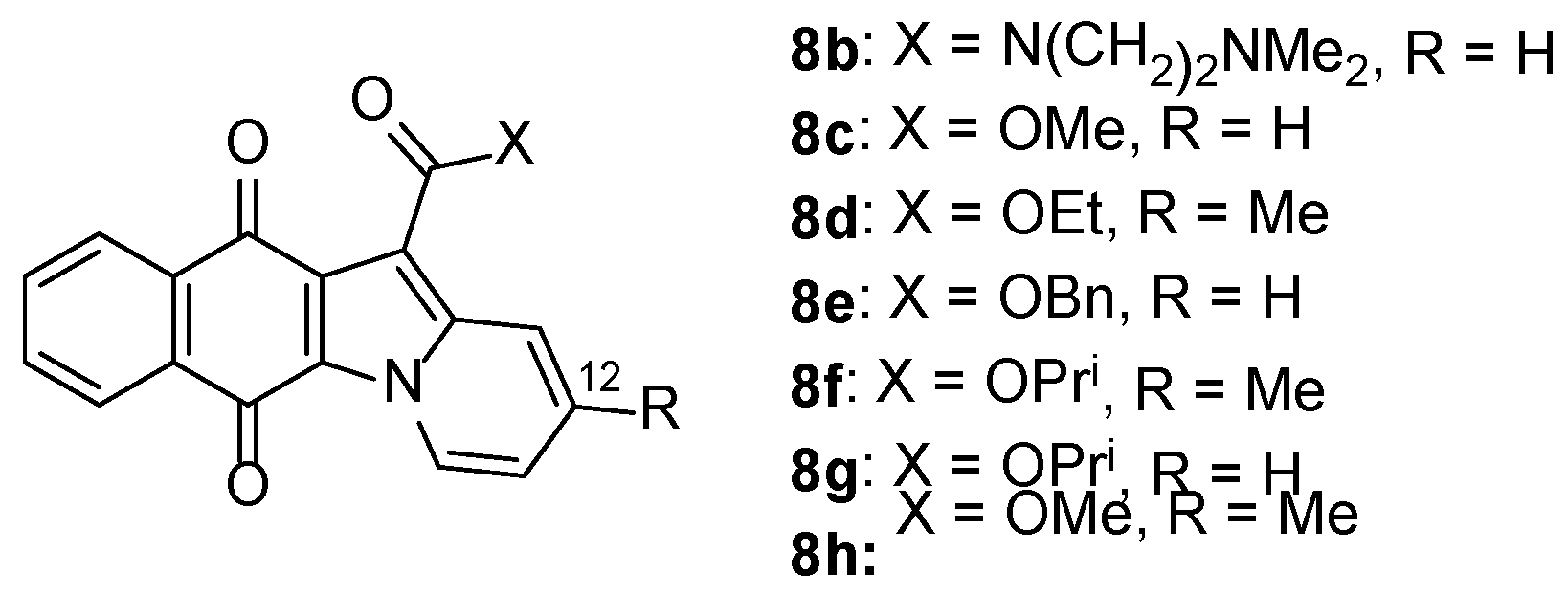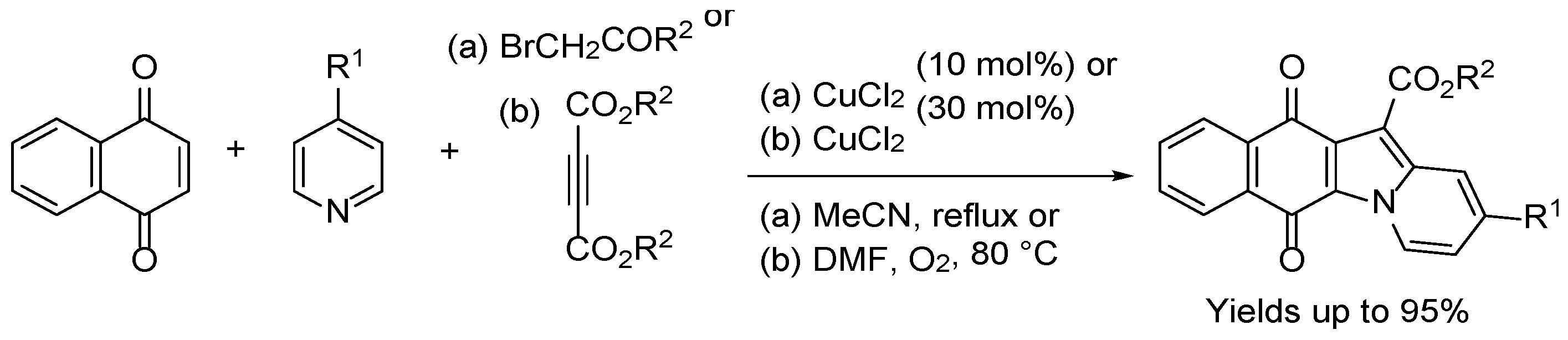Submitted:
05 June 2023
Posted:
06 June 2023
You are already at the latest version
Abstract
Keywords:
