1. INTRODUCTION
The World Health Organization (WHO) estimates that one-fourth of the world population is infected with
Mycobacterium tuberculosis, the microorganism that causes tuberculosis (TB). Estimates indicate that in 2021, 10.6 million people and 1.6 million fell ill and died with TB, respectively [
1]. Simultaneously, the global burden of diabetes mellitus (DM) is on the rise. In 2017, there were an estimated 425 million people globally with DM, with numbers predicted to increase to 629 million by 2045 [
2]. DM triples the risk of active tuberculosis (TB) [
3]. Patients with DM and TB have more severe clinical manifestations, a longer time to smear conversion, and a higher probability of treatment failure, mortality and recurrence or reinfection [
4,
5]. Thus, the increasing burden of DM worldwide may offset the global decrease in TB incidence.
In Mexico, TB continues to represent a public health problem aggravated by the emergence of DM and recently by the Covid-19 pandemic. In contrast to what is happening on a global scale, where a decrease in incidence has been observed, Mexico has experienced a 13% increase since 2015; the WHO estimates that during 2021 the TB rate was 25 (19-32) cases per 100,000 inhabitants.[
6] DM is a problem that has increased in recent decades. Adult-diagnosed diabetes prevalence in Mexico increased from 7% to 8.9% to 10.3% from 2006, 2012 to 2018 [
7,
8]. One-fifth of patients diagnosed with TB also suffer from DM. It is likely that the growing DM epidemic has had an impact on the rates of pulmonary TB [
9].
Most
M. tuberculosis infections can be avoided to progress to disease with tuberculosis preventive treatment (TPT).[
10] WHO's guidelines recommend treating TBI with:6 or 9 months of daily isoniazid (NIH), or a 3-month regimen of weekly rifapentine plus isoniazid (NIH), or a 3 month regimen of daily isoniazid plus rifampicin (RIF). Alternatively, a 1-month regimen of daily rifapentine plus isoniazid (NIH), or 4 months of daily RIF alone may also be offered. These schedules update previous guidelines which recommended 3 or 4 months of RIF [
11]. Recommendations for LTBI among patients with DM have not been determined since there is limited information regarding efficacy and safety [
2,
12]. TB preventive treatment (TPT) is one of the key interventions recommended by WHO to achieve the End TB Strategy targets, as upheld by the United Nations High Level Meeting on TB in September 2018. Experts have increasingly identified the need to treat LTBI to reach the End TB strategy targets of reducing deaths by 95% and cases by 90% by 2035 [
13]. Modeling studies have concluded that treating people with LTBI is the most effective way of reducing TB incidence [
14].
Given the magnitude and relevance of the comorbidity in Mexico, we considered it relevant to obtain information on the safety of LTBI therapy among patients with DM. This study aimed to assess adverse events (AEs) to LTBI treatment among patients with DM. Secondary objectives were to evaluate tolerability, adherence, the proportion of treatment completion, and hepatotoxicity in patients living with DM and LTBI.
2. METHODS
Study design, participants and randomization
We conducted an open-label, parallel-group, randomized, and controlled trial. We recruited consenting adults of 18 years of age or more, males and non-pregnant females, with a previous DM diagnosis and a documented positive tuberculin skin test (TST). From July 24th, 2017, to February 20th, 2019, we consecutively invited all patients listed in the outpatient registry according to the order assigned by the administrative department at the time of their admission to the outpatient clinic of a tertiary care center in Mexico City. If the patient accepted to participate and met eligibility criteria, he/she was randomized. Treatment allocation was achieved using a random-number generator in ratios of 1: 1 of the a priori calculated sample size (N=403). Participants were assigned to six months of INH 300mg/day plus pyridoxine 75mg or three months of RIF 600mg/day. Follow-up extended until May 24th, 2019. We excluded patients with active TB. We screened for active disease symptoms, chest X-Ray abnormalities, and mycobacteria in sputum or other appropriate samples. We ruled out pregnancy among 18-49 years old- women by a urine pregnancy test. Subjects receiving immunosuppressive therapy, with HIV infection, severe peripheral neuropathy of any cause, previous hepatic, or kidney disease, drinking habit above 70g/week (males) or 50g/week (females), or those with known allergy to INH, RIF or pyridoxine were not included. TST induration ≥10mm was considered positive. Personnel at the Instituto Nacional de Salud Pública (National Institute of Public Health) generated the random allocation sequence. Study personnel enrolled and registered participants, obtained consent, verified assignment, and administered treatment at the outpatient clinic in the study hospital.
Procedures
The follow-up time was six months for the INH group and three months for the RIF group. Clinical and laboratory evaluations were made on days 15, 30, 60, 90 (both groups), 120, and 180 (INH group). In every visit, a Michigan Neuropathy Screening Instrument was completed [
15]. Data were entered into a questionnaire, one for each visit.
