Submitted:
03 June 2023
Posted:
05 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemical Products
2.2. Preparation of Catalysts
2.3. Characterization Techniques
2.4. Catalytic Tests
3. Results and Discussion
3.1. Characterization
3.2. Catalytic Results and Discussion
4. Conclusion
Author Contributions
Conflicts of Interest
References
- Blay, V.; Epelde, E.; Miravalles, R.; Perea, L.A. Converting olefins to propene: Ethene to propene and olefin cracking. Catalysis Reviews 2018, 60, 278–335. [Google Scholar] [CrossRef]
- Cnudde, P.; De Wispelaere, K.; Vanduyfhuys, L.; Demuynck, R.; Van der Mynsbrugge, J.; Waroquier, M.; Van Speybroeck, V. How Chain Length and Branching Influence the Alkene Cracking Reactivity on H-ZSM-5. ACS Catalysis 2018, 8, 9579–9595. [Google Scholar] [CrossRef] [PubMed]
- Gholami, Z.; Gholami, F.; Tišler, Z.; Tomas, M.; Vakili, M. A Review on Production of Light Olefins via Fluid Catalytic Cracking. Energies 2021, 14. [Google Scholar] [CrossRef]
- Usman, A.; Siddiqui, M.A.B.; Hussain, A.; Aitani, A.; Al-Khattaf, S. Catalytic cracking of crude oil to light olefins and naphtha: Experimental and kinetic modeling. Chemical Engineering Research and Design 2017, 120, 121–137. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, J.; Putaj, P.; Caps, V.; Lefebvre, F.; Pelletier, J.; Basset, J.-M. Catalytic Oxidation of Light Alkanes (C1–C4) by Heteropoly Compounds. Chemical Reviews 2014, 114, 981–1019. [Google Scholar] [CrossRef] [PubMed]
- Owusu, P.A.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Engineering 2016, 3, 1167990. [Google Scholar] [CrossRef]
- Wu, X.; Tian, Z.; Guo, J. A review of the theoretical research and practical progress of carbon neutrality. Sustainable Operations and Computers 2022, 3, 54–66. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chemical Reviews 2014, 114, 10613–10653. [Google Scholar] [CrossRef]
- Monai, M.; Gambino, M.; Wannakao, S.; Weckhuysen, B.M.J.C.S.R. Propane to olefins tandem catalysis: a selective route towards light olefins production. 2021, 50, 11503–11529. [Google Scholar] [CrossRef]
- Melnikov, D.P.; Novikov, A.A.; Glotov, A.P.; Reshetina, M.V.; Smirnova, E.M.; Wang, H.Q.; Vinokurov, V.A. Dehydrogenation of Light Alkanes (A Review). Petroleum Chemistry 2022, 62, 1027–1046. [Google Scholar] [CrossRef]
- Ai-Zegbayer, Y.S.; Ai- Mayman, S.I.; Ai-Smarei, T.A. Oxidative Dehydrogenation of Ethane to Ethylene Over Mo-V-Nb Catalysts: Effect of Calcination Temperature and Type of Support. Journal of King Saud University - Engineering Sciences 2010, 22, 21–27. [Google Scholar] [CrossRef]
- Brik, Y.; Kacimi, M.; Ziyad, M.; Bozon-Verduraz, F. Titania-Supported Cobalt and Cobalt–Phosphorus Catalysts: Characterization and Performances in Ethane Oxidative Dehydrogenation. Journal of Catalysis 2001, 202, 118–128. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, J.; Chai, R.; Zhao, G.; Liu, Y.; Lu, Y. Oxidative dehydrogenation of ethane to ethylene: A promising CeO2-ZrO2-modified NiO-Al2O3/Ni-foam catalyst. Applied Catalysis A: General 2018, 550, 151–159. [Google Scholar] [CrossRef]
- Loukah, M.; Coudurier, G.; Vedrine, J.C.; Ziyad, M. Oxidative dehydrogenation of ethane on V- and Cr-based phosphate catalysts. Microporous Materials 1995, 4, 345–358. [Google Scholar] [CrossRef]
- El-Drissi, J.; Kacimi, M.; Loukah, M.; Ziyad, M.J.J.C.P. Activité de V2O5/TiO2 modifié par le phosphore dans la réaction de déshydrogénation oxydante de l’éthane en éthylène. 1997, 94, 1984-1992.
