Submitted:
23 May 2023
Posted:
25 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
ESI-Mass and FTIR Spectra
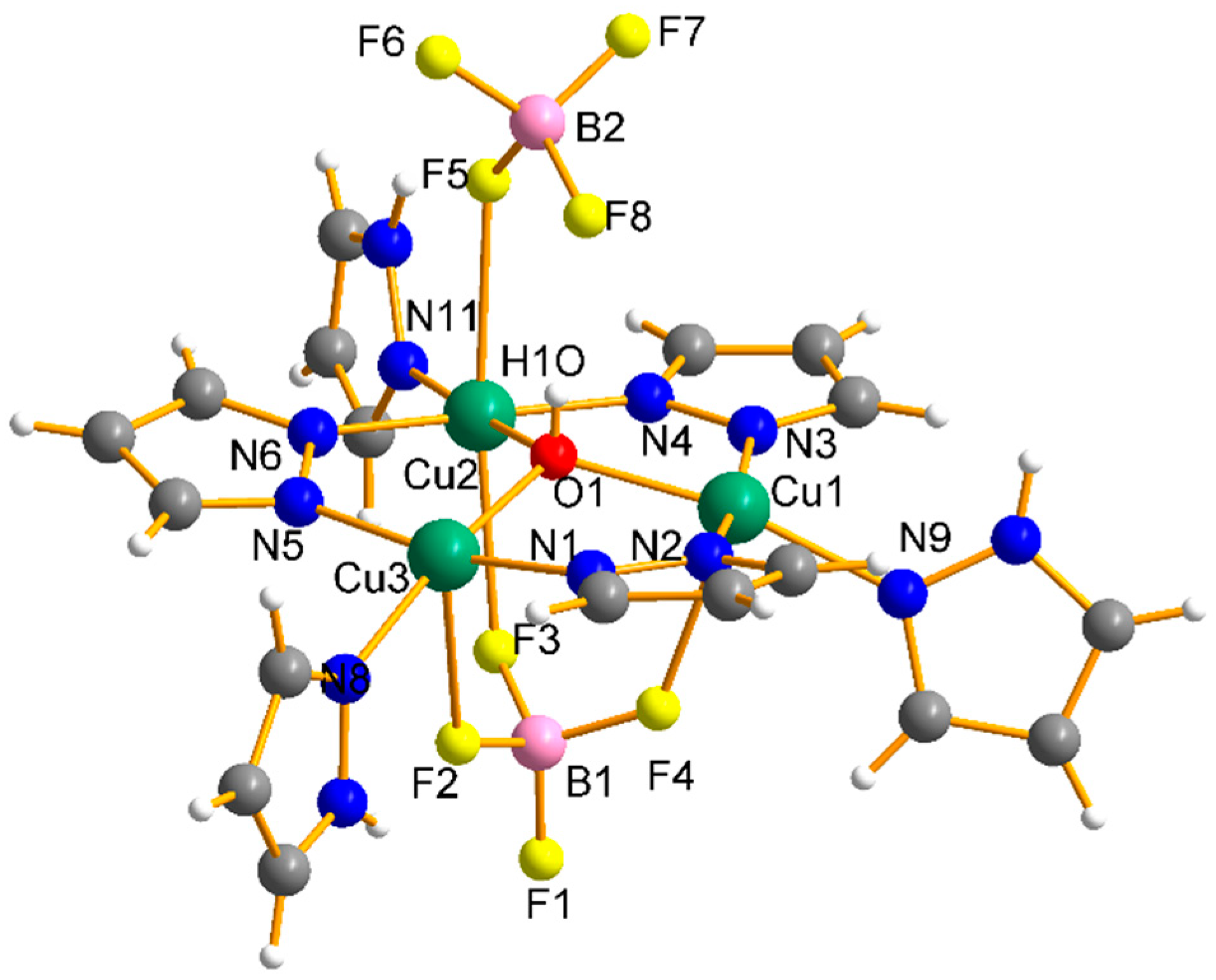
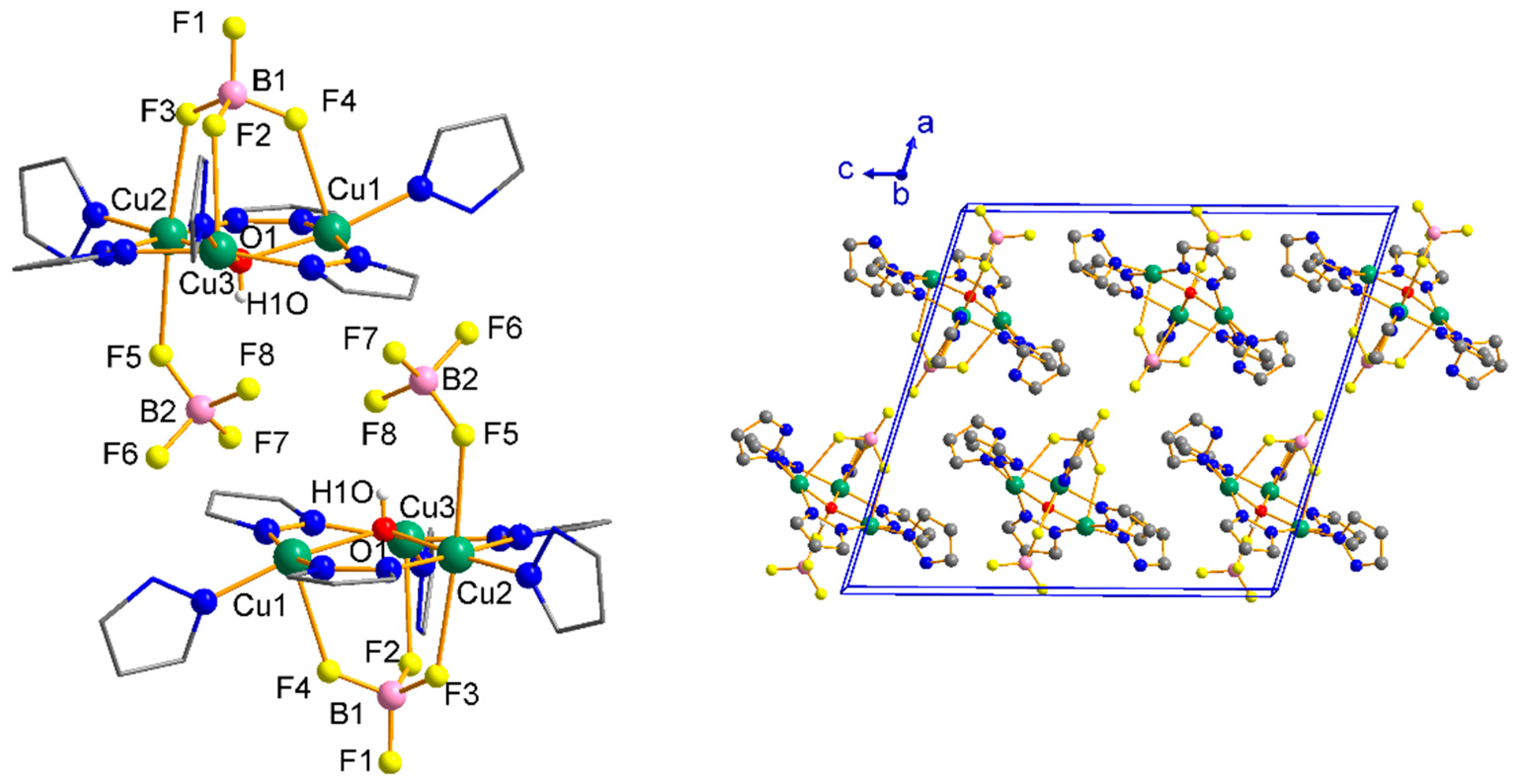
Structure Analysis
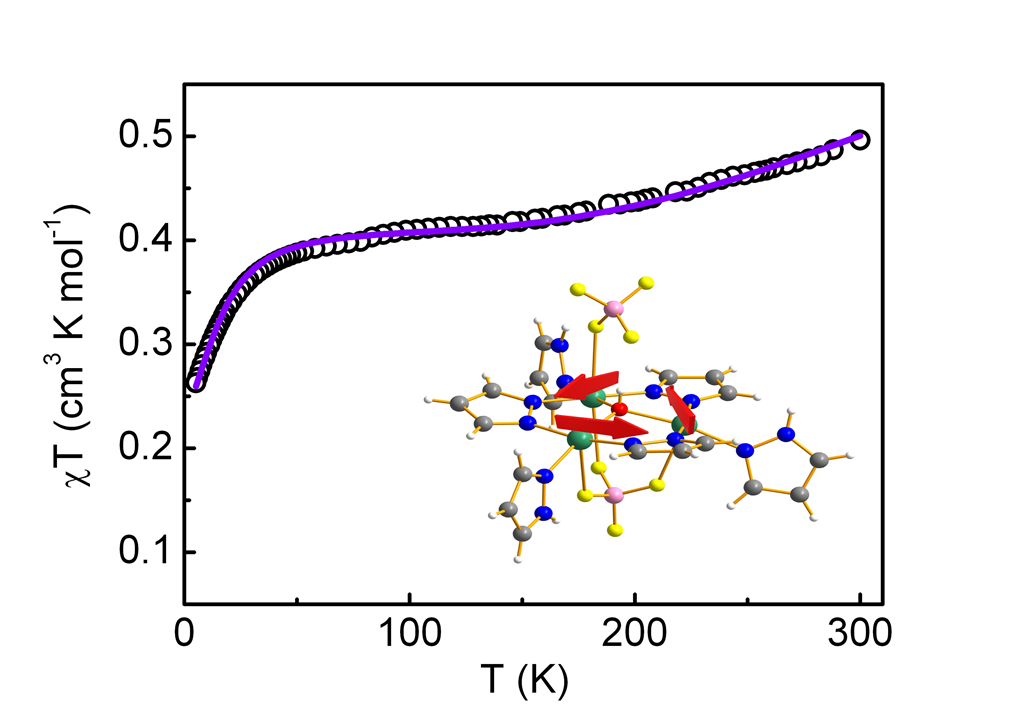
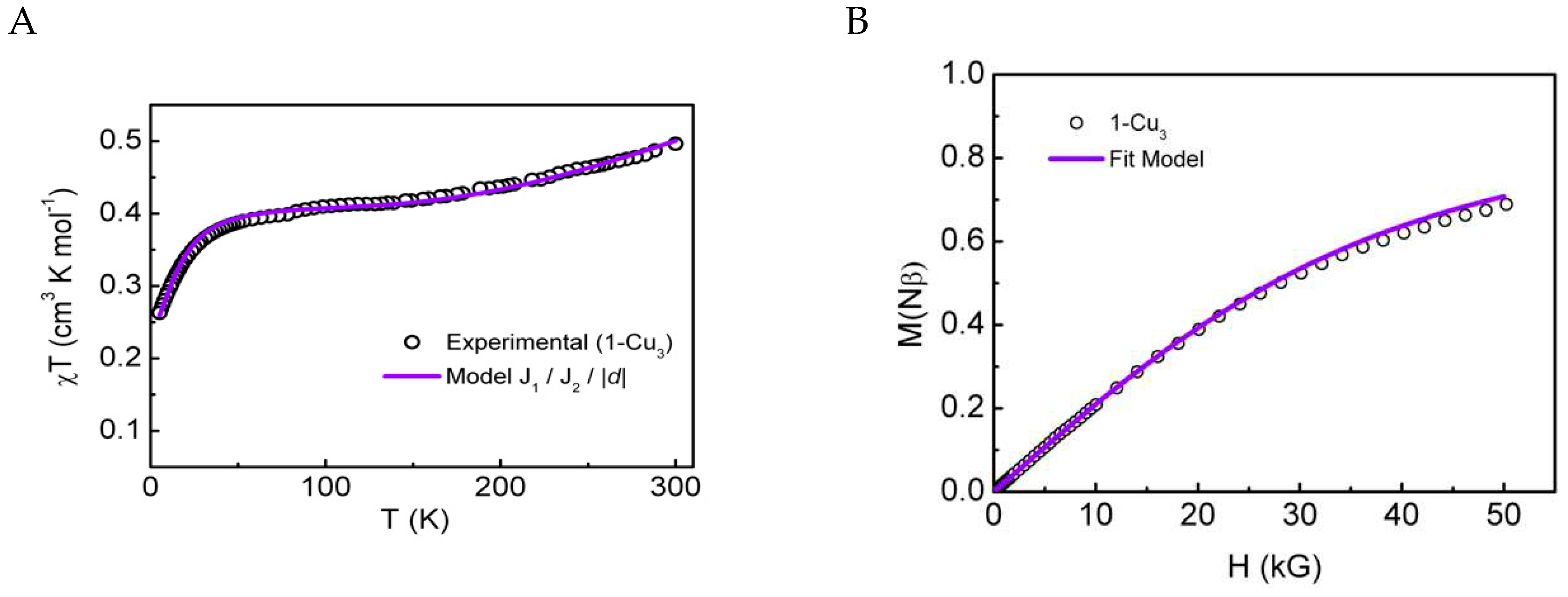
Magnetic Properties. dc Magnetic Measurements
3. Materials and Methods
Synthesis of [Cu3(μ3-OH)(pz)3(Hpz)3][BF4]2 (1-Cu3)
Physical Characterization
X-ray Diffraction
Magnetic Susceptibility Measurements
DFT Calculations of the Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scatena, R.; Massignani, S.; Lanza, A.E.; Zorzi, F.; Monari, M.; Nestola, F.; Pettinari, C.; Pandolfo, L. Synthesis of Coordination Polymers and Discrete Complexes from the Reaction of Copper(II) Carboxylates with Pyrazole: Role of Carboxylates Basicity. Cryst. Growth Des. 2022, 22, 1032–1044. [Google Scholar] [CrossRef]
- Di Nicola, C.; Karabach, Y.Y.; Kirillov, A.M.; Monari, M.; Pandolfo, L.; Pettinari, C.; Pombeiro, A.J.L. Supramolecular assemblies of trinuclear triangular copper(II) secondary building units through hydrogen bonds. Generation of different metal-organic frameworks, valuable catalysts for peroxidative oxidation of alkanes. Inorg. Chem. 2007, 46, 221–30. [Google Scholar] [CrossRef] [PubMed]
- Angaridis, P.A.; Baran, P.; Boča, R.; Cervantes-Lee, F.; Haase, W.; Mezei, G.; Raptis, R.G.; Werner, R. Synthesis and Structural Characterization of Trinuclear CuII −Pyrazolato Complexes Containing μ3-OH, μ3-O, and μ3-Cl Ligands. Magnetic Susceptibility Study of [PPN]2[(μ3-O)Cu3(μ-pz)3Cl3]. Inorg. Chem. 2002, 41, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Bhattacharya, S.; Goswami, A.; Adhikary, A.; Konar, S.; Mondal, R. Designing functional metal-organic frameworks by imparting a hexanuclear copper-based secondary building unit specific properties: Structural correlation with magnetic and photocatalytic activity. Cryst. Growth Des. 2014, 14, 6391–6398. [Google Scholar] [CrossRef]
- Condello, F.; Garau, F.; Lanza, A.; Monari, M.; Nestola, F.; Pandolfo, L.; Pettinari, C. Synthesis and structural characterizations of new coordination polymers generated by the interaction between the trinuclear triangular SBU [Cu3(μ3-OH)(μ-pz)3]2+ and 4,4′-Bipyridine. 3°. Cryst. Growth Des. 2015, 15, 4854–4862. [Google Scholar] [CrossRef]
- Rivera-Carrillo, M.; Chakraborty, I.; Raptis, R.G. Systematic synthesis of a metal organic framework based on triangular Cu3(μ3-OH) secondary building units: From a 0-D complex to a 1-D chain and a 3-D lattice. Cryst. Growth Des. 2010, 10, 2606–2612. [Google Scholar] [CrossRef]
