Submitted:
14 May 2023
Posted:
15 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
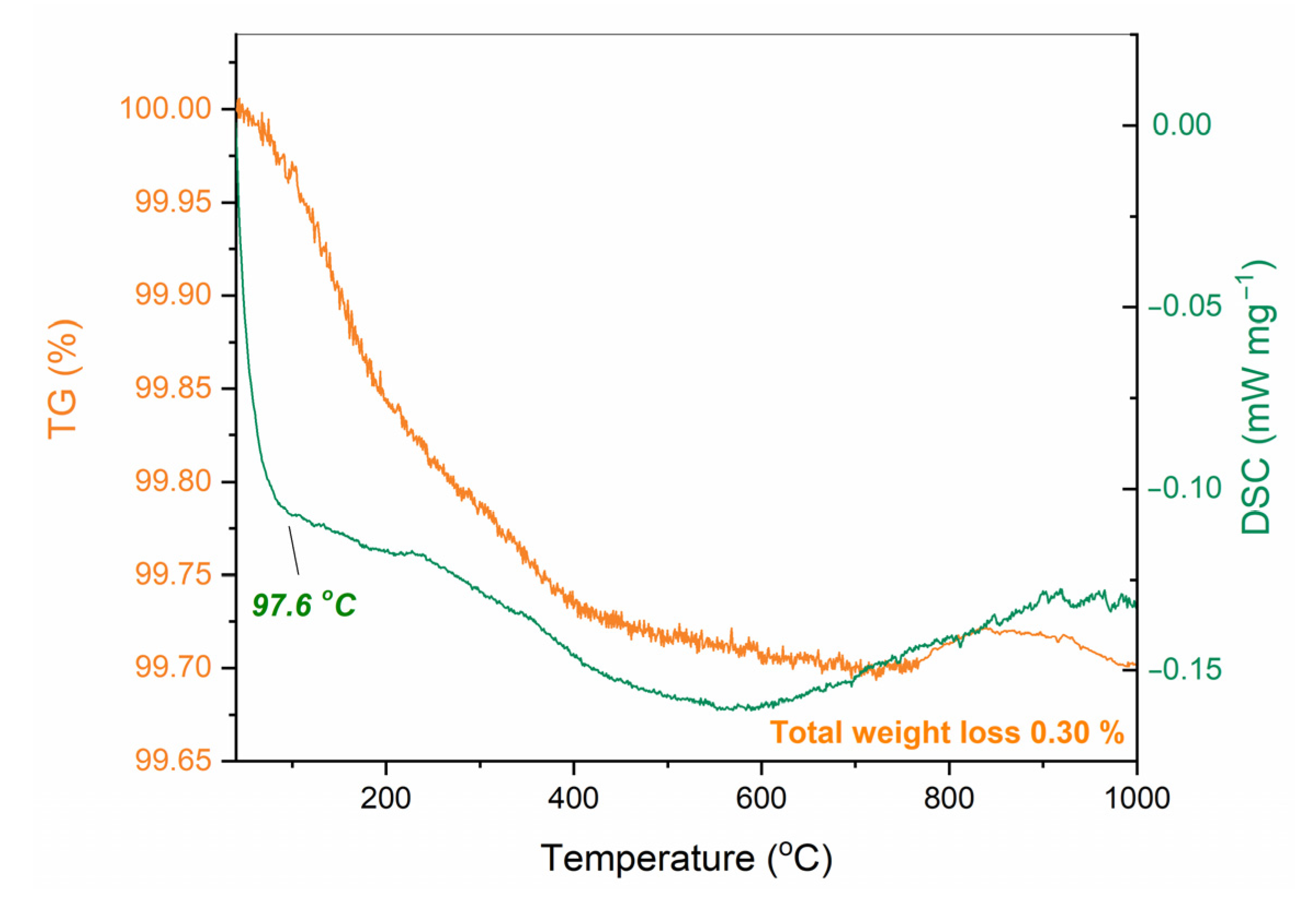
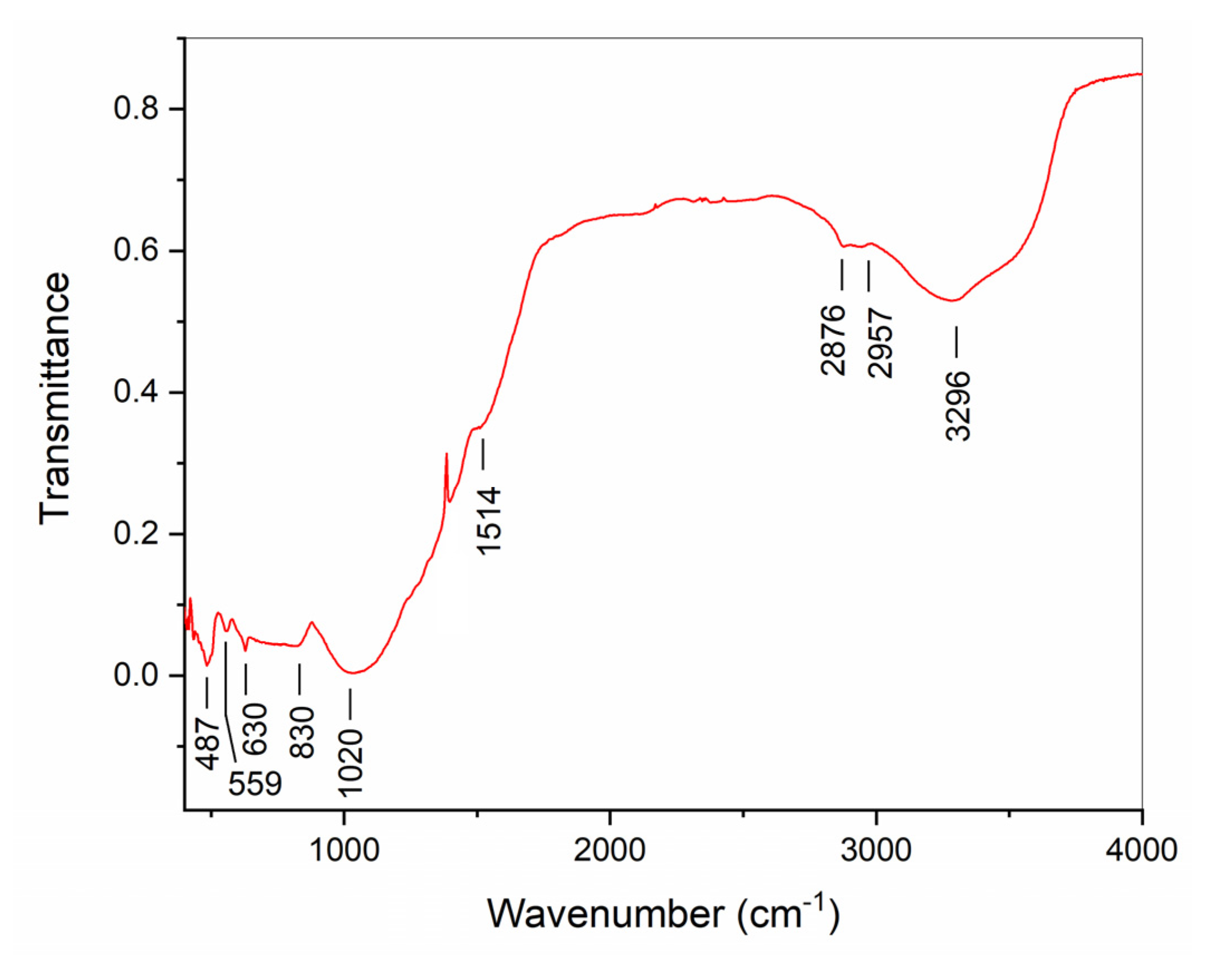
References
- Ohji, T.; Fukushima, M. Macro-Porous Ceramics: Processing and Properties. Int. Mater. Rev. 2012, 57, 115–131. [Google Scholar] [CrossRef]
- Hardy, D.; Green, D.J. Mechanical Properties of a Partially Sintered Alumina. J. Eur. Ceram. Soc. 1995, 15, 769–775. [Google Scholar] [CrossRef]
- Çelik, A.; Çağlar, G.; Çelik, Y. Fabrication of Porous Al2O3 Ceramics Using Carbon Black as a Pore Forming Agent by Spark Plasma Sintering. Ceram. Int. 2022, 48, 28181–28190. [Google Scholar] [CrossRef]
- González-Sánchez, M.; Rivero-Antúnez, P.; Cano-Crespo, R.; Morales-Flórez, V. Fabrication of Porous Alumina Structures by SPS and Carbon Sacrificial Template for Bone Regeneration. Materials 2022, 15, 1754. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ding, X.; Wang, Y.; Luo, Z.; Zhai, P. Fractal Dimension Analysis of Structure and Bending Strength of Porous Alumina Prepared Using Starch and Carbon Fiber as Pore-Forming Agents. Fractal Fract. 2022, 6, 574. [Google Scholar] [CrossRef]
- Biggemann, J.; Stumpf, M.; Fey, T. Porous Alumina Ceramics with Multimodal Pore Size Distributions. Materials 2021, 14, 3294. [Google Scholar] [CrossRef] [PubMed]
- Schelm, K.; Fey, T.; Dammler, K.; Betke, U.; Scheffler, M. Hierarchical-Porous Ceramic Foams by a Combination of Replica and Freeze Technique. Adv. Eng. Mater. 2019, 21, 1801362. [Google Scholar] [CrossRef]
- Scheithauer, U.; Kerber, F.; Füssel, A.; Holtzhausen, S.; Beckert, W.; Schwarzer, E.; Weingarten, S.; Michaelis, A. Alternative Process Routes to Manufacture Porous Ceramics—Opportunities and Challenges. Materials 2019, 12, 663. [Google Scholar] [CrossRef]
- He, X.; Zhou, X.; Su, B. 3D Interconnective Porous Alumina Ceramics via Direct Protein Foaming. Mater. Lett. 2009, 63, 830–832. [Google Scholar] [CrossRef]
- Devavarapu, S.; Chaudhuri, P.; Shrivastava, A.; Bhattacharyya, S. Processing of Porous Alumina by Foaming Method-Effect of Foaming Agent, Solid Loading and Binder. Ceram. Int. 2019, 45, 12264–12273. [Google Scholar] [CrossRef]
- Maria, J.-P.; Kang, X.; Floyd, R.D.; Dickey, E.C.; Guo, H.; Guo, J.; Baker, A.; Funihashi, S.; Randall, C.A. Cold Sintering: Current Status and Prospects. J. Mater. Res. 2017, 32, 3205–3218. [Google Scholar] [CrossRef]
