Submitted:
06 June 2023
Posted:
07 June 2023
You are already at the latest version
Abstract
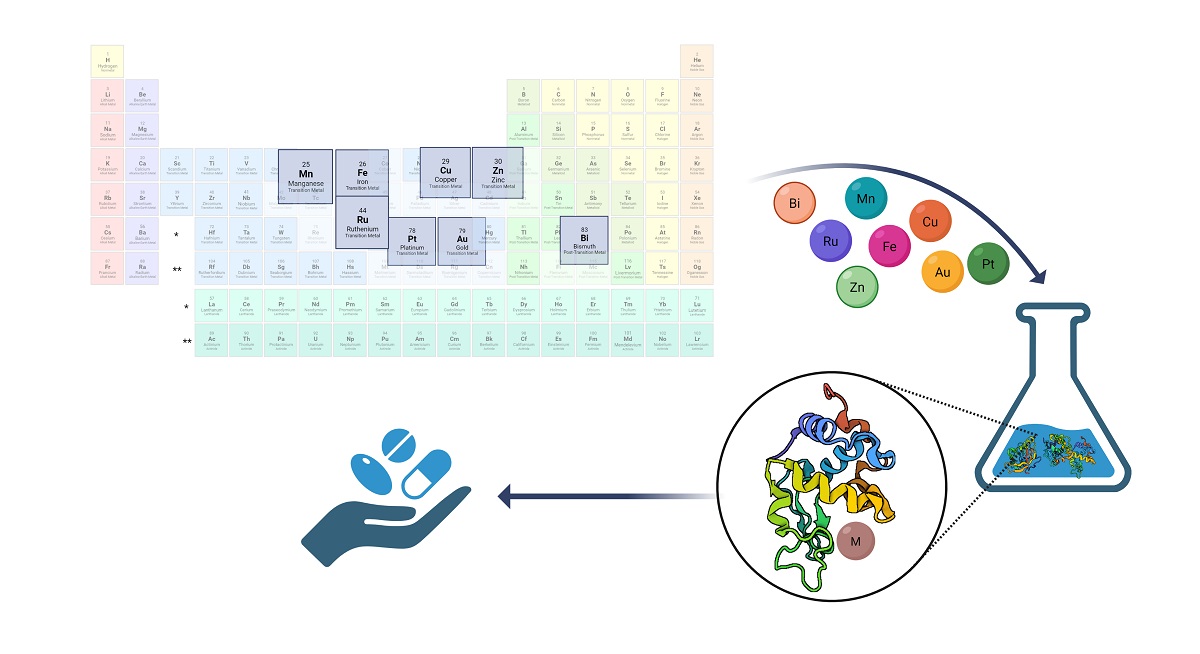
Keywords:
1. Introduction
2. Protein targets identification through proteomics approaches
2.1. Affinity-based strategies

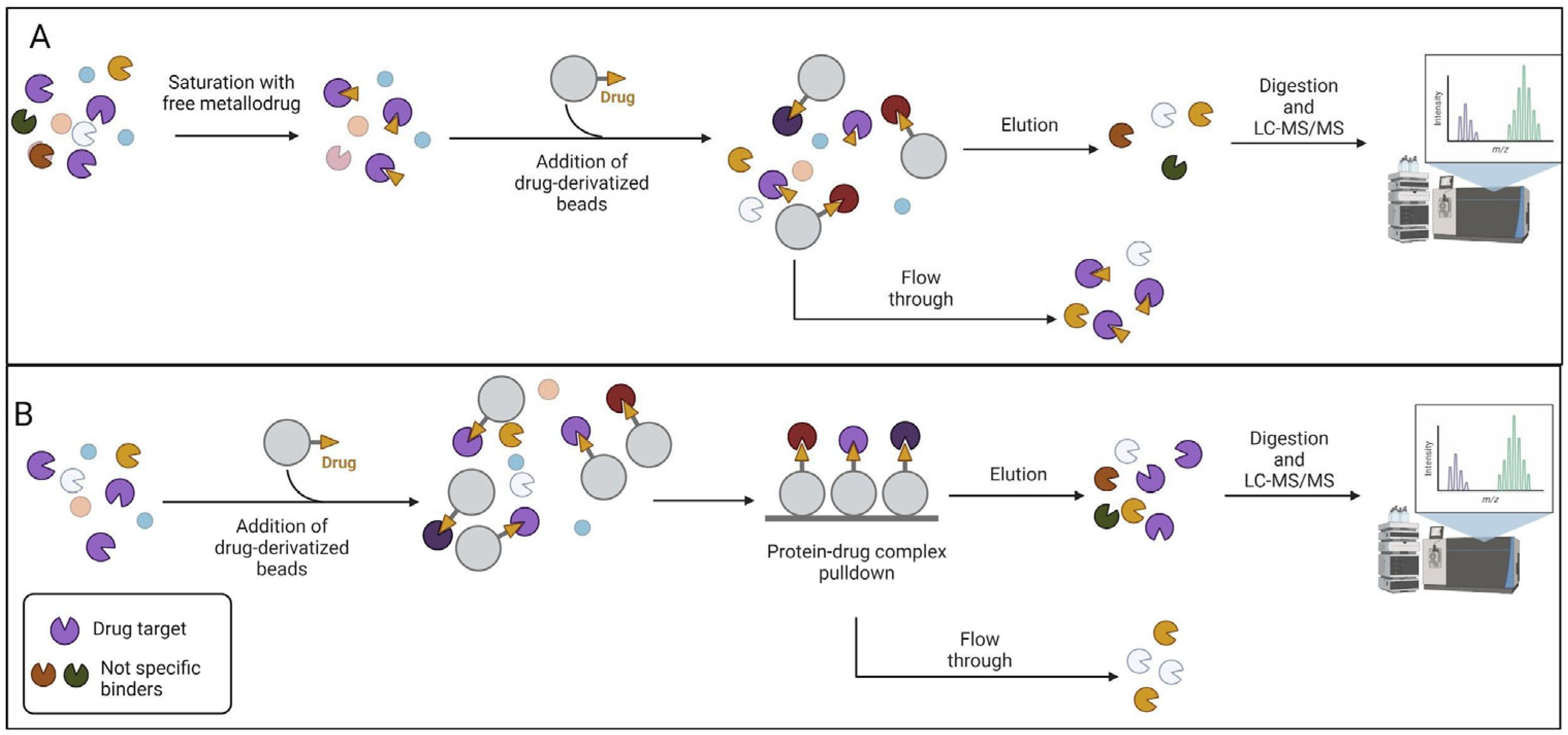
1.2. Label-free approaches
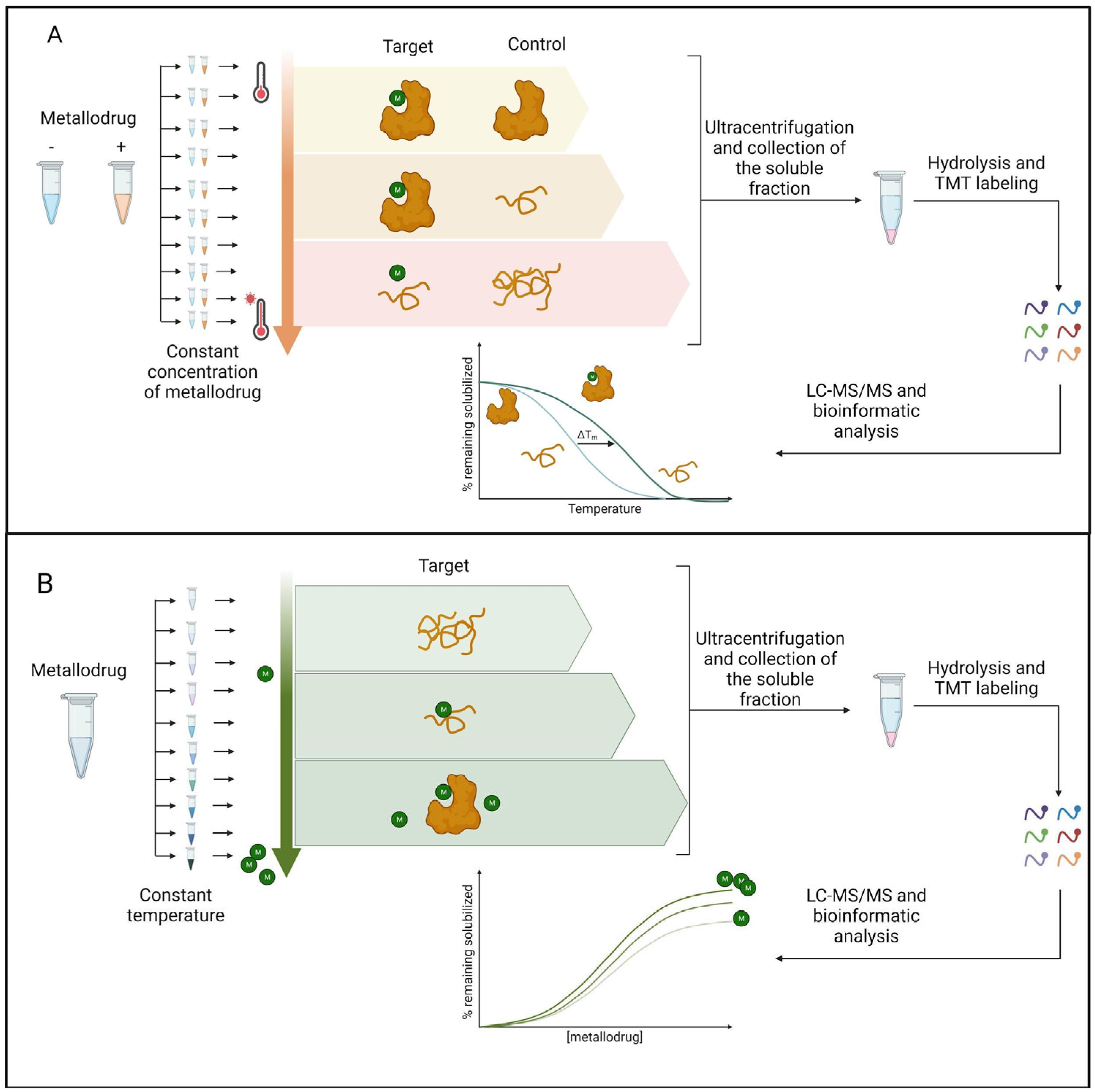
3. Methods to enhance metallodrugs efficiency administration
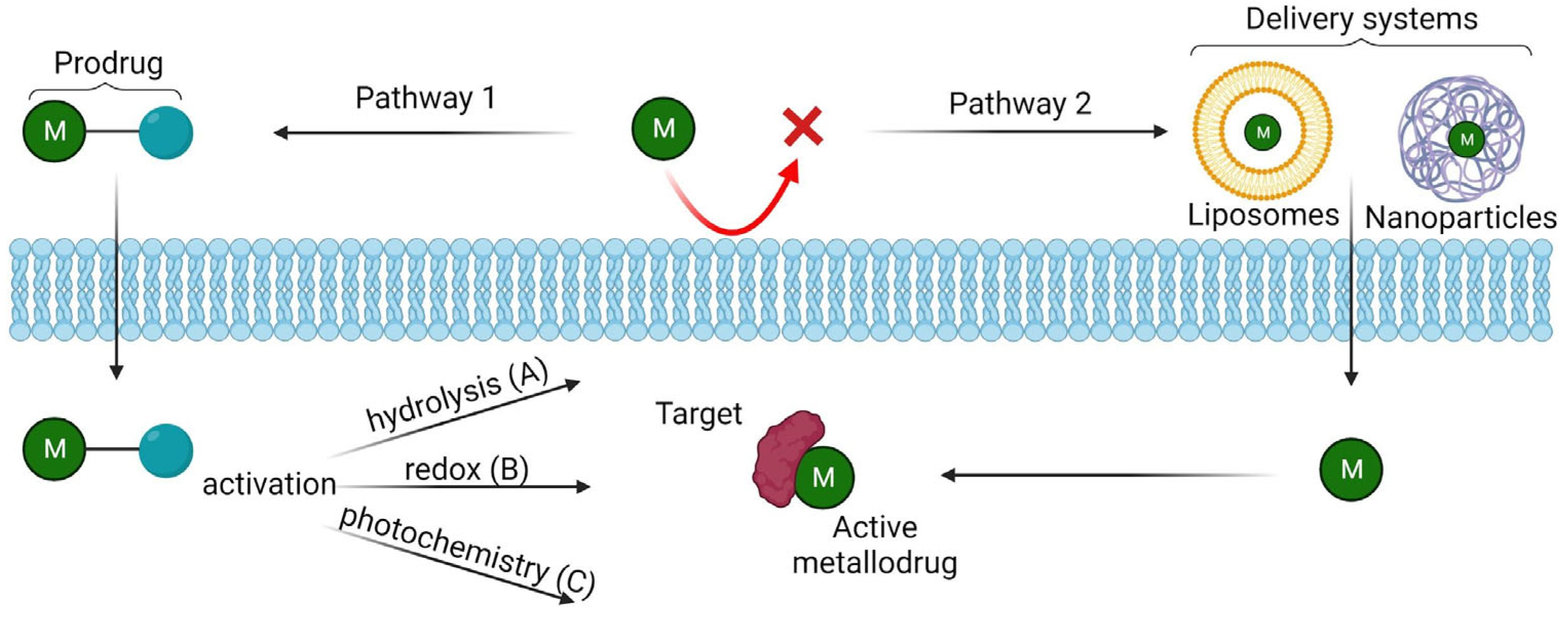
3.1. Pro-metallodrugs
- 1)
- Activation via hydrolysis
- 2)
- Redox activation
- 3)
- Photoactivation (Light-activatable metallodrugs)
3.2. Delivery systems
4. Conclusions and Future Directions
Author Contributions
Conflicts of Interest
References
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiological Reviews 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Mohammadi, S.; Farjami, M.; Suha, A.J.; Zarch, S.M.A.; Najafi, S.; Esmaeili, A. The Mitochondrial Calcium Signaling, Regulation, and Cellular Functions: A Novel Target for Therapeutic Medicine in Neurological Disorders. J Cell Biochem 2023, 124, 635–655. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Li, S.; Huang, X.; Chen, X.; Shan, H.; Zhang, M. The Role of Copper Homeostasis in Brain Disease. Int J Mol Sci 2022, 23, 13850. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of Copper on Mitochondrial Function and Metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef] [PubMed]
- Opazo, C.M.; Greenough, M.A.; Bush, A.I. Copper: From Neurotransmission to Neuroproteostasis. Front Aging Neurosci 2014, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, Y.-L.; Liu, X.-Z.; Shen, P.; Zheng, Y.-G.; Lan, X.-R.; Lu, C.-B.; Wang, J.-Z. Current Understanding of Metal Ions in the Pathogenesis of Alzheimer’s Disease. Transl Neurodegener 2020, 9, 10. [Google Scholar] [CrossRef]
- Haywood, S. Brain–Barrier Regulation, Metal (Cu, Fe) Dyshomeostasis, and Neurodegenerative Disorders in Man and Animals. Inorganics 2019, 7, 108. [Google Scholar] [CrossRef]
- Franz, K.J.; Metzler-Nolte, N. Introduction: Metals in Medicine. Chem. Rev. 2019, 119, 727–729. [Google Scholar] [CrossRef]
- Galib, null; Barve, M.; Mashru, M.; Jagtap, C.; Patgiri, B.J.; Prajapati, P.K. Therapeutic Potentials of Metals in Ancient India: A Review through Charaka Samhita. J Ayurveda Integr Med 2011, 2, 55–63. [CrossRef] [PubMed]