- Trif, M.; Troiani, F.; Stepanenko, D.; Loss, D. Spin-electric coupling in molecular magnets. Phys. Rev. Lett. 2008, 101, 1–4. [Google Scholar] [CrossRef]
- Troiani, F.; Stepanenko, D.; Loss, D. Hyperfine-induced decoherence in triangular spin-cluster qubits. Phys. Rev. B - Condens. Matter Mater. Phys. 2012, 86, 161409. [Google Scholar] [CrossRef]
- Belinsky, M.I. Spin Chirality of Cu3 and V3 Nanomagnets. 1. Rotation Behavior of Vector Chirality, Scalar Chirality, and Magnetization in the Rotating Magnetic Field, Magnetochiral Correlations. Inorg. Chem. 2016, 55, 4078–4090. [Google Scholar] [CrossRef]
- Belinsky, M.I. Spin Chirality of Cu3 and V3 Nanomagnets. 2. Frustration, Temperature, and Distortion Dependence of Spin Chiralities and Magnetization in the Rotating and Tilted Magnetic Fields. Inorg. Chem. 2016, 55, 4091–4109. [Google Scholar] [CrossRef]
- Kintzel, B.; Böhme, M.; Liu, J.; Burkhardt, A.; Mrozek, J.; Buchholz, A.; Ardavan, A.; Plass, W. Molecular electronic spin qubits from a spin-frustrated trinuclear copper complex. Chem. Commun. 2018, 54, 12934–12937. [Google Scholar] [CrossRef] [PubMed]
- Boudalis, A.K. Half-Integer Spin Triangles: Old Dogs, New Tricks. Chem. – A Eur. J. 2021, 27, 7022–7042. [Google Scholar] [CrossRef] [PubMed]
- Spielberg, E.T.; Gilb, A.; Plaul, D.; Geibig, D.; Hornig, D.; Schuch, D.; Buchholz, A.; Ardavan, A.; Plass, W. A Spin-Frustrated Trinuclear Copper Complex Based on Triaminoguanidine with an Energetically Well-Separated Degenerate Ground State. Inorg. Chem. 2015, 54, 3432–3438. [Google Scholar] [CrossRef]
- Sowrey, F.E.; Tilford, C.; Wocadlo, S.; Anson, C.E.; Powell, A.K.; Bennington, S.M.; Montfrooij, W.; Jayasooriya, U. a.; Cannon, R.D. Spin frustration and concealed asymmetry: structure and magnetic spectrum of [Fe3O(O2CPh)6(py)3]ClO4·py†. J. Chem. Soc. Dalt. Trans. 2001, 6, 862–866. [Google Scholar] [CrossRef]
- Tsukerblat, B.S.; Belinskii, M.I.; Fainzil´berg, V.E. Magnetochemistry and Spectroscopy of Transition Metals Exchange Clusters. Sov. Sci. Rev. B Chem. 1987, 9, 337–481. [Google Scholar]
- Toader, A.M.; Buta, M.C.; Cimpoesu, F.; Toma, A.-I.; Zalaru, C.M.; Cinteza, L.O.; Ferbinteanu, M. New Syntheses, Analytic Spin Hamiltonians, Structural and Computational Characterization for a Series of Tri-, Hexa- and Hepta-Nuclear Copper (II) Complexes with Prototypic Patterns. Chemistry (Easton). 2021, 3, 411–439. [Google Scholar] [CrossRef]
