Submitted:
30 May 2023
Posted:
01 June 2023
You are already at the latest version
Abstract
Keywords:
Developing of sensitization
Techniques to identify sensitization level and to stratify the risk
- (a)
- If the patient has no DSA and no cellular memory, the transplant is possible with low risk for AMR;
- (b)
- If at the time of transplantation, there is absence of DSA, but there is a potential cellular memory against donor HLA, the transplant is possible with risk for AMR increased. The cellular memory is possible if there are historical DSA and/or pregnancy or previous transplant with repeat antigens. Other possibilities are transfusions with no information on blood donors.
- (c)
- If at the time of transplantation there are DSA, but with negative flow, the transplant is possible with risk for acute AMR and acceptable medium-term graft survival.
- (d)
- If at the time of transplantation there are DSA with positive flow and negative CDC, the transplant is possible, but there is a very high risk for acute AMR and accelerated chronic AMR.
- (e)
- If at the time of transplantation there are DSA with positive CDC, the transplant is not possible and there is the need of desensitization before proceeding with transplant.
Incidence of hyper immune patients and graft survival with desensitization
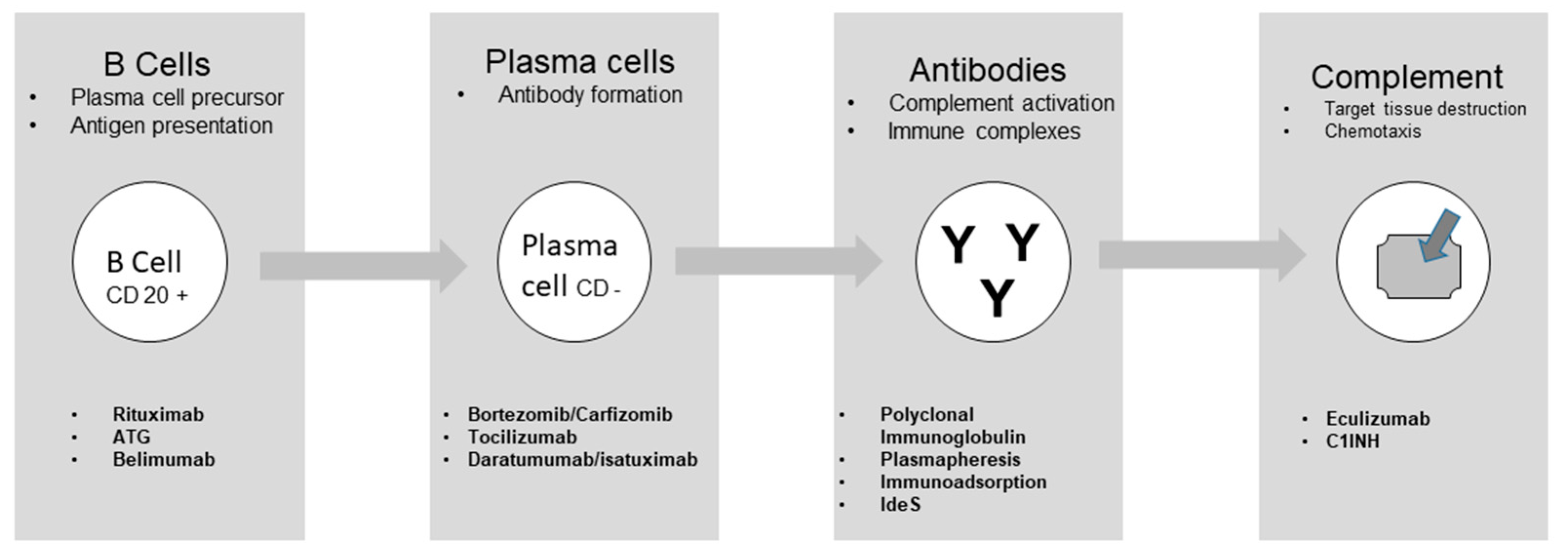
Desensitization strategies and drugs
Drugs acting on B cells
Drugs acting on Plasma cells
Drugs acting on antibodies
Drugs acting on complement
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salvadori M. Strategies for access to kidney transplantation for highly sensitized and incompatible patients Transplantology 2023; 4:85-89.
