Submitted:
21 May 2023
Posted:
23 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Conclusion
Author Contributions
Funding
Ethics approval and consent to participate
Consent for publication
Availability of data and material
Acknowledgments
Conflicts of Interest
References
- Trapp, B.D.; Peterson, J.; Ransohoff, R.M.; Rudick, R.; Mörk, S.; Bö, L. Axonal Transection in the Lesions of Multiple Sclerosis. New Engl. J. Med. 1998, 338, 278–285. [CrossRef]
- Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann Neurol. 2000;47(6):707-17. [CrossRef]
- Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology. 2014;83(3):278-86. [CrossRef]
- Hauser, S.L.; Bhan, A.K.; Gilles, F.; Kemp, M.; Kerr, C.; Weiner, H.L. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann. Neurol. 1986, 19, 578–587. [CrossRef]
- Olsson, T.; Zhi, W.W.; Höjeberg, B.; Kostulas, V.; Jiang, Y.P.; Anderson, G.; Ekre, H.P.; Link, H. Autoreactive T lymphocytes in multiple sclerosis determined by antigen-induced secretion of interferon-gamma.. J. Clin. Investig. 1990, 86, 981–985. [CrossRef]
- Ota, K.; Matsui, M.; Milford, E.L.; Mackin, G.A.; Weiner, H.L.; Hafler, D.A. T-cell recognition of an immuno-dominant myelin basic protein epitope in multiple sclerosis. Nature 1990, 346, 183–187. [CrossRef]
- Brück, W.; Sommermeier, N.; Bergmann, M.; Zettl, U.; Goebel, H.H.; Kretzschmar, H.A.; Lassmann, H. CHAPTER 14: Macrophages in Multiple Sclerosis. Immunobiology 1996, 195, 588–600. [CrossRef]
- Papadopoulos, D.; Dukes, S.; Patel, R.; Nicholas, R.; Vora, A.; Reynolds, R. Substantial Archaeocortical Atrophy and Neuronal Loss in Multiple Sclerosis. Brain Pathol. 2009, 19, 238–253. [CrossRef]
- Wegner, C.; Esiri, M.M.; Chance, S.A.; Palace, J.; Matthews, P.M. Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology 2006, 67, 960–967. [CrossRef]
- Bitsch, A.; Bruhn, H.; Vougioukas, V.; Stringaris, A.; Lassmann, H.; Frahm, J.; Brück, W. Inflammatory CNS Demyelination: Histopathologic Correlation with In Vivo Quantitative Proton MR Spectroscopy. 1999, 20, 1619–1627.
- Brown, J.W.L.; Coles, A.; Horakova, D.; Havrdova, E.; Izquierdo, G.; Prat, A.; Girard, M.; Duquette, P.; Trojano, M.; Lugaresi, A.; et al. Association of Initial Disease-Modifying Therapy With Later Conversion to Secondary Progressive Multiple Sclerosis. JAMA 2019, 321, 175–187. [CrossRef]
- Andersen, O.; Elovaara, I.; Färkkilä, M.; Hansen, H.J.; I Mellgren, S.; Myhr, K.-M.; Sandberg-Wollheim, M.; Sørensen, P.S. Multicentre, randomised, double blind, placebo controlled, phase III study of weekly, low dose, subcutaneous interferon beta-1a in secondary progressive multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2004, 75, 706–710. [CrossRef]
- Wolinsky, J.S.; Narayana, P.A.; O'Connor, P.; Coyle, P.K.; Ford, C.; Johnson, K.; Miller, A.; Pardo, L.; Kadosh, S.; Ladkani, D.; et al. Glatiramer acetate in primary progressive multiple sclerosis: Results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann. Neurol. 2007, 61, 14–24. [CrossRef]
- Hawker, K.; O'Connor, P.; Freedman, M.S.; Calabresi, P.A.; Antel, J.; Simon, J.; Hauser, S.; Waubant, E.; Vollmer, T.; Panitch, H.; et al. Rituximab in patients with primary progressive multiple sclerosis: Results of a randomized double-blind placebo-controlled multicenter trial. Ann. Neurol. 2009, 66, 460–471. [CrossRef]
- Barzegar, M.; Najdaghi, S.; Afshari-Safavi, A.; Nehzat, N.; Mirmosayyeb, O.; Shaygannejad, V. Early predictors of conversion to secondary progressive multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 54, 103115. [CrossRef]
- Kalincik, T.; Diouf, I.; Sharmin, S.; Malpas, C.; Spelman, T.; Horakova, D.; Havrdova, E.K.; Trojano, M.; Izquierdo, G.; Lugaresi, A.; et al. Effect of Disease-Modifying Therapy on Disability in Relapsing-Remitting Multiple Sclerosis Over 15 Years. Neurology 2020, 96, e783–e797. [CrossRef]
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nature Reviews Neuroscience. 2006;7(1):41-53. [CrossRef]
- Plumb, J.; McQuaid, S.; Mirakhur, M.; Kirk, J. Abnormal Endothelial Tight Junctions in Active Lesions and Normal-appearing White Matter in Multiple Sclerosis. Brain Pathol. 2002, 12, 154–169. [CrossRef]
- Kirk, J.; Plumb, J.; Mirakhur, M.; McQuaid, S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood–brain barrier leakage and active demyelination. J. Pathol. 2003, 201, 319–327. [CrossRef]
- Sheikh, M.H.; Henson, S.M.; Loiola, R.A.; Mercurio, S.; Colamatteo, A.; Maniscalco, G.T.; De Rosa, V.; McArthur, S.; Solito, E. Immuno-metabolic impact of the multiple sclerosis patients’ sera on endothelial cells of the blood-brain barrier. J. Neuroinflammation 2020, 17, 153. [CrossRef]
- Schreibelt, G.; Musters, R.J.P.; Reijerkerk, A.; de Groot, L.R.; van der Pol, S.M.A.; Hendrikx, E.M.L.; Döpp, E.D.; Dijkstra, C.D.; Drukarch, B.; de Vries, H.E. Lipoic Acid Affects Cellular Migration into the Central Nervous System and Stabilizes Blood-Brain Barrier Integrity. J. Immunol. 2006, 177, 2630–2637. [CrossRef]
- Goes, A.; Wouters, D.; Pol, S.M.A.; Huizinga, R.; Ronken, E.; Adamson, P.; Greenwood, J.; Dijkstra, C.D.; Vries, H.E. Reactive oxygen species enhance the migration of monocytes across the blood-brain barrier in vitro. FASEB J. 2001, 15, 1852–1854. [CrossRef]
- Gray, E.; Thomas, T.L.; Betmouni, S.; Scolding, N.; Love, S. Elevated myeloperoxidase activity in white matter in multiple sclerosis. Neurosci. Lett. 2008, 444, 195–198. [CrossRef]
- Agrawal, S.M.; Williamson, J.; Sharma, R.; Kebir, H.; Patel, K.; Prat, A.; Yong, V.W. Extracellular matrix metalloproteinase inducer shows active perivascular cuffs in multiple sclerosis. Brain 2013, 136, 1760–1777. [CrossRef]
- Bar-Or, A.; Nuttall, R.K.; Duddy, M.; Alter, A.; Kim, H.J.; Ifergan, I.; Pennington, C.J.; Bourgoin, P.; Edwards, D.R.; Yong, V.W. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain 2003, 126, 2738–2749. [CrossRef]
- Song, J.; Wu, C.; Korpos, E.; Zhang, X.; Agrawal, S.M.; Wang, Y.; Faber, C.; Schäfers, M.; Körner, H.; Opdenakker, G.; et al. Focal MMP-2 and MMP-9 Activity at the Blood-Brain Barrier Promotes Chemokine-Induced Leukocyte Migration. Cell Rep. 2015, 10, 1040–1054. [CrossRef]
- Kanesaka, T.; Mori, M.; Hattori, T.; Oki, T.; Kuwabara, S. Serum matrix metalloproteinase-3 levels correlate with disease activity in relapsing-remitting multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2006, 77, 185–188. [CrossRef]
- Cossins, J.A.; Clements, J.M.; Ford, J.; Miller, K.M.; Pigott, R.; Vos, W.; Van Der Valk, P.; De Groot, C.J.A. Enhanced expression of MMP-7 and MMP-9 in demyelinating multiple sclerosis lesions. Acta Neuropathol. 1997, 94, 590–598. [CrossRef]
- Maeda, A.; Sobel, R.A. Matrix Metalloproteinases in the Normal Human Central Nervous System, Microglial Nodules, and Multiple Sclerosis Lesions. J. Neuropathol. Exp. Neurol. 1996, 55, 300–309. [CrossRef]
- Alexander JS, Harris MK, Wells SR, Mills G, Chalamidas K, Ganta VC; et al. Alterations in serum MMP-8, MMP-9, IL-12p40 and IL-23 in multiple sclerosis patients treated with interferon-beta1b. Mult Scler. 2010;16(7):801-9. [CrossRef]
- Charabati, M.; Grasmuck, C.; Ghannam, S.; Bourbonnière, L.; Fournier, A.P.; Lécuyer, M.-A.; Tastet, O.; Kebir, H.; Rébillard, R.-M.; Hoornaert, C.; et al. DICAM promotes T H 17 lymphocyte trafficking across the blood-brain barrier during autoimmune neuroinflammation. Sci. Transl. Med. 2022, 14, eabj0473. [CrossRef]
- Jung YK, Jin JS, Jeong JH, Kim HN, Park NR, Choi JY. DICAM, a novel dual immunoglobulin domain containing cell adhesion molecule interacts with alphavbeta3 integrin. J Cell Physiol. 2008;216(3):603-14. [CrossRef]
- Han, S.-W.; Jung, Y.-K.; Lee, E.-J.; Park, H.-R.; Kim, G.-W.; Jeong, J.-H.; Han, M.-S.; Choi, J.-Y. DICAM inhibits angiogenesis via suppression of AKT and p38 MAP kinase signalling. Cardiovasc. Res. 2013, 98, 73–82. [CrossRef]
- Sen MK, Almuslehi MSM, Shortland PJ, Coorssen JR, Mahns DA. Revisiting the Pathoetiology of Multiple Sclerosis: Has the Tail Been Wagging the Mouse? Front Immunol. 2020;11:572186. [CrossRef]
- Huseby, E.S.; Liggitt, D.; Brabb, T.; Schnabel, B.; Öhlén, C.; Goverman, J. A Pathogenic Role for Myelin-Specific Cd8+ T Cells in a Model for Multiple Sclerosis. J. Exp. Med. 2001, 194, 669–676. [CrossRef]
- Viglietta, V.; Baecher-Allan, C.; Weiner, H.L.; Hafler, D.A. Loss of Functional Suppression by CD4+CD25+ Regulatory T Cells in Patients with Multiple Sclerosis. J. Exp. Med. 2004, 199, 971–979. [CrossRef]
- Jelcic I, Al Nimer F, Wang J, Lentsch V, Planas R, Jelcic I; et al. Memory B Cells Activate Brain-Homing, Autoreactive CD4(+) T Cells in Multiple Sclerosis. Cell. 2018;175(1):85-100.e23. [CrossRef]
- Titus HE, Chen Y, Podojil JR, Robinson AP, Balabanov R, Popko B; et al. Pre-clinical and Clinical Implications of “Inside-Out” vs. “Outside-In” Paradigms in Multiple Sclerosis Etiopathogenesis. Frontiers in Cellular Neuroscience. 2020;14. [CrossRef]
- Patsopoulos NA, Baranzini SE, Santaniello A, Shoostari P, Cotsapas C, Wong G; et al. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 2019;365(6460):eaav7188. [CrossRef]
- Ziaei, A.; Garcia-Miralles, M.; Radulescu, C.I.; Sidik, H.; Silvin, A.; Bae, H.; Bonnard, C.; Yusof, N.A.B.M.; Bardile, C.F.; Tan, L.J.; et al. Ermin deficiency leads to compromised myelin, inflammatory milieu, and susceptibility to demyelinating insult. Brain Pathol. 2022, 32, e13064. [CrossRef]
- Barnett, M.H.; Prineas, J.W. Relapsing and remitting multiple sclerosis: Pathology of the newly forming lesion. Ann. Neurol. 2004, 55, 458–468. [CrossRef]
- Kerlero de Rosbo N, Milo R, Lees MB, Burger D, Bernard CC, Ben-Nun A. Reactivity to myelin antigens in multiple sclerosis. Peripheral blood lymphocytes respond predominantly to myelin oligodendrocyte glycoprotein. J Clin Invest. 1993;92(6):2602-8. [CrossRef]
- Reindl, M.; Linington, C.; Brehm, U.; Egg, R.; Dilitz, E.; Deisenhammer, F.; Poewe, W.; Berger, T. Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: A comparative study. Brain 1999, 122, 2047–2056. [CrossRef]
- Gardinier, M.V.; Amiguet, P.; Linington, C.; Matthieu, J. Myelin/oligodendrocyte glycoprotein is a unique member of the immunoglobulin superfamily. J. Neurosci. Res. 1992, 33, 177–187. [CrossRef]
- Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296-301. [CrossRef]
- Lang, H.L.; Jacobsen, H.; Ikemizu, S.; Andersson, C.; Harlos, K.; Madsen, L.; Hjorth, P.; Sondergaard, L.; Svejgaard, A.; Wucherpfennig, K.; et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat. Immunol. 2002, 3, 940–943. [CrossRef]
- Holmøy, T.; Kvale, E..; Vartdal, F. Cerebrospinal fluid CD4+T cells from a multiple sclerosis patient cross-recognize Epstein-Barr virus and myelin basic protein. J. NeuroVirology 2004, 10, 278–283. [CrossRef]
- Garzelli, C.; E Taub, F.; E Scharff, J.; Prabhakar, B.S.; Ginsberg-Fellner, F.; Notkins, A.L. Epstein-Barr virus-transformed lymphocytes produce monoclonal autoantibodies that react with antigens in multiple organs. J. Virol. 1984, 52, 722–725. [CrossRef]
