Figure 1.
We report the case of a 45-year-old male patient with a history of heavy smoking (10 years, 20 cigarettes/day) underwent an 18F-fluorodeoxyglucose (FDG) PET/CT dynamic (chest) + static (whole-body) scan for diagnosis and pre-treatment staging. The abnormal serum tumor markers associated with lung cancer before 18F-FDG PET/CT were CYFRA21-1 18.57 ng/ml (<3.3) and CA125 64.60 U/ml (<35.0). Before the 18F-FDG injection, the patient had fasted for at least 6 hours and had a pre-scan glucose level of 4.4mmol/L. According to the body mass index, the chest region PET scans (dynamic) was initiated immediately after the injection of 18F-FDG (7.07mCi)from an intravenous indwelling needle. The total dynamic scans lasted for 65 minutes. Dynamic scan data were partitioned into 28 frames as follows: 6 × 10 s, 4 × 30 s, 4 × 60 s, 4 × 120 s, and 10 × 300 s. An additional whole-body static PET/CT scan was performed at the end of the dynamic acquisition. Quantitative parameters (Ki) was obtained by applying the irreversible two-tissue compartment model using in-house Matlab software. 18F-FDG PET/CT showed an FDG-avid mass in the upper lobe of the left lung (Figure 1 B and C, white arrow), size of 3.4×3.0cm, SUVmax of 22.4 (Figure 1 B), Ki of 0.0525 ml/g/min (Figure 1 C), FDG-avid LN in the left pulmonary hilar region (Figure 1 D and E, white arrow), size of 1.1×0.9cm, SUVmax of 5.7 (Figure 1 D), Ki of 0.0231 ml/g/min (Figure 1 E), and bone destruction with the mass formation in the right 8th rib (Figure 1 F and G, white arrow), size of 8.0×4.5cm, SUVmax of 9.4 (Figure 1 F), Ki of 0.0214 ml/g/min (Figure 1 G). Multiple metastases throughout the body (Figure 1 A), including retroperitoneal LN (size of 1.3×1.0cm, SUVmax of 8.3), left adrenal gland (size of 3.8×3.5cm, SUVmax of 8.4), bone of the sacrum (size of 3.6×3.1cm, SUVmax of 10.9), left subcutaneous lumbar region (size of 1.7×1.3cm, SUVmax of 7.6), and brain (size of 1.9×1.8cm, SUVmax of 9.1).
Figure 1.
We report the case of a 45-year-old male patient with a history of heavy smoking (10 years, 20 cigarettes/day) underwent an 18F-fluorodeoxyglucose (FDG) PET/CT dynamic (chest) + static (whole-body) scan for diagnosis and pre-treatment staging. The abnormal serum tumor markers associated with lung cancer before 18F-FDG PET/CT were CYFRA21-1 18.57 ng/ml (<3.3) and CA125 64.60 U/ml (<35.0). Before the 18F-FDG injection, the patient had fasted for at least 6 hours and had a pre-scan glucose level of 4.4mmol/L. According to the body mass index, the chest region PET scans (dynamic) was initiated immediately after the injection of 18F-FDG (7.07mCi)from an intravenous indwelling needle. The total dynamic scans lasted for 65 minutes. Dynamic scan data were partitioned into 28 frames as follows: 6 × 10 s, 4 × 30 s, 4 × 60 s, 4 × 120 s, and 10 × 300 s. An additional whole-body static PET/CT scan was performed at the end of the dynamic acquisition. Quantitative parameters (Ki) was obtained by applying the irreversible two-tissue compartment model using in-house Matlab software. 18F-FDG PET/CT showed an FDG-avid mass in the upper lobe of the left lung (Figure 1 B and C, white arrow), size of 3.4×3.0cm, SUVmax of 22.4 (Figure 1 B), Ki of 0.0525 ml/g/min (Figure 1 C), FDG-avid LN in the left pulmonary hilar region (Figure 1 D and E, white arrow), size of 1.1×0.9cm, SUVmax of 5.7 (Figure 1 D), Ki of 0.0231 ml/g/min (Figure 1 E), and bone destruction with the mass formation in the right 8th rib (Figure 1 F and G, white arrow), size of 8.0×4.5cm, SUVmax of 9.4 (Figure 1 F), Ki of 0.0214 ml/g/min (Figure 1 G). Multiple metastases throughout the body (Figure 1 A), including retroperitoneal LN (size of 1.3×1.0cm, SUVmax of 8.3), left adrenal gland (size of 3.8×3.5cm, SUVmax of 8.4), bone of the sacrum (size of 3.6×3.1cm, SUVmax of 10.9), left subcutaneous lumbar region (size of 1.7×1.3cm, SUVmax of 7.6), and brain (size of 1.9×1.8cm, SUVmax of 9.1).
