1. Introduction
Eye-tracking has been well established to be able to determine cognitive states in neurological health and injury, or with neurological disorders [
1,
2,
3]. Recently, devices that assess oculomotor eye movements have been developed to improve the objectivity and time taken in order to be able provide a neurological assessment. This in turn has placed oculomotor testing as a convenient technology to assess neurological injuries such as concussion.
Induced by biomechanical force directly or indirectly to the brain [
4], a concussion causes an assemblage of transient signs and symptoms representing complex pathophysiological processes across multiple cortical areas of the brain [
4]. Moreover, concussion is an evolving injury whereby symptoms can change (or worsen) over time reflecting the changing physiology as the brain recovers from the insult.
The current consensus on concussion in sport relies heavily on symptom reporting and presentation, prior to further testing such as neurocognitive assessment, and this may be open to manipulation or under-reporting [
5,
6]. For example, in elite sport, ~6% of male and female professional athletes in the Australian Football League have admitted to hiding symptoms of concussion [
7].
Consequently, there is increasing discussion and emerging evidence on the benefits of utilizing supplementary markers within the concussion assessment protocols [
8,
9,
10]. Incorporation of assessments such as oculomotor smooth pursuits provide objective screening to assist sports club medical staff who may suspect a concussion, or during the recovery phase after sport-related concussion [
11,
12].
While the physiological mechanisms of smooth pursuits is still yet to be fully understood, this involves saccadic and cognitive requirements of focus tracking on a moving object [
12,
13]. Multiple cortical and subcortical areas are involved in eye movement (see review [
14]), making eye-tracking a suitable proxy for a measurement of cognitive functioning after a concussion [
11,
15].
One eye-tracking device recently developed is the EyeGuide Focus. The system is portable and simple to utilize involving a 10-second assessment, quantifying oculomotor smooth pursuit or Dynamic Visio-Motor Synchronization [
16]. There are emerging studies employing the EyeGuide Focus system, not only across concussion management [
17,
18,
19] but also studies in fatigue [
20]. However, to date, there are no published studies on reliability of the system. The aim of this study was to determine intra-session reliability and differences in healthy adolescent and adult cohorts.
2. Materials and Methods
A sample of 75 healthy participants were recruited (adolescents: n=28; female=11, male=17, mean age 16.5 ± 1.4 years; adults n=47; female=22; male=25, mean age 26.7 ± 7.0 years). All participants were physically active, but not involved in elite sport. Participants had reported no history of concussions or other traumatic brain injury; and no pre-existing visual, neurological or psychiatric conditions, nor were on any medication. The sample size was determined using an a-prior power analysis (G*Power V3.1.9.6, Germany)[
21] for within-between groups repeated measures with an assumed effect size of
f =0.25; α
<0.05; power (1- β)=0.95 would require a minimum sample size of 44. Prior to testing, all participants (or their parent) completed voluntary informed consent. Protocols were approved by La Trobe University Human Research Ethics (HEC18207) conforming to the Declaration of Helsinki.
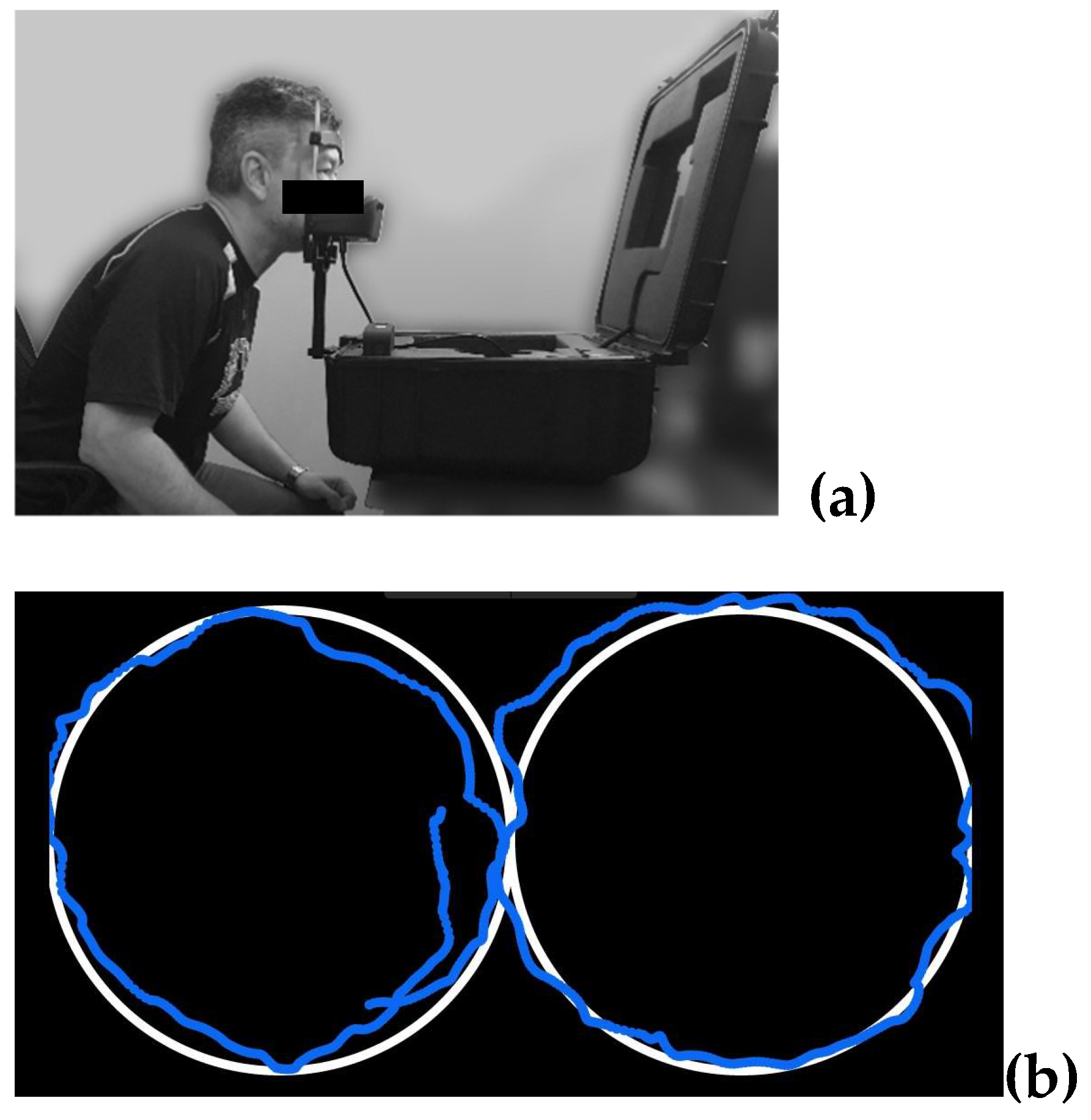
Oculomotor smooth-pursuits eye-tracking was completed using the EyeGuide Focus (EyeGuide, Texas, USA). The protocol, following EyeGuide protocols, involves the participant seated with their head placed in a chin/head rest, to reduce unnecessary head movements (
Figure 1a), and tracking a white dot against a black background moving anti-clockwise, then clockwise through one cycle of a horizontal “lazy 8” or “infinity-shape” path for 10-seconds (
Figure 1b). The fixed position digital camera follows the participant’s pupils, at 60 Hz frequency, comparing the position and movement speed of the pupils to the white dot stimulus.
