for phytoplankton using, besides K
d, the compensation depth, that corresponds to the bottom of the euphotic zone and is defined as the depth where PAR is 1% of I
0. Given that this amount of PAR is sufficient to sustain photosynthesis, the compensation depth is another metric widely used in phytoplankton research (e.g., [
27]). However, the euphotic layer and the mixed layer are usually neither coincident nor proportional, and whilst the euphotic depth depends on inherent optical properties of the water, the mixing depth is mainly affected by physical-meteorological forcing, bathymetry and even tides. For phytoplankton, it really does not matter how deep the euphotic zone is; what matters is the mean light intensity to which cells are exposed while being continuously mixed in the mixed layer. A useful approach, given by [
28], combines both Z
eu and Z
mix to characterize light availability for phytoplankton growth, in the form of a mixing depth to euphotic depth ratio (Z
mix:Z
eu). It is usually considered that when the mixing depth is more than 5 times the euphotic depth (Z
mix:Z
eu > 5), no net phytoplankton growth will occur.
Secondly, the light attenuation coefficient must be determined to calculate I
m. Two methods can be used, as referred above: vertical profiles of PAR in the water column or a function that relates K
d with Secchi depth. PAR measurements with a spherical quantum sensor are more reliable than the Secchi disk, which relies on the sensitivity of the human eye. But even vertical profiles of PAR are not exempt of problems. Several measurements of PAR intensity should be taken in the water column at specified depths (usually every meter), to adjust the exponential function. All measurements must made in the same conditions, considering intermittent cloud cover and making sure that the cable supporting the light sensor is always vertical. K
d is usually considered constant with depth, so only a few data points are necessary for an accurate estimate of K
d, especially in turbid systems, where light attenuation closely follows an exponential function and thus can be characterized by a single K
d [
11]. In clear waters light attenuation with depth may present a biphasic behaviour, characterized by two K
d values [
11].
It should be noted, however, that light attenuation in the water column may show significant daily variability. In shallow turbid ecosystems, significant and positive correlations between SPM and K
d are common, indicating that light attenuation is mainly controlled by suspended sediments [
29,
30], which in turn may show significant short-term variability associated to tidal cycles and river flow [
2]. For instance, SPM in the Guadiana estuary is usually higher during spring tides, due to stronger tidal currents and higher resuspension of bottom sediments [
30], and during flood, due to the resuspension of sediments deposited during the preceding long low tide slack [
31]. However, light attenuation coefficients are typically considered constant throughout the day, which may lead to erroneous estimates of I
m. In the Guadiana estuary, K
d varies along the semidiurnal cycle, with higher values during ebb and flood and lower at slack water. During an autumn 2008 spring tide, K
d ranged between 7.3 m
-1 during flood and 3.9 m
-1 three hours later, at high tide [
30]. Considering Z
m = 9.4 m and I
0 = 1,000 µmol photons m
-2 s
-1, I
m calculation based on K
d = 3.9 m
-1 would be 27 µmol photons m
-2 s
-1, whilst using k
d = 7.3 m
-1, I
m is 15 µmol photons m
-2 s
-1, roughly half of the first value.
Thirdly, incident light at the water surface (I0) shows a significant daily variability. If sampling is conducted in the early morning when incident solar radiation is lower, Im will be lower than if measurements were taken in the afternoon; likewise, sampling around noon will result in higher Im values. When these isolated estimates are taken as a proxy for the whole day, the mean light availability in the mixed layer over the light period may be severely under- or overestimated. Therefore, light measurements should consider the significant daily variability of solar radiation, to avoid inaccurate assessments of light limitation of phytoplankton growth. Ideally, Im estimates should use the mean radiation for the whole light period. Data on daily solar radiation should thus be obtained, either from a public database or by making continuous measurements of PAR at the water surface throughout the light period.
The mean light intensity in the mixed layer is the variable of interest to evaluate the underwater light environment for phytoplankton. However, to assess the occurrence of light limitation of phytoplankton growth using I
m, a previous knowledge on how a phytoplankton community responds to light is necessary. The effects of light on phytoplankton growth and community structure can be determined using bioassays where micro- or mesocosms of natural phytoplankton assemblages are exposed to different light intensities. The outcomes of these experiments can be extrapolated to the field and used to assess the occurrence of light limitation of phytoplankton growth. Light addition experiments also provide threshold I
m values that can be used to evaluate the occurrence of light limitation in natural phytoplankton communities (e.g., [
4,
8]). In addition, the measurement of carbon incorporation by phytoplankton (e.g., using the
14C method: [
32]) under different light intensities allows the determination of a light-response curve of photosynthesis, known as a photosynthesis-irradiance (P-E) curve, which describes the variability of photosynthetic characteristics of phytoplankton over a range of light conditions. Again, experimental PAR values can be compared to I
m values in the field, providing an array of information concerning the effects of light on phytoplankton, such as the saturating irradiance, photosynthetic efficiency, and the occurrence of photoinhibition.