- El-Idrissi, J.; Kacimi, M.; Bozon-Verduraz, F.; Ziyad, M. Oxidative dehydrogenation of ethane over Cr/TiO2 modified by phosphorus. Catalysis Letters 1998, 56, 221–225. [Google Scholar] [CrossRef]
- Benzaouak, A.; Mahir, H.; El Hamidi, A.; Kacimi, M.; Liotta, L.F. Investigation of Phosphorus Loaded V2O5/ZrO2 Catalysts for the Oxidative Dehydrogenation of Propane (ODH). Catalysts 2022, 12. [Google Scholar] [CrossRef]
- Munirathinam, R.; Pham Minh, D.; Nzihou, A. Effect of the Support and Its Surface Modifications in Cobalt-Based Fischer–Tropsch Synthesis. Industrial & Engineering Chemistry Research 2018, 57, 16137–16161. [Google Scholar] [CrossRef]
- Lögdberg, S.; Yang, J.; Lualdi, M.; Walmsley, J.C.; Järås, S.; Boutonnet, M.; Blekkan, E.A.; Rytter, E.; Holmen, A. Further insights into methane and higher hydrocarbons formation over cobalt-based catalysts with γ-Al2O3, α-Al2O3 and TiO2 as support materials. Journal of Catalysis 2017, 352, 515–531. [Google Scholar] [CrossRef]
- Dreyer, M.; Krebs, M.; Najafishirtari, S.; Rabe, A.; Friedel Ortega, K.; Behrens, M. The Effect of Co Incorporation on the CO Oxidation Activity of LaFe1−xCoxO3 Perovskites. Catalysts 2021, 11. [Google Scholar] [CrossRef]