Participants undertook testing in a quiet room, away from potential distractions. Participants were also allowed usual eyewear such as prescription contact lenses or glasses. Each participant completed three trials with a 30-second rest between each trial to avoid potential fatigue. A successful trial was defined as a ‘tick’ indicating that their score (see section 2.1) fell into the EyeGuide community range [
17]. If a trial was unsuccessful, that is their result was 1SD above the mean, the EyeGuide would indicate the trial was not successful, and participants were allowed to repeat that trial. If three successful trials were not able to be obtained after an arbitrary 10 attempts, the participant’s data was not included.
2.1. Data and Statistical Analysis
Eye-tracking score was calculated as the cumulative distance between the stimulus data and the pupil position [
22]. A greater error was indicated by a larger test score. Participants and test administrators were not blinded to test results, with a classification of scores provided at the end of the test (e.g., “Superior”, “High Average”, “Above Average”, “Average”, “Low Average”, “Impaired”, “Severely Impaired”), however exact test scores were not displayed. Data collected by the EyeGuide system was imported into Jamovi (V2.3, Sydney, Australia). Data was screened for normal distribution using the Shaprio-Wilk tests and found to be normally distributed (W=0.98,
p=0.18). Test-retest reliability was calculated via Intraclass Correlation Coefficient (ICC). ICC values were selected using the criteria by Koo and Li [
23] :
>0.91: excellent reliability, 0.75–0.9: good reliability, 0.51–0.74: moderate reliability, and ≤ 0.5: poor reliability. Data for ICC is presented as value (±95% CI), and comparisons between groups using mean (±SD). Cohen’s
d effect sizes were utilized to describe differences between group means [
24]. EyeGuide scores are reported in arbitrary units (AUs).
3. Results
All but three adult participants completed testing. In three adult participants data was not able to be collected due to EyeGuide being unsuccessful in tracking pupil movement.
3.1. Reliability Analyses
ICCs for all participants (n=72) showed good reliability across three trials (0.79, 95%CI: 0.70, 0.86). Sub-comparisons between trials 1 and 2, trials 1 and 3, and trials 2 and 3 showed good reliability (ICCs: 0.76 [95%CI: 0.62, 0.84], 0.77 [95%CI: 0.64, 0.85], 0.83 [95%CI: 0.73, 0.89], respectively).
ICCS in all adolescents showed overall good reliability (0.78, 95%CI: 0.62, 0.88) with moderate reliability seen between trails 1 and 2 (0.74, 95%CI: 0.51, 0.87) and between trials 1 and 3 (0.65, 95%CI: 0.35, 0.82). A good reliability was found between trials 2 and 3 (0.81, 95%CI: 0.63, 0.91). When comparing reliability by sex, males showed good overall reliability (0.81, 95%CI: 0.61, 0.82) and across trials 1 and 2, trials 1 and 3, and trials 2 and 3 (0.77 [95%CI: 0.47, 0.91], 0.81 [95%CI: 0.54, 0.92], 0.87 [95%CI: 0.67, 0.95] respectively). In females, overall good reliability was observed (0.76, 95%CI: 0.44, 0.92). A good reliability across trials 1 and 2, and trials 2 and 3 were seen (0.71 [95%CI: 0.22, 0.91], 0.77 [95%CI: 0.35, 0.93] respectively), but poor reliability was seen between trials 1 and 3 (0.48, 95%CI: 0.14, 0.83).
In all adults, ICCS showed overall good reliability (0.80, 95%CI: 0.67, 0.89) and good reliability between trails 1 and 2 (0.79, 95%CI: 0.61, 0.88), trials 2 and 3 (0.82, 95%CI: 0.67, 0.90), and between trials 1 and 3 (0.84, 95%CI: 0.71, 0.91). When comparing reliability by sex, adult males showed good reliability (0.79, 95%CI: 0.60, 0.90), and across trials 1 and 2, trials 2 and 3, and trials 1 and 3 (0.80 [95%CI: 0.58, 0.91], 0.80 [95%CI: 0.50, 0.91], 0.83 [95%CI: 0.63, 0.92] respectively). Similarly in females, overall good reliability was observed (0.86, 95%CI: 0.73, 0.94), and between trials 1 and 2, trials 2 and 3, and trials 1 and 3 were seen (0.86 [95%CI: 0.70, 0.94], 0.86 [95%CI: 0.70, 0.94], 0.84 [95%CI: 0.65, 0.93] respectively).