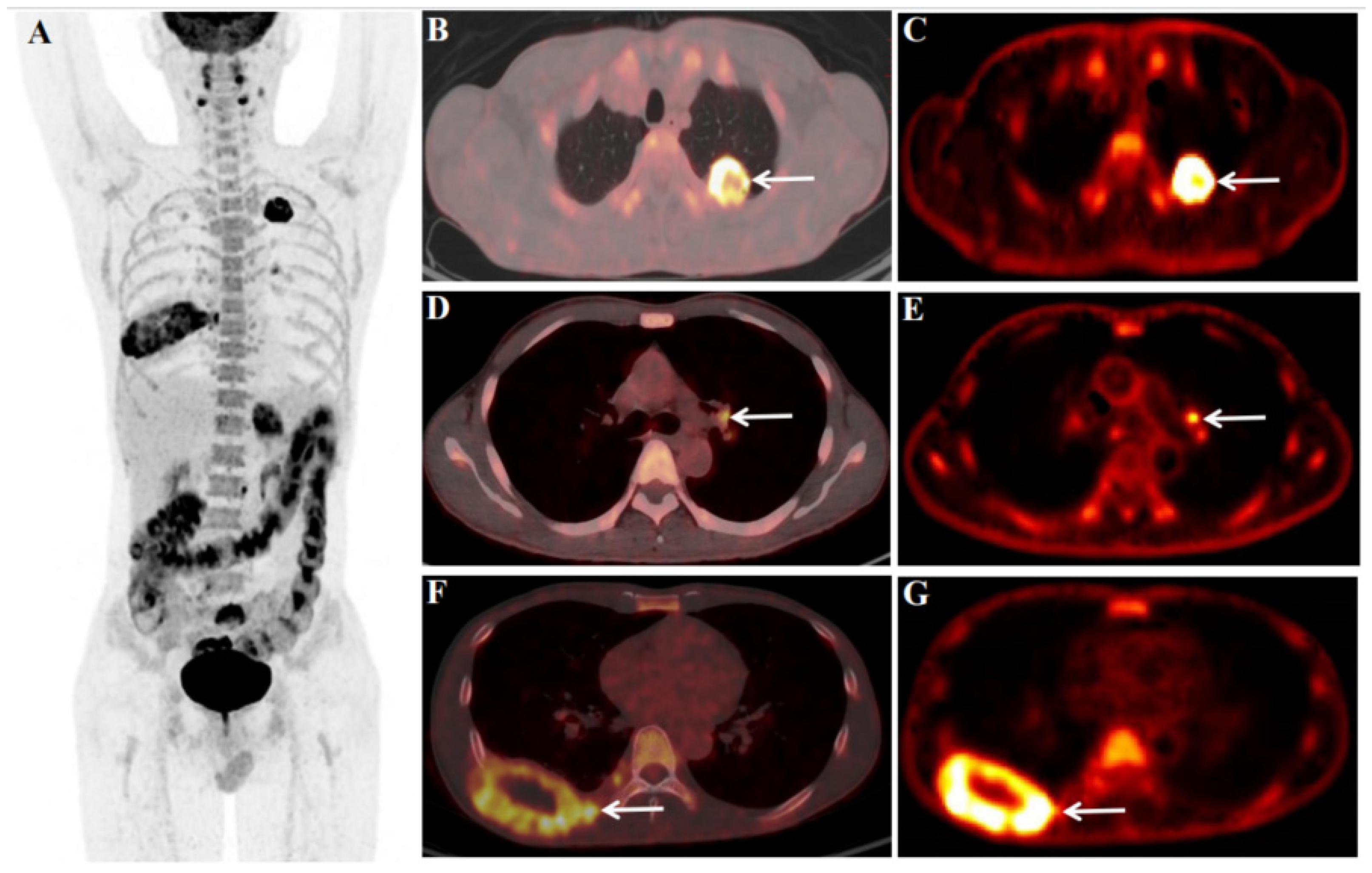
Figure 2.