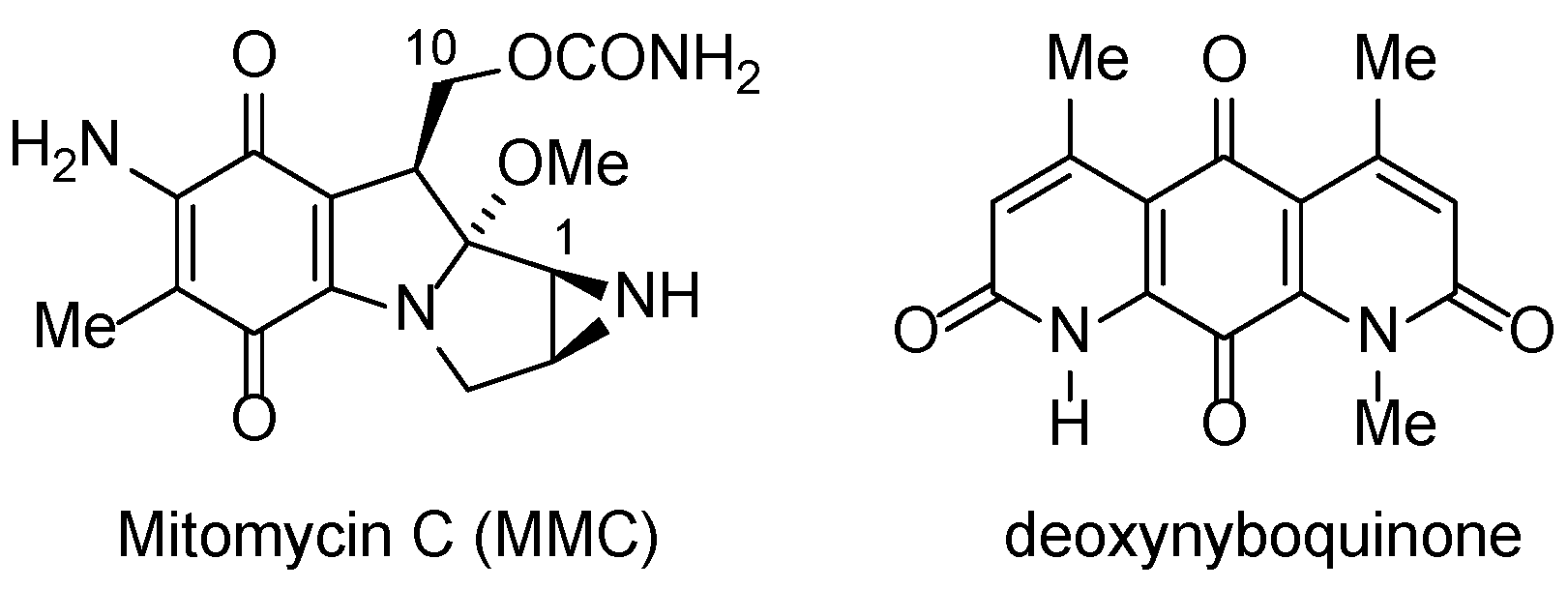
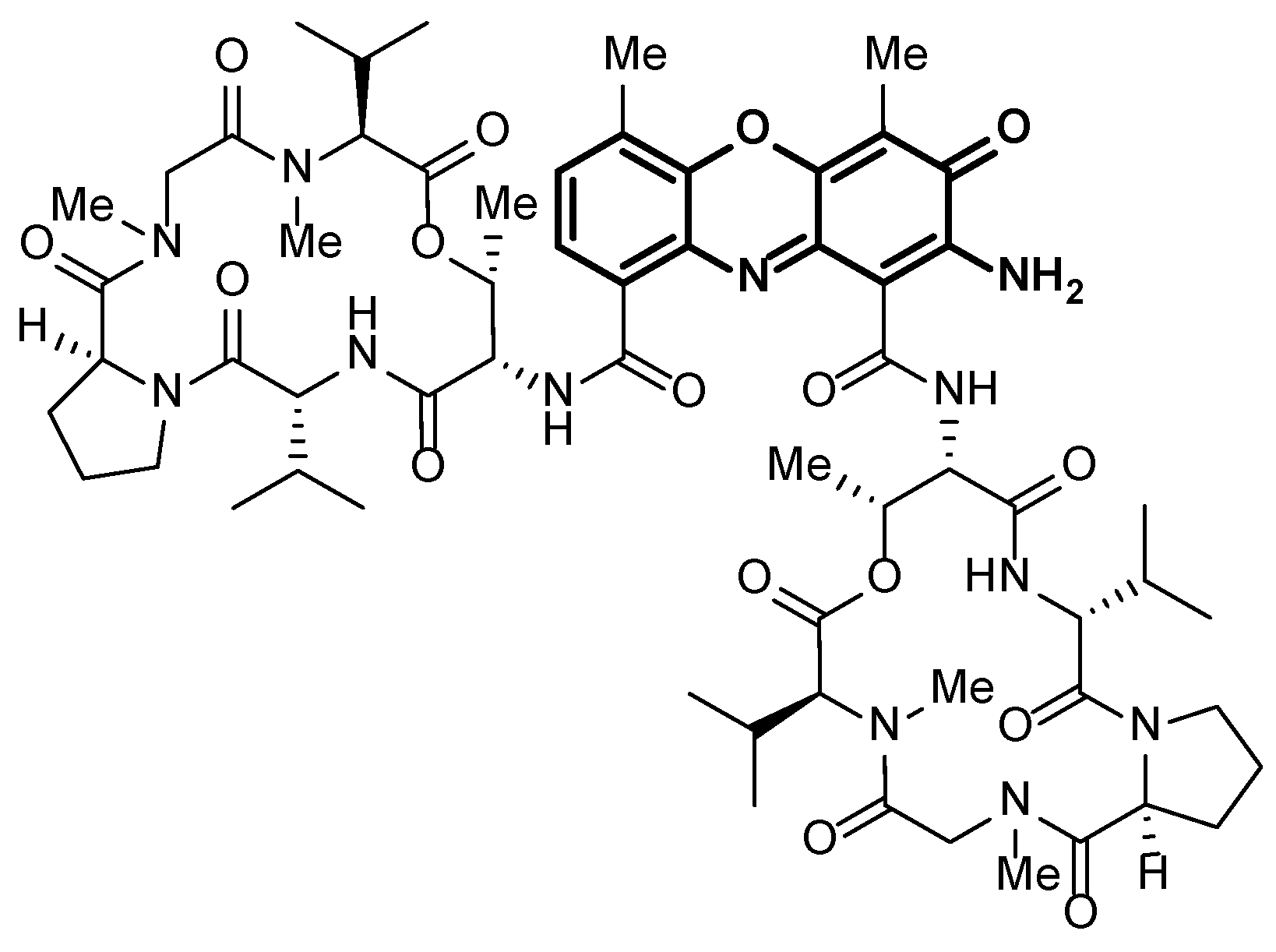
1. Introduction
2. Compound Search Methods
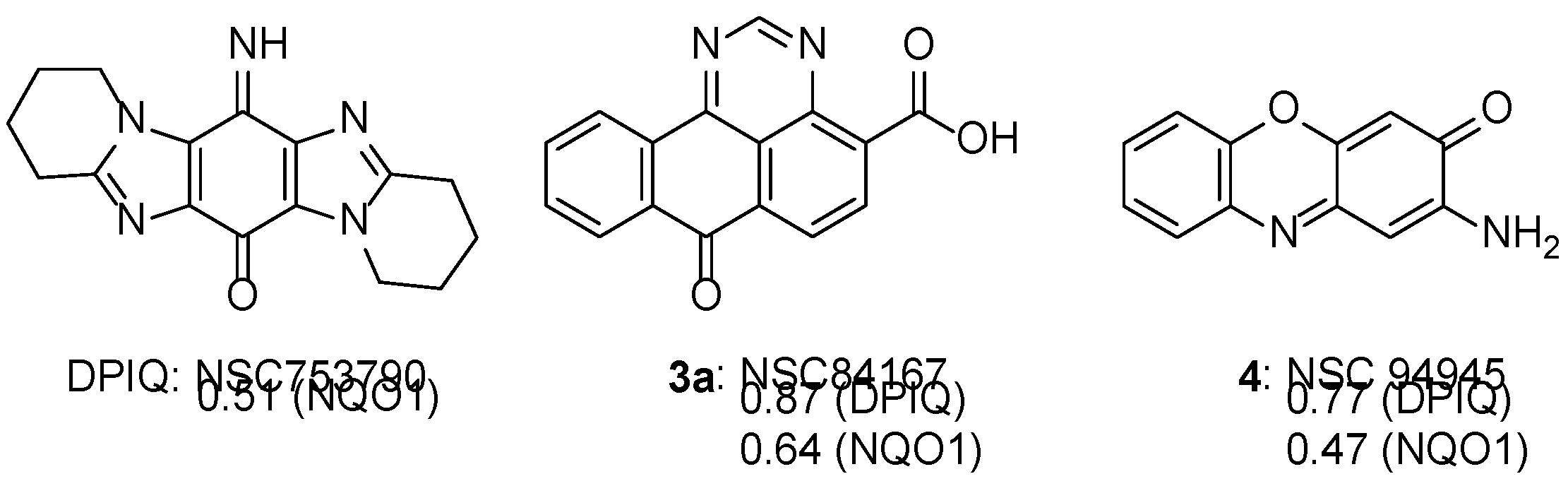
2.1. COMPARE using DPIQ and 5a as the Seed.
2.2. COMPARE using Molecular Target Expression
3. Discussion
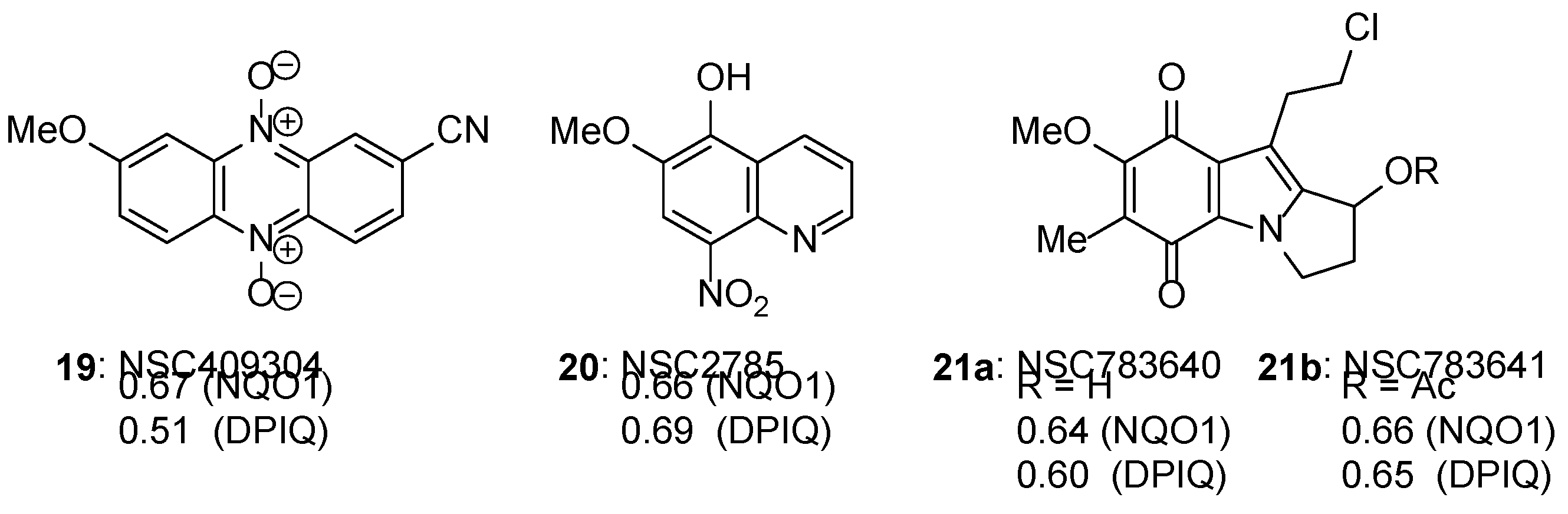
3.1. COMPARE Analysis: Strong Correlations to DPIQ as the Seed.
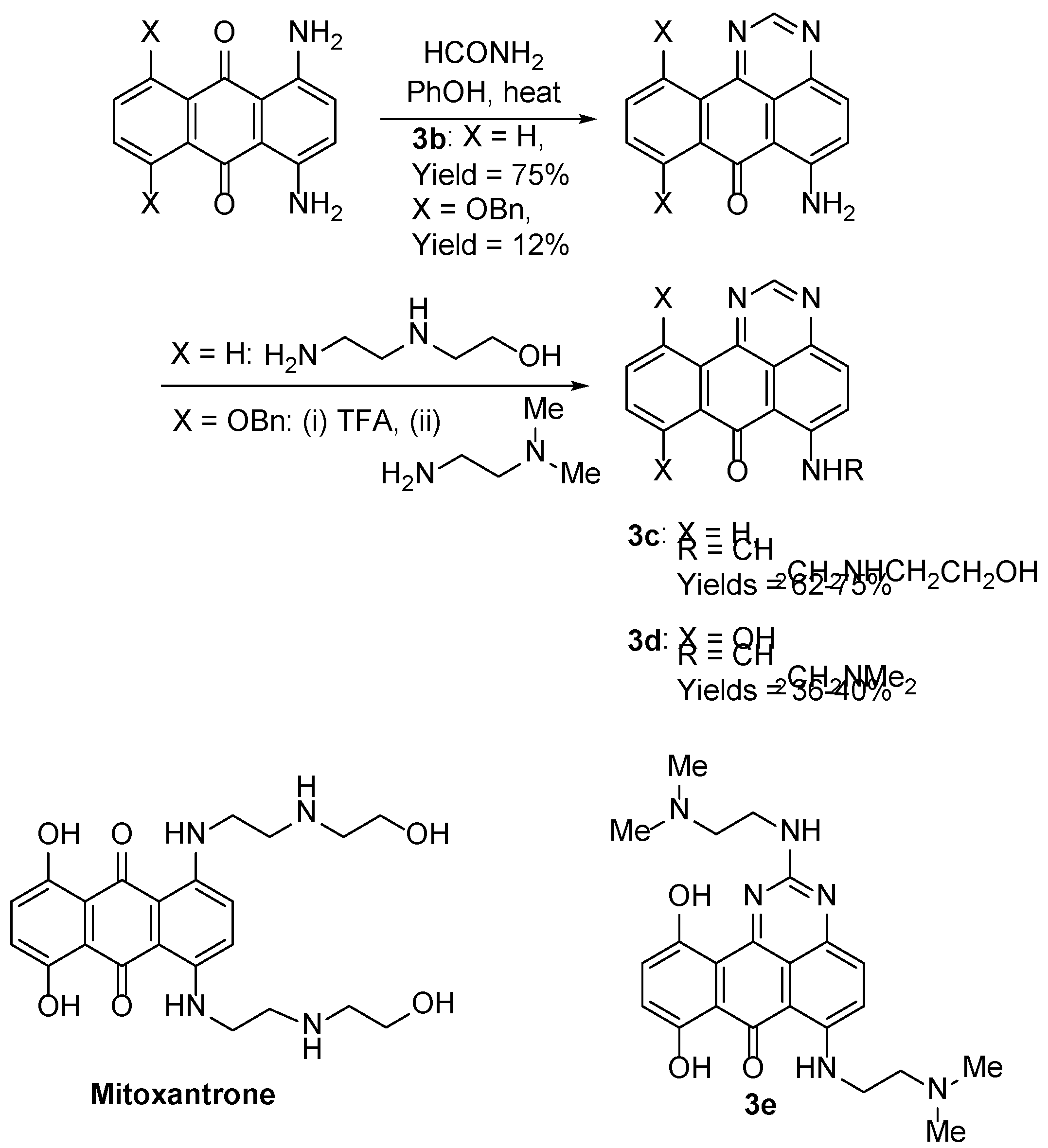
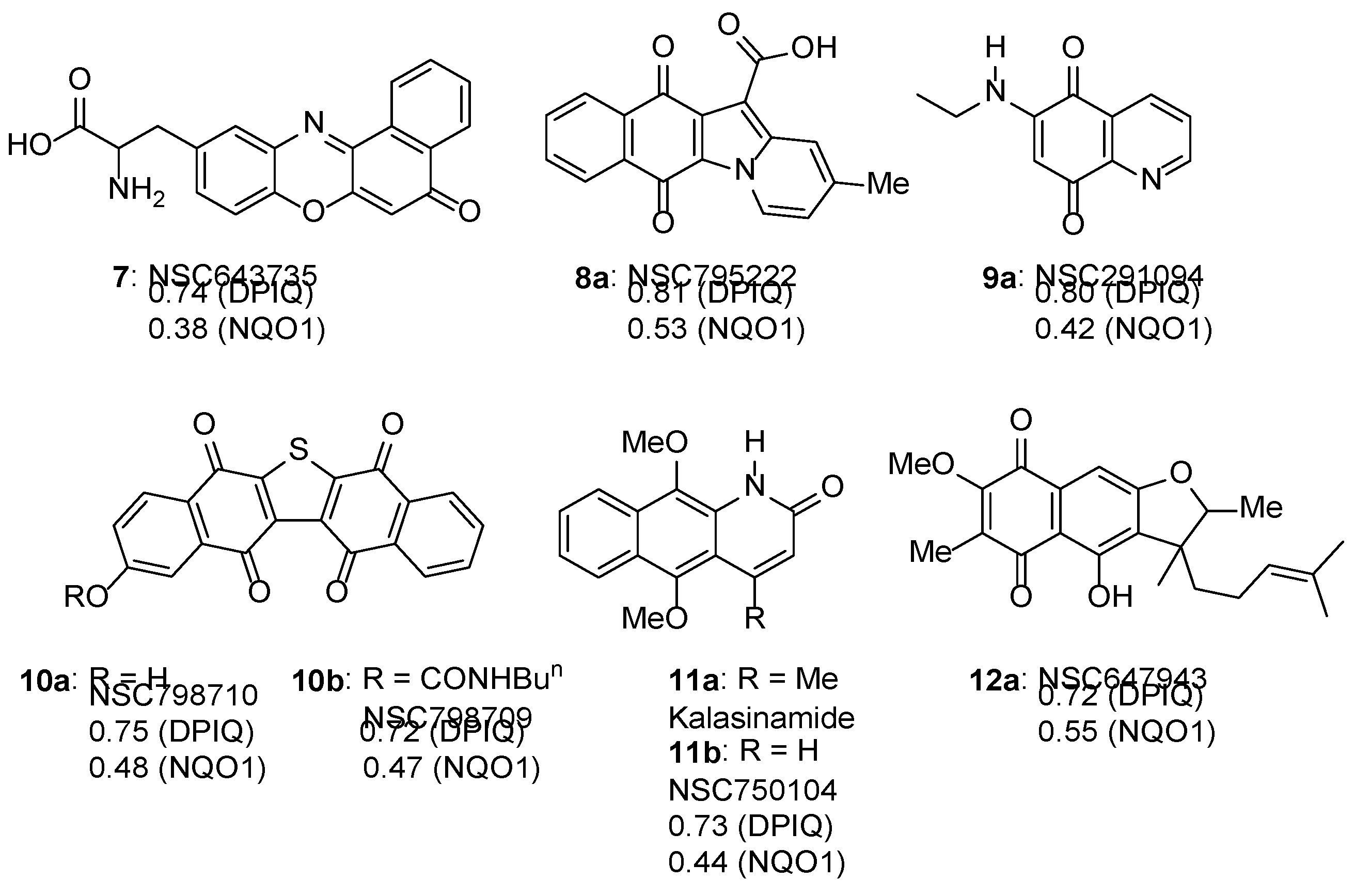
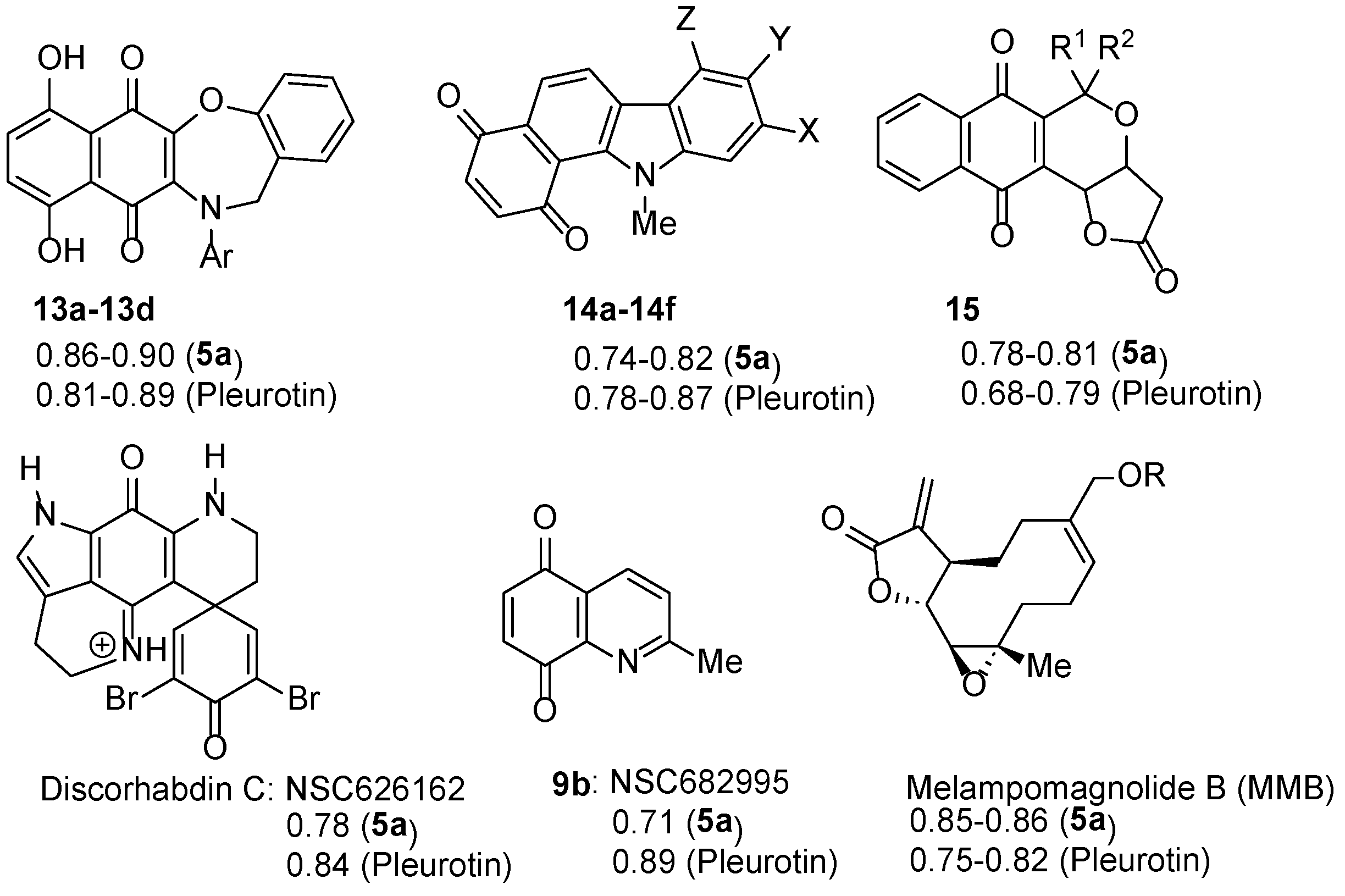
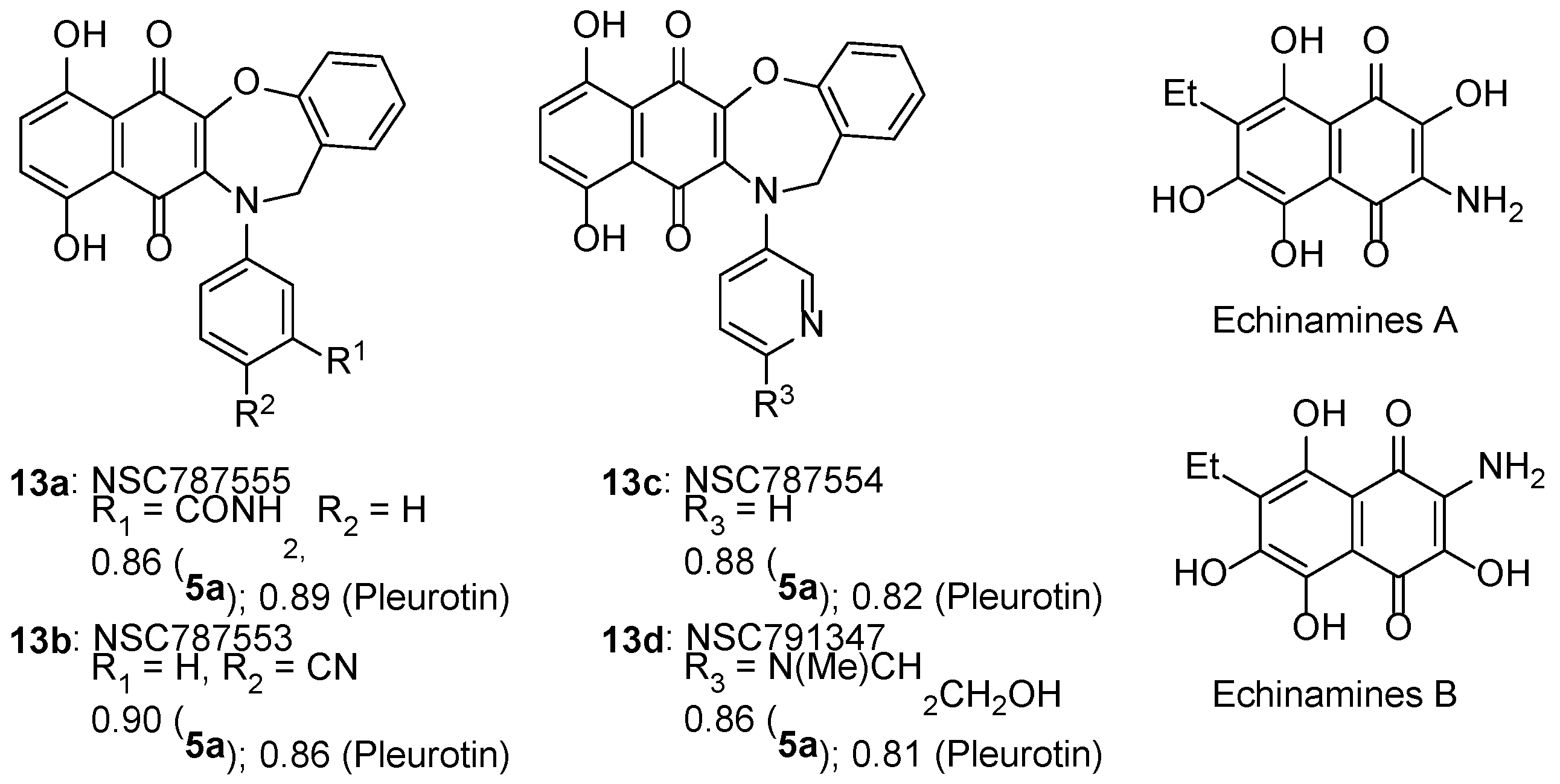
3.1.1. Benzo[e]perimidines
3.1.2. Phenoxazinones
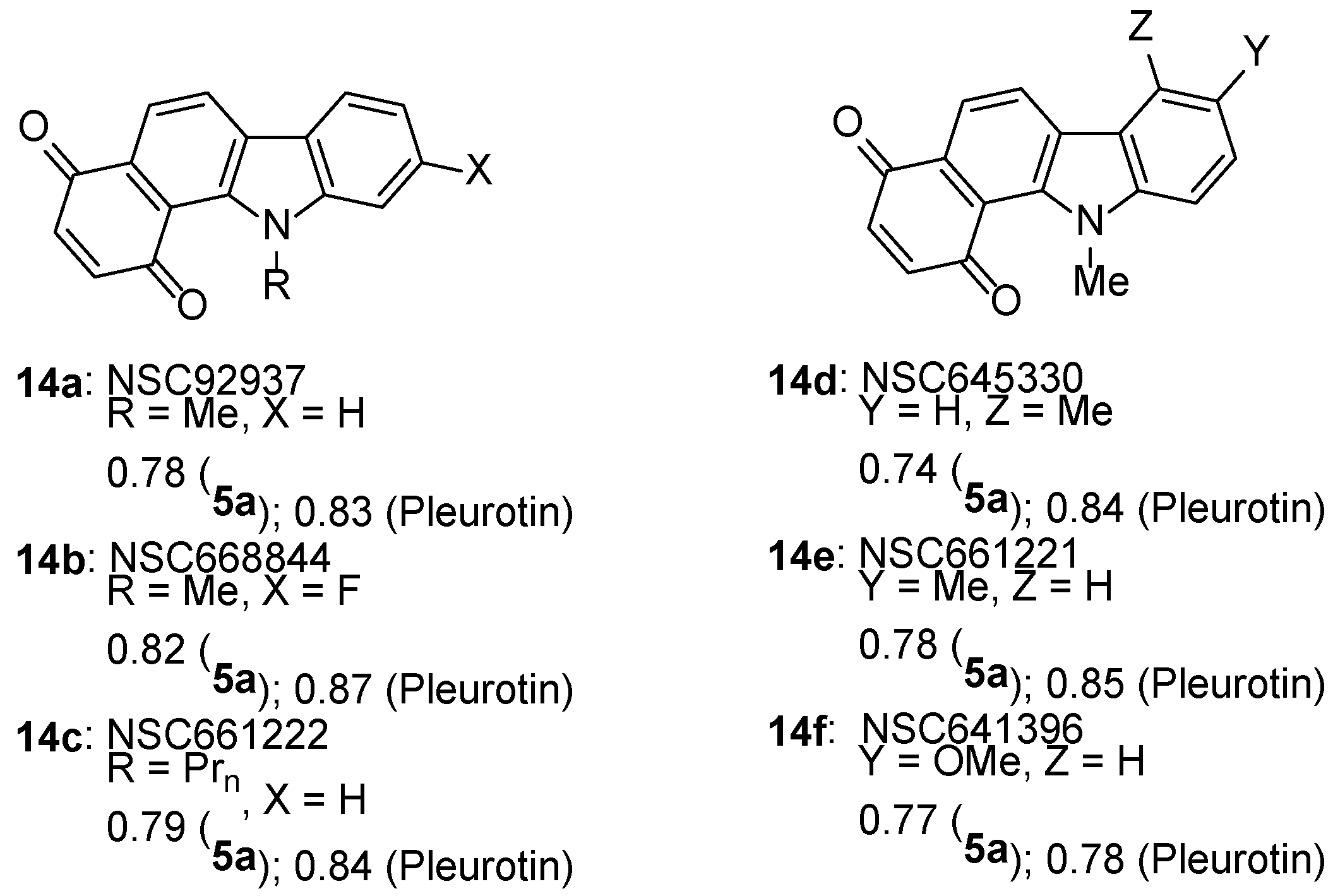
3.1.3. Benz[f]pyrido[1,2-a]indole-6,11-quinone
3.1.4. Analogues of Seriniquinone
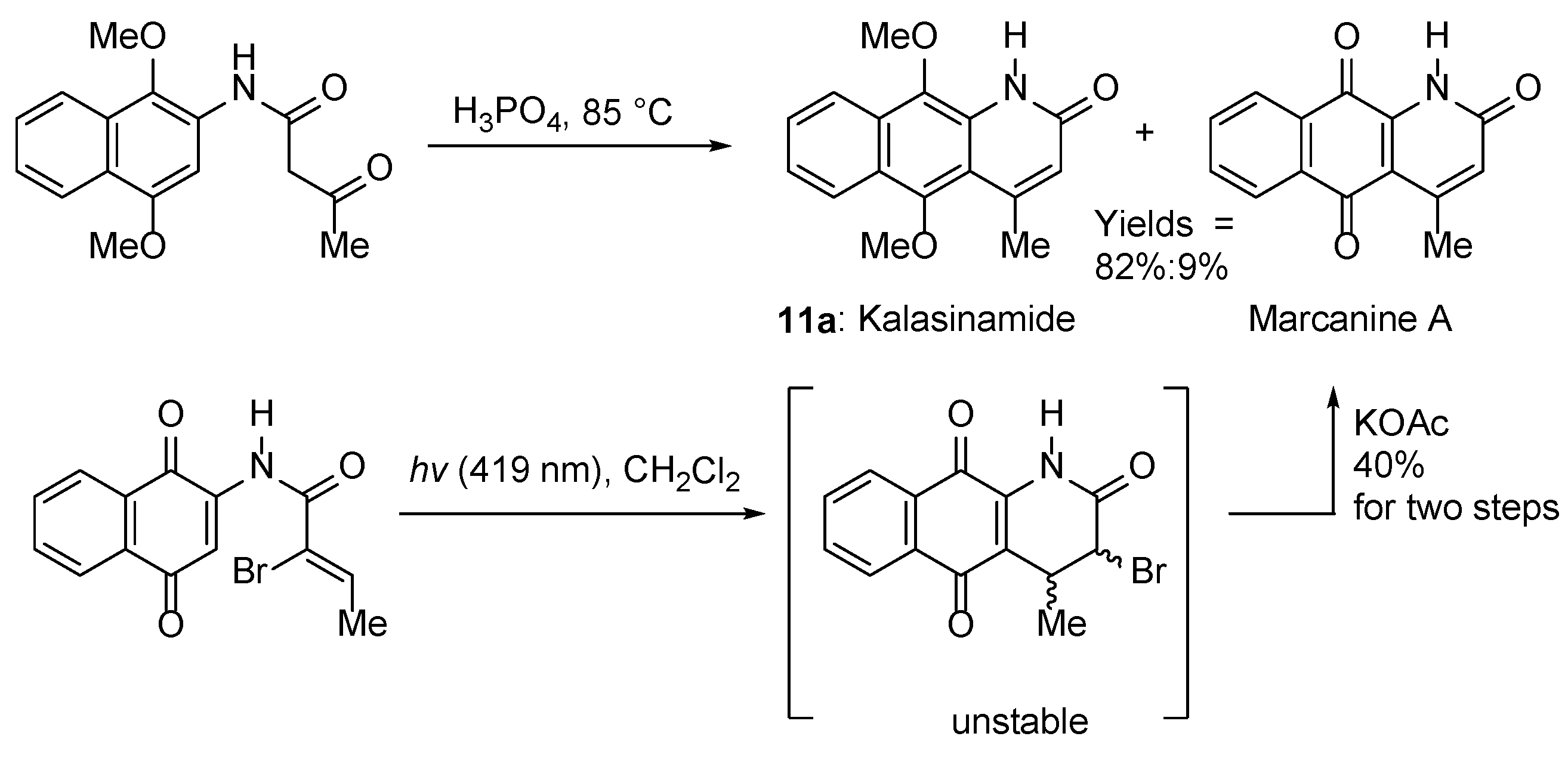
3.1.5. Azaanthracenone
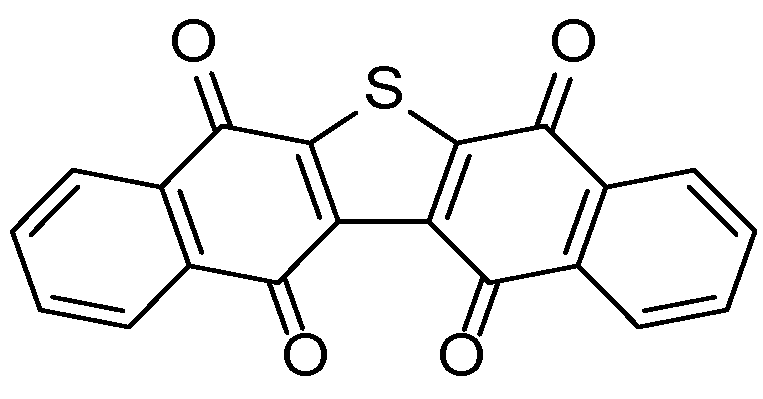
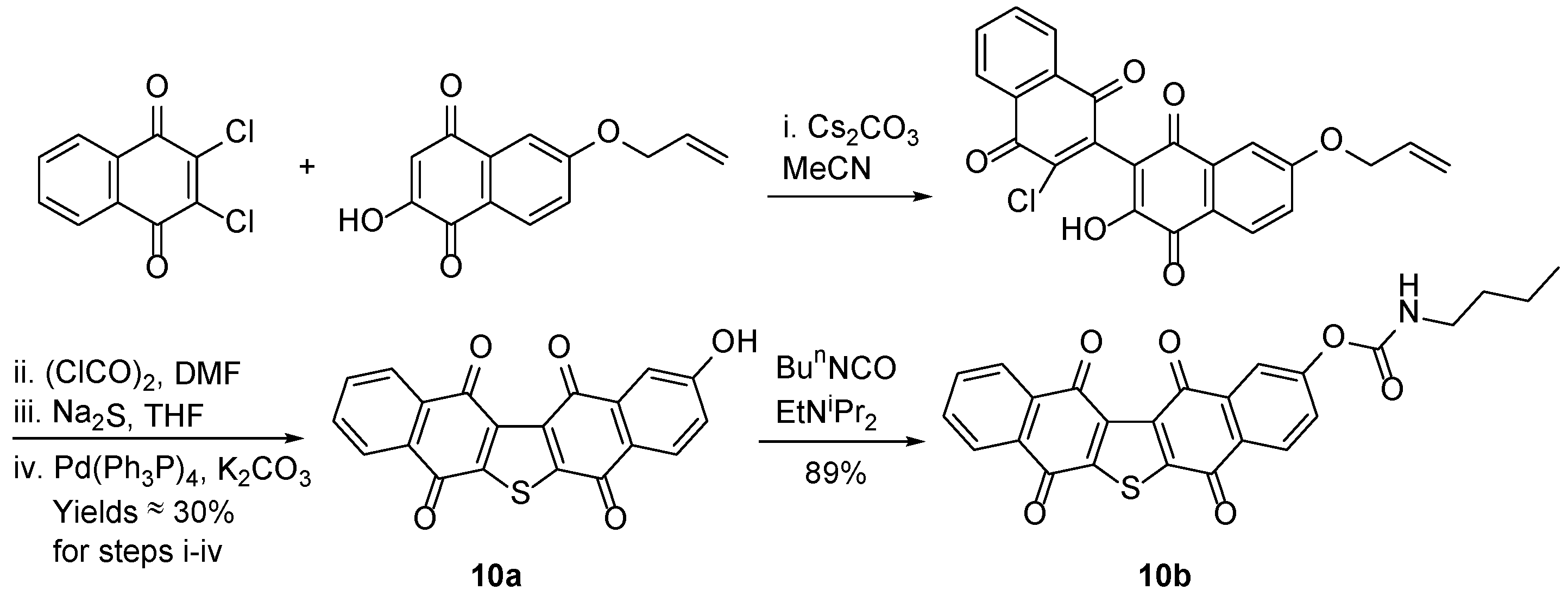
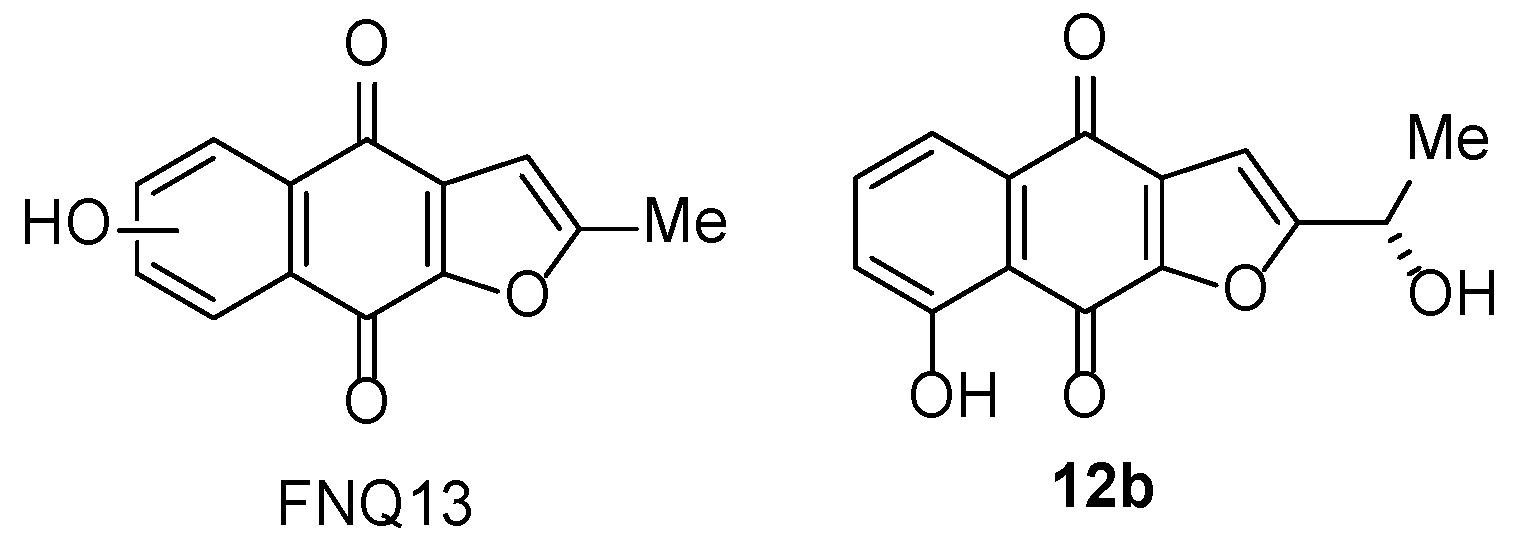
3.1.6. Furano[2,3-b]naphthoquinone (FNQ)
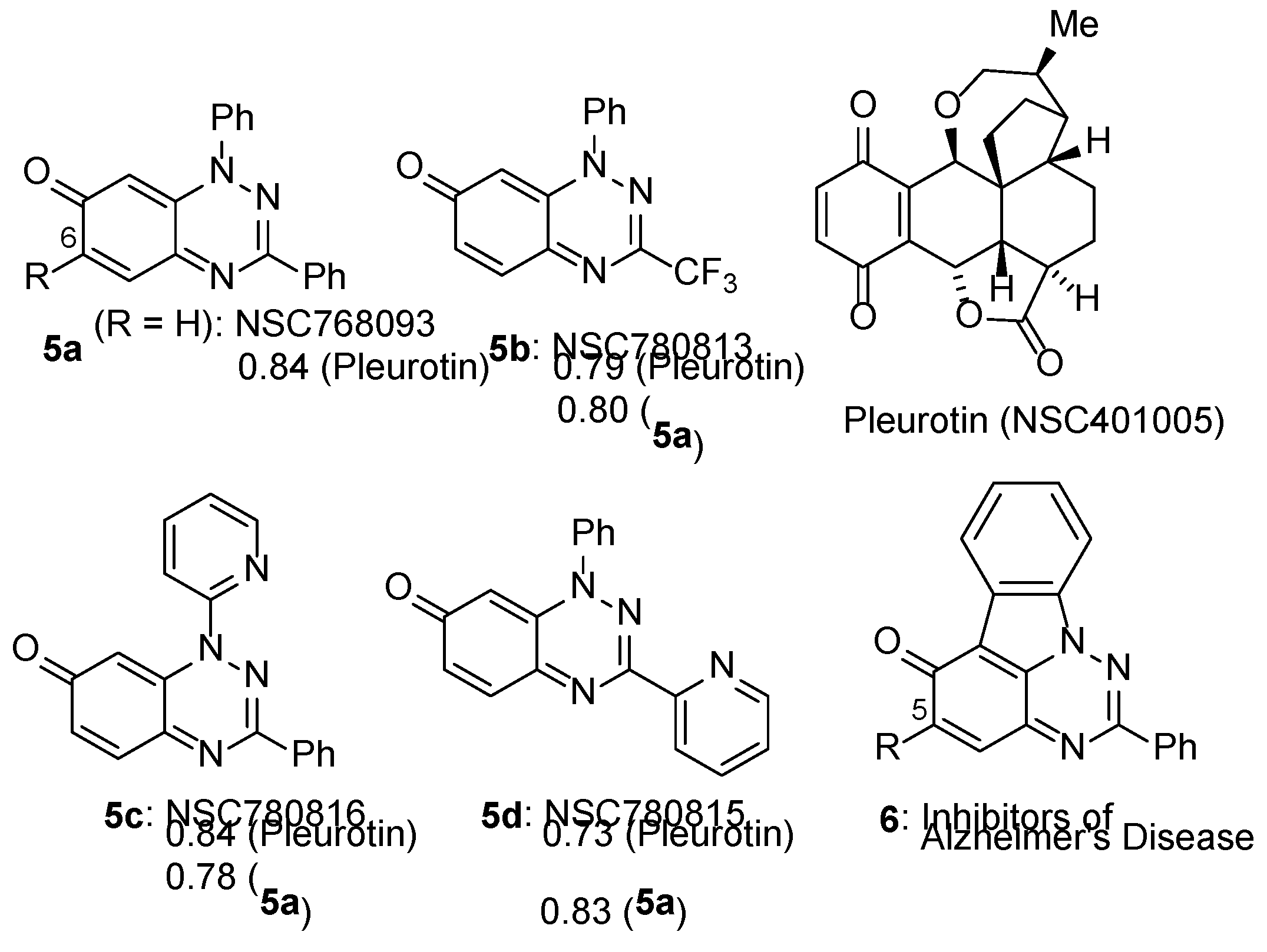
3.2. COMPARE Analysis: Strong Correlations to Benzo[1,2,4]trazin-7-ones 5a as the Seed.
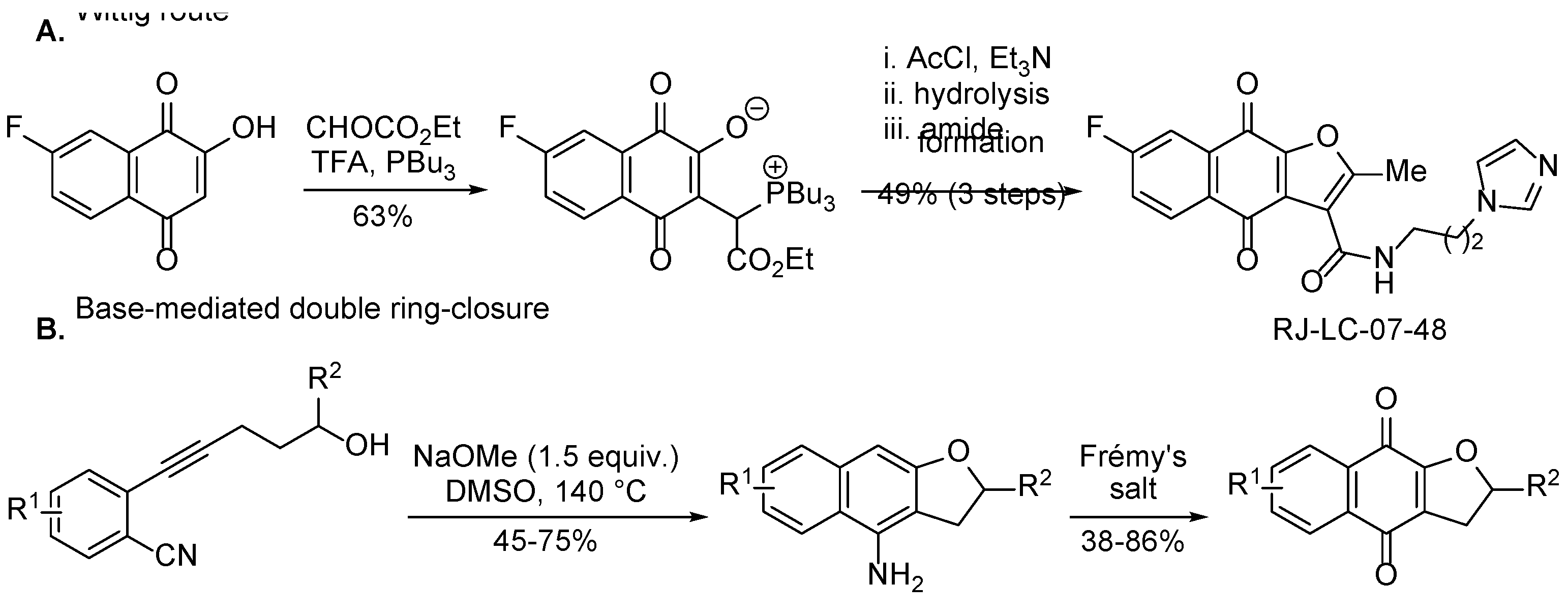
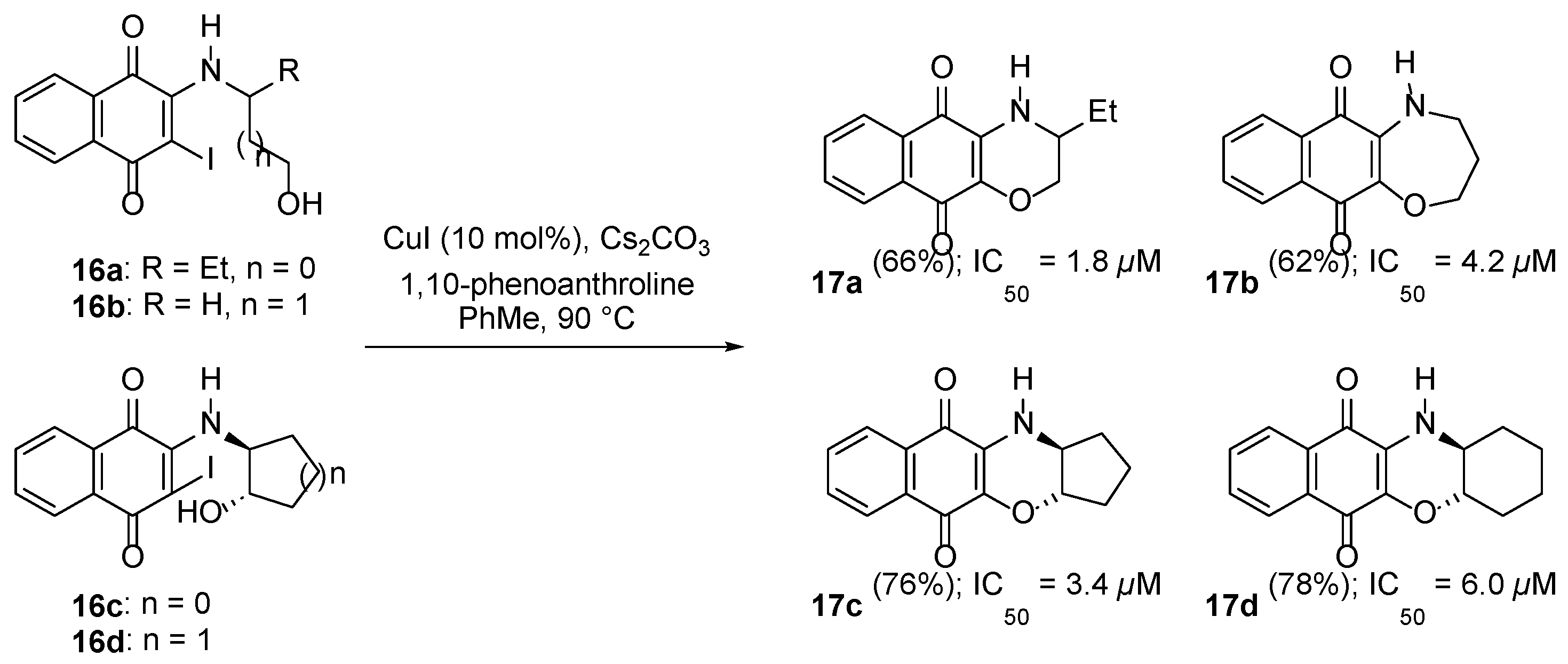
3.2.1. Naphtho[2,3-b][1,4]oxazepine-6,11-dione
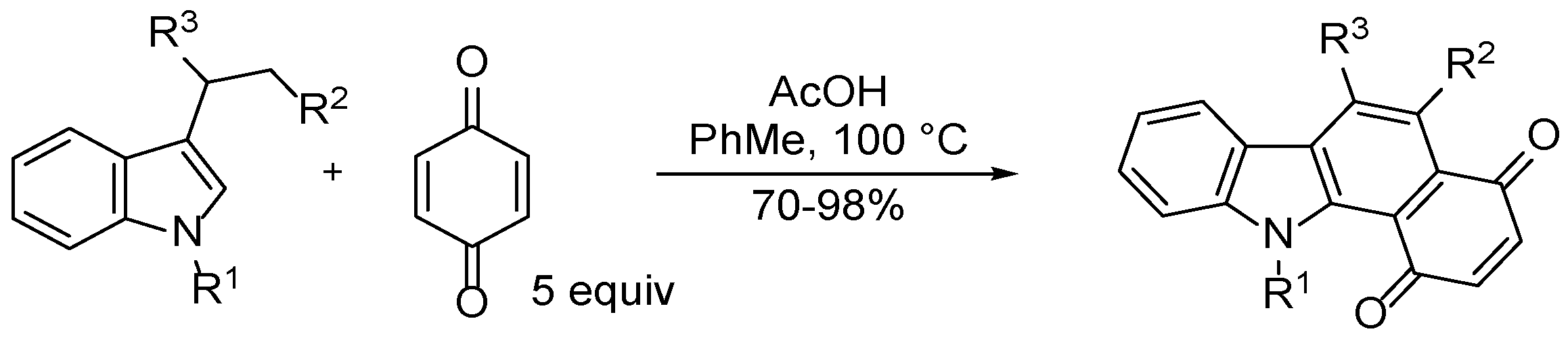
3.2.2. Benzo[a]carbazole-1,4-dione
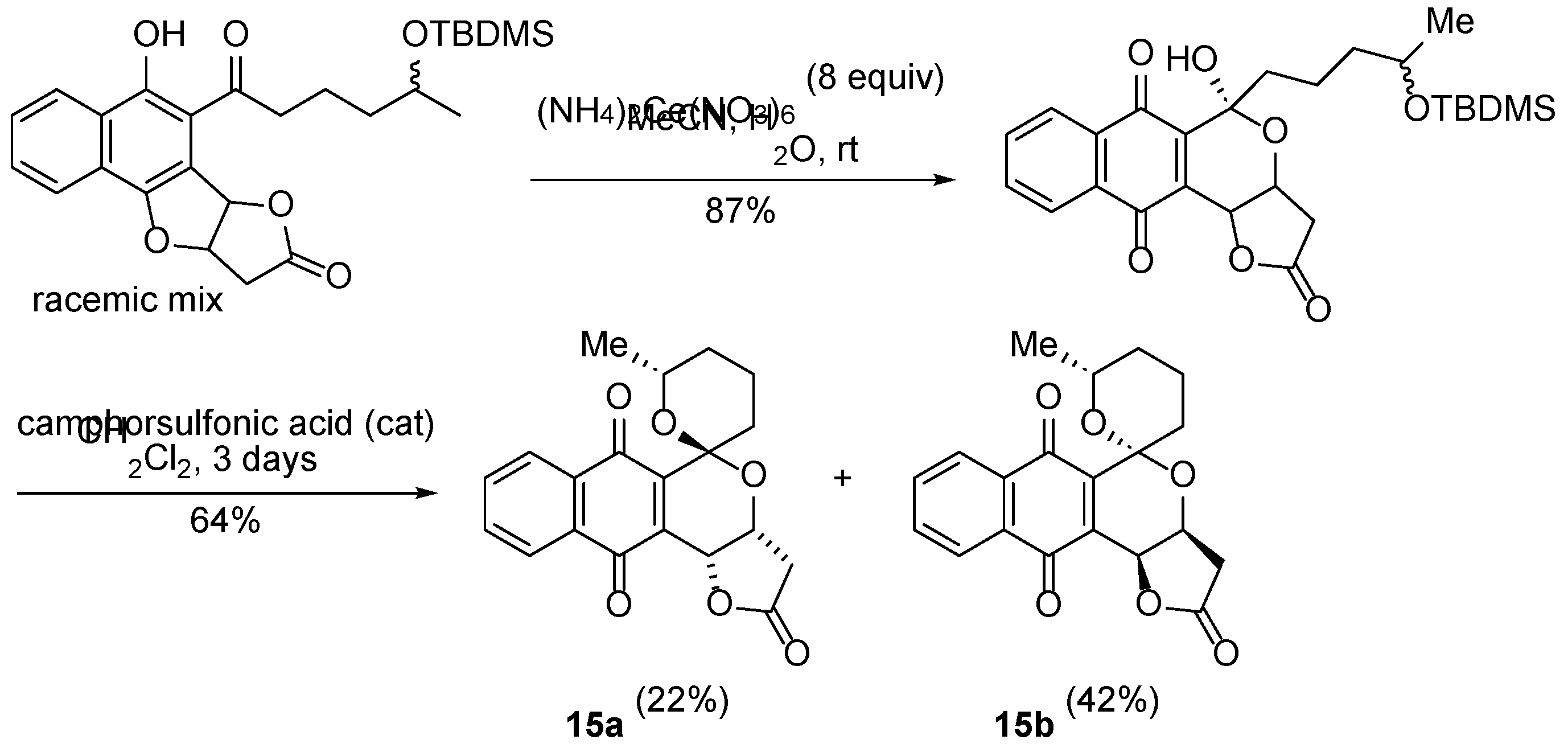
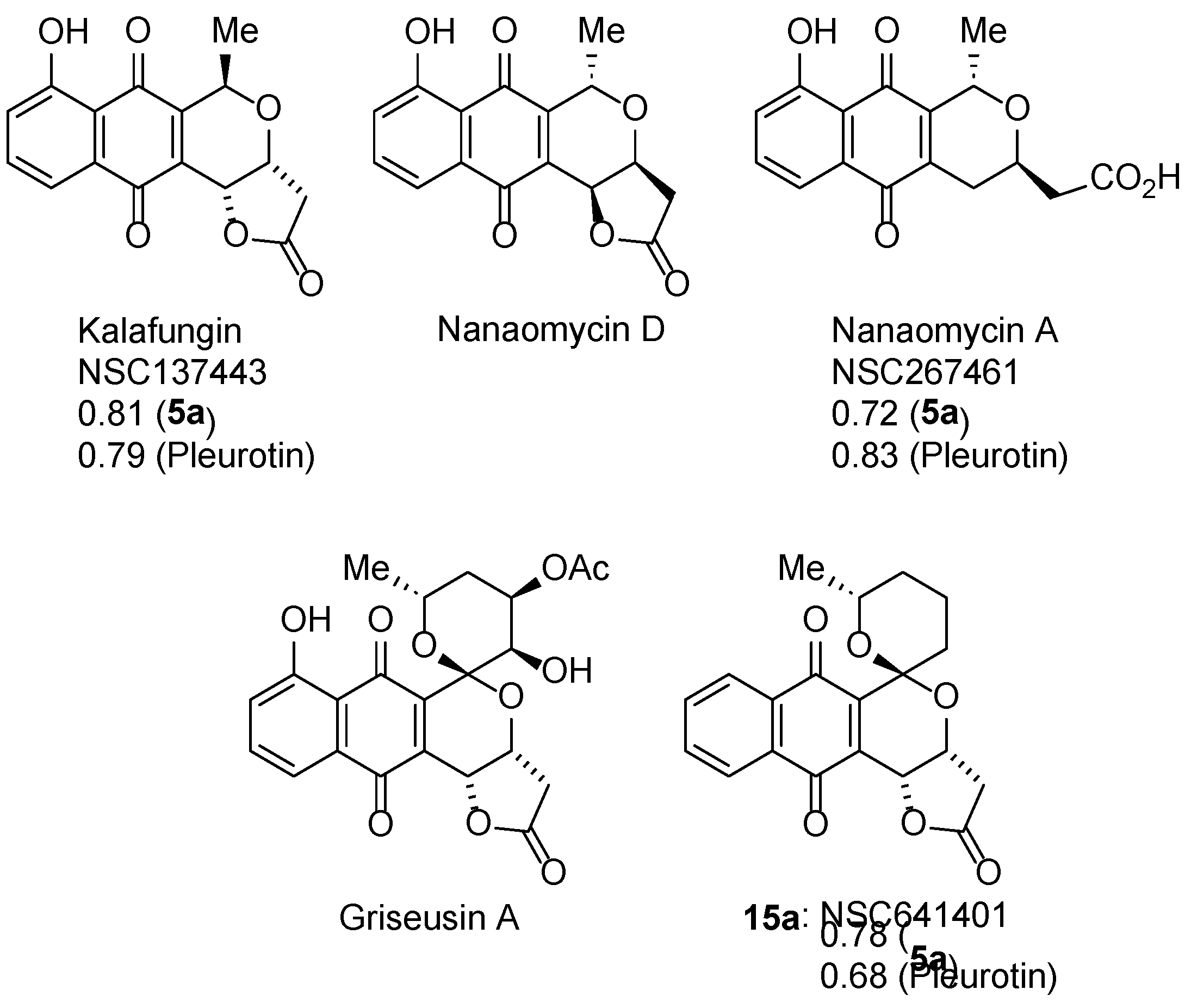
3.2.3. Pyranonaphthoquinones
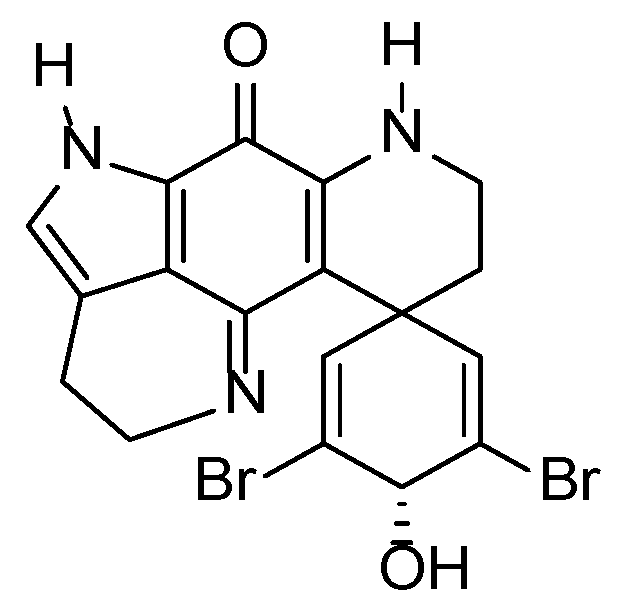
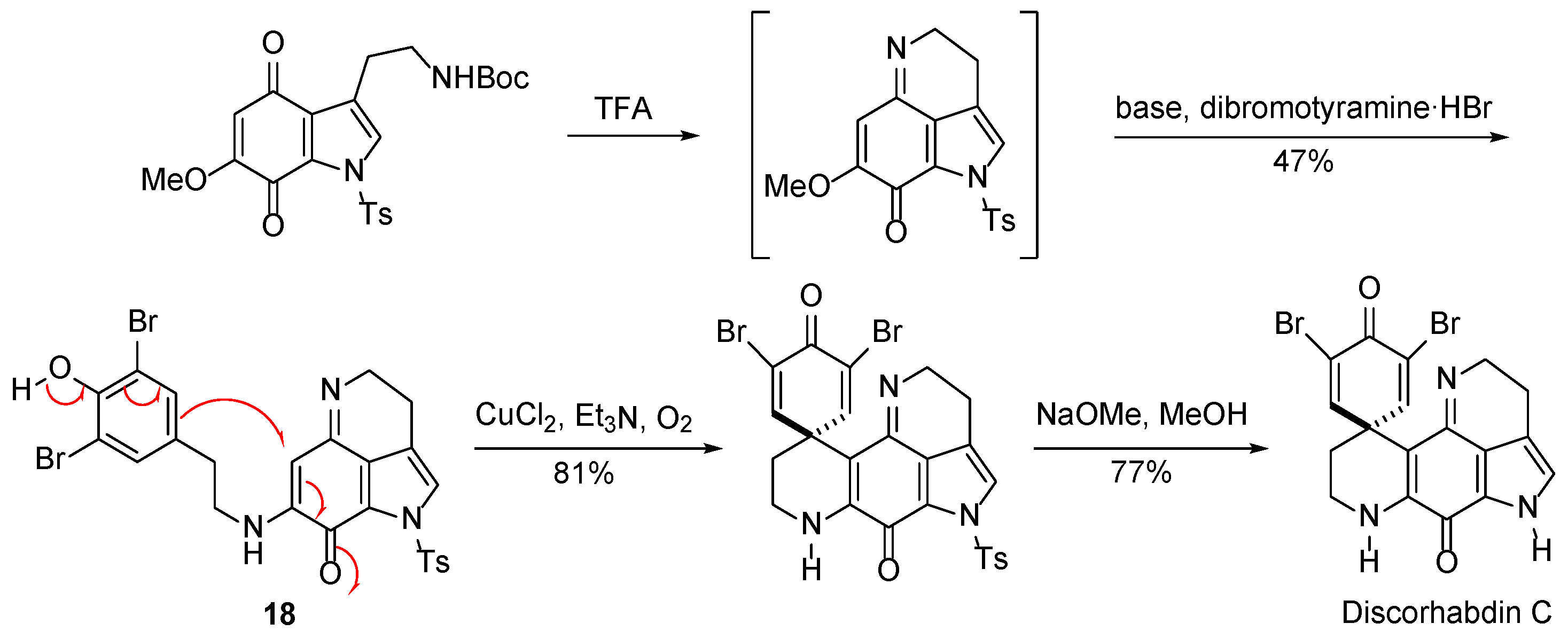
3.2.4. Discorhabdin C
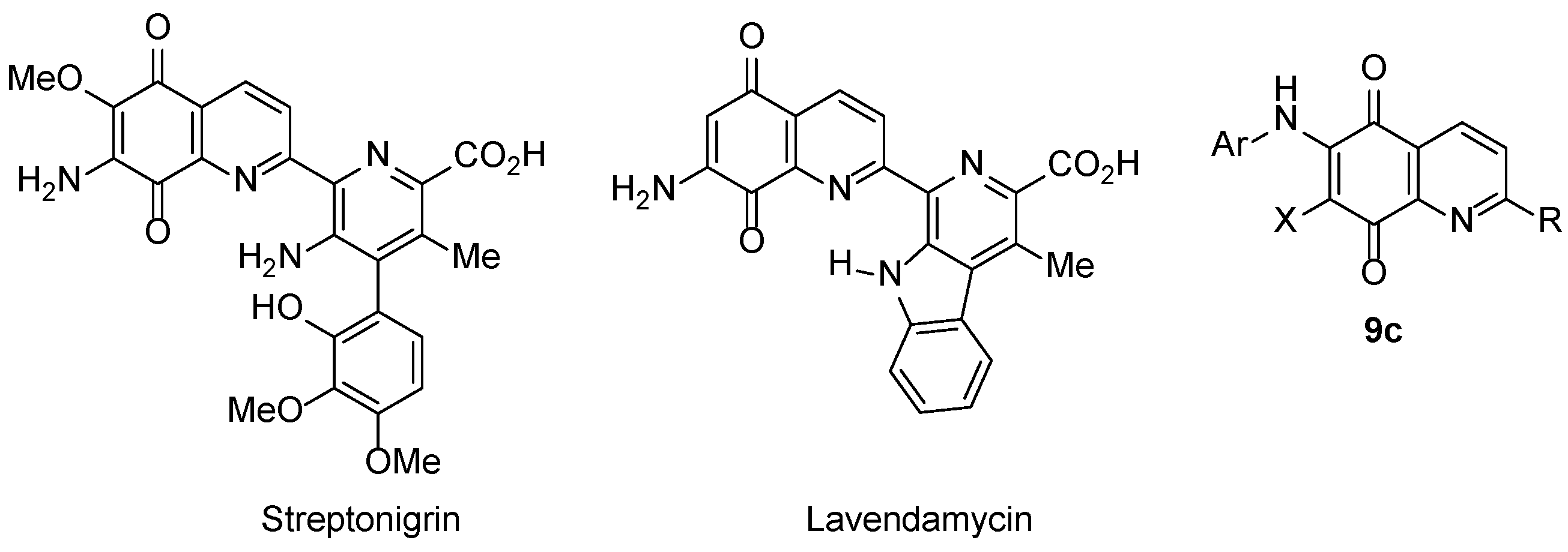