- Luque S, Lúcia M, Bestard O. Refinement of humoral immune monitoring in kidney transplantation: the role of “hidden” alloreactive memory B cells. Transpl Int. 2017 ; 30 : 955-968. [CrossRef]
- orija A, Favà A, Meneghini M, Crespo E, Bestard O. Novel insights into the pathobiology of humoral alloimmune memory in kidney transplantation. Curr Opin Organ Transplant. 2020; 25 :15-21. [CrossRef]
- Chong AS. New insights into the development of B cell responses: Implications for solid organ transplantation. Hum Immunol. 2019; 80 :378-384. /: :378-384 ttps.
- Cano-Romero FL, Laguna Goya R, Utrero-Rico A, Gómez-Massa E, Arroyo-Sánchez D, Suárez-Fernández P, Lora D, Andrés A, Castro-Panete MJ, Paz-Artal E. Longitudinal profile of circulating T follicular helper lymphocytes parallels anti-HLA sensitization in renal transplant recipients. Am J Transplant. 2019; 19 :89-97. [CrossRef]
- Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dörner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006; 6 :741-750.
- Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997; 388 :133-134.
- Muehlinghaus G, Cigliano L, Huehn S, Peddinghaus A, Leyendeckers H, Hauser AE, Hiepe F, Radbruch A, Arce S, Manz RA. Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood. 2005;105 :3965-3971. [CrossRef]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998; 392 : 565-568. [CrossRef]
- Bestard O, Couzi L, Crespo M, Kessaris N, Thaunat O. Stratifying the humoral risk of candidates to a solid organ transplantation: a proposal of the ENGAGE working group. Transpl Int. 2021; 34 :1005-1018. [CrossRef]
- Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969 ; 280 :735-739. [CrossRef]
- Bray RA, Tarsitani C, Gebel HM, Lee JH. Clinical cytometry and progress in HLA antibody detection. Methods Cell Biol. 2011; 103: 285-310. [CrossRef]
- Schlaf G, Pollok-Kopp B, Manzke T, Schurat O, Altermann W. Novel solid phase-based ELISA assays contribute to an improved detection of anti-HLA antibodies and to an increased reliability of pre- and post-transplant crossmatching. NDT Plus. 2010; 3 :527-538.
- Tait BD. Detection of HLA Antibodies in Organ Transplant Recipients - Triumphs and Challenges of the Solid Phase Bead Assay. Front Immunol. 2016; 7:570. [CrossRef]
- Zachary AA, Kopchaliiska D, Montgomery RA, Leffell MS. HLA-specific B cells: I. A method for their detection, quantification, and isolation using HLA tetramers. Transplantation. 2007; 83 :982-988. [CrossRef]
- Lúcia M, Luque S, Crespo E, Melilli E, Cruzado JM, Martorell J, Jarque M, Gil-Vernet S, Manonelles A, Grinyó JM, Bestard O. Preformed circulating HLA-specific memory B cells predict high risk of humoral rejection in kidney transplantation. Kidney Int. 2015; 88 :874-887.
- Karahan GE, Krop J, Wehmeier C, de Vaal YJH, Langerak-Langerak J, Roelen DL, Lardy NM, Bemelman FJ, Ten Berge IJM, Reinders MEJ, van Kooten C, Claas FHJ, Heidt S. An Easy and Sensitive Method to Profile the Antibody Specificities of HLA-specific Memory B Cells. Transplantation. 2019; 103 : 716-723. [CrossRef]
- Dahdal S, Saison C, Valette M, Bachy E, Pallet N, Lina B, Koenig A, Monneret G, Defrance T, Morelon E, Thaunat O. Residual Activatability of Circulating Tfh17 Predicts Humoral Response to Thymodependent Antigens in Patients on Therapeutic Immunosuppression. Front Immunol. 2019; 9 :3178. [CrossRef]
- Gobierno de España, Ministerio de Sanidad Organizatión National de Transplantes Informe 2020. [CrossRef]
- Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, Warren DS, Simpkins CE, Dagher NN, Singer AL, Zachary AA, Segev DL. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011; 365 :318-326.
