1. Introduction
Vaccines against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in liver transplant (LT) recipients induced decreased immunogenicity compared to the general population[
1,
2]. Although the administration of a booster (third) dose of mRNA-based vaccines increased the immune response rates produced by two vaccine doses by 20%[
3], up to 30% of LT patients remained unresponsive to the vaccination[
1,
2]. Thus, the administration of a fourth dose of anti-SARS-CoV-2 vaccines seems to be a useful way to increase immunogenicity in LT and in solid organ transplant recipients[
4]. Mycophenolate (MMF) plays a major role in reducing the probability of achieving positive antibody[
3,
5] and T cell[
6] immune responses following a booster dose of mRNA-based anti-SARS-CoV-2 vaccines. This could be explained by decreases in lymphocyte activation, interaction of T cells with antigen-presenting cells, and B cell memory responses caused by prolonged administration of MMF[
7]. Despite this evidence, no data are still available regarding the benefit of MMF interruption in increasing humoral and T cell-mediated immune responses after the fourth dose of anti-SARS-CoV-2 vaccine in nonresponsive LT recipients.
The aim of the present study was to evaluate whether temporary interruption of MMF might improve humoral and T cell-mediated immune responses after the fourth dose of anti-SARS-CoV-2 BNT16b2 vaccine in previously nonresponsive LT recipients. Furthermore, the incidence of symptomatic coronavirus-19 disease (COVID-19) was evaluated up to 6 months after the fourth vaccination dose.
2. Materials and methods
2.1.Ethics statement
All patients provided written informed consent to receive the fourth vaccine dose and to participate in this study, which conformed to the ethical guidelines of the Declaration of Helsinki as revised in 2013. The evaluation of the anonymized patient data was approved by the Ethical Committee of the Academic Hospital of Udine, where the study was performed.
2.2.Study design and selection of patients
Patients enrolled were selected from a recently reported cohort of nonresponsive LT recipients who completed three doses of the anti-SARS-CoV-2 BNT162b2 vaccine[
3]. All patients were taking MMF, and the fourth SARS-CoV-2 BNT16b2 vaccine dose was offered at a mean (range) of 226 (174-273) days following the third dose. Anti-SARS-CoV-2-N protein IgM and IgG and anti-spike glycoprotein-specific immunoglobulin G receptor-binding domain (s-RBD) antibodies were measured as previously reported[
3], before and twice between 2 and 6 months after the fourth vaccine dose. Anti-SARS-CoV-2 spike-specific T cell response was assessed by interferon-g release assay (Euroimmun Diagnostica, Italy) both at baseline and two months after the fourth vaccine dose. The T cell response was considered positive if interferon-g was measured at ≥12 pg/ml above the negative control.
An expert team of transplant hepatologists selected patients in which to propose the MMF interruption two weeks before and after the fourth vaccine dose administration. Patients who experienced acute cellular rejection or poor adherence to immunosuppressive treatment were excluded. During the MMF interruption period, tacrolimus monotherapy was maintained with a mean (range) serum level of 4.82 (3.4-6.49) ng/ml. Liver function tests were evaluated twice a month, up to 3 months after the vaccination.
3.Results
Among the 107 LT recipients from the original cohort previously described[
3], 9 (8.4%) were identified as vaccine nonresponsive. Two patients refused the fourth vaccine dose, and two tested positive for a past SARS-CoV-2 infection. Thus, 5 patients were included. The main clinical and demographic patient characteristics are reported in the
Table 1. The mean (range) time from LT to the fourth vaccine dose was 94 (19-314) months.
