Submitted:
22 May 2023
Posted:
22 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Isolation and Characterization of Eurycomalactone and Eurycomanone
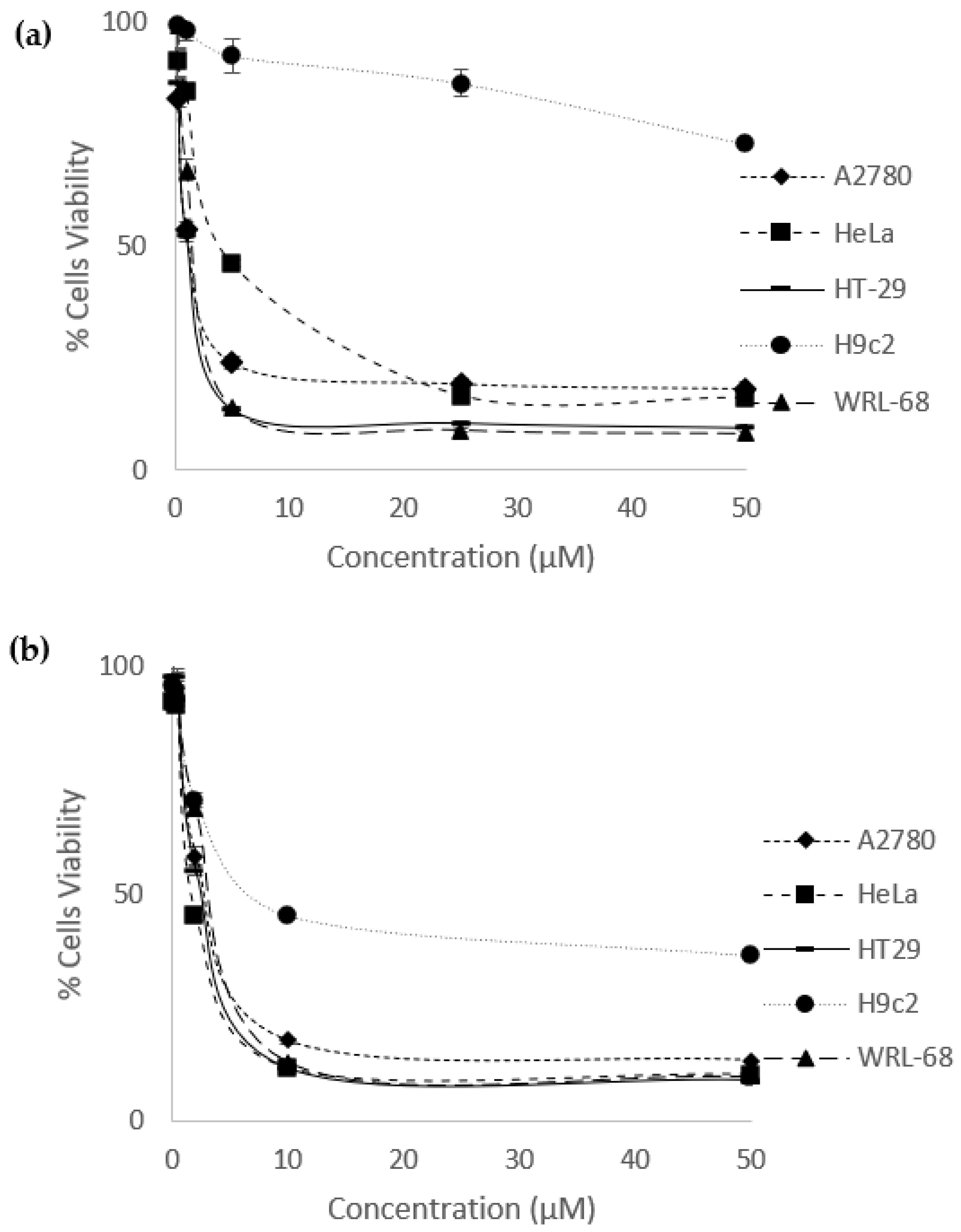
2.2. Percentage of Cells Viability and IC50 values
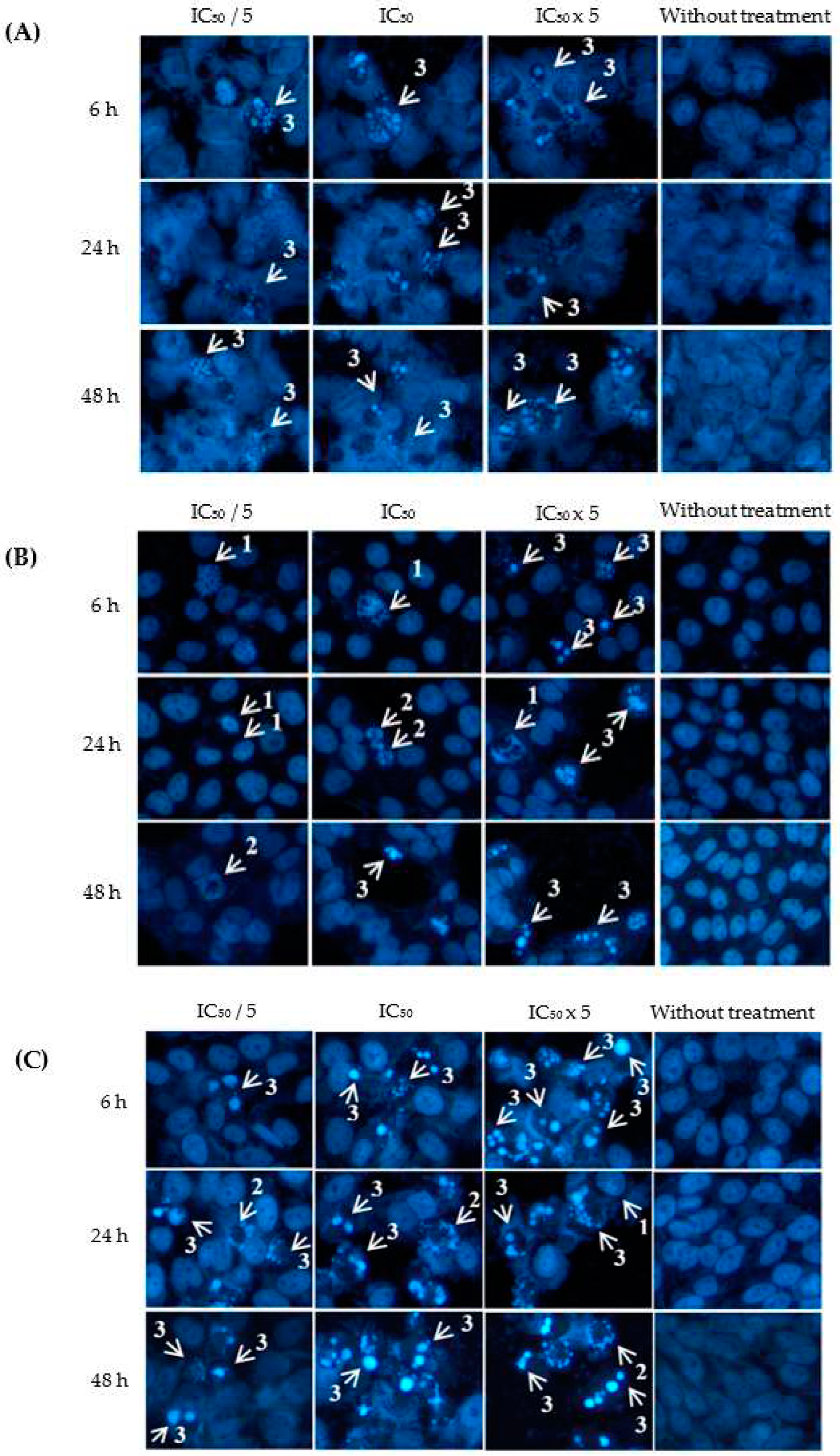
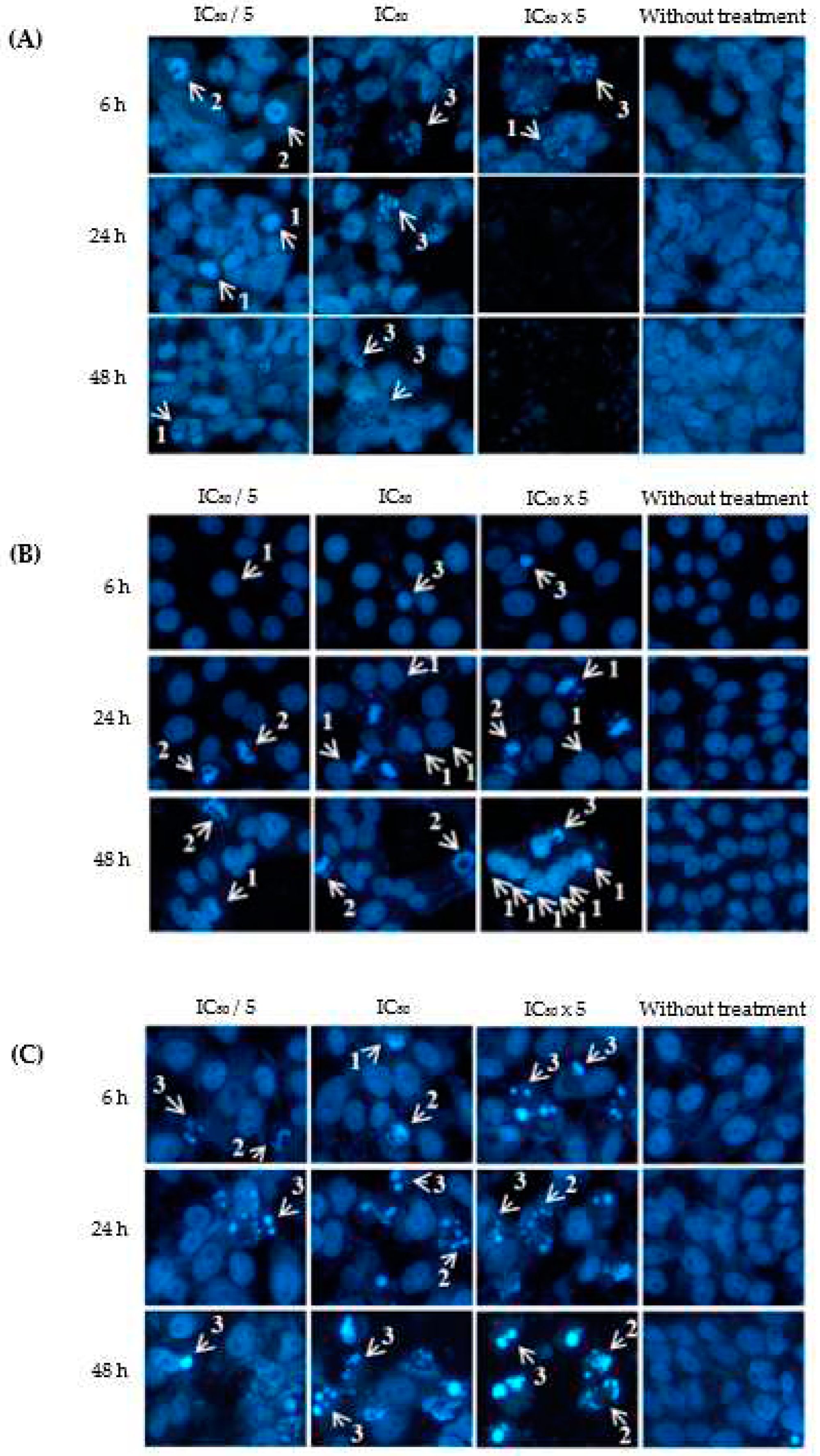
2.3. Apoptotic Effects of Eurycomanone and Eurycomalactone via Hoechst 33342 Assay
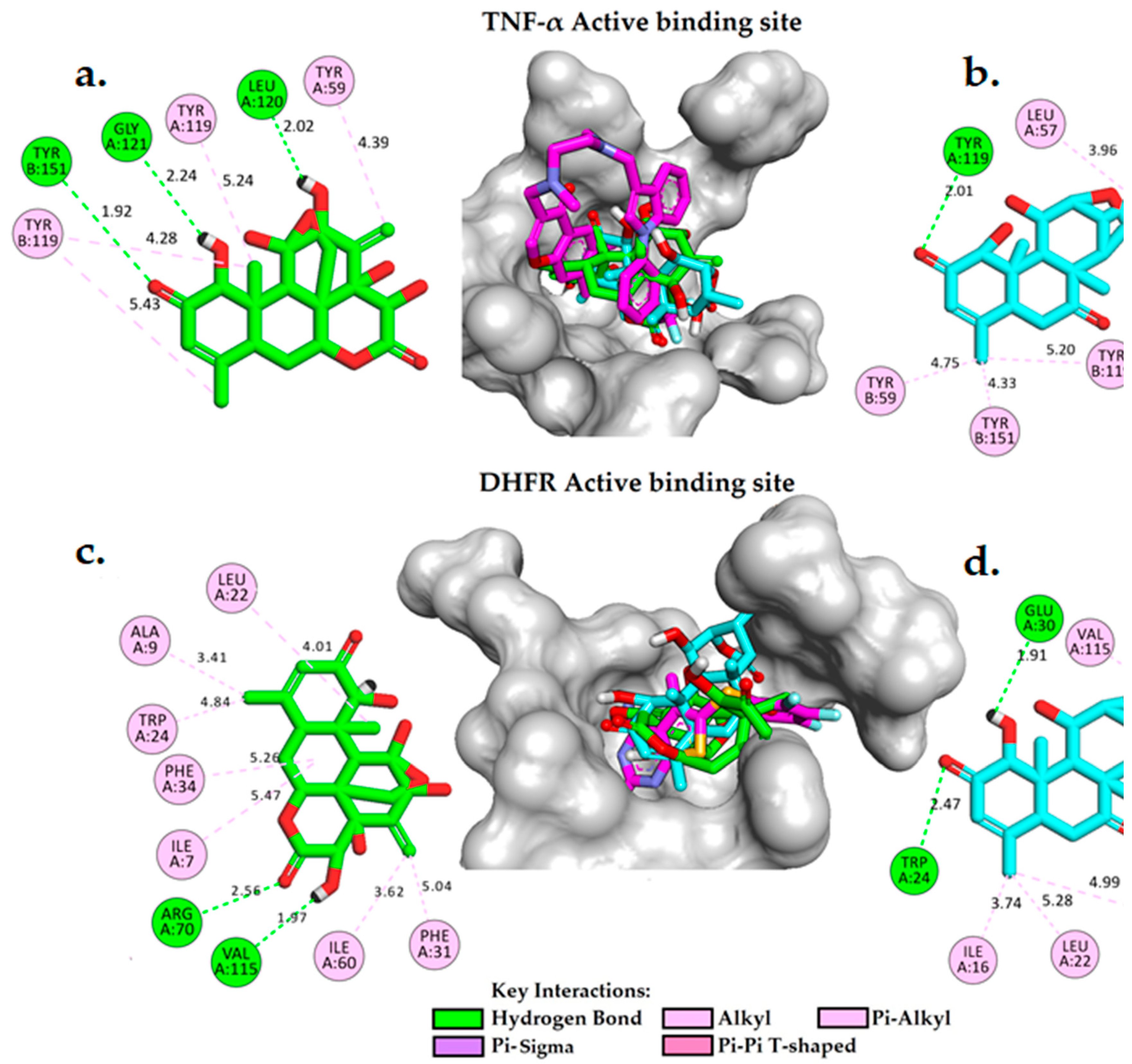
2.4. Molecular Docking Analysis
- ➢
- In the active binding site of DHFR (PDB ID: 5HQY), both eurycomanone and eurycomalactone exhibit free binding energies similar to the co-crystallized ligand. The calculated binding free energies for eurycomanone and eurycomalactone, which are -8.83 kcal/mol and -7.51 kcal/mol, respectively, suggest a promising potential for these compounds to bind to DHFR within its active binding site (Table 5).
- ➢
- Figure 5(c,d) illustrate the 2D and 3D molecular interactions of eurycomanone and eurycomalactone with DHFR. Eurycomanone is observed to establish two intermolecular hydrogen bonds with crucial residues in the active binding site of the enzyme (Figure 5c). The first hydrogen bond is formed between the carbonyl group of the 3-hydroxytetrahydro-2H-pyran-2-one ring and the guanidine group of ARG70 at a distance