Appendix A. Experimental Section
Unless otherwise specified, all reagents and starting materials were purchased from commercial sources and used as received, and the solvents were purified and dried using standard procedures. The chromatography solvents were technical grade and distilled prior to use. The NMR spectra were recorded with a Bruker Avance 500 spectrometer (500 MHz for 1H and 125 MHz for 13C) with CDCl3 as solvent with tetramethylsilane (TMS) as the internal standard at room temperature. Chemical shifts are given in δ relative to TMS, the coupling constants J are given in Hz. HRMS spectra were obtained with an Agilent 6200 using a quadrupole time-of-flight mass spectrometer equipped with an ESI source. The melting points were measured using SGWX-4 melting point apparatus and not corrected. The X-ray source used for the single crystal X-ray diffraction analysis of compound 3a and 5a was Mo Kα (λ = 0.71073 Å), and the thermal ellipsoid was drawn at the 30% probability level.
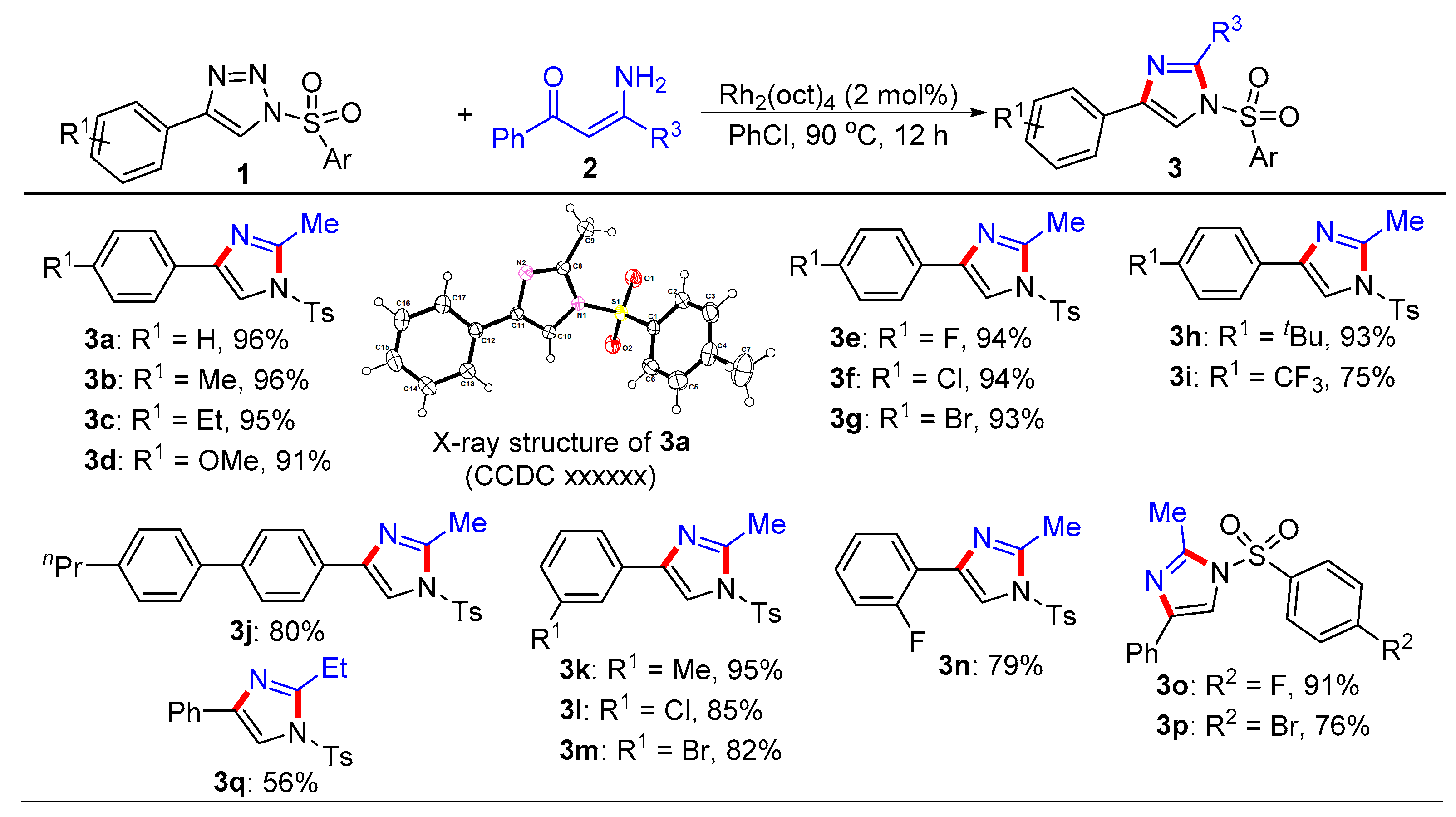
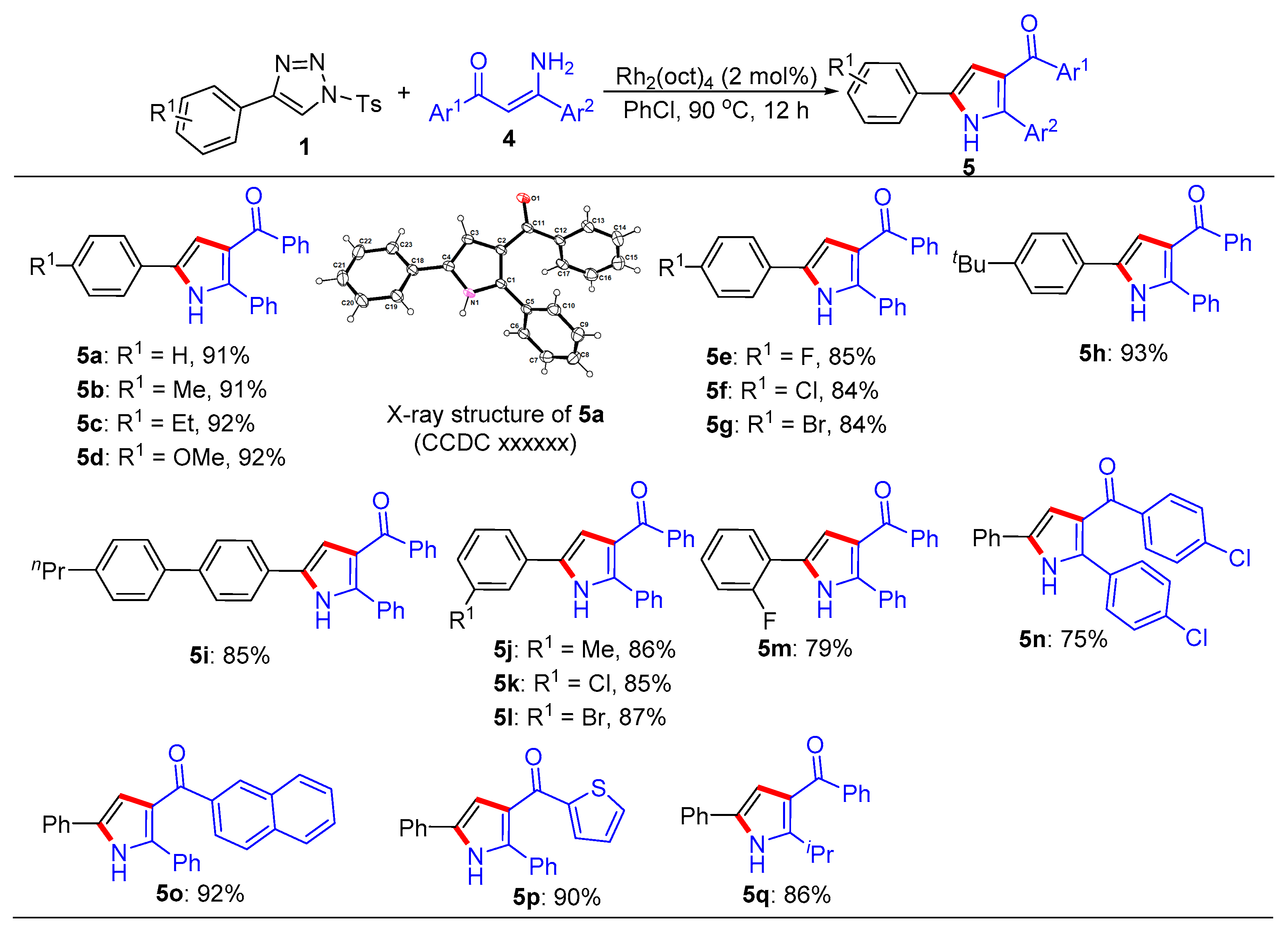
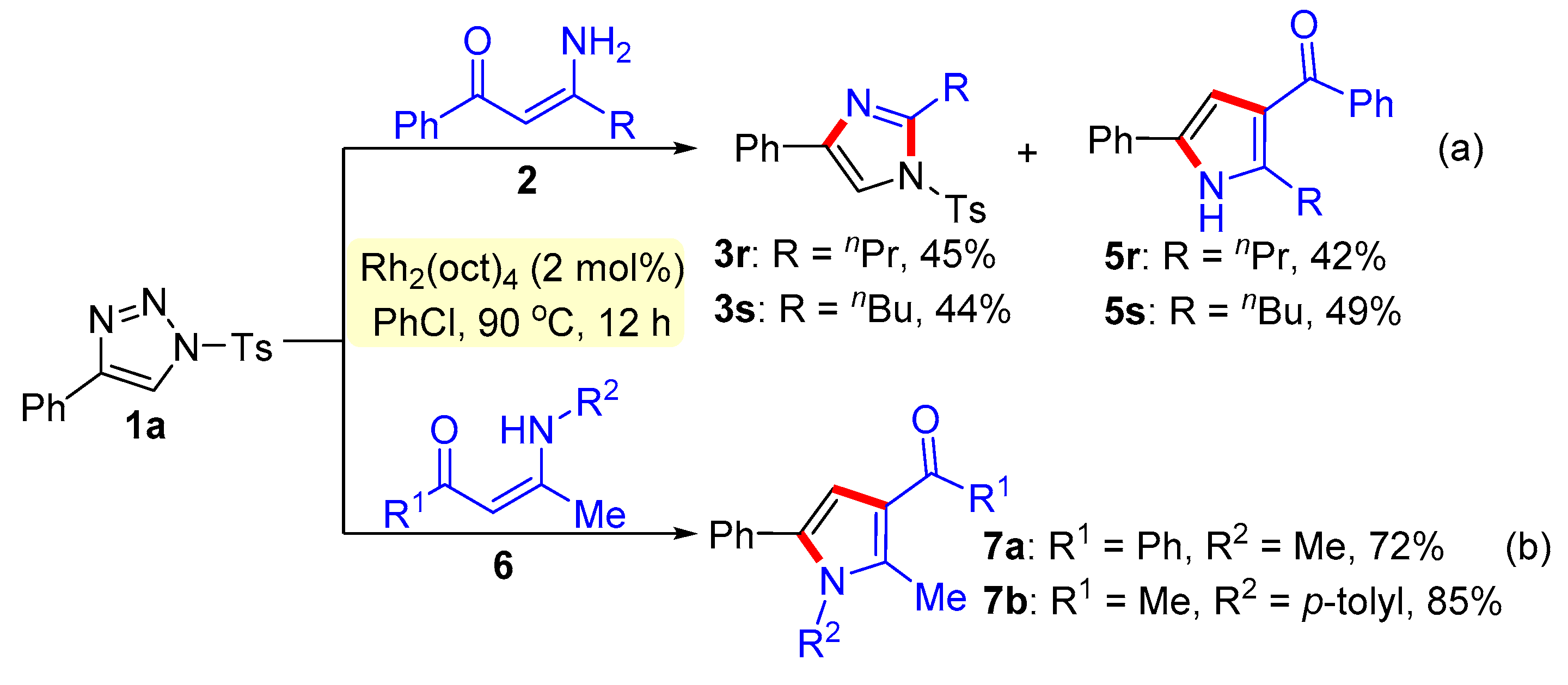
General procedure for the synthesis of trisubstituted imidazoles 3 and pyrroles 5. N-Sulfonyl-1H-1,2,3-triazoles 1 (0.2 mmol), β-enaminones 2 (0.2 mmol), and Rh2(oct)4 (2 mol%) were successively added to a Schlenk reaction tube. The reaction set was evacuated and backfilled with argon atmosphere for three times. Then chlorobenzene (2.0 mL) was added into the reaction tube through a syringe. The reaction mixture was stirred vigorously in an oil bath preheated to 90 °C for 12 hours. After the reaction was complete, the reaction mixture was cooled to room temperature, extracted with CH2Cl2 (3 × 10 mL), and washed with brine. The organic layers were combined, dried over Na2SO4 and then evaporated under vacuum. The residue was purified by flash column chromatography on silica gel (200-300 mesh) using ethyl acetate and petroleum ether (1:8, v/v) as the elution solvent to give desired products 3 or 5.