- Mathivathanan, L.; Boudalis, A.K.; Turek, P.; Pissas, M.; Sanakis, Y.; Raptis, R.G. Interactions between H-bonded [CuII3(μ3-OH)] triangles; A combined magnetic susceptibility and EPR study. Phys. Chem. Chem. Phys. 2018, 20, 17234–17244. [Google Scholar] [CrossRef]
- Shi, K.; Mathivathanan, L.; Herchel, R.; Boudalis, A.K.; Raptis, R.G. Supramolecular Assemblies of Trinuclear Copper(II)-Pyrazolato Units: A Structural, Magnetic and EPR Study. Chemistry (Easton). 2020, 2, 626–644. [Google Scholar] [CrossRef]
- Mezei, G.; Raptis, R.G.; Telser, J. Trinuclear, antiferromagnetically coupled CuII complex with an EPR spectrum of mononuclear CuII: Effect of alcoholic solvents. Inorg. Chem. 2006, 45, 8841–8843. [Google Scholar] [CrossRef]
- Mukherjee, P.; Drew, M.G.B.; Estrader, M.; Diaz, C.; Ghosh, A. Influence of counter anions on the structures and magnetic properties of trinuclear Cu(II) complexes containing a μ3-OH core and Schiff base ligands. Inorganica Chim. Acta 2008, 361, 161–172. [Google Scholar] [CrossRef]
- Cañon-Mancisidor, W.; Spodine, E.; Paredes-Garcia, V.; Venegas-Yazigi, D. Theoretical description of the magnetic properties of μ3-hydroxo bridged trinuclear copper(II) complexes. J. Mol. Model. 2013, 19, 2835–44. [Google Scholar] [CrossRef]
- Zhou, J.-H.; Liu, Z.; Li, Y.-Z.; Song, Y.; Chen, X.-T.; You, X.-Z. Synthesis, structures and magnetic properties of two copper(II) complexes with pyrazole and pivalate ligands. J. Coord. Chem. 2006, 59, 147–156. [Google Scholar] [CrossRef]
- Davydenko, Y.M.; Demeshko, S.; Pavlenko, V. a.; Dechert, S.; Meyer, F.; Fritsky, I.O. Synthesis, Crystal Structure, Spectroscopic and Magnetically Study of Two Copper(II) Complexes with Pyrazole Ligand. Zeitschrift für Anorg. und Allg. Chemie 2013, 639, 1472–1476. [Google Scholar] [CrossRef]
- Ferrer, S.; Lloret, F.; Pardo, E.; Clemente-Juan, J.M.; Liu-González, M.; García-Granda, S. Antisymmetric Exchange in Triangular Tricopper(II) Complexes: Correlation among Structural, Magnetic, and Electron Paramagnetic Resonance Parameters. Inorg. Chem. 2012, 51, 985–1001. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-J.; Liu, Y.-Z.; Wang, R.-L.; Fu, J.-W.; Xu, J.-Y.; Lou, J.-S. Synthesis, crystal structure and magnetic properties of a trinuclear Cu(II)-pyrazolate complex containing μ3-OH. J. Coord. Chem. 2009, 62, 311–318. [Google Scholar] [CrossRef]
- Shi, K.; Mathivathanan, L.; Boudalis, A.K.; Turek, P.; Chakraborty, I.; Raptis, R.G. Nitrite Reduction by Trinuclear Copper Pyrazolate Complexes: An Example of a Catalytic, Synthetic Polynuclear NO Releasing System. Inorg. Chem. 2019, 58, 7537–7544. [Google Scholar] [CrossRef] [PubMed]