At each visit, study personnel interviewed and examined the patients for adverse events, and they were instructed to contact study personnel in case new symptoms appeared between visits. Study personnel was trained to recognize, grade, evaluate and report adverse events following a standardized protocol before the initiation of the trial. If the treating physician decided to stop treatment due to a possible treatment-related adverse event, he/she filed a report within 24 hours. If treatment interruption was temporary (<48 hrs.) or symptoms of intolerance did not merit treatment interruption, they were not reported. Adverse events reports were collected until 30 days after the end of treatment. It comprised clinical management, laboratory results, patient response to drug withdrawal, and results of drug re-challenge if unsuccessful. The report was delivered to an adverse event manager who ensured there were no details revealing which drug the patient was receiving and ensured that the information was complete. If necessary, further information was requested from the reporting physician. The description of the event was then transmitted to an Adverse Event Safety Panel composed by three clinical and epidemiological experts who independently evaluated the events and were blinded to the study drug. Adverse events were categorized into one of ten types: drug interaction, rash, hepatotoxicity, gastrointestinal intolerance, hematological, pregnancy, dizziness, drug induced pancreatitis, seizure, and other. Classification of events was based on published criteria [
16,
17,
18]. Grade 3 hepatotoxicity was defined as liver aminotransferase levels that increased to 5 to 10 or 3 to 10 times the upper limit of normal (ULN) plus compatible symptoms. Grade 4 hepatotoxicity was defined as aminotransferase levels more than ten times the ULN. For all other adverse events we used the National Cancer Institute Common Terminology Criteria for Adverse Events. Relationship to the study drug was judged as none, unlikely, possible, or probable. If individuals with adverse events were hospitalized, these same experts determined if the hospitalization was indicated for the management of the event (yes or no). In the case of panel disagreement, a simple majority was used. If there was no majority, the panel members were asked to independently reassess. Treatment adherence was evaluated through pill count in every visit. We considered that the participant was compliant when he/she ingested ≥80% of the doses. Maximal allowed time to be off treatment was three weeks in both groups.
The presence and severity of diabetes complications were quantified using the Diabetes Complications Severity Index tool (DCSI) [
19]. Disease severity and comorbidity were measured using the Charlson Comorbidity Index (CCI) [
20,
21].
Outcomes
We only contemplated adverse events resulting in permanent treatment cessation and considered possibly or probably related to study drugs by the Safety Panel as outcomes in the statistical analysis. The primary outcome was grade 1–2 rash, recurrent grade 2 hepatotoxicity, or grade 3–5 adverse events. We included grade 1–2 rash within our primary outcome as health personnel are usually prone to interrupt medications if a rash develops, whereas for other mild adverse events, such as grade 1–2 hepatotoxicity, guidelines recommend continuation of treatment as these are generally transient [
18]. Secondary outcomes included grade 1–4 rash, grade 3–4 hepatotoxicity, grade 3–4 hematological events, and grade 3–5 nonhepatotoxic or non-rash adverse events.
Sample size calculation. The study was designed to enroll 403 participants with a power of 81% to detect a 5% difference (6% vs.1%) in the risk of permanent treatment interruption due to adverse drug effects at the end of treatment between groups (one-sided α level, 0.05). The expected permanent drug interruption due to the adverse impacts was based on prior Mexican data [
22]. It was assumed that 5% in each group would be lost to follow up. Due to a high rate of grade 3 or 4 hepatotoxic events leading to permanent treatment interruptions, the Adverse Event Safety Panel advised the research group to halt the study prematurely. With 68 subjects in the INH group, 63 in the RIF group, and 6 (8.8%) permanent treatment interruptions in the INH group vs. none in the RIF group, we rejected the null hypothesis with a power of 0.8 and α level of .05 (one-sided).
Statistical analysis
Descriptive analyses were performed using frequencies and percentages for qualitative variables and median and interquartile range for continuous quantitative variables. We described adverse events resulting in permanent treatment cessation and considered possibly or probably related to study drugs according to study arm and estimated the OR and 95% Cis.
A multivariate Cox proportional hazards model was developed to predict the risk of grade 3 and 4 adverse events, according to the treatment arm adjusting for socio-demographic and clinical variables. Proportional hazard ratios were obtained with a confidence level of 95%. Variables that did not improve the model's likelihood and did not affect the coefficient values were eliminated from the saturated model. Finally, the most parsimonious model was used.From this model, the proportional hazards assumption was evaluated for each of the variables and globally by means of the Shoenfeld residuals test.The goodness of fit of the model was evaluated using Cox Snell residuals. Statistical calculations were performed using stata software version 15.0.