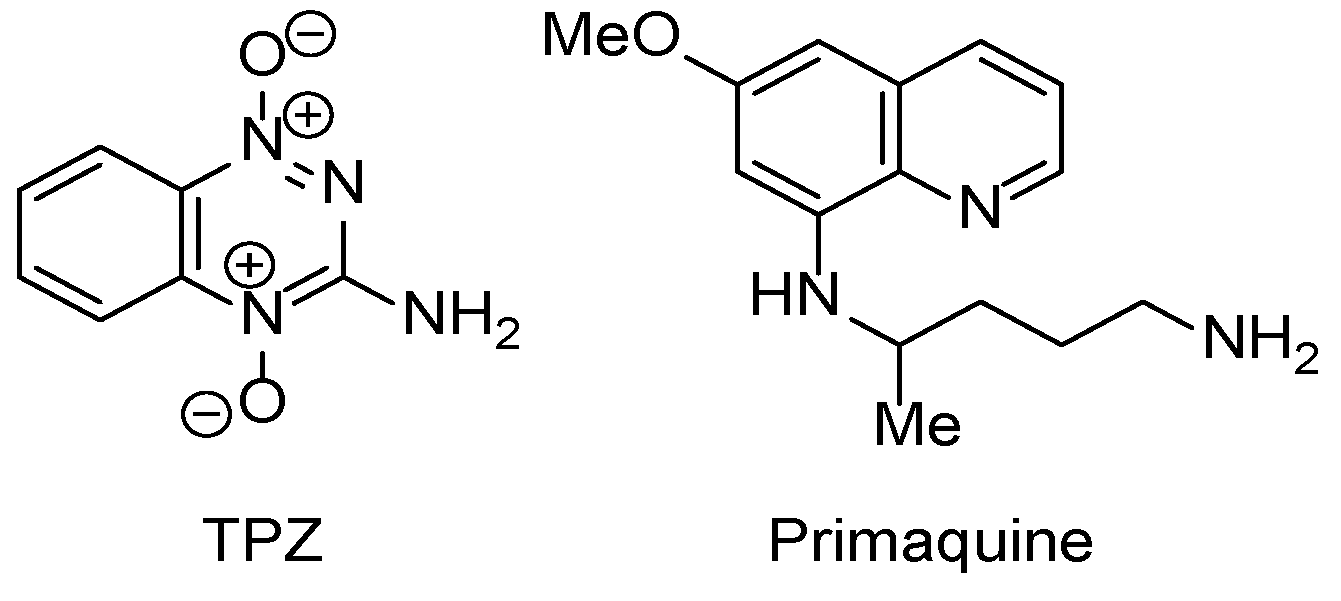
3.3. COMPARE Analysis: Strong Correlations to DPIQ and Benzo[1,2,4]trazin-7-one 5a as the Seed: Quinoline-5,8-diones
3.4. COMPARE Analysis using Molecular Target Expression
3.4.1. Compound Correlations to NQO1 expression
3.4.1. Compound Correlations to TrxR
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef]
- Holbeck, S.L. Update on NCI in vitro drug screen utilities. Eur. J. Cancer 2004, 40, 785–793. [Google Scholar] [CrossRef]
- Sharma, S.V.; Haber D., A.; Settleman, J. Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat. Rev. Cancer 2010, 10, 241–253. [Google Scholar] [CrossRef]
- Holbeck, S.L.; Camalier, R.; Crowell, J.A.; Govindharajulu, J.P.; Hollingshead, M.; Anderson, L.W.; Polley, E.; Rubinstein, L.; Srivastava, A.; Wilsker, D.; Collins, J.M.; Doroshow, J.H. The National Cancer Institute ALMANAC: A Comprehensive screening resource for the detection of anticancer drug pairs with enhanced therapeutic activity. Cancer Res. 2017, 77, 3564–3576. [Google Scholar] [CrossRef]
- Paull, K.D.; Shoemaker, R.H.; Hodes, L.; Monks, A.; Scudiero, D.A.; Rubinstein, L.; Plowman, J.; Boyd, M.R. Display and analysis of patterns of differential activity of drugs against human tumor cell lines: development of mean graph and COMPARE algorithm. J. Natl. Cancer Inst. 1989, 81, 1088–1092. [Google Scholar] [CrossRef]
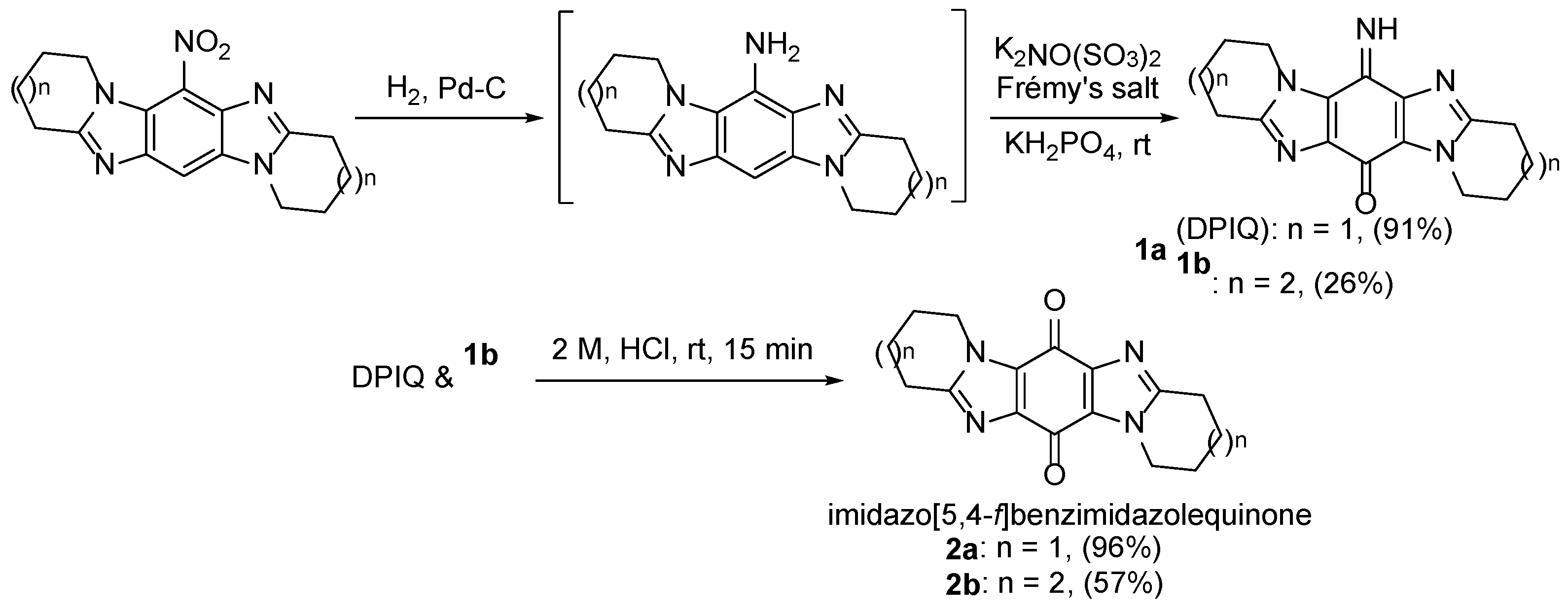
- Fagan, V. Synthesis and anti-cancer activity of novel imidazo[5,4-f]benzimidazolequinones. Ph.D., National University of Ireland Galway, Ireland, 2011.
