1. Introduction
Metallic trace elements (MTEs) are particularly abundant in urban soils; lead, copper, zinc, chromium, nickel, cadmium, mercury and arsenic are the main MTE pollutants in urban soils [
1,
2]. For instance, lead concentrations can be fourteen times higher in urban than in rural soils [
3]. For these reasons, soil organisms are likely to be affected by MTE pollution in urban soils. Yet, soil invertebrates fulfil numerous soil-based ecosystem services such as organic matter decomposition, regulation of microbial activity, soil structure, etc. [
4,
5]. Amongst others, earthworms are of primary importance for ecosystem functioning by modifying the availability of resources for other organisms through physical and chemical changes in their surrounding soil environment [
6]. Therefore, understanding how soil invertebrate populations respond to MTE pollution in urban soils is key to comprehend variations in soil ecosystem functioning in an increasingly urbanized world.
Both experimental and correlative studies at industrially and geologically MTE-contaminated sites demonstrated profound effects of soil MTE pollution on soil invertebrates. For instance, MTE soil pollution is associated with reduced density, biomass, diversity index and richness of soil invertebrates, including earthworms, close to smelters or mining sites [
7,
8,
9,
10]. Earthworms were virtually absent in a 2 km radius around the smelter in Avonmouth (England), likely as a result of soil zinc pollution [
11,
12]. Moreover, experimental studies on
Eisenia fetida and
Eisenia Andrei demonstrated negative effects of copper, cadmium, chromium, lead, nickel and zinc exposure on either survival, body mass maintenance, cocoon production and viability, growth or sexual development [
11,
12,
13,
14,
15,
16]. However, MTE concentrations at industrial sites are usually extremely high sites (e.g. 28000 ppm lead [
17], 2500 ppm copper [
18], 19000 ppm zinc [
9]. Similarly, MTE concentrations used in experimental studies often exceed the mean concentrations measured in this study in Parisian gardens [
11]. Maybe more importantly, toxicity assessments are based on the exposure to a single MTE and do not account for synergetic, antagonistic or additive effects between MTEs [
19]. Therefore, while MTE pollution may contribute to the lower species richness and the altered community composition of invertebrates in cities relative to less disturbed habitats [
20,
21], the effects of soil MTE pollution in urban environments on soil invertebrates has not been directly tested so far.
Soil MTE pollution may affect soil invertebrate spatial distribution by influencing habitat preference/avoidance [
22,
23]. Avoidance behavior toward MTE polluted habitats varies between species of invertebrates [
24]. For instance, several species of earthworms were shown to avoid copper and zinc enriched soils, although the concentration threshold determining departure was species-specific (Lukkari and Haimi 2005; Lukkari et al. 2005); interestingly, the threshold concentration for avoidance of arsenic contaminated soil was lower in individuals originating from uncontaminated soil, suggesting either acclimatization or adaptation mechanisms [
27]. The evolution of avoidance behavior, which implies the capacity to detect MTEs and to move away, depends on the cost of exploration and/or of disperal [
28]. In fragmented landscapes, such as urban environments [
29], the energy expenditure and mortality risk associated with dispersal can be particularly high [
28,
30]. Populations may then evolve alternative strategies to overcome the adverse influence of otherwise toxic MTE concentrations, which strategies involve the absorption, immobilization and/or excretion of MTEs. The evolution of MTE tolerant genotypes is well known in vascular plants [
31,
32]. In invertebrates, MTE exposure, although not in a context of urbanization, is associated with genetic and phenotypic variations (reviewed in Posthuma and Van Straalen 1993; Morgan et al. 2007; Janssens et al. 2009). For instance, wild populations of common fruit fly
Drosophila melanogaster that have been exposed to MTE pollution are endowed with a duplicated gene coding for metallothionein (Mtn), a protein involved in the binding, transport and detoxification of MTEs; this confers individuals a better tolerance to copper and cadmium exposure [
36]. In the earthworm
Dendrobaena octaedra, offspring had a higher survival rate when exposed to elevated cadmium concentrations if their parents originated from MTE polluted than from unpolluted forests [
37]. Experimentally comparing populations exposed, in their natural environment, to different levels of MTE pollution is an interesting avenue to gain insight on the effects of urbanization on soil invertebrates and to identify adaptation and/or acclimatization mechanisms shaping spatial distribution in cities [
38].
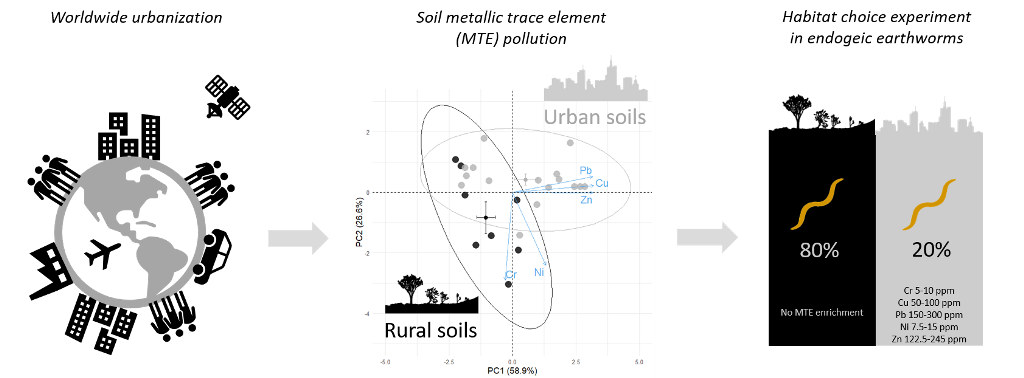
Using soils with MTE concentrations mimicking MTE pollution level in urban parks, I compared the effect of MTE soil pollution on habitat preference between three species of endogeic earthworms, namely Apporectodea caliginosa, Apporectodea icterica and Allolobophora chlorotica, originating either from urban or rural grasslands. The aim of this study was two-fold: to 1) understand whether soil MTE pollution explains earthworm movements and habitat selection and 2) test whether habitat preference depends on earthworm previous exposure to MTE-polluted soil. Earthworm response to soil MTE pollution was also characterized by measuring mortality, mobility, body mass maintenance and MTE accumulation.