- Massignani, S.; Scatena, R.; Lanza, A.; Monari, M.; Condello, F.; Nestola, F.; Pettinari, C.; Zorzi, F.; Pandolfo, L. Coordination polymers from mild condition reactions of copper(II) carboxylates with pyrazole (Hpz). Influence of carboxylate basicity on the self-assembly of the [Cu3(μ3-OH)(μ-pz)3]2+ secondary building unit. Inorganica Chim. Acta 2017, 455, 618–626. [Google Scholar] [CrossRef]
- Ahmed, B.M.; Mezei, G. From ordinary to extraordinary: Insights into the formation mechanism and pH-dependent assembly/disassembly of nanojars. Inorg. Chem. 2016, 55, 7717–7728. [Google Scholar] [CrossRef]
- Alsalme, A.; Ghazzali, M.; Khan, R.A.; Al-Farhan, K.; Reedijk, J. A novel trinuclear μ3-hydroxido-bridged Cu(II) compound; A molecular cluster, stabilized by hydrogen bonding, bridging pyrazolates, terminal pyrazoles, water and nitrate anions. Polyhedron 2014, 75, 64–67. [Google Scholar] [CrossRef]
- Di Nicola, C.; Garau, F.; Gazzano, M.; Monari, M.; Pandolfo, L.; Pettinari, C.; Pettinari, R. Reactions of a coordination polymer based on the triangular cluster [Cu3(μ3-OH)(μ-pz)3]2+ with strong acids. Crystal structure and supramolecular assemblies of new mono-, tri-, and hexanuclear complexes and coordination polymers. Cryst. Growth Des. 2010, 10, 3120–3131. [Google Scholar] [CrossRef]
- Casarin, M.; Cingolani, A.; Di Nicola, C.; Falcomer, D.; Monari, M.; Pandolfo, L.; Pettinari, C. The Different Supramolecular Arrangements of the Triangular [Cu3(μ3-OH)(μ-pz)3]2+ (pz = Pyrazolate) Secondary Building Units. Synthesis of a Coordination Polymer with Permanent Hexagonal Channels. Cryst. Growth Des. 2007, 7, 676–685. [Google Scholar] [CrossRef]
- Vynohradov, O.S.; Pavlenko, V.A.; Fritsky, I.O.; Gural’skiy, I.A.; Shova, S. Synthesis and Crystal Structure of Copper(II) 9-Azametallacrowns-3 with 4-Iodopyrazole. Russ. J. Inorg. Chem. 2020, 65, 1481–1488. [Google Scholar] [CrossRef]
- Angaroni, M.; Ardizzoia, G.A.; Beringhelli, T.; La Monica, G.; Gatteschi, D.; Masciocchi, N.; Moret, M. Oxidation reaction of [{Cu(Hpz)2Cl}2](Hpz = pyrazole): synthesis of the trinuclear copper(II) hydroxo complexes [Cu3(OH)(pz)3(Hpz)2Cl2]·solv (solv = H2O or tetrahydrofuran). Formation, magnetic properties, and X-ray crystal structure of [Cu3(OH)(pz)3(py)2CI2]·py (py = pyridine). J. Chem. Soc. Dalt. Trans. 1990; 3305–3309. [Google Scholar] [CrossRef]
- Liu, X.; Nie, Y.; Tang, Q.; Tian, A.; Hu, Z.; Yan, J.; Zhang, S. Pyrazole-based trinuclear and mononuclear complexes: synthesis, characterization, DNA interactions and cytotoxicity studies. Transit. Met. Chem. 2021, 46, 481–494. [Google Scholar] [CrossRef]