Ethical considerations
The study was approved by the Ethics Committee and Research Ethics Committee (Comités de Ética y de Ética en Investigación), with reference number 1878. Financing was received from the Mexican Council of Science and Technology (Consejo Nacional de Ciencia y Tecnología) with reference number 247582. ClinicalTrials.gov registration number NCT03278483.
Role of the funding source
The study's funder had no role in study design, data collection, data analysis, data interpretation, or report writing. The corresponding author had full access to all the study data and had final responsibility for submitting it for publication.
3. RESULTS
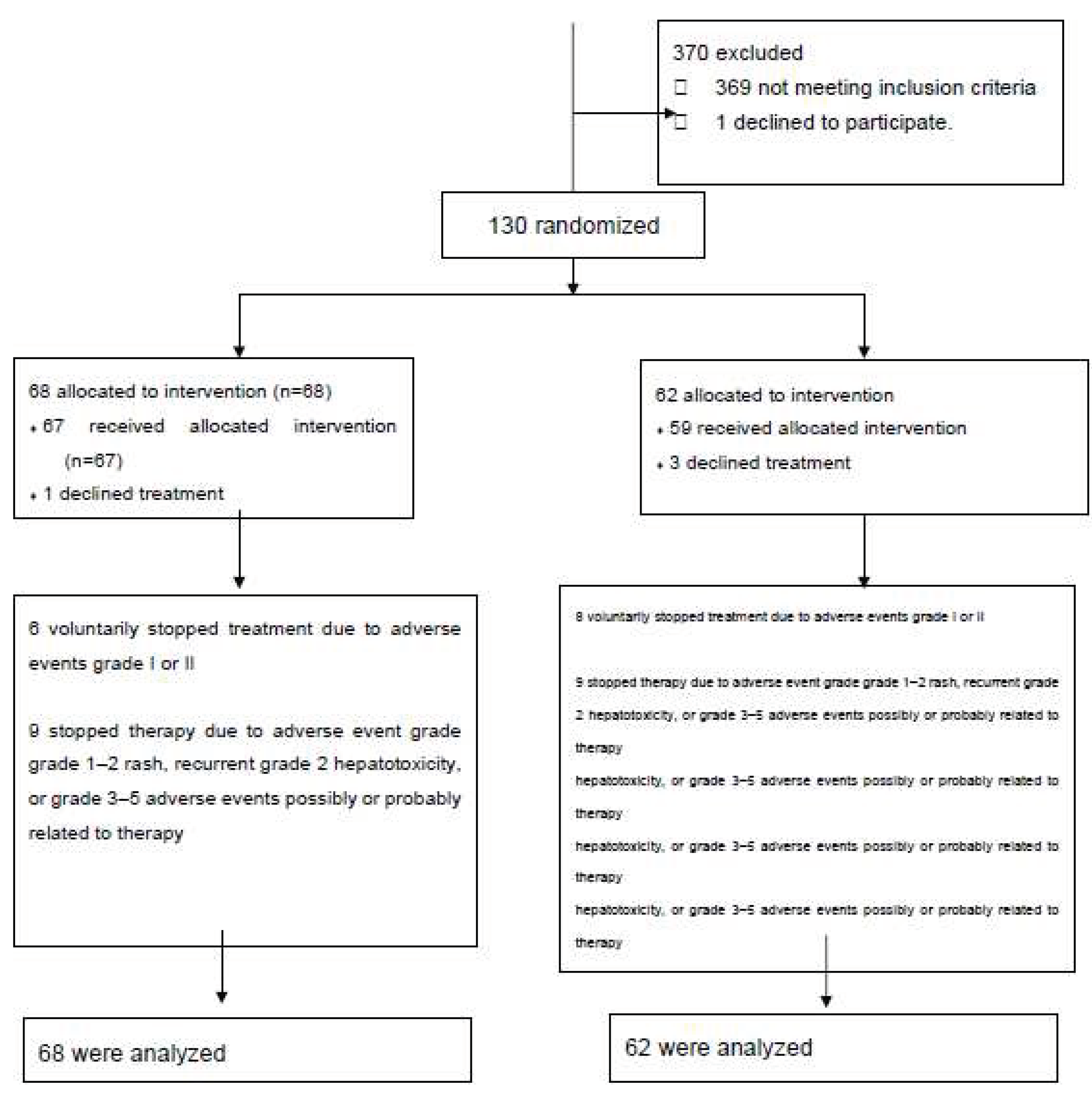
We studied 131 subjects; 68 were allocated to the INH group and 63 to the RIF group. Participant flow is shown in
Figure 1. Of the 68 patients assigned to INH, one refused to take the drug so 67 received the medication. Six patients dropped out of the study against study personnel advice due to grade 1 or 2 gastrointestinal intolerance grade... Nine patients met our definition of adverse effect and were advised to interrupt medication. Of the 62 patients allocated to RIF, three patients refused to take the medication so 59 received the drug. Eight patients dropped out of the study against study personnel advice due to grade 1 or 2 gastrointestinal intolerance. Nine patients discontinued medication due to adverse effects according to our definition. We analyzed 68 patients in the INH group and 62 patients in the RIF group.