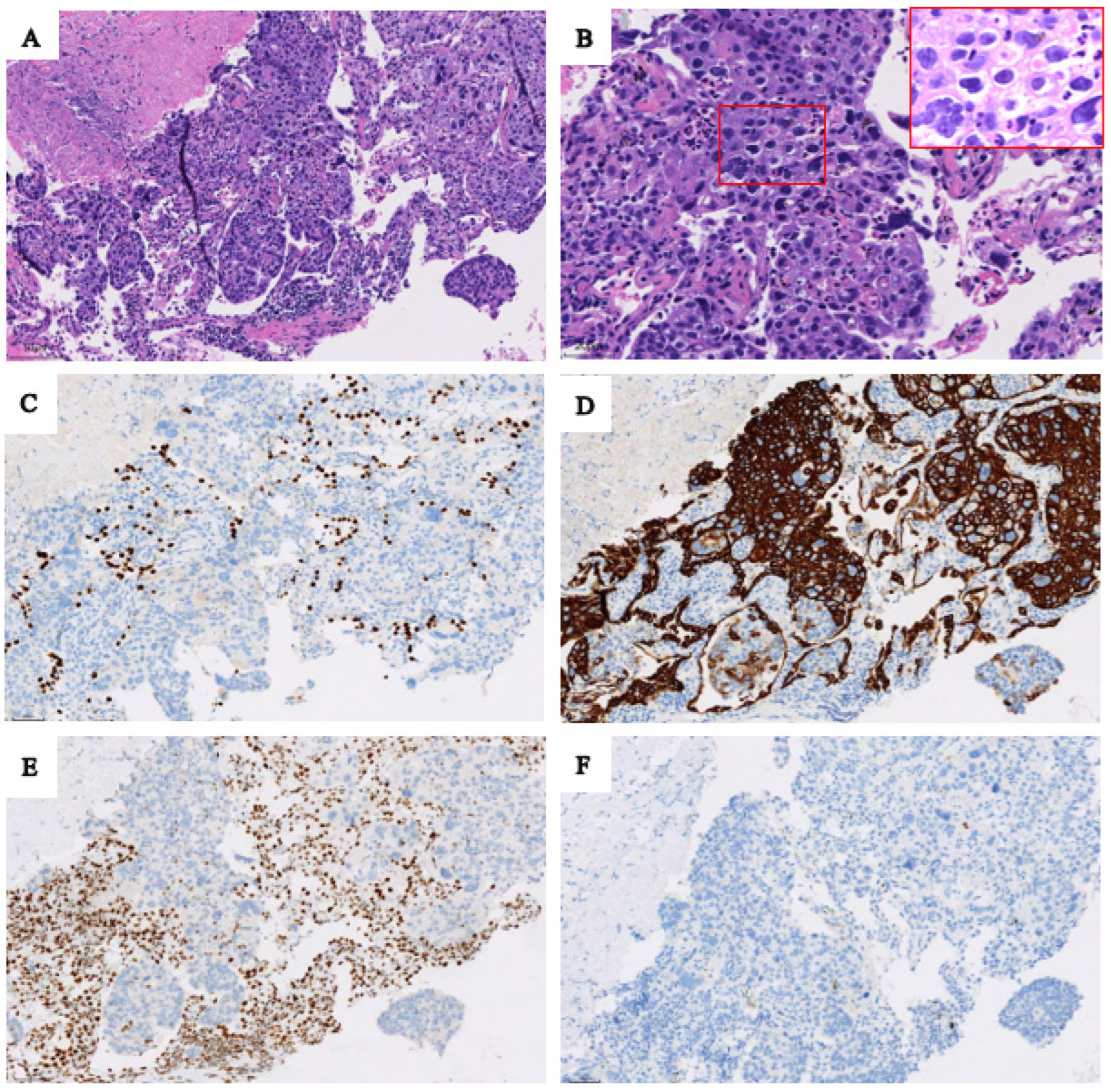
Based on the 18F-FDG PET/CT findings, the patient underwent a puncture biopsy of a mass in the upper lobe of the left lung. Pathological and immunohistochemical findings confirmed SMARCA4-deficient NSCLC. Hematoxylin-eosin staining (original magnifications×200 [Figure 2 A]) showed the tumor cells arranged in sheets and lobules, and (Figure 2 B, original magnifications×400) showed sheets of large pleomorphic tumor cells with prominent nucleoli. At immunohistochemistry (original magnification×200), tumor cells were not express TTF1 (Figure 2 C) with alveolar epithelial cells as internal positive controls; diffuse expression of cytokeratin 7 (Figure 2 D) was seen in tumor cells, and loss of BRG1 protein (Figure 2 E) in tumor cells with mesenchymal cells and inflammatory cells serving as internal positive controls, and P63 (Figure 2 F) was a negative expression in tumor cells.
Figure 2.
Based on the 18F-FDG PET/CT findings, the patient underwent a puncture biopsy of a mass in the upper lobe of the left lung. Pathological and immunohistochemical findings confirmed SMARCA4-deficient NSCLC. Hematoxylin-eosin