- Dreyer, M.; Rabe, A.; Budiyanto, E.; Friedel Ortega, K.; Najafishirtari, S.; Tüysüz, H.; Behrens, M. Dynamics of Reactive Oxygen Species on Cobalt-Containing Spinel Oxides in Cyclic CO Oxidation. Catalysts 2021, 11. [Google Scholar] [CrossRef]
- Aaddane, A.; Kacimi, M.; Ziyad, M. Oxidative dehydrogenation of ethane and propane over magnesium-cobalt phosphates CoxMg3-x(PO4)2. Catalysis Letters 2001, 73, 47–53. [Google Scholar] [CrossRef]
- Aaddane, A.; Kacimi, M.; Ziyad, M. Oxidative dehydrogenation of ethane and propane over Ca-Co-P catalysts. In Proceedings of the Journal De Physique. IV : JP; 2004; pp. 151–154. [Google Scholar]
- Concepción, P.; Blasco, T.; López Nieto, J.M.; Vidal-Moya, A.; Martínez-Arias, A. Preparation, characterization and reactivity of V- and/or Co-containing AlPO-18 materials (VCoAPO-18) in the oxidative dehydrogenation of ethane. Microporous and Mesoporous Materials 2004, 67, 215–227. [Google Scholar] [CrossRef]