- Langer, J.; Hoffmann, M.J.; Guillon, O. Direct Comparison between Hot Pressing and Electric Field-Assisted Sintering of Submicron Alumina. Acta Mater. 2009, 57, 5454–5465. [Google Scholar] [CrossRef]
- Bocanegra-Bernal, M.H. Hot Isostatic Pressing (HIP) Technology and Its Applications to Metals and Ceramics. J. Mater. Sci. 2004, 39, 6399–6420. [Google Scholar] [CrossRef]
- Oghbaei, M.; Mirzaee, O. Microwave versus Conventional Sintering: A Review of Fundamentals, Advantages and Applications. J. of Alloys Compd. 2010, 494, 175–189. [Google Scholar] [CrossRef]
- Munir, Z.A.; Quach, D.V.; Ohyanagi, M. Electric Current Activation of Sintering: A Review of the Pulsed Electric Current Sintering Process: Electric Current Activation of Sintering. J. Am. Ceram. Soc. 2011, 94, 1–19. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The Effect of Electric Field and Pressure on the Synthesis and Consolidation of Materials: A Review of the Spark Plasma Sintering Method. J. Mater. Sci 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Rubinovskii, N.A.; Shornikov, D.P.; Tenishev, A.V.; Zaluzhnui, A.G.; Zholnin, A.G. Effect of Aluminum Oxide Powder Particle Size on Spark Plasma Sintering Results. Glass Ceram. 2019, 76, 94–98. [Google Scholar] [CrossRef]
- Wang, S.W.; Chen, L.D.; Hirai, T. Densification of Al2O3 Powder Using Spark Plasma Sintering. J. Mater. Res. 2000, 15, 982–987. [Google Scholar] [CrossRef]
- Young, C.; Zhang, C.; Nisar, A.; Boesl, B.; Agarwal, A. Spark Plasma Sintered Porous Aluminum Oxide for Filtration Applications. Ceram. Int. 2021, 47, 21822–21827. [Google Scholar] [CrossRef]
- Roussel, N.; Lallemant, L.; Chane-Ching, J.-Y.; Guillemet-Fristch, S.; Durand, B.; Garnier, V.; Bonnefont, G.; Fantozzi, G.; Bonneau, L.; Trombert, S.; et al. Highly Dense, Transparent α-Al2O3 Ceramics From Ultrafine Nanoparticles Via a Standard SPS Sintering. J. Am. Ceram. Soc. 2013, 96, 1039–1042. [Google Scholar] [CrossRef]
- Guo, J.; Guo, H.; Baker, A.L.; Lanagan, M.T.; Kupp, E.R.; Messing, G.L.; Randall, C.A. Cold Sintering: A Paradigm Shift for Processing and Integration of Ceramics. Angew. Chem. Int. Ed. 2016, 55, 11457–11461. [Google Scholar] [CrossRef] [PubMed]
- Ibn-Mohammed, T.; Randall, C.A.; Mustapha, K.B.; Guo, J.; Walker, J.; Berbano, S.; Koh, S.C.L.; Wang, D.; Sinclair, D.C.; Reaney, I.M. Decarbonising Ceramic Manufacturing: A Techno-Economic Analysis of Energy Efficient Sintering Technologies in the Functional Materials Sector. J. Eur. Ceram. Soc. 2019, 39, 5213–5235. [Google Scholar] [CrossRef]