staining (original magnifications×200 [Figure 2 A]) showed the tumor cells arranged in sheets and lobules, and (Figure 2 B, original magnifications×400) showed sheets of large pleomorphic tumor cells with prominent nucleoli. At immunohistochemistry (original magnification×200), tumor cells were not express TTF1 (Figure 2 C) with alveolar epithelial cells as internal positive controls; diffuse expression of cytokeratin 7 (Figure 2 D) was seen in tumor cells, and loss of BRG1 protein (Figure 2 E) in tumor cells with mesenchymal cells and inflammatory cells serving as internal positive controls, and P63 (Figure 2 F) was a negative expression in tumor cells.
Figure 3.
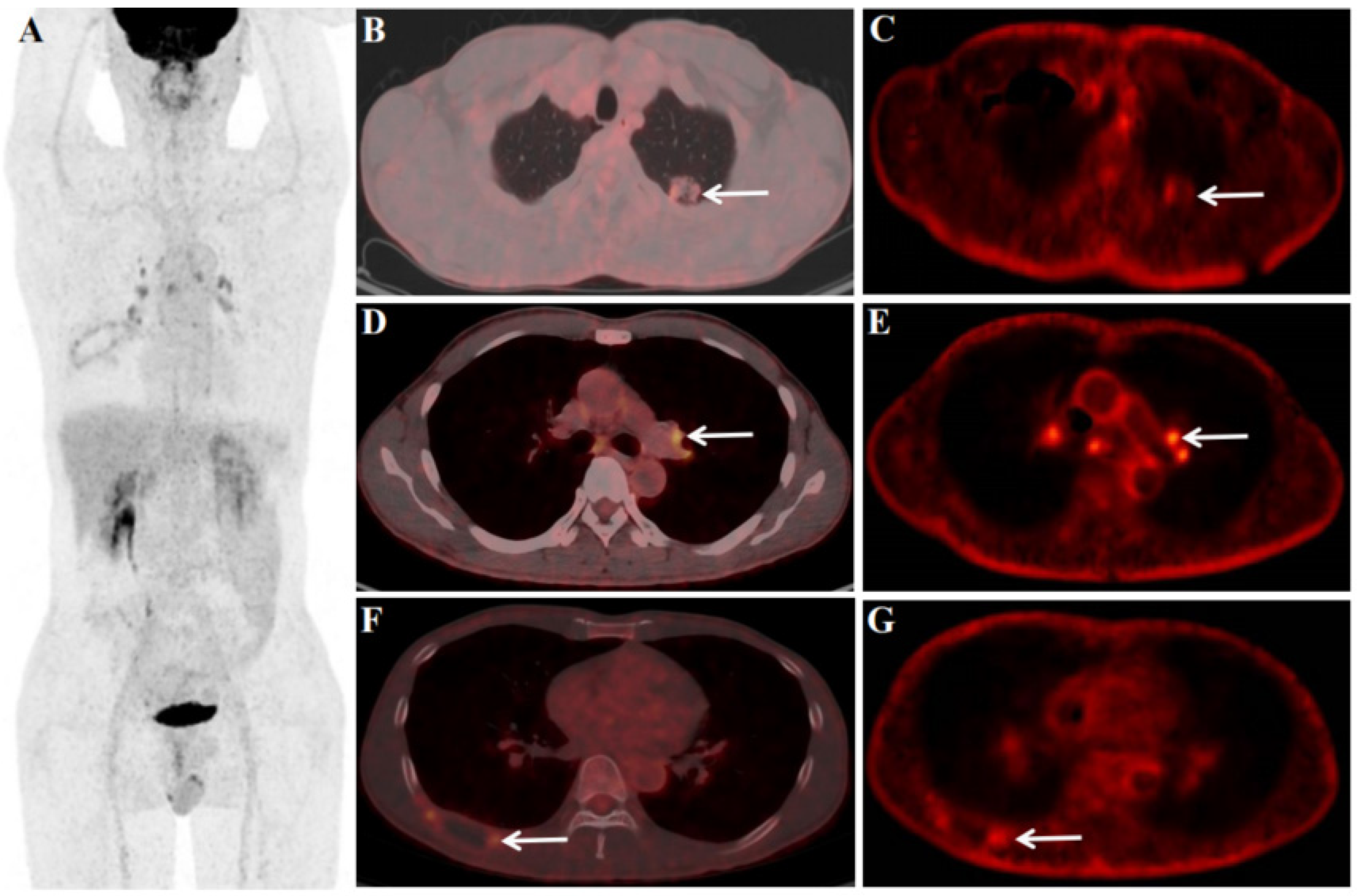
After 4 cycles of immune checkpoint inhibitors(ICI)and chemotherapy, the patient underwent an 18F-FDG PET/CT scan ( including dynamic scan), the aim is to perform an efficacy evaluation. After treatment, 18F-FDG PET/CT dynamic + static scan showed a left lung primary foci were smaller than before and FDG uptake was reduced, size of 2.1×1.2cm, SUVmax of 2.2 (Figure 3 B), Ki of 0.0070 ml/g/min (Figure 3 C), ΔSUVmax (pre-treatment