- Serafini, B.; Rosicarelli, B.; Franciotta, D.; Magliozzi, R.; Reynolds, R.; Cinque, P.; Andreoni, L.; Trivedi, P.; Salvetti, M.; Faggioni, A.; et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J. Exp. Med. 2007, 204, 2899–2912. [CrossRef]
- Esfahani, B.S.; Gharesouran, J.; Ghafouri-Fard, S.; Talebian, S.; Arsang-Jang, S.; Omrani, M.D.; Taheri, M.; Rezazadeh, M. Down-regulation of ERMN expression in relapsing remitting multiple sclerosis. Metab. Brain Dis. 2019, 34, 1261–1266. [CrossRef]
- Oveland, E.; Ahmad, I.; Lereim, R.R.; Kroksveen, A.C.; Barsnes, H.; Guldbrandsen, A.; Myhr, K.-M.; Bø, L.; Berven, F.S.; Wergeland, S. Cuprizone and EAE mouse frontal cortex proteomics revealed proteins altered in multiple sclerosis. Sci. Rep. 2021, 11, 1–16. [CrossRef]
- Schirmer, L.; Velmeshev, D.; Holmqvist, S.; Kaufmann, M.; Werneburg, S.; Jung, D.; Vistnes, S.; Stockley, J.H.; Young, A.; Steindel, M.; et al. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 2019, 573, 75–82. [CrossRef]
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117(2):145-52. [CrossRef]
- Ajami, B.; Bennett, J.L.; Krieger, C.; McNagny, K.M.; Rossi, F.M.V. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 2011, 14, 1142–1149. [CrossRef]
- Mildner, A.; Schmidt, H.; Nitsche, M.; Merkler, D.; Hanisch, U.-K.; Mack, M.; Heikenwalder, M.; Brück, W.; Priller, J.; Prinz, M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 2007, 10, 1544–1553. [CrossRef]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.M.; Liu, Y.-J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [CrossRef]
- Geissmann, F.; Jung, S.; Littman, D.R. Blood Monocytes Consist of Two Principal Subsets with Distinct Migratory Properties. Immunity 2003, 19, 71–82. [CrossRef]
- Yona, S.; Kim, K.-W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate Mapping Reveals Origins and Dynamics of Monocytes and Tissue Macrophages under Homeostasis. Immunity 2013, 38, 79–91. [CrossRef]
- Meghraoui-Kheddar A, Barthelemy S, Boissonnas A, Combadière C. Revising CX3CR1 Expression on Murine Classical and Non-classical Monocytes. Front Immunol. 2020;11:1117. [CrossRef]
- Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N; et al. Localization of fractalkine and CX3CR1 mRNAs in rat brain: Does fractalkine play a role in signaling from neuron to microglia? FEBS Lett. 1998;429(2):167-72. [CrossRef]
- Harrison, J.K.; Jiang, Y.; Chen, S.; Xia, Y.; Maciejewski, D.; McNamara, R.K.; Streit, W.J.; Salafranca, M.N.; Adhikari, S.; Thompson, D.A.; et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl. Acad. Sci. USA 1998, 95, 10896–10901. [CrossRef]
- Lampron, A.; Larochelle, A.; Laflamme, N.; Préfontaine, P.; Plante, M.-M.; Sánchez, M.G.; Yong, V.W.; Stys, P.K.; Tremblay, M.; Rivest, S. Inefficient clearance of myelin debris by microglia impairs remyelinating processes. J. Exp. Med. 2015, 212, 481–495. [CrossRef]
- Jing, X.; Yao, Y.; Wu, D.; Hong, H.; Feng, X.; Xu, N.; Liu, Y.; Liang, H. IFP35 family proteins promote neuroinflammation and multiple sclerosis. Proc. Natl. Acad. Sci. 2021, 118. [CrossRef]
- Peferoen, L.A.; Vogel, D.Y.; Ummenthum, K.; Breur, M.; Heijnen, P.D.; Gerritsen, W.H.; Peferoen-Baert, R.M.; van der Valk, P.; Dijkstra, C.D.; Amor, S. Activation Status of Human Microglia Is Dependent on Lesion Formation Stage and Remyelination in Multiple Sclerosis. J. Neuropathol. Exp. Neurol. 2015, 74, 48–63. [CrossRef]
- Jäckle, K.; Zeis, T.; Schaeren-Wiemers, N.; Junker, A.; van der Meer, F.; Kramann, N.; Stadelmann, C.; Brück, W. Molecular signature of slowly expanding lesions in progressive multiple sclerosis. Brain 2020, 143, 2073–2088. [CrossRef]
- Grajchen, E.; Wouters, E.; van de Haterd, B.; Haidar, M.; Hardonnière, K.; Dierckx, T.; Van Broeckhoven, J.; Erens, C.; Hendrix, S.; Kerdine-Römer, S.; et al. CD36-mediated uptake of myelin debris by macrophages and microglia reduces neuroinflammation. J. Neuroinflammation 2020, 17, 1–14. [CrossRef]
- Grajchen, E.; Hendriks, J.J.A.; Bogie, J.F.J. The physiology of foamy phagocytes in multiple sclerosis. Acta Neuropathol. Commun. 2018, 6, 1–21. [CrossRef]
- Epstein, L.; Prineas, J.; Raine, C. Attachment of myelin to coated pits on macrophages in experimental allergic encephalomyelitis. J. Neurol. Sci. 1983, 61, 341–348. [CrossRef]
- Marta, C.B.; Bansal, R.; Pfeiffer, S.E. Microglial Fc receptors mediate physiological changes resulting from antibody cross-linking of myelin oligodendrocyte glycoprotein. J. Neuroimmunol. 2008, 196, 35–40. [CrossRef]
- Melief, J.; Schuurman, K.G.; van de Garde, M.D.B.; Smolders, J.; van Eijk, M.; Hamann, J.; Huitinga, I. Microglia in normal appearing white matter of multiple sclerosis are alerted but immunosuppressed. Glia 2013, 61, 1848–1861. [CrossRef]
- Hendrickx, D.A.; Koning, N.; Schuurman, K.G.; van Strien, M.E.; van Eden, C.G.; Hamann, J.; Huitinga, I. Selective Upregulation of Scavenger Receptors in and Around Demyelinating Areas in Multiple Sclerosis. J. Neuropathol. Exp. Neurol. 2013, 72, 106–118. [CrossRef]
- Reichert, F.; Rotshenker, S. Complement-receptor-3 and scavenger-receptor-AI/II mediated myelin phagocytosis in microglia and macrophages. Neurobiol. Dis. 2003, 12, 65–72. [CrossRef]
- Ulvestad, E.; Williams, K.; Vedeler, C.; Antel, J.; Nyland, H.; Mørk, S.; Matre, R. Reactive microglia in multiple sclerosis lesions have an increased expression of receptors for the Fc part of IgG. J. Neurol. Sci. 1994, 121, 125–131. [CrossRef]
- Van der Goes, A.; Kortekaas, M.; Hoekstra, K.; Dijkstra, C.D.; Amor, S. The role of anti-myelin (auto)-antibodies in the phagocytosis of myelin by macrophages. J. Neuroimmunol. 1999, 101, 61–67. [CrossRef]
- Karni, A.; Bakimer-Kleiner, R.; Abramsky, O.; Ben-Nun, A. Elevated levels of antibody to myelin oligodendrocyte glycoprotein is not specific for patients with multiple sclerosis.. Arch. Neurol. 1999, 56, 311–315. [CrossRef]
- Lampasona, V.; Franciotta, D.; Furlan, R.; Zanaboni, S.; Fazio, R.; Bonifacio, E.; Comi, G.; Martino, G. Similar low frequency of anti-MOG IgG and IgM in MS patients and healthy subjects. Neurology 2004, 62, 2092–2094. [CrossRef]
- Loveless, S.; Neal, J.W.; Howell, O.W.; Harding, K.E.; Sarkies, P.; Evans, R.; Bevan, R.J.; Hakobyan, S.; Harris, C.L.; Robertson, N.P.; et al. Tissue microarray methodology identifies complement pathway activation and dysregulation in progressive multiple sclerosis. Brain Pathol. 2017, 28, 507–520. [CrossRef]
- Levy-Barazany, H.; Frenkel, D. Expression of Scavenger receptor A on antigen presenting cells is important for CD4+ T-cells proliferation in EAE mouse model. J. Neuroinflammation 2012, 9, 120–120. [CrossRef]
- Healy, L.M.; Perron, G.; Won, S.-Y.; Michell-Robinson, M.A.; Rezk, A.; Ludwin, S.K.; Moore, C.S.; Hall, J.A.; Bar-Or, A.; Antel, J.P. MerTK Is a Functional Regulator of Myelin Phagocytosis by Human Myeloid Cells. J. Immunol. 2016, 196, 3375–3384. [CrossRef]
- Nagata, K.; Ohashi, K.; Nakano, T.; Arita, H.; Zong, C.; Hanafusa, H.; Mizuno, K. Identification of the Product of Growth Arrest-specific Gene 6 as a Common Ligand for Axl, Sky, and Mer Receptor Tyrosine Kinases. J. Biol. Chem. 1996, 271, 30022–30027. [CrossRef]
- Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C; et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80(4):661-70. [CrossRef]
- Ma, G.Z.M.; Stankovich, J.; Kilpatrick, T.J.; Binder, M.D.; Field, J.; The Australia and New Zealand Multiple Sclerosis Genetics Consortium (ANZgene) , Polymorphisms in the Receptor Tyrosine Kinase MERTK Gene Are Associated with Multiple Sclerosis Susceptibility. PLoS ONE 2011, 6, e16964. [CrossRef]
- Binder, M.D.; Cate, H.S.; Prieto, A.L.; Kemper, D.; Butzkueven, H.; Gresle, M.M.; Cipriani, T.; Jokubaitis, V.G.; Carmeliet, P.; Kilpatrick, T.J. Gas6 Deficiency Increases Oligodendrocyte Loss and Microglial Activation in Response to Cuprizone-Induced Demyelination. J. Neurosci. 2008, 28, 5195–5206. [CrossRef]
- Weinger, J.G.; Omari, K.M.; Marsden, K.; Raine, C.S.; Shafit-Zagardo, B. Up-Regulation of Soluble Axl and Mer Receptor Tyrosine Kinases Negatively Correlates with Gas6 in Established Multiple Sclerosis Lesions. Am. J. Pathol. 2009, 175, 283–293. [CrossRef]
- Shen, K.; Reichelt, M.; Kyauk, R.V.; Ngu, H.; Shen, Y.-A.A.; Foreman, O.; Modrusan, Z.; Friedman, B.A.; Sheng, M.; Yuen, T.J. Multiple sclerosis risk gene Mertk is required for microglial activation and subsequent remyelination. Cell Rep. 2021, 34, 108835. [CrossRef]
- Cignarella, F.; Filipello, F.; Bollman, B.; Cantoni, C.; Locca, A.; Mikesell, R.; Manis, M.; Ibrahim, A.; Deng, L.; Benitez, B.A.; et al. TREM2 activation on microglia promotes myelin debris clearance and remyelination in a model of multiple sclerosis. Acta Neuropathol. 2020, 140, 513–534. [CrossRef]
- Ennerfelt, H.; Frost, E.L.; Shapiro, D.A.; Holliday, C.; Zengeler, K.E.; Voithofer, G.; Bolte, A.C.; Lammert, C.R.; Kulas, J.A.; Ulland, T.K.; et al. SYK coordinates neuroprotective microglial responses in neurodegenerative disease. Cell 2022, 185, 4135–4152.e22. [CrossRef]
- Yeh FL, Hansen DV, Sheng M. TREM2, Microglia, and Neurodegenerative Diseases. Trends in Molecular Medicine. 2017;23(6):512-33. [CrossRef]
- Bennett, M.L.; Bennett, F.C.; Liddelow, S.A.; Ajami, B.; Zamanian, J.L.; Fernhoff, N.B.; Mulinyawe, S.B.; Bohlen, C.J.; Adil, A.; Tucker, A.; et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. USA 2016, 113, E1738–E1746. [CrossRef]
- Satoh, J.; Kino, Y.; Asahina, N.; Takitani, M.; Miyoshi, J.; Ishida, T.; Saito, Y. TMEM119 marks a subset of microglia in the human brain. Neuropathology 2015, 36, 39–49. [CrossRef]
- Butovsky, O.; Jedrychowski, M.P.; Moore, C.S.; Cialic, R.; Lanser, A.J.; Gabriely, G.; Koeglsperger, T.; Dake, B.; Wu, P.M.; E Doykan, C.; et al. Identification of a unique TGF-β–dependent molecular and functional signature in microglia. Nat. Neurosci. 2013, 17, 131–143. [CrossRef]
- Kenkhuis, B.; Somarakis, A.; Kleindouwel, L.R.; van Roon-Mom, W.M.; Höllt, T.; van der Weerd, L. Co-expression patterns of microglia markers Iba1, TMEM119 and P2RY12 in Alzheimer's disease. Neurobiol. Dis. 2022, 167, 105684. [CrossRef]
- Zrzavy, T.; Hametner, S.; Wimmer, I.; Butovsky, O.; Weiner, H.L.; Lassmann, H. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 2017, 140, 1900–1913. [CrossRef]
- van Wageningen TA, Vlaar E, Kooij G, Jongenelen CAM, Geurts JJG, van Dam AM. Regulation of microglial TMEM119 and P2RY12 immunoreactivity in multiple sclerosis white and grey matter lesions is dependent on their inflammatory environment. Acta Neuropathol Commun. 2019;7(1):206. [CrossRef]
- Ziebell, J.M.; E Taylor, S.; Cao, T.; Harrison, J.L.; Lifshitz, J. Rod microglia: Elongation, alignment, and coupling to form trains across the somatosensory cortex after experimental diffuse brain injury. J. Neuroinflammation 2012, 9, 247–247. [CrossRef]