2. Methods
2.1. Subjects and housing
Free-living adult earthworms from three endogeic species –
Apporectodea caliginosa,
Apporectodea icterica and
Allolobophora chlorotica (green morph) – were collected from February to May 2016 in gardens, squares or grasslands at 16 urban sites in Paris, and 8 rural sites across France (based on land use; see Table A1). At each urban site, they were collected from three different locations less than 200 m apart. The rural and urban soils (0-30 cm deep) were similar in terms of physico-chemical characteristics other than MTEs (i.e. pH, CO
3, TOC, OM, N
org, ρ, P205), except for the proportion of bigger particles (> 6.3 mm) being more abundant in rural than in urban sites (see Table A3). Earthworms were identified in the lab using morphological characteristics [
39,
40]. To reduce potential stress effects of capture on earthworm behavior, earthworms were kept in acclimatizing terrariums (12 cm x 10 cm x 8 cm) filled with suitable soil (i.e. grassland soil sampled from a brown earth at the Research Institute for Development (Bondy, France; 48°54’N, 2°29’E) hosting large earthworm populations); they were kept at a maximum density of 10 earthworms per terrarium, at 20°C during the day and night. Acclimatization lasted at least 3 weeks but varied between individuals depending on their capture and trial dates. Twice a month, acclimatizing terrariums were humidified and enriched with a cupped hand of homogenized horse dropping spread on the surface of the soil. At least a week before the start of the trial, earthworms were transferred in MTE-less control soil (see §2.2) enriched with homogenized horse dropping, in a similar way as acclimatizing terrariums. Each individual earthworm was used only once during the experiment. The species names used herein are conformed to the Fauna Europaea web site (
http://www.faunaeur.org/index.php).
2.2. Terrarium set-up for behavioral trials
Behavioral trials were carried out in terrariums (25 x 25 x 1cm) with transparent walls to allow easy location of the earthworms and observation of the galleries (see §2.3). The two sides of the terrariums were filled separately with MTE-enriched (MTE-rich M++ or MTE-poor M+) or MTE-less soil (M-); the two soils were held apart by a divider which was subsequently removed. The soils were slightly compacted whenever needed to obtain a visually homogeneous and similar soil density among and between terrariums. To ensure that MTE-enriched and MTE-less soils differed only in MTE concentrations, artificial soils were created; they consisted of 70% sand, 20% clay and 10% sphagnum. Forty-eight hours before the start of the trial, dry soil was transferred into soil preparation buckets where soils were humidified up to 25% of the dry mass with distilled water (M-), or MTE enriched solutions (chromium sulphate, copper sulphate, lead acetate, nickel acetate and zinc chloride; Sigma-Aldrich, St-Louis, USA) to obtain a MTE-rich soil (M++: Cr 10 ppm, Cu 100 ppm, Pb 300 ppm, Ni 15 ppm and Zn 245 ppm) and a MTE-poor soil (M+: half concentrated compared to the MTE-rich soil) (
Figure 1). MTE concentrations were chosen to mimic average MTE levels in soils of public green spaces in Paris (personal data from Florence Dubs collected in 2015), which concentrations are in the range of concentrations measured in urban soils worldwide [
1,
2,
41]. MTE salts were chosen for their solubility in water. Although not significantly so, MTE enriched soils (both M++ and M+) had systematically lower pH, carbon to nitrate ratio and assimilable phosphorus (see Table A2). However, the pH (from 5.42 to 6.55) always remained very close to the 6.0 ± 0.5 pH advised in the OECD guideline for the testing of chemicals.
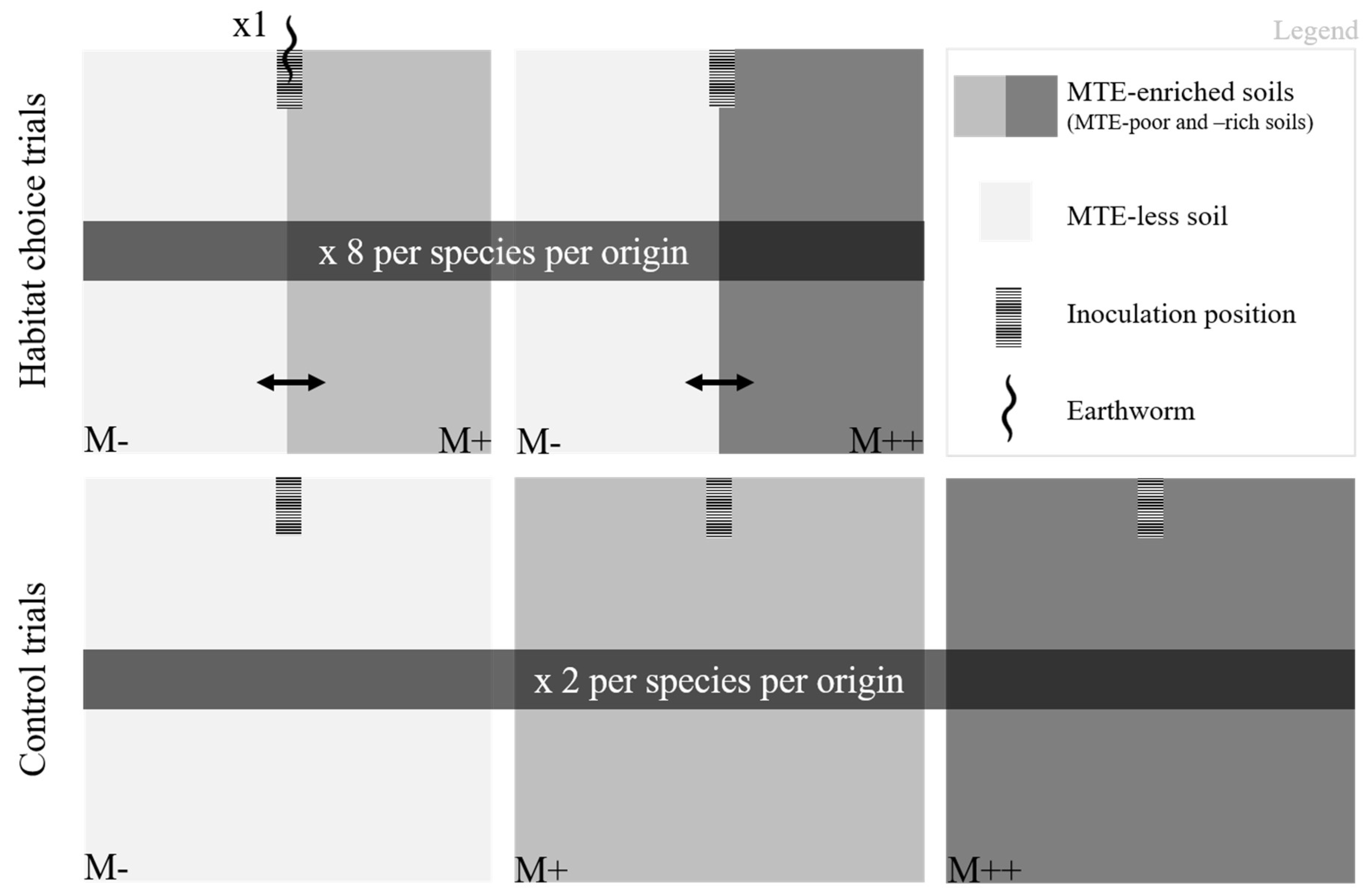
To test whether earthworm habitat preference depends on MTE exposure, the two sections were filled with a MTE-enriched and with the MTE-less soil (M+/M- or M++/M-); the two soils were alternatively on the right or on the left of the terrarium. I carried out a total of 96 habitat choice trials: eight trials per species (
A. caliginosa,
A. icterica and
A. chlorotica) per origin (urban and rural) per MTE exposure (M-/M+ and M-/M++) (
Figure 1). In addition, to ensure that earthworm location within the terrarium results from a choice rather than from impaired mobility caused by MTE exposure, both sections were filled with a MTE-enriched (M+/M+ or M++/M++) or the MTE-less soil (M-/M-;
Figure 1). I carried out a total of 36 control trials: two per species per origin per MTE exposure.