- Boudalis, A.K.; Rogez, G.; Heinrich, B.; Raptis, R.G.; Turek, P. Towards ionic liquids with tailored magnetic properties: Bmim+ salts of ferro- and antiferromagnetic CuII3 triangles. Dalt. Trans. 2017, 46, 12263–12273. [Google Scholar] [CrossRef]
- Razali, M.R.; Urbatsch, A.; Deacon, G.B.; Batten, S.R. Transition metal complexes of the small cyano anion dicyanonitromethanide [C(CN)2(NO2)]- Dedicated to George Christou on the occasion of his 60th birthday. Polyhedron 2013, 64, 352–364. [Google Scholar] [CrossRef]
- Rivera-Carrillo, M.; Chakraborty, I.; Mezei, G.; Webster, R.D.; Raptis, R.G. Tuning of the [Cu3(μ-O)]4+/5+ redox couple: Spectroscopic evidence of charge delocalization in the mixed-valent [Cu3(μ-O)]5+ species. Inorg. Chem. 2008, 47, 7644–7650. [Google Scholar] [CrossRef]
- Lang, J.; Hewer, J.M.; Meyer, J.; Schuchmann, J.; van Wüllen, C.; Niedner-Schatteburg, G. Magnetostructural correlation in isolated trinuclear iron(III) oxo acetate complexes. Phys. Chem. Chem. Phys. 2018, 20, 16673–16685. [Google Scholar] [CrossRef]
- Antkowiak, M.; Kamieniarz, G.; Florek, W. Comment on “Magnetostructural correlations in isolated trinuclear iron(III) oxo acetate complexes” by J. Lang, J. M. Hewer, J. Meyer, J. Schuchmann, C. van Wüllen and G. Niedner-Schatteburg,: Phys. Chem. Chem. Phys., 2018, 20, 16673. Phys. Chem. Chem. Phys. 2019, 21, 504. [Google Scholar] [CrossRef]
- van Wüllen, C.; Lang, J.; Niedner-Schatteburg, G. Reply to the ‘Comment on “Magnetostructural correlations in isolated trinuclear iron(III) oxo acetate complexes”’ by M. Antkowiak, G. Kamieniarz and W. Florek, Phys. Chem. Chem. Phys., 2018, 20, DOI: 10.1039/C8CP04691C. Phys. Chem. Chem. Phys. 2019, 21, 505–506. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Afrati, T.; Dendrinou-Samara, C.; Raptopoulou, C.; Terzis, A.; Tangoulis, V.; Tsipis, A.; Kessissoglou, D.P. Experimental and theoretical study of the antisymmetric magnetic behavior of copper inverse-9-metallacrown-3 compounds. Inorg. Chem. 2008, 47, 7545–7555. [Google Scholar] [CrossRef]