- Fagan, V.; Bonham, S.; Carty, M.P.; Saenz-Méndez, P.; Eriksson, L.A.; Aldabbagh, F. COMPARE analysis of the toxicity of an iminoquinone derivative of the imidazo[5,4-f]benzimidazoles with NAD(P)H:quinone oxidoreductase 1 (NQO1) activity and computational docking of quinones as NQO1 substrates. Bioorg. Med. Chem. 2012, 20, 3223–3232. [Google Scholar] [CrossRef]
- Fagan, V.; Bonham, S.; McArdle, P.; Carty, M.P.; Aldabbagh, F. Synthesis and toxicity of new ring-fused imidazo[5,4-f]benzimidazolequinones and mechanism using amine N-oxide cyclization. Eur. J. Org. Chem. 2012, 1967–1975. [Google Scholar] [CrossRef]
- Fagan, V.; Bonham, S.; Carty, M.P.; Aldabbagh, F. One-pot double intramolecular homolytic aromatic substitution routes to dialicyclic ring fused imidazobenzimidazolequinones and preliminary analysis of anticancer activity. Org. Biomol. Chem. 2010, 8, 3149–3156. [Google Scholar] [CrossRef]
- Sweeney, M.; Coyle, R.; Kavanagh, P.; Berezin, A.A.; Lo Re, D.; Zissimou, G.A.; Koutentis, P.A.; Carty, M.P.; Aldabbagh, F. Discovery of anti-cancer activity for benzo[1,2,4]triazin-7-ones: Very strong correlation to pleurotin and thioredoxin reductase inhibition. Bioorg. Med. Chem. 2016, 24, 3565–3570. [Google Scholar] [CrossRef]
- Keane, L. -A. J.; Mirallai, S.I.; Sweeney, M.; Carty, M.P.; Zissimou, G.A.; Berezin, A.A.; Koutentis, P.A.; Aldabbagh, F. Anti-cancer activity of phenyl and pyrid-2-yl 1,3-substituted benzo[1,2,4]triazin-7-ones and stable free radical precursors. Molecules 2018, 23, 574. [Google Scholar] [CrossRef]