- Čapek, L.; Vaněk, L.; Adam, J.; Smoláková, L. Dehydrogenation of ethane over vanadium, cobalt and nickel based catalysts. Studies in Surface Science and Catalysis 2008, 174, 1175–1178. [Google Scholar] [CrossRef]
- Alifanti, M.; Bueno, G.; Parvulescu, V.; Parvulescu, V.I.; Cortés Corberán, V. Oxidation of ethane on high specific surface SmCoO3 and PrCoO3 perovskites. Catalysis Today 2009, 143, 309–314. [Google Scholar] [CrossRef]
- Tian, X.; Zheng, C.; Li, F.; Zhao, H. Co and Mo Co-doped Fe2O3for Selective Ethylene Production via Chemical Looping Oxidative Dehydrogenation. ACS Sustainable Chemistry and Engineering 2021, 9, 8002–8011. [Google Scholar] [CrossRef]
- Xiao, F.; Guo, D.; Zhao, F.; Zhao, Y.; Wang, S.; Ma, X. Catalytic oxidative dehydrogenation of ethane using carbon dioxide as a soft oxidant over Co-HMS catalysts to ethylene. Asia-Pacific Journal of Chemical Engineering 2022, 17. [Google Scholar] [CrossRef]
- Povari, S.; Alam, S.; Somannagari, S.; Nakka, L.; Chenna, S. Oxidative Dehydrogenation of Ethane with CO2 over the Fe-Co/Al2O3 Catalyst: Experimental Data Assisted AI Models for Prediction of Ethylene Yield. Industrial and Engineering Chemistry Research 2023, 62, 2573–2582. [Google Scholar] [CrossRef]