- Trapp BD, Wujek JR, Criste GA, Jalabi W, Yin X, Kidd GJ; et al. Evidence for synaptic stripping by cortical microglia. Glia. 2007;55(4):360-8. [CrossRef]
- van der Poel M, Ulas T, Mizee MR, Hsiao C-C, Miedema SSM, Adelia; et al. Transcriptional profiling of human microglia reveals grey–white matter heterogeneity and multiple sclerosis-associated changes. Nature Communications. 2019;10(1):1139. [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [CrossRef]
- Ioannou, M.S.; Jackson, J.; Sheu, S.-H.; Chang, C.-L.; Weigel, A.V.; Liu, H.; Pasolli, H.A.; Xu, C.S.; Pang, S.; Matthies, D.; et al. Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell 2019, 177, 1522–1535.e14. [CrossRef]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization.. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [CrossRef]
- Qi, G.; Mi, Y.; Shi, X.; Gu, H.; Brinton, R.D.; Yin, F. ApoE4 Impairs Neuron-Astrocyte Coupling of Fatty Acid Metabolism. Cell Rep. 2021, 34, 108572–108572. [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [CrossRef]
- Holley, J.E.; Gveric, D.; Newcombe, J.; Cuzner, M.L.; Gutowski, N.J. Astrocyte characterization in the multiple sclerosis glial scar. Neuropathol. Appl. Neurobiol. 2003, 29, 434–444. [CrossRef]
- Simpson, J.E.; Ince, P.G.; Lace, G.; Forster, G.; Shaw, P.J.; Matthews, F.; Savva, G.; Brayne, C.; Wharton, S.B. Astrocyte Phenotype in Relation to Alzheimer-Type Pathology in the Ageing Brain. Neurobiol. Aging 2010, 31, 578–590. [CrossRef]
- Diaz-Castro, B.; Bernstein, A.M.; Coppola, G.; Sofroniew, M.V.; Khakh, B.S. Molecular and functional properties of cortical astrocytes during peripherally induced neuroinflammation. Cell Rep. 2021, 36, 109508. [CrossRef]
- Ponath, G.; Lincoln, M.R.; Levine-Ritterman, M.; Park, C.; Dahlawi, S.; Mubarak, M.; Sumida, T.; Airas, L.; Zhang, S.; Isitan, C.; et al. Enhanced astrocyte responses are driven by a genetic risk allele associated with multiple sclerosis. Nat. Commun. 2018, 9, 5337. [CrossRef]
- Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S; et al. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202(1):145-56. [CrossRef]
- Brambilla R, Persaud T, Hu X, Karmally S, Shestopalov VI, Dvoriantchikova G; et al. Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. Journal of immunology (Baltimore, Md : 1950). 2009;182(5):2628-40. [CrossRef]
- Kirkley KS, Popichak KA, Hammond SL, Davies C, Hunt L, Tjalkens RB. Genetic suppression of IKK2/NF-κB in astrocytes inhibits neuroinflammation and reduces neuronal loss in the MPTP-Probenecid model of Parkinson's disease. Neurobiol Dis. 2019;127:193-209. [CrossRef]
- Kang, Z.; Altuntas, C.Z.; Gulen, M.F.; Liu, C.; Giltiay, N.; Qin, H.; Liu, L.; Qian, W.; Ransohoff, R.M.; Bergmann, C.; et al. Astrocyte-Restricted Ablation of Interleukin-17-Induced Act1-Mediated Signaling Ameliorates Autoimmune Encephalomyelitis. Immunity 2010, 32, 414–425. [CrossRef]
- Wheeler, M.A.; Clark, I.C.; Tjon, E.C.; Li, Z.; Zandee, S.E.J.; Couturier, C.P.; Watson, B.R.; Scalisi, G.; Alkwai, S.; Rothhammer, V.; et al. MAFG-driven astrocytes promote CNS inflammation. Nature 2020, 578, 593–599. [CrossRef]
- Bi, F.; Huang, C.; Tong, J.; Qiu, G.; Huang, B.; Wu, Q.; Li, F.; Xu, Z.; Bowser, R.; Xia, X.-G.; et al. Reactive astrocytes secrete lcn2 to promote neuron death. Proc. Natl. Acad. Sci. 2013, 110, 4069–4074. [CrossRef]
- Jones, E.V.; Bernardinelli, Y.; Zarruk, J.G.; Chierzi, S.; Murai, K.K. SPARC and GluA1-Containing AMPA Receptors Promote Neuronal Health Following CNS Injury. Front. Cell. Neurosci. 2018, 12, 22. [CrossRef]
- Guttenplan, K.A.; Weigel, M.K.; Prakash, P.; Wijewardhane, P.R.; Hasel, P.; Rufen-Blanchette, U.; Münch, A.E.; Blum, J.A.; Fine, J.; Neal, M.C.; et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature 2021, 599, 102–107. [CrossRef]
- Chung, W.-S.; Clarke, L.E.; Wang, G.X.; Stafford, B.K.; Sher, A.; Chakraborty, C.; Joung, J.; Foo, L.C.; Thompson, A.; Chen, C.; et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 2013, 504, 394–400. [CrossRef]
- Konishi, H.; Okamoto, T.; Hara, Y.; Komine, O.; Tamada, H.; Maeda, M.; Osako, F.; Kobayashi, M.; Nishiyama, A.; Kataoka, Y.; et al. Astrocytic phagocytosis is a compensatory mechanism for microglial dysfunction. EMBO J. 2020, 39, e104464. [CrossRef]
- Batiuk, M.Y.; Martirosyan, A.; Wahis, J.; de Vin, F.; Marneffe, C.; Kusserow, C.; Koeppen, J.; Viana, J.F.; Oliveira, J.F.; Voet, T.; et al. Identification of region-specific astrocyte subtypes at single cell resolution. Nat. Commun. 2020, 11, 1–15. [CrossRef]
- Clark, I.C.; Wheeler, M.A.; Lee, H.-G.; Li, Z.; Sanmarco, L.M.; Thaploo, S.; Polonio, C.M.; Shin, S.W.; Scalisi, G.; Henry, A.R.; et al. Identification of astrocyte regulators by nucleic acid cytometry. Nature 2023, 614, 326–333. [CrossRef]
- Wheeler, M.A.; Jaronen, M.; Covacu, R.; Zandee, S.E.; Scalisi, G.; Rothhammer, V.; Tjon, E.C.; Chao, C.-C.; Kenison, J.E.; Blain, M.; et al. Environmental Control of Astrocyte Pathogenic Activities in CNS Inflammation. Cell 2019, 176, 581–596.e18. [CrossRef]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015, 161, 1202–1214. [CrossRef]
- Amamoto R, Garcia MD, West ER, Choi J, Lapan SW, Lane EA; et al. Probe-Seq enables transcriptional profiling of specific cell types from heterogeneous tissue by RNA-based isolation. Elife. 2019;8. [CrossRef]
- Niu J, Tsai HH, Hoi KK, Huang N, Yu G, Kim K; et al. Aberrant oligodendroglial-vascular interactions disrupt the blood-brain barrier, triggering CNS inflammation. Nat Neurosci. 2019;22(5):709-18. [CrossRef]
- Tanaka, R.; Iwasaki, Y.; Koprowski, H. Ultrastructural studies of perivascular cuffing cells in multiple sclerosis brain.. 1975, 81, 467–78.
- Dal-Bianco, A.; Grabner, G.; Kronnerwetter, C.; Weber, M.; Kornek, B.; Kasprian, G.; Berger, T.; Leutmezer, F.; Rommer, P.S.; Trattnig, S.; et al. Long-term evolution of multiple sclerosis iron rim lesions in 7 T MRI. Brain 2021, 144, 833–847. [CrossRef]
- Frischer, J.M.; Weigand, S.D.; Guo, Y.; Kale, N.; Parisi, J.E.; Pirko, I.; Mandrekar, J.; Bramow, S.; Metz, I.; Brück, W.; et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann. Neurol. 2015, 78, 710–721. [CrossRef]
- Bosch, A.v.D.; Fransen, N.; Mason, M.; Rozemuller, A.J.; Teunissen, C.; Smolders, J.; Huitinga, I. Neurofilament Light Chain Levels in Multiple Sclerosis Correlate With Lesions Containing Foamy Macrophages and With Acute Axonal Damage. Neurol. - Neuroimmunol. Neuroinflammation 2022, 9. [CrossRef]
- Raine, C.S.; Wu, E. Multiple sclerosis: Remyelination in acute lesions.. 1993, 52, 199–204.
- Prineas, J.W.; Kwon, E.E.; Cho, E.; Sharer, L.R.; Barnett, M.H.; Oleszak, E.L.; Hoffman, B.; Morgan, B.P. Immunopathology of secondary-progressive multiple sclerosis. Ann. Neurol. 2001, 50, 646–657. [CrossRef]
- Kuhlmann, T.; Ludwin, S.; Prat, A.; Antel, J.; Brück, W.; Lassmann, H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2016, 133, 13–24. [CrossRef]
- Sobel, R.A.; Ahmed, A.S. White Matter Extracellular Matrix Chondroitin Sulfate/Dermatan Sulfate Proteoglycans in Multiple Sclerosis. J. Neuropathol. Exp. Neurol. 2001, 60, 1198–1207. [CrossRef]
- Kornek, B.; Storch, M.K.; Weissert, R.; Wallstroem, E.; Stefferl, A.; Olsson, T.; Linington, C.; Schmidbauer, M.; Lassmann, H. Multiple Sclerosis and Chronic Autoimmune Encephalomyelitis: A Comparative Quantitative Study of Axonal Injury in Active, Inactive, and Remyelinated Lesions. Am. J. Pathol. 2000, 157, 267–276. [CrossRef]
- Prineas JW, Connell F. Remyelination in multiple sclerosis. Ann Neurol. 1979;5(1):22-31. [CrossRef]
- Prineas, J.W.; Barnard, R.O.; Kwon, E.E.; Sharer, L.R.; Cho, E. Multiple sclerosis: Remyelination of nascent lesions: Remyelination of nascent lesions. Ann. Neurol. 1993, 33, 137–151. [CrossRef]
- Bramow, S.; Frischer, J.M.; Lassmann, H.; Koch-Henriksen, N.; Lucchinetti, C.F.; Sørensen, P.S.; Laursen, H. Demyelination versus remyelination in progressive multiple sclerosis. Brain 2010, 133, 2983–2998. [CrossRef]
- Magliozzi, R.; Howell, O.; Vora, A.; Serafini, B.; Nicholas, R.; Puopolo, M.; Reynolds, R.; Aloisi, F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2006, 130, 1089–1104. [CrossRef]
- Serafini, B.; Rosicarelli, B.; Magliozzi, R.; Stigliano, E.; Aloisi, F. Detection of Ectopic B-cell Follicles with Germinal Centers in the Meninges of Patients with Secondary Progressive Multiple Sclerosis. Brain Pathol. 2004, 14, 164–174. [CrossRef]
- Hochmeister, S.; Grundtner, R.; Bauer, J.; Engelhardt, B.; Lyck, R.; Gordon, G.; Korosec, T.; Kutzelnigg, A.; Berger, J.J.; Bradl, M.; et al. Dysferlin Is a New Marker for Leaky Brain Blood Vessels in Multiple Sclerosis. J. Neuropathol. Exp. Neurol. 2006, 65, 855–865. [CrossRef]
- Lassmann, H. Targets of therapy in progressive MS. Mult. Scler. J. 2017, 23, 1593–1599. [CrossRef]
- Kuhle, J.; Kropshofer, H.; Haering, D.A.; Kundu, U.; Meinert, R.; Barro, C.; Dahlke, F.; Tomic, D.; Leppert, D.; Kappos, L. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019, 92, e1007–e1015. [CrossRef]
- Novakova L, Zetterberg H, Sundström P, Axelsson M, Khademi M, Gunnarsson M; et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89(22):2230-7. [CrossRef]
- Abdelhak, A.; Huss, A.; Kassubek, J.; Tumani, H.; Otto, M. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci. Rep. 2018, 8, 14798. [CrossRef]
- Hinsinger, G.; Galéotti, N.; Nabholz, N.; Urbach, S.; Rigau, V.; Demattei, C.; Lehmann, S.; Camu, W.; Labauge, P.; Castelnovo, G.; et al. Chitinase 3-like proteins as diagnostic and prognostic biomarkers of multiple sclerosis. Mult. Scler. J. 2015, 21, 1251–1261. [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [CrossRef]
- Ziemssen, T.; Akgün, K.; Brück, W. Molecular biomarkers in multiple sclerosis. J. Neuroinflamm. 2019, 16, 272. [CrossRef]
- Lebrun-Frénay, C.; Rollot, F.; Mondot, L.; Zephir, H.; Louapre, C.; Le Page, E.; Durand-Dubief, F.; Labauge, P.; Bensa, C.; Thouvenot, E.; et al. Risk Factors and Time to Clinical Symptoms of Multiple Sclerosis Among Patients With Radiologically Isolated Syndrome. JAMA Netw. Open 2021, 4, e2128271–e2128271. [CrossRef]
- Lebrun-Frenay, C.; Kantarci, O.; Siva, A.; Sormani, M.P.; Pelletier, D.; Okuda, D.T.; 10-year RISC study group on behalf of SFSEP, OFSEP Radiologically Isolated Syndrome: 10-Year Risk Estimate of a Clinical Event. Ann. Neurol. 2020, 88, 407–417. [CrossRef]
- Naseri, A.; Nasiri, E.; Sahraian, M.A.; Daneshvar, S.; Talebi, M. Clinical Features of Late-Onset Multiple Sclerosis: A Systematic Review and Meta-analysis. Mult. Scler. Relat. Disord. 2021, 50, 102816. [CrossRef]
- Kuhle, J.; Disanto, G.; Dobson, R.; Adiutori, R.; Bianchi, L.; Topping, J.; Bestwick, J.; Meier, U.-C.; Marta, M.; Costa, G.D.; et al. Conversion from clinically isolated syndrome to multiple sclerosis: A large multicentre study. Mult. Scler. J. 2015, 21, 1013–1024. [CrossRef]