- Conboy, D.; Aldabbagh, F. 6-Imino-1,2,3,4,8,9,10,11-octahydropyrido[1,2-a]pyridoimidazo[4,5-f]benzimidazole-13-one: synthesis and cytotoxicity evaluation. Molbank 2020, (1), m1118. [Google Scholar] [CrossRef]
- Fitzsimmons, S.A.; Workman, P.; Grever, M.; Paull, K.; Camalier, R. ; Lewis. A. D. Reductase enzyme expression across the National Cancer Institute tumor cell line panel: correlation with sensitivity to mitomycin C and EO9. J. Natl. Cancer Inst. 1996, 88, 259–269. [Google Scholar]
- Catto, M.; Berezin, A.A.; Lo Re, D.; Loizou, G.; Demetriades, M.; De Stradis, A.; Campagna, F.; Koutentis, P.A.; Carotti, A. Design, synthesis and biological evaluation of benzo[e][1,2,4]triazin-7(1H)-one and [1,2,4]triazino[5,6,1-jk]carbazol-6-one derivatives as dual inhibitors of beta-amyloid aggregation and acetyl/butyryl cholinesterase. Eur. J. Med. Chem. 2012, 58, 84–97. [Google Scholar] [CrossRef]
- Welsh, S.J.; Williams, R.R.; Birmingham, A.; Newman, D.J.; Kirkpatrick, D.L.; Powis, G. The thioredoxin redox inhibitors 1-methylpropyl 2-imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1α and vascular endothelial growth factor formation. Mol. Cancer Ther. 2003, 2, 235–243. [Google Scholar]
- Saikolappan, S.; Kumar, B.; Shishodia, G.; Koul, S.; Koul, H.K. Reactive oxygen species and cancer: a complex interaction. Cancer Lett. 2019, 452, 132–143. [Google Scholar] [CrossRef]