- Floyd, R.D.; Lowum, S.; Maria, J.-P. Cold Sintering Zinc Oxide with a Crystalline Zinc Acetate Dihydrate Mass Transport Phase. J. Mater. Sci. 2020, 55, 15117–15129. [Google Scholar] [CrossRef]
- Dargatz, B.; Gonzalez-Julian, J.; Bram, M.; Jakes, P.; Besmehn, A.; Schade, L.; Röder, R.; Ronning, C.; Guillon, O. FAST/SPS Sintering of Nanocrystalline Zinc Oxide—Part I: Enhanced Densification and Formation of Hydrogen-Related Defects in Presence of Adsorbed Water. J. Eur. Ceram. Soc. 2016, 36, 1207–1220. [Google Scholar] [CrossRef]
- Dargatz, B.; Gonzalez-Julian, J.; Bram, M.; Shinoda, Y.; Wakai, F.; Guillon, O. FAST/SPS Sintering of Nanocrystalline Zinc Oxide—Part II: Abnormal Grain Growth, Texture and Grain Anisotropy. J. Eur. Ceram. Soc. 2016, 36, 1221–1232. [Google Scholar] [CrossRef]
- Ivakin, Yu.; Smirnov, A.; Kholodkova, A.; Vasin, A.; Kormilicin, M.; Kornyushin, M.; Stolyarov, V. Comparative Study of Cold Sintering Process and Autoclave Thermo-Vapor Treatment on a ZnO Sample. Crystals 2021, 11, 71. [Google Scholar] [CrossRef]
- Bang, S.H.; Tsuji, K.; Ndayishimiye, A.; Dursun, S.; Seo, J.; Otieno, S.; Randall, C.A. Toward a Size Scale-up Cold Sintering Process at Reduced Uniaxial Pressure. J. Am. Ceram. Soc. 2020, 103, 2322–2327. [Google Scholar] [CrossRef]
- Hérisson de Beauvoir, T.; Dursun, S.; Gao, L.; Randall, C. New Opportunities in Metallization Integration in Cofired Electroceramic Multilayers by the Cold Sintering Process. ACS Appl. Electron. Mater. 2019, 1, 1198–1207. [Google Scholar] [CrossRef]
- Jing, Y.; Luo, N.; Wu, S.; Han, K.; Wang, X.; Miao, L.; Wei, Y. Remarkably Improved Electrical Conductivity of ZnO Ceramics by Cold Sintering and Post-Heat-Treatment. Ceram. Int. 2018, 44, 20570–20574. [Google Scholar] [CrossRef]
- Funahashi, S.; Guo, J.; Guo, H.; Wang, K.; Baker, A.L.; Shiratsuyu, K.; Randall, C.A. Demonstration of the Cold Sintering Process Study for the Densification and Grain Growth of ZnO Ceramics. J. Am. Ceram. Soc. 2017, 100, 546–553. [Google Scholar] [CrossRef]
- Ma, J.-P.; Chen, X.-M.; Ouyang, W.-Q.; Wang, J.; Li, H.; Fang, J.-L. Microstructure, Dielectric, and Energy Storage Properties of BaTiO3 Ceramics Prepared via Cold Sintering. Ceram. Int. 2018, 44, 4436–4441. [Google Scholar] [CrossRef]
- Tsuji, K.; Ndayishimiye, A.; Lowum, S.; Floyd, R.; Wang, K.; Wetherington, M.; Maria, J.-P.; Randall, C.A. Single Step Densification of High Permittivity BaTiO3 Ceramics at 300 oC. J. Eur. Ceram. Soc. 2020, 40, 1280–1284. [Google Scholar] [CrossRef]