Table 1 describes characteristics of enrolled patients. The median age was 57 years (IQR 50-62 years), similar between both groups. Subjects in the INH group had a longer interval since DM diagnosis (13 years vs. ten years, p=0.045). Body mass index, gender, and exposure to alcohol and drugs were equally distributed between groups. Diabetes therapy, the number of medications used, baseline liver enzymes, and glycated hemoglobin were also similar between both groups (
Table 1). Kidney disease was present at similar rates in both groups, although significantly longer in the INH group (7 vs. four years, p=0.049). Retinopathy was reported in 19% of subjects (25/131), more frequent also in the INH group (27.9% vs. 8.0%, p=0.007). Diagnosis of cataracts was reported in 20.5% of subjects in the INH group, while 9.5% of the RIF group, p=0.079. DCSI and other comorbidities were similar between groups (
Table 1).
The Safety Panel judged that 18 events resulting in permanent treatment cessation were possibly or probably related to study drugs, 9 (13.23%) in the INH group and 9 (14.51%) in the RIF group; frequency was not significantly different between groups,
Table 2. However, when categories of adverse events were analyzed, all hepatotoxicity (one recurrent and 6 grade 3 or 4) occurred in the INH group while the majority of grade 3 or 4 gastrointestinal intolerance occurred in the RIF arm. The multivariate analysis revealed that occurrence of adverse effects was associated with characteristics of patients (sex and chronic renal disease). (
Table 3).
The multivariate Cox model revealed that the treatment arm was not associated with adverse events resulting in permanent treatment cessation and considered possibly or probably related to study drugs. We found that in our study population patient characteristics such as chronic kidney disease and female sex were significantly associated with this outcome (
Table 3).
Ninety four (72.31%) patients ingested 80% or more of prescribed pills, with no difference between arms (52 (76.41%) INH group vs 42 (67.74%) RIF group =0.26).
4. DISCUSSION
The present study aimed to assess adverse events due to tuberculosis preventive treatment among patients with DM. We prematurely halted the study based on recommendations of the Adverse Event Safety Panel. There was no difference between arms in the overall frequency of adverse events resulting in permanent treatment cessation and considered possibly or probably related to study drugs. However, the INH group had significantly more permanent treatment interruptions due to grade 2 recurrent or grade 3 or 4 hepatoxicity, while the RIF arm had more treatment interruptions due to grade 3 or 4 gastrointestinal intolerance. Overall adherence to treatment was 72.31%, with no difference between groups.
Previous reports on INH preventive therapy among patients with DM date from more than 50 years ago and had limited results [
23,
24]. Most studies have included patients with DM as part of larger study populations, and to our knowledge, there is a single study that investigated treatment completion under programmatic conditions [
25].
Severe adverse events related to INH in patients with LTBI without DM have been reported with variable frequency among studies and range between 3.3% to 8.2% [
26]. Several studies of tuberculosis preventive treatment have been conducted in Mexico but, to our knowledge, none have only included patients with DM; rates of hepatotoxicity in these studies have been similar to what has been described in the literature [
22,
27,
28]. Comparison between studies is limited due to the lack of uniformity between definitions used to grade adverse events and thresholds for drug discontinuation. These effects can be classified as hepatotoxicity, gastrointestinal intolerance, hematological, and allergic such as dermatitis. Regarding hepatotoxicity, recommendations are to stop treatment in ALT elevations of ≥3x ULN or ≥5x with or without symptoms [
18].
We found that the INH group presented more hepatotoxicity events than the RIF group, DM's effect on drug metabolism has been studied for decades [
29], showing in animal models and some trials in humans that poor glycemic control is associated with a higher rate of adverse events from co-administered drugs, mainly through changes in enzymes such as p450 cytochrome and protein binding dysfunction from glycosylation [
30,
31,
32]. We observed more grade 3 and 4 hepatoxic events among participants receiving daily INH for six months than what was followed by Huang and collaborators who administered daily INH for nine months (8.8% (6/68) vs. 3.2% 2/62) to patients living with DM under programmatic conditions [
25]. Our study population had a higher proportion of women who have been described as a higher risk of liver enzyme derangements with INH administration [
18,
33,
34]. Our study's frequency of severe liver toxicity in the INH group (8.8%) and none in the RIF group was similar to that seen in other case series of patients living with HIV. Hepatotoxicity has been observed in up to 18% of subjects [
33]. Menzies and collaborators reported a rate of 3.7% and 0.7% among subjects taking INH and RIF, respectively, although their population was younger and <30% had comorbid conditions [
35]. In another study, Menzies and collaborators compared over 6,000 subjects, randomized to 9 months of INH versus four months of RIF, observing grade 3-4 hepatotoxicity in 1.8% vs. 0.3% RIF groups, respectively. Most of these events led to treatment interruption. The study population in this study was also younger [
36].