- Wang, L.-L.; Sun, Y.-M.; Yu, Z.-Y.; Qi, Z.-N.; Liu, C.-B. Theoretical Investigation on Triagonal Symmetry Copper Trimers: Magneto-Structural Correlation and Spin Frustration. J. Phys. Chem. A 2009, 113, 10534–10539. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Solomon, E.I. Ground-state electronic and magnetic properties of a μ3-oxo-bridged trinuclear Cu(II) complex: correlation to the native intermediate of the multicopper oxidases. Inorg. Chem. 2005, 44, 8076–86. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O. V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2 : a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Schafer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted quality for atoms Li to Kr Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Jaguar Jaguar 5.5. version 5.5; Schrödinger, LLC Portland, OR 2003.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. G09. Gaussian 09 (Revision D.2), Gaussian, Inc., Pittsburgh, PA 2009.
- Ruiz, E.; Alvarez, S.; Cano, J.; Polo, V. About the calculation of exchange coupling constants using density-functional theory: the role of the self-interaction error. J. Chem. Phys. 2005, 123, 164110. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E. Theoretical Study of the Exchange Coupling in Large Polynuclear Transition Metal Complexes Using DFT Methods. Struct. Bond. 2004, 113, 71–102. [Google Scholar] [CrossRef]
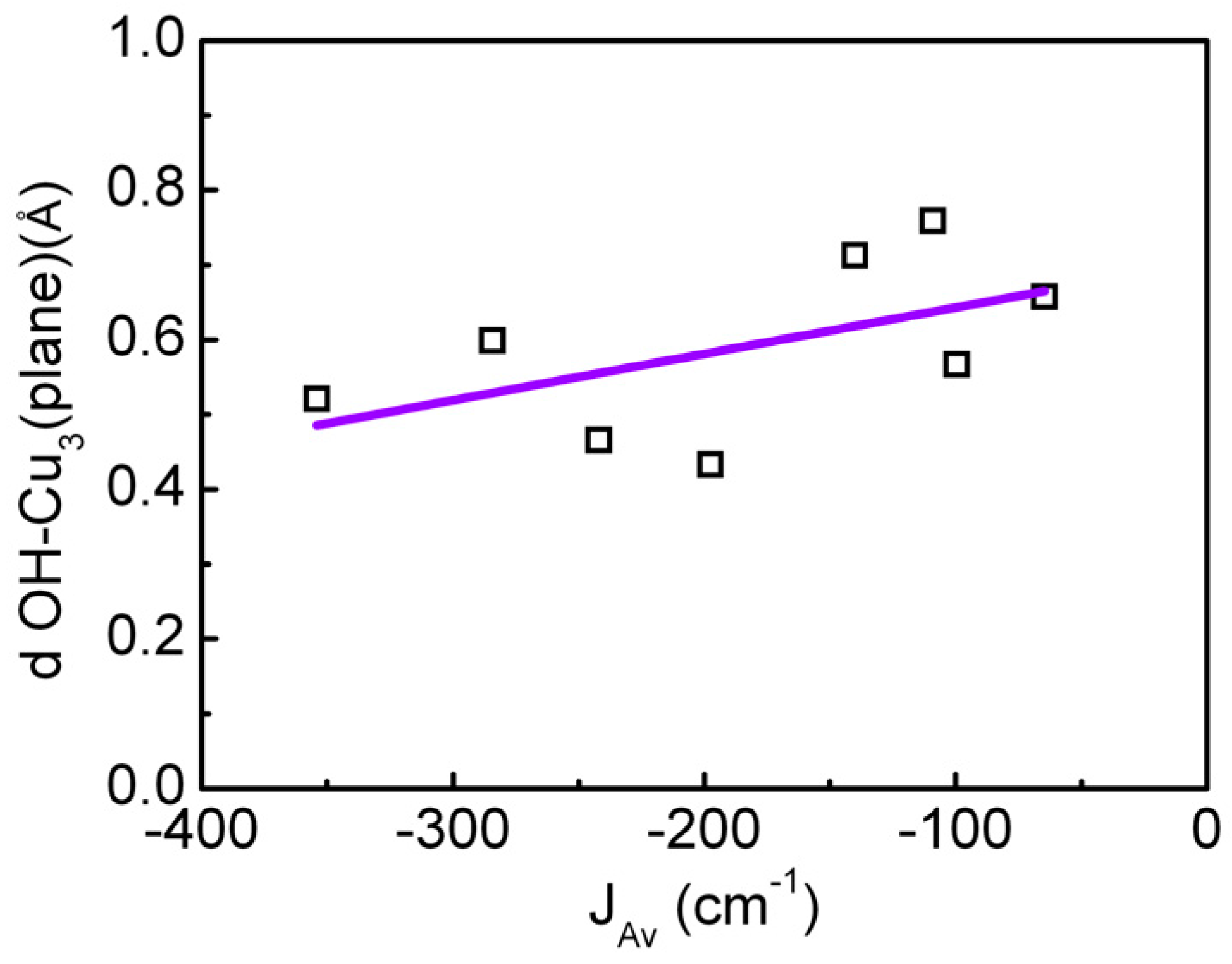
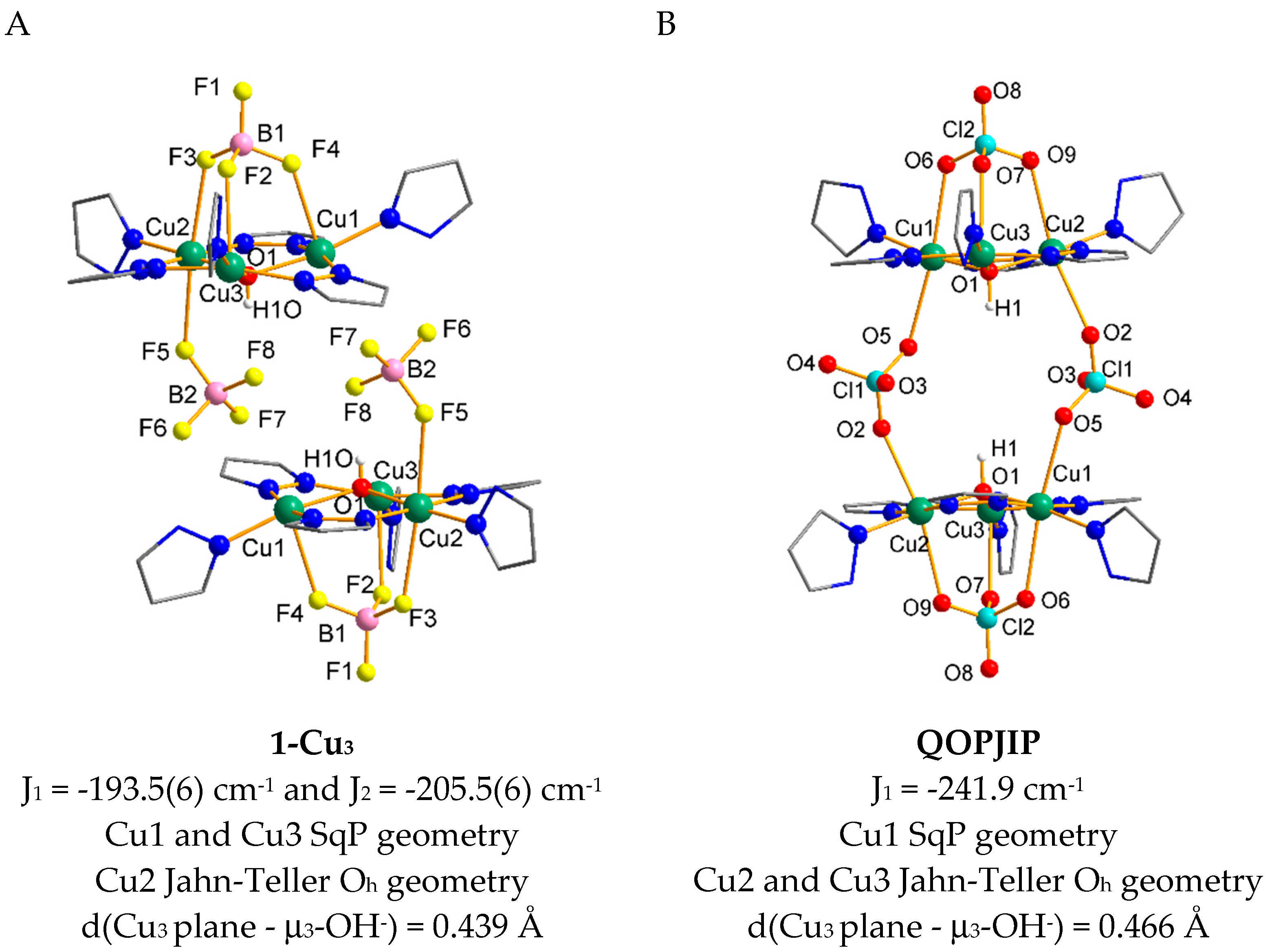
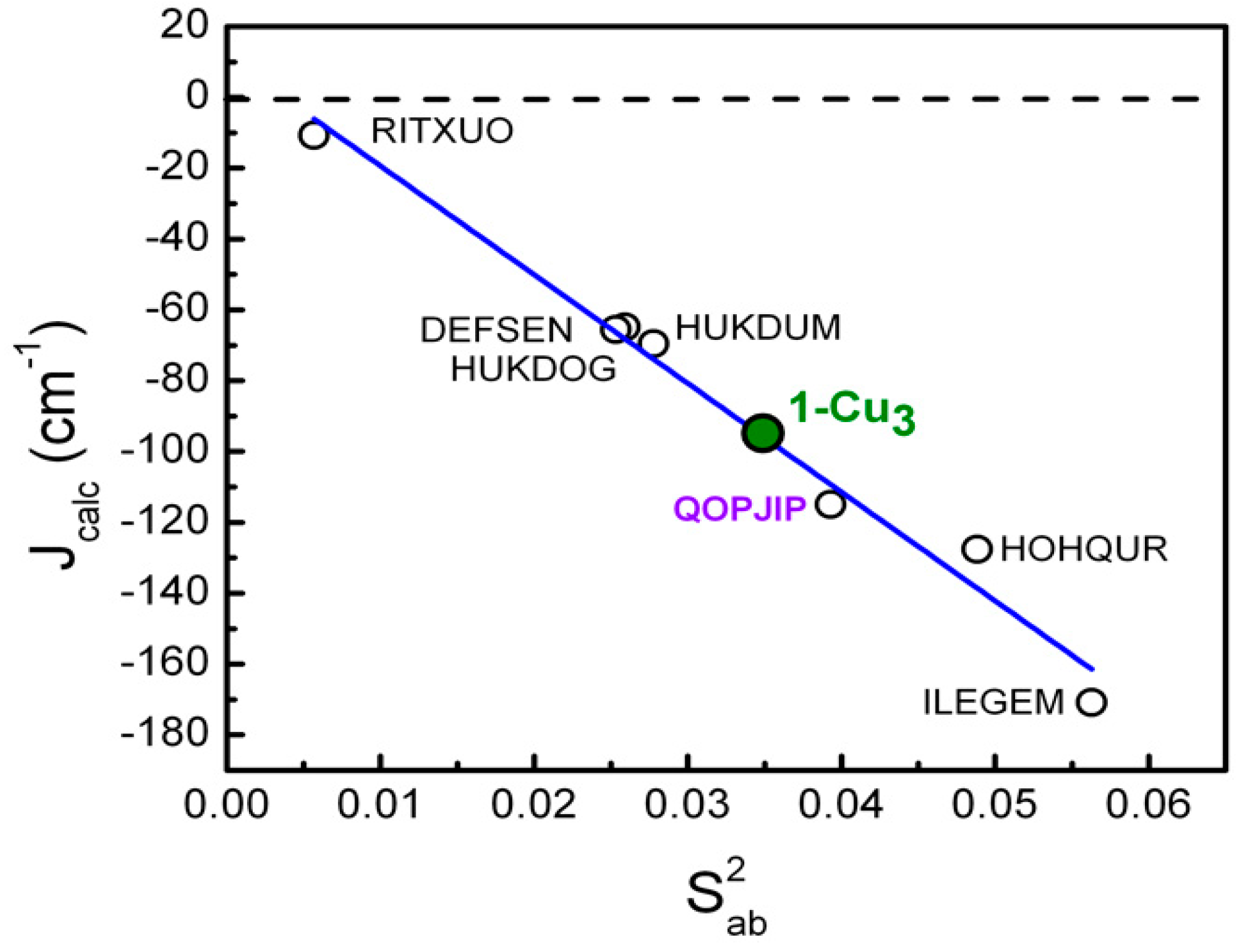
| CCDC Code |
CuII-CuII (Å) | Cu3(plane)-OH (Å) | Cun-OH (Å) | Cun-N(pz) (Å) | Cun-1-Cun-Cun+1 (°) | Cun-OH-Cun+1 (°) | Ref. |
|---|---|---|---|---|---|---|---|
| 1-Cu3 | 3.3740(8) 3.3574(8) 3.3702(9) |
0.439 | 2.005(3) 1.978(3) 1.995(3) |
1.942(4) to 1.965(3) | 59.71(2) 60.09(2) 60.20(2) |
115.8(2) 115.3(2) 114.8(2) |
This work |
| AMACIC | 3.3020(6) 3.2561(5) 3.3927(6) |
0.553 | 1.977(2) 2.001(2) 2.005(2) |
1.932(3) to 1.947(2) | 58.19(1) 62.30(1) 59.51(1) |
112.20(9) 116.9(1) 108.73(9) |
[27] |
| ASUNIN | 3.3456(1) 3.3266(6) 3.3456(1) |
0.510 | 2.011(1) 1.932(5) 2.042(5) |
1.918(3) to 1.952(1) | 59.62(1) 60.19(1) 60.19(1) |
116.1(1) 113.6(2) 111.3(1) |
[28] |
| BOFLEP | 3.349(2) 3.239(2) 3.355(2) |
0.580 | 2.005(4) 2.001(4) 1.995(3) |
1.924(5) to 1.958(4) | 57.78(2) 61.20(2) 61.02(2) |
113.5(2) 108.3(2) 114.1(2) |
[29] |
| DEFSEN | 3.384(1) 3.2503(9) 3.2950(9) |
0.567 | 1.975(3) 2.008(3) 2.000(2) |
1.928(4) to 1.948(4) | 58.22(2) 59.52(2) 62.26(2) |
116.3(1) 108.4(1) 112.0(1) |
[22] |
| DIBXOC | 3.2972(5) 3.2972(5) 3.3843(4) |
0.609 | 2.008(2) 2.030(2) 2.008(2) |
1.946(2) to 1.1.957 | 59.12(1) 61.76(1) 59.12(1) |
109.5(1) 109.5(1) 114.9(1) |
[31] |
| EGIXOK | 3.3540(5) 3.3874(6) 3.4036(6) |
0.363 | 1.979(2) 1.993(2) 1.985(3) |
1.921(3) to 1.941(2) | 60.16(1) 60.64(1) 59.19(1) |
115.2(2) 116.7(2) 118.3(2) |
[26] |
| EGIXUQ | 3.268(1) 3.379(1) 3.350(1) |