- Sada, T.; Ndayishimiye, A.; Fan, Z.; Fujioka, Y.; Randall, C.A. Surface Modification of BaTiO3 with Catechol Surfactant and Effects on Cold Sintering. J. Appl. Phys. 2021, 129, 184102. [Google Scholar] [CrossRef]
- Guo, N.; Shen, H.-Z.; Shen, P. One-Step Synthesis and Densification of BaTiO3 by Reactive Cold Sintering. Scr. Mater. 2022, 213, 114628. [Google Scholar] [CrossRef]
- Ma, J.; Li, H.; Wang, H.; Lin, C.; Wu, X.; Lin, T.; Zheng, X.; Yu, X. Composition, Microstructure and Electrical Properties of K0.5Na0.5NbO3 Ceramics Fabricated by Cold Sintering Assisted Sintering. J. Eur. Ceram. Soc. 2019, 39, 986–993. [Google Scholar] [CrossRef]
- Chi, M.; Ma, W.; Guo, J.; Wu, J.; Li, T.; Wang, S.; Zhang, P. Effect of NaCl on the Microstructure and Electrical Properties of K0.5Na0.5NbO3 Ceramics Prepared by Cold Sintering Process. J. Mater. Sci: Mater. Electron. 2019, 30, 21435–21443. [Google Scholar] [CrossRef]
- Cong, L.; Huajing, W.; Jianzhang, M.; Baoyu, D.; Xiao, W.; Tengfei, L.; Xinghua, Z.; Xing, Y. Effect of Dwell Time on Cold Sintering Assisted Sintering Based Highly Transparent 0.9K0.5Na0.5NbO3-0.1LiBiO3 Ceramics. J. Alloys Compd. 2020, 826, 154249. [Google Scholar] [CrossRef]
- Tsuji, K.; Fan, Z.; Bang, S.H.; Dursun, S.; Trolier-McKinstry, S.; Randall, C.A. Cold Sintering of the Ceramic Potassium Sodium Niobate, (K0.5Na0.5)NbO3, and Influences on Piezoelectric Properties. J. Eur. Ceram. Soc. 2022, 42, 105–111. [Google Scholar] [CrossRef]
- Wang, D.; Guo, H.; Morandi, C.S.; Randall, C.A.; Trolier-McKinstry, S. Cold Sintering and Electrical Characterization of Lead Zirconate Titanate Piezoelectric Ceramics. APL Materials 2018, 6, 016101. [Google Scholar] [CrossRef]
- Wang, D.; Tsuji, K.; Randall, C.A.; Trolier-McKinstry, S. Model for the Cold Sintering of Lead Zirconate Titanate Ceramic Composites. J. Am. Ceram. Soc. 2020, 103, 4894–4902. [Google Scholar] [CrossRef]
- Wang, D.; Dursun, S.; Gao, L.; Morandi, C.S.; Randall, C.A.; Trolier-McKinstry, S. Fabrication of Bimorph Lead Zirconate Titanate Thick Films on Metal Substrates via the Cold Sintering-Assisted Process. Acta Mater. 2020, 195, 482–490. [Google Scholar] [CrossRef]
- Induja, I.J.; Sebastian, M.T. Microwave Dielectric Properties of Cold Sintered Al2O3-NaCl Composite. Mater. Lett. 2018, 211, 55–57. [Google Scholar] [CrossRef]
- Suleiman, B.; Zhang, H.; Ding, Y.; Li, Y. Microstructure and Mechanical Properties of Cold Sintered Porous Alumina Ceramics. Ceram. Int. 2022, 48, 13531–13540. [Google Scholar] [CrossRef]