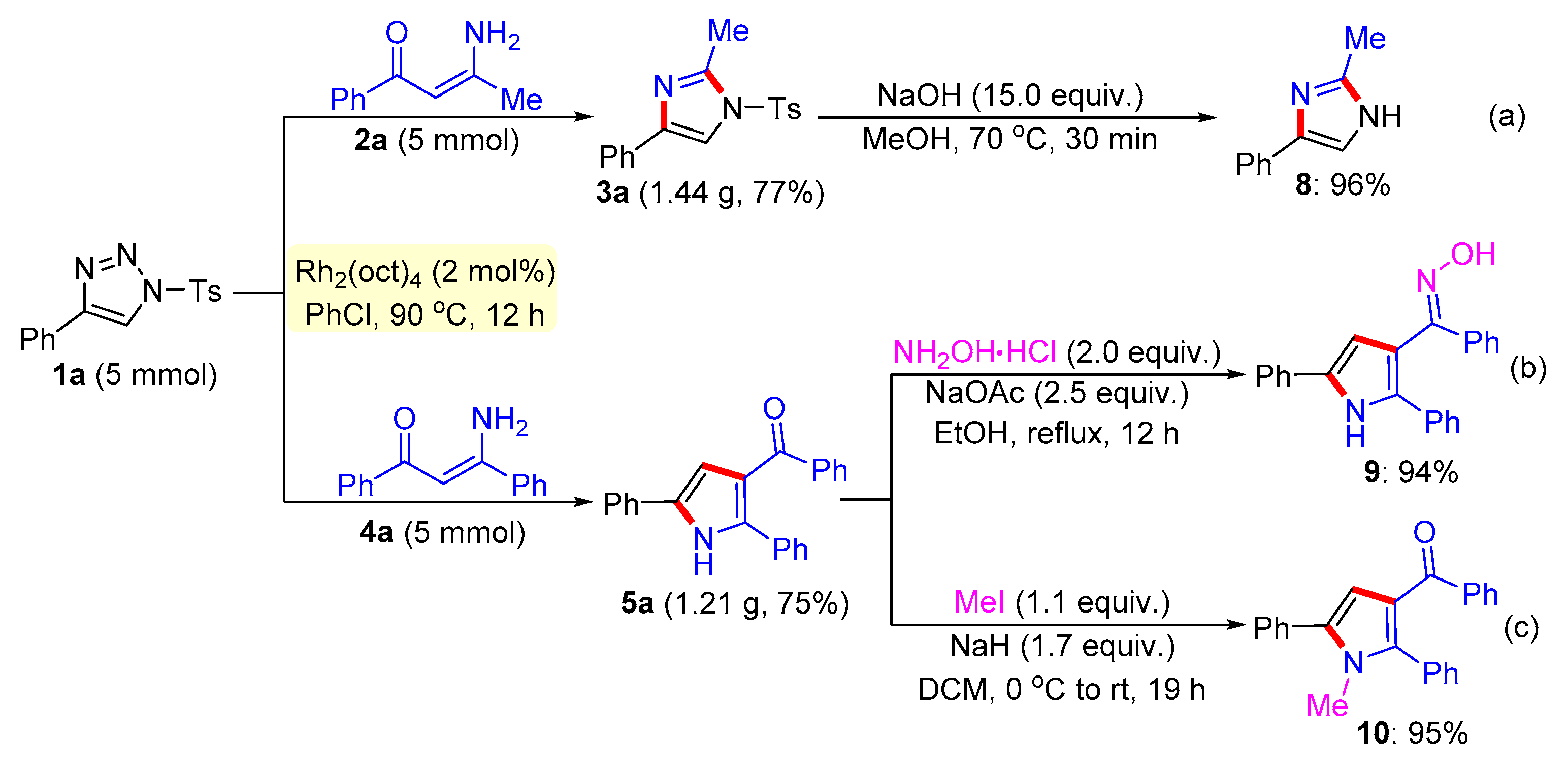
General procedure for the synthesis of compound 8. 2-Methyl-4-phenyl-1-tosyl-1H-imidazole 3a (0.15 mmol) and NaOH (2.25 mmol) were successively added to a Schlenk reaction tube. The reaction set was evacuated and backfilled with argon atmosphere for three times. Then methanol (2.0 mL) was added into the reaction tube through a syringe. The reaction mixture was stirred vigorously in an oil bath preheated to 70 °C for 30 minutes. After the reaction was complete, the reaction mixture was cooled to room temperature, extracted with CH2Cl2 (3 × 10 mL), and washed with brine. The organic layers were combined, dried over Na2SO4 and then evaporated under vacuum. The residue was purified by flash column chromatography on silica gel (200-300 mesh) using ethyl acetate and petroleum ether (1:3, v/v) as the elution solvent to give desired product 8 in 96% yield.
General procedure for the synthesis of compound 9. A mixture of (2,5-diphenyl-1H-pyrrol-3-yl)(phenyl)methanone 5a (0.2 mmol), hydroxylamine hydrochloride (0.4 mmol), and sodium acetate (0.5 mmol) were added to a round-bottomed flask with reflux condenser. Ethanol (4 mL) was then added and the reaction mixture was stirred vigorously at reflux in oil bath with stirring 12 hours. After quenching with water, the residue was extracted with ethyl acetate twice. The combined layer was washed with brine, dried over Na2SO4 and then evaporated under vacuum. The residue was purified by flash column chromatography on silica gel (200-300 mesh) using ethyl acetate and petroleum ether (1:8, v/v) as the elution solvent to give desired product 9 in 94% yield.
General procedure for the synthesis of compound 10. NaH (60% in mineral oil, 0.5 mmol, 1.7 equiv.) was added to a solution of the 5a (0.25 mmol) in DCM (4 mL) at 0 °C in portions. After stirring for 5 min at 0 °C, MeI (0.22 mmol, 1.1 equiv.) was added drop-wise and the reaction mixture was allowed to warm to room temperature and stirred for another 19 h. After quenching with water, the residue was extracted with ethyl acetate twice. The combined organic layer was washed with brine, dried over Na2SO4, filtrated and concentrated, and purified by column chromatography to afford 10 in 95% yield.
2-Methyl-4-phenyl-1-tosyl-1H-imidazole (3a). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 96% yield (60 mg); mp 122-124 °C; 1H NMR (500 MHz, CDCl3) δ 7.81 (d, J = 8.5 Hz, 2H), 7.73 (d, J = 7.0 Hz, 2H), 7.67 (s, 1H), 7.39-7.35 (m, 4H), 7.25 (d, J = 7.5 Hz, 1H), 2.57 (s, 3H), 2.44 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 146.5, 146.4, 141.0, 135.5, 132.7, 130.8, 129.1, 128.2, 127.8, 125.6, 114.4, 22.1, 15.6; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C17H17N2O2S 313.1005; Found 313.1006.
2-Methyl-4-(p-tolyl)-1-tosyl-1H-imidazole (3b). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 96% yield (62 mg); mp 60-62 °C; 1H NMR (500 MHz, CDCl3) δ 7.80 (d, J = 8.5 Hz, 2H), 7.62 (d, J = 8.0 Hz, 3H), 7.35 (d, J = 8.0 Hz, 2H), 7.18 (d, J = 8.0 Hz, 2H), 2.57 (s, 3H), 2.44 (s, 3H), 2.35 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 146.4, 146.3, 141.0, 138.0, 135.4, 130.7, 129.9, 129.8, 127.7, 125.5, 113.9, 22.1, 21.7, 15.6; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H19N2O2S 327.1162; Found 327.1170.
4-(4-Ethylphenyl)-2-methyl-1-tosyl-1H-imidazole (3c). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 95% yield (64 mg); mp 77-79 °C; 1H NMR (500 MHz, CDCl3) δ 7.80 (d, J = 8.5 Hz, 2H), 7.65 (d, J = 8.0 Hz, 3H), 7.34 (d, J = 8.0 Hz, 2H), 7.21 (d, J = 8.0 Hz, 2H), 2.64 (q, J = 7.5 Hz, 2H), 2.57 (s, 3H), 2.42 (s, 3H), 1.23 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 146.4, 146.3, 144.4, 141.0, 135.4, 130.8, 130.1, 128.6, 127.7, 125.5, 113.9, 29.1, 22.1, 15.9, 15.6; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C19H21N2O2S 341.1318; Found 341.1319.