0.148 | 1.936(5) 1.943(4) 1.913(5) |
1.914(5) to 1.942(5) | 61.39(2) 60.50(2) 58.11(2) |
114.8(2) 121.0(2) 122.4(2) |
[26] |
| EHOLIZ | 3.389(5) 3.389(5) 3.389(5) |
0.274 | 2.046(10) 1.941(10) 1.941(10) |
1.92(1) to 1.97(2) | 60.0(1) 60.0(1) 60.0(1) |
116(1) 122(1) 116(1) |
[32] |
| JEWWEO | 3.3416(8) 3.3825(8) 3.3502(7) |
0.461 | 1.988(3) 2.010(3) 1.982(3) |
1.923(4) to 1.943(4) | 60.73(2) 59.76(2) 59.51(2) |
113.4(1) 115.9(2) 115.1(2) |
[2] |
| JEWWIS | 3.387(1) 3.309(1) 3.350(1) |
0.486 | 1.976(6) 2.021(5) 1.985(6) |
1.919(7) to 1.952(8) | 58.84(3) 60.03(3) 61.13(3) |
115.9(3) 111.4(3) 115.5(3) |
[2] |
| MUZQUU | 3.3696(5) 3.3461(5) 3.3788(5) |
0.455 | 1.982(2) 2.003(2) 2.001(2) |
1.947(3) to 1.960(2) | 59.45(1) 60.41(1) 60.14(1) |
115.45(9) 113.39(9) 116.05(9) |
[6] |
| *QIMSIQ-a | 3.2977(4) 3.1704(4) 3.3126(4) |
0.688 | 2.016(2) 2.012(2) 1.987(2) |
1.938(2) to 1.959(2) | 57.32(1) 61.58(1) 61.10(1) |
109.91(7) 104.90(7) 111.68(8) |
[23] |
| *QIMSIQ-b | 3.3911(4) 3.3023(4) 3.3214(4) |
0.512 | 2.000(1) 1.994(2) 1.989(2) |
1.944(2) to 1.959(2) | 58.93(1) 59.48(1) 61.59(1) |
116.20(8) 111.99(7) 112.72(7) |
[23] |
| *QIMSOW-a | 3.2559(7) 3.342(1) 3.2345(9) |
0.713 | 1.992(3) 2.032(3) 2.044(3) |
1.941(4) to 1.958(4) | 61.98(2) 58.69(2) 59.32(2) |
108.0(1) 110.2(1) 106.6)1) |
[23] |
| *QIMSOW-b | 3.2045(6) 3.1837(8) 3.2007(9) |
0.759 | 1.985(3) 2.011(2) 1.990(3) |
1.948(3) to 1.960(4) | 59.61(2) 60.13(2) 60.25(2) |
106.6(1) 105.4(1) 107.3(1) |
[23] |
| QOPJIP | 3.355(1) 3.386(1) 3.368(1) |
0.466 | 1.994(5) 2.000(4) 2.007(5) |
1.929(6) to 1.958(6) | 59.94(3) 60.49(3) 59.57(3) |
114.3(2) 114.4(2) 115.6(2) |
[25] |
| QUSMEX | 3.344(2) 3.286(2) 3.392(2) |
0.475 | 1.955(8) 2.017(6) 1.992(9) |
1.933(9) to 1.978(9) | 58.39(4) 61.53(4) 60.07(4) |
114.7(4) 110.1(4) 118.5(4) |
[30] |
| QUSMIB | 3.289(2) 3.289(2) 3.289(2) |
0.489 | 1.961(1) 1.962(1) 1.960(1) |
1.89(1) to 1.930(8) | 60.00(4) 60.00(4) 60.00(4) |
114.0(1) 114.0(1) 114.0(1) |
[30] |
| QUSMUN | 3.3550(5) 3.3615(5) 3.3439(6) |
0.471 | 1.985(2) 2.005(2) 1.987(2) |
1.937(2) to 1.951(2) | 60.24(1) 59.72(1) 60.04(1) |
114.42(9) 114.68(9) 114.65(9) |
[30] |
| RETQUD | 3.3833(6) 3.3629(6) 3.3769(5) |
0.542 | 2.026(2) 2.028(3) 2.013(2) |
1.942(3) to 1.961(2) | 59.66(1) 60.07(1) 60.26(1) |
113.1(1) 112.7(1) 113.5(1) |
[19] |
| RETRAK | 3.365(1) 3.3650(9) 3.3886(8) |
0.565 | 2.023(3) 2.041(3) 2.019(2) |
1.933(3) to 1.962(5) | 59.77(2) 60.46(2) 59.77(2) |
111.8(1) 111.9(1) 113.9(1) |
[19] |
| RETREO | 3.3442(6) 3.3975(6) 3.3022(7) |
0.625 | 2.024(2) 2.033(2) 2.038(2) |