- Mukherjee, A.; Sadler, P.J. Metals in Medicine: Therapeutic Agents. In Wiley Encyclopedia of Chemical Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; p. wecb333. ISBN 978-0-470-04867-2. [Google Scholar]
- Sodhi, R.K. Metal Complexes in Medicine: An Overview and Update from Drug Design Perspective. CTOIJ 2019, 14. [Google Scholar] [CrossRef]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of Cell Division in Escherichia Coli by Electrolysis Products from a Platinum Electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.P.; Holtkamp, H.U.; Hartinger, C.G. 13. Antitumor Metallodrugs That Target Proteins. In Metallo-Drugs: Development and Action of Anticancer Agents; Sigel, A., Sigel, H., Freisinger, E., Sigel, R.K.O., Eds.; De Gruyter: Berlin, Boston, 2018; pp. 351–386. ISBN 978-3-11-047073-4. [Google Scholar]
- Khoury, A.; Deo, K.M.; Aldrich-Wright, J.R. Recent Advances in Platinum-Based Chemotherapeutics That Exhibit Inhibitory and Targeted Mechanisms of Action. J Inorg Biochem 2020, 207, 111070. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Yu, J.; Pan, Y.; Zhang, N.; Qi, Y.; Huang, Y. Recent Advances of Platinum-Based Anticancer Complexes in Combinational Multimodal Therapy. Adv Healthc Mater 2023, e2300253. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Guo, Z. Functionalization of Platinum Complexes for Biomedical Applications. Acc Chem Res 2015, 48, 2622–2631. [Google Scholar] [CrossRef]
- Barabas, K.; Milner, R.; Lurie, D.; Adin, C. Cisplatin: A Review of Toxicities and Therapeutic Applications. Vet Comparative Oncology 2008, 6, 1–18. [Google Scholar] [CrossRef]
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Williams, C.J.; Whitehouse, J.M. Cis-Platinum: A New Anticancer Agent. BMJ 1979, 1, 1689–1691. [Google Scholar] [CrossRef]
- Coffetti, G.; Moraschi, M.; Facchetti, G.; Rimoldi, I. The Challenging Treatment of Cisplatin-Resistant Tumors: State of the Art and Future Perspectives. Molecules 2023, 28, 3407. [Google Scholar] [CrossRef]
- Florio, D.; La Manna, S.; Annunziata, A.; Iacobucci, I.; Monaco, V.; Di Natale, C.; Mollo, V.; Ruffo, F.; Monti, M.; Marasco, D. Ruthenium Complexes Bearing Glucosyl Ligands Are Able to Inhibit the Amyloid Aggregation of Short Histidine-Peptides. Dalton Trans. 2023, 10.1039.D3DT01110K. [Google Scholar] [CrossRef]
- Paul, N.P.; Galván, A.E.; Yoshinaga-Sakurai, K.; Rosen, B.P.; Yoshinaga, M. Arsenic in Medicine: Past, Present and Future. Biometals 2023, 36, 283–301. [Google Scholar] [CrossRef]