2.3. Trial procedure
In order not to influence earthworm choice for one of the two sides of the terrariums, a hole of 2cm of depth was perpendicularly dug from the top of the soil such as it was in the middle of the terrarium. For each trial, a single worm was introduced to the hole, anterior end first (
Figure 1). The terrarium was immediately closed and let undisturbed in a dark room at 20°C. During five consecutive days, earthworm location was marked (i.e. a cross was drawn on the terrarium at the middle of the earthworm’s body) every two hours from 9am to 5pm, resulting in 23 to 25 location marks per trial. At the end of both control and choice trials, the galleries were traced on both sides of the terrarium; a picture of the empty terrarium – allowing to simultaneously see the galleries on both sides – was taken. Total gallery length in the MTE-enriched and MTE-less soils (i.e. the total length of all galleries formed on each side of the terrarium) was measured using the SmartRoot package in ImageJ [
42]. Each earthworm was weighed before and at the end of a trial to measure mass change, calculated as the difference between post and pre-trial mass divided by pre-trial mass. At the end of the choice trials, earthworms were moved back in an acclimatizing terrarium; they were released in their environment at the end of the study. Earthworms from the control trials were individually kept fasting during 24h in a petri dish containing a wet sponge, then froze at -20°C until MTE analysis (see §2.4).
Earthworm movements were measured using five variables: mobility, total gallery length and side choice (in control trials), and specificity and soil choice (in choice trials). For each control trials (M-/M-, M+/M+ and M++/M++), mobility was calculated as the number of non-overlapping location marks. In addition, side choice was measured by counting the number of locations on the left and the right section of the terrarium to verify that earthworm movements were homogeneous within the terrarium. For each choice trial (M+/M- and M++/M-), specificity for the MTE-less soil was defined as 0 when the individual visited the MTE-enriched soil at least once and as 1 when all the locations were in the MTE-less soil. Moreover, soil choice was measured by (i) counting the number of locations, and (ii) measuring the size of the galleries in the MTE-enriched soil (M+ or M++) and in the MTE-less soil (M-); the locations in the middle of the terrarium were excluded.
2.4. MTE analyses
In earthworm native soils – To ensure that wild earthworms collected in rural and urban environments were exposed to different level of MTE pollution, soil of each site was collected and MTE concentrations (i.e. chromium, copper, lead, nickel and zinc) were measured. For urban sites, soil was collected at three different locations not more than 200 m apart. Briefly, a 30 x 30 x 30 cm block of soil was extracted and from which at least 1 L of soil was sampled from the topsoil (from 0 to 5 cm below the surface) and from the subsoil (from 10 to 30 cm below the surface), separately. MTE analyses were carried out by the Laboratoire d’Agronomie de la ville de Paris (France): soil samples were dried at 40°C, 2 mm sieved and 150 µm ground with a ball mill (MM400 Retsch). A total of 0.5 g per sample was then digested simultaneously in 1.2 mL HNO3 (65%) and 3.5 mL HCl (37%) during 16h at room temperature, then 2h at 100°C. Ultrapure water was added to reach a final volume of 50 mL and left to decant during 8h. Total chromium, copper, lead, nickel and zinc concentrations were determined using an inductively coupled plasma optical emission spectrometer (iCAP 7000 ICP-OES, Thermo Electron, USA). The ICP-OES conditions were as follows: plasma power 1150 W, nebulize gaz flow 0.5 L/min, auxiliary gas flow 0.5 L/min. The ICP-OS was calibrated before performing measurements with the use of Cr, Cu, Ni, Pb and Zn standard solutions (1000 ± 2 mg dm− 3 in 5% HNO3) from Chem-Lab. Each MTE was measured three times per sample. Relative standard deviation between the three measurements per sample were all below 10%. The blanks and certified reference materials (Internal Soil Reference Materials ISRM1 and ISRM2) were prepared and analyzed using the same methods as the samples. Calibration and blank samples were analyzed every ten measurements. To estimate earthworm exposure to MTEs at each site, MTE concentrations in the topsoil and the subsoil were averaged. For urban sites, MTE concentrations at the three locations, less than 200 m apart within the site, were averaged.
In artificial soils – To test the validity of my MTE supplementation protocol, the MTE-less (M-), -poor (M+) and -rich soils (M++) were sampled twice during the study. Forty-eight hours after their preparation, ca. 1L of soil was taken from the soil preparation buckets. The samples MTE concentrations were measured using the same protocol as described above.
In earthworms – Earthworms that were used in the control trials were defrosted and dried for 48h at 50°C. They were weighed to the nearest 0.1 mg then digested simultaneously in 1 mL HNO3 (68%) and 1 mL HF (40%) during 24h at 80°C. The product of digestion was transferred into plastic tubes and ultrapure water was added to reach a final 1% acid concentration. Total chromium, copper, lead, nickel and zinc concentrations were determined using an inductively coupled plasma mass spectrometer (NexION 300D ICP Mass Spectrometer, Perkin Elmer SCIEX, USA) in the Biological and Chemical Research Centre (Faculty of Chemistry, University of Warsaw, Poland). A conventional Mainhardt nebulizer and a quartz cyclonic spray chamber were used for sample introduction. The ICP-MS conditions were as follows: plasma power 1100 W, plasma argon flow 15 L/min, auxiliary gas flow 1.21 L/min, nebulizer gas flow 0.86 L/min. The ICP-MS was calibrated before performing measurements with the use of multi standard solutions (ICP Calibration Standard from Merck). During the measurements, the parameters of calibration were checked using the standard containing mercury at the concentration of 1 μg/L in 1% nitric acid. Each isotope was measured three times and each sample was analysed two times. Relative standard deviation between the three measurement per isotope and between the two measurements per sample were all below 10%. The blanks and Certified Reference Materials (CRMs; trace elements in water 1643f from LGC Standards and SPS-SW1 batch 112 from SpectraPure Standards) were prepared and analysed using the same methods as the samples.