- Koirala, R.; Safonova, O.V.; Pratsinis, S.E.; Baiker, A. Effect of cobalt loading on structure and catalytic behavior of CoOx/SiO2 in CO2-assisted dehydrogenation of ethane. Applied Catalysis A: General 2018, 552, 77–85. [Google Scholar] [CrossRef]
- Brik, Y.; Kacimi, M.; Bozon-Verduraz, F.; Ziyad, M. Characterization and Comparison of the Activity of Boron-Modified Co/TiO2 Catalysts in Butan-2-ol Conversion and Oxidative Dehydrogenation of Ethane. Journal of Catalysis 2002, 211, 470–481. [Google Scholar] [CrossRef]
- Advanced materials in catalysis / edited by James, J. Burton and Robert L. Garten; Academic Press: New York, 1977. [Google Scholar]
- Blasse, G. Optical electron transfer between metal ions and its consequences. In Proceedings of the Complex Chemistry, Berlin, Heidelberg, 1991; 1991//; pp. 153–187. [Google Scholar]
- Verberckmoes, A.A.; Weckhuysen, B.M.; Schoonheydt, R.A. Spectroscopy and coordination chemistry of cobalt in molecular sieves1Dedicated to Professor Lovat V.C. Rees in recognition and appreciation of his lifelong devotion to zeolite science and his outstanding achievements in this field.1. Microporous and Mesoporous Materials 1998, 22, 165–178. [Google Scholar] [CrossRef]