- Florio, D.; Iacobucci, I.; Ferraro, G.; Mansour, A.M.; Morelli, G.; Monti, M.; Merlino, A.; Marasco, D. Role of the Metal Center in the Modulation of the Aggregation Process of Amyloid Model Systems by Square Planar Complexes Bearing 2-(2’-Pyridyl)Benzimidazole Ligands. Pharmaceuticals (Basel) 2019, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, A.M.; Habtemariam, A.; Sadler, P.J. Activation Mechanisms for Organometallic Anticancer Complexes. In Medicinal Organometallic Chemistry; Jaouen, G., Metzler-Nolte, N., Eds.; Topics in Organometallic Chemistry; Springer Berlin Heidelberg: Berlin, Heidelberg, 2010; Vol. 32, pp. 21–56. ISBN 978-3-642-13184-4. [Google Scholar]
- Sullivan, M.P.; Holtkamp, H.U.; Hartinger, C.G. Antitumor Metallodrugs That Target Proteins. Met Ions Life Sci 2018, 18, /books/9783110470734/9783110470734-019/9783110470734-019.xml. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Sun, H. Metalloproteomics for Biomedical Research: Methodology and Applications. Annu Rev Biochem 2022, 91, 449–473. [Google Scholar] [CrossRef]
- Kostova, I. Ruthenium Complexes as Anticancer Agents. CMC 2006, 13, 1085–1107. [Google Scholar] [CrossRef]
- Huang, H.; Cao, K.; Kong, Y.; Yuan, S.; Liu, H.; Wang, Y.; Liu, Y. A Dual Functional Ruthenium Arene Complex Induces Differentiation and Apoptosis of Acute Promyelocytic Leukemia Cells. Chem. Sci. 2019, 10, 9721–9728. [Google Scholar] [CrossRef]
- Moreno-Alcántar, G.; Picchetti, P.; Casini, A. Gold Complexes in Anticancer Therapy: From New Design Principles to Particle-Based Delivery Systems. Angew Chem Int Ed 2023, 62, e202218000. [Google Scholar] [CrossRef] [PubMed]
- Ahrweiler-Sawaryn, M.-C.; Biswas, A.; Frias, C.; Frias, J.; Wilke, N.L.; Wilke, N.; Berkessel, A.; Prokop, A. Novel Gold(I) Complexes Induce Apoptosis in Leukemia Cells via the ROS-Induced Mitochondrial Pathway with an Upregulation of Harakiri and Overcome Multi Drug Resistances in Leukemia and Lymphoma Cells and Sensitize Drug Resistant Tumor Cells to Apoptosis in Vitro. Biomed Pharmacother 2023, 161, 114507. [Google Scholar] [CrossRef]
- Ferraro, M.G.; Piccolo, M.; Misso, G.; Santamaria, R.; Irace, C. Bioactivity and Development of Small Non-Platinum Metal-Based Chemotherapeutics. Pharmaceutics 2022, 14, 954. [Google Scholar] [CrossRef]
- Barhamand, B.A. Difficulties Encountered in Implementing Guidelines for Handling Antineoplastics in the Physician’s Office. Cancer Nurs 1986, 9, 138–143. [Google Scholar] [CrossRef]