of 2.56 Å, while the second hydrogen bond is formed between the hydroxyl group of the 3-hydroxytetrahydro-2H-pyran-2-one at a distance of 1.67 Å. In the case of eurycomalactone, the 6-hydroxy-4-methylcyclohex-2-en-1-one also forms two intermolecular hydrogen bonds with TRP24 at a distance of 2.47 Å and with GLU30 at a distance of 1.91 Å (Figure 5d). Along with these hydrogen bonds, both eurycomanone and eurycomalactone are stabilized in the active site of DHFR through hydrophobic interactions with several residues, including ILE7, ALA9, LEU22, TRP24, PHE31, PHE34, and ILE60 for eurycomanone, and VAL8, ILE16, LEU22, PHE34, and VAL115 for eurycomalactone. These interactions bear substantial resemblance to those observed with the original co-crystallized ligand, indicating that eurycomanone and eurycomalactone may inhibit DHFR through similar mechanisms or contribute to the inhibitory activity of the compounds.
2.5. Lipinski’s Rule and ADMET of Eurycomanone and Eurycomalactone
3. Materials and Methods
3.1. Isolation and Characterisation of Eurycomanone and Eurycomalactone
3.2. Cell Culture and Treatments
3.3. Cells Viability Assay
3.4. Apoptotic Hoechst 33342 Assay
3.5. Molecular Docking Simulation and ADMET Predictions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO) WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All. 2020.