3.2. Comparisons Across Trials and Groups
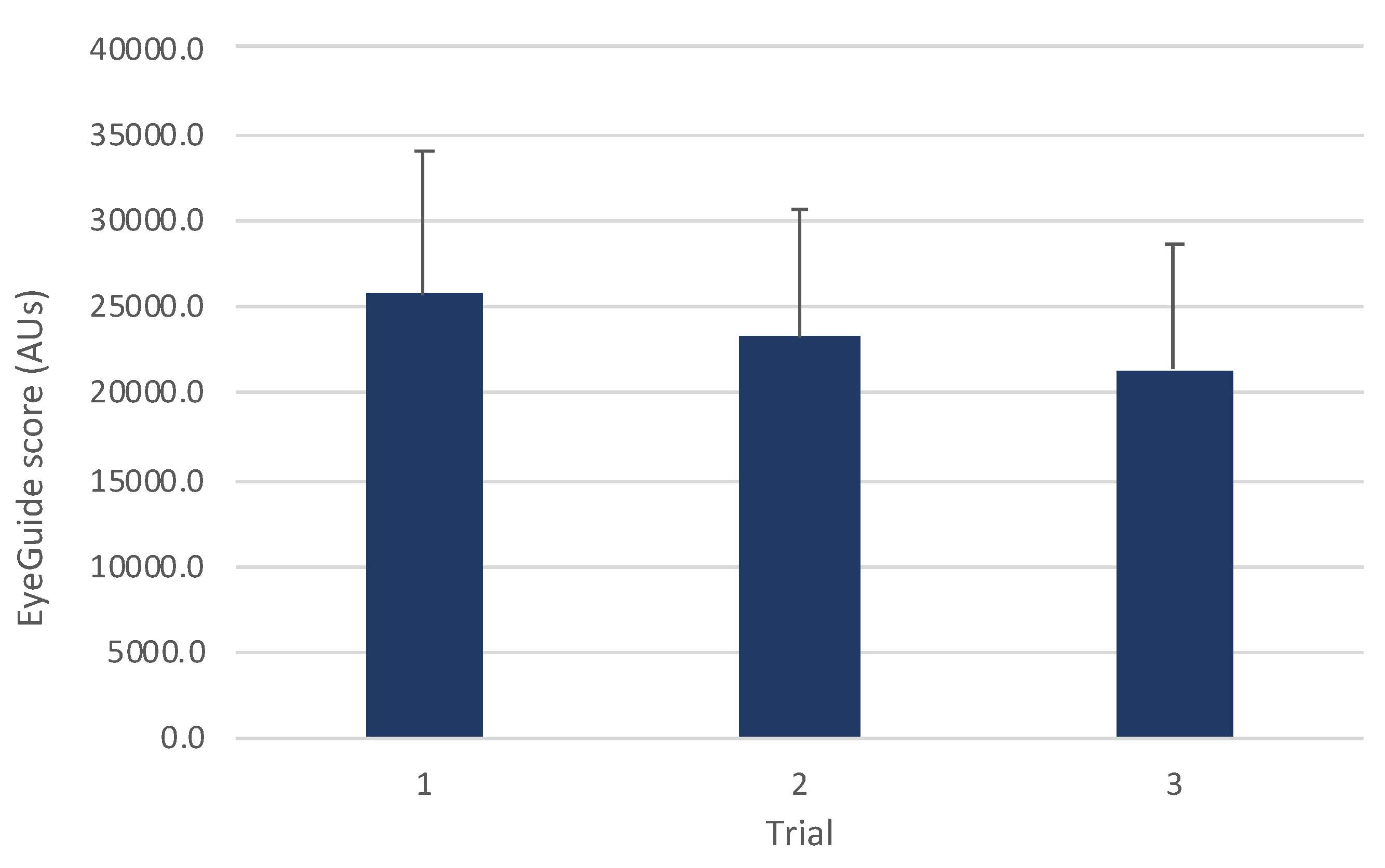
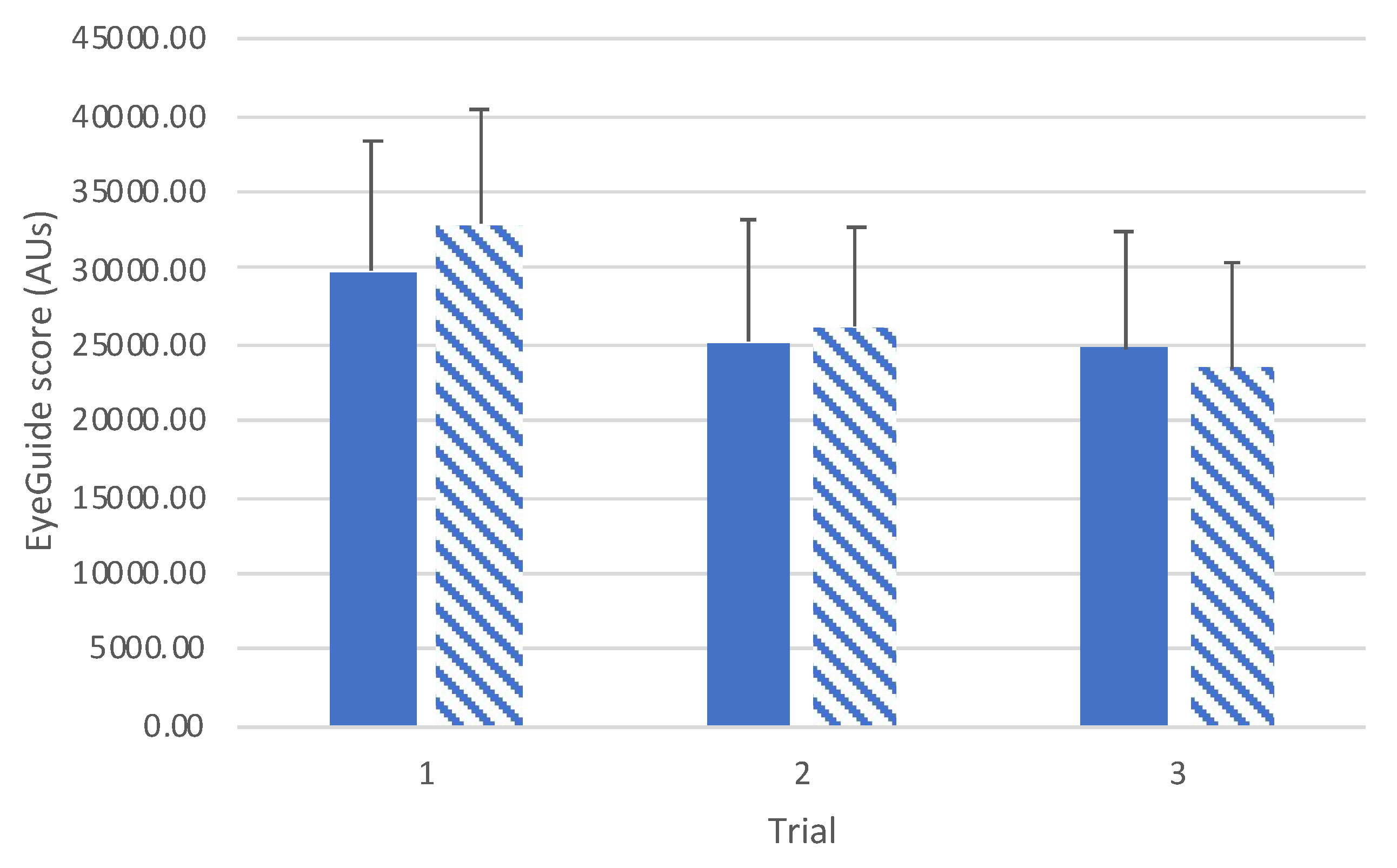
A one-way ANOVA for all participants (n=72) showed a significant improvement in scores with subsequent trials (
F(2,140)=28.2,
p<0.001;