4-(4-Methoxyphenyl)-2-methyl-1-tosyl-1H-imidazole (3d). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 91% yield (62 mg); mp 66-68 °C; 1H NMR (500 MHz, CDCl3) δ 7.80 (d, J = 8.5 Hz, 2H), 7.65 (d, J = 9.0 Hz, 2H), 7.57 (s, 1H), 7.34 (d, J = 8.0 Hz, 2H), 6.90 (d, J = 9.0 Hz, 2H), 3.81 (s, 3H), 2.56 (s, 3H), 2.42 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 159.8, 146.4, 146.3, 140.8, 135.4, 130.7, 127.7, 126.9, 125.5, 114.5, 113.2, 55.7, 22.1, 15.6; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H19N2O3S 343.1111; Found 343.1112.
4-(4-Fluorophenyl)-2-methyl-1-tosyl-1H-imidazole (3e). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 94% yield (62 mg); mp 107-109 °C; 1H NMR (500 MHz, CDCl3) δ 7.81 (d, J = 8.5 Hz, 2H), 7.69 (dd, J = 8.5, 5.0 Hz, 2H), 7.61 (s, 1H), 7.36 (d, J = 8.0 Hz, 2H), 7.06 (t, J = 8.5 Hz, 2H), 2.56 (s, 3H), 2.44 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 162.9 (d, JC-F = 246.3 Hz), 146.6, 146.5, 140.1, 135.3, 130.8, 129.0, 127.8, 127.3 (d, JC-F = 8.0 Hz), 116.0 (d, JC-F = 21.6 Hz), 114.0, 22.1, 15.6; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C17H16FN2O2S 331.0911; Found 331.0909.
4-(4-Chlorophenyl)-2-methyl-1-tosyl-1H-imidazole (3f). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 94% yield (65 mg); mp 107-109 °C; 1H NMR (500 MHz, CDCl3) δ 7.80 (d, J = 7.5 Hz, 2H), 7.66-7.65 (m, 3H), 7.36-7.32 (m, 4H), 2.56 (s, 3H), 2.43 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 146.6, 146.6, 139.9, 135.2, 133.8, 131.3, 130.8, 129.2, 127.8, 126.8, 114.5, 22.1, 15.5; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C17H16ClN2O2S 347.0616; Found 347.0612.
4-(4-Bromophenyl)-2-methyl-1-tosyl-1H-imidazole (3g). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 93% yield (72 mg); mp 104-106 °C; 1H NMR (500 MHz, CDCl3) δ 7.80 (d, J = 8.5 Hz, 2H), 7.67 (s, 1H), 7.60 (d, J = 8.5 Hz, 2H), 7.48 (d, J = 8.5 Hz, 2H), 7.35 (d, J = 8.5 Hz, 2H), 2.56 (s, 3H), 2.42 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 146.7, 146.6, 139.9, 135.2, 132.2, 131.7, 130.8, 127.8, 127.1, 122.0, 114.6, 22.1, 15.6; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C17H16BrN2O2S 391.0110; Found 391.0109.
4-(4-(tert-Butyl)phenyl)-2-methyl-1-tosyl-1H-imidazole (3h). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 93% yield (68 mg); mp 69-71 °C; 1H NMR (500 MHz, CDCl3) δ 7.79 (d, J = 7.0 Hz, 2H), 7.69-7.64 (m, 3H), 7.39 (d, J = 7.0 Hz, 2H), 7.34 (d, J = 8.0 Hz, 2H), 2.57 (s, 3H), 2.42 (s, 3H), 1.32 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 151.3, 146.4, 146.4, 141.0, 135.5, 130.7, 129.9, 127.7, 126.0, 125.3, 114.0, 35.0, 31.7, 22.1, 15.6; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C21H25N2O2S 369.1631; Found 369.1634.
2-Methyl-1-tosyl-4-(4-(trifluoromethyl)phenyl)-1H-imidazole (3i). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 75% yield (57 mg); mp 76-78 °C; 1H NMR (500 MHz, CDCl3) δ 7.84-7.82 (m, 4H), 7.76 (s, 1H), 7.62 (d, J = 8.0 Hz, 2H), 7.38 (d, J = 8.0 Hz, 2H), 2.58 (s, 3H), 2.45 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 146.8, 139.5, 136.2, 135.1, 130.9, 129.9 (q, JC-F = 32.5 Hz), 128.5, 127.9, 126.6, 126.1(q, JC-F = 3.8 Hz), 124.6(q, JC-F = 270.0 Hz), 115.6, 22.1, 15.5.; HRMS (ESI-TOF) m/z: [M + H] + Calcd for C18H16F3N2O2S 381.0879; Found 381.0878.
2-Methyl-4-(4'-propyl-[1,1'-biphenyl]-4-yl)-1-tosyl-1H-imidazole (3j). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 80% yield (69 mg); mp 94-96 °C; 1H NMR (500 MHz, CDCl3) δ 7.83-7.79 (m, 4H), 7.71(s, 1H), 7.61 (d, J = 8.5 Hz, 2H), 7.54 (d, J = 8.0 Hz, 2H), 7.36 (d, J = 8.0 Hz, 2H), 7.26 (d, J = 8.5 Hz, 2H), 2.63 (t, J = 7.5 Hz, 2H), 2.59 (s, 3H), 1.72-1.64 (m, 2H), 0.98 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 146.5, 146.5, 142.4, 140.9, 140.7, 138.4, 135.4, 131.4, 130.8, 129.3, 127.8, 127.6, 127.1, 125.9, 114.3, 38.1, 25.0, 22.1, 15.6, 14.3; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C26H27N2O2S 431.1788; Found 431.1781.
2-Methyl-4-(m-tolyl)-1-tosyl-1H-imidazole (3k). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 95% yield (62 mg); mp 107-109 °C; 1H NMR (500 MHz, CDCl3) δ 7.80 (d, J = 8.5 Hz, 2H), 7.67 (s, 1H), 7.58 (s, 1H), 7.51 (d, J = 7.5 Hz, 1H), 7.35 (d, J = 8.0 Hz, 2H), 7.26 (t, J = 7.5 Hz, 1H), 7.09 (d, J = 7.5 Hz, 1H), 2.57 (s, 3H), 2.43 (s, 3H), 2.37 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 146.5, 146.4, 141.0, 138.8, 135.4, 132.5, 130.8, 129.0, 127.8, 126.2, 122.6, 114.3, 22.1, 21.8, 15.6; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H19N2O2S 327.1162; Found 327.1163.