- Ashdown, M.L.; Robinson, A.P.; Yatomi-Clarke, S.L.; Ashdown, M.L.; Allison, A.; Abbott, D.; Markovic, S.N.; Coventry, B.J. Chemotherapy for Late-Stage Cancer Patients: Meta-Analysis of Complete Response Rates. F1000 Research 2015, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dinić, J.; Efferth, T.; García-Sosa, A.T.; Grahovac, J.; Padrón, J.M.; Pajeva, I.; Rizzolio, F.; Saponara, S.; Spengler, G.; Tsakovska, I. Repurposing Old Drugs to Fight Multidrug Resistant Cancers. Drug Resist. Updates 2020, 52, 100713. [Google Scholar] [CrossRef] [PubMed]
- El-Awady, E.S.E.; Moustafa, Y.M.; Abo-Elmatty, D.M.; Radwan, A. Cisplatin-Induced Cardiotoxicity: Mechanisms and Cardioprotective Strategies. Eur. J. Pharmacol. 2011, 650, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Heppner, G.H. Tumor Heterogeneity. Cancer Res. 1984, 44, 2259–2265. [Google Scholar] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Kulip, J.; Wong, K.M. Simaroubaceae. In Tree Flora of Sabah and Sarawak Volume One; Soepadmo, E., Wong, K.M., Eds.; Ampang Press Sdn. Bhd.: Kuala Lumpur, 1995. [Google Scholar]
- Burkill, L.H. A Dictionary of the Economic Products of the Malay Peninsula; Ministry of Agriculture and Cooperatives, Kuala Lumpur., 1966; Vol. I & II.