SUVmax - treatment SUVmax/pre-treatment SUVmax) of 90.18%, and ΔKi (pre-treatment Ki - treatment Ki/pre-treatment Ki) of 86.67%. For the LNs, most of the LNs in the mediastinal region and pulmonary hilar regions were larger and FDG-avid than before after treatment. Among them, although the left pulmonary hilar LN was larger than before, the FDG uptake and Ki were reduced than before, size of 1.2×0.8cm, SUVmax of 4.0 (Figure 3 D), Ki of 0.0060 ml/g/min (Figure 3 E), ΔSUVmax of 29.83%, and ΔKi of 74.03%. The right 8th rib was also significantly smaller than before treatment, and FDG uptake was reduced, size of 4.5×1.8 cm, SUVmax of 2.8 (Figure 3 F), Ki of 0.0068 ml/g/min (Figure 3 G), ΔSUVmax of 70.21%, and ΔKi of 68.22%. In addition, distant metastases were smaller than before and FDG uptake was reduced, including the retroperitoneal LN (not clearly shown), left adrenal gland (size of 1.8×1.2cm, SUVmax of 2.1, ΔSUVmax of 75.0%), bone of the sacrum (size of 3.5×3.0cm, SUVmax of 2.1, ΔSUVmax of 80.73%), left subcutaneous lumbar region (size of 0.9×0.8cm, SUVmax of 1.4, ΔSUVmax of 81.58%), and brain (size of 1.0×0.8cm, not uptake seen).