The higher frequency of INH hepatoxicity in our study may have several explanations. Information from the Mexican Health and Nutrition Survey 2016 [
37] revealed that in 2016 prevalence of previously diagnosed DM was 9.4% (95% CI 8.3%-10.8%), representing approximately 6.4 million people with this condition. People are diagnosed on average at 49 years of age [
38]. Our study population was almost a decade older, probably because our institution is a referral center. As it is well known, age has been associated with a higher risk of hepatotoxicity [
39]. Another condition that may have favored hepatotoxicity was the pre-existence of the undiagnosed and aggravated non-alcoholic fatty liver since our study population was mostly overweight. Finally, the median of co-ingested drugs was above polypharmacy definitions (between 3 and 6 drugs per day); therefore, drug-drug interactions might have occurred [
40]. Statin use was reported in 75%, and alcohol use in 44%.
Our trial's treatment adherence rate was similar to previously reported studies [
35,
36]. However, adherence in our study was lower than what was observed by Huang and collaborators; they found that once-weekly INH and rifapentine for 12 weeks (3HP) or daily INH for nine months (9H) was administered to patients with DM with a completion rate of 80% or more. The lower adherence might be explained by a greater frequency of drug interactions due to polypharmacy in our study population.
Gastrointestinal intolerance was the most frequent adverse event in the RIF group. Rifampicin gastrointestinal intolerance is due to hypersensitivity [
41,
42]. In our patient population this was probably aggravated by diabetic gatroenteropathy and interaction with other drugs [
43,
44].
The median of glycated hemoglobin was 8.65%, with similar values between groups, which informs our study population's poor glycemic control, which has also been observed in other low and medium-income countries [
45,
46,
47]. On the one hand, the latter underlies the relevance of treating this population for LTBI to avoid their increased risk of reactivation and the poor outcomes these patients show when presenting with active tuberculosis.
The main limitation of our study was its small study sample, but we halted the study by recommendation of the Adverse Event Safety Panel on observation of adverse events.. The small sample size did not allow us to evaluate the interaction of study drugs and other patients' characteristics such as age, sex, additional medications, or metabolic control over hepatotoxicity. Another limitation was that patients in the INH group had longer intervals since DM diagnosis than the RIF group due to single-block and premature halting of our trial. Therefore, the higher rate of hepatoxicity might be due to diabetes-associated complications in the INH group. However, the DCSI values, BUN and creatinine levels, Charlson scores, other comorbidities, frequency, and interval since diagnoses of DM-associated complications, age, and gender were similar between both groups. Another limitation was the open-label design of our study. To minimize the possible bias, we used standard definitions for adverse effects based on laboratory results. Notably, members of the Adverse Event Safety Panel blindly evaluated study outcomes. The main advantage of our study was the fact that all participants had been previously diagnosed with DM. Consequently, subjects in our study are older than those in previous literature, making our results more widely applicable to older populations suffering from diabetes. Finally, our results may be generalized to similar regions with a high prevalence of DM and TB and large populations of elderly patients living with uncontrolled DM.
In conclusion, our study shows that, in a setting where the association of DM and TB constitutes a severe public health problem, tuberculosis preventive treatment with INH is not safe enough to be considered a universal indication to patients with DM and LTBI. On the other hand, the frequency of gastrointestinal intolerance among the group receiving RIF also precludes adherence among patients receiving this drug. Previous considerations of the potential risks of hepatotoxicity due to polypharmacy and comorbidities, as well as gastric intolerance due to polypharmacy and preexistent gastric disease related to DM, are both confirmed in our study. These results underline the need to search for alternatives to LTBI treatment in DM patients with better safety profiles, such as rifapentine.