- Rehman, S.U.; Choe, K.; Yoo, H.H. Review on a Traditional Herbal Medicine, Eurycoma Longifolia Jack (Tongkat Ali): Its Traditional Uses, Chemistry, Evidence-Based Pharmacology and Toxicology. Molecules 2016, 21, 331. [Google Scholar] [CrossRef]
- Girish, S.; Kumar, S.; Aminudin, N.; Hashim, N.M. Comparison of Apoptotic Responses in Blastocystis Sp. upon Treatment with Tongkat Ali and Metronidazole. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Low, B.S.; Teh, C.H.; Yuen, K.H.; Chan, K.L. Physicochemical Effects of the Major Quassinoids in a Standardized Eurycoma longifolia Extract (Fr 2) on the Bioavailability and Pharmacokinetic Properties, and Their Implications for Oral Antimalarial Activity. Nat. Prod. Commun. 2011, 6, 337–341. [Google Scholar] [CrossRef]
- Tada, H.; Yasuda, F.; Otani, K.; Doteuchi, M.; Ishihara, Y.; Shiro, M. New Antiulcer Quassinoids from Eurycoma longifolia. Eur. J. Med. Chem. 1991, 26, 345–349. [Google Scholar] [CrossRef]
- Chan, K.L.; O’Neill, M.J.; Phillipson, J.D.; Warhurst, D.C. Plants as Sources of Antimalarial Drugs. Part 3. Eurycoma longifolia. Planta Med. 1986, 2, 105–107. [Google Scholar] [CrossRef]
- Tran, T.V.A.; Malainer, C.; Schwaiger, S.; Atanasov, A.G.; Heiss, E.H.; Dirsch, V.M.; Stuppner, H. NF-ΚB Inhibitors from Eurycoma longifolia. J. Nat. Prod. 2014, 77, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Choonong, R.; Ruangdachsuwan, S.; Churod, T.; Palabodeewat, S.; Punyahathaikul, S.; Juntarapornchai, S.; Ketsuwan, K.; Komaikul, J.; Masrinoul, P.; Kitisripanya, T.; et al. Evaluating the in Vitro Efficacy of Quassinoids from Eurycoma longifolia and Eurycoma harmandiana against Common Cold Human Coronavirus OC43 and SARS-CoV-2 Using In-Cell Enzyme-Linked Immunosorbent Assay. J. Nat. Prod. 2022. [Google Scholar] [CrossRef]
- Haiat, S.; Billard, C.; Quiney, C.; Ajchenbaum-Cymbalista, F.; Kolb, J.P. Role of BAFF and APRIL in Human B-Cell Chronic Lymphocytic Leukaemia. Immunology 2006, 118, 281–292. [Google Scholar] [CrossRef]
- Lim, B.; Greer, Y.; Lipkowitz, S.; Takebe, N. Novel Apoptosis-Inducing Agents for the Treatment of Cancer, a New Arsenal in the Toolbox. Cancers 2019, 11, 1087. [Google Scholar] [CrossRef] [PubMed]
- Niknejad, H.; Khayat-Khoei, M.; Peirovi, H.; Abolghasemi, H. Human Amniotic Epithelial Cells Induce Apoptosis of Cancer Cells: A New Anti-Tumor Therapeutic Strategy. Cytotherapy 2014, 16, 33–40. [Google Scholar] [CrossRef] [PubMed]
- van Horssen, R.; ten Hagen, T.L.M.; Eggermont, A.M.M. TNF-α in Cancer Treatment: Molecular Insights, Antitumor Effects, and Clinical Utility. Oncologist 2006, 11, 397–408. [Google Scholar] [CrossRef]
- Balkwill, F. Tumour Necrosis Factor and Cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Zidi, I.; Mestiri, S.; Bartegi, A.; Amor, N. Ben TNF-α and Its Inhibitors in Cancer. Med. Oncol. 2009, 27, 185–198. [Google Scholar] [CrossRef]
- Zia, K.; Ashraf, S.; Jabeen, A.; Saeed, M.; Nur-e-Alam, M.; Ahmed, S.; Al-Rehaily, A.J.; Ul-Haq, Z. Identification of Potential TNF-α Inhibitors: From in Silico to in Vitro Studies. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Marriott, J.B.; Westby, M.; Dalgleish, A.G. Therapeutic Potential of TNF-α Inhibitors Old and New. Drug Discov. Today 1997, 2, 273–282. [Google Scholar] [CrossRef]
- Neradil, J.; Pavlasova, G.; Veselska, R. New Mechanisms for an Old Drug; DHFR-and Non-DHFR-Mediated Effects of Methotrexate in Cancer Cells. Klin. Oncol. 2012, 25, S87–S92. [Google Scholar]
- Srivastava, V.; Kumar, A.; Mishra, B.N.; Siddiqi, M.I. Molecular Docking Studies on DMDP Derivatives as Human DHFR Inhibitors. Bioinformation 2008, 3, 188. [Google Scholar] [CrossRef]
- Raimondi, M.V.; Randazzo, O.; Franca, M. La; Barone, G.; Vignoni, E.; Rossi, D.; Collina, S. DHFR Inhibitors: Reading the Past for Discovering Novel Anticancer Agents. Molecules 2019, 24, 1140. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Drozdowska, D. Recent Design and Structure-Activity Relationship Studies on the Modifications of DHFR Inhibitors as Anticancer Agents. Curr. Med. Chem. 2019, 28, 910–939. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, J.; Liao, M.; Yang, Y.; Wang, Y.; Yuan, Y.; Ouyang, L. Folate-Mediated One-Carbon Metabolism: A Targeting Strategy in Cancer Therapy. Drug Discov. Today 2021, 26, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.C.; Maddocks, O.D.K. One-Carbon Metabolism in Cancer. Br. J. Cancer 2017, 116, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Shuvalov, O.; Petukhov, A.; Daks, A.; Fedorova, O.; Vasileva, E.; Barlev, N.A. One-Carbon Metabolism and Nucleotide Biosynthesis as Attractive Targets for Anticancer Therapy. Oncotarget 2017, 8, 23977. [Google Scholar] [CrossRef]
- Nurhanan, M.Y.; Nor Datiakma, M.A.; Muhammad Haffiz, J.; Sui Kiong, L.; Nor Hasnida, H.; Nor Jannah, S. The In Vitro Anti-Cancer Activities and Mechanisms of Action of 9-Methoxycanthin-6-One from Eurycoma longifolia in Selected Cancer Cell Lines. Molecules 2022, 27, 585. [Google Scholar]
- Shailima Vardhini, R.D. Insilico Analysis of Protein-Ligand Docking of DHFR (Dihydro Folate Reductase) and Quassinoids. Int. J. Comput. Appl. 2013, 62, 14–19. [Google Scholar]