- Farhadi, S.; Javanmard, M.; Nadri, G. Characterization of Cobalt Oxide Nanoparticles Prepared by the Thermal Decomposition. Acta Chimica Slovenica 2016, 63. [Google Scholar] [CrossRef]
- Vennari, C.E.; Williams, Q. A high-pressure Raman study of FeTiO3 ilmenite: Fermi resonance as a manifestation of Fe-Ti charge transfer. Physics and Chemistry of Minerals 2021, 48, 34. [Google Scholar] [CrossRef]
- Peng, W.; Ziqi, W.; Zhongqing, Y.; Zhilei, L.; Jingyu, R.; Mingnv, G. Selective catalytic and kinetic studies on oxydehydrogenation of ethane with CO2 over lanthanide metal catalysts. Comptes Rendus. Chimie 2020, 23, 33–46. [Google Scholar] [CrossRef]
- Zeng, T.; Sun, G.; Miao, C.; Yan, G.; Ye, Y.; Yang, W.; Sautet, P. Stabilizing Oxidative Dehydrogenation Active Sites at High Temperature with Steam: ZnFe2O4-Catalyzed Oxidative Dehydrogenation of 1-Butene to 1,3-Butadiene. ACS Catalysis 2020, 10, 12888–12897. [Google Scholar] [CrossRef]
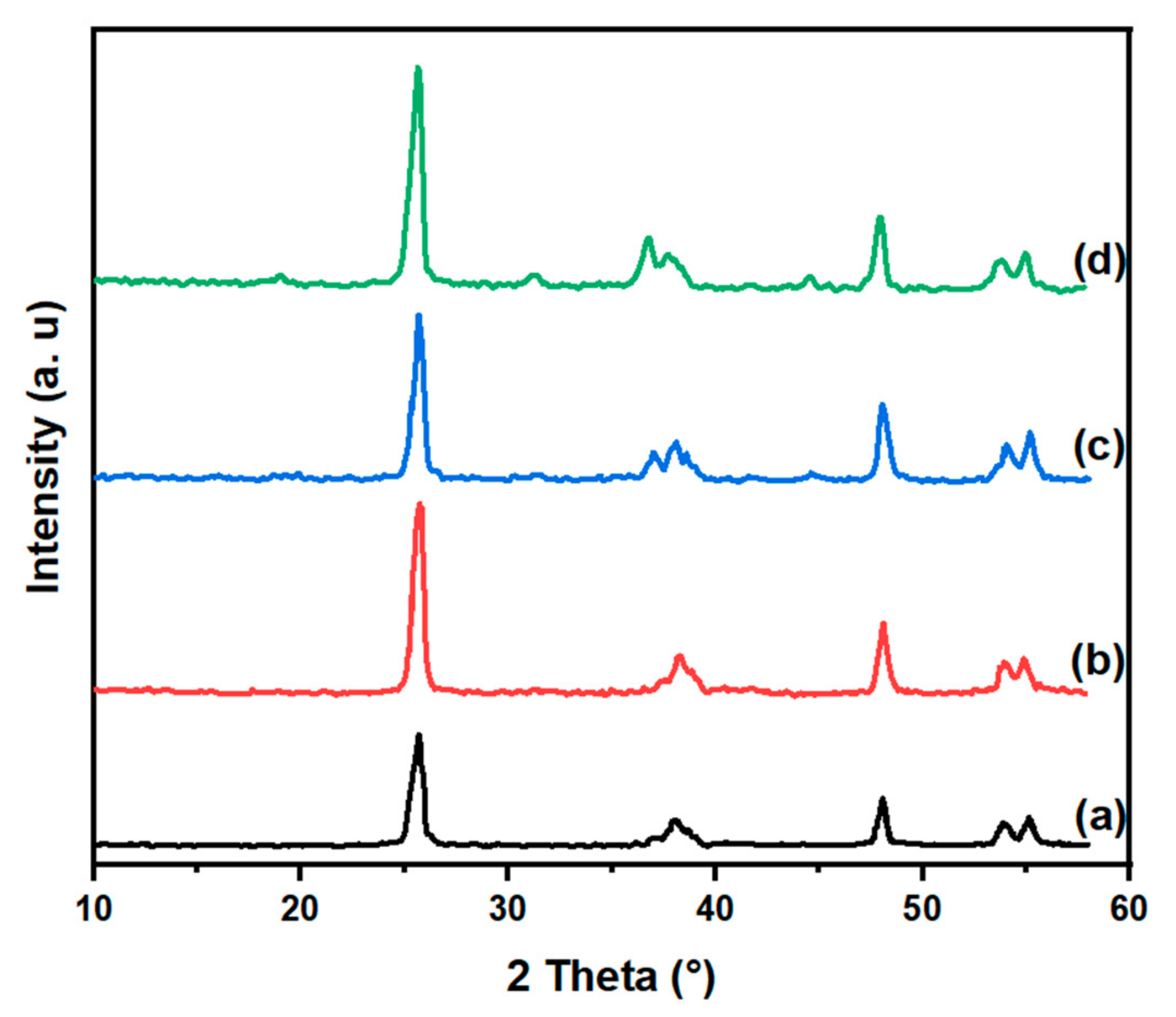


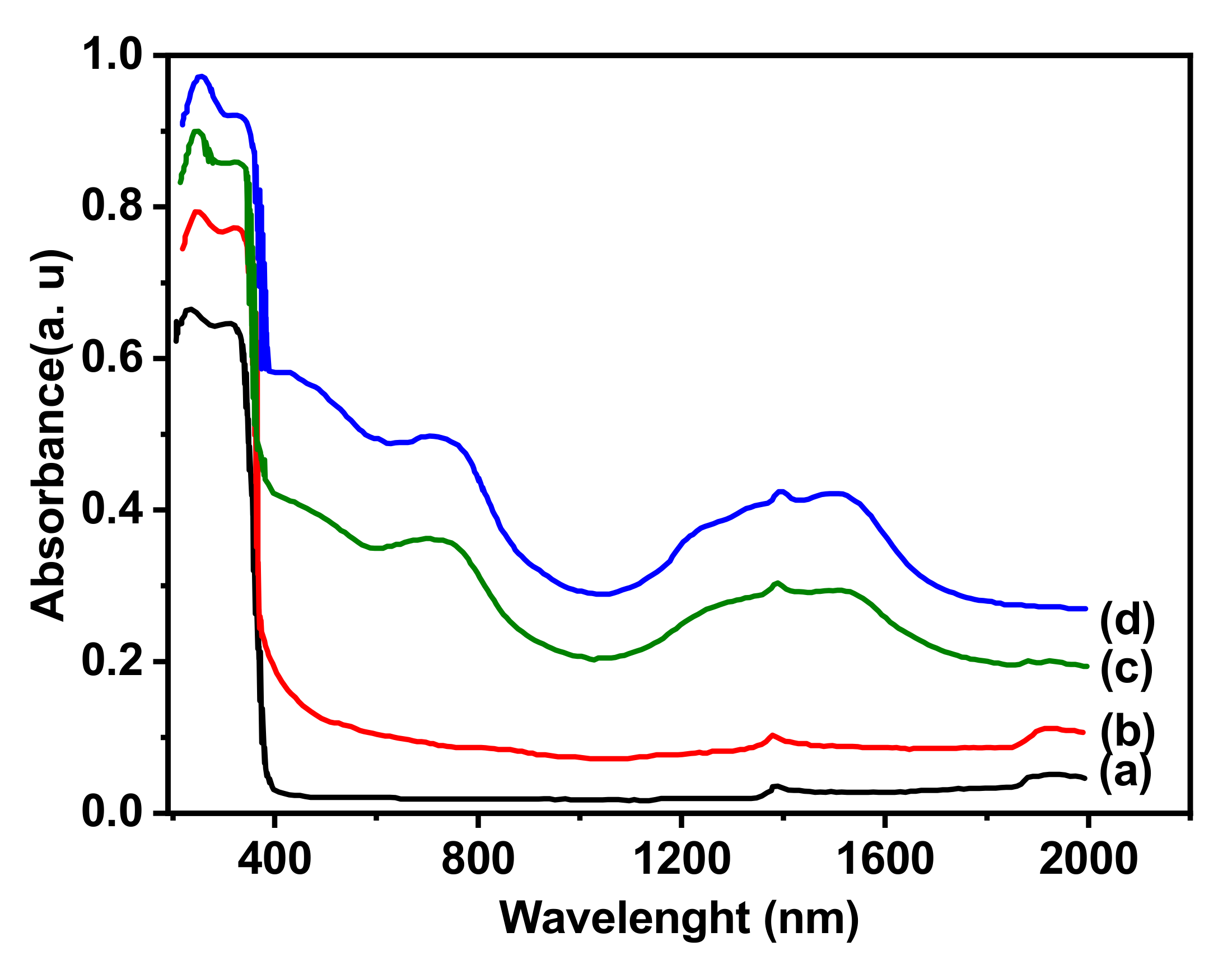