Authorship statement:
KTG, PTG, NMR, LFR, GDS, MBV, EFG, , PCH, JSO, CAS, LGG and APL conceptualized the study; KTG, NMR, PTG, GDS, PCH, VJS, PLC, LGG, and APL obtained, analyzed, or interpreted data; KTG, PTG, LFR, GDS, MMH, VJS, and PLC curated data; KTG, PTG, JSO, CAS, LGG and APL drafted the work or revised it critically for important intellectual content; all authors approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgments
We want to thank Brenda P. Castillo-Marmolejo, Corazon de J. Barrientos-Flores, Michelle Marvin-Huergo, Carlos A. Castelan-Garcia and Adolfo Marín for their invaluable support in the follow-up of patients and data collection. This work was supported by the Mexican Secretariat of Health and the Mexican Council of Science and Technology (2015-PDN I0002-Fondo Institucional 247582).
Competing Interests
None declared.
References
- World Health Organization. Global tuberculosis control: WHO report 2020. https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131. Accessed March, 2021.
- Lin, Y.; Harries, A.; Kumar, A.; Critchley, J.; van Crevel, R.; Owiti, P.; Dlodlo, R.; Dejgaard, A. Management of diabetes mellitus-tuberculosis: a guide to the essential practice. 2019.
- Jeon, C.Y.; Murray, M.B. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS medicine 2008, 5, e152. [CrossRef]
- Baker, M.A.; Harries, A.D.; Jeon, C.Y.; Hart, J.E.; Kapur, A.; Lonnroth, K.; Ottmani, S.E.; Goonesekera, S.D.; Murray, M.B. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 2011, 9, 81. [CrossRef]
- Jimenez-Corona, M.E.; Cruz-Hervert, L.P.; Garcia-Garcia, L.; Ferreyra-Reyes, L.; Delgado-Sanchez, G.; Bobadilla-Del-Valle, M.; Canizales-Quintero, S.; Ferreira-Guerrero, E.; Baez-Saldana, R.; Tellez-Vazquez, N.; et al. Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax 2013, 68, 214-220. [CrossRef]
- World Health Organization. Global tuberculosis report 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022. Accessed. May, 2023.
- Meza, R.; Barrientos-Gutierrez, T.; Rojas-Martinez, R.; Reynoso-Noverón, N.; Palacio-Mejia, L.S.; Lazcano-Ponce, E.; Hernández-Ávila, M. Burden of type 2 diabetes in Mexico: past, current and future prevalence and incidence rates. Prev Med 2015, 81, 445-450. [CrossRef]
- Shamah-Levy T, Vielma-Orozco E, Heredia-Hernández O, Romero-Martínez M, Mojica-Cuevas J, Cuevas-Nasu L, Santaella-Castell JA, Rivera-Dommarco J. Encuesta Nacional de Salud y Nutrición 2018-19: Resultados Nacionales. Cuernavaca, México: Instituto Nacional de Salud Pública, 2020. https://ensanut.insp.mx/encuestas/ensanut2018/informes.php. Accessed Mat 2023.
- Delgado-Sanchez, G.; Garcia-Garcia, L.; Castellanos-Joya, M.; Cruz-Hervert, P.; Ferreyra-Reyes, L.; Ferreira-Guerrero, E.; Hernandez, A.; Ortega-Baeza, V.M.; Montero-Campos, R.; Sulca, J.A.; et al. Association of Pulmonary Tuberculosis and Diabetes in Mexico: Analysis of the National Tuberculosis Registry 2000-2012. PloS one 2015, 10, e0129312. [CrossRef]
- Sterling, T.R.; Njie, G.; Zenner, D.; Cohn, D.L.; Reves, R.; Ahmed, A.; Menzies, D.; Horsburgh, C.R., Jr.; Crane, C.M.; Burgos, M.; et al. Guidelines for the Treatment of Latent Tuberculosis Infection: Recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep 2020, 69, 1-11. [CrossRef]
- World Health Organization. Guidelines on the management of latent tuberculosis infection. 2015; WHO/HTM/TB. http://apps.who.int/iris/bitstream/10665/136471/1/9789241548908_eng.pdf?ua=1&ua=1. Accessed March, 2021.
- World Health Organization. Collaborative framework for care and control of Tuberculosis and Diabetes . 2011. http://www.ncbi.nlm.nih.gov/pubmed/17158327. Accessed March 2021.
- World Health Organization. The END TB strategy. Global strategy and targets for tuberculosis prevention, care and control after 2015. Geneva: WHO; 2016. https://www.who.int/tb/strategy/End_TB_Strategy.pdf?ua=1. Accessed March, 2021.
- Dye, C.; Glaziou, P.; Floyd, K.; Raviglione, M. Prospects for tuberculosis elimination. Annu Rev Public Health 2013, 34, 271-286. [CrossRef]
- Feldman, E.L.; Stevens, M.J.; Thomas, P.K.; Brown, M.B.; Canal, N.; Greene, D.A. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes care 1994, 17, 1281-1289. [CrossRef]
- National Commission of Sanitary Risks. [Comisión Federal de Riesgos Sanitarios]. Guidelines for pharmacovigilance in clinical research. [Guia de farmacovigilancia en investigación clínica]. https://www.gob.mx/cofepris/documentos/guias-lineamientos-y-requerimientos-de-farmacovigilancia. Accessed February, 2021.