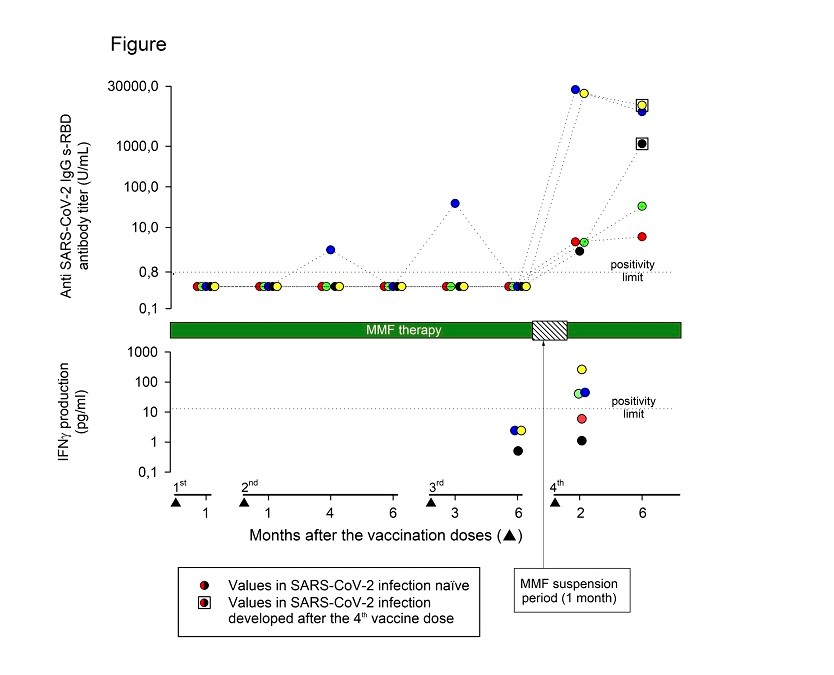
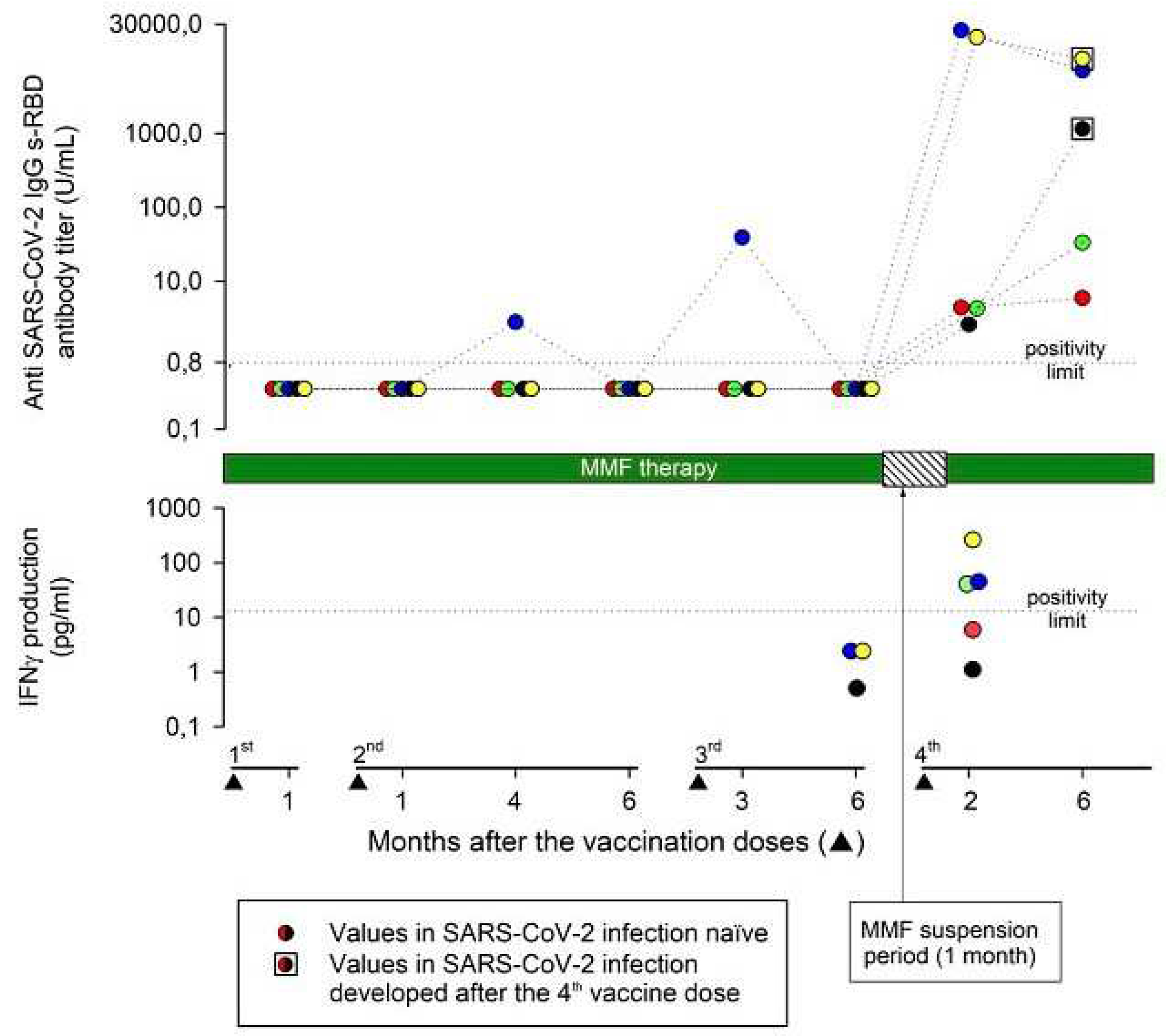
After a mean (range) time of 78 (62-113) days after the fourth vaccine dose, all patients tested positive for IgG anti-SARS-CoV-2-s-RBD antibodies. The mean (range) anti-SARS-CoV-2 s-RBD antibody titer was 8944 (2.6-24745) UI/ml. Two patients developed a strong and three a weak (mean antibody titer of 22355 and 3.8 U/ml, respectively) antibody response. No demographic or clinical differences between strong and weak responders were evident. A further anti-SARS-CoV-2 s-RBD antibody titer was measured at a mean (range) time of 193 (140-245) days after the fourth vaccine dose. All patients maintained a positive antibody response to vaccination, but the mean (range) antibody titer decreased to 3690 (5.9-10201) U/ml (
Figure 1).
Measurements of the vaccine-induced SARS-CoV-2 spike-specific T cell response were available at the time of the fourth vaccine dose in 3/5 patients and were negative in all patients. Two months after the fourth vaccine dose, 3/5 patients (one negative before the fourth vaccine dose) developed a positive T cell immune response, while two patients remained negative (
Figure 1). Interestingly, 2/3 patients who developed a positive T cell immune response presented the highest anti-SARS-CoV-2 s-RBD IgG antibody titers both at 2 months and up to 6 months after the fourth vaccine dose.
Two male patients developed symptomatic COVID-19 three and four months after the fourth vaccine dose respectively. The anti-SARS-CoV-2 s-RBD antibody titer, measured at the time of COVID-19 resolution and compared to that recorded two months after the vaccine dose, increased from 2.6 U/ml to 1142 U/ml in one patient and decreased from 19965 U/ml to 10201 U/ml in the other (
Figure 1). In one patient, the T cell immune response was negative both before and after the fourth vaccine dose, and he presented a low (2.6 U/ml) anti-SARS-CoV-2 s-RBD antibody titer 2 months after the fourth vaccine dose. In the other patient, the T cell immune response was negative only before the fourth vaccine dose, and he presented a very high (19965 U/ml) antibody titer 2 months after the fourth vaccine dose.