Figure 3.
After 4 cycles of immune checkpoint inhibitors(ICI)and chemotherapy, the patient underwent an 18F-FDG PET/CT scan ( including dynamic scan), the aim is to perform an efficacy evaluation. After treatment, 18F-FDG PET/CT dynamic + static scan showed a left lung primary foci were smaller than before and FDG uptake was reduced, size of 2.1×1.2cm, SUVmax of 2.2 (Figure 3 B), Ki of 0.0070 ml/g/min (Figure 3 C), ΔSUVmax (pre-treatment SUVmax - treatment SUVmax/pre-treatment SUVmax) of 90.18%, and ΔKi (pre-treatment Ki - treatment Ki/pre-treatment Ki) of 86.67%. For the LNs, most of the LNs in the mediastinal region and pulmonary hilar regions were larger and FDG-avid than before after treatment. Among them, although the left pulmonary hilar LN was larger than before, the FDG uptake and Ki were reduced than before, size of 1.2×0.8cm, SUVmax of 4.0 (Figure 3 D), Ki of 0.0060 ml/g/min (Figure 3 E), ΔSUVmax of 29.83%, and ΔKi of 74.03%. The right 8th rib was also significantly smaller than before treatment, and FDG uptake was reduced, size of 4.5×1.8 cm, SUVmax of 2.8 (Figure 3 F), Ki of 0.0068 ml/g/min (Figure 3 G), ΔSUVmax of 70.21%, and ΔKi of 68.22%. In addition, distant metastases were smaller than before and FDG uptake was reduced, including the retroperitoneal LN (not clearly shown), left adrenal gland (size of 1.8×1.2cm, SUVmax of 2.1, ΔSUVmax of 75.0%), bone of the sacrum (size of 3.5×3.0cm, SUVmax of 2.1, ΔSUVmax of 80.73%), left subcutaneous lumbar region (size of 0.9×0.8cm, SUVmax of 1.4, ΔSUVmax of 81.58%), and brain (size of 1.0×0.8cm, not uptake seen).
SMARCA4-deficient non-small cell lung cancer (NSCLC) accounts for 3%-6% of all NSCLCs [
1]. It is only in recent years has emerged as a distinct NSCLC subset [
2]. SMARCA4-deficient NSCLC is prevalent in men aged 40-50 years and shows a strong association with smoking [
3]. It is highly aggressive, rapidly progressive, and has a poor prognosis [
4,
5,
6]. There are very limited descriptions of SMARCA4-deficient NSCLC morphology as well as
18F-FDG PET/CT features. To our knowledge, we are the first to present a dynamic imaging and metabolic parameter profile for SMARCA4-deficient NSCLC.
Effective treatment for SMARCA4-deficient NSCLC has not been established. Little is known about the efficacy of ICIs in SMARCA4-deficient NSCLC. In 2019, Tomoyuki Naito et al. reported the first case of SMARCA4-deficient NSCLC successfully treated with nivolumab [
7]. In the case we reported, the patient also received 4 cycles of ICIs (Tislelizumab).