- Hall, I.H.; Liou, Y.F.; Okano, M.; Lee, K.H. Antitumor Agents XLVI: In Vitro Effects of Esters of Brusatol, Bisbrusatol, and Related Compounds on Nucleic Acid and Protein Synthesis of P-388 Lymphocytic Leukemia Cells. J. Pharm. Sci. 1982, 71, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, T.; Dou, Y.; Huang, Y.; Qu, C.; Gao, J.; Huang, Z.; Xie, Y.; Huang, P.; Lin, Z.; et al. Brusatol Ameliorates 2, 4, 6-Trinitrobenzenesulfonic Acid-Induced Experimental Colitis in Rats: Involvement of NF-ΚB Pathway and NLRP3 Inflammasome. Int. Immunopharmacol. 2018, 64, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Olsen, N.J.; Spurlock, C.F.; Aune, T.M. Methotrexate Induces Production of IL-1 and IL-6 in the Monocytic Cell Line U937. Arthritis Res. Ther. 2014, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Esim, O.; Oztuna, A.; Sarper, M.; Hascicek, C. Chitosan-Coated Bovine Serum Albumin Nanocarriers Mediate Efficient Delivery of Methotrexate in Breast Cancer Therapeutics. J. Drug Deliv. Sci. Technol. 2022, 77, 103906. [Google Scholar] [CrossRef]
- Balkwill, F. TNF-α in Promotion and Progression of Cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Lahrita, L.; Hirosawa, R.; Kato, E.; Kawabata, J. Isolation and Lipolytic Activity of Eurycomanone and Its Epoxy Derivative from Eurycoma Longifolia. Bioorg Med. Chem. 2017, 25, 4829–4834. [Google Scholar] [CrossRef] [PubMed]
- Miyake, K.; Tezuka, Y.; Awale, S.; Li, F.; Kadota, S. Quassinoids from Eurycoma Longifolia. J. Nat. Prod. 2009, 72, 2135–2140. [Google Scholar] [CrossRef]
- Thigpen, J.T.; Bertelsen, K.; Eisenhauer, E.A.; Hacker, N.E.; Lund, B.; Sessa, C. Long-Term Follow-up of Patients with Advanced Ovarian Carcinoma Treated with Chemotherapy. Ann. Oncol. 1993, 4, 35–40. [Google Scholar] [CrossRef]
- Go, R.S.; Adjei, A.A. Review of the Comparative Pharmacology and Clinical Activity of Cisplatin and Carboplatin. J. Clin. Oncol. 1999, 17, 409–422. [Google Scholar] [CrossRef]
- Köhne-Wömpner, C.H.; Schmoll, H.J.; Harstrick, A.; Rustum, Y.M. Chemotherapeutic Strategies in Metastatic Colorectal Cancer: An Overview of Current Clinical Trials. Semin. Oncol. 1992, 19, 105–125. [Google Scholar]
- Malaviya, A.N.; Sharma, A.; Agarwal, D.; Kapoor, S.; Garg, S.; Sawhney, S. Low-Dose and High-Dose Methotrexate Are Two Different Drugs in Practical Terms. Int. J. Rheum. Dis. 2010, 13, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Boik, J.C.; Newman, R.A. Structure-Activity Models of Oral Clearance, Cytotoxicity, and LD50: A Screen for Promising Anticancer Compounds. BMC Pharmacol. 2008, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kardono, L.B.S.; Angerhofer, C.K.; Tsauri, S.; Padmawinata, K.; Pezzuto, J.M.; Douglas Kinghorn, A. Cytotoxic and Antimalarial Constituents of the Roots of Eurycoma longifolia. J. Nat. Prod. 1991, 54, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.C.; Damu, A.G.; Lee, K.H.; Wu, T.S. Cytotoxic and Antimalarial Constituents from the Roots of Eurycoma Longifolia. Bioorg Med. Chem. 2004, 12, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, H.; Kishi, E.; Morita, H.; Takeya, K. Cytotoxic Quassinoids and Tirucallane-Type Triterpenes from the Woods of Eurycoma Longifolia. Chem Pharm Bull (Tokyo) 1992, 40, 1053–1055. [Google Scholar] [CrossRef]
- Miyake, K.; Li, F.; Tezuka, Y.; Awale, S.; Kadota, S. Cytotoxic Activity of Quassinoids from Eurycoma Longifolia. Nat. Prod. Commun. 2010, 5, 1009–1012. [Google Scholar] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W.; et al. Calculating Structures and Free Energies of Complex Molecules: Combining Molecular Mechanics and Continuum Models. Acc. Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef]
- Al-Thiabat, M.G.; Saqallah, F.G.; Gazzali, A.M.; Mohtar, N.; Yap, B.K.; Choong, Y.S.; Wahab, H.A. Heterocyclic Substitutions Greatly Improve Affinity and Stability of Folic Acid towards FRα. an In Silico Insight. Molecules 2021, 26, 1079. [Google Scholar] [CrossRef]
- Al-Thiabat, M.G.; Gazzali, A.M.; Mohtar, N.; Murugaiyah, V.; Kamarulzaman, E.E.; Yap, B.K.; Rahman, N.A.; Othman, R.; Wahab, H.A. Conjugated β-Cyclodextrin Enhances the Affinity of Folic Acid towards FRα: Molecular Dynamics Study. Molecules 2021, 26, 5304. [Google Scholar] [CrossRef]
- Larue, L.; Kenzhebayeva, B.; Al-Thiabat, M.G.; Jouan–Hureaux, V.; Mohd–Gazzali, A.; Wahab, H.A.; Boura, C.; Yeligbayeva, G.; Nakan, U.; Frochot, C.; et al. TLyp–1: A Peptide Suitable to Target NRP–1 Receptor. Bioorg Chem. 2023, 130, 106200. [Google Scholar] [CrossRef]
- Amir Rawa, M.S.; Al-Thiabat, M.G.; Nogawa, T.; Futamura, Y.; Okano, A.; Wahab, H.A. Naturally Occurring 8ß,13ß-Kaur-15-En-17-al and Anti-Malarial Activity from Podocarpus polystachyus Leaves. Pharmaceuticals 2022, 15, 902. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration Pediatric Oncology Drug Approvals | FDA Available online:. Available online: https://www.fda.gov/about-fda/oncology-center-excellence/pediatric-oncology-drug-approvals (accessed on 17 March 2023).
- U.S. National Library of Medicine Safety and Efficacy of Oral Methotrexate Tablets Combined with Immunotherapy During Radiotherapy for Unresectable/Metastatic Solid Tumors: A Single-Center, Prospective Study Available online:. Available online: https://clinicaltrials.gov/ct2/show/NCT05522582?term=methotrexate&cond=cancer&draw=4&rank=19 (accessed on 17 March 2023).
- U.S. Food & Drug Administration Drug Approval Package: Alimta (Pemetrexed) NDA #021677 Available online:. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/021677s000_alimtatoc.cfm (accessed on 16 March 2023).
- U.S. National Library of Medicine Early Phase I Window of Opportunity Biomarker and Safety Trial to Test Pyrimethamine as an Inhibitor of NRF2 in HPV-Negative, Locally Advanced Head and Neck Squamous Cell Carcinoma Available online:. Available online: https://clinicaltrials.gov/ct2/show/NCT05678348?term=DHFR+inhibitor+clinical+trials&draw=2&rank=1 (accessed on 17 March 2023).
- U.S. Available online: https://clinicaltrials.gov/ct2/show/NCT00003789 (accessed on 16 March 2023).