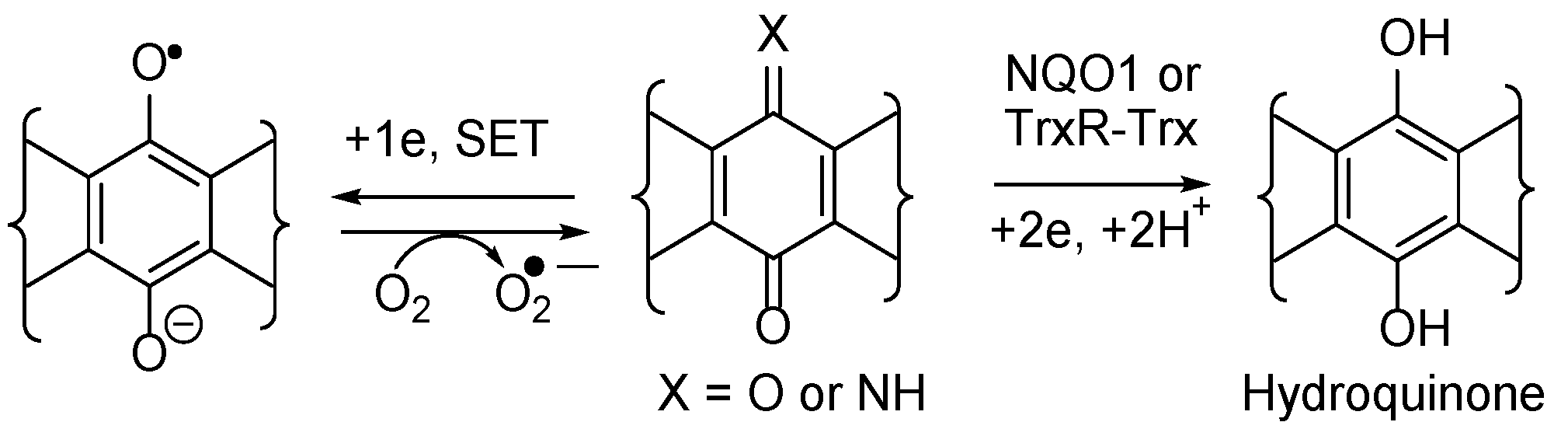
- Bolton, J.L.; Dunlap, T. Formation and biological targets of quinones: Cytotoxic versus cytoprotective effects. Chem. Res. Toxicol. 2017, 30, 13–37. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, D.; Ma, K.; Wu, X.; Hao, H.; Jiang, S. NAD(P)H:quinone oxidoreductase 1 (NQO1) as a therapeutic and diagnostic target in cancer. J. Med. Chem. 2018, 61, 6983–7003. [Google Scholar] [CrossRef]
- Bian, M.; Fan, R.; Zhao, S.; Liu, W. Targeting the thioredoxin system as a strategy for cancer therapy. J. Med. Chem. 2019, 62, 7309–7321. [Google Scholar] [CrossRef]
- Tomasz, M.; Lipman, R.; Chowdary, D.; Pawlak, J.; Verdine, G.L.; Nakanishi, K. Isolation and structure of a covalent cross-link adduct between mitomycin C and DNA. Science 1987, 235, 1204–1208. [Google Scholar] [CrossRef]
- Parkinson, E.I.; Bair, J.S.; Cismesia, M.; Hergenrother, P.J. Efficient NQO1 substrates are potent and selective anticancer agents. ACS Chem. Biol. 2013, 8, 2173–2183. [Google Scholar] [CrossRef]
- Li, R.; Bianchet, M.A.; Talalay, P.; Amzel, L.M. NAD(P)H:quinone reductase, a flavoprotein involved in cancer chemoprotection and chemotherapy: mechanism of the two-electron reduction. Proc. Natl. Acad. Sci. U. S. A. 1995, 92, 8846–8850. [Google Scholar] [CrossRef]
- Coyle, R. Barton esters for iniator-free radical cyclisation with heteroaromatic substitution and anti-cancer evaluation of benzo[e][1,2,4]triazin-7-ones. Ph.D., National University of Ireland Galway, Ireland, 2014.