- Kostulas VK, Link H, Lefvert AK. Oligoclonal IgG bands in cerebrospinal fluid. Principles for demonstration and interpretation based on findings in 1114 neurological patients. Arch Neurol. 1987;44(10):1041-4. [CrossRef]
- Chu, A.B.; Sever, J.L.; Madden, D.L.; Iivanainen, M.; Leon, M.; Wallen, W.; Brooks, B.R.; Lee, Y.J.; Houff, S. Oligoclonal IgG bands in cerebrospinal fluid in various neurological diseases. Ann. Neurol. 1983, 13, 434–439. [CrossRef]
- Simonsen, C.S.; Flemmen, H..; Lauritzen, T.; Berg-Hansen, P.; Moen, S.M.; Celius, E.G. The diagnostic value of IgG index versus oligoclonal bands in cerebrospinal fluid of patients with multiple sclerosis. Mult. Scler. J. - Exp. Transl. Clin. 2020, 6. [CrossRef]
- Mermelstein M, Naftali J, Wilf-Yarkoni A, Lotan I, Hellmann MA, Steiner I. Repeated lumbar puncture in search of oligoclonal bands – What is the yield? Journal of the Neurological Sciences. 2022;439:120298. [CrossRef]
- Lee, M.K.; Cleveland, D.W. Neurofilament function and dysfunction: Involvement in axonal growth and neuronal disease. Curr. Opin. Cell Biol. 1994, 6, 34–40. [CrossRef]
- Hoffman, P.N.; Cleveland, D.W.; Griffin, J.W.; Landes, P.W.; Cowan, N.J.; Price, D.L. Neurofilament gene expression: A major determinant of axonal caliber.. Proc. Natl. Acad. Sci. USA 1987, 84, 3472–3476. [CrossRef]
- Friede, R.L.; Samorajski, T. Axon caliber related to neurofilaments and microtubules in sciatic nerve fibers of rats and mice. Anat. Rec. 1970, 167, 379–387. [CrossRef]
- Yuan, A.; Sasaki, T.; Rao, M.V.; Kumar, A.; Kanumuri, V.; Dunlop, D.S.; Liem, R.K.; Nixon, R.A. Neurofilaments Form a Highly Stable Stationary Cytoskeleton after Reaching a Critical Level in Axons. J. Neurosci. 2009, 29, 11316–11329. [CrossRef]
- Kuhle, J.; Leppert, D.; Petzold, A.; Regeniter, A.; Schindler, C.; Mehling, M.; Anthony, D.; Kappos, L.; Lindberg, R. Neurofilament heavy chain in CSF correlates with relapses and disability in multiple sclerosis. Neurology 2011, 76, 1206–1213. [CrossRef]
- Malmeström, C.; Haghighi, S.; Rosengren, L.; Andersen, O.; Lycke, J. Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology 2003, 61, 1720–1725. [CrossRef]
- Semra, Y.; Seidi, O.; Sharief, M. Heightened intrathecal release of axonal cytoskeletal proteins in multiple sclerosis is associated with progressive disease and clinical disability. J. Neuroimmunol. 2002, 122, 132–139. [CrossRef]
- Kuhle, J.; Malmeström, C.; Axelsson, M.; Plattner, K.; Yaldizli, .; Derfuss, T.; Giovannoni, G.; Kappos, L.; Lycke, J. Neurofilament light and heavy subunits compared as therapeutic biomarkers in multiple sclerosis. Acta Neurol. Scand. 2013, 128, e33–e36. [CrossRef]
- Kuhle, J.; Plattner, K.; Bestwick, J.P.; Lindberg, R.L.; Ramagopalan, S.V.; Norgren, N.; Nissim, A.; Malaspina, A.; Leppert, D.; Giovannoni, G.; et al. A comparative study of CSF neurofilament light and heavy chain protein in MS. Mult. Scler. J. 2013, 19, 1597–1603. [CrossRef]
- Ferreira-Atuesta, C.; Reyes, S.; Giovanonni, G.; Gnanapavan, S. The Evolution of Neurofilament Light Chain in Multiple Sclerosis. Front. Neurosci. 2021, 15. [CrossRef]
- Khalil, M.; Pirpamer, L.; Hofer, E.; Voortman, M.M.; Barro, C.; Leppert, D.; Benkert, P.; Ropele, S.; Enzinger, C.; Fazekas, F.; et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat. Commun. 2020, 11, 1–9. [CrossRef]
- Gaetani, L.; Blennow, K.; Calabresi, P.; Di Filippo, M.; Parnetti, L.; Zetterberg, H. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 2019, 90, 870–881. [CrossRef]
- Engelborghs, S.; Niemantsverdriet, E.; Struyfs, H.; Blennow, K.; Brouns, R.; Comabella, M.; Dujmovic, I.; Van Der Flier, W.; Frölich, L.; Galimberti, D.; et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimer’s Dement.: Diagn. Assess. Dis. Monit. 2017, 8, 111–126. [CrossRef]
- Kuhle, J.; Barro, C.; Andreasson, U.; Derfuss, T.; Lindberg, R.; Sandelius, .; Liman, V.; Norgren, N.; Blennow, K.; Zetterberg, H. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. cclm 2016, 54, 1655–1661. [CrossRef]
- Rissin, D.M.; Kan, C.W.; Campbell, T.G.; Howes, S.C.; Fournier, D.R.; Song, L.; Piech, T.; Patel, P.P.; Chang, L.; Rivnak, A.J.; et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 2010, 28, 595–599. [CrossRef]
- Barro, C.; Benkert, P.; Disanto, G.; Tsagkas, C.; Amann, M.; Naegelin, Y.; Leppert, D.; Gobbi, C.; Granziera, C.; Yaldizli, .; et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018, 141, 2382–2391. [CrossRef]
- Disanto, G.; Barro, C.; Benkert, P.; Naegelin, Y.; Schädelin, S.; Giardiello, A.; Zecca, C.; Blennow, K.; Zetterberg, H.; Leppert, D.; et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 2017, 81, 857–870. [CrossRef]
- Aharoni, R.; Eilam, R.; Lerner, S.; Shavit-Stein, E.; Dori, A.; Chapman, J.; Arnon, R. Neuroprotective Effect of Glatiramer Acetate on Neurofilament Light Chain Leakage and Glutamate Excess in an Animal Model of Multiple Sclerosis. Int. J. Mol. Sci. 2021, 22, 13419. [CrossRef]
- Salzer J, Svenningsson A, Sundström P. Neurofilament light as a prognostic marker in multiple sclerosis. Mult Scler. 2010;16(3):287-92. [CrossRef]
- Williams, T.; Zetterberg, H.; Chataway, J. Neurofilaments in progressive multiple sclerosis: A systematic review. J. Neurol. 2020, 268, 1–11. [CrossRef]
- Ferraro, D.; Guicciardi, C.; De Biasi, S.; Pinti, M.; Bedin, R.; Camera, V.; Vitetta, F.; Nasi, M.; Meletti, S.; Sola, P. Plasma neurofilaments correlate with disability in progressive multiple sclerosis patients. Acta Neurol. Scand. 2019, 141, 16–21. [CrossRef]
- Mañé-Martínez, M.; Olsson, B.; Bau, L.; Matas, E.; Cobo-Calvo, .; Andreasson, U.; Blennow, K.; Romero-Pinel, L.; Martínez-Yélamos, S.; Zetterberg, H. Glial and neuronal markers in cerebrospinal fluid in different types of multiple sclerosis. J. Neuroimmunol. 2016, 299, 112–117. [CrossRef]
- Novakova, L.; Axelsson, M.; Malmeström, C.; Imberg, H.; Elias, O.; Zetterberg, H.; Nerman, O.; Lycke, J. Searching for neurodegeneration in multiple sclerosis at clinical onset: Diagnostic value of biomarkers. PLoS ONE 2018, 13, e0194828. [CrossRef]
- Burman, J.; Zetterberg, H.; Fransson, M.; Loskog, A.S.; Raininko, R.; Fagius, J. Assessing tissue damage in multiple sclerosis: A biomarker approach. Acta Neurol. Scand. 2014, 130, 81–89. [CrossRef]
- Chatterjee, M.; Koel-Simmelink, M.J.; Verberk, I.M.; Killestein, J.; Vrenken, H.; Enzinger, C.; Ropele, S.; Fazekas, F.; Khalil, M.; E Teunissen, C. Contactin-1 and contactin-2 in cerebrospinal fluid as potential biomarkers for axonal domain dysfunction in multiple sclerosis. Mult. Scler. J. - Exp. Transl. Clin. 2018, 4. [CrossRef]
- Hendricks, R.; Baker, D.; Brumm, J.; Davancaze, T.; Harp, C.; Herman, A.; Von Büdingen, H.-C.; Townsend, M.; Fischer, S.K. Establishment of neurofilament light chain Simoa assay in cerebrospinal fluid and blood. Bioanalysis 2019, 11, 1405–1418. [CrossRef]
- Dhiman K, Gupta VB, Villemagne VL, Eratne D, Graham PL, Fowler C; et al. Cerebrospinal fluid neurofilament light concentration predicts brain atrophy and cognition in Alzheimer's disease. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2020;12(1):e12005. [CrossRef]
- Rosengren LE, Karlsson JE, Karlsson JO, Persson LI, Wikkelsø C. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem. 1996;67(5):2013-8. [CrossRef]
- Saraste, M.; Bezukladova, S.; Matilainen, M.; Tuisku, J.; Rissanen, E.; Sucksdorff, M.; Laaksonen, S.; Vuorimaa, A.; Kuhle, J.; Leppert, D.; et al. High serum neurofilament associates with diffuse white matter damage in MS. Neurol. - Neuroimmunol. Neuroinflammation 2021, 8. [CrossRef]
- Meeker, K.L.; Butt, O.H.; Gordon, B.A.; Fagan, A.M.; Schindler, S.E.; Morris, J.C.; Benzinger, T.L.; Ances, B.M. Cerebrospinal fluid neurofilament light chain is a marker of aging and white matter damage. Neurobiol. Dis. 2022, 166, 105662–105662. [CrossRef]
- Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, Alvarez-Cermeño JC; et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol. 2019;76(9):1035-48. [CrossRef]
- Pérez-Miralles, F.; Prefasi, D.; García-Merino, A.; Gascón-Giménez, F.; Medrano, N.; Castillo-Villalba, J.; Cubas, L.; Alcalá, C.; Gil-Perotín, S.; Gómez-Ballesteros, R.; et al. CSF chitinase 3-like-1 association with disability of primary progressive MS. Neurol. - Neuroimmunol. Neuroinflammation 2020, 7. [CrossRef]
- Malmeström C, Axelsson M, Lycke J, Zetterberg H, Blennow K, Olsson B. CSF levels of YKL-40 are increased in MS and replaces with immunosuppressive treatment. J Neuroimmunol. 2014;269(1-2):87-9. [CrossRef]
- Kušnierová, P.; Zeman, D.; Hradílek, P.; Zapletalová, O.; Stejskal, D. Determination of chitinase 3-like 1 in cerebrospinal fluid in multiple sclerosis and other neurological diseases. PLoS ONE 2020, 15, e0233519. [CrossRef]
- Matute-Blanch, C.; Calvo-Barreiro, L.; Carballo-Carbajal, I.; Gonzalo, R.; Sanchez, A.; Vila, M.; Montalban, X.; Comabella, M. Chitinase 3-like 1 is neurotoxic in primary cultured neurons. Sci. Rep. 2020, 10, 7118. [CrossRef]
- Schneider, R.; Bellenberg, B.; Gisevius, B.; Hirschberg, S.; Sankowski, R.; Prinz, M.; Gold, R.; Lukas, C.; Haghikia, A. Chitinase 3–like 1 and neurofilament light chain in CSF and CNS atrophy in MS. Neurol. - Neuroimmunol. Neuroinflammation 2021, 8. [CrossRef]
- Cantó, E.; Tintoré, M.; Villar, L.M.; Costa, C.; Nurtdinov, R.; Álvarez-Cermeño, J.C.; Arrambide, G.; Reverter, F.; Deisenhammer, F.; Hegen, H.; et al. Chitinase 3-like 1: Prognostic biomarker in clinically isolated syndromes. Brain 2015, 138, 918–931. [CrossRef]
- Håkansson, I.; Tisell, A.; Cassel, P.; Blennow, K.; Zetterberg, H.; Lundberg, P.; Dahle, C.; Vrethem, M.; Ernerudh, J. Neurofilament levels, disease activity and brain volume during follow-up in multiple sclerosis. J. Neuroinflammation 2018, 15, 1–10. [CrossRef]
- Cantó, E.; Reverter, F.; Morcillo-Suárez, C.; Matesanz, F.; Fernández, O.; Izquierdo, G.; Vandenbroeck, K.; Rodríguez-Antigüedad, A.; Urcelay, E.; Arroyo, R.; et al. Chitinase 3-like 1 plasma levels are increased in patients with progressive forms of multiple sclerosis. Mult. Scler. J. 2011, 18, 983–990. [CrossRef]
- Gil-Perotin, S.; Castillo-Villalba, J.; Cubas-Nuñez, L.; Gasque, R.; Hervas, D.; Gomez-Mateu, J.; Alcala, C.; Perez-Miralles, F.; Gascon, F.; Dominguez, J.A.; et al. Combined Cerebrospinal Fluid Neurofilament Light Chain Protein and Chitinase-3 Like-1 Levels in Defining Disease Course and Prognosis in Multiple Sclerosis. Front. Neurol. 2019, 10, 1008. [CrossRef]
- Correale, J.; Fiol, M. Chitinase effects on immune cell response in neuromyelitis optica and multiple sclerosis. Mult. Scler. J. 2010, 17, 521–531. [CrossRef]
- Wang, Z.; Wang, R.; Li, Y.; Li, M.; Zhang, Y.; Jiang, L.; Fan, J.; Wang, Q.; Yang, D. Plasma Neurofilament Light Chain as a Predictive Biomarker for Post-stroke Cognitive Impairment: A Prospective Cohort Study. Front. Aging Neurosci. 2021, 13. [CrossRef]
- Evangelou N, Esiri MM, Smith S, Palace J, Matthews PM. Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol. 2000;47(3):391-5.