Dynamic 18F-FDG PET/CT (dPET/CT) extracts physiological parameters which can better reveal the pathophysiological mechanisms of diseases. However, studies evaluating the efficacy of ICIs in lung cancer were seldom seen. In this case, the enlarged LNs in the mediastinum and pulmonary hilar region after treatment raised our attention. Therefore, we studied the pre-treatment and post-treatment ratios of static and dynamic parameters for chest lesions (including the upper lobe of the left lung, LN in the left pulmonary hilar region, and the right 8th rib). The results showed that both the primary left lung cancer focus (ΔSUVmax of 90.18%, and ΔKi of 85.52%) and the right 8th rib ratio were high (ΔSUVmax of 70.21%, and ΔKi of 68.22%), but the left hilar LN ΔSUVmax was low (29.83%) and ΔKi was high (74.03%), and the trend of ΔKi remained consistent with the primary focus. Therefore, whether dynamic metabolic parameters (Ki) can give more metabolic information for the assessment of ICI efficacy deserves further investigation and is something we are studying.
Author Contributions
X W and Y L were responsible for writing and guidance. Xx Y provided pathology support and pictures. S J provided internal medicine support and guidance. Yr Z organized the data and figures. All authors have read and agreed to the published version of the manuscript.
Funding
This research was sponsored by the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen (E010322003, SZ2020MS008), and Shenzhen High–level Hospital Construction Fund.
Institutional Review Board Statement
The study was approved by the ethics committee of Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences (KYLH2022-1).
Informed Consent Statement
All patients signed a written informed consent before the 18FDG PET/CT imaging with the Declaration of Helsinki.
Data Availability Statement
None.
Conflicts of Interest
All the authors have participated in the writing and revision of this article and take public responsibility for its content. The present publication is approved by all authors and by the responsible authorities where the work was carried out. All the authors confirm the fact that the article is not under consideration for publication elsewhere and has no conflicts of interest.
References
- Nambirajan A, Singh V, Bhardwaj N, Mittal S, Kumar S, Jain D. SMARCA4/BRG1-Deficient Non-Small Cell Lung Carcinomas: A Case Series and Review of the Literature. Arch Pathol Lab Med. 2021 Jan 1;145(1):90-98. [CrossRef]
- Agaimy A, Fuchs F, Moskalev EA, Sirbu H, Hartmann A, Haller F. SMARCA4-deficient pulmonary adenocarcinoma: clinicopathological, immunohistochemical, and molecular characteristics of a novel aggressive neoplasm with a consistent TTF1neg/CK7pos/HepPar-1pos immunophenotype. Virchows Arch. 2017 Nov;471(5):599-609. [CrossRef]
- Naito T, Udagawa H, Umemura S, Sakai T, Zenke Y, Kirita K, et al. Non-small cell lung cancer with loss of expression of the SWI/SNF complex is associated with aggressive clinicopathological features, PD-L1-positive status, and high tumor mutation burden. Lung Cancer. 2019 Dec; 138:35-42. Epub 2019 Oct 13. [CrossRef]
- Schoenfeld AJ, Bandlamudi C, Lavery JA, Montecalvo J, Namakydoust A, Rizvi H, et al. The Genomic Landscape of SMARCA4 Alterations and Associations with Outcomes in Patients with Lung Cancer. Clin Cancer Res. 2020 Nov 1;26(21):5701-5708. [CrossRef]
- Nambirajan A, Singh V, Bhardwaj N, Mittal S, Kumar S, Jain D. SMARCA4/BRG1-Deficient Non-Small Cell Lung Carcinomas: A Case Series and Review of the Literature. Arch Pathol Lab Med. 2021 Jan 1;145(1):90-98. [CrossRef]
- Imielinski M, Berger AH, Hammerman PS, Hernandez B, Pugh TJ, Hodis E, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012 Sep 14;150(6):1107-20.
- Naito T, Umemura S, Nakamura H, Zenke Y, Udagawa H, Kirita K, et al. Successful treatment with nivolumab for SMARCA4-deficient non-small cell lung carcinoma with a high tumor mutation burden: A case report. Thorac Cancer. 2019 May;10(5):1285-1288. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).