- Orandi BJ, Garonzik-Wang JM, Massie AB, Zachary AA, Montgomery JR, Van Arendonk KJ, Stegall MD, Jordan SC, Oberholzer J, Dunn TB, Ratner LE, Kapur S, Pelletier RP, Roberts JP, Melcher ML, Singh P, Sudan DL, Posner MP, El-Amm JM, Shapiro R, Cooper M, Lipkowitz GS, Rees MA, Marsh CL, Sankari BR, Gerber DA, Nelson PW, Wellen J, Bozorgzadeh A, Gaber AO, Montgomery RA, Segev DL. Quantifying the risk of incompatible kidney transplantation: a multicenter study. Am J Transplant. 2014; 14 :1573-1580. [CrossRef]
- Manook M, Koeser L, Ahmed Z, Robb M, Johnson R, Shaw O, Kessaris N, Dorling A, Mamode N. Post-listing survival for highly sensitised patients on the UK kidney transplant waiting list: a matched cohort analysis. Lancet. 2017 ; 389 :727-734.
- Schwaiger E, Eskandary F, Kozakowski N, Bond G, Kikić Ž, Yoo D, Rasoul-Rockenschaub S, Oberbauer R, Böhmig GA. Deceased donor kidney transplantation across donor-specific antibody barriers: predictors of antibody-mediated rejection. Nephrol Dial Transplant. 2016; 31 :1342-1351. [CrossRef]
- Amrouche L, Aubert O, Suberbielle C, Rabant M, Van Huyen JD, Martinez F, Sberro-Soussan R, Scemla A, Tinel C, Snanoudj R, Zuber J, Cavalcanti R, Timsit MO, Lamhaut L, Anglicheau D, Loupy A, Legendre C. Long-term Outcomes of Kidney Transplantation in Patients With High Levels of Preformed DSA: The Necker High-Risk Transplant Program. Transplantation. 2017; 101: 2440-2448. 2448. [CrossRef]
- Lorenz M, Regele H, Schillinger M, Kletzmayr J, Haidbauer B, Derfler K, Druml W, Böhmig GA. Peritransplant immunoadsorption: a strategy enabling transplantation in highly sensitized crossmatch-positive cadaveric kidney allograft recipients. Transplantation. 2005; 79 :696-701.
- Morath C, Beimler J, Opelz G, Scherer S, Schmidt J, Macher-Goeppinger S, Klein K, Sommerer C, Schwenger V, Zeier M, Süsal C. Living donor kidney transplantation in crossmatch-positive patients enabled by peritransplant immunoadsorption and anti-CD20 therapy. Transpl Int. 2012; 25: 506-517. [CrossRef]
- Fehr T, Gaspert A. Antibody-mediated kidney allograft rejection: therapeutic options and their experimental rationale. Transpl Int. 2012; 25 :623-632. [CrossRef]
- Jordan SC, Ammerman N, Choi J, Huang E, Peng A, Sethi S, Najjar R, Toyoda M, Lim K, Louie S, Vo A. Novel Therapeutic Approaches to Allosensitization and Antibody-mediated Rejection. Transplantation. 2019; 103 :262-272.
- Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, Peng A, Villicana R, Jordan SC. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008; 359 :242-251. [CrossRef]
- Ramos EJ, Pollinger HS, Stegall MD, Gloor JM, Dogan A, Grande JP. The effect of desensitization protocols on human splenic B-cell populations in vivo. Am J Transplant. 2007; 7 :402-407. [CrossRef]
- Dhilleswara RV, Belimumab: therapeutic mechanism and current status of clinical trials Biomed Res 2018; 29: 3034-3039. 3: RV, Belimumab: therapeutic mechanism and current status of clinical trials Biomed Res 2018; 29. [CrossRef]
- Treml JF, Hao Y, Stadanlick JE, Cancro MP. The BLyS family: toward a molecular understanding of B cell homeostasis. Cell Biochem Biophys. 2009; 53 :1-16.
- Mackay F, Schneider P. Cracking the BAFF code Nat Rev Immunol. 2009 ; 9 :491-502. [CrossRef]
- Dubey AK, Handu SS, Dubey S, Sharma P, Sharma KK, Ahmed QM. Belimumab: First targeted biological treatment for systemic lupus erythematosus. J Pharmacol Pharmacother. 2011; 2 :317-319.
- NCT01025193 Clinical trial.gov Naji A University of Pennsylvania Desensitization With Belimumab in Sensitized Patients Awaiting Kidney Transplant. Accessed May 19, 2023. [CrossRef]
- NCT01536379 Clinical trial.gov GSK Investigational Site Cambridge UK A Study of Belimumab in the Prevention of Kidney Transplant Rejection. Accessed May 19, 2023.