- Dai, B.; Augustine, J.J.; Kang, Y.; Roife, D.; Li, X.; Deng, J.; Tan, L.; Rusling, L.A.; Weinstein, J.N.; Lorenzi, P.L.; Kim, M.P.; Fleming, J.B. Compound NSC84167 selectively targets NRF2-activated pancreatic cancer by inhibiting asparagine synthesis pathway. Cell Death Dis. 2021, 12, 693. [Google Scholar] [CrossRef]
- Lister, A.; NedjadI, T.; Kitteringham, N.R.; Campbell, F.; Costello, E.; Lloyd, B.; Copple, I.M.; Williams, S.; Owen, A.; Neoptolemos, J.P.; Goldring, C.E.; Park, B.K. Nrf2 is overexpressed in pancreatic cancer: implications for cell proliferation and therapy. Mol. Cancer 2011, 10, 37. [Google Scholar] [CrossRef]
- Stefañska, B.; Dzieduszycka, M.; Martelli, S.; Tarasiuk, J.; Bontemps-Gracz, M.; Borowski, E. 6-[(Aminoalkyl)amino]-substituted 7H-benzo[e]perimidin-7-ones as novel antineoplastic agents. Synthesis and biological evaluation. J. Med. Chem. 1993, 36, 38–41. [Google Scholar] [CrossRef]
- Stefañska, B.; Dzieduszycka, M.; Bontemps-Gracz, M.M.; Borowski, E.; Martelli, S.; Supino, R.; Pratesi, G.; De Cesare, M.; Zunino, F. Kuśnierczyk, H.; Radzikowski, C. 8,11-Dihydroxy-6-[(aminoalkyl)amino]-7H-benzo[e]perimidin-7-ones with activity in multidrug-resistant cell lines: synthesis and antitumor evaluation. J. Med. Chem. 1999, 42, 3494–3501. [Google Scholar] [CrossRef]
- Dzieduszycka, M.; Martelli, S.; Arciemiuk, M.; Bontemps-Gracz, M.M.; Kupieca, A.; Borowski, E. Effect of modification of 6-[(Aminoalkyl)amino]-7H-benzo[e]perimidin-7-ones on their cytotoxic activity toward sensitive and multidrug resistant tumor cell lines. Synthesis and biological evaluation. Bioorg. Med. Chem. 2002, 10, 1025–1035. [Google Scholar] [CrossRef]
- Anzai, K.; Isono, K.; Okuma, K.; Suzuki, S. The new antibiotics, questiomycins A and B. J. Antibiot. (Tokyo) 1960, 13, 125–132. [Google Scholar]
- Kozlovsky, A.G.; Zhelifonova, V.P.; Antipova, T.V.; Adanin, V.M. Novikova, N.D.; Deshevaya, E.A.; Schlegel, B.; Dahse, H.M.; Gollmik, F.; Grafe, U. Penicillium expansum, a resident fungal strain of the orbital complex Mir, producing xanthocyllin X and questiomycin A. Appl. Biochem. Microbiol. 2004, 40, 344–349. [Google Scholar] [CrossRef]
- Bitzer, J.; Große, T.; Wang, L.; Lang, S.; Beil, W.; Zeeck, A. New aminophenoxazinones from a marine Halomonas sp.: fermentation, structure elucidation, and biological activity. J. Antibiot. 2006, 59, 86–92. [Google Scholar] [CrossRef]
- Hanawa, T.; Osaki, T.; Manzoku, T.; Fukuda, M.; Kawakami, H.; Tomoda, A.; Kamiya, S. In vitro antibacterial activity of Phx-3 against Helicobacter pylori. Biol. Pharm. Bull. 2010, 33, 188–191. [Google Scholar] [CrossRef]
- Guo, S.; Hu, H.; Bilal, M.; Zhang, X. Production of antibacterial questiomycin A in metabolically engineered Pseudomonas chlororaphis HT66. J. Agric. Food Chem. 2022, 70, 7742–7750. [Google Scholar] [CrossRef]
- Barry III, C.E.; Nayar, P.G.; Begley, T.P. Phenoxazinone synthase: mechanism for the formation of the phenoxazinone chromophore of actinomycin1. Biochem. 1989, 28, 6323–6333. [Google Scholar] [CrossRef]
- Le Roes-Hill, M.; Goodwin, C.; Burton, S. Phenoxazinone synthase: what’s in a name? Trends Biotechnol. 2009, 27, 248–258. [Google Scholar] [CrossRef]
- Nakachi, T.; Tabuchi, T.; Takasaki, A.; Arai, S.; Miyazawa, K.; Tomoda, A. Anticancer activity of phenoxazines produced by bovine erythrocytes on colon cancer cells. Oncol. Rep. 2010, 23, 1517–1522. [Google Scholar]
- Hongo, T.; Miyano-Kurosaki, N.; Kurosaki, K.; Hata, A.; Harigae, S.; Tomoda, A. 2-Aminophenoxazine-3-one prevents pulmonary metastasis of mouse B16 melanoma cells in mice. J. Pharmacol. Sci. 2010, 114, 63–68. [Google Scholar] [CrossRef]
- Kohno, K.; Miyake, M.; Sano, O.; Tanaka-Kataoka, M.; Yamamoto, S.; Koya-Miyata, S.; Arai, N.; Fujii, M.; Watanabe, H.; Ushio, S.; Iwaki, K.; Fukuda, S. Anti-inflammatory and immunomodulatory properties of 2-amino-3H-phenoxazin-3-one. Biol. Pharm. Bull. 2008, 31, 1938–1945. [Google Scholar] [CrossRef]
- Baindara, P.; Mandal, S.M. Bacteria and bacterial anticancer agents as a promising alternative for cancer therapeutics. Biochimie 2020, 177, 164–189. [Google Scholar] [CrossRef]
- Bhansali, K.G.; Kook, A.M. Synthesis and characterization of a series of 5H-benzo[a]phenoxazin-5-one derivatives as potential antiviral/antitumor agents. Heterocycles 1993, 36, 1239–1251. [Google Scholar] [CrossRef]
- Chau, N.-M.; Rogers, P.; Aherne, W.; Carroll, V.; Collins, I.; McDonald, E.; Workman, P.; Ashcroft, M. Identification of novel small molecule inhibitors of hypoxia-inducible factor-1 that differentially block hypoxia-inducible factor-1 activity and hypoxia-inducible factor-1α induction in response to hypoxic stress and growth factors. Cancer Res. 2005, 65, 4918–4928. [Google Scholar] [CrossRef]
- Lv, X.; Li, J.; Zhang, C.; Hu, T.; Li, S.; He, S.; Yan, H.; Tan, Y.; Lei, M.; Wen, M.; Zuo, J. The role of hypoxia-inducible factors in tumor angiogenesis and cell metabolism. Genes Dis. 2017, 4, 19–24. [Google Scholar] [CrossRef]
- Li, Z.; You, Q.; Zhang, X. Small-Molecule modulators of the hypoxia-inducible factor pathway: development and therapeutic applications. J. Med. Chem. 2019, 62, 5725–5749. [Google Scholar] [CrossRef]
- Sharma, A.; Arambula, J.F.; Koo, S.; Kumar, R.; Singh, H.; Sessler, J.L.; Kim, J.-S. Hypoxia-targeted drug delivery. Chem. Soc. Rev. 2019, 48, 771–813. [Google Scholar] [CrossRef]
- Faig, M.; Bianchet, M.A.; Talalay, P.; Chen, S.; Winski, S.; Ross, D.; Amzel, L.M. Structures of recombinant human and mouseNAD(P)H:quinone oxidoreductases: species comparison and structural changes with substrate binding and release. Proc. Natl. Acad. Sci. USA 2000, 97, 3177–3182. [Google Scholar] [CrossRef]
- Song, Y.; Wang, S.; Zhao, M.; Yang, X.; Yu, B. Strategies targeting protein tyrosine phosphatase SHP2 for cancer therapy. J. Med. Chem. 2022, 65, 3066–3079. [Google Scholar] [CrossRef]
- Homan, K.T.; Balasubramaniam, D.; Zabell, A.P. R.; Wiest, O.; Helquist, P.; Stauffacher, C.V. Identification of novel inhibitors for a low molecular weight protein tyrosine phosphatase via virtual screening. Bioorg. Med. Chem. 2010, 18, 5449–5456. [Google Scholar] [CrossRef]
- Defant, A.; Guella, G.; Mancini, I. Synthesis and in vitro cytotoxicity evaluation of novel naphthindolizinedione derivatives. Arch. Pharm. Chem. Life Sci. 2007, 340, 147–153. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Y.; Pan, L.; Yang, Y.; Zheng, Q.; Hu, Y.; Wang, Y.; Zhang, L.; Sun, Y.; Li, Z.; Meng, X. Design, synthesis and structure-activity relationship study of novel naphthoindolizine and indolizinoquinoline-5,12-dione derivatives as IDO1 inhibitors. Bioorg. Med. Chem. 2018, 26, 4886–4897. [Google Scholar] [CrossRef]
- Feng, X.; Liao, D.; Liu, D.; Ping, A.; Li, Z.; Bian, J. Development of indoleamine 2,3-dioxygenase 1 inhibitors for cancer therapy and beyond: A recent perspective. J. Med. Chem. 2020, 63, 15115–15139. [Google Scholar] [CrossRef]
- Ryu, C.-K.; Yoon, J.-H.; Song, A.L.; Im, H.A.; Kim, J.Y.; Kim, A. Synthesis and antifungal evaluation of pyrido[1,2-a]indole-1,4-diones and benzo[f]pyrido[1,2-a]indole-6,11-diones. Bioorg. Med. Chem. 2012, 22, 497–499. [Google Scholar] [CrossRef]
- Székely, R.; Rengifo-Gonzalez, M.; Singh, V.; Riabova, O.; Benjak, A.; Piton, J.; Gimino, M.; Kornobus, E.; Mizrahi, V.; Johnsson, K.; Manina, G.; Makarov, V.; Cole, S.T. 6,11-Dioxobenzo[f]pyrido[1,2-a]indoles kill mycobacterium tuberculosis by targeting iron−sulfur protein Rv0338c (IspQ), a putative redox sensor. ACS Infect. Dis. 2020, 6, 3015–3025. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.-W. Copper(II)-catalyzed synthesis of benzo[f]pyrido[1,2-a]indole-6,11-dione derivatives via naphthoquinone difunctionalization reaction. J. Org. Chem. 2012, 77, 1191–1197. [Google Scholar] [CrossRef]
- Sun, J.; Wang, F.; Hu, H.; Wang, X.; Wu, H.; Liu, Y. Copper(II)-catalyzed carbon−carbon triple bond cleavage of internal alkynes for the synthesis of annulated indolizines. J. Org. Chem. 2014, 79, 3992–3998. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Iwamoto, A.; Kitao, T. Reaction of 2,3-dichloro-1,4-naphthoquinone with dithiooxamide. Synthesis of dibenzo[b,i]thianthrene-5,7,12,14-tetrone. J. Heterocycl. Chem. 1991, 28, 1445–1447. [Google Scholar] [CrossRef]
- Trzoss, L.; Fukuda, T.; Costa-Lotufo, L.V.; Jimenez, P.; La Clair, J.J.; Fenical, W. Seriniquinone, a selective anticancer agent, induces cell death by autophagocytosis, targeting the cancer-protective protein dermcidin. Proc. Natl. Acad. Sci. USA 2014, 111, 14687–14692. [Google Scholar] [CrossRef]
- Hammons, J.C.; Trzoss, L.; Jimenez, P.C.; Hirata, A.S.; Costa-Lotufo, L.V.; La Clair, J.J.; Fenical, W. Advance of seriniquinone analogues as melanoma agents. ACS Med. Chem. Lett. 2019, 10, 186–190. [Google Scholar] [CrossRef]
- Burian, M.; Schittek, B. The secrets of dermcidin action. Int. J. Med. Microbiol. 2015, 305, 283–286. [Google Scholar] [CrossRef]
- Nagao, H.; Ninomiya, M.; Sugiyama, H.; Itabashi, A.; Uno, K.; Tanaka, K.; Koketsu, M. Comparative analysis of p-terphenylquinone and seriniquinone derivatives as reactive oxygen species-modulating agents. Bioorg. Med. Chem. Lett. 2022, 76, 128992. [Google Scholar] [CrossRef] [PubMed]
- Tuchinda, P.; Pohmakotr, M.; Munyoo, B.; Reutrakul, V.; Santisuk, T. An azaanthracene alkaloid from Polyalthia suberosa. Phytochem. 2000, 53, 1079–1082. [Google Scholar] [CrossRef]
- Gandy, M.N.; Piggott, M.J. Synthesis of kalasinamide. A putative plant defense photoxin. J. Nat. Prod. 2008, 71, 866–868. [Google Scholar] [CrossRef]
- Lang, S.; Groth, U. Total syntheses of cytotoxic, naturally occurring kalasinamide, geovanine, and marcanine A. Angew Chem. Int. Ed. 2009, 48, 911–913. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Kong, M.; Chen, R.-Y.; Yu, D.Q. Alkaloids from the roots of Goniothalamus griffithii. J. Nat. Prod. 1999, 62, 1050–1052. [Google Scholar] [CrossRef]
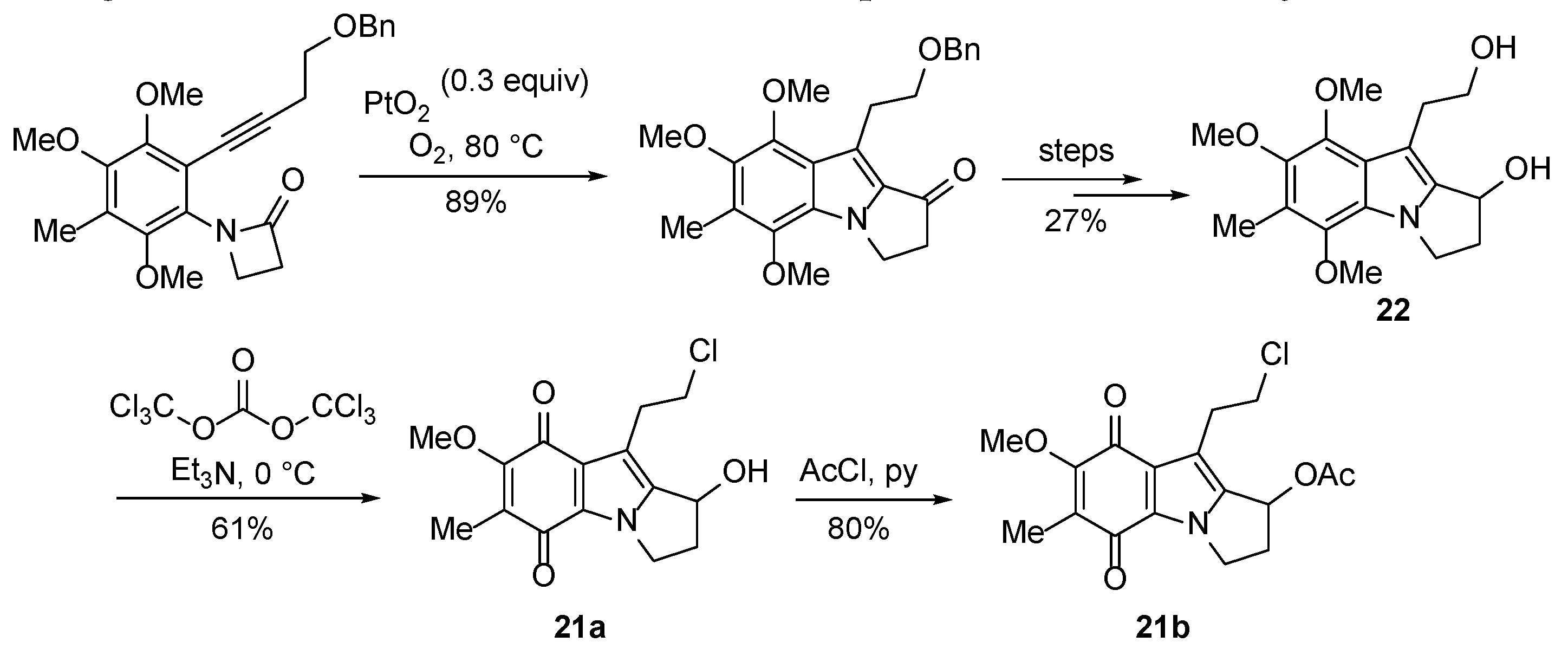
- Yin, J.; Landward, M.B.; Rainier, J.D. Photoelectrocyclization reactions of amidonaphthoquinones. J. Org. Chem. 2020, 85, 4298–4311. [Google Scholar] [CrossRef]
- Soonthornchareonnon, N.; Suwanborirux, K.; Bavovada, R.; Patarapanich, C.; Cassady, J.M. New cytotoxic 1-azaanthraquinones and 3-aminonaphthoquinone from the stem bark of Goniothalamus marcanii. J. Nat. Prod. 1999, 62, 1390–1394. [Google Scholar] [CrossRef]
- Sedmera, P.; Pospisil, S.; Novak, J. New furanonaphthoquinone from Streptomyces cinnamonensis. J. Nat. Prod. 1991, 54, 870–872. [Google Scholar] [CrossRef]
- Bringmann, G.; Haagen, Y.; Gulder, T.A. M.; Gulder, T.; Heide, L. Biosynthesis of the isoprenoid moieties of furanonaphthoquinone I and endophenazine A in Streptomyces cinnamonensis DSM 1042. J. Org. Chem. 2007, 72, 4198–4204. [Google Scholar] [CrossRef]
- Simamura, E.; Hirai, K.-I.; Shimada, H.; Koyama, J.; Niwa, Y.; Shimizu, S. Furanonaphthoquinones cause apoptosis of cancer cells by inducing the production of reactive oxygen species by the mitochondrial voltage-dependent anion channel. Cancer Biol. Ther. 2006, 5, 1523–1529. [Google Scholar] [CrossRef]
- Tahara, T.; Watanabe, A.; Yutani, M.; Yamano, Y.; Sagara, M.; Nagai, S.; Saito, K.; Yamashita, M.; Ihara, M.; Iida, A. STAT3 inhibitory activity of naphthoquinones isolated from Tabebuia avellanedae. Bioorg. Med. Chem. 2020, 28, 115347. [Google Scholar] [CrossRef]
- Feng, X.; Qiu, X.; Huang, H.; Wang, J.; Xu, X.; Xu, P.; Ge, R.; Liu, X.; Li, Z.; Bian, J. Palladium(II)-catalyzed reaction of Lawsones and propargyl carbonates: construction of 2,3-furanonaphthoquinones and evaluation as potential indoleamine 2,3-dioxygenase inhibitors. J. Org. Chem. 2018, 83, 8003–8010. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: from bench to clinic. Sig. Transduct. Target Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Wu, Z.-Z.; Jang, Y.-J.; Lee, C.-J.; Lin, W. A versatile and practical method for the regioselective synthesis of polysubstituted furanonapthoquinones. Org. Biomol. Chem. 2013, 11, 828–834. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Wu, H.-Y.; Hsu, Y.-L.; Cheng, T.-J. R.; Liu, J.-H.; Huang, R.-J.; Hsiao, T.-H.; Wang, C.-J.; Hung, P.-F.; Lan, A.; Pan, S.-H.; Chein, R.-J.; Wong, C.-H.; Yang, P.-C. Suppression of drug-resistant non-small-cell lung cancer with inhibitors targeting minichromosomal maintenance protein. J. Med. Chem. 2020, 63, 3172–3187. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-J.; Chen, C.-C.; Tsai, C.-W.; Wu, M.J. Base-mediated cyclization reaction of 2-(5-hydroxy-1-pentynyl)benzonitriles to 4-amino-2,3-dihydronaphtho[2,3-b]furanes and synthesis of furanonaphthoquinones. J. Org. Chem. 2016, 81, 3882–3889. [Google Scholar] [CrossRef] [PubMed]
- El-Feraly, F.S. Melampolides from Magnolia grandiflora. Phytochem. (Elsevier) 1984, 23, 2372–2374. [Google Scholar] [CrossRef]
- Nasim, S.; Pei, S.; Hagen, F.K.; Jordan, C.T.; Crooks, P.A. Melampomagnolide B: a new antileukemic sesquiterpene. Bioorg. Med. Chem. 2011, 19, 1515–1519. [Google Scholar] [CrossRef]
- Janganati, V.; Ponder, J.; Jordan, C.T.; Borrelli, M.J.; Penthala, N.R.; Crooks, P.A. Dimers of melampomagnolide B exhibit potent anticancer activity against hematological and solid tumor cells. J. Med. Chem. 2015, 58, 8896–8906. [Google Scholar] [CrossRef] [PubMed]
- Penthala, N.R.; Balasubramaniam, M.; Dachavaram, S.S.; Morris, E.J.; Bhat-Nakshatri, P.; Ponder, J.; Jordan, C.T.; Nakshatri, H.; Crooks, P.A. Antitumor properties of novel sesquiterpene lactone analogs as NFκB inhibitors that bind to the IKKβ ubiquitin-like domain (ULD). Eur. J. Med. Chem. 2021, 224, 113675. [Google Scholar] [CrossRef] [PubMed]
- Mischenko, N.P.; Fedoreyev, S.A.; Pokhilo, N.D.; Anufriev, V.P.; Denisenko, V.A.; Glazunov, V.P. Echinamines A and B, first aminated hydroxynaphthazarins from the sea urchin scaphechinus mirabilis. J. Nat. Prod. 2005, 68, 1390–1393. [Google Scholar] [CrossRef]
- Mischenko, N.P.; Krylova, N.V.; Iunikhina, O.V.; Vasileva, E.A.; Likhatskaya, G.N.; Pislyagin, E.A.; Tarbeeva, D.V.; Dmitrenok, P.S.; Fedoreyev, S.A. A. Antiviral potential of sea urchin aminated spinochromes against herpes simplex virus type 1. Mar. Drugs 2020, 18, 550. [Google Scholar]
- You, H.; Vegi, S.R.; Lagishetti, C.; Chen, S.; Reddy, R.S.; Yang, X.; Guo, J.; Wang, C.; He, Y. Synthesis of bioactive 3,4-dihydro-2H-naphtho[2,3-b][1,4]oxazine-5,10-dione and 2,3,4,5-tetrahydro-1H-naphtho[2,3-b]azepine-6,11-dione derivatives via the copper-catalyzed intramolecular coupling reaction. J. Org. Chem. 2018, 83, 4119–4130. [Google Scholar] [CrossRef]
- Kirkpatrick, D.L.; Powis, G. Inhibitors of redox signaling for restoration of apoptosis and inhibition of abnormal cell proliferation. WO200000 October 2, 2000.
- Liu, L.; Richard, J.; Kim, S.; Wojcik, E.J. Small molecule screen for candidate antimalarials targeting plasmodium kinesin-5. J. Biol. Chem. 2014, 289, 16601–16614. [Google Scholar] [CrossRef] [PubMed]
- Tomek, P.; Palmer, B.D.; Flanagan, J.U.; Sun, C.; Raven, E.L.; Ching, L.-M. Discovery and evaluation of inhibitors to the immunosuppressive enzyme indoleamine 2,3-dioxygenase 1 (IDO1): probing the active site-inhibitor interactions. Eur. J. Med. Chem. 2017, 126, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Soni, I.; Kelkar, D.S.; Dharmaraja, A.T.; Sankar, R.K.; Beniwal, G.; Rajendran, A.; Tamhankar, S.; Chopra, S.; Kamat, S.S.; Chakrapani, H. Chemoproteomics of an indole-based quinone epoxide identifies druggable vulnerabilities in vancomycin-resistant staphylococcus aureus. J. Med. Chem. 2019, 62, 6785–6795. [Google Scholar] [CrossRef] [PubMed]
- Bergamasco, R.; Porter, Q.N.; Yap, C. Vinylindenes and some heteroanalogues in the Diels-Alder reaction. V. 3-Vinylindoles and quinones. Aust. J. Chem. 1978, 31, 1841–1844. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Konala, A.; Lin, L.; Chiang, T.-T.; Huang, C.-Y; Yang, T.-H.; Kavala, V.; Yao, C.-F. Synthesis of benzo[a]carbazole derivatives from 3-ethylindoles by exploiting the dual character of benzoquinone as an oxidizing agent and dienophile. Chem. Commun. 2016, 52, 7870–7873. [Google Scholar] [CrossRef] [PubMed]
- Brimble, M.A.; Duncalf, L.J.; Nairn, M.R. Pyranonaphthoquinone antibiotics—isolation, structure and biological activity. Nat. Prod. Rep. 1999, 16, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Naysmith, B.J.; Hume, P.A.; Sperry, J.; Brimble, M.A. Pyranonaphthoquinones – isolation, biology and synthesis: an update. Nat. Prod. Rep. 2017, 34, 25–61. [Google Scholar] [CrossRef]
- Bergy, M.E. Kalafungin, a new broad spectrum antibiotic: isolation and characterization. J. Antibiot. 1968, 21, 454–457. [Google Scholar] [CrossRef]
- Kakinuma, S.; Ikeda, H.; Omura, S.; Hopwood, D.A. Biosynthesis of kalafungin in Streptomyces tanashiensis. J. Antibiot. 1990, 43, 391–396. [Google Scholar] [CrossRef]
- Omura, S.; Tanaka, H.; Okada, Y.; Marumo, H. Isolation and structure of nanaomycin D, an enantiomer of the antibiotic kalafungin. J. Chem. Soc. Chem. Commun. 1976, 320–321. [Google Scholar]
- Heapy, A.M.; Patterson, A.V.; Smaill, J.B.; Jamieson, S.M. F.; Guise, C.P.; Sperry, J.; Hume, P.A.; Rathwell, K.; Brimble, M.A. Synthesis and cytotoxicity of pyranonaphthoquinone natural product analogues under bioreductive conditions. Bioorg. Med. Chem. 2013, 21, 7971–7980. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Minami-Kakinuma, S.; Omura, S. Biosynthesis of nanaomycin. III. Nanaomycin A formation from nanaomycin D by nanaomycin D reductase via a hydroquinone. J. Antibiot. 1982, 35, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Salaski, E.J.; Krishnamurthy, G.; Ding, W.-D.; Yu, K.; Insaf, S.S.; Eid, C.; Shim, J.; Levin, J.I.; Tabei, K.; Toral-Barza, L.; Zhang, W.-G.; McDonald, L.A.; Honores, E.; Hanna, C.; Yamashita, A.; Johnson, B.; Li, Z.; Laakso, L.; Powell, D.; Mansour, T.S. Pyranonaphthoquinone lactones: A new class of AKT selective kinase inhibitors alkylate a regulatory loop cysteine. J. Med. Chem. 2009, 52, 2181–2184. [Google Scholar] [CrossRef] [PubMed]
- Kuck, D.; Caulfield, T.; Lyko, F.; Medina-Franco, J.L. Nanaomycin A selectively inhibits DNMT3B and reactivates silenced tumor suppressor genes in human cancer cells. Mol. Cancer Ther. 2010, 9, 3015–3023. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, N.; Kobayashi, M.; Wakisaka, Y.; Kawamura, Y.; Mayama, M.; Matsumoto, K. New antibiotics, griseusins A and B. Isolation and characterization. J. Antibiot. 1976, 29, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Masamoto, K.; Takeuchi, Y.; Takeda, K.; Yoshii, E. Synthesis of pentacyclic structure of griseusin A. Heterocycles 1981, 16, 1659–1664. [Google Scholar]
- Brimble, M.A.; Nairn, M.R. Synthesis of the Griseusin A ring system. J. Chem. Soc., Perkin Trans 1990, 1, 169–171. [Google Scholar] [CrossRef]
- Brimble, M.A.; Nairn, M.R. Synthesis of a pyranonaphthoquinone-spiroacetal. J. Chem. Soc., Perkin Trans. 1992, 1, 579–583. [Google Scholar] [CrossRef]
- Brimble, M.A.; Nairn, M.R.; Park, J. Synthesis of Analogues of Griseusin A. Org. Lett. 1999, 1, 1459–1462. [Google Scholar] [CrossRef]
- Perry, N.B.; Blunt, J.W.; McCombs, J.D.; Munro, M.H. G. Discorhabdin C, a highly cytotoxic pigment from a sponge of the genus Latrunculia. J. Org. Chem. 1986, 51, 5476–5478. [Google Scholar] [CrossRef]
- Hu, J.-F.; Fan, H.; Xiong, J.; Wu, S.-B. Discorhabdins and pyrroloiminoquinone-related alkaloids. Chem. Rev. 2011, 111, 5465–5491. [Google Scholar] [CrossRef]
- Copp, B.R.; Fulton, K.F.; Perry, N.B.; Blunt, J.W.; Munro, M.H. G. Natural and synthetic derivatives of discorhabdin C, a cytotoxic pigment from the New Zealand sponge Latrunculia cf. bocagei. J. Org. Chem. 1994, 59, 8233–8238. [Google Scholar] [CrossRef]
- Na, M.K.; Ding, Y.; Wang, B.; Tekwani, B.L.; Schinazi, R.F.; Franzblau, S.; Kelly, M.; Stone, R.; Li, X.-C.; Ferreira, D.; Hamann, M.T. Anti-infective discorhabdins from a deep-water Alaskan sponge of the genus Latrunculia. J. Nat. Prod. 2010, 73, 383–387. [Google Scholar] [CrossRef]
- Goey, A.K. L.; Chau, C.H.; Sissung, T.M.; Cook, K.M.; Venzon, D.J.; Castro, A.; Ransom, T.R.; Henrich, C.J.; McKee, T.C.; McMahon, J.B.; Grkovic, T.; Cadelis, M.M.; Copp, B.R.; Gustafson, K.R.; Figg, W.D. Screening and biological effects of marine pyrroloiminoquinone alkaloids: potential inhibitors of the HIF-1α/p300 interaction J. Nat. Prod. 2016, 79, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Mąkosza, M.; Stalewski, J.; Maslennikova, O.S. Synthesis of 7,8-dimethoxy-4-oxo-1,3,4,5-tetrahydropyrrolo[4,3,2-de]quinoline. A key intermediate en route to makaluvamines, discorhabdin C and other marine alkaloids of this group via vicarious nucleophilic substitution of hydrogen. Synthesis 1997, 1131–1133. [Google Scholar] [CrossRef]
- Roberts, D.; Joule, J.A.; Bros, M.A.; Alvarez, M. Synthesis of pyrrolo[4,3,2-de]quinolines from 6,7-dimethoxy-4-methylquinoline. Formal total syntheses of damirones A and B, batzelline C, isobatzelline C, discorhabdin C, and makaluvamines A-D. J. Org. Chem. 1997, 62, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Tohma, H.; Inagaki, M.; Hatanaka, K.; Yakura, T. Total synthesis of discorhabdin C: a general aza spiro dienone formation from O-silylated phenol derivatives using a hypervalent iodine reagent. J. Am. Chem. Soc. 1992, 114, 2175–2180. [Google Scholar] [CrossRef]
- Sadanandan, E.V.; Pillai, S.K.; Lakshmikantham, M.V.; Billimoria, A.D.; Culpepper, J.S.; Cava, M.P. Efficient syntheses of the marine alkaloids makaluvamine D and discorhabdin C: The 4,6,7-trimethoxyindole approach. J. Org. Chem. 1995, 60, 1800–1805. [Google Scholar] [CrossRef]
- Aubart, K.M.; Heathcock, C.H. A Biomimetic approach to the discorhabdin alkaloids: total syntheses of discorhabdins C and E and dethiadiscorhabdin D. J. Org. Chem. 1999, 64, 16–22. [Google Scholar] [CrossRef]
- Kublak, G.G.; Confalone, P.N. The preparation of the aza-spirobicyclic system of discorhabdin C via an intramolecular phenolate alkylation. Tetrahedron Lett. 1990, 31, 3845–3848. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Bebenek, E.; Chrobak, E.; Boryczka, S. 5,8-Quinolinedione scaffold as a promising moiety of bioactive agents. Molecules 2019, 24, 4115. [Google Scholar] [CrossRef] [PubMed]
- Bolzán, A.D.; Bianchi, M.S. Genotoxicity of streptonigrin: a review. Mutat. Res. 2001, 488, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.V.; Cullen, W.P. Streptonigrin, an antitumor substance, in: H. Welch, F. Martilhanez (Eds.), Antibiotics Annual: 1959–1960, Medical Encyclopedia, Inc., New York, 1960, pp. 950–953.