The clinical course of COVID-19 in these two patients was mild, and neither of them required hospital admission. No severe side effects of vaccination were recorded, and liver function tests remained unchanged during the entire follow-up.
4.Discussion
To our knowledge, this is the first study that evaluated the impact of temporary MMF interruption on both humoral and T cell immune responses following the fourth BNT162b2 vaccine dose in previously nonresponsive LT patients. The decision to temporarily suspend MMF could be controversial, as it could increase the risk of rejection. However, the duration of MMF suspension was very short and was adopted more than 1.5 years after LT. After this period the clinical guidelines suggest that patients can be maintained on tacrolimus monotherapy with target serum levels near 5 mg/ml[
8], as reported in our study.
All enrolled patients developed a positive antibody response, and 3/5 patients developed a positive T cell immune response after the fourth vaccine dose. The positive impact of MMF interruption in increasing the vaccine-induced immunogenicity provided by our study was not recorded in kidney transplanted patients[
9]. These different results could depend on the shorter MMF interruption period adopted in kidney transplant recipients (2 weeks) compared to our patients. Our data seem to be in agreement to those recently reported in a group of 10 LT patients nonresponsive to the third anti-SARS-CoV-2 vaccine dose, in whom MMF administration was interrupted before the fourth vaccine dose[
10]. All patients presented a positive anti spike IgG antibodies following 2 to 4 weeks after the fourth vaccine dose. However, it is important to note that 4 patients were already responsive, albeit with a low antibody titer, to the third vaccination, thus the final percentage of vaccine responsive patients could be overestimated. Moreover, the antibody titer was measured only 4 weeks after the fourth vaccine dose and T-cell response was not evaluated in this study.