- Banham GD, Flint SM, Torpey N, Lyons PA, Shanahan DN, Gibson A, Watson CJE, O’Sullivan AM, Chadwick JA, Foster KE, Jones RB, Devey LR, Richards A, Erwig LP, Savage CO, Smith KGC, Henderson RB, Clatworthy MR. Belimumab in kidney transplantation: an experimental medicine, randomised, placebo-controlled phase 2 trial. Lancet. 2018 Jun 30;391(10140):2619-2630.
- Ravetch jv, Bolland S IgG Fc receptors Ann Rev Immunol 2001; 19: 275-290.
- Pearse RN SHIP recruitment attenuates FcγIIB-induced apoptosis. Immunity 1999; 10: 753-760.
- Mackay M, Stanevsky A, Wang T, Aranow C, Li M, Koenig S, J Ravetch JV, Diamond B Selective dysregulation of the FcgammaIIB receptor on memory B cells in SLE J Exp Med 2006; 203 :2157-2164.
- Xiang Z, Cutler AJ, Brownlie RJ, Fairfax K, Lawlor KE, Severinson E, Walker EU, Manz RA, Tarlinton DM, Smith KG. FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nat Immunol. 2007; 8 : 419-429.
- Diwan TS, Raghavaiah S, Burns JM, Kremers WK, Gloor JM, Stegall MD. The impact of proteasome inhibition on alloantibody-producing plasma cells in vivo. Transplantation. 2011; 9 :536-541.
- Perry DK, Burns JM, Pollinger HS, Amiot BP, Gloor JM, Gores GJ, Stegall MD. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009; 9 : 201-209. [CrossRef]
- Ejaz NS, Shields AR, Alloway RR, Sadaka B, Girnita AL, Mogilishetty G, Cardi M, Woodle ES. Randomized controlled pilot study of B cell-targeted induction therapy in HLA sensitized kidney transplant recipients. Am J Transplant. 2013 ;13 :3142-3154. [CrossRef]
- Eskandary F, Regele H, Baumann L, Bond G, Kozakowski N, Wahrmann M, Hidalgo LG, Haslacher H, Kaltenecker CC, Aretin MB, Oberbauer R, Posch M, Staudenherz A, Handisurya A, Reeve J, Halloran PF, Böhmig GA. A Randomized Trial of Bortezomib in Late Antibody-Mediated Kidney Transplant Rejection. J Am Soc Nephrol. 2018; 29 :591-605. [CrossRef]
- Gandolfi S, Laubach JP, Hideshima T, Chauhan D, Anderson KC, Richardson PG. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017; 36: 561-584. [CrossRef]
- NCT 02442648 Clinical Trial.gov. Woodle ES B-Cell Targeted Desensitization With Carfilzomib for Preformed Anti-HLA Antibodies in Patients Awaiting Kidney Transplantation. Accessed May 22, 2023. 22 May. [CrossRef]
- Tremblay S, Driscoll JJ, Rike-Shields A, Hildeman DA, Alloway RR, Girnita AL, Brailey PA, Woodle ES. A prospective, iterative, adaptive trial of carfilzomib-based desensitization. Am J Transplant. 2020 ;20 :411-421.