4-(3-Chlorophenyl)-2-methyl-1-tosyl-1H-imidazole (3l). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 85% yield (59 mg); mp 83-85 °C; 1H NMR (500 MHz, CDCl3) δ 7.81 (d, J = 8.0 Hz, 2H), 7.73 (s, 1H), 7.68 (s, 1H), 7.59 (d, J = 7.5 Hz, 1H), 7.37 (d, J = 8.5 Hz, 2H), 7.29 (t, J = 8.0 Hz, 1H), 7.24 (d, J = 8.0 Hz, 1H), 2.56 (s, 3H), 2.44 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 146.7, 139.7, 135.1, 134.6, 130.8, 130.3, 128.1, 127.8, 125.7, 123.6, 115.0, 22.1, 15.5; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C17H16ClN2O2S 347.0616; Found 347.0625.
4-(3-Bromophenyl)-2-methyl-1-tosyl-1H-imidazole (3m). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 82% yield (64 mg); mp 79-81 °C; 1H NMR (500 MHz, CDCl3) δ 7.89 (s, 1H), 7.80 (d, J = 8.5 Hz, 2H), 7.67 (s, 1H), 7.64 (d, J = 7.5 Hz, 1H), 7.39-7.35 (m, 3H), 7.22 (t, J = 8.0 Hz, 1H), 2.56 (s, 3H), 2.43 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 146.7, 146.6, 139.5, 135.2, 134.8, 131.0, 130.8, 130.6, 128.6, 127.8, 124.1, 123.3, 115.0, 22.1, 15.5; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C17H16BrN2O2S 391.0110; Found 391.0119.
4-(2-Fluorophenyl)-2-methyl-1-tosyl-1H-imidazole (3n). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 79% yield (52 mg); mp 57-59 °C; 1H NMR (500 MHz, CDCl3) δ 8.03 (t, J = 7.5 Hz, 1H), 7.85 (d, J = 4.0 Hz, 1H), 7.82 (d, J = 8.0 Hz, 2H), 7.35 (d, J = 8.0 Hz, 2H), 7.23 (t, J = 7.0 Hz, 1H), 7.17 (t, J = 7.5 Hz, 1H), 7.10 (t, J = 10 Hz, 1H), 2.58 (s, 3H), 2.43 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 160.2 (d, JC-F = 247.9 Hz), 146.5, 145.9, 135.4, 134.5, 130.8, 129.1 (d, JC-F = 8.5 Hz), 128.2 (d, JC-F = 3.6 Hz), 127.8, 124.7 (d, JC-F = 3.6 Hz), 120.6 (d, JC-F = 12.5 Hz), 118.4 (d, JC-F = 15.4 Hz), 116.0 (d, JC-F = 21.5 Hz), 22.1, 15.5; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C17H16FN2O2S 331.0911; Found 331.0914.
1-((4-Fluorophenyl)sulfonyl)-2-methyl-4-phenyl-1H-imidazole (3o). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 91% yield (57 mg); mp 107-109 °C; 1H NMR (500 MHz, CDCl3) δ 7.97-7.94 (m, 2H), 7.73 (d, J = 7.5 Hz, 2H), 7.67 (s, 1H), 7.38 (t, J = 8.0 Hz, 2H), 7.29 (t, J = 7.5 Hz, 1H), 7.24 (t, J = 8.0 Hz, 2H), 2.58 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 166.6 (d, JC-F = 257.5 Hz), 146.4, 141.3, 134.4 (d, JC-F = 2.8 Hz), 132.5, 130.7 (d, JC-F = 9.8 Hz), 129.1, 128.4, 125.6, 117.7 (d, JC-F = 22.9 Hz), 114.2, 15.7; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C16H14FN2O2S 317.0755; Found 317.0760.
1-((4-Bromophenyl)sulfonyl)-2-methyl-4-phenyl-1H-imidazole (3p). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 76% yield (57 mg); mp 97-99 ℃; 1H NMR (500 MHz, CDCl3) δ 7.78 (d, J = 9.0 Hz, 2H), 7.72 (t, J = 9.0 Hz, 4H), 7.65 (s, 1H), 7.38 (d, J = 7.5 Hz, 2H), 7.29 (d, J = 7.5 Hz, 1H), 2.58 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 146.5, 141.4, 137.3, 133.6, 132.4, 130.6, 129.1, 129.1, 128.4, 125.6, 114.2, 15.7; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C16H14BrN2O2S 376.9954; Found 376.9952.
2-Ethyl-4-phenyl-1-tosyl-1H-imidazole (3q). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 56% yield (37 mg); mp 49-51 °C; 1H NMR (500 MHz, CDCl3) δ 7.79 (d, J = 8.5 Hz, 2H), 7.75 (d, J = 7.0 Hz, 2H), 7.67 (s, 1H), 7.39-7.34 (m, 4H), 7.28 (d, J = 7.5 Hz, 1H), 2.90 (q, J = 7.5 Hz, 2H), 2.43 (s, 3H), 1.32 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 151.4, 146.4, 140.9, 135.7, 132.9, 130.7, 129.0, 128.1, 127.7, 125.6, 114.3, 22.4, 22.1, 12.5; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H19N2O2S 327.1162; Found 327.1163.
4-Phenyl-2-propyl-1-tosyl-1H-imidazole (3r). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a colorless oil in 45% yield (31 mg); 1H NMR (500 MHz, CDCl3) δ 7.79 (d, J = 8.5 Hz, 2H), 7.75 (d, J = 7.0 Hz, 2H), 7.66 (s, 1H), 7.38-7.33 (m, 4H), 7.27 (t, J = 7.5 Hz, 1H), 2.85 (t, J = 7.5 Hz, 2H), 2.43 (s, 3H), 1.81-1.74 (m, 2H), 0.98 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 150.4, 146.4, 141.0, 135.8, 132.9, 130.7, 129.0, 128.1, 127.6, 125.6, 114.3, 30.8, 22.1, 21.9, 14.3; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C19H21N2O2S 341.1318; Found 341.1312.
2-Butyl-4-phenyl-1-tosyl-1H-imidazole (3s). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a colorless oil in 44% yield (31 mg); 1H NMR (500 MHz, CDCl3) δ 7.79 (d, J = 8.5 Hz, 2H), 7.75 (d, J = 7.5 Hz, 2H), 7.67 (s, 1H), 7.38-7.33 (m, 4H), 7.28 (d, J = 7.5 Hz, 1H), 2.87 (t, J = 8 Hz, 2H), 2.43 (s, 3H), 1.72-1.69 (m, 2H), 1.42-1.37 (m, 2H), 0.91 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 150.5, 146.4, 140.9, 135.7, 132.9, 130.7, 129.1, 128.2, 127.7, 125.6, 114.3, 30.5, 28.6, 22.9, 22.1, 14.2; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C20H23N2O2S 355.1475; Found 355.1482.