- Akmal, M.; Hassan, M.; Afzal, M.; Ryu, H.J. Novel Approach to Sintering Hydroxyapatite-Alumina Nanocomposites at 300 °C. Mater. Chem. Phys. 2021, 260, 124187. [Google Scholar] [CrossRef]
- Hérisson de Beauvoir, T.; Estournès, C. Translucent γ-AlOOH and γ-Al2O3 Glass-Ceramics Using the Cold Sintering Process. Scr. Mater. 2021, 194, 113650. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, X.; Guo, J.; Liang, J.; Sun, J.; Yang, Y.; Yang, L.; Liao, R.; Randall, C.A. Thermal-Assisted Cold Sintering Study of Al2O3 Ceramics: Enabled with a Soluble γ-Al2O3 Intermediate Phase. J. Eur. Ceram. Soc. 2023, 43, 478–485. [Google Scholar] [CrossRef]
- Herisson de Beauvoir, T.; Sangregorio, A.; Cornu, I.; Elissalde, C.; Josse, M. Cool-SPS: An Opportunity for Low Temperature Sintering of Thermodynamically Fragile Materials. J. Mater. Chem. C 2018, 6, 2229–2233. [Google Scholar] [CrossRef]
- Schwarz, S.; Thron, A.M.; Rufner, J.; Benthem, K.; Guillon, O. Low Temperature Sintering of Nanocrystalline Zinc Oxide: Effect of Heating Rate Achieved by Field Assisted Sintering/Spark Plasma Sintering. J. Am. Ceram. Soc. 2012, 95, 2451–2457. [Google Scholar] [CrossRef]
- Gonzalez-Julian, J.; Neuhaus, K.; Bernemann, M.; Pereira da Silva, J.; Laptev, A.; Bram, M.; Guillon, O. Unveiling the Mechanisms of Cold Sintering of ZnO at 250 °C by Varying Applied Stress and Characterizing Grain Boundaries by Kelvin Probe Force Microscopy. Acta Mater. 2018, 144, 116–128. [Google Scholar] [CrossRef]
- Nur, K.; Mishra, T.P.; da Silva, J.G.P.; Gonzalez-Julian, J.; Bram, M.; Guillon, O. Influence of Powder Characteristics on Cold Sintering of Nano-Sized ZnO with Density above 99 %. J. Eur. Ceram. Soc. 2021, 41, 2648–2662. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, X.; Kang, S.; Guo, J.; Chen, Z.; Long, Y.; Zeng, Q.; Sun, J.; Yang, L.; Liao, R.; et al. Microstructural Evolution of ZnO via Hybrid Cold Sintering/Spark Plasma Sintering. J. Eur. Ceram. Soc. 2022, 42, 5738–5746. [Google Scholar] [CrossRef]
- Ivakin, Yu.D.; Smirnov, A.V.; Kurmysheva, A.Yu.; Kharlanov, A.N.; Solís Pinargote, N.W.; Smirnov, A.; Grigoriev, S.N. The Role of the Activator Additives Introduction Method in the Cold Sintering Process of ZnO Ceramics: CSP/SPS Approach. Materials 2021, 14, 6680. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, V.B.; Panasyuk, G.P.; Voroshilov, I.L.; Boudova, G.P.; Danchevskaya, M.N.; Torbin, S.N.; Ivakin, Yu.D. New Ecologically Pure Technologies of Fine-Crystalline Materials. Ind. Eng. Chem. Res. 1996, 35, 3721–3725. [Google Scholar] [CrossRef]