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), v4.0. 2009. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf Accessed February, 2020.
- Saukkonen, J.J.; Cohn, D.L.; Jasmer, R.M.; Schenker, S.; Jereb, J.A.; Nolan, C.M.; Peloquin, C.A.; Gordin, F.M.; Nunes, D.; Strader, D.B.; et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med 2006, 174, 935-952. [CrossRef]
- Glasheen, W.P.; Renda, A.; Dong, Y. Diabetes Complications Severity Index (DCSI)-Update and ICD-10 translation. J Diabetes Complications 2017, 31, 1007-1013. [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005, 43, 1130-1139. [CrossRef]
- Deyo, R.; Cherkin, D.; Ciol, M. .Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992, 45, 613-619.
- Gordin, F.; Chaisson, R.E.; Matts, J.P.; Miller, C.; de Lourdes Garcia, M.; Hafner, R.; Valdespino, J.L.; Coberly, J.; Schechter, M.; Klukowicz, A.J.; et al. Rifampin and pyrazinamide vs isoniazid for prevention of tuberculosis in HIV-infected persons: an international randomized trial. Terry Beirn Community Programs for Clinical Research on AIDS, the Adult AIDS Clinical Trials Group, the Pan American Health Organization, and the Centers for Disease Control and Prevention Study Group. JAMA 2000, 283, 1445-1450. [CrossRef]
- Lesnichii, A.V.; Karpina, L.Z. Tuberculosis chemoprophylaxis for diabetics: are the benefits of isoniazid worth the risk. Probl Tuberk 1969, 47 1-3.
- Smith, B.M.; Schwartzman, K.; Bartlett, G.; Menzies, D. Adverse events associated with treatment of latent tuberculosis in the general population. CMAJ 2011, 183, E173-179. [CrossRef]
- Huang, H.L.; Huang, W.C.; Lin, K.D.; Liu, S.S.; Lee, M.R.; Cheng, M.H.; Chin, C.S.; Lu, P.L.; Sheu, C.C.; Wang, J.Y.; et al. Completion Rate and Safety of Programmatic Screening and Treatment for Latent Tuberculosis Infection in Elderly Patients with Poorly Controlled Diabetic Mellitus: A Prospective Multicenter Study. Clin Infect Dis 2021, Published Online First 3 March 2021. doi:2010.1093/cid/ciab2209. [CrossRef]
- Pease, C.; Hutton, B.; Yazdi, F.; Wolfe, D.; Hamel, C.; Barbeau, P.; Skidmore, B.; Alvarez, G.G. A systematic review of adverse events of rifapentine and isoniazid compared to other treatments for latent tuberculosis infection. Pharmacoepidemiol Drug Saf 2018, 27, 557-566. [CrossRef]
- Ostrosky-Zeichner, L.; Rangel-Frausto, M.S.; García-Romero, E.; Vázquez, A.; Ibarra, M.J.; Ponce de León-Rosales, S. [Tuberculosis in health personnel: importance of surveillance and control programs]. Salud Publica Mex 2000, 42, 48-52.
- Bourlon, C.; Camacho-Hernández, R.; Fierro-Angulo, O.M.; Acosta-Medina, A.A.; Bourlon, M.T.; Niembro-Ortega, M.D.; Gonzalez-Lara, M.F.; Sifuentes-Osornio, J.; Ponce-de-León, A. Latent Tuberculosis in Hematopoietic Stem Cell Transplantation: Diagnostic and Therapeutic Strategies to Prevent Disease Activation in an Endemic Population. Biol Blood Marrow Transplant 2020, 26, 1350-1354. [CrossRef]
- Daintith, H.; Stevenson, I.H.; O'Malley, K. Influence of diabetes mellitus on drug metabolism in man. Int J Clin Pharmacol Biopharm 1976, 13, 55-58.