2.5. Statistical analyses
Statistical analyses were performed using R software (version 3.5.1).
MTE concentrations in natural soils – To ensure that earthworms collected in urban and rural soils were, in their natural environment, exposed to different level of MTE pollution, chromium, copper, lead, nickel and zinc concentrations in urban and rural soils where earthworms have been collected were compared using linear models (R built-in ‘lm’ function) with the log-transformed MTE concentration as the dependent variable and the origin (urban vs. rural) as the explanatory variable.
MTE-induced health impairments (control trials) – To investigate MTE exposure health effects on earthworms, I performed linear mixed-effects models (‘lmer’ function from the ‘lme4’ package (Bates et al., 2015)) with either i) mobility, ii) log-transformed gallery length or iii) mass loss as the dependent variable, and MTE exposure (M-, M++ or M++), earthworm origin (rural vs. urban) and species (A. caliginosa, A. chlorotica and A. icterica), and their two-way interactions as explanatory variables; the low sample size (i.e. two individuals per species per MTE exposure) prevented to test for the three-way interaction. The capture site was added as random intercept. Moreover, to insure that earthworm movements were homogeneous within the terrarium, I performed a generalized mixed-effects model (‘glmer’ function from the ‘lme4’ package) with the number of locations in the left section over the total number of locations (binomial distributions) as the dependent variable, and MTE exposure, earthworm origin and species, and their two way interactions as the explanatory variables. The capture site was added as random intercept. The trial identity was also added as random intercept to correct for overdispersion.
MTE accumulation in earthworms (control trials) – To test whether MTE accumulation differed in response to MTE exposure and depending on the species and the origin of the earthworm, I performed similar models as previously described to test MTE-induced health impairments, with log-transformed MTE concentrations (chromium, copper, lead, nickel or zinc) as explanatory variables.
MTE-linked habitat preference (choice trials) – To explain the variability in earthworms movements in response to MTE exposure, I performed generalized mixed-effects models (‘glmer’ function) with the proportion of locations or galleries in the MTE-enriched soil (M+ or M++) as the dependent variable (binomial distributions), and MTE exposure (M+/M- vs. M++/M-), earthworm origin and species, and their two and three-way interactions as the explanatory variables. The capture site was added as random intercept. The trial identity was also added as random intercept to correct for overdispersion. More than half of the earthworms did not visit the MTE-enriched soil. Visiting or not the MTE-enriched soil may reflect different habitat selection strategies (e.g. strict avoidance of MTE polluted soils associated with high sensory capacities vs. tolerance to MTE pollution associated with a benefit for exploratory behavior). Therefore, in a second step, I analysed separately the behaviour of earthworms that visited the MTE-less soil only and the ones that visited both soils. Variation in specificity for the MTE-less soil was investigated using a similar generalized mixed-effects model as described above. However, the low sample size prevented to test for the three-way interaction. In individuals that visited both MTE-less and MTE-enriched soils, I investigated both soil choice (i.e. the proportion of locations or galleries in the MTE-enriched soil), using a similar generalized mixed-effects model as describe above, and learning – defined as the variation of earthworm location between the MTE-less and -enriched soils over the course of the trial – using a generalized mixed-effects model with earthworm location (in MTE-less or MTE-enriched soil) as the dependent variable (binomial distribution) and time (the number of location marks; from 1 to 23), MTE exposure, earthworm origin and species, and the two-way interactions with time as the explanatory variables. The capture site and the trial identity were added as random intercepts. The low sample size prevented to test for other interactions between the explanatory variables.
Linear mixed-effects models and generalized mixed-effects models were fitted using the restricted maximum likelihood (REML) and the Laplace approximation of the maximum likelihood methods, respectively. For each model, I performed a backward stepwise selection using the AIC. A Type III Wald chisquare test Anova was used to determine the significance of the retained variables in the final models. Contrasts among groups were tested using least-square mean pairwise comparisons (contrast function of the ‘lsmeans’ package; Lenth 2016).
Figure 1.
Schematic representation of the experimental design. The study consisted of a total of (i) 96 choice trials – i.e. eight trials per species (Apporectodea caliginosa, Apporectodea icterica and Allolobophora chlorotica) per origin (urban and rural) and per MTE exposure (M-/M+ and M-/M++), and 36 control trials – i.e. two trials per species per origin and per MTE exposure (M-, M+ and M++).
Figure 1.
Schematic representation of the experimental design. The study consisted of a total of (i) 96 choice trials – i.e. eight trials per species (Apporectodea caliginosa, Apporectodea icterica and Allolobophora chlorotica) per origin (urban and rural) and per MTE exposure (M-/M+ and M-/M++), and 36 control trials – i.e. two trials per species per origin and per MTE exposure (M-, M+ and M++).