- Filippi, M.; Campi, A.; Dousset, V.; Baratti, C.; Martinelli, V.; Canal, N.; Scotti, G.; Comi, G. A Magnetization Transfer Imaging Study of Normal-Appearing White Matter in Multiple Sclerosis. Neurology 1995, 45, 478–482. [CrossRef]
- Zivadinov, R.; Uher, T.; Hagemeier, J.; Vaneckova, M.; Ramasamy, D.P.; Tyblova, M.; Bergsland, N.; Seidl, Z.; Dwyer, M.G.; Krasensky, J.; et al. A serial 10-year follow-up study of brain atrophy and disability progression in RRMS patients. Mult. Scler. J. 2016, 22, 1709–1718. [CrossRef]
- Eijlers, A.J.; Dekker, I.; Steenwijk, M.D.; Meijer, K.A.; Hulst, H.E.; Pouwels, P.J.; Uitdehaag, B.M.; Barkhof, F.; Vrenken, H.; Schoonheim, M.M.; et al. Cortical atrophy accelerates as cognitive decline worsens in multiple sclerosis. Neurology 2019, 93, e1348–e1359. [CrossRef]
- Warntjes, J.; Tisell, A.; Håkansson, I.; Lundberg, P.; Ernerudh, J. Improved Precision of Automatic Brain Volume Measurements in Patients with Clinically Isolated Syndrome and Multiple Sclerosis Using Edema Correction. Am. J. Neuroradiol. 2017, 39, 296–302. [CrossRef]
- Cheriyan, J.; Kim, S.; Wolansky, L.J.; Cook, S.D.; Cadavid, D. Impact of Inflammation on Brain Volume in Multiple Sclerosis. Arch. Neurol. 2012, 69, 82. [CrossRef]
- Scahill, R.I.; Frost, C.; Jenkins, R.; Whitwell, J.L.; Rossor, M.N.; Fox, N.C. A Longitudinal Study of Brain Volume Changes in Normal Aging Using Serial Registered Magnetic Resonance Imaging. Arch. Neurol. 2003, 60, 989–94. [CrossRef]
- Ghione, E.; Bergsland, N.; Dwyer, M.G.; Hagemeier, J.; Jakimovski, D.; Paunkoski, I.; Ramasamy, D.P.; Carl, E.; Hojnacki, D.; Kolb, C.; et al. Aging and Brain Atrophy in Multiple Sclerosis. J. Neuroimaging 2019, 29, 527–535. [CrossRef]
- Filippi, M.; Cercignani, M.; Inglese, M.; Horsfield, M.; Comi, G. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology 2001, 56, 304–311. [CrossRef]
- de Sitter A, Steenwijk MD, Ruet A, Versteeg A, Liu Y, van Schijndel RA; et al. Performance of five research-domain automated WM lesion segmentation methods in a multi-center MS study. Neuroimage. 2017;163:106-14. [CrossRef]
- van Walderveen MA, Lycklama ANGJ, Adèr HJ, Jongen PJ, Polman CH, Castelijns JA; et al. Hypointense lesions on T1-weighted spin-echo magnetic resonance imaging: Relation to clinical characteristics in subgroups of patients with multiple sclerosis. Arch Neurol. 2001;58(1):76-81. [CrossRef]
- Fisniku, L.K.; Brex, P.A.; Altmann, D.R.; Miszkiel, K.A.; Benton, C.E.; Lanyon, R.; Thompson, A.J.; Miller, D.H. Disability and T2 MRI lesions: A 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain 2008, 131, 808–817. [CrossRef]
- Klistorner, S.; Barnett, M.H.; Yiannikas, C.; Barton, J.; Parratt, J.; You, Y.; Graham, S.L.; Klistorner, A. Expansion of chronic lesions is linked to disease progression in relapsing–remitting multiple sclerosis patients. Mult. Scler. J. 2020, 27, 1533–1542. [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [CrossRef]
- Tillema, J.-M.; Pirko, I. Neuroradiological evaluation of demyelinating disease. Ther. Adv. Neurol. Disord. 2013, 6, 249–268. [CrossRef]
- Li DK, Held U, Petkau J, Daumer M, Barkhof F, Fazekas F; et al. MRI T2 lesion burden in multiple sclerosis: A plateauing relationship with clinical disability. Neurology. 2006;66(9):1384-9. [CrossRef]
- Mostert, J.P.; Koch, M.W.; Steen, C.; Heersema, D.J.; De Groot, J.C.; De Keyser, J. T2 lesions and rate of progression of disability in multiple sclerosis. Eur. J. Neurol. 2010, 17, 1471–1475. [CrossRef]
- Ciccarelli, O.; Brex, P.A.; Thompson, A.J.; Miller, D.H. Disability and lesion load in MS: A reassessment with MS functional composite score and 3D fast FLAIR.. J. Neurol. 2002, 249, 18–24. [CrossRef]
- Radue, E.-W.; O’connor, P.; Polman, C.H.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Mueller-Lenke, N.; Agoropoulou, C.; Holdbrook, F.; de Vera, A.; et al. Impact of Fingolimod Therapy on Magnetic Resonance Imaging Outcomes in Patients With Multiple Sclerosis. Arch. Neurol. 2012, 69, 1259–1269. [CrossRef]
- Comi, G.; Filippi, M.; Wolinsky, J.S. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging--measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group.. 2001, 49, 290–7. [CrossRef]
- Ebers, G.C. Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet 1998, 352, 1498–1504. [CrossRef]
- Elliott, C.; Belachew, S.; Wolinsky, J.S.; Hauser, S.L.; Kappos, L.; Barkhof, F.; Bernasconi, C.; Fecker, J.; Model, F.; Wei, W.; et al. Chronic white matter lesion activity predicts clinical progression in primary progressive multiple sclerosis. Brain 2019, 142, 2787–2799. [CrossRef]
- Kocsis, K.; Szabó, N.; Tóth, E.; Király, A.; Faragó, P.; Kincses, B.; Veréb, D.; Bozsik, B.; Boross, K.; Katona, M.; et al. Two Classes of T1 Hypointense Lesions in Multiple Sclerosis With Different Clinical Relevance. Front. Neurol. 2021, 12. [CrossRef]
- Truyen, L.; van Waesberghe, J.H.; van Walderveen, M.; van Oosten, B.W.; Polman, C.H.; Hommes, O.R.; Ader, H.J.; Barkhof, F. Accumulation of hypointense lesions ("black holes") on T 1 spin-echo MRI correlates with disease progression in multiple sclerosis. Neurology 1996, 47, 1469–1476. [CrossRef]
- Bagnato, F.; Jeffries, N.; Richert, N.D.; Stone, R.D.; Ohayon, J.M.; McFarland, H.F.; Frank, J.A. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain 2003, 126, 1782–1789. [CrossRef]
- Andermatt, S.; Papadopoulou, A.; Radue, E.-W.; Sprenger, T.; Cattin, P. Tracking the Evolution of Cerebral Gadolinium-Enhancing Lesions to Persistent T1 Black Holes in Multiple Sclerosis: Validation of a Semiautomated Pipeline. J. Neuroimaging 2017, 27, 469–475. [CrossRef]
- Elskamp, I.v.D.; Lembcke, J.; Dattola, V.; Beckmann, K.; Pohl, C.; Hong, W.; Sandbrink, R.; Wagner, K.; Knol, D.; Uitdehaag, B.; et al. Persistent T1 hypointensity as an MRI marker for treatment efficacy in multiple sclerosis. Mult. Scler. J. 2008, 14, 764–769. [CrossRef]
- van Waesberghe JH, van Walderveen MA, Castelijns JA, Scheltens P, Lycklama à Nijeholt GJ, Polman CH; et al. Patterns of lesion development in multiple sclerosis: Longitudinal observations with T1-weighted spin-echo and magnetization transfer MR. AJNR Am J Neuroradiol. 1998;19(4):675-83.
- Filippi, M.; Rovaris, M.; Rocca, M.A.; Sormani, M.P.; Wolinsky, J.S.; Comi, G. Glatiramer acetate reduces the proportion of new MS lesions evolving into "black holes". Neurology 2001, 57, 731–733. [CrossRef]
- Oommen VV, Tauhid S, Healy BC, Chua AS, Malik MT, Diaz-Cruz C; et al. The Effect of Fingolimod on Conversion of Acute Gadolinium-Enhancing Lesions to Chronic T1 Hypointensities in Multiple Sclerosis. Journal of neuroimaging : Official journal of the American Society of Neuroimaging. 2016;26(2):184-7. [CrossRef]
- Beckers, L.; Ory, D.; Geric, I.; Declercq, L.; Koole, M.; Kassiou, M.; Bormans, G.; Baes, M. Increased Expression of Translocator Protein (TSPO) Marks Pro-inflammatory Microglia but Does Not Predict Neurodegeneration. Mol. Imaging Biol. 2017, 20, 94–102. [CrossRef]
- Sadigh G, Saindane AM, Waldman AD, Lava NS, Hu R. Comparison of Unenhanced and Gadolinium-Enhanced Imaging in Multiple Sclerosis: Is Contrast Needed for Routine Follow-Up MRI? AJNR Am J Neuroradiol. 2019;40(9):1476-80.
- Bruschi, N.; Boffa, G.; Inglese, M. Ultra-high-field 7-T MRI in multiple sclerosis and other demyelinating diseases: From pathology to clinical practice. Eur. Radiol. Exp. 2020, 4, 1–13. [CrossRef]
- Luchetti, S.; Fransen, N.L.; van Eden, C.G.; Ramaglia, V.; Mason, M.; Huitinga, I. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: A retrospective autopsy cohort analysis. Acta Neuropathol. 2018, 135, 511–528. [CrossRef]
- Sbardella, E.; Tona, F.; Petsas, N.; Pantano, P. DTI Measurements in Multiple Sclerosis: Evaluation of Brain Damage and Clinical Implications. Mult. Scler. Int. 2013, 2013, 1–11. [CrossRef]
- Budde, M.D.; Kim, J.H.; Liang, H.; Russell, J.H.; Cross, A.H.; Song, S. Axonal injury detected by in vivo diffusion tensor imaging correlates with neurological disability in a mouse model of multiple sclerosis. NMR Biomed. 2007, 21, 589–597. [CrossRef]
- Nishioka, C.; Liang, H.-F.; Barsamian, B.; Sun, S.-W. Sequential phases of RGC axonal and somatic injury in EAE mice examined using DTI and OCT. Mult. Scler. Relat. Disord. 2018, 27, 315–323. [CrossRef]
- Naismith, R.T.; Xu, J.; Tutlam, N.T.; Snyder, A.; Benzinger, T.; Shimony, J.; Shepherd, J.; Trinkaus, K.; Cross, A.H.; Song, S.-K. Disability in optic neuritis correlates with diffusion tensor-derived directional diffusivities. Neurology 2009, 72, 589–594. [CrossRef]
- Budde, M.D.; Xie, M.; Cross, A.H.; Song, S.-K. Axial Diffusivity Is the Primary Correlate of Axonal Injury in the Experimental Autoimmune Encephalomyelitis Spinal Cord: A Quantitative Pixelwise Analysis. J. Neurosci. 2009, 29, 2805–2813. [CrossRef]
- Andersen, O.; Hildeman, A.; Longfils, M.; Tedeholm, H.; Skoog, B.; Tian, W.; Zhong, J.; Ekholm, S.; Novakova, L.; Runmarker, B.; et al. Diffusion tensor imaging in multiple sclerosis at different final outcomes. Acta Neurol. Scand. 2017, 137, 165–173. [CrossRef]
- Gujar SK, Maheshwari S, Björkman-Burtscher I, Sundgren PC. Magnetic resonance spectroscopy. J Neuroophthalmol. 2005;25(3):217-26.
- Tognarelli, J.M.; Dawood, M.; Shariff, M.I.; Grover, V.P.; Crossey, M.M.; Cox, I.J.; Taylor-Robinson, S.D.; McPhail, M.J. Magnetic Resonance Spectroscopy: Principles and Techniques: Lessons for Clinicians. J. Clin. Exp. Hepatol. 2015, 5, 320–328. [CrossRef]
- De Stefano, N.; Narayanan, S.; Francis, S.J.; Smith, S.; Mortilla, M.; Tartaglia, M.C.; Bartolozzi, M.L.; Guidi, L.; Federico, A.; Arnold, D.L. Diffuse Axonal and Tissue Injury in Patients With Multiple Sclerosis With Low Cerebral Lesion Load and No Disability. Arch. Neurol. 2002, 59, 1565–1571. [CrossRef]
- Tartaglia, M.C.; Narayanan, S.; De Stefano, N.; Arnaoutelis, R.; Antel, S.B.; Francis, S.J.; Santos, A.C.; Lapierre, Y.; Arnold, D.L. Choline is increased in pre-lesional normal appearing white matter in multiple sclerosis. J. Neurol. 2002, 249, 1382–1390. [CrossRef]
- Suhy J, Rooney WD, Goodkin DE, Capizzano AA, Soher BJ, Maudsley AA; et al. 1H MRSI comparison of white matter and lesions in primary progressive and relapsing-remitting MS. Mult Scler. 2000;6(3):148-55. [CrossRef]
- Hannoun S, Bagory M, Durand-Dubief F, Ibarrola D, Comte JC, Confavreux C; et al. Correlation of diffusion and metabolic alterations in different clinical forms of multiple sclerosis. PLoS ONE. 2012;7(3):e32525. [CrossRef]
- Inglese, M.; Li, B.S.; Rusinek, H.; Babb, J.S.; Grossman, R.I.; Gonen, O. Diffusely elevated cerebral choline and creatine in relapsing-remitting multiple sclerosis. Magn. Reson. Med. 2003, 50, 190–195. [CrossRef]
- Vrenken, H.; Barkhof, F.; Uitdehaag, B.; Castelijns, J.; Polman, C.; Pouwels, P. MR spectroscopic evidence for glial increase but not for neuro-axonal damage in MS normal-appearing white matter. Magn. Reson. Med. 2005, 53, 256–266. [CrossRef]
- Li, B.S.; Wang, H.; Gonen, O. Metabolite ratios to assumed stable creatine level may confound the quantification of proton brain MR spectroscopy. Magn. Reson. Imaging 2003, 21, 923–928. [CrossRef]
- Bagory, M.; Durand-Dubief, F.; Ibarrola, D.; Confavreux, C.; Sappey-Marinier, D. "Absolute" quantification in Magnetic Resonance Spectroscopy: Validation of a clinical protocol in multiple sclerosis. 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. LOCATION OF CONFERENCE, FranceDATE OF CONFERENCE; pp. 3458–3461.