- Ivakin, Yu.D.; Danchevskaya, M.N.; Muravieva, G.P. Induced Formation of Corundum Crystals in Supercritical Water Fluid. Russ. J. Phys. Chem. B 2015, 9, 1082–1094. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R. Beta-Alumina. In Concise Encyclopedia of Advanced Ceramic Materials; Elsevier, 1991; pp. 32–35 ISBN 978-0-08-034720-2.
- Danchevskaya, M.N.; Ivakin, Yu.D.; Torbin, S.N.; Panasyuk, G.P.; Belan, V.N.; Voroshilov, I.L. Scientific Basis of Technology of Fine-Crystalline Quartz and Corundum. High Press. Res. 2001, 20, 229–239. [Google Scholar] [CrossRef]
- Sato, T. Thermal Decomposition of Aluminium Hydroxides. J. Therm. Anal. 1987, 32, 61–70. [Google Scholar] [CrossRef]
- Lamouri, S.; Hamidouche, M.; Bouaouadja, N.; Belhouchet, H.; Garnier, V.; Fantozzi, G.; Trelkat, J.F. Control of the γ-Alumina to α-Alumina Phase Transformation for an Optimized Alumina Densification. Bol. Soc. Esp. Ceram. V. 2017, 56, 47–54. [Google Scholar] [CrossRef]
- Panasyuk, G.P.; Kozerozhets, I.V.; Semenov, E.A.; Danchevskaya, M.N.; Azarova, L.A.; Belan, V.N. Thermodynamics and Kinetics of γ-Al2O3 and AlOOH Transformations under Hydrothermal Conditions. Inorg. Mater. 2019, 55, 920–928. [Google Scholar] [CrossRef]
- Kozerozhets, I.V.; Panasyuk, G.P.; Semenov, E.A.; Danchevskaya, M.N.; Azarova, L.A.; Simonenko, N.P. Transformations of Nanosized Boehmite and γ-Аl2О3 upon Heat Treatment. Russ. J. Inorg. Chem. 2020, 65, 587–591. [Google Scholar] [CrossRef]
- Maryashkin, A.V.; Ivakin, Yu.D.; Danchevskaya, M.N.; Murav’eva, G.P.; Kirikova, M.N. Synthesis of Corundum Doped with Cerium in Supercritical Water Fluid. Moscow Univ. Chem. Bull. 2011, 66, 290–298. [Google Scholar] [CrossRef]
- Kiss, A.B.; Keresztury, G.; Farkas, L. Raman and i.r. Spectra and Structure of Boehmite (y-AlOOH). Evidence for the Recently Discarded D172h Space Group. Spectrochim. 1980, 36A, 653–658. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Zamani, R.; Beiygie, E.; Nekouei, H. Synthesis of Micro-Mesopores Flowerlike γ-Al2O3 Nano-Architectures. J. Serb. Chem. Soc. 2014, 79, 1007–1017. [Google Scholar] [CrossRef]
- Sun, T.; Zhuo, Q.; Chen, Y.; Wu, Z. Synthesis of Boehmite and Its Effect on Flame Retardancy of Epoxy Resin. High Perform. Polym. 2015, 27, 100–104. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Ruan, H.D.; Frost, R.L. Thermal Decomposition of Bauxite Minerals: Infrared Emission Spectroscopy of Gibbsite, Boehmite and Diaspore. J. Mater. Sci. 2002, 37, 1121–1129. [Google Scholar] [CrossRef]