- Zanca, C.; Cozzolino, F.; Quintavalle, C.; Di Costanzo, S.; Ricci-Vitiani, L.; Santoriello, M.; Monti, M.; Pucci, P.; Condorelli, G. PED Interacts with Rac1 and Regulates Cell Migration/Invasion Processes in Human Non-Small Cell Lung Cancer Cells. J Cell Physiol 2010, 225, 63–72. [Google Scholar] [CrossRef]
- Fusco, S.; Aulitto, M.; Iacobucci, I.; Crocamo, G.; Pucci, P.; Bartolucci, S.; Monti, M.; Contursi, P. The Interaction between the F55 Virus-Encoded Transcription Regulator and the RadA Host Recombinase Reveals a Common Strategy in Archaea and Bacteria to Sense the UV-Induced Damage to the Host DNA. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms 2020, 1863, 194493. [Google Scholar] [CrossRef] [PubMed]
- Iacobucci, I.; Monaco, V.; Cozzolino, F.; Monti, M. From Classical to New Generation Approaches: An Excursus of -Omics Methods for Investigation of Protein-Protein Interaction Networks. J Proteomics 2021, 230, 103990. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, F.; Iacobucci, I.; Monaco, V.; Monti, M. Protein-DNA/RNA Interactions: An Overview of Investigation Methods in the -Omics Era. J Proteome Res 2021, 20, 3018–3030. [Google Scholar] [CrossRef] [PubMed]
- Kumara, B.N.; Kalimuthu, P.; Prasad, K.S. Synthesis, Properties and Potential Applications of Photoluminescent Carbon Nanoparticles: A Review. Anal Chim Acta 2023, 1268, 341430. [Google Scholar] [CrossRef]
- Xia, Y.; Fu, S.; Ma, Q.; Liu, Y.; Zhang, N. Application of Nano-Delivery Systems in Lymph Nodes for Tumor Immunotherapy. Nanomicro Lett 2023, 15, 145. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Moghanian, A.; Rashvand, A.; Miri, A.K.; Hamzehlou, S.; Baino, F.; Mozafari, M.; Wang, A.Z. Nanostructured Bioactive Glasses: A Bird’s Eye View on Cancer Therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2023, e1905. [Google Scholar] [CrossRef] [PubMed]
- Peña, Q.; Wang, A.; Zaremba, O.; Shi, Y.; Scheeren, H.W.; Metselaar, J.M.; Kiessling, F.; Pallares, R.M.; Wuttke, S.; Lammers, T. Metallodrugs in Cancer Nanomedicine. Chem Soc Rev 2022, 51, 2544–2582. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, I.I.; Lineva, V.I.; Tarasova, I.A.; Gorshkov, M.V. Mass Spectrometry-Based Chemical Proteomics for Drug Target Discoveries. Biochemistry (Mosc) 2022, 87, 983–994. [Google Scholar] [CrossRef]
- Skos, L.; Borutzki, Y.; Gerner, C.; Meier-Menches, S.M. Methods to Identify Protein Targets of Metal-Based Drugs. Current Opinion in Chemical Biology 2023, 73, 102257. [Google Scholar] [CrossRef]