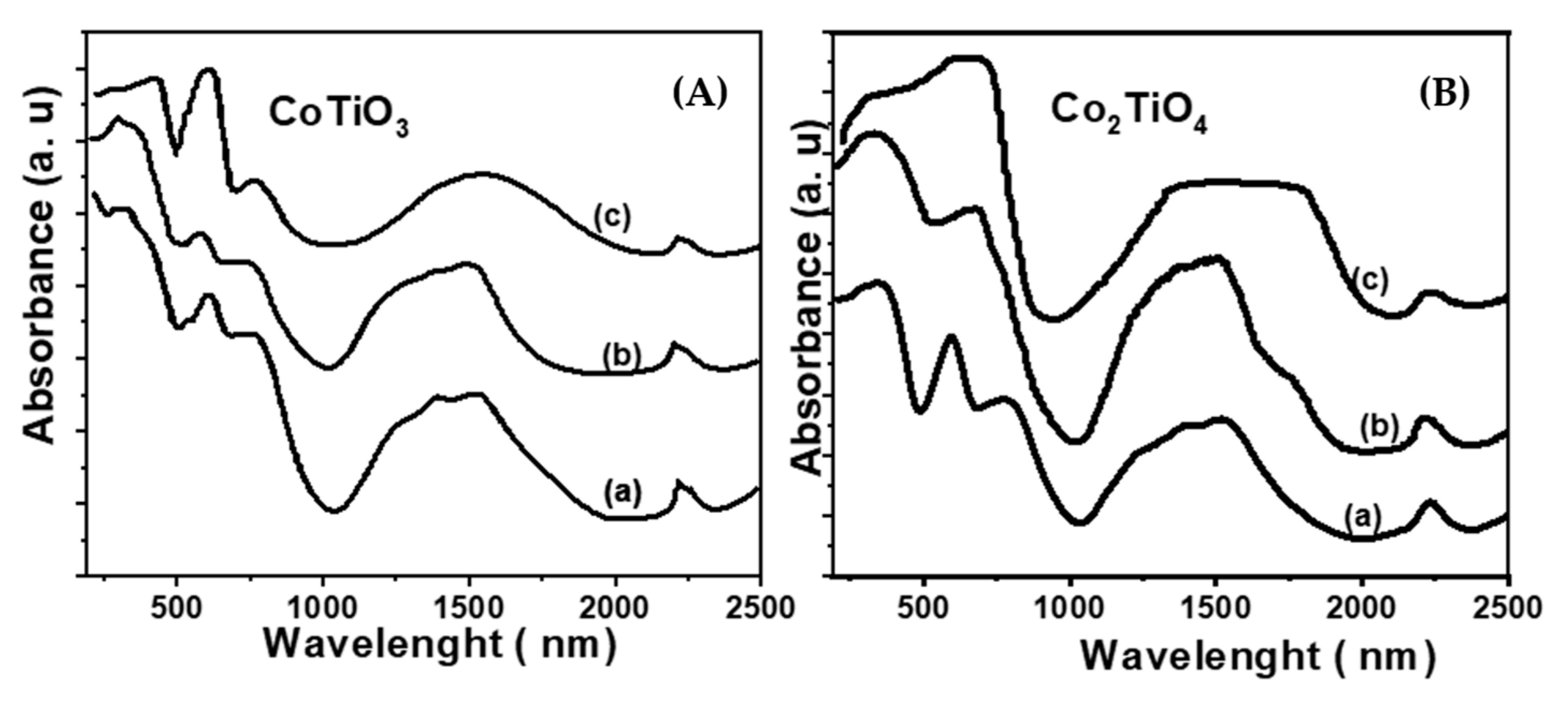
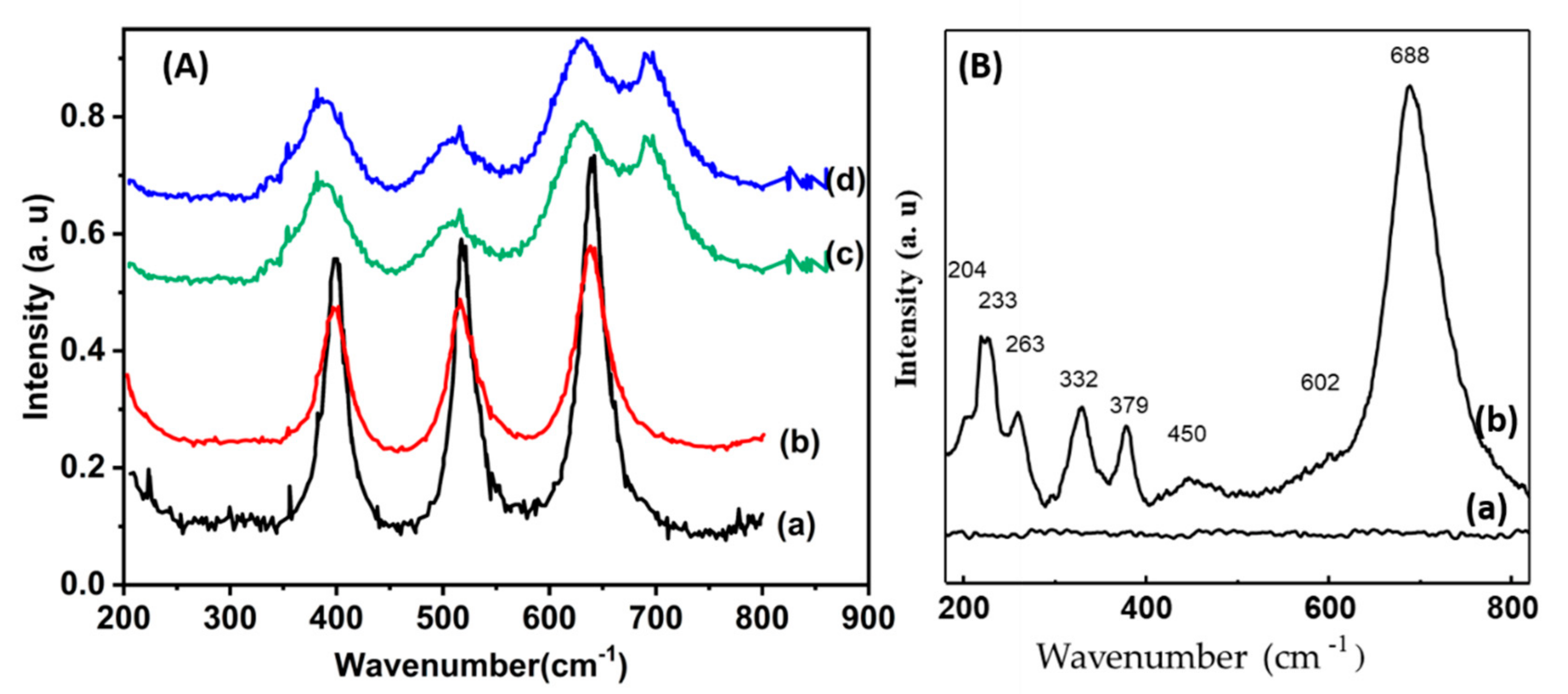
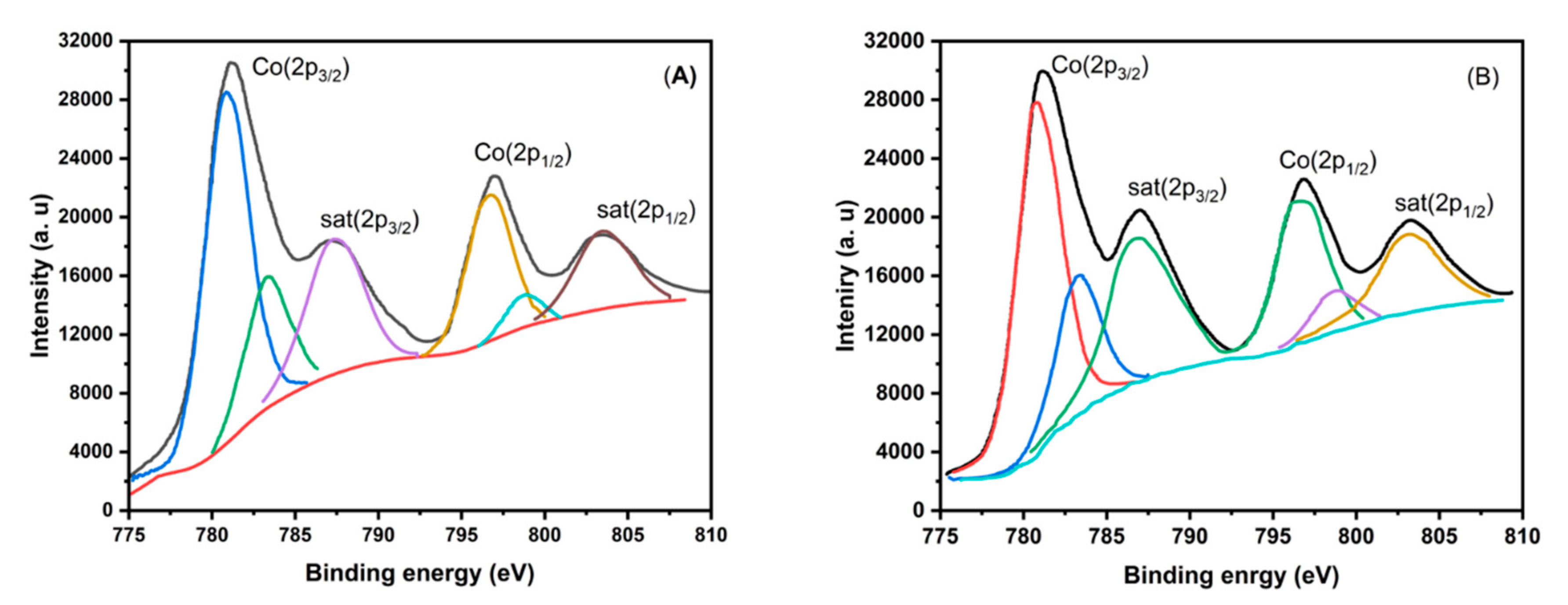
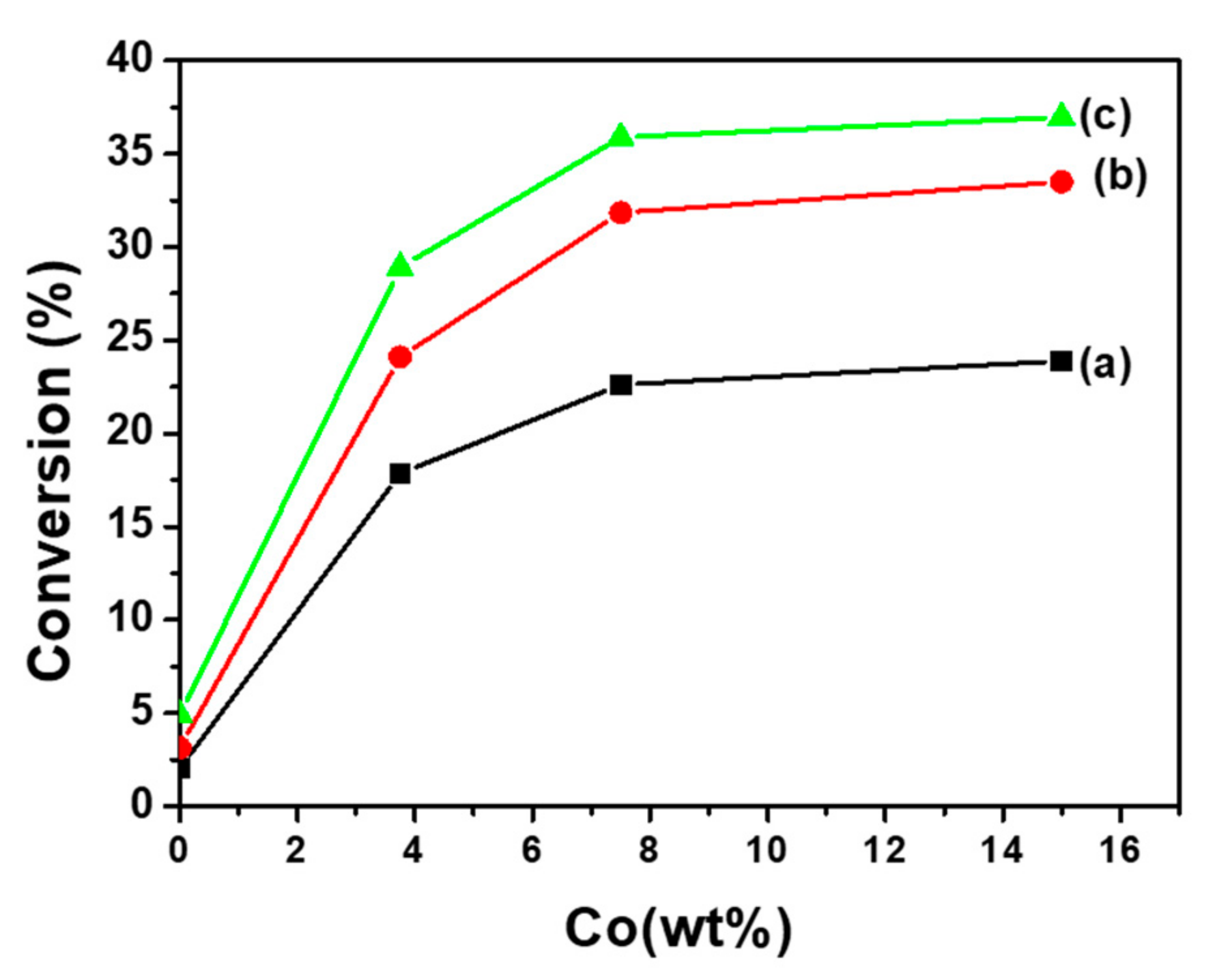
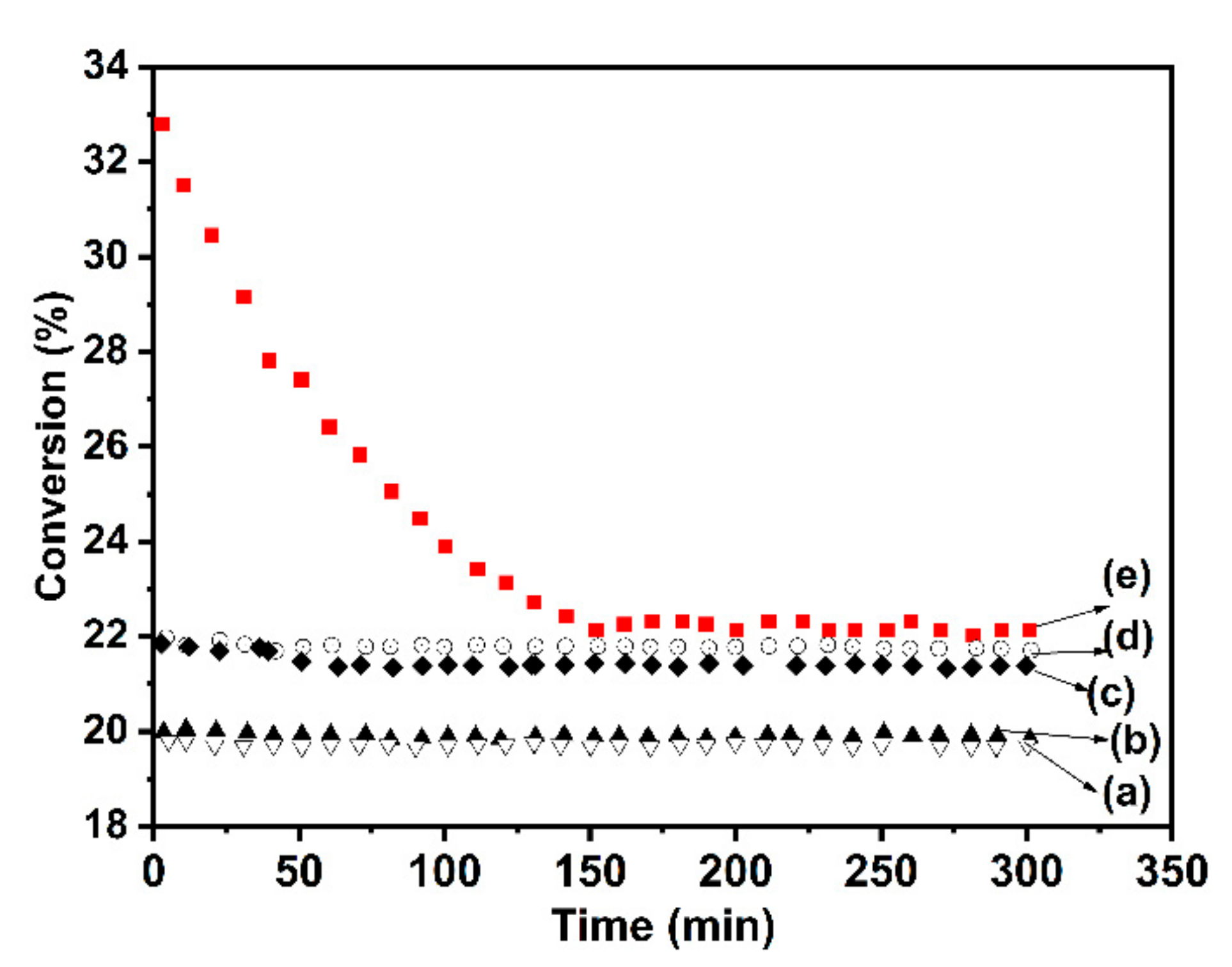
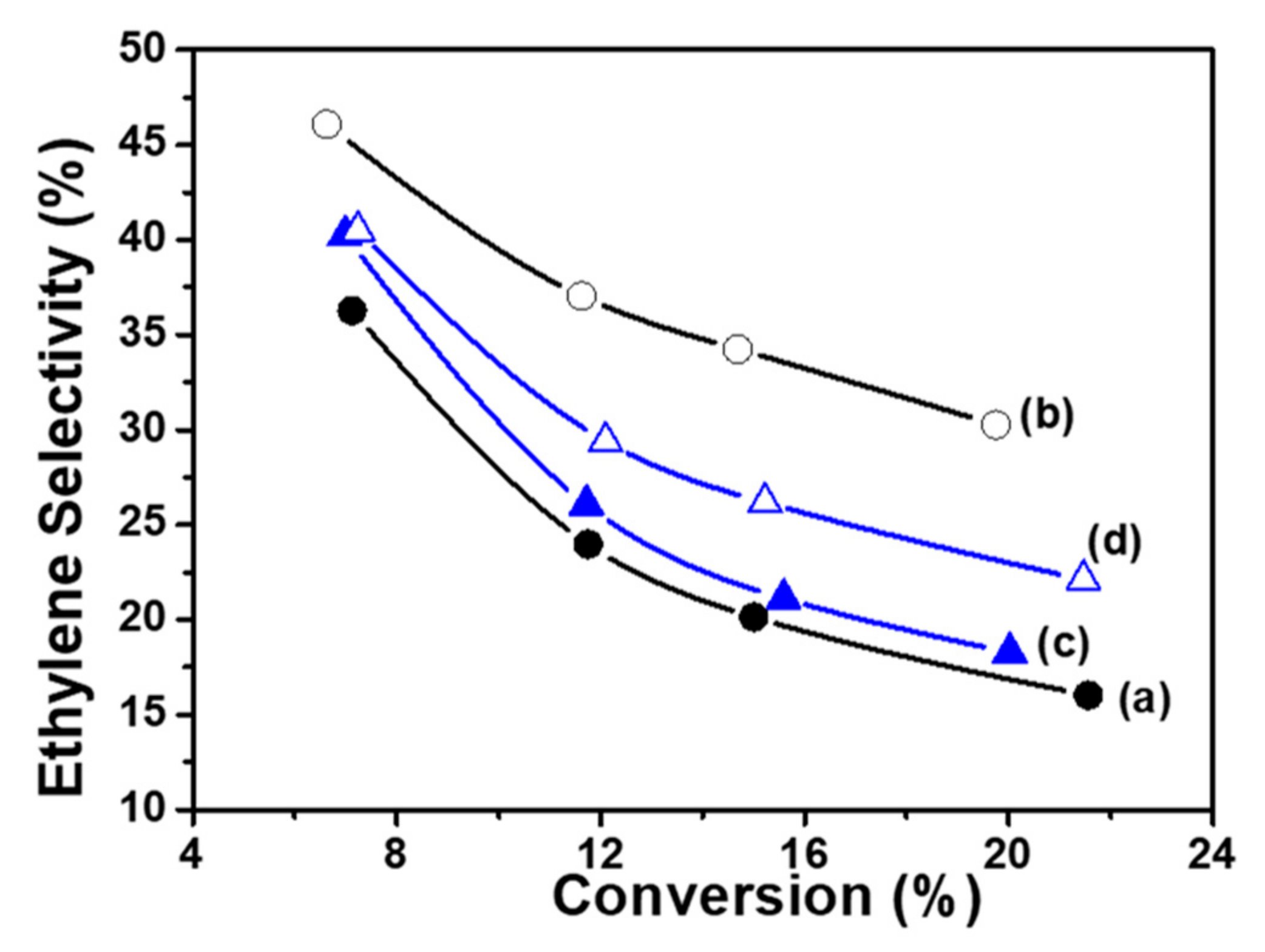
| Samples | Co(3.8)/TiO2 | Co(7.5)/TiO2 | Co(15)/TiO2 | CoTiO3 | Co2TiO4 |
| Surface area BET(m2/g) | 65 | 60 | 56 | a 46 | a 45 |
| - | - | - | b 9.4 | b10 |
| samples | Co2p3/2 | ΔEc | |
| B.E. S/M* ratio | |||
| Co(3.8)/TiO2 | 781.0 | 0.42 | 15.4 |
| Co(7.5)/TiO2 | 780.2 | 0.27 | 15.2 |
| Co(15)/TiO2 | 779.9 | 0.19 | 15.1 |
| Co2TiO4 | 781 | 0.40 | 15.8 |
| CoTiO3 | 781.2 | 0.47 | 15.9 |
| Co3O4 | 779.9 | 0.18 | 15.1 |
| CoO | 780.4 | 0.4 | 16.0 |
| Samples calcined at 550°C | ||||||||
| Reaction Temperature | CoTiO3 | Co2TiO4 | ||||||
| αg | αene | Sene | SCOx | αg | αene | Sene | SCOx | |
| 450°C | 12.9 | 4.1 | 32 | 72 | 13.8 | 3.8 | 28 | 72 |
| 500°C | 16.8 | 5.2 | 31 | 69 | 16.8 | 4.5 | 27 | 69 |
| 550°C | 19.9 | 6 | 30 | 70 | 21.4 | 4.6 | 21.5 | 78.5 |
| Activation Energy (kJ.mol-1) | 21.6 | 21.6 | ||||||
| Samples calcined at 1150°C | ||||||||