- Hayslip, J.; Dressler, E.V.; Weiss, H.; Taylor, T.J.; Chambers, M.; Noel, T.; Miriyala, S.; Keeney, J.T.R.; Ren, X.; Sultana, R.; et al. Plasma TNF-α and Soluble TNF Receptor Levels after Doxorubicin with or without Co-Administration of Mesna-A Randomized, Cross-Over Clinical Study. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine Oncolytic Adenovirus TILT-123 With Pembrolizumab as Treatment for Ovarian Cancer Available online:. Available online: https://clinicaltrials.gov/ct2/show/NCT05271318?term=TNF-%CE%B1+clinical+trials+on+cancer&draw=3&rank=11 (accessed on 16 March 2023).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- van de Waterbeemd, H.; Gifford, E. ADMET in Silico Modelling: Towards Prediction Paradise? Nat. Rev. Drug Discov. 2003, 2, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Li Di Edward Kerns, B.H.; York, N.; Diego, S. Drug-Like Properties Concepts, Structure, Design, and Methods from ADME to Toxicity Optimization; 2016; ISBN 9780128010761.
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An Integrated Online Platform for Accurate and Comprehensive Predictions of ADMET Properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.N.; Dong, J.; Deng, Y.H.; Zhu, M.F.; Wen, M.; Yao, Z.J.; Lu, A.P.; Wang, J.B.; Cao, D.S. ADME Properties Evaluation in Drug Discovery: Prediction of Caco-2 Cell Permeability Using a Combination of NSGA-II and Boosting. J. Chem. Inf. Model. 2016, 56, 763–773. [Google Scholar] [CrossRef]
- Low, B.S.; Ng, B.H.; Choy, W.P.; Yuen, K.H.; Chan, K.L. Bioavailability and Pharmacokinetic Studies of Eurycomanone from Eurycoma longifolia. Planta Med. 2005, 71, 803–807. [Google Scholar] [CrossRef]
- Furst, D.E.; Koehnke, R.; Burmeister, L.F.; Kohler, J.; Cargill, I. Increasing Methotrexate Effect with Increasing Dose in the Treatment of Resistant Rheumatoid Arthritis. J. Rheumatol. 1989, 16, 313–320. [Google Scholar]
- Colom, H.; Farré, R.; Soy, D.; Peraire, C.; Cendros, J.M.; Pardo, N.; Torrent, M.; Domenech, J.; Mangues, M.A. Population Pharmacokinetics of High-Dose Methotrexate after Intravenous Administration in Pediatric Patients with Osteosarcoma. Ther. Drug Monit. 2009, 31, 76–85. [Google Scholar] [CrossRef]
- Wang, N.N.; Huang, C.; Dong, J.; Yao, Z.J.; Zhu, M.F.; Deng, Z.K.; Lv, B.; Lu, A.P.; Chen, A.F.; Cao, D.S. Predicting Human Intestinal Absorption with Modified Random Forest Approach: A Comprehensive Evaluation of Molecular Representation, Unbalanced Data, and Applicability Domain Issues. RSC Adv. 2017, 7, 19007–19018. [Google Scholar] [CrossRef]
- Clark, D.E. In Silico Prediction of Blood–Brain Barrier Permeation. Drug Discov. Today 2003, 8, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Aggarwal, V.; Sharma, M.; Dhingra, N. Lantadenes Targeting NF-ΚB in Cancer: Molecular Docking and ADMET Predictions Article In. Int. J. Pharma Bio Sci. 2021. [Google Scholar]
- Kazmi, S.R.; Jun, R.; Yu, M.S.; Jung, C.; Na, D. In Silico Approaches and Tools for the Prediction of Drug Metabolism and Fate: A Review. Comput. Biol. Med. 2019, 106, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Ingelman-Sundberg, M. Human Drug Metabolising Cytochrome P450 Enzymes: Properties and Polymorphisms. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 369, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tiong, K.H.; Abd-Rashid, B.A.; Ismail, Z.; Ismail, R.; Mak, J.W.; Ong, C.E. Effect of Eurycomanone on Cytochrome P450 Isoforms CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2E1 and CYP3A4 in Vitro. J. Nat. Med. 2014, 68, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Beaumont, K.; Maurer, T.S.; Di, L. Relevance of Half-Life in Drug Design. J. Med. Chem. 2018, 61, 4273–4282. [Google Scholar] [CrossRef]
- Yang, H.; Sun, L.; Li, W.; Liu, G.; Tang, Y. In Silico Prediction of Chemical Toxicity for Drug Design Using Machine Learning Methods and Structural Alerts. Front. Chem. 2018, 6, 30. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; Mcmahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. JNCI J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Nurhanan, M.; Nor Azah, M.; Zunoliza, A.; Siti Humeriah, A.; Siti Syarifah, M.; Nor Hayati, A. In Vitro Anticancer Activity and High-Performance Liquid Chromatography Profiles of Aquilaria Subintegra Fruit and Seed Extracts. J. Trop. For. Sci. 2017, 29, 208–214. [Google Scholar]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the Worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 2003, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Cody, V.; Gangjee, A. RCSB PDB - 5HPB: Human Dihydrofolate Reductase Complex with NADPH and 5-Methyl-6-(Phenylthio-4’trifluoromethyl)Thieno[2,3-d]Pyrimidine-2,4-Diamine Available online:. Available online: https://www.rcsb.org/structure/5hpb (accessed on 26 November 2022).
- He, M.M.; Smith, A.S.; Oslob, J.D.; Flanagan, W.M.; Braisted, A.C.; Whitty, A.; Cancilla, M.T.; Wang, J.; Lugovskoy, A.A.; Yoburn, J.C.; et al. Medicine: Small-Molecule Inhibition of TNF-α. Science (1979) 2005, 310, 1022–1025. [Google Scholar]
- Biovia, D.S. Discovery Studio Visualizer Available online:. Available online: https://discover.3ds.com/discovery-studio-visualizer-download (accessed on 26 November 2022).
- Eswar, N.; Eramian, D.; Webb, B.; Shen, M.Y.; Sali, A. Protein Structure Modeling with MODELLER. Methods Mol. Biol. 2008, 426, 145–159. [Google Scholar] [PubMed]
- Dolinsky, T.J.; Czodrowski, P.; Li, H.; Nielsen, J.E.; Jensen, J.H.; Klebe, G.; Baker, N.A. PDB2PQR: Expanding and Upgrading Automated Preparation of Biomolecular Structures for Molecular Simulations. Nucleic Acids Res. 2007, 35, W522–W525. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.H.M.; SØndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical p K a Predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and Better Reference Data for Improved All-Atom Structure Validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound Databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Rowinska-Zyrek, M.; Salerno, M.; Kozlowski, H. Neurodegenerative Diseases – Understanding Their Molecular Bases and Progress in the Development of Potential Treatments. Coord. Chem. Rev. 2015, 284, 298–312. [Google Scholar] [CrossRef]
- Rowland, M.; Tozer, T.N.; Derendorf, H.; Hochhaus, G. Chapter 1. Therapeutic Relevance. Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications; 4th ed.; Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia, 2011; ISBN 9780781750097. [Google Scholar]
- Shargel, L.; Andrew, B.; Wu-Pong, S. Applied Biopharmaceutics & Pharmacokinetics ; Appleton & Lange Stamford, 1999; Vol. 264.