- Steel, T.R.; Hartinger, C.G. Metalloproteomics for Molecular Target Identification of Protein-Binding Anticancer Metallodrugs. Metallomics 2020, 12, 1627–1636. [Google Scholar] [CrossRef]
- Ziegler, S.; Pries, V.; Hedberg, C.; Waldmann, H. Target Identification for Small Bioactive Molecules: Finding the Needle in the Haystack. Angew. Chem. Int. Ed. 2013, 52, 2744–2792. [Google Scholar] [CrossRef]
- Iacobucci, I.; Monaco, V.; Canè, L.; Bibbò, F.; Cioffi, V.; Cozzolino, F.; Guarino, A.; Zollo, M.; Monti, M. Spike S1 Domain Interactome in Non-Pulmonary Systems: A Role beyond the Receptor Recognition. Front Mol Biosci 2022, 9, 975570. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Sepe, R.; Cozzolino, F.; Piccolo, C.; Iannone, C.; Iacobucci, I.; Pucci, P.; Monti, M.; Fusco, A. The Complex CBX7-PRMT1 Has a Critical Role in Regulating E-Cadherin Gene Expression and Cell Migration. Biochim Biophys Acta Gene Regul Mech 2019, 1862, 509–521. [Google Scholar] [CrossRef]
- Cozzolino, F.; Vezzoli, E.; Cheroni, C.; Besusso, D.; Conforti, P.; Valenza, M.; Iacobucci, I.; Monaco, V.; Birolini, G.; Bombaci, M.; et al. ADAM10 Hyperactivation Acts on Piccolo to Deplete Synaptic Vesicle Stores in Huntington’s Disease. Hum Mol Genet 2021, 30, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Babak, M.V.; Meier, S.M.; Huber, K.V.M.; Reynisson, J.; Legin, A.A.; Jakupec, M.A.; Roller, A.; Stukalov, A.; Gridling, M.; Bennett, K.L.; et al. Target Profiling of an Antimetastatic RAPTA Agent by Chemical Proteomics: Relevance to the Mode of Action. Chem. Sci. 2015, 6, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, M.; Gao, F.; Wei, W.; Qian, Y.; Liu, H.-K.; Zhao, J. Imaging of a Clickable Anticancer Iridium Catalyst. Journal of Inorganic Biochemistry 2018, 180, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Zhao, X.; Wei, W.; Zhao, J. Imaging and Proteomic Study of a Clickable Iridium Complex. Metallomics 2019, 11, 1344–1352. [Google Scholar] [CrossRef]
- Neuditschko, B.; King, A.P.; Huang, Z.; Janker, L.; Bileck, A.; Borutzki, Y.; Marker, S.C.; Gerner, C.; Wilson, J.J.; Meier-Menches, S.M. An Anticancer Rhenium Tricarbonyl Targets Fe−S Cluster Biogenesis in Ovarian Cancer Cells. Angew Chem Int Ed 2022, 61. [Google Scholar] [CrossRef]