Figure 2). Post hoc comparisons revealed significant differences between trials 1 and 2 (
t(71)=4.12,
p<0.001,
d=0.33), trials 1 and 3 (
t(71)=7.21,
p<0.001,
d=0.57), and trials 2 and 3 (
t(71)=
t71=3.69,
p=0.001,
d=0.26).
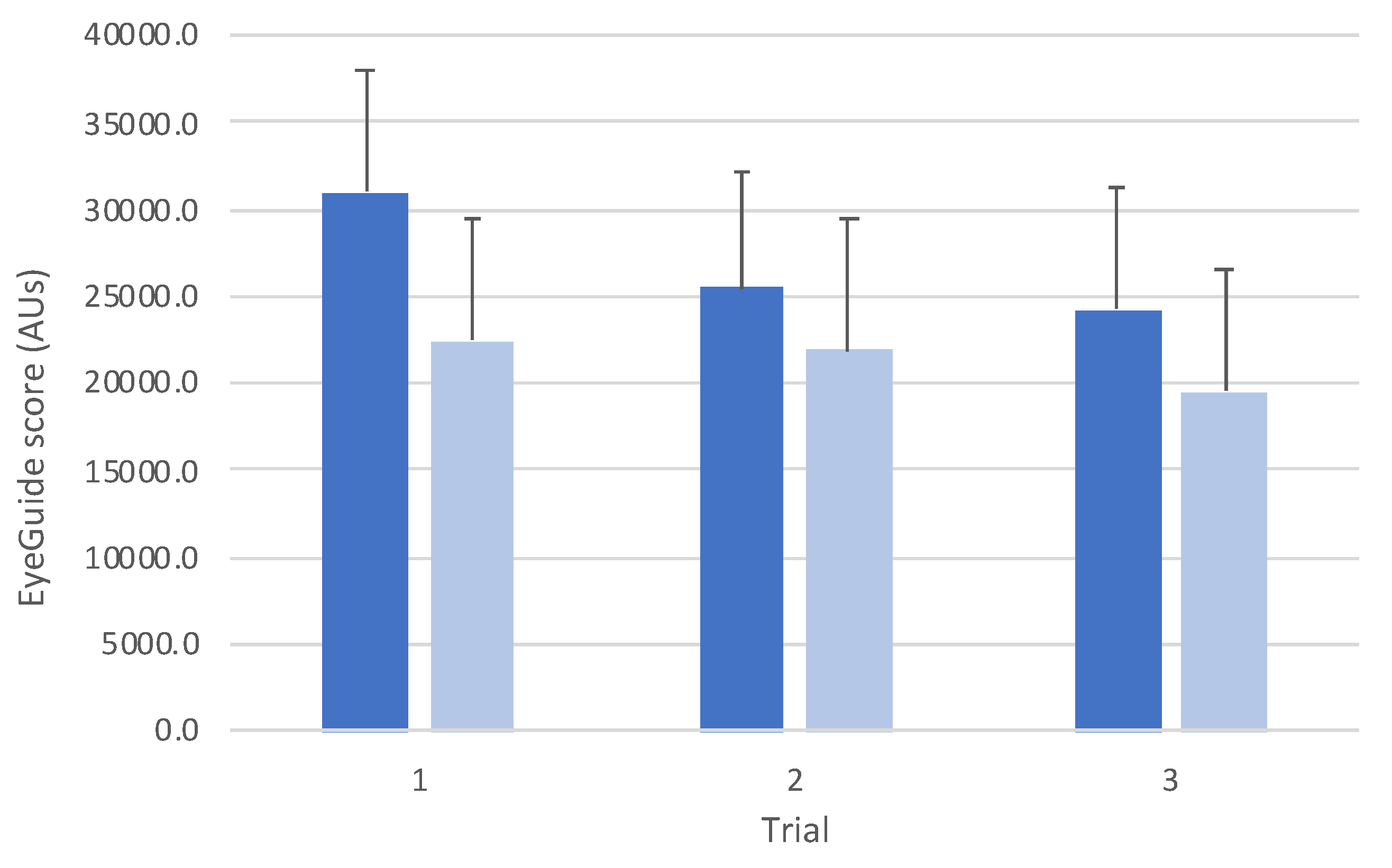
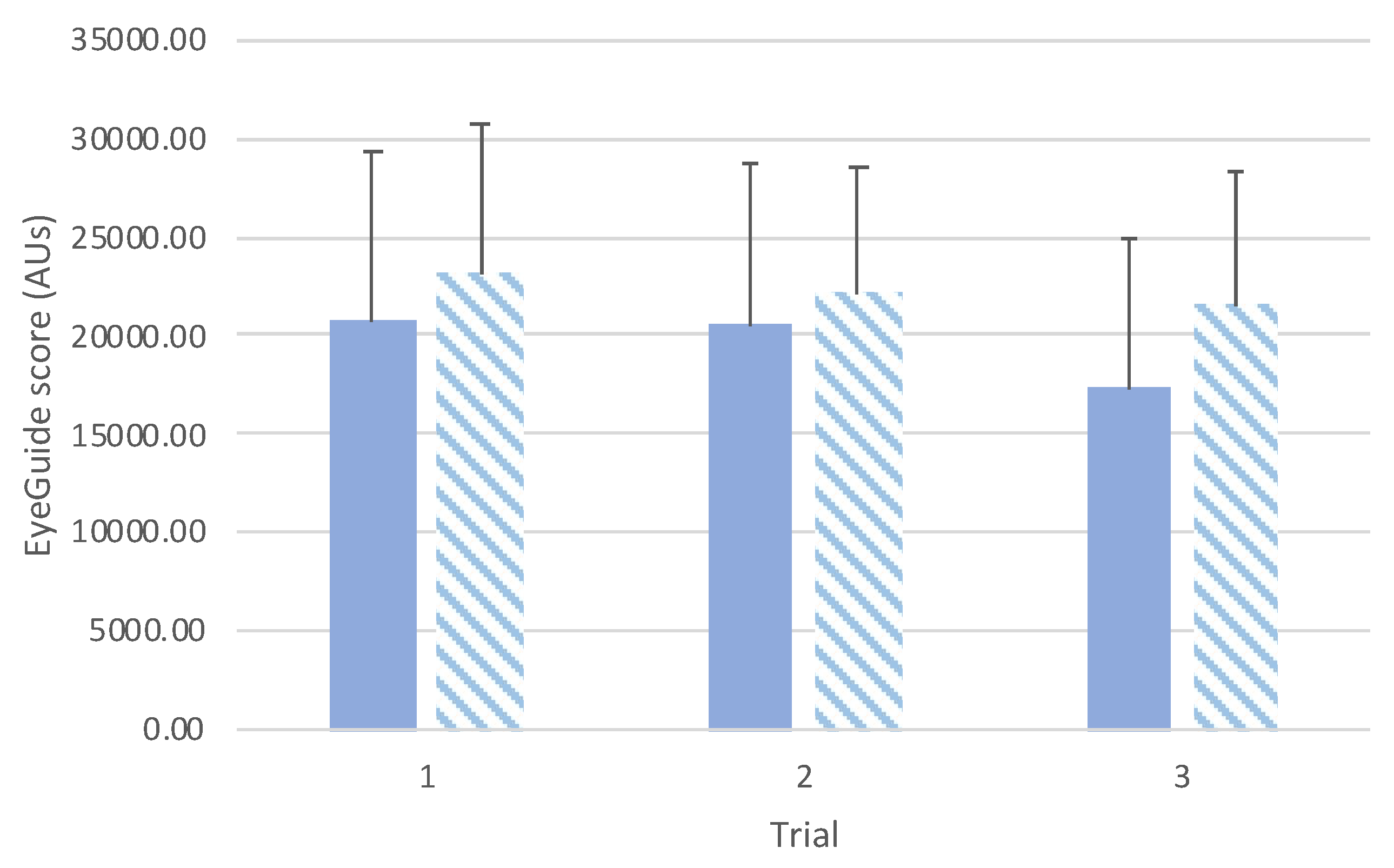
Comparison between groups revealed an interaction effect between groups (
F(2,140)=10.3,
p<0.001;
Figure 3). Post hoc testing between groups showed that there was a statistically significant difference for trial 1 (
t(70)=4.97,
p<0.001,
d=1.20), but no differences for trial 2 (
t(70)=2.11,
p=0.58,
d=0.52) or trial 3 (
t(70)=2.79,
p=0.10,
d=0.68).