- Vo AA, Choi J, Kim I, Louie S, Cisneros K, Kahwaji J, Toyoda M, Ge S, Haas M, Puliyanda D, Reinsmoen N, Peng A, Villicana R, Jordan SC. A Phase I/II Trial of the Interleukin-6 Receptor-Specific Humanized Monoclonal (Tocilizumab) + Intravenous Immunoglobulin in Difficult to Desensitize Patients. Transplantation. 2015 ; 99 : 2356-2363. [CrossRef]
- Doberer K, Duerr M, Halloran PF, Eskandary F, Budde K, Regele H, Reeve J, Borski A, Kozakowski N, Reindl-Schwaighofer R, Waiser J, Lachmann N, Schranz S, Firbas C, Mühlbacher J, Gelbenegger G, Perkmann T, Wahrmann M, Kainz A, Ristl R, Halleck F, Bond G, Chong E, Jilma B, Böhmig GA. A Randomized Clinical Trial of Anti-IL-6 Antibody Clazakizumab in Late Antibody-Mediated Kidney Transplant Rejection. J Am Soc Nephrol. 2021; 32 : 708-722. [CrossRef]
- Mease PJ, Gottlieb AB, Berman A, Drescher E, Xing J, Wong R, Banerjee S. The Efficacy and Safety of Clazakizumab, an Anti-Interleukin-6 Monoclonal Antibody, in a Phase IIb Study of Adults With Active Psoriatic Arthritis. Arthritis Rheumatol. 2016 ; 68 : 2163-2173. [CrossRef]
- Kaufman GP, Schrier SL, Lafayette RA, Arai S, Witteles RM, Liedtke M. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood. 2017; 130 : 900-902. [CrossRef]
- Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, Doyen C, Lucio P, Nagy Z, Kaplan P, Pour L, Cook M, Grosicki S, Crepaldi A, Liberati AM, Campbell P, Shelekhova T, Yoon SS, Iosava G, Fujisaki T, Garg M, Chiu C, Wang J, Carson R, Crist W, Deraedt W, Nguyen H, Qi M, San-Miguel J; ALCYONE Trial Investigators. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N Engl J Med. 2018; 378 :518-528. [CrossRef]
- Kwun J, Matignon M, Manook M, Guendouz S, Audard V, Kheav D, Poullot E, Gautreau C, Ezekian B, Bodez D, Damy T, Faivre L, Menouche D, Yoon J, Park J, Belhadj K, Chen D, Bilewski AM, Yi JS, Collins B, Stegall M, Farris AB, Knechtle S, Grimbert P Daratumumab in Sensitized Kidney Transplantation: Potentials and Limitations of Experimental and Clinical Use. J Am Soc Nephrol. 2019; 30 : 1206-1219.
- Joher N, Matignon M, Grimbert P. HLA Desensitization in Solid Organ Transplantation: Anti-CD38 to Across the Immunological Barriers. Front Immunol. 2021;12 :688301.
- Doberer K, Kläger J, Gualdoni GA, Mayer KA, Eskandary F, Farkash EA, Agis H, Reiter T, Reindl-Schwaighofer R, Wahrmann M, Cohen G, Haslacher H, Bond G, Simonitsch-Klupp I, Halloran PF, Böhmig GA. CD38 Antibody Daratumumab for the Treatment of Chronic Active Antibody-mediated Kidney Allograft Rejection. Transplantation. 2021; 105 :451-457. [CrossRef]
- Spica D, Junker T, Dickenmann M, Schaub S, Steiger J, Rüfli T, Halter J, Hopfer H, Holbro A, Hirt-Minkowski P. Daratumumab for Treatment of Antibody-Mediated Rejection after ABO-Incompatible Kidney Transplantation. Case Rep Nephrol Dial. 2019; 9 :149-157. [CrossRef]
- Aguilera Agudo C, Gómez Bueno M, Krsnik Castello I. Daratumumab for Antibody-mediated Rejection in Heart Transplant-A Novel Therapy: Successful Treatment of Antibody-mediated Rejection. Transplantation. 2021;105 : e30-e31. [CrossRef]
- Glotz D, Antoine C, Julia P, Suberbielle-Boissel C, Boudjeltia S, Fraoui R, Hacen C, Duboust A, Bariety J. Desensitization and subsequent kidney transplantation of patients using intravenous immunoglobulins (IVIg). Am J Transplant. 2002; 2 :758-760.
- Jordan SC, Tyan D, Stablein D, McIntosh M, Rose S, Vo A, Toyoda M, Davis C, Shapiro R, Adey D, Milliner D, Graff R, Steiner R, Ciancio G, Sahney S, Light J. Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end-stage renal disease: report of the NIH IG02 trial. J Am Soc Nephrol. 2004; 15 :3256-3562. [CrossRef]
- Montgomery RA, Zachary AA, Racusen LC, Leffell MS, King KE, Burdick J, Maley WR, Ratner LE. Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation. 2000; 70 :887-895.