- Forli, S.; Olson, A.J. A Force Field with Discrete Displaceable Waters and Desolvation Entropy for Hydrated Ligand Docking. J. Med. Chem. 2012, 55, 623–638. [Google Scholar] [CrossRef]
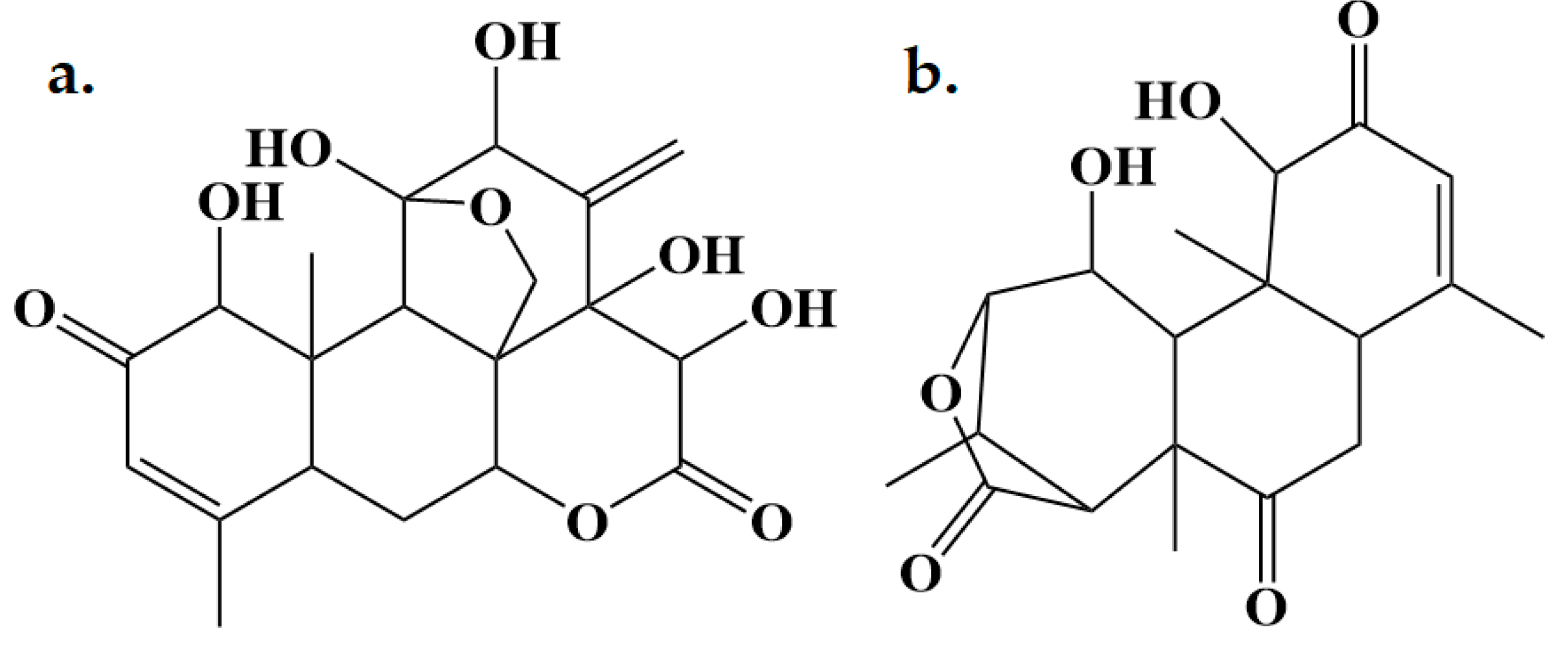
| Eurycomanone (1) | Eurycomalactone (2) | ||||
|---|---|---|---|---|---|
| No | 1H δ ppm | 13C δ ppm | No | 1H δ ppm | 13C δ ppm |
| 1 | 4.03 (1H, d, 8 Hz) | 81.40 | 1 | 4.05 (1H, s) | 81.27 |
| 2 | - | 197.89 | 2 | - | 197.41 |
| 3 | 6.14 (1H, d, 1.5 Hz) | 162.98 | 3 | 6.13 (1H, s) | 124.39 |
| 4 | - | 126.48 | 4 | - | 162.17 |
| 5 | 2.36 (1H, td, 2.4 Hz) | 48.14 | 5 | 2.91 (1H, m) | 49.38 |
| 6 | 2.08 (2H, m) | 42.58 | 6 | 2.79 (2H, m) | 36.21 |
| 7 | - | 199.32 | 7 | - | 205.56 |
| 8 | - | 53.02 | 8 | - | 51.16 |
| 9 | 2.02 (1H, t, 2.7, 13.3 Hz) | 50.42 | 9 | 1.88 (1H, d, 3.2 Hz) | 49.06 |
| 10 | - | 46.34 | 10 | - | 46.90 |
| 11 | 4.53 (1H, t, 6.8 Hz) | 68.10 | 11 | 4.78 (1H, t, 4 Hz) | 69.75 |
| 12 | 4.59 (1H, d, 8 Hz) | 72.21 | 12 | 4.39 (1H, d, 4.4 Hz) | 83.16 |
| 13 | - | 119.80 | 13 | 2.89 (1H, m) | 32.33 |
| 14 | 3.25 (1H,d, 12.5 Hz) | 76.28 | 14 | 3.02 (1H, m) | 52.88 |
| 15 16 |
5.25 (1H,t, 2.5, 18.7 Hz) - |
79.81 174.35 |
15 | - | 176.31 |
| 4-CH3 | 1.81 (3H, s) | 10.81 | 4-CH3 | 1.64 (3H, s) | 23.64 |
| 10-CH3 | 2.00 (3H, s) | 26.11 | 8-CH3 | 1.96 (3H, s) | 21.97 |
| 8-CH2 | 2.07 (2H, m) | 84.95 | 10-CH3 | 1.27 (3H, s) | 12.19 |
| 13’ | 7.6 (2H,s) | 108.71 | 13-CH3 | 1.18 (3H, d, 6.4 Hz) | 32.33 |
| Compound | A2780 | HeLa | HT-29 | H9C2 | WRL-68 |
|---|---|---|---|---|---|
| Eurycomanone | 1.37 ± 0.13 | 4.58 ± 0.090 | 1.22 ± 0.11 | >50 | 1.34 ± 0.046 |
| Eurycomalactone | 2.46 ± 0.081 | 1.60 ± 0.12 | 2.21 ± 0.049 | 7.00 ± 0.43 | 2.71 ± 0.042 |
| Cisplatin | 1.77 ± 0.018 | 1.54 ± 0.12 | 1.38 ± 0.037 | 14.07 ± 1.14 | 1.13 ± 0.098 |
| Methotrexate | 0.016 ± 0.00050 | 0.094 ± 0.0043 | 0.059 ± 0.0010 | >50 | 0.015 ± 0.00041 |
| Concentrations/ Incubation Time |
IC50/5 | IC50 | IC50 x 5 |
|---|---|---|---|
| Cell Line: A2780 | |||
| 6 h | 4.30 ± 0.34a/ x | 8.48 ± 0.60ab/ x | 13.01 ± 0.29ac,bc/ x |
| 24 h | 7.11 ± 1.60a/ xy | 13.13 ± 1.30ab/ y | 28.90 ± 0.93ac,bc/ xy |
| 48 h | 14.72 ± 0.59a/ xz,yz | 31.74 ± 3.19ab/ xz,yz | 100.00 ± 0.00ac,bc/ xz,yz |
| Cell Line: HT-29 | |||
| 6 h | 4.08 ± 0.81a/ x | 5.24 ± 0.17b/ x | 5.97 ± 0.35ac/ x |
| 24 h | 4.18 ± 0.21a/ y | 6.30 ± 1.01b/ y | 10.95 ± 0.71ac,bc/ y |
| 48 h | 4.24 ± 0.20a/ z | 14.80 ± 0.56ab/ xz,yz | 26.20 ± 1.48ac,bc/ xz,yz |
| Cell Line: HeLa | |||
| 6 h | 8.42 ± 0.20a/ x | 19.98 ± 0.76ab/ x | 41.37 ± 0.24ac,bc/ x |
| 24 h | 13.76 ± 1.26a/ xy | 52.57 ± 1.40ab/ y | 94.56 ± 1.16ac,bc/ xy |
| 48 h | 16.79 ± 0.92a/ xz,yz | 61.16 ± 0.63ab/ xz,yz | 100.00 ± 0.00ac,bc/ xz,yz |