- Narayana PA. Magnetic resonance spectroscopy in the monitoring of multiple sclerosis. Journal of neuroimaging : Official journal of the American Society of Neuroimaging. 2005;15(4 Suppl):46S-57S. [CrossRef]
- Larsson, H.B.W.; Christiansen, P.; Jensen, M.; Frederiksen, J.; Heltberg, A.; Olesen, J.; Henriksen, O. Localized in vivo proton spectroscopy in the brain of patients with multiple sclerosis. Magn. Reson. Med. 1991, 22, 23–31. [CrossRef]
- De Stefano, N.; Narayanan, S.; Francis, G.S.; Arnaoutelis, R.; Tartaglia, M.C.; Antel, J.P.; Matthews, P.M.; Arnold, D.L. Evidence of Axonal Damage in the Early Stages of Multiple Sclerosis and Its Relevance to Disability. Arch. Neurol. 2001, 58, 65–70. [CrossRef]
- Simone, I.; Tortorella, C.; Federico, F.; Liguori, M.; Lucivero, V.; Giannini, P.; Carrara, D.; Bellacosa, A.; Livrea, P. Axonal damage in multiple sclerosis plaques: A combined magnetic resonance imaging and 1H-magnetic resonance spectroscopy study. J. Neurol. Sci. 2001, 182, 143–150. [CrossRef]
- Aboul-Enein, F.; Krššák, M.; Höftberger, R.; Prayer, D.; Kristoferitsch, W. Reduced NAA-Levels in the NAWM of Patients with MS Is a Feature of Progression. A Study with Quantitative Magnetic Resonance Spectroscopy at 3 Tesla. PLoS ONE 2010, 5, e11625. [CrossRef]
- Rigotti DJ, Inglese M, Kirov, II, Gorynski E, Perry NN, Babb JS; et al. Two-year serial whole-brain N-acetyl-L-aspartate in patients with relapsing-remitting multiple sclerosis. Neurology. 2012;78(18):1383-9. [CrossRef]
- Sun, J.; Song, H.; Yang, Y.; Zhang, K.; Gao, X.; Li, X.; Ni, L.; Lin, P.; Niu, C. Metabolic changes in normal appearing white matter in multiple sclerosis patients using multivoxel magnetic resonance spectroscopy imaging. Medicine 2017, 96, e6534. [CrossRef]
- Tourbah, A.; Stievenart, J.; Gout, O.; Fontaine, B.; Liblau, R.; Lubetzki, C.; Cabanis, E.; Lyon-Caen, O. Localized proton magnetic resonance spectroscopy in relapsing remitting versus secondary progressive multiple sclerosis. Neurology 1999, 53, 1091–1091. [CrossRef]
- Tedeschi, G.; Bonavita, S.; McFarland, H.; Richert, N.; Duyn, J.; Frank, J. Proton MR spectroscopic imaging in multiple sclerosis. Neuroradiology 2002, 44, 37–42. [CrossRef]
- Cucurella MG, Rovira A, Río J, Pedraza S, Tintoré MM, Montalbán X; et al. Proton magnetic resonance spectroscopy in primary and secondary progressive multiple sclerosis. NMR Biomed. 2000;13(2):57-63. [CrossRef]
- Ruiz-Peña, J.L.; Piñero, P.; Sellers, G.; Argente, J.; Casado, A.; Foronda, J.; Uclés, A.; Izquierdo, G. Magnetic resonance spectroscopy of normal appearing white matter in early relapsing-remitting multiple sclerosis: Correlations between disability and spectroscopy. BMC Neurol. 2004, 4, 8–8. [CrossRef]
- Wu, X.; Hanson, L.G.; Skimminge, A.; Sorensen, P.S.; Paulson, O.B.; Mathiesen, H.K.; Blinkenberg, M. CorticalN-acetyl aspartate is a predictor of long-term clinical disability in multiple sclerosis. Neurol. Res. 2014, 36, 701–708. [CrossRef]
- Britze, J.; Frederiksen, J.L. Optical coherence tomography in multiple sclerosis. Eye 2018, 32, 884–888. [CrossRef]
- Mehmood A, Ali W, Song S, Din ZU, Guo RY, Shah W; et al. Optical coherence tomography monitoring and diagnosing retinal changes in multiple sclerosis. Brain Behav. 2021;11(10):e2302. [CrossRef]
- Toussaint, D.; Périer, O.; Verstappen, A.; Bervoets, S. Clinicopathological study of the visual pathways, eyes, and cerebral hemispheres in 32 cases of disseminated sclerosis.. 1983, 3, 211–20.
- Cruz-Herranz, A.; Balk, L.J.; Oberwahrenbrock, T.; Saidha, S.; Martinez-Lapiscina, E.H.; Lagreze, W.A.; Schuman, J.S.; Villoslada, P.; Calabresi, P.; Balcer, L.; et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology 2016, 86, 2303–2309. [CrossRef]
- Straatsma BR, Foos RY, Heckenlively JR, Taylor GN. Myelinated Retinal Nerve Fibers. American Journal of Ophthalmology. 1981;91(1):25-38.
- Ratchford, J.N.; Saidha, S.; Sotirchos, E.S.; Oh, J.A.; Seigo, M.A.; Eckstein, C.; Durbin, M.K.; Oakley, J.D.; Meyer, S.A.; Conger, A.; et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology 2013, 80, 47–54. [CrossRef]
- Klistorner, A.; Sriram, P.; Vootakuru, N.; Wang, C.; Barnett, M.H.; Garrick, R.; Parratt, J.; Levin, N.; Raz, N.; Van der Walt, A.; et al. Axonal loss of retinal neurons in multiple sclerosis associated with optic radiation lesions. Neurology 2014, 82, 2165–2172. [CrossRef]
- Shen, J.; Yang, Q.; Yu, D.; Wu, J.; Zhu, Y.; Guo, W. Vulnerability study of myelinated and unmyelinated nerve fibers in acute ocular hypertension in rabbit. Mol. Med. Rep. 2017, 16, 6794–6802. [CrossRef]
- Albrecht, P.; Ringelstein, M.; Müller, A.; Keser, N.; Dietlein, T.; Lappas, A.; Foerster, A.; Hartung, H.; Aktas, O.; Methner, A. Degeneration of retinal layers in multiple sclerosis subtypes quantified by optical coherence tomography. Mult. Scler. J. 2012, 18, 1422–1429. [CrossRef]
- Davies, E.C.; Galetta, K.M.; Sackel, D.J.; Talman, L.S.; Frohman, E.M.; A Calabresi, P.; Galetta, S.L.; Balcer, L.J. Retinal Ganglion Cell Layer Volumetric Assessment by Spectral-Domain Optical Coherence Tomography in Multiple Sclerosis: Application of a High-Precision Manual Estimation Technique. J. Neuro-Ophthalmology 2011, 31, 260–264. [CrossRef]
- Walter, S.D.; Ishikawa, H.; Galetta, K.M.; Sakai, R.E.; Feller, D.J.; Henderson, S.B.; Wilson, J.A.; Maguire, M.G.; Galetta, S.L.; Frohman, E.; et al. Ganglion Cell Loss in Relation to Visual Disability in Multiple Sclerosis. Ophthalmology 2012, 119, 1250–1257. [CrossRef]
- Talman, L.S.; Bisker, E.R.; Sackel, D.J.; Long, D.A.; Galetta, K.M.; Ratchford, J.N.; Lile, D.J.; Farrell, S.K.; Loguidice, M.J.; Remington, G.; et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann. Neurol. 2010, 67, 749–760. [CrossRef]
- Garcia-Martin E, Ara JR, Martin J, Almarcegui C, Dolz I, Vilades E; et al. Retinal and Optic Nerve Degeneration in Patients with Multiple Sclerosis Followed up for 5 Years. Ophthalmology. 2017;124(5):688-96. [CrossRef]
- Paul, F.; Calabresi, P.A.; Barkhof, F.; Green, A.J.; Kardon, R.; Sastre-Garriga, J.; Schippling, S.; Vermersch, P.; Saidha, S.; Gerendas, B.S.; et al. Optical coherence tomography in multiple sclerosis: A 3-year prospective multicenter study. Ann. Clin. Transl. Neurol. 2021, 8, 2235–2251. [CrossRef]
- Bock, M.; Brandt, A.U.; Dörr, J.; Pfueller, C.F.; Ohlraun, S.; Zipp, F.; Paul, F. Time domain and spectral domain optical coherence tomography in multiple sclerosis: A comparative cross-sectional study. Mult. Scler. J. 2010, 16, 893–896. [CrossRef]
- Behbehani, R.; Abu Al-Hassan, A.; Al-Salahat, A.; Sriraman, D.; Oakley, J.D.; Alroughani, R. Optical coherence tomography segmentation analysis in relapsing remitting versus progressive multiple sclerosis. PLoS ONE 2017, 12, e0172120. [CrossRef]
- Garcia-Martin, E.; Polo, V.; Larrosa, J.M.; Marques, M.L.; Herrero, R.; Martin, J.; Ara, J.R.; Fernandez, J.; Pablo, L.E. Retinal Layer Segmentation in Patients with Multiple Sclerosis Using Spectral Domain Optical Coherence Tomography. Ophthalmology 2014, 121, 573–579. [CrossRef]
- Cordon, B.; Vilades, E.; Orduna, E.; Satue, M.; Perez-Velilla, J.; Sebastian, B.; Polo, V.; Larrosa, J.M.; Pablo, L.E.; Garcia-Martin, E. Angiography with optical coherence tomography as a biomarker in multiple sclerosis. PLoS ONE 2020, 15, e0243236. [CrossRef]
- Ulusoy, M.O.; Horasanlı, B.; Işık-Ulusoy, S. Optical coherence tomography angiography findings of multiple sclerosis with or without optic neuritis. Neurol. Res. 2020, 42, 319–326. [CrossRef]
- Giovannoni, G.; Lai, M.; Thorpe, J.; Kidd, D.; Chamoun, V.; Thompson, A.J.; Miller, D.H.; Feldmann, M.; Thompson, E.J. Longitudinal study of soluble adhesion molecules in multiple sclerosis. Neurology 1997, 48, 1557–1565. [CrossRef]
- Kirbas, S.; Turkyilmaz, K.; Anlar, O.; Tufekci, A.; Durmus, M. Retinal Nerve Fiber Layer Thickness in Patients With Alzheimer Disease. J. Neuro-Ophthalmology 2013, 33, 58–61. [CrossRef]
- Moreno-Ramos T, Benito-León J, Villarejo A, Bermejo-Pareja F. Retinal nerve fiber layer thinning in dementia associated with Parkinson's disease, dementia with Lewy bodies, and Alzheimer's disease. J Alzheimers Dis. 2013;34(3):659-64. [CrossRef]
- Cipollini V, Abdolrahimzadeh S, Troili F, De Carolis A, Calafiore S, Scuderi L; et al. Neurocognitive Assessment and Retinal Thickness Alterations in Alzheimer Disease: Is There a Correlation? J Neuroophthalmol. 2020;40(3):370-7. [CrossRef]
- Nutma, E.; Gebro, E.; Marzin, M.C.; van der Valk, P.; Matthews, P.M.; Owen, D.R.; Amor, S. Activated microglia do not increase 18 kDa translocator protein (TSPO) expression in the multiple sclerosis brain. Glia 2021, 69, 2447–2458. [CrossRef]
- Bezukladova, S.; Tuisku, J.; Matilainen, M.; Vuorimaa, A.; Nylund, M.; Smith, S.; Sucksdorff, M.; Mohammadian, M.; Saunavaara, V.; Laaksonen, S.; et al. Insights into disseminated MS brain pathology with multimodal diffusion tensor and PET imaging. Neurol. - Neuroimmunol. Neuroinflammation 2020, 7. [CrossRef]
- Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F; et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: Quantitative in vivo imaging of microglia as a measure of disease activity. Brain. 2000;123(11):2321-37. [CrossRef]
- Nutma, E.; A Stephenson, J.; Gorter, R.P.; de Bruin, J.; Boucherie, D.M.; Donat, C.K.; Breur, M.; van der Valk, P.; Matthews, P.M.; Owen, D.R.; et al. A quantitative neuropathological assessment of translocator protein expression in multiple sclerosis. Brain 2019, 142, 3440–3455. [CrossRef]
- Debruyne, J.C.; Versijpt, J.; Van Laere, K.J.; De Vos, F.; Keppens, J.; Strijckmans, K.; Achten, E.; Slegers, G.; Dierckx, R.A.; Korf, J.; et al. PET visualization of microglia in multiple sclerosis patients using [11C]PK11195. Eur. J. Neurol. 2003, 10, 257–264. [CrossRef]
- Datta G, Colasanti A, Kalk N, Owen D, Scott G, Rabiner EA; et al. (11)C-PBR28 and (18)F-PBR111 Detect White Matter Inflammatory Heterogeneity in Multiple Sclerosis. J Nucl Med. 2017;58(9):1477-82. [CrossRef]