(2,5-Diphenyl-1H-pyrrol-3-yl)(phenyl)methanone (5a). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 91% yield (59 mg); mp 81-83 °C; 1H NMR (500 MHz, CDCl3) δ 8.98 (s, 1H), 7.80 (d, J = 7.0 Hz, 2H), 7.54 (d, J = 7.5 Hz, 2H), 7.45-7.42 (m, 3H), 7.39 (t, J = 7.5 Hz, 2H), 7.32 (t, J = 7.5 Hz, 2H), 7.29-7.24 (m, 4H), 6.84 (d, J = 3.0 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 192.9, 139.8, 138.3, 132.3, 132.1, 131.8, 130.1, 129.5, 128.9, 128.8, 128.5, 128.3, 127.5, 124.5, 122.3, 110.9; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C23H18NO 324.1383; Found 324.1382.
Phenyl(2-phenyl-5-(p-tolyl)-1H-pyrrol-3-yl)methanone (5b). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 91% yield (61 mg); mp 81-83 °C; 1H NMR (500 MHz, CDCl3) δ 9.12 (s, 1H), 7.79 (d, J = 8.0 Hz, 2H), 7.47 – 7.38 (m, 5H), 7.31 (t, J = 7.7 Hz, 2H), 7.21 - 7.17 (m, 5H), 6.78 (s, 1H), 2.36 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.1, 139.8, 138.1, 137.3, 132.5, 132.2, 132.1, 130.1, 130.1, 129.1, 128.9, 128.7, 128.4, 128.3, 124.6, 122.2, 110.4, 21.6; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C24H20NO 338.1539; Found 338.1545.
(5-(4-Ethylphenyl)-2-phenyl-1H-pyrrol-3-yl)(phenyl)methanone (5c). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 92% yield (64 mg); mp 71-73 °C; 1H NMR (500 MHz, CDCl3) δ 8.92 (s, 1H), 7.80 (d, J = 7.0 Hz, 2H), 7.47-7.42 (m, 5H), 7.32 (t, J = 7.5 Hz, 2H), 7.26-7.22 (m, 5H), 6.81 (d, J = 3.0 Hz, 1H), 2.67 (q, J = 7.5 Hz, 2H), 1.26 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 193.0, 143.8, 139.8, 138.0, 132.5, 132.2, 132.1, 130.1, 129.3, 128.9, 128.9, 128.7, 128.4, 128.3, 124.6, 122.2, 110.4, 29.0, 15.9; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C25H22NO 352.1693; Found 352.1689.
(5-(4-Methoxyphenyl)-2-phenyl-1H-pyrrol-3-yl)(phenyl)methanone (5d). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 92% yield (65 mg); mp 111-113 °C; 1H NMR (500 MHz, CDCl3) δ 8.96 (s, 1H), 7.79 (d, J = 7.5 Hz, 2H), 7.46 (d, J = 8.5 Hz, 2H), 7.41 (d, J = 7.0 Hz, 3H), 7.31 (t, J = 7.5 Hz, 2H), 7.22 (d, J = 7.5 Hz, 3H), 6.92 (d, J = 8.5 Hz, 2H), 6.72 (d, J = 3.0 Hz, 1H), 3.82 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.1, 159.3, 139.9, 137.9, 132.4, 132.3, 132.1, 130.1, 128.9, 128.7, 128.3, 128.3, 126.0, 124.8, 122.2, 114.9, 109.8, 55.8; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C24H20NO 354.1489; Found 354.1489.
(5-(4-Fluorophenyl)-2-phenyl-1H-pyrrol-3-yl)(phenyl)methanone (5e). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 85% yield (58 mg); mp 104-106 °C; 1H NMR (500 MHz, CDCl3) δ 8.93 (s, 1H), 7.78 (d, J = 7.0 Hz, 2H), 7.50 (dd, J = 9.0, 5.0 Hz, 2H), 7.45-7.41 (m, 3H), 7.31 (t, J = 7.5 Hz, 2H), 7.24-7.22 (m, 3H), 7.08 (t, J = 8.5 Hz, 2H), 6.76 (d, J = 7.5 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 192.9, 162.4 (d, JC-F = 245.0 Hz), 139.7, 138.3, 132.2, 132.1, 131.5, 130.1, 128.8 (d, JC-F = 6.3 Hz), 128.6, 128.3, 128.2, 126.4, 126.3, 122.4, 116.5 (d, JC-F = 22.5 Hz), 110.7; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C23H17FNO 342.1289; Found 342.1287.
(5-(4-Chlorophenyl)-2-phenyl-1H-pyrrol-3-yl)(phenyl)methanone (5f). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 84% yield (60 mg); mp 112-114 ℃; 1H NMR (500 MHz, CDCl3) δ 9.04 (s, 1H), 7.77 (d, J = 7.0Hz, 2H), 7.46 – 7.42 (m, 3H), 7.39 (dd, J = 6.5, 3.0 Hz, 2H), 7.35-7.30 (m, 4H), 7.23 – 7.19 (m, 3H), 6.79 (d, J = 3.0 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 192.9, 139.6, 138.6, 133.1, 132.2, 131.9, 131.3, 130.4, 130.1, 129.6, 128.9, 128.8, 128.6, 128.3, 125.8, 122.4, 111.2; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C23H17ClNO 358.0993; Found 358.1002.
(5-(4-Bromophenyl)-2-phenyl-1H-pyrrol-3-yl)(phenyl)methanone (5g). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 84% yield (67 mg); mp 127-129 °C; 1H NMR (500 MHz, CDCl3) δ 8.77 (s, 1H), 7.79 (d, J = 8.5 Hz, 2H), 7.52 (d, J = 8.5 Hz, 2H), 7.46-7.43 (m, 3H), 7.40 (d, J = 8.5 Hz, 2H), 7.33 (t, J = 7.5 Hz, 2H), 7.29-7.27 (m, 3H), 6.85 (d, J = 7.5 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 193.0, 139.6, 138.7, 132.5, 132.3, 131.9, 131.3, 130.8, 130.1, 128.9, 128.7, 128.6, 128.3, 126.1, 122.4, 121.1, 111.3; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C23H17BrNO 402.0488; Found 402.0489.
(5-(4-(tert-Butyl)phenyl)-2-phenyl-1H-pyrrol-3-yl)(phenyl)methanone (5h). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 93% yield (70 mg); mp 98-100 °C; 1H NMR (500 MHz, CDCl3) δ 8.89 (s, 1H), 7.80 (d, J = 8.0 Hz, 2H), 7.49 – 7.40 (m, 7H), 7.32 (t, J = 8.0 Hz, 2H), 7.28 – 7.23 (m, 3H), 6.82 (s, 1H), 1.34 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 192.9, 150.7, 139.8, 138.0, 132.4, 132.3, 132.1, 130.1, 129.1, 128.9, 128.8, 128.4, 128.3, 126.4, 124.3, 122.3, 110.5, 35.0, 31.7; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C27H26NO 380.2009; Found 380.2008.