- Doyle, T.W.; Balitz, D.M.; Grulich, R.E.; Nettleton, D.E.; Gould, S.J.; Tann, C.-H.; Moews, A.E. Structure determination of lavendamycin, a new antitumor antibiotic from Streptomyces lavendulae. Tetrahedron Lett. 1981, 22, 4595–4598. [Google Scholar] [CrossRef]
- Behforouz, M.; Cai, W.; Stocksdale, M.G.; Lucas, J.S.; Jung, J.; Briere, D.; Wang, A.; Katen, K.S.; Behforouz, N.C. Novel lavendamycin analogues as potent HIV-reverse transcriptase inhibitors: Synthesis and evaluation of anti-reverse transcriptase activity of amide and ester analogues of lavendamycin. J. Med. Chem. 2003, 46, 5773–5780. [Google Scholar] [CrossRef]
- Hassani, M.; Cai, W.; Koelsch, K.H.; Holley, D.C.; Rose, A.S.; Olang, F.; Lineswala, J.P.; Holloway, W.G.; Gerdes, J.M.; Behforouz, M.; Beall, H.D. Lavendamycin antitumor agents: Structure-based design, synthesis, and NAD(P)H:quinone oxidoreductase 1 (NQO1) model validation with molecular docking and biological studies. J. Med. Chem. 2008, 51, 3104–3115. [Google Scholar] [CrossRef]
- Porter, T.H.; Skelton, F.S.; Folkers, K. Synthesis of new alkylamino- and alkylaminomethyl-5,8-quinolinequinones as inhibitors of Coenzyme Q and as antimalarials. J. Med. Chem. 1971, 14, 1029–1033. [Google Scholar] [CrossRef]
- Cossy, J.; Belotti, D.; Brisson, M.; Skoko, J.J.; Wipf, P.; Lazo, J.S. Biological evaluation of newly synthesized quinoline-5,8-quinones as Cdc25B inhibitors. Bioorg. Med. Chem. 2006, 14, 6283–6287. [Google Scholar] [CrossRef]
- Santoso, K.T.; Menorca, A.; Cheung, C.-Y.; Cook, G.M.; Stocker, B.L.; Timmer, M.S. M. The synthesis and evaluation of quinolinequinones as anti-mycobacterial agents. Bioorg. Med. Chem. 2019, 27, 3532–3545. [Google Scholar] [CrossRef]
- Egu, S.A.; Ibezim, A.; Onoabedje, E.A.; Okoro, U.C. Biological and in silico evaluation of quinolinedione and naphthoquinone derivatives as potent antibacterial agents. ChemistrySelect 2017, 2, 92222–9226. [Google Scholar] [CrossRef]
- Ciftci, H.I.; Bayrak, N.; Yıldız, M.; Yıldırım, H.; Sever, B.; Tateishi, H.; Otsuka, M.; Fujita, M.; Tuyun, A.F. Design, synthesis and investigation of the mechanism of action underlying anti-leukemic effects of the quinolinequinones as LY83583 analogs. Bioorg. Chem. 2021, 114, 105160. [Google Scholar] [CrossRef]
- Ryu, C.-K.; Jeong, H.-J.; Lee, S.K.; You, H.J.; Choi, K.U.; Shim, J.Y.; Heo, Y.H.; Lee, C.-O. Effects of 6-arylamino-5,8-quinolinediones and 6-chloro-7-arylamino-5,8-isoquinolinediones on NAD(P)H:quinone oxidoreductase (NQO1) activity and their cytotoxic potential. Arch. Pharm. Res. 2001, 24, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Fryatt, T.; Pettersson, H.I.; Gardipee, W.T.; Bray, K.C.; Green, S.J.; Slawin, A.M. Z.; Beall, H.D.; Moody, C.J. Novel quinolinequinone antitumor agents: structure-metabolism studies with NAD(P)H:quinone oxidoreductase (NQO1). Bioorg. Med. Chem. 2004, 12, 1667–1687. [Google Scholar] [CrossRef] [PubMed]
- Schlager, J.J.; Powis, G. Cytosolic NAD(P)H:(quinone-acceptor)oxidoreductase in human normal and tumor tissue: effects of cigarette smoking and alcohol. Int. J. Cancer 1990, 45, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Cerecetto, H.; González, M.; Lavaggi, M.L.; Azqueta, A.; Lopez de Cerain, A.; Monge, A. Phenazine 5,10-dioxide derivatives as hypoxic selective cytotoxins. J. Med. Chem. 2005, 48, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Lavaggi, M.L.; Cabrera, M.; Pintos, C.; Arredondo, C.; Pachon, G.; Rodriguez, J.; Raymondo, S.; Pacheco, J.P.; Cascante, M.; Olea-Azar, C.; Lopez de Cerain, A.; Monge, A.; Cerecetto, H.; Gonzalez, M. Novel phenazine 5,10-dioxides release •OH in simulated hypoxia and induce reduction of tumour volume in vivo. ISRN Pharmacol. 2011, 314209. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.B.; Williamson, S.K. Tirapazamine: a novel agent targeting hypoxic tumor cells. Expert Opin. Invest. Drugs 2009, 18, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Abi-Jaoudeh, N.; Dayyani, F.; Chen, P.J.; Fernando, D.; Fidelman, N.; Javan, H.; Liang, P. -C.; Hwang, J. -I.; Imagawa, D.K. Phase I trial on arterial embolization with hypoxia activated tirapazamine for unresectable hepatocellular carcinoma. J. Hepatocell. Carcinoma 2021, 8, 421–434. [Google Scholar] [CrossRef]
- Chen, E.H.; Tanabe, K.; Saggiomo, A.J.; Nodiff, E.A. Modifications of primaquine as antimalarials. 4. 5-Alkoxy derivatives of primaquine. J. Med. Chem. 1987, 30, 1193–1199. [Google Scholar] [CrossRef]
- Shelke, Y.G.; Hande, P.E.; Gharpure, S.J. Recent advances in the synthesis of pyrrolo[1,2-a]indoles and their derivatives. Org. Biomol. Chem. 2021, 19, 7544–7574. [Google Scholar] [CrossRef]
- Bass, P.D.; Gubler, D.A.; Judd, T.C.; Williams, R.M. Mitomycinoid alkaloids: Mechanism of action, biosynthesis, total syntheses, and synthetic approaches. Chem. Rev. 2013, 113, 6816–6863. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Zhang, L. Formal synthesis of 7-methoxymitosene and synthesis of its analog via a key PtCl2-catalyzed cycloisomerization. Org. Lett. 2012, 14, 3736–3739. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Touve, M.; Barnes, J.; Reich, N.; Zhang, L. Synthesis-enabled probing of mitosene structural space leads to improved IC50 over mitomycin C. Angew. Chem. Int. Ed. 2014, 53, 9302–9305. [Google Scholar] [CrossRef] [PubMed]
- Ayala, C.E.; Villalpando, A.; Nguyen, A.L.; McCandless, G.T.; Kartika, R. Chlorination of aliphatic primary alcohols via triphosgene-triethylamine activation. Org. Lett. 2012, 14, 3676–3679. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M. Hydrogen peroxide and hydrohalic acid mediated synthesis of halogenated benzimidazolequinones and anti-cancer evaluation of benzotriazinones. Ph.D., National University of Ireland Galway, Ireland, 2019.
- Lee, S.-R.; Yang, K.-S.; Kwon, J.; Lee, C.; Jeong, W.; Rhee, S, G. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 2002, 277, 20336–20342. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.L.; Pitt, A.R.; Spickett, C.M. Approaches to investigating the protein interactome of PTEN. J. Proteome Res. 2021, 20, 60–77. [Google Scholar] [CrossRef]
- Meuillet, E.J.; Mahadevan, D.; Berggren, M.; Coon, A.; Powis, G. Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN’s lipid phosphatase activity and membrane binding: a mechanism for the functional loss of PTENs tumor suppressor activity. Arch. Biochem. Biophys. 2004, 429, 123–133. [Google Scholar] [CrossRef]
- Duan, D.; Zhang, J.; Yao, J.; Liu, Y.; Fang, J. Targeting thioredoxin reductase by parthenolide contributes to inducing apoptosis of HeLa Cells. J. Biol. Chem. 2016, 291, 10021–10031. [Google Scholar] [CrossRef]
- Madkour, M.M.; Anbar, H.S.; El-Gamal, M.I. Current status and future prospects of p38a/MAPK14 kinase and its inhibitors. Eur. J. Med. Chem. 2021, 213, 113216. [Google Scholar] [CrossRef]
- Paz, M.M.; Zhang, X.; Lu, J.; Holmgren, A. A new mechanism of action for the anticancer drug mitomycin C: mechanism-based inhibition of thioredoxin reductase. Chem. Res. Toxicol. 2012, 25, 1502–1511. [Google Scholar] [CrossRef]
- Newsome, J.J.; Swann, E.; Hassani, M.; Bray, K.C.; Slawin, A.M. Z.; Beall, H.D.; Mood, C.J. Indolequinone antitumour agents: correlation between quinone structure and rate of metabolism by recombinant human NAD(P)H:quinone oxidoreductase. Org. Biomol. Chem. 2007, 5, 1629–1640. [Google Scholar] [CrossRef]
- Yan, C.; Siegel, D.; Newsome, J.J.; Chilloux, A.; Moody, C.J.; Ross, D. Antitumor indolequinones induced apoptosis in human pancreatic cancer cells via inhibition of thioredoxin reductase and activation of redox signaling. Mol. Pharmacol. 2012, 81, 401–410. [Google Scholar] [CrossRef] [PubMed]
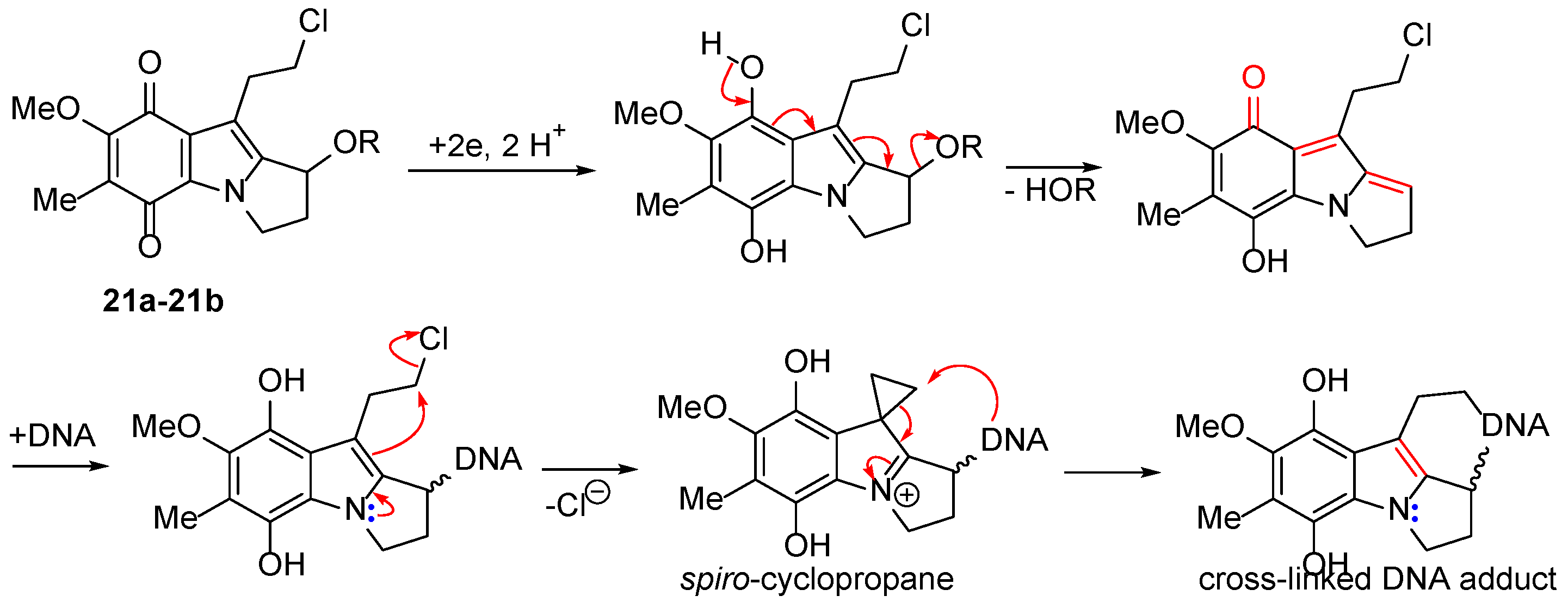
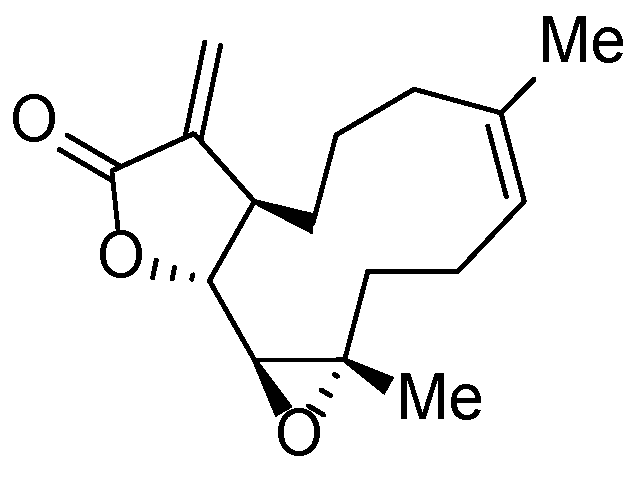
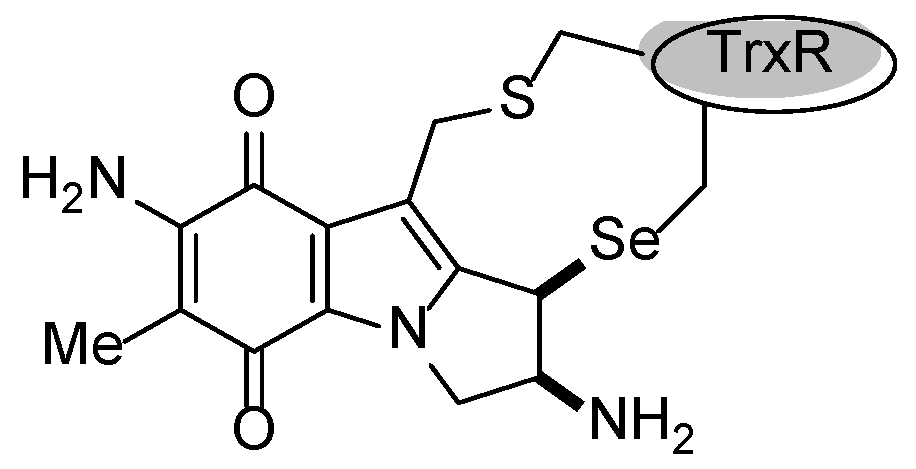
| Compound | NQO1 | TrxR |
|---|---|---|
| 5a | -0.37 | -0.24 |
| 5b | -0.30 | -0.12 |
| Pleurotin | -0.27 | -0.21 |
| 14b | -0.48 | -0.22 |
| Kalafungin | -0.27 | -0.24 |
| Discorhabdin C | -0.29 | -0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).