Two patients in our study developed symptomatic COVID-19 despite a positive antibody response to the fourth vaccine dose, and one patient developed symptomatic COVID-19 despite a positive T cell immune response. A recent report indicated that 10% of SOT recipients vaccinated with four doses developed COVID-19 during the Omicron wave[
11,
12]. Interestingly, it seems that the fourth vaccine dose failed to induce significant neutralization of the Omicron variant in SOT, indicating 10- to 20-fold higher titers are required to neutralize this heavily mutated variant in vitro[
12]. These findings suggest that additional dosing of the original vaccines in SOT recipients may not generate robust protection against infection in the form of neutralizing antibodies against the Omicron variant or future variants evolved from Omicron.
Our study has some limitations. The number of patients enrolled was small, but no other studies with the same study design have been reported so far. Furthermore, neutralizing antibody measurements and characterization of SARS-CoV-2 variants in the two breakthrough infections were not evaluated. The lack of a control group of patients not responding to the third vaccination who maintained MMF therapy during the fourth dose vaccination is another limitation of the study. However, like what has been reported in the literature[
1,
2,
4,
6,
12,
13,
14,
15], the immunogenicity of vaccination with the fourth dose would likely have been lower than that measured in the patients enrolled in our study.
In summary, suspending MMF prior to administration of the fourth dose of anti-SARS-CoV-2 mRNA vaccine in LT recipients who failed to respond to the third dose seems feasible and safe. If confirmed in larger studies, this procedure could restore the vaccine-induced humoral and T cell-mediated immune responses in a large portion of previously non responder LT recipients. Although the achievement of restored immunogenicity to vaccination was not associated with a full protection of SARS-CoV-2 infection, COVID-19 occurrence in these patients was always clinically uneventful.
Funding
This research received no external funding.
Institutional Review Board Statement
All patients provided written informed consent to receive the fourth vaccine dose and to participate in this study, which conformed to the ethical guidelines of the Declaration of Helsinki as revised in 2013. The evaluation of the anonymized patient data was approved by the Ethical Committee of the Academic Hospital of Udine, where the study was performed.
Conflict of Interests
The authors declare no conflict of interest.
Study concept and design
(PT, EFa), acquisition of data (EFo, DB, EFu, SC, AC, MF, FC), analysis and interpretation of data (PT, EFa), drafting of the manuscript (PT), critical revision of the manuscript for important intellectual content (EFa). All authors have made a significant contribution to this study and have approved the final manuscript.
Data sharing statement
The clinical and laboratory data used to support the findings of this study are included within the article.
References
- Yoo, J.J.; Yon, D.K.; Lee, S.W.; Shin, J.I.; Kim, B.K. Humoral Immunogenicity to SARS-CoV-2 Vaccination in Liver Transplant Recipients: A Systematic Review and Meta-Analysis. Int J Biol Sci 2022, 18, 5849–5857. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Mok, C.H.; Mao, X.; Zhang, R.; Hung, I.F.; Seto, W.K.; Yuen, M.F. COVID-19 vaccine immunogenicity among chronic liver disease patients and liver transplant recipients: A meta-analysis. Clin Mol Hepatol 2022, 28, 890–911. [Google Scholar] [CrossRef] [PubMed]
- Toniutto, P.; Cussigh, A.; Cmet, S.; Bitetto, D.; Fornasiere, E.; Fumolo, E.; Fabris, M.; D'Aurizio, F.; Fabris, C.; Grillone, L.; et al. Immunogenicity and safety of a third dose of anti-SARS-CoV-2 BNT16b2 vaccine in liver transplant recipients. Liver Int 2022. [Google Scholar] [CrossRef] [PubMed]