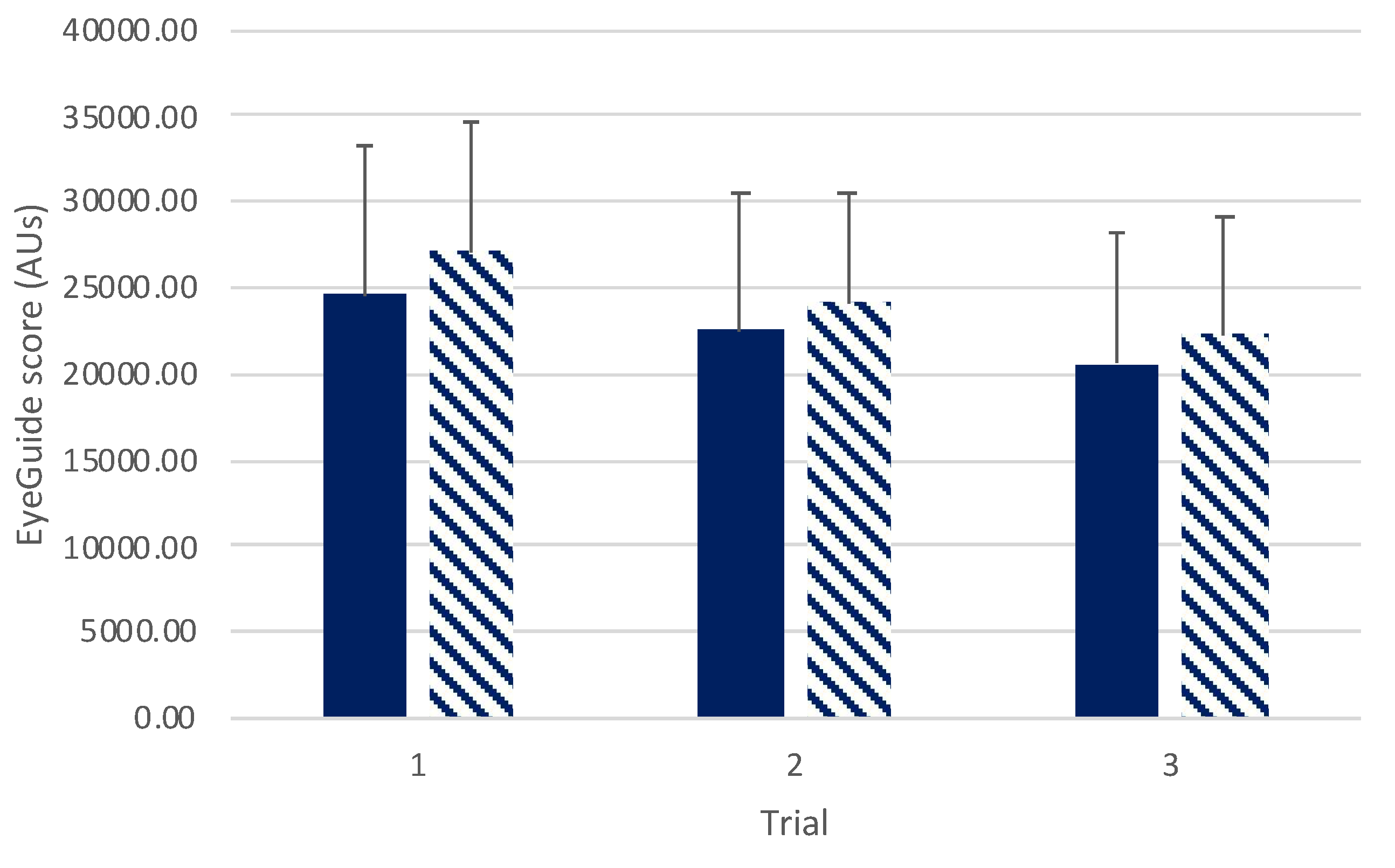
There was no interaction effect found between sexes across trials (
F(2,140)=0.36,
p=0.69;
Figure 4). A main effect was observed for trials (
F(2,140)=29.08,
p<0.001) with post hoc testing revealing significant difference between trials 1 and 2 (
t(70)=4.16,
p<0.001,
d=0.34), and trials 1 and 3 (
t(70)=7.21,
p<0.001,
d=0.58), and between trials 2 and 3 (
t(70)=3.64,
p=0.002,
d=0.26). No differences were seen between sexes (
F(1,70)=1.38,
p=0.24).
Sub analyses between sexes were conducted in adolescent and adult populations separately (
Figure 5 and
Figure 6 respectively). In the adolescent group (Figure
5) there was no interaction effect found (
F(2,52)=2.55,
p=0.09) or main effects between groups (
F(1,26)=0.16,
p=0.69). A main effect across trials was observed (
F(2,52)=30.45,
p<0.001) with post hoc comparisons showing significant differences between trials 1 and 2 (
t(26)=5.77,
p<0.001,
d=0.87), and trials 1 and 3 (
t(70)=6.55,
p<0.001,
d=1.04). No difference was seen between trials 2 and 3 (
t(70)=1.84,
p=0.23,
d=0.12).
Similarly in the adult group (
Figure 6), no interaction effect was observed (
F(2,84)=0.99,
p=0.37) or main effect between groups (
F(1,42)=3.14,
p=0.08). A main effect across trials was observed (
F(2,84)=11.58,
p<0.001) with post hoc comparisons showing significant differences between trials 1 and 3 (
t(42)=4.78,
p<0.001,
d=0.36), and trials 2 and 3 (
t(42)=3.52, ,
d=0.27). No difference was seen between trials 1 and 2 (
t(42)=0.98,
p=0.99,
d=0.08).
4. Discussion
To the best of the authors’ knowledge, this is the first study to examine the reliability of the EyeGuide Focus as a tool for oculomotor pursuits. Overall findings suggest that the EyeGuide Focus was found to have good reliability, but familiarisation effects were observed in both healthy adolescent and adult cohorts with subsequent attempts. Of note, familiarisation effects in adolescents contributed to the moderate reliability in subsequent trials.