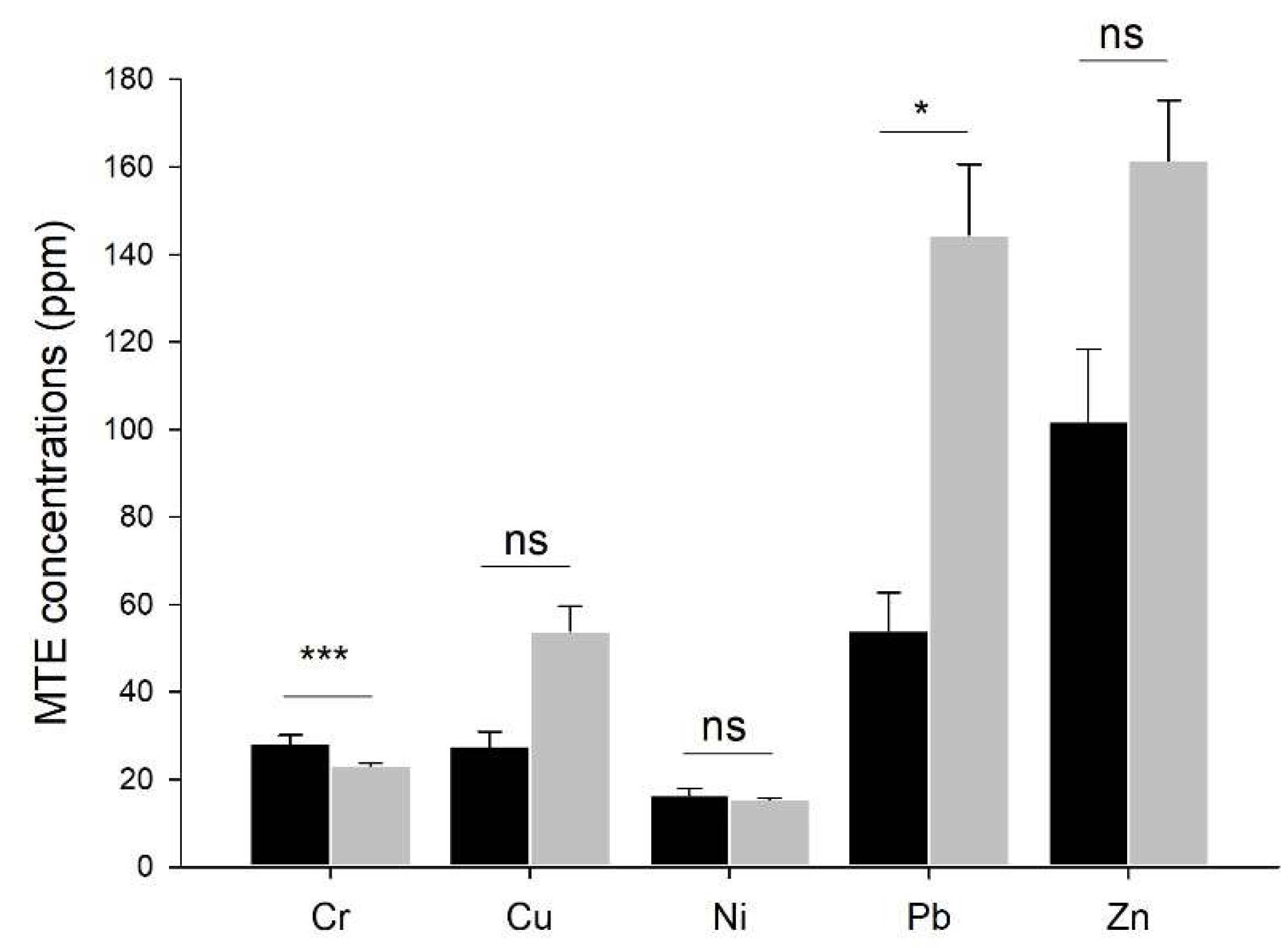
Figure 2.
MTE concentrations in natural soils. Mean ± se chromium, copper, nickel, lead, and zinc concentrations in the soils from rural and urban origins where earthworms were samples. Significant differences of MTE concentrations between the two origins are highlighted by asterisks; they account for the capture site.
Figure 2.
MTE concentrations in natural soils. Mean ± se chromium, copper, nickel, lead, and zinc concentrations in the soils from rural and urban origins where earthworms were samples. Significant differences of MTE concentrations between the two origins are highlighted by asterisks; they account for the capture site.
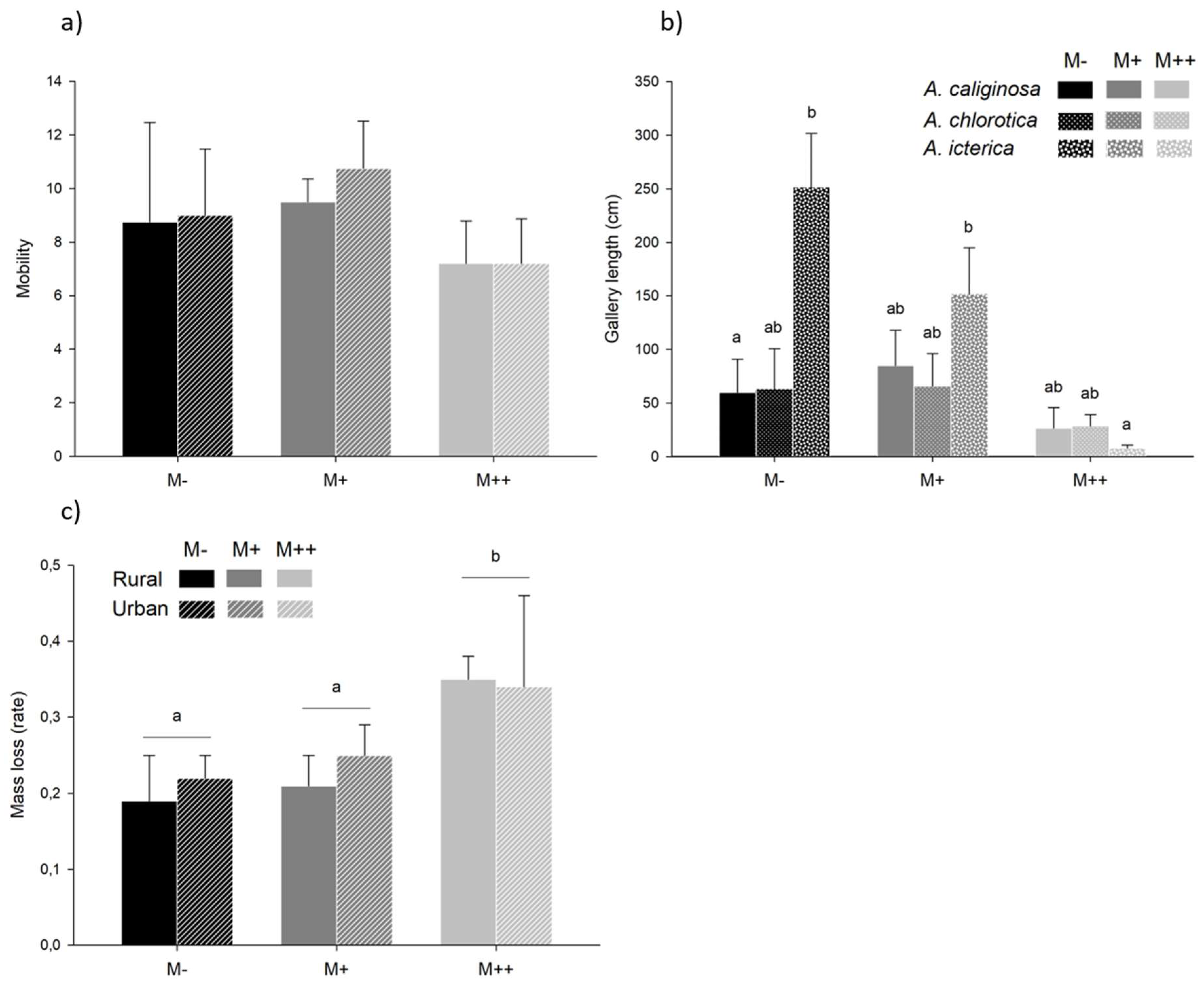
Figure 3.