- Mamode N, Bestard O, Claas F, Furian L, Griffin S, Legendre C, Pengel L, Naesens M. European Guideline for the Management of Kidney Transplant Patients With HLA Antibodies: By the European Society for Organ Transplantation Working Group. Transpl Int. 2022 ; 35 : 10511. [CrossRef]
- Chen X, Wang Y, Dong P, Wang J, Yu X, Yu B. Efficacy of Combined Desensitization Therapy Based on Protein A Immunoadsorption on Anti-human Leukocyte Antigen Antibodies in Sensitized Kidney Transplant Recipients: A Retrospective Study. Cureus. 2022 ; 14 : e28661. [CrossRef]
- Kälble F, Süsal C, Pego da Silva L, Speer C, Benning L, Nusshag C, Pham L, Tran H, Schaier M, Sommerer C, Beimler J, Mehrabi A, Zeier M, Morath C. Living Donor Kidney Transplantation in Patients With Donor-Specific HLA Antibodies After Desensitization With Immunoadsorption. Front Med (Lausanne). 2021; 8 :781491. [CrossRef]
- Junker T, Volken T, Stehle G, Drexler B, Infanti L, Buser A, Passweg J, Schaub S, Dickenmann M, Halter J, Holbro A. Safety and Feasibility of Immunoadsorption with Heparin Anticoagulation in Preparation of ABO-Incompatible Kidney Transplantation: A Retrospective Single-Center Study. Transfus Med Hemother. 2023; 50 : 76-87.
- von Pawel-Rammingen U, Björck L. IdeS and SpeB: immunoglobulin-degrading cysteine proteinases of Streptococcus pyogenes. Curr Opin Microbiol. 2003 ; 6 :50-55. [CrossRef]
- Järnum S, Bockermann R, Runström A, Winstedt L, Kjellman C. The Bacterial Enzyme IdeS Cleaves the IgG-Type of B Cell Receptor (BCR), Abolishes BCR-Mediated Cell Signaling, and Inhibits Memory B Cell Activation. J Immunol. 2015 ; 195 : 5592-5601. [CrossRef]
- Jordan SC, Lorant T, Choi J, Kjellman C, Winstedt L, Bengtsson M, Zhang X, Eich T, Toyoda M, Eriksson BM, Ge S, Peng A, Järnum S, Wood KJ, Lundgren T, Wennberg L, Bäckman L, Larsson E, Villicana R, Kahwaji J, Louie S, Kang A, Haas M, Nast C, Vo A, Tufveson G. IgG Endopeptidase in Highly Sensitized Patients Undergoing Transplantation. N Engl J Med. 2017; 377 :442-453.
- Vo AA, Choi J, Cisneros K, Reinsmoen N, Haas M, Ge S, Toyoda M, Kahwaji J, Peng A, Villicana R, Jordan SC. Benefits of rituximab combined with intravenous immunoglobulin for desensitization in kidney transplant recipients. Transplantation. 2014; 98 :312-319. [CrossRef]
- Zachary AA, Lucas DP, Montgomery RA, Leffell MS. Rituximab prevents an anamnestic response in patients with cryptic sensitization to HLA. Transplantation. 2013; 95 : 701-704. [CrossRef]
- Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, Cosio FG, Gandhi MJ, Kremers W, Gloor JM. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11 :2405-2413. [CrossRef]
- Cornell LD, Schinstock CA, Gandhi MJ, Kremers WK, Stegall MD. Positive crossmatch kidney transplant recipients treated with eculizumab: outcomes beyond 1 year. Am J Transplant. 2015; 15 :1293-1302. [CrossRef]
- Marks WH, Mamode N, Montgomery RA, Stegall MD, Ratner LE, Cornell LD, Rowshani AT, Colvin RB, Dain B, Boice JA, Glotz D; C10-001 Study Group. Safety and efficacy of eculizumab in the prevention of antibody-mediated rejection in living-donor kidney transplant recipients requiring desensitization therapy: A randomized trial. Am J Transplant. 2019; 19 :2876-2888. [CrossRef]
- Morgan BP, Harris CL. Complement, a target for therapy in inflammatory and degenerative diseases. Nat Rev Drug Discov. 2015; 14 :857-877. [CrossRef]
- Sharp JA, Whitley PH, Cunnion KM, Krishna NK. Peptide inhibitor of complement c1, a novel suppressor of classical pathway activation: mechanistic studies and clinical potential. Front Immunol. 2014 ; 5 : 406. [CrossRef]
- Gadek JE, Hosea SW, Gelfand JA, Santaella M, Wickerhauser M, Triantaphyllopoulos DC, Frank MM. Replacement therapy in hereditary angioedema: successful treatment of acute episodes of angioedema with partly purified C1 inhibitor. N Engl J Med. 1980; 302 : 542-546. [CrossRef]
- Zanichelli A, Mansi M, Periti G, Cicardi M. Therapeutic management of hereditary angioedema due to C1 inhibitor deficiency. Expert Rev Clin Immunol. 2013 ; 9 :477-488. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