- Hajduchova, Z. Adsorption of Dodecylbenzenesulfonic Acid on the Alumina Particles in the Preparation of Alumina Foam. Ceram. - Silik. [CrossRef]
- Santhiya, D.; Subramanian, S.; Natarajan, K.A.; Malghan, S.G. Surface Chemical Studies on Alumina Suspensions Using Ammonium Poly(Methacrylate). Colloids Surf. 2000, 164, 143–154. [Google Scholar] [CrossRef]
- Colomban, P. Vibrational Characterization of the Various Forms of (Solvated or Unsolvated) Mobile Proton in the Solid State. Advantages, Limitations and Open Questions. Solid State Ion. 2023, 393, 116187. [Google Scholar] [CrossRef]
- Wang, L.; Hu, J.; Cheng, Y.; Fu, Z.; Shen, Z.; Xiong, Y. Defect Formation by Order Coalescence in Vermicular Grains during Alumina Phase Transformation. Scr. Mater. 2015, 107, 59–62. [Google Scholar] [CrossRef]
- Jiménez-Morales, F.; Rivero-Antúnez, P.; González-Sánchez, M.; Garrido-Regife, L.; Morales-Flórez, V. The Evolution of Vermicular Structures and Sintering Behavior of Alumina. In Cellular Automata; Chopard, B., Bandini, S., Dennunzio, A., Arabi Haddad, M., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, 2022; ISBN 978-3-031-14925-2. [Google Scholar]
- Dutta, S.; Kim, T.B.; Krentz, T.; Vinci, R.P.; Chan, H.M. Sol-Gel-Derived Single-Crystal Alumina Coatings with Vermicular Structure: Rapid Communications of the American Ceramic Society. J. Am. Ceram. Soc. 2011, 94, 340–343. [Google Scholar] [CrossRef]
- Guo, J.; Floyd, R.; Lowum, S.; Maria, J.-P.; Herisson de Beauvoir, T.; Seo, J.-H.; Randall, C.A. Cold Sintering: Progress, Challenges, and Future Opportunities. Annu. Rev. Mater. Res. 2019, 49, 275–295. [Google Scholar] [CrossRef]
- Grasso, S.; Biesuz, M.; Zoli, L.; Taveri, G.; Duff, A.I.; Ke, D.; Jiang, A.; Reece, M.J. A Review of Cold Sintering Processes. Adv. Appl. Ceram. 2020, 119, 115–143. [Google Scholar] [CrossRef]
- Kholodkova, A.A.; Danchevskaya, M.N.; Ivakin, Yu.D.; Muravieva, G.P.; Tyablikov, A.S. Crystalline Barium Titanate Synthesized in Sub- and Supercritical Water. J. Supercrit. Fluids 2016, 117, 194–202. [Google Scholar] [CrossRef]
- Ivakin, Yu.D.; Danchevskaya, M.N.; Muravieva, G.P. Recrystallization of Zinc Oxide in a Sub- and Supercritical Water Medium. Russ. J. Phys. Chem. B 2019, 13, 1189–1200. [Google Scholar] [CrossRef]