Reliability in EyeGuide was comparable to other eye movement cognitive tests (e.g., King-Devick). For example, studies in adolescent populations have reported moderate to good ICCs of 0.81 (95%CI: 0.73, 0.87), and 0.91 (95%CI: 0.86, 0.95) [
25,
26]; and in adults with good ICCs of 0.95 (95% CI: 0.91, 0.97) and 0.91 (95%CI: 0.80, 0.96) [
27,
28]. The data in the present study is also consistent to previously published EyeGuide data. In 2017 Kelly [
17] reported in adolescents (12 – 18 years) a mean score of 29633.05 (± 9209.83) AUs and this is comparable to our results for trial 1 (30976.57 ± 7099.87 AUs). In adults the mean from trial 1 (22503.33 ± 7014.32 AUs) was equivalent to ‘high average’ to the EyeGuide normative range (21066.64 ± 1858.57 AUs) [
17]. Of interest, the baseline range in the current study being classified as ‘high average’ was above the baseline reported, ‘average’ classification, in a cohort of surgeons (no raw data presented however) by Puckett et al [
20]. However, in studies by Kelly [
17] and Puckett et al [
20], only a single baseline trial was recorded.
The finding of familiarization/learning effects in this sample is interesting but not surprising. Well described in cognitive psychology, learning/familiarization effects are noteworthy improvements that occur with repetition of a task until scores no longer notably change and achieve stability [
29]. The results in this study concur with previous findings in other eye-movement tests (e.g. King-Devick) with improvements in subsequent trials [
30]. Consequently, based upon the results of this study, it is suggested that a minimum of three successful trials are completed with the best score used as the player’s baseline.
The data also showed that, overall, adults performed testing with ~20% less error than adolescents (
Figure 3). Similar to studies in other eye-movement tests that show improved performance with age [
31], these differences reflect developmental changes in eye-movement and cognition more generally. Mechanisms contributing to differences between adolescents and mature oculomotor systems include longer saccade latencies, reduced smooth pursuit gain, and increased general variability reflecting maturation differences between groups [
32,
33].
The concern that reliability is affected by improvement in scores, suggesting familiarization effects, should not necessarily be interpreted as a limitation but useful in developing standardized protocols for future use. The diminishing familiarisation effect observed between trials 2 and 3 suggest that three trials should suffice to determine a baseline. Given data is collected within a 10-second time frame, this should not be too onerous on both operator and participant. However, further studies are suggested that could determine the optimal number of baseline trials to reduce familiarization effects, particularly in junior athletes undertaking baseline concussion testing.
Further limitations of this study include the relatively young adult population utilized in this study. We focussed on a younger adult sample to emulate the type of adult population likely to be using EyeGuide Focus (athletic cohorts). However, concussion is not a ‘young person injury’ and indeed many older athletes participate in contact sports and are at risk of concussion. Future studies should consider assessing reliability in an older population cohort.
While previous studies have investigated the effect of workplace fatigue [
20] future studies should also look at the effect of acute exercise and fatigue effects on oculomotor smooth pursuit tracking. Other eye-movement tests have found influence of exercise on outcomes [
34], and with these systems being suggested as a ‘sideline’ assessment tool to aid medical decisions, it is not yet known the effect of fatigue on eye-movement performance outcomes.
In conclusion this study has demonstrated reliability with the EyeGuide Focus, suggesting that this system is suitable for concussion recognition. However, with familiarization effects apparent, baseline determination in adults and adolescents should employ a standardized protocol of a minimum of three trials.
Author Contributions
Conceptualization, AJP; methodology, AJP; data analysis, AJP.; writing—original draft preparation, AJP, ED, LR, DK; writing—review and editing, AJP, ED, LR, DK; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Human Research Ethics Committee) of La Trobe University (HEC18207 and 6 July 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon reasonable request from an authorised institution.
Acknowledgments
AJP has previously received partial research funding from the Erasmus+ strategic partnerships program (2019-1-IE01-KA202-051555), Sports Health Check Charity (Australia), Australian Football League, Impact Technologies Inc., and Samsung Corporation, and is remunerated for expert advice to medico-legal practices.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wolf, A.; Ueda, K. Contribution of eye-tracking to study cognitive impairments among clinical populations. Frontiers in Psychology 2021, 12, 590986. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, Z.; Gu, Y.; Liu, H.; Wang, P. The effectiveness of eye tracking in the diagnosis of cognitive disorders: A systematic review and meta-analysis. PLoS One 2021, 16, e0254059. [Google Scholar] [CrossRef]
- Snegireva, N.; Derman, W.; Patricios, J.; Welman, K.E. Eye tracking technology in sports-related concussion: a systematic review and meta-analysis. Physiol Meas 2018, 39, 12TR01. [Google Scholar] [CrossRef]
- Harmon, K.G.; Clugston, J.R.; Dec, K.; Hainline, B.; Herring, S.; Kane, S.F.; Kontos, A.P.; Leddy, J.J.; McCrea, M.; Poddar, S.K. American Medical Society for Sports Medicine position statement on concussion in sport. Br J Sports Med 2019, 53, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Ferdinand Pennock, K.; McKenzie, B.; McClemont Steacy, L.; Mainwaring, L. Under-reporting of sport-related concussions by adolescent athletes: a systematic review. Int Rev Sport Exerc Psychol 2020, 1–27. [Google Scholar] [CrossRef]
- Pearce, A.J.; Young, J.A.; Parrington, L.; Aimers, N. Do as I say: Contradicting beliefs and attitudes towards sports concussion in Australia. J Sports Sci 2017, 35, 1911–1919. [Google Scholar] [CrossRef]
- Assocation, A.F.L.P. Insights and Impact Report (Edition 1); 2022.