- Neuditschko, B.; Legin, A.A.; Baier, D.; Schintlmeister, A.; Reipert, S.; Wagner, M.; Keppler, B.K.; Berger, W.; Meier-Menches, S.M.; Gerner, C. Interaction with Ribosomal Proteins Accompanies Stress Induction of the Anticancer Metallodrug BOLD-100/KP1339 in the Endoplasmic Reticulum. Angew Chem Int Ed 2021, 60, 5063–5068. [Google Scholar] [CrossRef]
- Hu, D.; Liu, Y.; Lai, Y.-T.; Tong, K.-C.; Fung, Y.-M.; Lok, C.-N.; Che, C.-M. Anticancer Gold(III) Porphyrins Target Mitochondrial Chaperone Hsp60. Angew. Chem. Int. Ed. 2016, 55, 1387–1391. [Google Scholar] [CrossRef]
- Wang, S.; Tian, Y.; Wang, M.; Wang, M.; Sun, G.; Sun, X. Advanced Activity-Based Protein Profiling Application Strategies for Drug Development. Front. Pharmacol. 2018, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Lu, K. Chemoproteomics: Towards Global Drug Target Profiling. ChemBioChem 2020, 21, 3189–3191. [Google Scholar] [CrossRef] [PubMed]
- Jenmalm Jensen, A.; Cornella Taracido, I. Affinity-Based Chemoproteomics for Target Identification. In Methods and Principles in Medicinal Chemistry; Plowright, A.T., Ed.; Wiley, 2019; pp. 25–49. ISBN 978-3-527-34529-8. [Google Scholar]
- Savitski, M.M.; Reinhard, F.B.M.; Franken, H.; Werner, T.; Savitski, M.F.; Eberhard, D.; Molina, D.M.; Jafari, R.; Dovega, R.B.; Klaeger, S.; et al. Tracking Cancer Drugs in Living Cells by Thermal Profiling of the Proteome. Science 2014, 346, 1255784. [Google Scholar] [CrossRef] [PubMed]
- Saei, A.A.; Gullberg, H.; Sabatier, P.; Beusch, C.M.; Johansson, K.; Lundgren, B.; Arvidsson, P.I.; Arnér, E.S.J.; Zubarev, R.A. Comprehensive Chemical Proteomics for Target Deconvolution of the Redox Active Drug Auranofin. Redox Biology 2020, 32, 101491. [Google Scholar] [CrossRef]
- Mateus, A.; Kurzawa, N.; Becher, I.; Sridharan, S.; Helm, D.; Stein, F.; Typas, A.; Savitski, M.M. Thermal Proteome Profiling for Interrogating Protein Interactions. Molecular Systems Biology 2020, 16, e9232. [Google Scholar] [CrossRef]
- Hu, D.; Yang, C.; Lok, C.; Xing, F.; Lee, P.; Fung, Y.M.E.; Jiang, H.; Che, C. An Antitumor Bis(N-Heterocyclic Carbene)Platinum(II) Complex That Engages Asparagine Synthetase as an Anticancer Target. Angew. Chem. Int. Ed. 2019, 58, 10914–10918. [Google Scholar] [CrossRef]
- Chernobrovkin, A.; Marin-Vicente, C.; Visa, N.; Zubarev, R.A. Functional Identification of Target by Expression Proteomics (FITExP) Reveals Protein Targets and Highlights Mechanisms of Action of Small Molecule Drugs. Sci Rep 2015, 5, 11176. [Google Scholar] [CrossRef]