Health impairment. Mean ± se a) mobility (calculated as the number of non-overlapping locations at the end of a trial) in earthworms from rural and urban origin exposed to MTE-less (M-), -low (M+) and -rich soils (M++), b) gallery length in A. caliginosa, A. chlorotica and A. icterica exposed to MTE-less, -low and rich soils, and c) mass loss (calculated as the difference between post and pre-trial mass divided by pre-trial mass) in earthworms from rural and urban origin after exposure to MTE-less, -low and rich soils. Significant differences between species and MTE exposure (in b), and between MTE exposure (in c) are highlighted by different letters. Mobility did not significantly differ between MTE exposure, origin and species.
Figure 3.
Health impairment. Mean ± se a) mobility (calculated as the number of non-overlapping locations at the end of a trial) in earthworms from rural and urban origin exposed to MTE-less (M-), -low (M+) and -rich soils (M++), b) gallery length in A. caliginosa, A. chlorotica and A. icterica exposed to MTE-less, -low and rich soils, and c) mass loss (calculated as the difference between post and pre-trial mass divided by pre-trial mass) in earthworms from rural and urban origin after exposure to MTE-less, -low and rich soils. Significant differences between species and MTE exposure (in b), and between MTE exposure (in c) are highlighted by different letters. Mobility did not significantly differ between MTE exposure, origin and species.
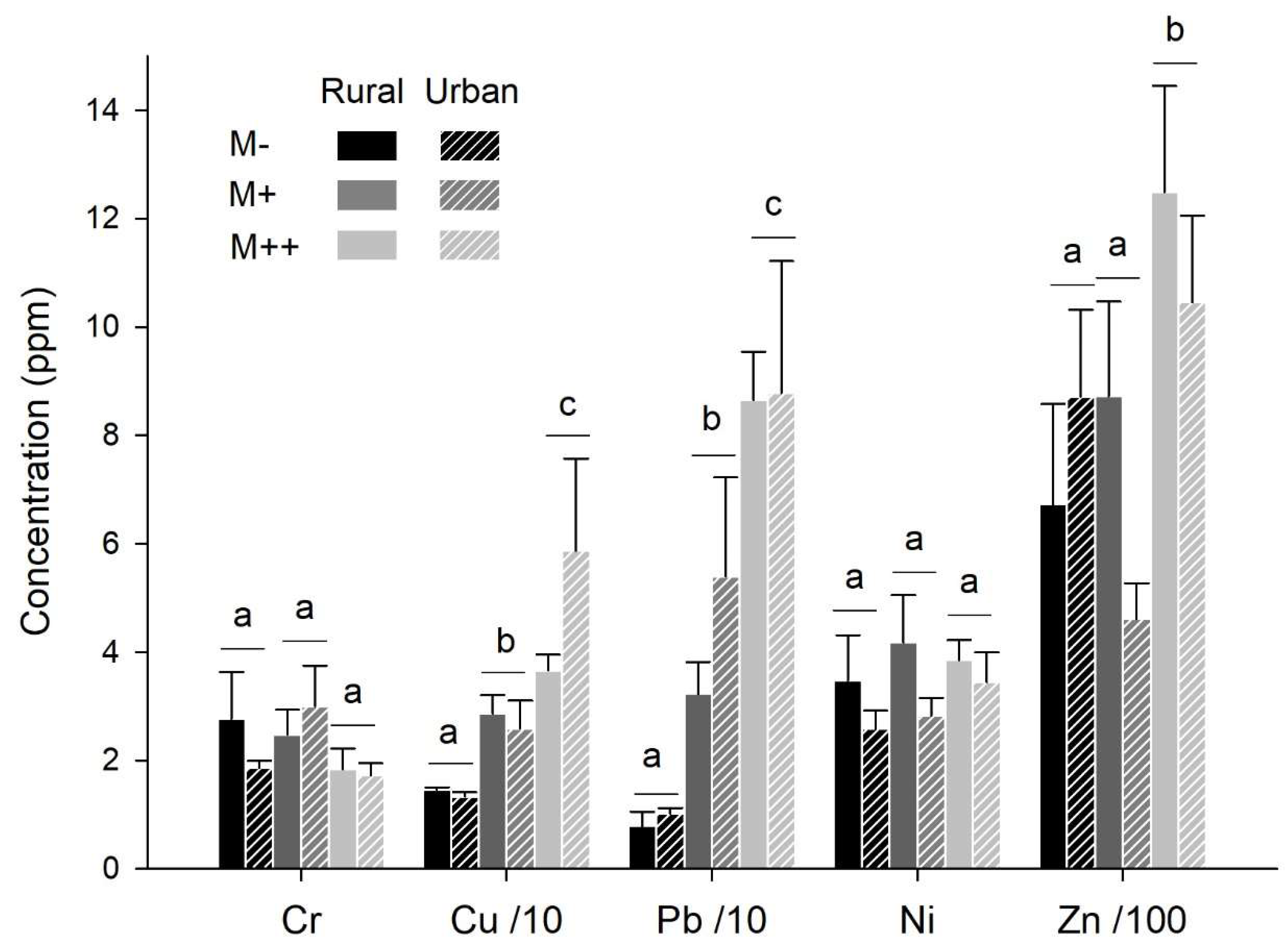
Figure 4.
MTE accumulation. Mean ± se chromium, copper, nickel, lead, and zinc concentrations in earthworms from rural and urban origin and exposed to MTE-less (M-), -poor (M+) and rich soils (M++). For graphical purpose, copper and lead concentrations have been divided by 10 and zinc concentrations by 100. For each MTE, significant differences of concentrations between MTE exposure (M-, M+ and M++) are highlighted by different letters. MTE concentrations did not vary according to earthworm origin.
Figure 4.
MTE accumulation. Mean ± se chromium, copper, nickel, lead, and zinc concentrations in earthworms from rural and urban origin and exposed to MTE-less (M-), -poor (M+) and rich soils (M++). For graphical purpose, copper and lead concentrations have been divided by 10 and zinc concentrations by 100. For each MTE, significant differences of concentrations between MTE exposure (M-, M+ and M++) are highlighted by different letters. MTE concentrations did not vary according to earthworm origin.