- Kozawa, T.; Onda, A.; Yanagisawa, K. Accelerated Formation of Barium Titanate by Solid-State Reaction in Water Vapour Atmosphere. J. Eur. Ceram. Soc. 2009, 29, 3259–3264. [Google Scholar] [CrossRef]
- Ivakin, Yu.D.; Danchevskaya, M.N.; Muravieva, G.P. Influence of Molar Ratio Water Fluid / Al2O3 on Solid Phase Composition. Proceedings of the 9th Meeting of Supercritical Fluids, Trieste, Italy, 04; 24. 20 June. [CrossRef]
- Okumiya, M.; Yamaguchi, G; Yamada, O. ; Ono, S. Formation of κ- and κ'-aluminium oxide from the dehydration of tohdite (5Al2O3·H2O). Bull. Chem. Soc. Jpn. 1971, 44, 418–423. [Google Scholar] [CrossRef]
- De Bellis, J.; Ochoa- Hernández, C.; Farès, C.; Petersen, H.; Ternienden, J.; Weidenthaler, C.; Amrute, A.P.; Schüth, F. Surface and Bulk Chemistry of Mechanochemically Synthesized Tohdite Nanoparticles. J. Am. Chem. Soc. 2022, 144, 9421–9433. [Google Scholar] [CrossRef]
- Dong, Y.; Jiang, H.; Chen, A.; Yang, T.; Zou, T.; Xu, D. Porous Al2O3 Ceramics with Spontaneously Formed Pores and Enhanced Strength Prepared by Indirect Selective Laser Sintering Combined with Reaction Bonding. Ceram. Int. 2020, 46, 15159–15166. [Google Scholar] [CrossRef]
- Xu, C.; Liu, H.; Yang, H.; Yang, L. A Green Biocompatible Fabrication of Highly Porous Functional Ceramics with High Strength and Controllable Pore Structures. J. Mater. Sci. Technol. 2016, 32, 729–732. [Google Scholar] [CrossRef]
- Smirnov, A.V.; Kornyushin, M.V.; Kholodkova, A.A.; Melnikov, S.A.; Stepanov, A.D.; Fesik, E.V.; Ivakin, Y.D. Cold Sintering Process of Zinc Oxide Ceramics: Powder Preparation and Sintering Conditions Effects on Final Microstructure. Inorganics 2022, 10, 197. [Google Scholar] [CrossRef]

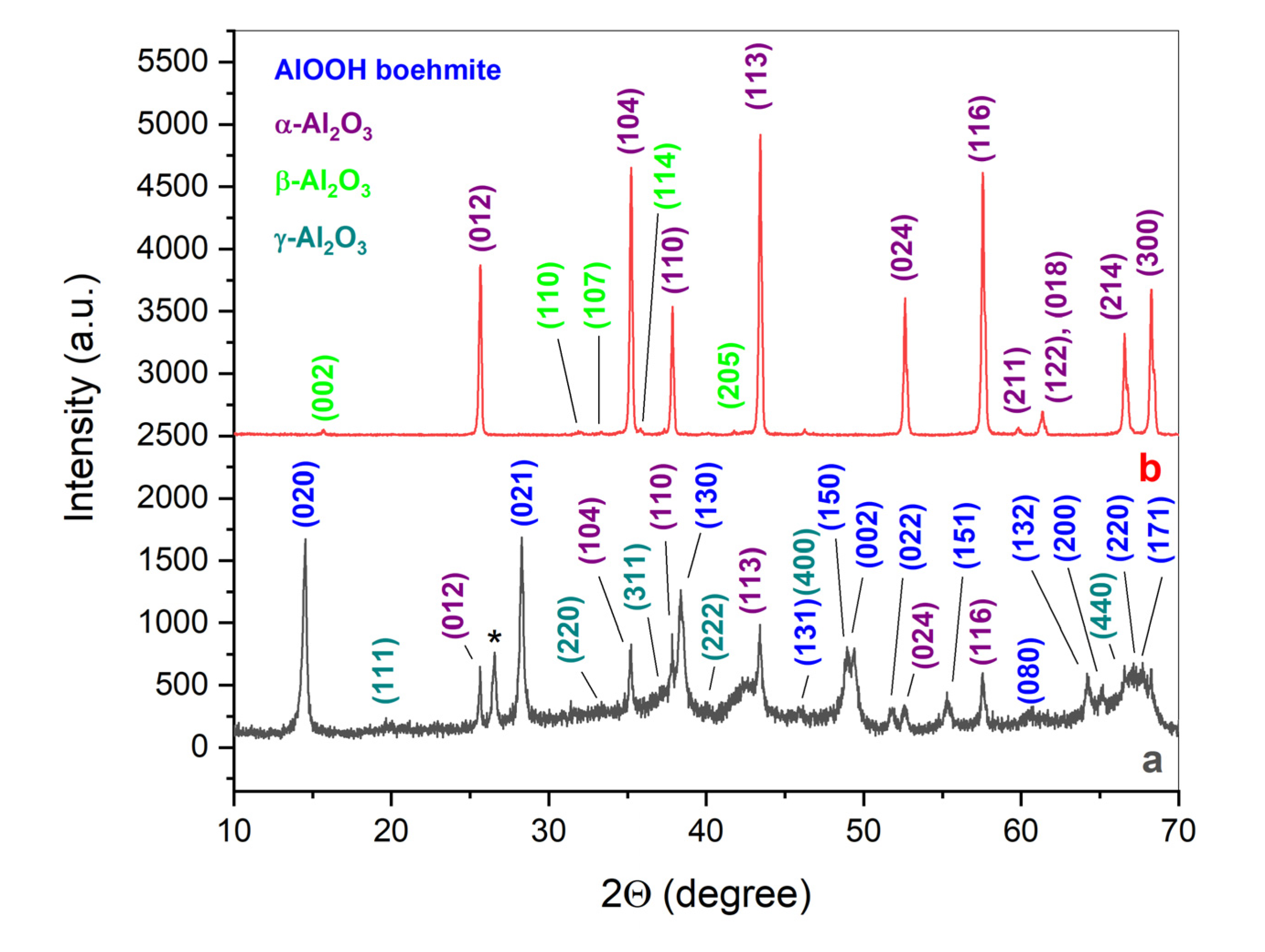
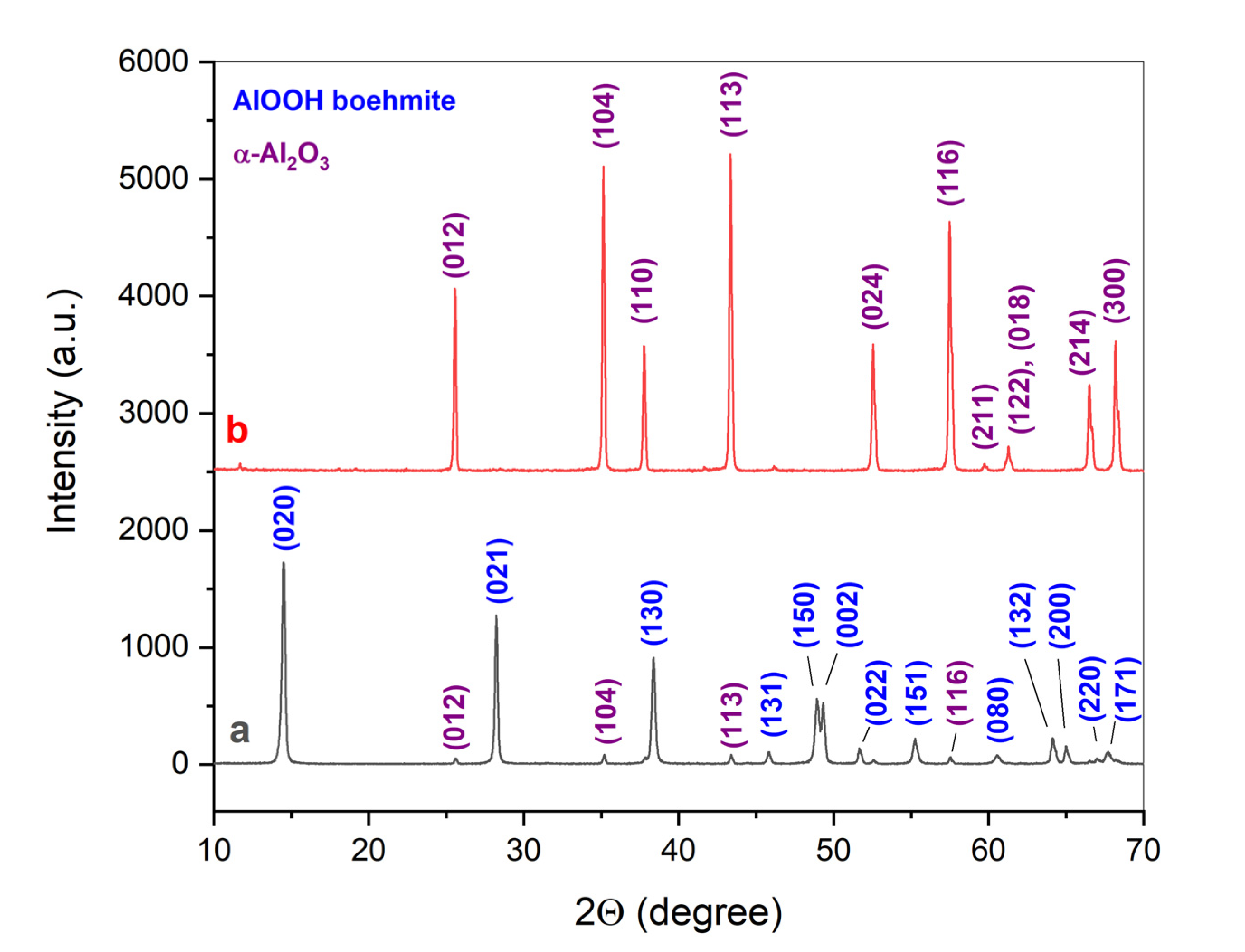
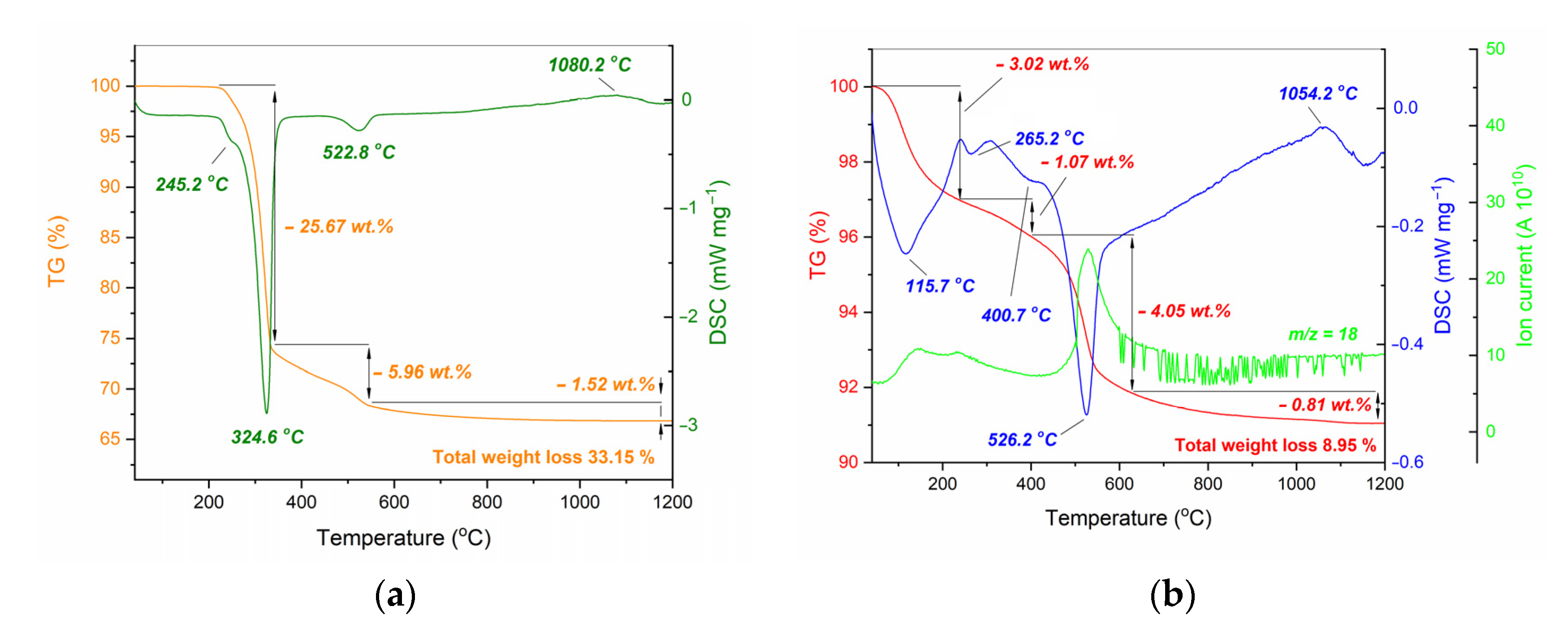
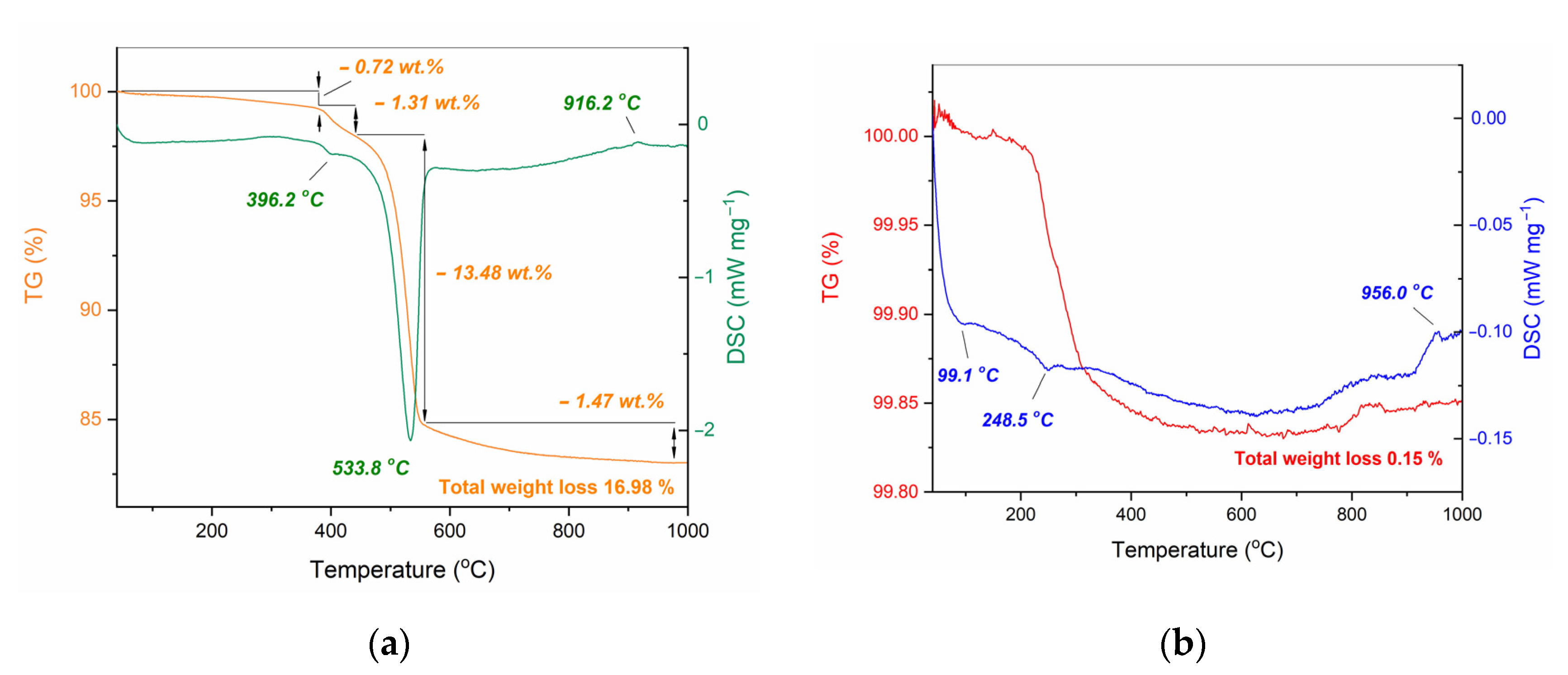
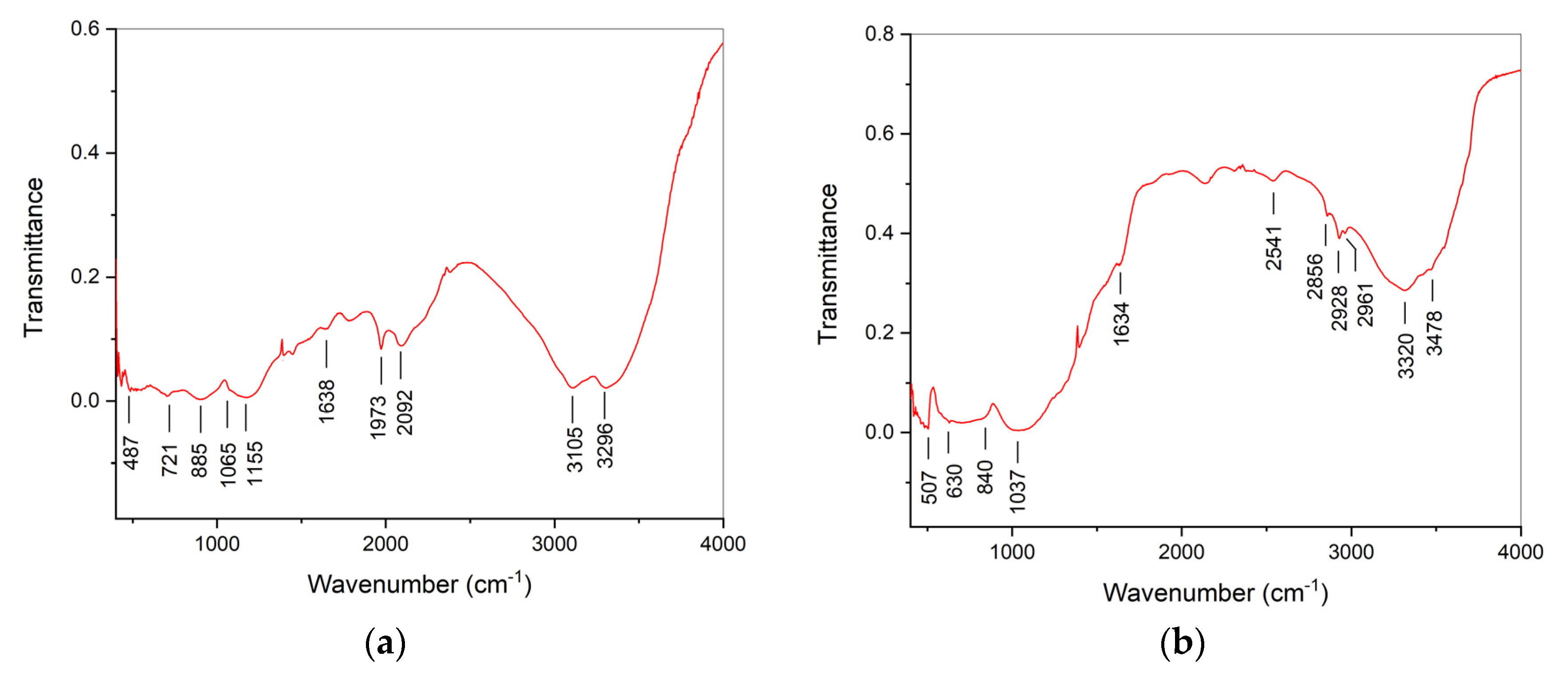
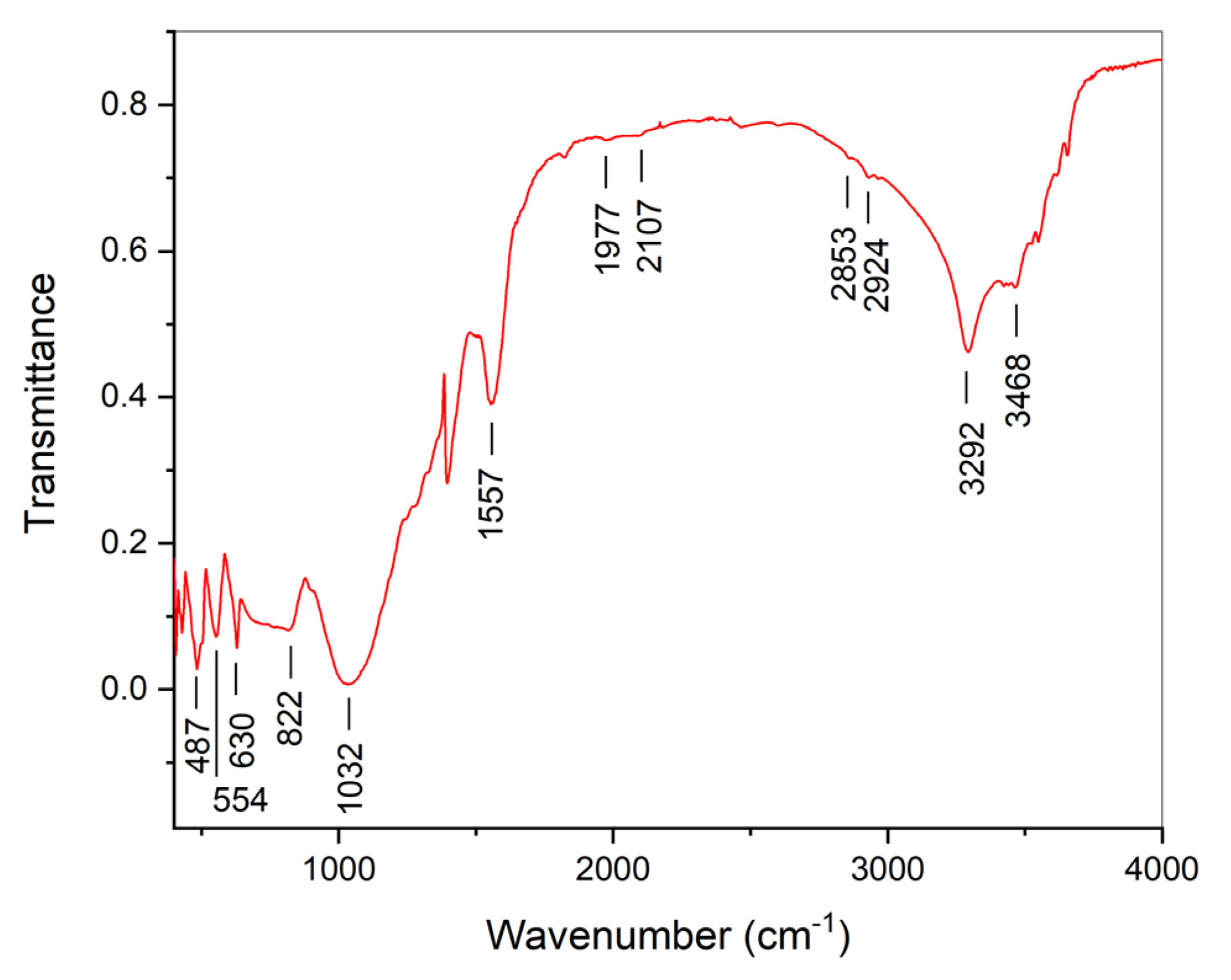

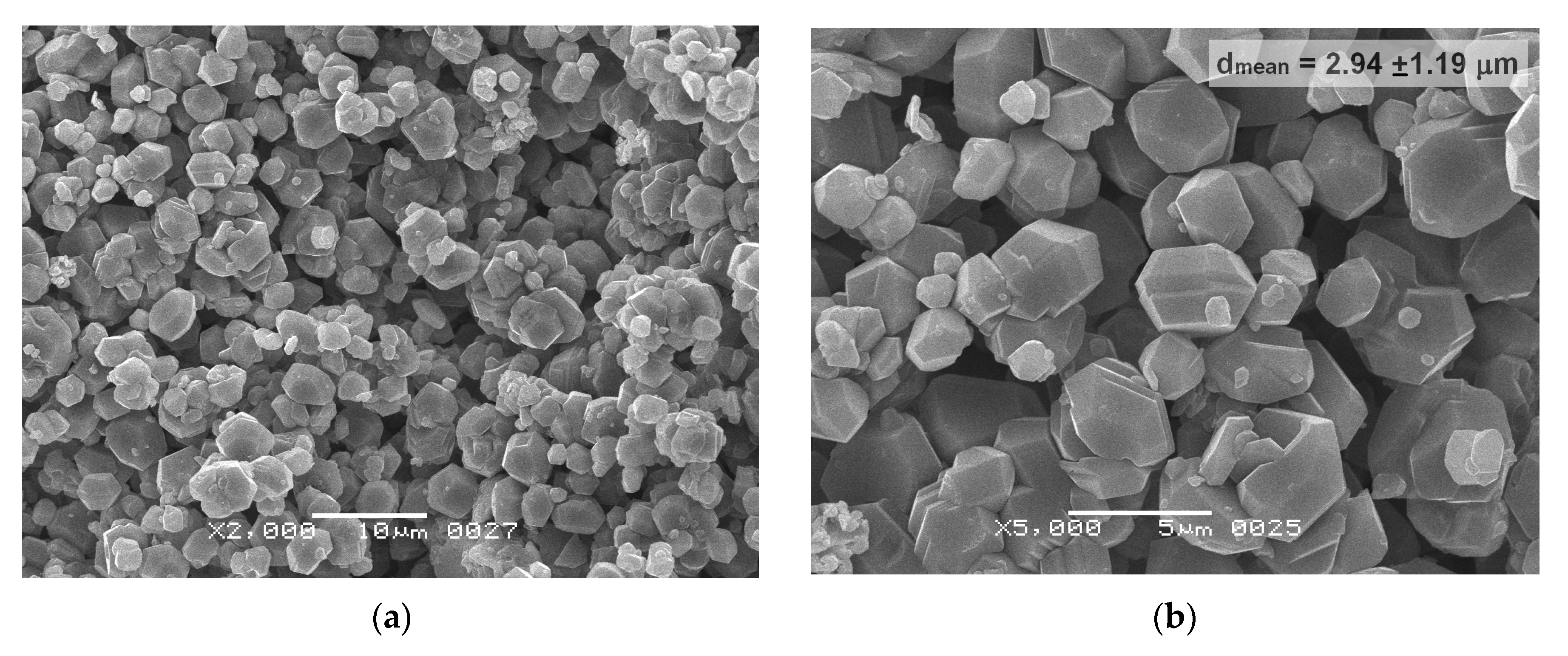
| Initial powder | Samples after CSP-SPS | Samples after annealing in air |
|---|---|---|
| γ-Al(OH)3 (HA-P) | HA-CS | HA-CS-A |
| γ-AlOOH (BO-P) | BO-CS | - |
| Sample | Density after CSP-SPS (g cm-3) | Porosity after CSP-SPS (%) | Density after annealing (g cm-3) | Porosity after annealing (%) |
|---|---|---|---|---|
| HA-CS 1 | 1.57 | 51.9 | 1.52 | 60.5 |
| HA-CS 2 | 1.53 | 53.3 | 1.46 | 60.2 |
| HA-CS 3 | 1.53 | 52.9 | 1.50 | 60.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