- Lee, R.F.S.; Chernobrovkin, A.; Rutishauser, D.; Allardyce, C.S.; Hacker, D.; Johnsson, K.; Zubarev, R.A.; Dyson, P.J. Expression Proteomics Study to Determine Metallodrug Targets and Optimal Drug Combinations. Sci Rep 2017, 7, 1590. [Google Scholar] [CrossRef]
- Strickland, E.C.; Geer, M.A.; Tran, D.T.; Adhikari, J.; West, G.M.; DeArmond, P.D.; Xu, Y.; Fitzgerald, M.C. Thermodynamic Analysis of Protein-Ligand Binding Interactions in Complex Biological Mixtures Using the Stability of Proteins from Rates of Oxidation. Nat Protoc 2013, 8, 148–161. [Google Scholar] [CrossRef]
- Park, C.; Marqusee, S. Pulse Proteolysis: A Simple Method for Quantitative Determination of Protein Stability and Ligand Binding. Nat Methods 2005, 2, 207–212. [Google Scholar] [CrossRef]
- Lomenick, B.; Jung, G.; Wohlschlegel, J.A.; Huang, J. Target Identification Using Drug Affinity Responsive Target Stability (DARTS). Curr Protoc Chem Biol 2011, 3, 163–180. [Google Scholar] [CrossRef]
- Feng, F.; Zhang, W.; Chai, Y.; Guo, D.; Chen, X. Label-Free Target Protein Characterization for Small Molecule Drugs: Recent Advances in Methods and Applications. Journal of Pharmaceutical and Biomedical Analysis 2023, 223, 115107. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Wang, R.; Wu, K.; Jiang, H.; Du, Z. Elucidation of the Mechanism of Action for Metal Based Anticancer Drugs by Mass Spectrometry-Based Quantitative Proteomics. Molecules 2019, 24, 581. [Google Scholar] [CrossRef]
- Roberts, E.A.; Sarkar, B. Metalloproteomics: Focus on Metabolic Issues Relating to Metals. Current Opinion in Clinical Nutrition and Metabolic Care 2014, 17, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem Sci 2020, 11, 12888–12917. [Google Scholar] [CrossRef] [PubMed]
- Coverdale, J.; Laroiya-McCarron, T.; Romero-Canelón, I. Designing Ruthenium Anticancer Drugs: What Have We Learnt from the Key Drug Candidates? Inorganics 2019, 7, 31. [Google Scholar] [CrossRef]
- Ravera, M.; Gabano, E.; Zanellato, I.; Rangone, B.; Perin, E.; Ferrari, B.; Bottone, M.G.; Osella, D. Cis,Cis,Trans -[Pt IV Cl 2 (NH 3 ) 2 (Perillato) 2 ], a Dual-Action Prodrug with Excellent Cytotoxic and Antimetastatic Activity. Dalton Trans. 2021, 50, 3161–3177. [Google Scholar] [CrossRef]
- Kenche, V.B.; Hung, L.W.; Perez, K.; Volitakes, I.; Ciccotosto, G.; Kwok, J.; Critch, N.; Sherratt, N.; Cortes, M.; Lal, V.; et al. Development of a Platinum Complex as an Anti-Amyloid Agent for the Therapy of Alzheimer’s Disease. Angew. Chem. Int. Ed. 2013, 52, 3374–3378. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, L.; Zhang, P.; Zhao, H.; Zhou, Q. The Development of Ru(II)-Based Photoactivated Chemotherapy Agents. Molecules 2021, 26, 5679. [Google Scholar] [CrossRef]