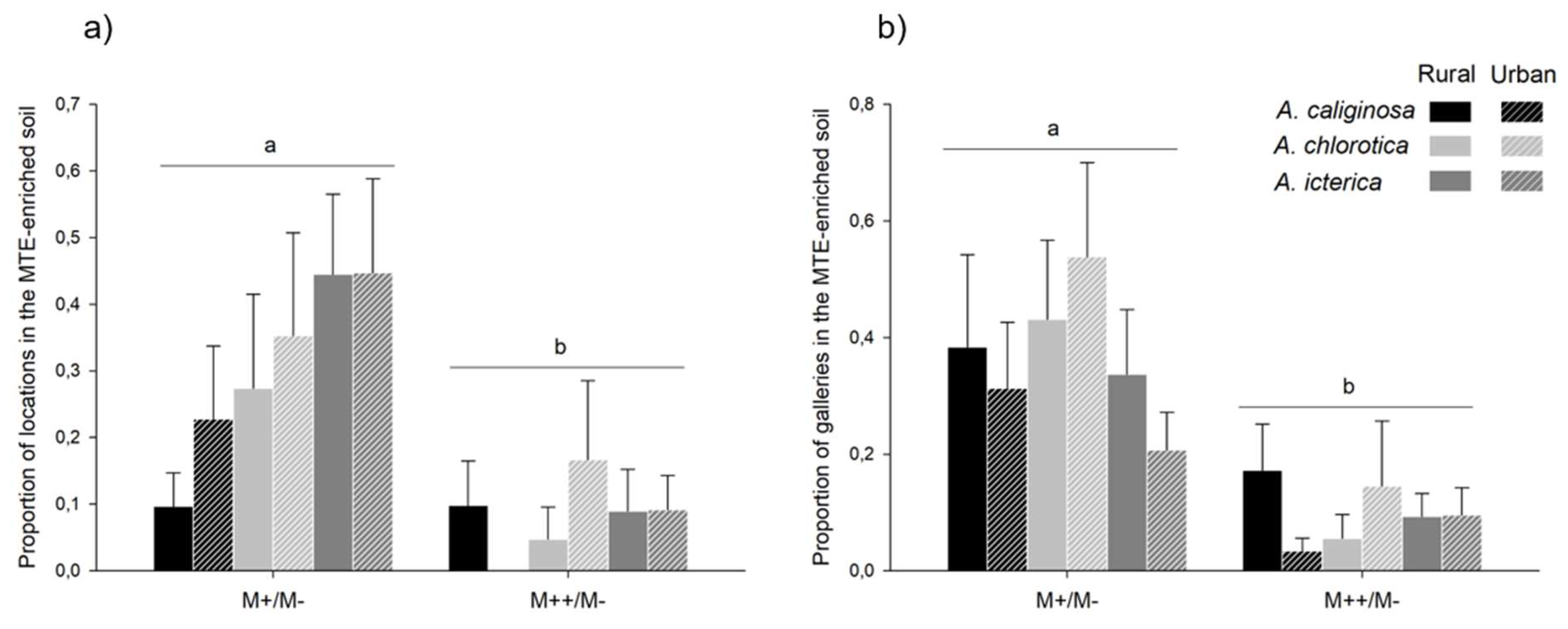
Figure 5.
Habitat preference. Mean ± se proportion of a) locations, b) galleries in the MTE-enriched soil in earthworms from rural and urban origin and exposed to MTE-poor and –rich soils. Significant differences between MTE exposure are highlighted by different letters. Earthworm movements did not significantly vary according to earthworm origin and species.
Figure 5.
Habitat preference. Mean ± se proportion of a) locations, b) galleries in the MTE-enriched soil in earthworms from rural and urban origin and exposed to MTE-poor and –rich soils. Significant differences between MTE exposure are highlighted by different letters. Earthworm movements did not significantly vary according to earthworm origin and species.
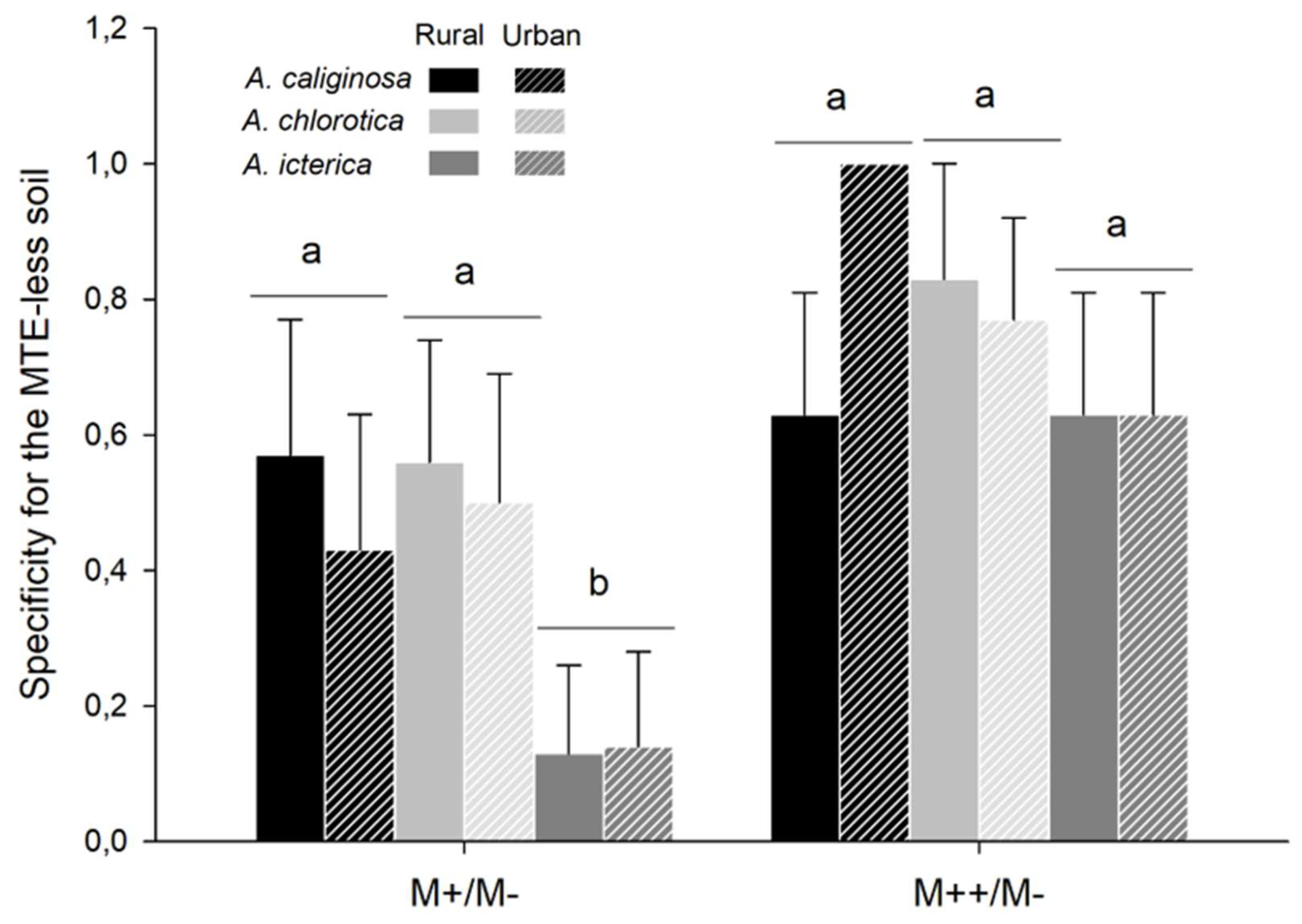
Figure 6.