- Vignal, N.; Cisternino, S.; Rizzo-Padoin, N.; San, C.; Hontonnou, F.; Gelé, T.; Declèves, X.; Sarda-Mantel, L.; Hosten, B. [18F]FEPPA a TSPO Radioligand: Optimized Radiosynthesis and Evaluation as a PET Radiotracer for Brain Inflammation in a Peripheral LPS-Injected Mouse Model. Molecules 2018, 23, 1375. [CrossRef]
- Ikawa, M.; Lohith, T.G.; Shrestha, S.; Telu, S.; Zoghbi, S.S.; Castellano, S.; Taliani, S.; Da Settimo, F.; Fujita, M.; Pike, V.W.; et al. 11C-ER176, a Radioligand for 18-kDa Translocator Protein, Has Adequate Sensitivity to Robustly Image All Three Affinity Genotypes in Human Brain. J. Nucl. Med. 2017, 58, 320–325. [CrossRef]
- Rissanen, E.; Tuisku, J.; Rokka, J.; Paavilainen, T.; Parkkola, R.; Rinne, J.O.; Airas, L. In Vivo Detection of Diffuse Inflammation in Secondary Progressive Multiple Sclerosis Using PET Imaging and the Radioligand 11C-PK11195. J. Nucl. Med. 2014, 55, 939–944. [CrossRef]
- Ching, A.S.C.; Kuhnast, B.; Damont, A.; Roeda, D.; Tavitian, B.; Dollé, F. Current paradigm of the 18-kDa translocator protein (TSPO) as a molecular target for PET imaging in neuroinflammation and neurodegenerative diseases. Insights into Imaging 2011, 3, 111–119. [CrossRef]
- Narayanaswami, V.; Dahl, K.; Bernard-Gauthier, V.; Josephson, L.; Cumming, P.; Vasdev, N. Emerging PET Radiotracers and Targets for Imaging of Neuroinflammation in Neurodegenerative Diseases: Outlook Beyond TSPO. Mol. Imaging 2018, 17. [CrossRef]
- Owen, D.R.; Narayan, N.; Wells, L.; Healy, L.; Smyth, E.; A Rabiner, E.; Galloway, D.; Williams, J.B.; Lehr, J.; Mandhair, H.; et al. Pro-inflammatory activation of primary microglia and macrophages increases 18 kDa translocator protein expression in rodents but not humans. J. Cereb. Blood Flow Metab. 2017, 37, 2679–2690. [CrossRef]
- Driscoll I, Troncoso JC, Rudow G, Sojkova J, Pletnikova O, Zhou Y; et al. Correspondence between in vivo (11)C-PiB-PET amyloid imaging and postmortem, region-matched assessment of plaques. Acta Neuropathol. 2012;124(6):823-31. [CrossRef]
- Bodini, B.; Veronese, M.; García-Lorenzo, D.; Battaglini, M.; Poirion, E.; Chardain, A.; Freeman, L.; Louapre, C.; Tchikviladze, M.; Papeix, C.; et al. Dynamic Imaging of Individual Remyelination Profiles in Multiple Sclerosis. Ann. Neurol. 2016, 79, 726–738. [CrossRef]
- Stankoff B, Freeman L, Aigrot MS, Chardain A, Dollé F, Williams A; et al. Imaging central nervous system myelin by positron emission tomography in multiple sclerosis using [methyl-¹¹C]-2-(4'-methylaminophenyl)- 6-hydroxybenzothiazole. Ann Neurol. 2011;69(4):673-80. [CrossRef]
- Zeydan, B.; Lowe, V.J.; Schwarz, C.G.; A Przybelski, S.; Tosakulwong, N.; Zuk, S.M.; Senjem, M.L.; Gunter, J.L.; O Roberts, R.; Mielke, M.M.; et al. Pittsburgh compound-B PET white matter imaging and cognitive function in late multiple sclerosis. Mult. Scler. J. 2017, 24, 739–749. [CrossRef]
- Pietroboni, A.M.; Carandini, T.; Colombi, A.; Mercurio, M.; Ghezzi, L.; Giulietti, G.; Scarioni, M.; Arighi, A.; Fenoglio, C.; De Riz, M.A.; et al. Amyloid PET as a marker of normal-appearing white matter early damage in multiple sclerosis: Correlation with CSF β-amyloid levels and brain volumes. Eur. J. Nucl. Med. 2018, 46, 280–287. [CrossRef]
- Montalban, X.; Arnold, D.L.; Weber, M.S.; Staikov, I.; Piasecka-Stryczynska, K.; Willmer, J.; Martin, E.C.; Dangond, F.; Syed, S.; Wolinsky, J.S.; et al. Placebo-Controlled Trial of an Oral BTK Inhibitor in Multiple Sclerosis. New Engl. J. Med. 2019, 380, 2406–2417. [CrossRef]
- Reich, D.S.; Arnold, D.L.; Vermersch, P.; Bar-Or, A.; Fox, R.J.; Matta, A.; Turner, T.; Wallström, E.; Zhang, X.; Mareš, M.; et al. Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: A phase 2b, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2021, 20, 729–738. [CrossRef]
- Magliozzi, R.; Howell, O.W.; Reeves, C.; Roncaroli, F.; Nicholas, R.; Serafini, B.; Aloisi, F.; Reynolds, R. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann. Neurol. 2010, 68, 477–493. [CrossRef]
- Gibiansky, E.; Petry, C.; Mercier, F.; Günther, A.; Herman, A.; Kappos, L.; Hauser, S.; Yamamoto, Y.; Wang, Q.; Model, F.; et al. Ocrelizumab in relapsing and primary progressive multiple sclerosis: Pharmacokinetic and pharmacodynamic analyses of OPERA I, OPERA II and ORATORIO. Br. J. Clin. Pharmacol. 2020, 87, 2511–2520. [CrossRef]
- Hauser, S.L.; Waubant, E.; Arnold, D.L.; Vollmer, T.; Antel, J.; Fox, R.J.; Bar-Or, A.; Panzara, M.; Sarkar, N.; Agarwal, S.; et al. B-Cell Depletion with Rituximab in Relapsing–Remitting Multiple Sclerosis. N. Engl. J. Med. 2008, 358, 676–688. [CrossRef]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [CrossRef]
- Baert L, Benkhoucha M, Popa N, Ahmed MC, Manfroi B, Boutonnat J; et al. A proliferation-inducing ligand-mediated anti-inflammatory response of astrocytes in multiple sclerosis. Ann Neurol. 2019;85(3):406-20. [CrossRef]
- Pellerin, K.; Rubino, S.J.; Burns, J.C.; A Smith, B.; McCarl, C.-A.; Zhu, J.; Jandreski, L.; Cullen, P.; Carlile, T.M.; Li, A.; et al. MOG autoantibodies trigger a tightly-controlled FcR and BTK-driven microglia proliferative response. Brain 2021, 144, 2361–2374. [CrossRef]
- Martin, E.; Aigrot, M.-S.; Grenningloh, R.; Stankoff, B.; Lubetzki, C.; Boschert, U.; Zalc, B. Bruton’s Tyrosine Kinase Inhibition Promotes Myelin Repair. Brain Plast. 2020, 5, 123–133. [CrossRef]
- A Back, S.; Tuohy, T.M.F.; Chen, H.; Wallingford, N.; Craig, A.; Struve, J.; Luo, N.L.; Banine, F.; Liu, Y.; Chang, A.; et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat. Med. 2005, 11, 966–972. [CrossRef]
- Winkler, C.W.; Foster, S.C.; Matsumoto, S.G.; Preston, M.A.; Xing, R.; Bebo, B.F.; Banine, F.; Berny-Lang, M.A.; Itakura, A.; McCarty, O.J.T.; et al. Hyaluronan Anchored to Activated CD44 on Central Nervous System Vascular Endothelial Cells Promotes Lymphocyte Extravasation in Experimental Autoimmune Encephalomyelitis. J. Biol. Chem. 2012, 287, 33237–33251. [CrossRef]
- Kuipers HF, Rieck M, Gurevich I, Nagy N, Butte MJ, Negrin RS; et al. Hyaluronan synthesis is necessary for autoreactive T-cell trafficking, activation, and Th1 polarization. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(5):1339-44. [CrossRef]
- Preston, M.; Gong, X.; Su, W.; Matsumoto, S.G.; Banine, F.; Winkler, C.; Foster, S.; Xing, R.; Struve, J.; Dean, J.; et al. Digestion products of the PH20 hyaluronidase inhibit remyelination. Ann. Neurol. 2013, 73, 266–280. [CrossRef]
- Asher, R.A.; Morgenstern, D.A.; Fidler, P.S.; Adcock, K.H.; Oohira, A.; Braistead, J.E.; Levine, J.M.; Margolis, R.U.; Rogers, J.H.; Fawcett, J.W. Neurocan Is Upregulated in Injured Brain and in Cytokine-Treated Astrocytes. J. Neurosci. 2000, 20, 2427–2438. [CrossRef]
- Lau, L.W.; Keough, M.B.; Haylock-Jacobs, S.; Cua, R.; Döring, A.; Sloka, S.; Stirling, D.P.; Rivest, S.; Yong, V.W. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann. Neurol. 2012, 72, 419–432. [CrossRef]
- Stephenson, E.L.; Mishra, M.K.; Moussienko, D.; Laflamme, N.; Rivest, S.; Ling, C.-C.; Yong, V.W. Chondroitin sulfate proteoglycans as novel drivers of leucocyte infiltration in multiple sclerosis. Brain 2018, 141, 1094–1110. [CrossRef]
- Rolls, A.; Cahalon, L.; Bakalash, S.; Avidan, H.; Lider, O.; Schwartz, M. A sulfated disaccharide derived from chondroitin sulfate proteoglycan protects against inflammation-associated neurodegeneration. FASEB J. 2006, 20, 547–549. [CrossRef]
- Zhou, J.; Nagarkatti, P.; Zhong, Y.; Nagarkatti, M. Immune modulation by chondroitin sulfate and its degraded disaccharide product in the development of an experimental model of multiple sclerosis. J. Neuroimmunol. 2010, 223, 55–64. [CrossRef]
- Keough, M.B.; Rogers, J.A.; Zhang, P.; Jensen, S.K.; Stephenson, E.L.; Chen, T.; Hurlbert, M.G.; Lau, L.W.; Rawji, K.S.; Plemel, J.R.; et al. An inhibitor of chondroitin sulfate proteoglycan synthesis promotes central nervous system remyelination. Nat. Commun. 2016, 7, 11312–11312. [CrossRef]
- Graham, J.B.; Neubauer, D.; Xue, Q.-S.; Muir, D. Chondroitinase applied to peripheral nerve repair averts retrograde axonal regeneration. Exp. Neurol. 2007, 203, 185–195. [CrossRef]
- Bradbury, E.J.; Moon, L.D.F.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.W.; McMahon, S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–640. [CrossRef]
- Rosenzweig, E.S.; Salegio, E.A.; Liang, J.J.; Weber, J.L.; Weinholtz, C.A.; Brock, J.H.; Moseanko, R.; Hawbecker, S.; Pender, R.; Cruzen, C.L.; et al. Chondroitinase improves anatomical and functional outcomes after primate spinal cord injury. Nat. Neurosci. 2019, 22, 1269–1275. [CrossRef]
- Siebert JR, Osterhout DJ. The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J Neurochem. 2011;119(1):176-88. [CrossRef]
- Lee, H.; McKeon, R.J.; Bellamkonda, R.V. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 2009, 107, 3340–3345. [CrossRef]
- Führmann, T.; Anandakumaran, P.N.; Payne, S.L.; Pakulska, M.M.; Varga, B.V.; Nagy, A.; Tator, C.; Shoichet, M.S. Combined delivery of chondroitinase ABC and human induced pluripotent stem cell-derived neuroepithelial cells promote tissue repair in an animal model of spinal cord injury. Biomed. Mater. 2017, 13, 024103. [CrossRef]
- Kalamakis, G.; Brüne, D.; Ravichandran, S.; Bolz, J.; Fan, W.; Ziebell, F.; Stiehl, T.; Catalá-Martinez, F.; Kupke, J.; Zhao, S.; et al. Quiescence Modulates Stem Cell Maintenance and Regenerative Capacity in the Aging Brain. Cell 2019, 176, 1407–1419.e14. [CrossRef]
- Khacho, M.; Clark, A.; Svoboda, D.S.; Azzi, J.; MacLaurin, J.G.; Meghaizel, C.; Sesaki, H.; Lagace, D.C.; Germain, M.; Harper, M.-E.; et al. Mitochondrial Dynamics Impacts Stem Cell Identity and Fate Decisions by Regulating a Nuclear Transcriptional Program. Cell Stem Cell 2016, 19, 232–247. [CrossRef]
- Mimeault M, Batra SK. Great Promise of Tissue-Resident Adult Stem/Progenitor Cells in Transplantation and Cancer Therapies. In: López-Larrea C, López-Vázquez A, Suárez-Álvarez B, editors. Stem Cell Transplantation. New York, NY: Springer US; 2012. p. 171-86.