Phenyl(2-phenyl-5-(4'-propyl-[1,1'-biphenyl]-4-yl)-1H-pyrrol-3-yl)methanone (5i). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 85% yield (72 mg); mp 149-151 °C; 1H NMR (500 MHz, CDCl3) δ 9.43 (s, 1H), 7.80 (d, J = 7.5 Hz, 2H), 7.59 (s, 4H), 7.52 (d, J = 8.0 Hz, 2H), 7.44 (t, J = 7.5 Hz, 1H), 7.41-7.36 (m, 2H), 7.32 (t, J = 7.5 Hz, 2H), 7.25 (d, J = 8.0 Hz, 2H), 7.20 – 7.15 (m, 3H), 6.85 (d, J = 3.0 Hz, 1H), 2.64 (d, J = 8.0 Hz, 2H), 1.69 (m, 2H), 0.99 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 193.3, 142.5, 140.0, 139.8, 138.7, 138.2, 132.3, 132.2, 132.1, 130.5, 130.2, 129.4, 129.0, 128.7, 128.4, 128.3, 127.8, 127.1, 125.0, 122.3, 110.9, 38.1, 25.0, 14.3; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C32H28NO 442.2165; Found 442.2170.
Phenyl(2-phenyl-5-(m-tolyl)-1H-pyrrol-3-yl)methanone (5j). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 86% yield (58 mg); mp 118-120 °C; 1H NMR (500 MHz, CDCl3) δ 9.08 (s, 1H), 7.80 (d, J = 7.5 Hz, 2H), 7.43 (d, J = 6.0 Hz, 3H), 7.37-7.31 (m, 4H), 7.27 (d, J = 7.5 Hz, 1H), 7.22 (t, J = 6.0 Hz, 3H), 7.08 (d, J = 7.5 Hz, 1H), 6.82 (d, J = 2.5 Hz, 1H), 2.38 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.1, 139.8, 139.0, 138.4, 132.5, 132.2, 132.1, 131.8, 130.1, 129.3, 128.9, 128.7, 128.4, 128.3, 125.4, 122.2, 121.7, 110.8, 21.9; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C24H20NO 338.1539; Found 338.1531.
(5-(3-Chlorophenyl)-2-phenyl-1H-pyrrol-3-yl)(phenyl)methanone (5k). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 85% yield (60 mg); mp 83-85 °C; 1H NMR (500 MHz, CDCl3) δ 9.19 (s, 1H), 7.78 (d, J = 7.5 Hz, 2H), 7.51 (s, 1H), 7.44 (t, J = 7.5 Hz, 1H), 7.41-7.38 (m, 3H), 7.34-7.27 (m, 3H), 7.23 – 7.18 (m, 4H), 6.80 (d, J = 2.5 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 193.0, 139.6, 138.9, 135.4, 133.6, 132.3, 131.8, 130.9, 130.6, 130.1, 128.9, 128.7, 128.6, 128.4, 127.3, 124.6, 122.6, 122.3, 111.7; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C23H17ClNO 358.0993; Found 358.0992.
(5-(3-Bromophenyl)-2-phenyl-1H-pyrrol-3-yl)(phenyl)methanone (5l). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 87% yield (69 mg); mp 99-101 °C; 1H NMR (500 MHz, CDCl3) δ 9.10 (s, 1H), 7.78 (d, J = 7.0 Hz, 2H), 7.67 (s, 1H), 7.46 – 7.40 (m, 4H), 7.37 (d, J = 8.0 Hz, 1H), 7.33 (t, J = 8.0 Hz, 2H), 7.25 – 7.21 (m, 4H), 6.81 (d, J = 2.5 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 192.9, 139.6, 138.9, 133.9, 132.3, 131.8, 130.9, 130.7, 130.2, 130.1, 128.9, 128.8, 128.7, 128.4, 127.4, 123.6, 123.1, 122.4, 111.7; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C23H17BrNO 402.0488; Found 402.0489.
(5-(2-Fluorophenyl)-2-phenyl-1H-pyrrol-3-yl)(phenyl)methanone (5m). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 79% yield (54 mg); mp 77-79 °C; 1H NMR (500 MHz, CDCl3) δ 9.39 (s, 1H), 7.81 (d, J = 7.0 Hz, 2H), 7.64 (t, J = 8.0 Hz, 1H), 7.48 - 7.44 (m, 3H), 7.34 (t, J = 8.0 Hz, 2H), 7.29 - 7.26 (m, 3H), 7.23 – 7.13 (m, 3H), 6.98 (d, J = 3.0 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ192.8, 159.2 (d, JC-F = 124.1 Hz), 139.7, 138.3, 132.2, 132.0, 130.1, 128.8, 128.6, 128.57 (d, JC-F = 8.5 Hz), 128.4, 127.3 (d, JC-F = 4.0 Hz), 127.1, 125.3 (d, JC-F = 3.0 Hz), 121.7, 119.4, 119.3, 116.8 (d, JC-F = 23.8 Hz), 112.7; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C23H17FNO 342.1289; Found 342.1281.
(4-Chlorophenyl)(2-(4-chlorophenyl)-5-phenyl-1H-pyrrol-3-yl) methanone (5n). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 75% yield (58 mg); mp 94-96 °C; 1H NMR (500 MHz, CDCl3) δ 8.75 (s, 1H), 7.77 (d, J = 9.0 Hz, 2H), 7.53 (d, J = 7.0 Hz, 2H), 7.46 (d, J = 8.5 Hz, 2H), 7.42 (t, J = 8.0 Hz, 2H), 7.34 (m, 4H), 6.80 (d, J = 2.5 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 191.2, 138.7, 138.0, 136.8, 134.8, 132.7, 131.4, 130.5, 130.0, 129.6, 129.2, 128.8, 127.9, 124.6, 122.3, 110.8; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C23H16Cl2NO 392.0603; Found 392.0612.
(2,5-Diphenyl-1H-pyrrol-3-yl) (naphthalen-2-yl) methanone (5o). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 92% yield (68 mg); mp 107-109 °C; 1H NMR (500 MHz, CDCl3) δ 8.81 (s, 1H), 8.34 (s, 1H), 7.94 (d, J = 8.5 Hz, 1H), 7.83 (t, J = 8.0 Hz, 3H), 7.60 – 7.52 (m, 5H), 7.49 (d, J = 7.0 Hz, 1H), 7.42 (t, J = 8.0 Hz, 2H), 7.31 – 7.26 (m, 3H), 7.22 (d, J = 7.5 Hz, 1H), 6.92 (d, J = 2.5 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 192.7, 138.1, 137.0, 135.4, 132.7, 132.3, 132.2, 131.8, 131.7, 129.7, 129.5, 128.9, 128.8, 128.6, 128.2, 128.2, 128.1, 127.6, 126.8, 126.1, 124.5, 122.6, 111.0; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C27H20NO 374.1539; Found 374.1537.
(2,5-Diphenyl-1H-pyrrol-3-yl) (thiophen-2-yl) methanone (5p). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 90% yield (59 mg); mp 86-88 °C; 1H NMR (500 MHz, CDCl3) δ 8.77 (s, 1H), 7.66 (d, J = 3.5 Hz, 1H), 7.60 – 7.54 (m, 5H), 7.42 (t, J = 8.0 Hz, 2H), 7.36 (t, J = 7.0 Hz, 2H), 7.33 – 7.28 (m, 2H), 7.04 (dd, J = 5.0, 4.0 Hz, 1H), 6.99 (d, J = 3.0 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 184.1, 145.9, 137.4, 134.1, 133.2, 132.4, 132.1, 131.8, 129.5, 129.0, 128.7, 128.6, 128.0, 127.6, 124.6, 122.3, 110.2; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C21H16NOS 330.0947; Found 330.0955.