Specificity. Mean ± se specificity for the MTE-less soil in earthworms from rural and urban origin and exposed to MTE-poor and -rich soils. For graphical purpose, significant differences between MTE exposure and species are highlighted by different letters. However, only the simple effects of MTE exposure and species significantly explained specificity; the interaction between the two variables was not retained in the model.
Figure 6.
Specificity. Mean ± se specificity for the MTE-less soil in earthworms from rural and urban origin and exposed to MTE-poor and -rich soils. For graphical purpose, significant differences between MTE exposure and species are highlighted by different letters. However, only the simple effects of MTE exposure and species significantly explained specificity; the interaction between the two variables was not retained in the model.
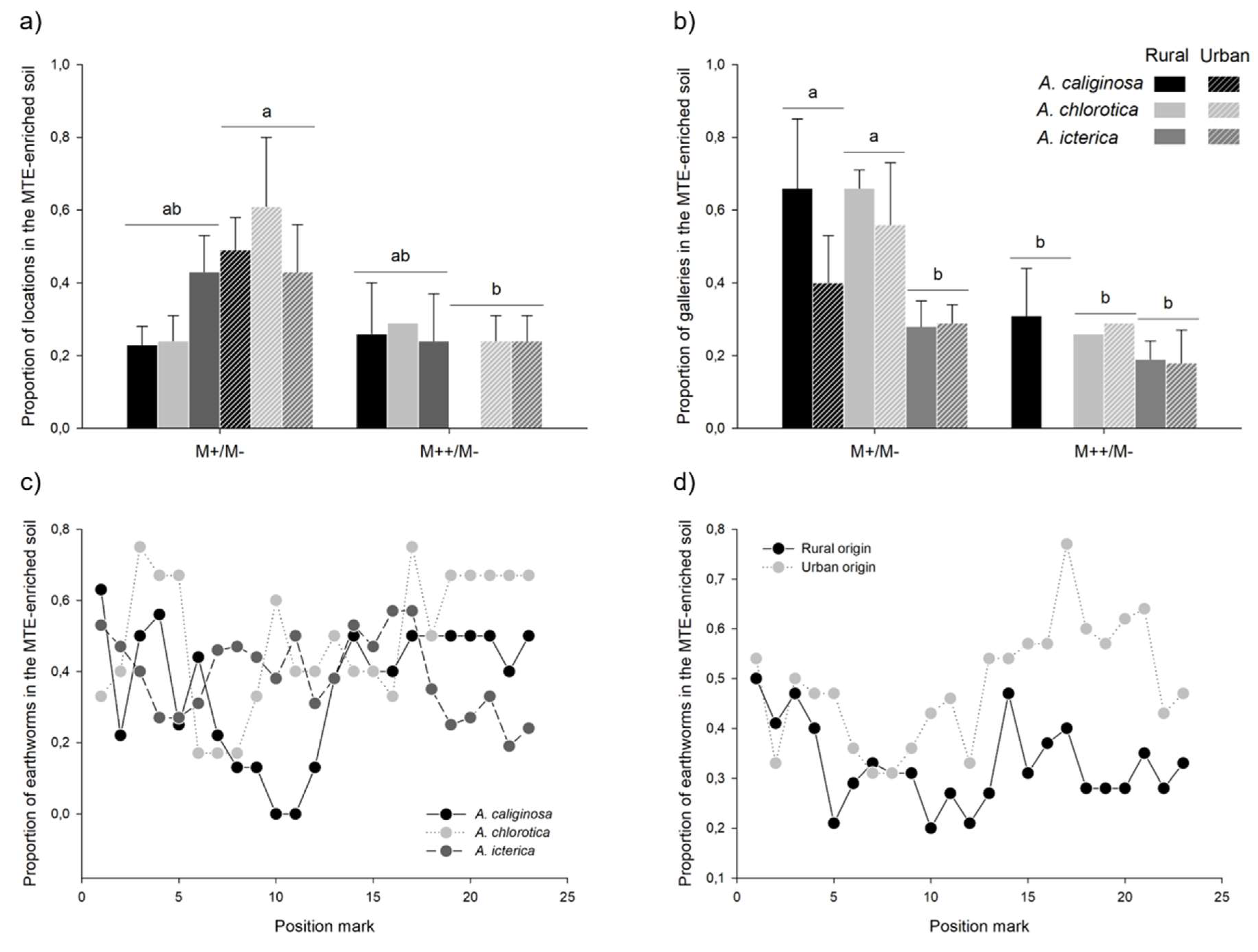
Figure 7.
Choice mechanism. Mean ± se proportion of a) locations and b) galleries in the MTE-enriched, and of earthworms in the MTE-enriched soil according to c) earthworm species and d) origin in earthworms that visited both MTE-enriched and -less soils. For graphical purpose, significant differences between MTE exposure and earthworm origin, and MTE exposure and species are highlighted by different letters, respectively in a) and b). However, only the simple effects of MTE exposure and species significantly explained the proportion of galleries in the MTE-enriched soil; the interaction between the two variables was not retained in the model.
Figure 7.
Choice mechanism. Mean ± se proportion of a) locations and b) galleries in the MTE-enriched, and of earthworms in the MTE-enriched soil according to c) earthworm species and d) origin in earthworms that visited both MTE-enriched and -less soils. For graphical purpose, significant differences between MTE exposure and earthworm origin, and MTE exposure and species are highlighted by different letters, respectively in a) and b). However, only the simple effects of MTE exposure and species significantly explained the proportion of galleries in the MTE-enriched soil; the interaction between the two variables was not retained in the model.
Table 1.
MTE concentrations in artificial soils.
Table 1.
MTE concentrations in artificial soils.
| |
Chromium |
Copper |
Lead |
Nickel |
Zinc |
| M- |
5.5±0.2 (BDL)
|
5.5±5.5 (BDL)
|
38.8±6.3 (BDL)
|
BDL (BDL)
|
12.5±12.5 (BDL)
|
| M+ |
6.2±0.4 (5.0)
|
42.0±3.0 (50.0)
|
154.3±9.8 (150.0)
|
2.9±2.8 (7.5)
|
106.0±7.0 (122.5)
|
| M++ |
7.1±0.1 (10.0)
|
69.5±7.5 (100.0)
|
243.8±28.8 (300.0)
|
7.9±0.0 (15.0)
|
189.5±12.5 (245.0)
|