| Concentrations/ Incubation Time (h) |
IC50/5 | IC50 | IC50 x 5 |
|---|---|---|---|
| Cell Line: A2780 | |||
| 6 h | 3.84 ± 0.10a/ x | 4.26 ± 0.64b/ x | 15.81 ± 0.38ac,bc/ x |
| 24 h | 4.12 ± 0.16a/ y | 6.29 ± 0.21ab/ y | Cells died and completely detachedac,bc/ xy |
| 48 h | 5.16 ± 0.065a/ xz,yz | 7.93 ± 2.28b/ xz | Cells died and completely detachedac,bc/ xz |
| Cell Line: HT-29 | |||
| 6 h | 3.79 ± 0.45a/ x | 4.51 ± 0.23b/ x | 6.52 ± 1.22ac,bc/ x |
| 24 h | 7.71 ± 0.51a/ xy | 12.41 ± 0.77ab/ xy | 20.21 ± 1.52ac,bc/ xy |
| 48 h | 8.86 ± 0.68a/ xz | 14.28 ± 0.84ab/ xz,yz | 100.00 ± 0.00ac,bc/ xz,yz |
| Cell Line: HeLa | |||
| 6 h | 5.45 ± 0.23a/ x | 17.67 ± 0.77ab/ x | 31.87 ± 2.19ac,bc/ x |
| 24 h | 8.94 ± 0.21a/ xy | 32.00 ± 1.57ab/ xy | 62.20 ± 1.35ac,bc/ xy |
| 48 h | 14.78 ± 0.12a/ xz,yz | 36.71 ± 1.19ab/ xz,yz | 100.00 ± 0.00ac,bc/ xz,yz |
| Compound | TNF-α | DHFR | ||
|---|---|---|---|---|
| *ΔGbind (kcal/mol) |
Ki (Micromolar uM) |
*ΔGbind (kcal/mol) |
Ki (Micromolar uM) |
|
| Eurycomanone | -8.83 | 0.34 | -8.05 | 1.25 |
| Eurycomalactone | -7.51 | 3.11 | -8.87 | 0.32 |
| *124037103 | ***** | ***** | -8.19 | 0.99 |
| *5327044 | -7.93 | 1.53 | ***** | ***** |
| Compounds | *M.W (g/mol) | *Hacc | *Hdon | *logP |
|---|---|---|---|---|
| Eurycomanone | 408.14 | 9 | 4 | 0.215 |
| Eurycomalactone | 348.16 | 6 | 1 | 0.655 |
| Methotrexate | 454.17 | 13 | 7 | -2.747 |
| Property | Model Name | Predicted Value | Comment | ||
|---|---|---|---|---|---|
| Eurycomanone | Eurycomalactone | Methotrexate | |||
| Absorption | Papp (Caco-2 Permeability) cm/s | -5.54 | -5.01 | -6.73 | * Papp ideal value is > −5.15 cm/s |
| HIA (Human Intestinal Absorption) % | 5.78 | 4.01 | 3.70 | * HIA idea value is < 30% | |
| Distribution | *PPB (Plasma Protein Binding) % | 52.15 | 52.66 | 55.23 | * PPB ideal value is < 90% |
| Cross BBB (Blood Brain Barrier) | No | Yes | No | ||
| Metabolism | CYP1A2 substrate | No | No | No | |
| CYP2C19 substrate | No | No | No | ||
| CYP2C9 substrate | No | No | No | ||
| CYP2D6 substrate | No | No | No | ||
| CYP1A2 inhibitor | No | No | No | ||
| CYP2C19 inhibitor | No | No | No | ||
| CYP2C9 inhibitor | No | No | No | ||
| CYP3A4 inhibitor | No | No | No | ||
| Excretion | *CL (Clearance Rate) mL/min/kg | 1.75 | 2.31 | 2.52 | ● High: CL >15 mL/min/kg ● Moderate: CL 5-15 mL/min/kg ● Low: CL <5 mL/min/kg |
| T ½ (Half Lifetime) hr | 0.03 | 0.18 | 0.89 | ● Long half-life: >3h ● Short half-life: <3h |
|
| Toxicity | H-HT (Human Hepatotoxicity) | + | + | +++ |
+ Low risk to be toxic. ++ Moderate risk to be toxic. +++ High risk to be toxic. |
| AMES (Ames Mutagenicity) | ++ | + | + | ||
| Carcinogenicity | + | + | ++ | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