- Perrier, Q.; Lupo, J.; Gerster, T.; Augier, C.; Falque, L.; Rostaing, L.; Pelletier, L.; Bedouch, P.; Blanc, M.; Saint-Raymond, C.; et al. SARS-CoV-2 anti-spike antibodies after a fourth dose of COVID-19 vaccine in adult solid-organ transplant recipients. Vaccine 2022, 40, 6404–6411. [Google Scholar] [CrossRef] [PubMed]
- Meunier, L.; Sanavio, M.; Dumortier, J.; Meszaros, M.; Faure, S.; Ursic Bedoya, J.; Echenne, M.; Boillot, O.; Debourdeau, A.; Pageaux, G.P. Mycophenolate mofetil decreases humoral responses to three doses of SARS-CoV-2 vaccine in liver transplant recipients. Liver Int 2022, 42, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Harberts, A.; Schaub, G.M.; Ruether, D.F.; Duengelhoef, P.M.; Brehm, T.T.; Karsten, H.; Fathi, A.; Jahnke-Triankowski, J.; Fischer, L.; Addo, M.M.; et al. Humoral and Cellular Immune Response After Third and Fourth SARS-CoV-2 mRNA Vaccination in Liver Transplant Recipients. Clin Gastroenterol Hepatol 2022, 20, 2558–2566.e2555. [Google Scholar] [CrossRef] [PubMed]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. JAMA 2021, 325, 1784–1786. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.; Levitsky, J.; Aqel, B.; O'Grady, J.; Hemibach, J.; Rinella, M.; Fung, J.; Ghabril, M.; Thomason, R.; Burra, P.; et al. International Liver Transplantation Society Consensus Statement on Immunosuppression in Liver Transplant Recipients. Transplantation 2018, 102, 727–743. [Google Scholar] [CrossRef] [PubMed]
- Regele, F.; Heinzel, A.; Hu, K.; Raab, L.; Eskandary, F.; Fae, I.; Zelzer, S.; Bohmig, G.A.; Bond, G.; Fischer, G.; et al. Stopping of Mycophenolic Acid in Kidney Transplant Recipients for 2 Weeks Peri-Vaccination Does Not Increase Response to SARS-CoV-2 Vaccination-A Non-randomized, Controlled Pilot Study. Front Med (Lausanne) 2022, 9, 914424. [Google Scholar] [CrossRef] [PubMed]
- Meunier, L.; Malezieux, E.; Ursic Bedoya, J.; Faure, S.; Echenne, M.; Debourdeau, A.; Meszaros, M.; Pageaux, G.P. Mycophenolate mofetil discontinuation increases severe acute respiratory syndrome coronavirus 2 vaccine response in nonresponder liver transplantation recipients: A proof of concept. Liver Transpl 2023, 29, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Alejo, J.L.; Chiang, T.P.Y.; Bowles Zeiser, L.; Kim, J.D.; Mitchell, J.; Avery, R.K.; Tobian, A.A.R.; Abedon, R.R.; Levan, M.L.; Warren, D.S.; et al. Incidence and Severity of COVID-19 Among Vaccinated Solid Organ Transplant Recipients During the Omicron Wave. Transplantation 2022, 106, e413–e415. [Google Scholar] [CrossRef] [PubMed]
- Karaba, A.H.; Johnston, T.S.; Aytenfisu, T.Y.; Akinde, O.; Eby, Y.; Ruff, J.E.; Abedon, A.T.; Alejo, J.L.; Blankson, J.N.; Cox, A.L.; et al. A Fourth Dose of COVID-19 Vaccine Does Not Induce Neutralization of the Omicron Variant Among Solid Organ Transplant Recipients With Suboptimal Vaccine Response. Transplantation 2022, 106, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Alejo, J.L.; Mitchell, J.; Chiang, T.P.; Abedon, A.T.; Boyarsky, B.J.; Avery, R.K.; Tobian, A.A.R.; Levan, M.L.; Massie, A.B.; Garonzik-Wang, J.M.; et al. Antibody Response to a Fourth Dose of a SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Transplantation 2021, 105, e280–e281. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Abravanel, F.; Marion, O.; Romieu-Mourez, R.; Couat, C.; Del Bello, A.; Izopet, J. Assessment of 4 Doses of SARS-CoV-2 Messenger RNA-Based Vaccine in Recipients of a Solid Organ Transplant. JAMA Netw Open 2021, 4, e2136030. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.; Alejo, J.L.; Chiang, T.P.Y.; Kim, J.; Chang, A.; Abedon, A.T.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Levan, M.L.; et al. Antibody Response to a Fourth Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: An Update. Transplantation 2022, 106, e338–e340. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).