- Pearce, A.J.; Hoy, K.; Rogers, M.A.; Corp, D.T.; Davies, C.B.; Maller, J.J.; Fitzgerald, P.B. Acute motor, neurocognitive and neurophysiological change following concussion injury in Australian amateur football. A prospective multimodal investigation. J Sci Med Sport 2015, 18, 500–506. [Google Scholar] [CrossRef]
- Dimou, S.; Lagopoulos, J. Toward objective markers of concussion in sport: a review of white matter and neurometabolic changes in the brain after sports-related concussion. J Neurotraum 2014, 31, 413–424. [Google Scholar] [CrossRef]
- Daly, E.; Pearce, A.J.; Finnegan, E.; Cooney, C.; McDonagh, M.; Scully, G.; McCann, M.; Doherty, R.; White, A.; Phelan, S.; et al. An assessment of current concussion identification and diagnosis methods in sports settings: a systematic review. BMC Sports Sci Med Rehabilitation 2022, 14, 125. [Google Scholar] [CrossRef]
- Fraser, C.L.; Mobbs, R. Visual effects of concussion: a review. Clin Exp Ophthalmol 2022, 50, 104–109. [Google Scholar] [CrossRef]
- Maruta, J.; Suh, M.; Niogi, S.N.; Mukherjee, P.; Ghajar, J. Visual tracking synchronization as a metric for concussion screening. J Head Trauma Rehabil 2010, 25, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Behling, S.; Lisberger, S.G. Different mechanisms for modulation of the initiation and steady-state of smooth pursuit eye movements. J Neurophysiol 2020, 123, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Ono, S. The neuronal basis of on-line visual control in smooth pursuit eye movements. Vision Res 2015, 110, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Zahid, A.B.; Hubbard, M.E.; Lockyer, J.; Podolak, O.; Dammavalam, V.M.; Grady, M.; Nance, M.; Scheiman, M.; Samadani, U.; Master, C.L. Eye tracking as a biomarker for concussion in children. Clin J Sport Med 2020, 30, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Maruta, J.; Suh, M.; Niogi, S.N.; Mukherjee, P.; Ghajar, J. Visual tracking synchronization as a metric for concussion screening. The Journal of head trauma rehabilitation 2010, 25, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M. Technical Report of the Use of a Novel Eye Tracking System to Measure Impairment Associated with Mild Traumatic Brain Injury. Cureus 2017, 9, e1251–e1251. [Google Scholar] [CrossRef]
- Lee, J.; Wei, B.; Blakely, S.; Baronia, B.C. Diagnosis and management of mild TBI via eye tracking. Neurology 2022, 98, S14–S14. [Google Scholar] [CrossRef]
- Murray, N.G.; Szekely, B.; Islas, A.; Munkasy, B.; Gore, R.; Berryhill, M.; Reed-Jones, R.J. Smooth pursuit and saccades after sport-related concussion. J Neurotraum 2020, 37, 340–346. [Google Scholar] [CrossRef]
- Puckett, Y.; Caballero, B.; Dissanaike, S.; Richmond, R.; Ronaghan, C.A. Surgeons maintain better focus working 12-hour shifts compared to 24-hour calls. J Surg Educ 2021, 78, 1280–1285. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Jahnke, N.A.; Still, J.B.; Gamel, G.L.; Baronia, B.C. Method and system for cognitive function testing. 2019.
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Erlbaum: Hillsdale, NJ, 1988. [Google Scholar]
- Oberlander, T.J.; Olson, B.L.; Weidauer, L. Test-retest reliability of the King-Devick test in an adolescent population. J Athl Train 2017, 52, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Worts, P.R.; Schatz, P.; Burkhart, S.O. Test performance and test-retest reliability of the vestibular/ocular motor screening and King-Devick test in adolescent athletes during a competitive sport season. Am J Sports Med 2018, 46, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; King, D.; Pearce, A. A reliability and comparative analysis of the new randomized King-Devick test. J Neuroophthalmol 2020, 40. [Google Scholar] [CrossRef] [PubMed]
- Hecimovich, M.; King, D.; Dempsey, A.R.; Murphy, M. The King-Devick test is a valid and reliable tool for assessing sport-related concussion in Australian football: A prospective cohort study. J Sci Med Sport 2018, 21, 1004–1007. [Google Scholar] [CrossRef]
- Tao, M.; Yang, D.; Liu, W. Learning effect and its prediction for cognitive tests used in studies on indoor environmental quality. Energy Build 2019, 197, 87–98. [Google Scholar] [CrossRef]
- Galetta, K.M.; Liu, M.; Leong, D.F.; Ventura, R.E.; Galetta, S.L.; Balcer, L.J. The King-Devick test of rapid number naming for concussion detection: meta-analysis and systematic review of the literature. Concussion 2016, 1, CNC8. [Google Scholar] [CrossRef]
- Gallagher, D.; King, D.; Hume, P.; Clark, T.; Pearce, A.; Gissane, C. Annual baseline King-Devick oculomotor function testing Is needed due to scores varying by age. Sports 2021, 9, 166. [Google Scholar] [CrossRef]
- Luna, B.; Velanova, K.; Geier, C.F. Development of eye-movement control. Brain Cogn 2008, 68, 293–308. [Google Scholar] [CrossRef]
- Doettl, S.M.; McCaslin, D.L. Oculomotor Assessment in Children. Semin Hear 2018, 39, 275–287. [Google Scholar] [CrossRef]
- Rist, B.; Cohen, A.; Pearce, A.J. King-Devick performance following moderate and high exercise intensity bouts. Int J Exerc Sci 2017, 10, 619–628. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).