| Reaction Temperature | CoTiO3 | Co2TiO4 | ||||||
| αg | αene | Sene | SCOx | αg | αene | Sene | SCOx | |
| 450°C | 13.1 | 2.5 | 19 | 71 | 13.8 | 2.6 | 19 | 81 |
| 500°C | 17 | 3 | 18 | 72 | 17.7 | 3.3 | 19 | 81 |
| 550°C | 21.6 | 3.8 | 16 | 84 | 19.7 | 3.8 | 18 | 82 |
| Activation Energy (kJ.mol-1) | 24.5 | 24.4 | ||||||
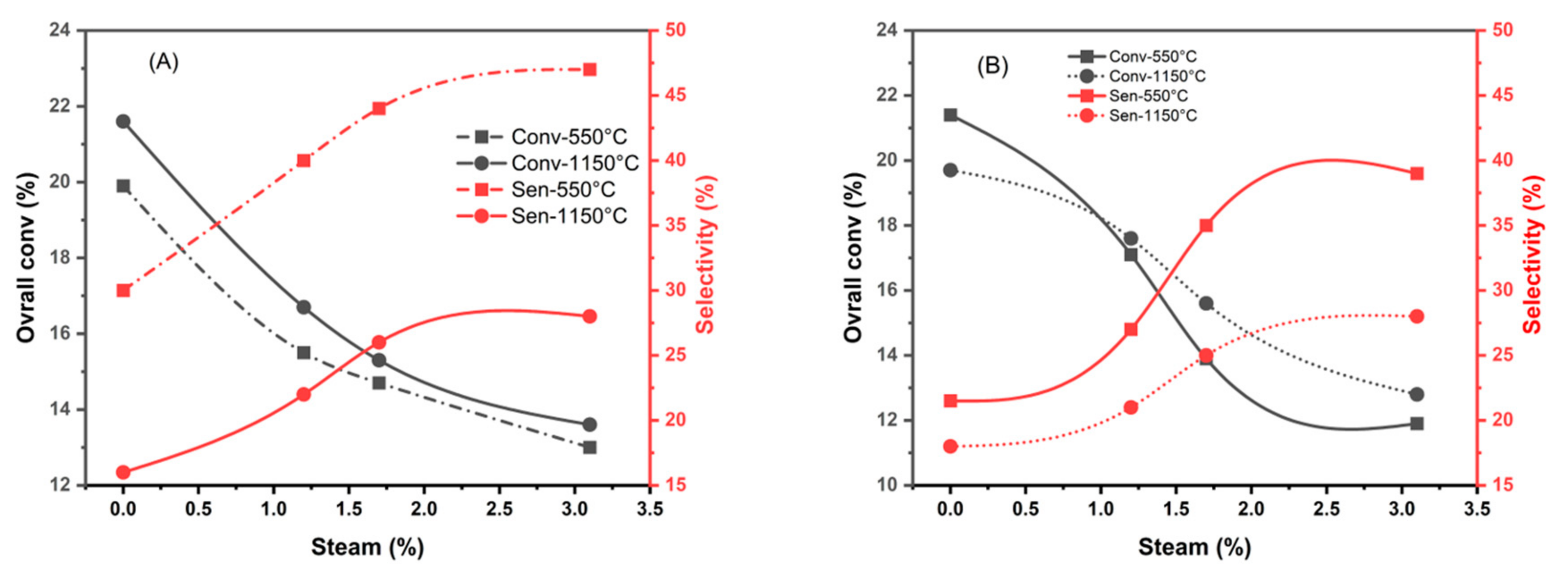
| Samples calcined at 550°C | |||||||||
| %H2O | CoTiO3 | Co2TiO4 | |||||||
| αg | αene | Sene | SCOx | αg | αene | Sene | SCOx | ||
| Dry mixture | 19.9 | 6 | 30 | 70 | 21.4 | 4.6 | 21.5 | 78.5 | |
| 1.2% | 15.5 | 6.2 | 40 | 60 | 17.1 | 4.7 | 27 | 73 | |
| 1.7% | 14.7 | 6.5 | 44 | 56 | 13.9 | 4.9 | 35 | 65 | |
| 3.1% | 13 | 6.1 | 47 | 53 | 11.9 | 4.6 | 39 | 61 | |
| Samples calcined at 1150°C | |||||||||
| %H2O | CoTiO3 | Co2TiO4 | |||||||
| αg | αene | Sene | SCOx | αg | αene | Sene | SCOx | ||
| Dry mixture | 21.6 | 3.8 | 16 | 84 | 19.7 | 3.8 | 18 | 82 | |
| 1.2% | 16.7 | 3.7 | 22 | 78 | 17.6 | 3.7 | 21 | 79 | |
| 1.7% | 15.3 | 4 | 26 | 74 | 15.6 | 3.9 | 25 | 75 | |
| 3.1% | 13.6 | 3.8 | 28 | 72 | 12.8 | 3.6 | 28 | 72 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