- Kulkarni, G.S.; Lilge, L.; Nesbitt, M.; Dumoulin-White, R.J.; Mandel, A.; Jewett, M.A.S. A Phase 1b Clinical Study of Intravesical Photodynamic Therapy in Patients with Bacillus Calmette-Guérin–Unresponsive Non–Muscle-Invasive Bladder Cancer. European Urology Open Science 2022, 41, 105–111. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.-S.; Liu, Y.-C.; Wang, N.; Zeng, X.-T.; Zhang, L.-L. Emerging Photodynamic/Sonodynamic Therapies for Urological Cancers: Progress and Challenges. J Nanobiotechnology 2022, 20, 437. [Google Scholar] [CrossRef]
- Karges, J. Clinical Development of Metal Complexes as Photosensitizers for Photodynamic Therapy of Cancer. Angew Chem Int Ed Engl 2022, 61, e202112236. [Google Scholar] [CrossRef] [PubMed]
- Poursharifi, M.; Wlodarczyk, M.T.; Mieszawska, A.J. Nano-Based Systems and Biomacromolecules as Carriers for Metallodrugs in Anticancer Therapy. Inorganics 2018, 7, 2. [Google Scholar] [CrossRef]
- Boulikas, T. Clinical Overview on Lipoplatin: A Successful Liposomal Formulation of Cisplatin. Expert Opin Investig Drugs 2009, 18, 1197–1218. [Google Scholar] [CrossRef] [PubMed]
- Blunden, B.M.; Stenzel, M.H. Incorporating Ruthenium into Advanced Drug Delivery Carriers - an Innovative Generation of Chemotherapeutics. J. Chem. Technol. Biotechnol. 2015, 90, 1177–1195. [Google Scholar] [CrossRef]
- Fischer, B.; Heffeter, P.; Kryeziu, K.; Gille, L.; Meier, S.M.; Berger, W.; Kowol, C.R.; Keppler, B.K. Poly(Lactic Acid) Nanoparticles of the Lead Anticancer Ruthenium Compound KP1019 and Its Surfactant-Mediated Activation. Dalton Trans. 2014, 43, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.R.; Colquhoun, A.; Wu, X.Y.; de Oliveira Silva, D. Synthesis of Terpolymer-Lipid Encapsulated Diruthenium(II,III)-Anti-Inflammatory Metallodrug Nanoparticles to Enhance Activity against Glioblastoma Cancer Cells. J Inorg Biochem 2020, 205, 110984. [Google Scholar] [CrossRef]
- Monti, D.M.; Ferraro, G.; Merlino, A. Ferritin-Based Anticancer Metallodrug Delivery: Crystallographic, Analytical and Cytotoxicity Studies. Nanomedicine 2019, 20, 101997. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiological Reviews 2015, 95, 749–784. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