- Snethen, H.; Love, S.; Scolding, N. Disease-responsive neural precursor cells are present in multiple sclerosis lesions. Regen. Med. 2008, 3, 835–847. [CrossRef]
- Xing, Y.L.; Röth, P.T.; Stratton, J.A.S.; Chuang, B.H.; Danne, J.; Ellis, S.L.; Ng, S.W.; Kilpatrick, T.J.; Merson, T.D. Adult Neural Precursor Cells from the Subventricular Zone Contribute Significantly to Oligodendrocyte Regeneration and Remyelination. J. Neurosci. 2014, 34, 14128–14146. [CrossRef]
- Brown, C.; McKee, C.; Halassy, S.; Kojan, S.; Feinstein, D.L.; Chaudhry, G.R. Neural stem cells derived from primitive mesenchymal stem cells reversed disease symptoms and promoted neurogenesis in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Stem Cell Res. Ther. 2021, 12, 1–21. [CrossRef]
- Yang, J.; Jiang, Z.; Fitzgerald, D.C.; Ma, C.; Yu, S.; Li, H.; Zhao, Z.; Li, Y.; Ciric, B.; Curtis, M.; et al. Adult neural stem cells expressing IL-10 confer potent immunomodulation and remyelination in experimental autoimmune encephalitis. J. Clin. Investig. 2009, 119, 3678–3691. [CrossRef]
- Einstein, O.; Karussis, D.; Grigoriadis, N.; Mizrachi-Kol, R.; Reinhartz, E.; Abramsky, O.; Ben-Hur, T. Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Mol. Cell. Neurosci. 2003, 24, 1074–1082. [CrossRef]
- Aharonowiz, M.; Einstein, O.; Fainstein, N.; Lassmann, H.; Reubinoff, B.; Ben-Hur, T. Neuroprotective Effect of Transplanted Human Embryonic Stem Cell-Derived Neural Precursors in an Animal Model of Multiple Sclerosis. PLoS ONE 2008, 3, e3145. [CrossRef]
- Guan Y, Jiang Z, Ciric B, Rostami AM, Zhang GX. Upregulation of chemokine receptor expression by IL-10/IL-4 in adult neural stem cells. Exp Mol Pathol. 2008;85(3):232-6. [CrossRef]
- Yang, J.; Yan, Y.; Ma, C.-G.; Kang, T.; Zhang, N.; Gran, B.; Xu, H.; Li, K.; Ciric, B.; Zangaladze, A.; et al. Accelerated and enhanced effect of CCR5-transduced bone marrow neural stem cells on autoimmune encephalomyelitis. Acta Neuropathol. 2012, 124, 491–503. [CrossRef]
- Sher, F.; Amor, S.; Gerritsen, W.; Baker, D.; Jackson, S.L.; Boddeke, E.; Copray, S. Intraventricularly Injected Olig2-NSCs Attenuate Established Relapsing–Remitting EAE in Mice. Cell Transplant. 2012, 21, 1883–1897. [CrossRef]
- Moore, C.S.; Cui, Q.-L.; Warsi, N.M.; Durafourt, B.A.; Zorko, N.; Owen, D.R.; Antel, J.P.; Bar-Or, A. Direct and Indirect Effects of Immune and Central Nervous System–Resident Cells on Human Oligodendrocyte Progenitor Cell Differentiation. J. Immunol. 2015, 194, 761–772. [CrossRef]
- Imamura, O.; Arai, M.; Dateki, M.; Oishi, K.; Takishima, K. Donepezil-induced oligodendrocyte differentiation is mediated through estrogen receptors. J. Neurochem. 2019, 155, 494–507. [CrossRef]
- Peruzzotti-Jametti L, Bernstock JD, Vicario N, Costa ASH, Kwok CK, Leonardi T; et al. Macrophage-Derived Extracellular Succinate Licenses Neural Stem Cells to Suppress Chronic Neuroinflammation. Cell Stem Cell. 2018;22(3):355-68.e13. [CrossRef]
- Littlewood-Evans, A.; Sarret, S.; Apfel, V.; Loesle, P.; Dawson, J.; Zhang, J.; Muller, A.; Tigani, B.; Kneuer, R.; Patel, S.; et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J. Exp. Med. 2016, 213, 1655–1662. [CrossRef]
- Yang, J.; Yan, Y.; Ciric, B.; Yu, S.; Guan, Y.; Xu, H.; Rostami, A.; Zhang, G.-X. Evaluation of Bone Marrow- and Brain-Derived Neural Stem Cells in Therapy of Central Nervous System Autoimmunity. Am. J. Pathol. 2010, 177, 1989–2001. [CrossRef]
- Xie, C.; Li, X.; Zhou, X.; Li, Z.; Zhang, Y.; Zhao, L.; Hao, Y.; Zhang, G.-X.; Guan, Y. TGFβ1 transduction enhances immunomodulatory capacity of neural stem cells in experimental autoimmune encephalomyelitis. Brain, Behav. Immun. 2018, 69, 283–295. [CrossRef]
- McIntyre, L.L.; Greilach, S.A.; Othy, S.; Sears-Kraxberger, I.; Wi, B.; Ayala-Angulo, J.; Vu, E.; Pham, Q.; Silva, J.; Dang, K.; et al. Regulatory T cells promote remyelination in the murine experimental autoimmune encephalomyelitis model of multiple sclerosis following human neural stem cell transplant. Neurobiol. Dis. 2020, 140, 104868–104868. [CrossRef]
- Harris, V.K.; Stark, J.; Vyshkina, T.; Blackshear, L.; Joo, G.; Stefanova, V.; Sara, G.; Sadiq, S.A. Phase I Trial of Intrathecal Mesenchymal Stem Cell-derived Neural Progenitors in Progressive Multiple Sclerosis. EBioMedicine 2018, 29, 23–30. [CrossRef]
- Aigrot, M.; Barthelemy, C.; Moyon, S.; Dufayet-Chaffaud, G.; Izagirre-Urizar, L.; Gillet-Legrand, B.; Tada, S.; Bayón-Cordero, L.; Chara, J.; Matute, C.; et al. Genetically modified macrophages accelerate myelin repair. EMBO Mol. Med. 2022, 14, e14759. [CrossRef]
- Atkins, H.L.; Bowman, M.; Allan, D.; Anstee, G.; Arnold, D.L.; Bar-Or, A.; Bence-Bruckler, I.; Birch, P.; Bredeson, C.; Chen, J.; et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: A multicentre single-group phase 2 trial. Lancet 2016, 388, 576–585. [CrossRef]
- Burt RK, Balabanov R, Burman J, Sharrack B, Snowden JA, Oliveira MC; et al. Effect of Nonmyeloablative Hematopoietic Stem Cell Transplantation vs Continued Disease-Modifying Therapy on Disease Progression in Patients With Relapsing-Remitting Multiple Sclerosis: A Randomized Clinical Trial. JAMA. 2019;321(2):165-74. [CrossRef]
- Marin-Bañasco, C.; Marin-Bañasco, C.; Benabdellah, K.; Benabdellah, K.; Melero-Jerez, C.; Melero-Jerez, C.; Oliver, B.; Oliver, B.; Pinto-Medel, M.J.; Pinto-Medel, M.J.; et al. Gene therapy with mesenchymal stem cells expressing IFN-ß ameliorates neuroinflammation in experimental models of multiple sclerosis. Br. J. Pharmacol. 2017, 174, 238–253. [CrossRef]
- Blurton-Jones, M.; Spencer, B.; Michael, S.; A Castello, N.; A Agazaryan, A.; Davis, J.L.; Müller, F.-J.; Loring, J.F.; Masliah, E.; LaFerla, F.M. Neural stem cells genetically-modified to express neprilysin reduce pathology in Alzheimer transgenic models. Stem Cell Res. Ther. 2014, 5, 46–46. [CrossRef]
- Suzuki, M.; McHugh, J.; Tork, C.; Shelley, B.; Hayes, A.; Bellantuono, I.; Aebischer, P.; Svendsen, C.N. Direct Muscle Delivery of GDNF With Human Mesenchymal Stem Cells Improves Motor Neuron Survival and Function in a Rat Model of Familial ALS. Mol. Ther. 2008, 16, 2002–2010. [CrossRef]
- Eichler, F.; Duncan, C.; Musolino, P.L.; Orchard, P.J.; De Oliveira, S.; Thrasher, A.J.; Armant, M.; Dansereau, C.; Lund, T.C.; Miller, W.P.; et al. Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy. New Engl. J. Med. 2017, 377, 1630–1638. [CrossRef]
- De Ravin, S.S.; Wu, X.; Moir, S.; Kardava, L.; Anaya-O’brien, S.; Kwatemaa, N.; Littel, P.; Theobald, N.; Choi, U.; Su, L.; et al. Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency. Sci. Transl. Med. 2016, 8, 335ra57–335ra57. [CrossRef]
- Bak, R.O.; Dever, D.P.; Porteus, M.H. CRISPR/Cas9 genome editing in human hematopoietic stem cells. Nat. Protoc. 2018, 13, 358–376. [CrossRef]
- Walmsley, A.R.; Mir, A.K. Targeting the Nogo-A Signalling Pathway to Promote Recovery Following Acute CNS Injury. Curr. Pharm. Des. 2007, 13, 2470–2484. [CrossRef]
- Petratos, S.; Ozturk, E.; Azari, M.F.; Kenny, R.; Lee, J.Y.; Magee, K.A.; Harvey, A.R.; McDonald, C.; Taghian, K.; Moussa, L.; et al. Limiting multiple sclerosis related axonopathy by blocking Nogo receptor and CRMP-2 phosphorylation. Brain 2012, 135, 1794–1818. [CrossRef]
- Niederöst, B.; Oertle, T.; Fritsche, J.; McKinney, R.A.; Bandtlow, C.E. Nogo-A and Myelin-Associated Glycoprotein Mediate Neurite Growth Inhibition by Antagonistic Regulation of RhoA and Rac1. 2002, 22, 10368–10376. [CrossRef]
- Wang, X.; Ba, K.W.B.; Basso, D.M.; Strittmatter, S.M. Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann. Neurol. 2006, 60, 540–549. [CrossRef]
- Wang X, Lin J, Arzeno A, Choi JY, Boccio J, Frieden E; et al. Intravitreal delivery of human NgR-Fc decoy protein regenerates axons after optic nerve crush and protects ganglion cells in glaucoma models. Invest Ophthalmol Vis Sci. 2015;56(2):1357-66. [CrossRef]
- Seita, J.; Weissman, I.L. Hematopoietic stem cell: Self-renewal versus differentiation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 640–653. [CrossRef]
- Peterson, C.W.; Adair, J.E.; Wohlfahrt, M.E.; Deleage, C.; Radtke, S.; Rust, B.; Norman, K.K.; Norgaard, Z.K.; Schefter, L.E.; Sghia-Hughes, G.M.; et al. Autologous, Gene-Modified Hematopoietic Stem and Progenitor Cells Repopulate the Central Nervous System with Distinct Clonal Variants. Stem Cell Rep. 2019, 13, 91–104. [CrossRef]
- Ruckh, J.M.; Zhao, J.-W.; Shadrach, J.L.; van Wijngaarden, P.; Rao, T.N.; Wagers, A.J.; Franklin, R.J. Rejuvenation of Regeneration in the Aging Central Nervous System. Cell Stem Cell 2012, 10, 96–103. [CrossRef]
- Fournier, A.E.; Gould, G.C.; Liu, B.P.; Strittmatter, S.M. Truncated Soluble Nogo Receptor Binds Nogo-66 and Blocks Inhibition of Axon Growth by Myelin. 2002, 22, 8876–8883. [CrossRef]
- Kotter, M.R.; Li, W.-W.; Zhao, C.; Franklin, R.J.M. Myelin Impairs CNS Remyelination by Inhibiting Oligodendrocyte Precursor Cell Differentiation. J. Neurosci. 2006, 26, 328–332. [CrossRef]
- Ye S, Theotokis P, Lee JY, Kim MJ, Nheu D, Ellen O; et al. Nogo receptor-Fc delivered by hematopoietic cells enhances neurorepair in a multiple sclerosis model. Brain Communications. 2023. [CrossRef]
- Rüther, B.J.; Scheld, M.; Dreymueller, D.; Clarner, T.; Kress, E.; Brandenburg, L.; Swartenbroekx, T.; Hoornaert, C.; Ponsaerts, P.; Fallier-Becker, P.; et al. Combination of cuprizone and experimental autoimmune encephalomyelitis to study inflammatory brain lesion formation and progression. Glia 2017, 65, 1900–1913. [CrossRef]
- Scheld, M.; Rüther, B.J.; Große-Veldmann, R.; Ohl, K.; Tenbrock, K.; Dreymüller, D.; Fallier-Becker, P.; Zendedel, A.; Beyer, C.; Clarner, T.; et al. Neurodegeneration Triggers Peripheral Immune Cell Recruitment into the Forebrain. J. Neurosci. 2016, 36, 1410–1415. [CrossRef]
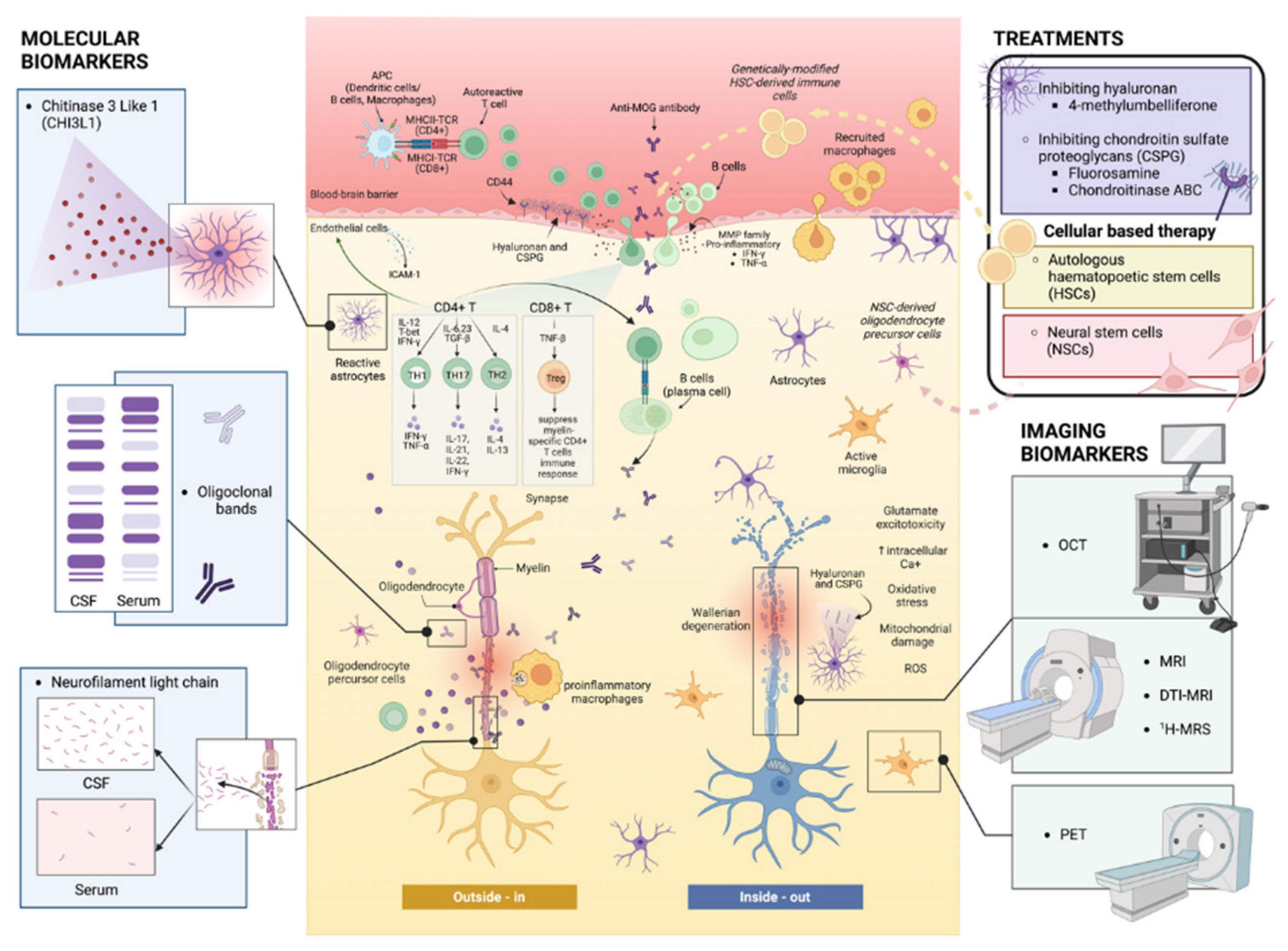
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