(2-Isopropyl-5-phenyl-1H-pyrrol-3-yl) (phenyl)methanone (5q). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 86% yield (50 mg); mp 85-87 °C; 1H NMR (500 MHz, CDCl3) δ 8.79 (s, 1H), 7.84 (d, J = 7.0 Hz, 2H), 7.53 (t, J = 7.0 Hz, 1H), 7.48 – 7.44 (m, 4H), 7.36 (t, J = 7.5 Hz, 2H), 7.23 (t, J = 7.5 Hz, 1H), 6.64 (d, J = 3.0 Hz, 1H), 3.87 (m, 1H), 1.37 (d, J = 7.0 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ 192.8, 147.8, 141.1, 132.2, 131.6, 129.8, 129.5, 129.4, 128.5, 127.2, 124.3, 120.2, 110.1, 26.8, 22.4; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C20H20NO 290.1539; Found 290.1530.
Phenyl(5-phenyl-2-propyl-1H-pyrrol-3-yl) methanone (5r). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 42% yield (24 mg); mp 74-76 °C; 1H NMR (500 MHz, CDCl3) δ 8.68 (s, 1H), 7.87 – 7.82 (m, 2H), 7.53 (t, J = 7.5 Hz, 1H), 7.49 – 7.44 (m, 4H), 7.39 – 7.34 (m, 2H), 7.23 (t, J = 7.5 Hz, 1H), 6.66 (d, J = 3.0 Hz, 1H), 3.02 (t, J = 7.5 Hz, 2H), 1.76 (m, 2H), 1.60 (s, 3H), 1.01 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 192.7, 142.4, 141.0, 132.1, 131.6, 130.0, 129.5, 129.4, 128.5, 127.1, 124.2, 121.2, 109.8, 30.1, 23.1, 14.4; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C20H20NO 290.1539; Found 290.1542.
(2-Butyl-5-phenyl-1H-pyrrol-3-yl) ( phenyl)methanone (5s). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 49% yield (30 mg); mp 74-76 °C; 1H NMR (500 MHz, CDCl3) δ 9.24 (s, 1H), 7.85 (d, J = 8.0 Hz, 2H), 7.55 - 7.45 (m, 5H), 7.34 (t, J = 7.0 Hz, 2H), 7.21 (t, J = 7.5 Hz, 1H), 6.67 (s, 1H), 3.01 (t, J = 7.5 Hz, 2H), 1.7.-1.64 (m, 2H), 1.39 - 1.32 (m, 2H), 0.89 (t, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 192.7, 142.7, 140.7, 131.8, 131.3, 129.8, 129.1, 129.0, 128.1, 126.6, 123.9, 120.6, 109.4, 31.7, 27.5, 22.6, 14.0, 13.9; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C21H22NO 304.1696; Found 304.1702.
(1,2-Dimethyl-5-phenyl-1H-pyrrol-3-yl) (phenyl)methanone (7a). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 72% yield (40 mg); mp 96-98 °C; 1H NMR (500 MHz, CDCl3) δ 7.64 (d, J = 7.0 Hz, 2H), 7.26 (t, J = 7.5Hz, 1H), 7.14 (t, J = 7.5 Hz, 2H), 7.04 (q, J = 8.0 Hz, 4H), 6.98 (d, J = 6.5 Hz, 1H), 6.64 (s, 1H), 3.62 (s, 3H), 2.38 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 194.4, 140.0, 135.8, 135.6, 131.9, 130.2, 128.8, 128.2, 128.0, 126.2, 125.9, 120.2, 120.1, 34.2, 11.7; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C19H18NO 276.1383; Found 276.1388.
1-(2-Methyl-5-phenyl-1-(p-tolyl)-1H-pyrrol-3-yl) ethan-1-one (7b). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a yellow solid in 85% yield (49 mg); mp 66-68 °C; 1H NMR (500 MHz, CDCl3) δ 7.38 (d, J = 4.0 Hz, 4H), 7.32 – 7.27 (m, 3H), 7.21 (d, J = 8.5 Hz, 2H), 6.64 (s, 1H), 2.42 (s, 3H), 2.39 (s, 3H), 2.07 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 198.1, 138.5, 136.6, 136.5, 135.8, 130.3, 129.7, 128.7, 127.2, 126.6, 126.4, 122.8, 121.1, 31.5, 21.5, 13.3; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C20H20NO 290.1539; Found 290.1538.
2-Methyl-4-phenyl-1H-imidazole (8). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 3) to afford a white solid in 96% yield (30 mg); mp 57-59 °C; 1H NMR (500 MHz, CDCl3) δ 7.67 (d, J = 7.0 Hz, 2H), 7.37 (s, 1H), 7.30 (t, J = 7.5Hz, 2H), 7.13 (t, J = 7.5 Hz, 1H), 2.29 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 145.7, 138.2, 133.2, 129.1, 127.2, 125.1, 115.6, 14.2; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C10H11N2 159.0917; Found 159.091.
(E)-(2,5-Diphenyl-1H-pyrrol-3-yl) (phenyl)methanone oxime (9). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a white solid in 94% yield (63 mg); mp 95-97 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.44 (s, 1H), 11.19 (s, 1H), 7.79 (d, J = 7.5 Hz, 2H), 7.49 (d, J = 7.5 Hz, 4H), 7.37 (t, J = 8.0 Hz, 2H), 7.26-7.23 (m, 5H), 7.19 (t, J = 7.5 Hz, 1H), 7.12 (t, J = 7.5 Hz, 1H), 6.52 (d, J = 3.0 Hz, 1H); 13C NMR (125 MHz, DMSO-d6) δ 153.7, 137.7, 133.2, 132.8, 131.6, 129.6, 129.5, 129.1, 129.0, 128.8, 127.4, 127.2, 127.0, 126.5, 124.8, 115.1, 109.2; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C23H19N2O 339.1492; Found 339.1496.
(1-Methyl-2,5-diphenyl-1H-pyrrol-3-yl) (phenyl)methanone (10). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1: 8) to afford a colorless oil in 98% yield (66 mg); 1H NMR (500 MHz, CDCl3) δ 7.76 (d, J = 7.0 Hz, 2H), 7.50 (d, J = 8.0 Hz, 2H), 7.45 (t, J = 8.0 Hz, 2H), 7.40 – 7.35 (m, 5H), 7.34 (s, 1H), 7.32 – 7.27 (m, 3H), 6.67 (s, 1H), 3.49 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 192.3, 140.8, 140.1, 135.7, 132.9, 132.3, 131.6, 131.2, 129.8, 129.4, 129.0, 128.6, 128.5, 128.1, 128.1, 122.3, 112.3, 34.3; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C24H20NO 338.1539; Found 338.1544.