1.936(3) to 1.957(3) | 61.48(1) 58.65(1) 59.87(1) |
111.0(1) 113.1(1) 108.7(1) |
[19] |
| RUYGEX | 3.4471(9) 3.206(1) 3.4227(9) |
0.524 | 1.987(3) 2.024(3) 2.035(3) |
1.940(4) to 1.953(4) | 55.55(2) 62.01(2) 62.44(2) |
118.5(1) 104.4(1) 117.3(1) |
[3] |
| RUYGIB | 3.2473(8) 3.4007(6) 3.4305(8) |
0.507 | 2.014(3) 2.017(2) 1.989(2) |
1.933(4) to 1.952(4) | 61.16(1) 62.08(1) 56.76(1) |
107.3(1) 116.2(1) 118.0(1) |
[3] |
| RUYHEY | 3.414(1) 3.253(1) 3.277(1) |
0.613 | 2.012(5) 2.006(4) 2.016(3) |
1.929(7) to 1.950(5) | 58.15(3) 58.82(3) 63.03(3) |
116.4(2) 108.0(2) 108.9(2) |
[3] |
| SIJKOL | 3.112(1) 3.321(1) 3.321(1) |
0.658 | 2.000(1) 2.000(1) 1.977(1) |
1.942(1) to 1.967(4) | 62.06(1) 62.06(1) 55.88(1) |
102.2(1) 113.3(1) 113.3(1) |
[33] |
| UZIWEI | 3.3695(6) 3.2840(5) 3.2953(5) |
0.595 | 1.998(2) 2.004(2) 2.104(2) |
1.937(2) to 1.952(2) | 59.03(1) 59.36(1) 61.61(1) |
114.68(8) 109.64(8) 110.42(8) |
[34] |
| VAZCOR | 3.1913(9) 3.391(1) 3.353(1) |
0.599 | 2.032(4) 2.030(4) 1.959(3) |
1.933(6) to 1.960(5) | 62.36(2) 61.16(2) 56.49(2) |
103.6(2) 116.4(2) 114.3(2) |
[35] |
| VIMYEX | 3.2639(7) 3.1851(8) 3.299(1) |
0.712 | 2.027(2) 1.991(2) 2.003(2) |
1.935(2) to 1.950(2) | 58.06(1) 61.52(1) 60.41(1) |
108.67(7) 105.79(7) 109.9387) |
[36] |
| XOKXAX | 3.347(1) 3.403(1) 3.320(1) |
0.491 | 1.998(4) 2.000(4) 2.000(4) |
1.939(5) to 1.963(6) | 61.38(2) 58.92(2) 59.70(2) |
113.6(2) 116.6(2) 112.3(2) |
[37] |
| YIFGIG | 3.3500(8) 3.2440(7) 3.3519(6) |
0.521 | 1.978(2) 1.968(2) 2.008(2) |
1.928(2) to 1.953(2) | 57.90(1) 61.08(1) 61.02(1) |
116.20(9) 109.37(9) 114.48(9) |
[17] |
| CCDC Code |
d(CuII-CuII) (Å) | Cu3(plane)-OH (Å) | J(CuII···CuII) (cm-1) |
g | zJ´ (cm-1) |
|GZ| (cm-1) |
Ref |
|---|---|---|---|---|---|---|---|
| 1-Cu3 | 3.3740(8) 3.3574(8) 3.3702(9) |
0.439 | -193.5(6) -205.5(6) |
2.09 | - | 28 | This work |
| BOFLEP# | 3.349(2) 3.239(2) 3.355(2) |
0.580 | - | - | - | - | [29] |
| DEFSEN | 3.384(1) 3.2503(9) 3.2950(9) |
0.567 | -117.7 -90.3 |
2.047 | -3.0 | - | [22] |
| QISOW-a* | 3.2559(7) 3.342(1) 3.2345(9) |
0.713 | -140 | 2.07 | - | - | [23] |
| QISOW-b* | 3.2045(6) 3.1837(8) 3.2007(9) |
0.759 | -109 | 2.07 | - | - | [23] |
| QOPJIP | 3.355(1) 3.386(1) 3.368(1) |
0.466 | -241.9 | 2.07 | -23.0 | - | [25] |
| SIJKOL | 3.112(1) 3.321(1) 3.321(1) |
0.658 | -148 -23 |
2.17 | - | - | [33] |
| VAZCOR | 3.1913(9) 3.391(1) 3.353(1) |
0.599 | -298 -257 |
2.12 | -0.37 | 18.2 | [35] |
| YIFGIG | 3.3500(8) 3.2440(7) 3.3519(6) |
0.521 | -392 -278 |
2.09 | - | 31.2 | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