- Ruslami, R.; Nijland, H.M.; Adhiarta, I.G.; Kariadi, S.H.; Alisjahbana, B.; Aarnoutse, R.E.; van Crevel, R. Pharmacokinetics of antituberculosis drugs in pulmonary tuberculosis patients with type 2 diabetes. Antimicrob Agents Chemother 2010, 54, 1068-1074. [CrossRef]
- Yang, Y.; Liu, X. Imbalance of Drug Transporter-CYP450s Interplay by Diabetes and Its Clinical Significance. Pharmaceutics 2020, 12. [CrossRef]
- Niazi, A.K.; Kalra, S. Diabetes and tuberculosis: a review of the role of optimal glycemic control. J Diabetes Metab Disord 2012, 11, 28. [CrossRef]
- Ena, J.; Valls, V. Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: a meta-analysis. Clin Infect Dis 2005, 40, 670-676. [CrossRef]
- LoBue, P.A.; Moser, K.S. Use of isoniazid for latent tuberculosis infection in a public health clinic. Am J Respir Crit Care Med 2003, 168, 443-447. [CrossRef]
- Menzies, D.; Long, R.; Trajman, A.; Dion, M.J.; Yang, J.; Al Jahdali, H.; Memish, Z.; Khan, K.; Gardam, M.; Hoeppner, V.; et al. Adverse events with 4 months of rifampin therapy or 9 months of isoniazid therapy for latent tuberculosis infection: a randomized trial. Ann Intern Med 2008, 149, 689-697. [CrossRef]
- Menzies, D.; Adjobimey, M.; Ruslami, R.; Trajman, A.; Sow, O.; Kim, H.; Obeng Baah, J.; Marks, G.B.; Long, R.; Hoeppner, V.; et al. Four Months of Rifampin or Nine Months of Isoniazid for Latent Tuberculosis in Adults. N Engl J Med 2018, 379, 440-453. [CrossRef]
- Shamah, T.; Cuevas, L.; Rivera, J.; Hernández, M. Encuesta Nacional de Salud y Nutrición 2016. Instituto Nacional de Salud Pública: Cuernavaca, México 2016.
- Rojas-Martinez, R.; Basto-Abreu, A.; Aguilar-Salinas, C.A.; Zarate-Rojas, E.; Villalpando, S.; Barrientos-Gutierrez, T. [Prevalence of previously diagnosed diabetes mellitus in Mexico.]. Salud Publica Mex 2018, 60, 224-232. [CrossRef]
- Fitzgerald, D.; Sterling, T.; Haas, D. Mycobacterium tuberculosis. In Mandell, Douglas, And Bennett’s Principles and Practice of Infectious Diseases, Ninth Edition, Mandell, J., Dolin, R., Blaser, M., Eds.; Elsevier, Inc: Philadelphia, 2020; Volume 2, pp. 2985-3021.
- Magro, L.; Moretti, U.; Leone, R. Epidemiology and characteristics of adverse drug reactions caused by drug-drug interactions. Expert Opin Drug Saf 2012, 11, 83-94. [CrossRef]
- Grosset, J.; Leventis, S. Adverse effects of rifampin. Rev Infect Dis 1983, 5 Suppl 3, S440-450. [CrossRef]
- Abulfathi, A.A.; Decloedt, E.H.; Svensson, E.M.; Diacon, A.H.; Donald, P.; Reuter, H. Clinical Pharmacokinetics and Pharmacodynamics of Rifampicin in Human Tuberculosis. Clin Pharmacokinet 2019, 58, 1103-1129. [CrossRef]
- Zawada, A.E.; Moszak, M.; Skrzypczak, D.; Grzymisławski, M. Gastrointestinal complications in patients with diabetes mellitus. Adv Clin Exp Med 2018, 27, 567-572. [CrossRef]
- Soldevila-Boixader, L.; Murillo, O.; Waibel, F.W.A.; Huber, T.; Schoni, M.; Lalji, R.; Uckay, I. The Epidemiology of Antibiotic-Related Adverse Events in the Treatment of Diabetic Foot Infections: A Narrative Review of the Literature. Antibiotics (Basel) 2023, 12. [CrossRef]
- Basto-Abreu, A.; Barrientos-Gutierrez, T.; Rojas-Martinez, R.; Aguilar-Salinas, C.A.; Lopez-Olmedo, N.; De la Cruz-Gongora, V.; Rivera-Dommarco, J.; Shamah-Levy, T.; Romero-Martinez, M.; Barquera, S.; et al. [Prevalence of diabetes and poor glycemic control in Mexico: results from Ensanut 2016.]. Salud Publica Mex 2020, 62, 50-59. [CrossRef]
- Borgharkar, S.S.; Das, S.S. Real-world evidence of glycemic control among patients with type 2 diabetes mellitus in India: the TIGHT study. BMJ Open Diabetes Res Care 2019, 7, e000654. [CrossRef]
- Ayele, B.H.; Roba, H.S.; Beyene, A.S.; Mengesha, M.M. Prevalent, uncontrolled, and undiagnosed diabetes mellitus among urban adults in Dire Dawa, Eastern Ethiopia: A population-based cross-sectional study. SAGE Open Med 2020, 8, 2050312120975235. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).