Submitted:
11 May 2023
Posted:
12 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
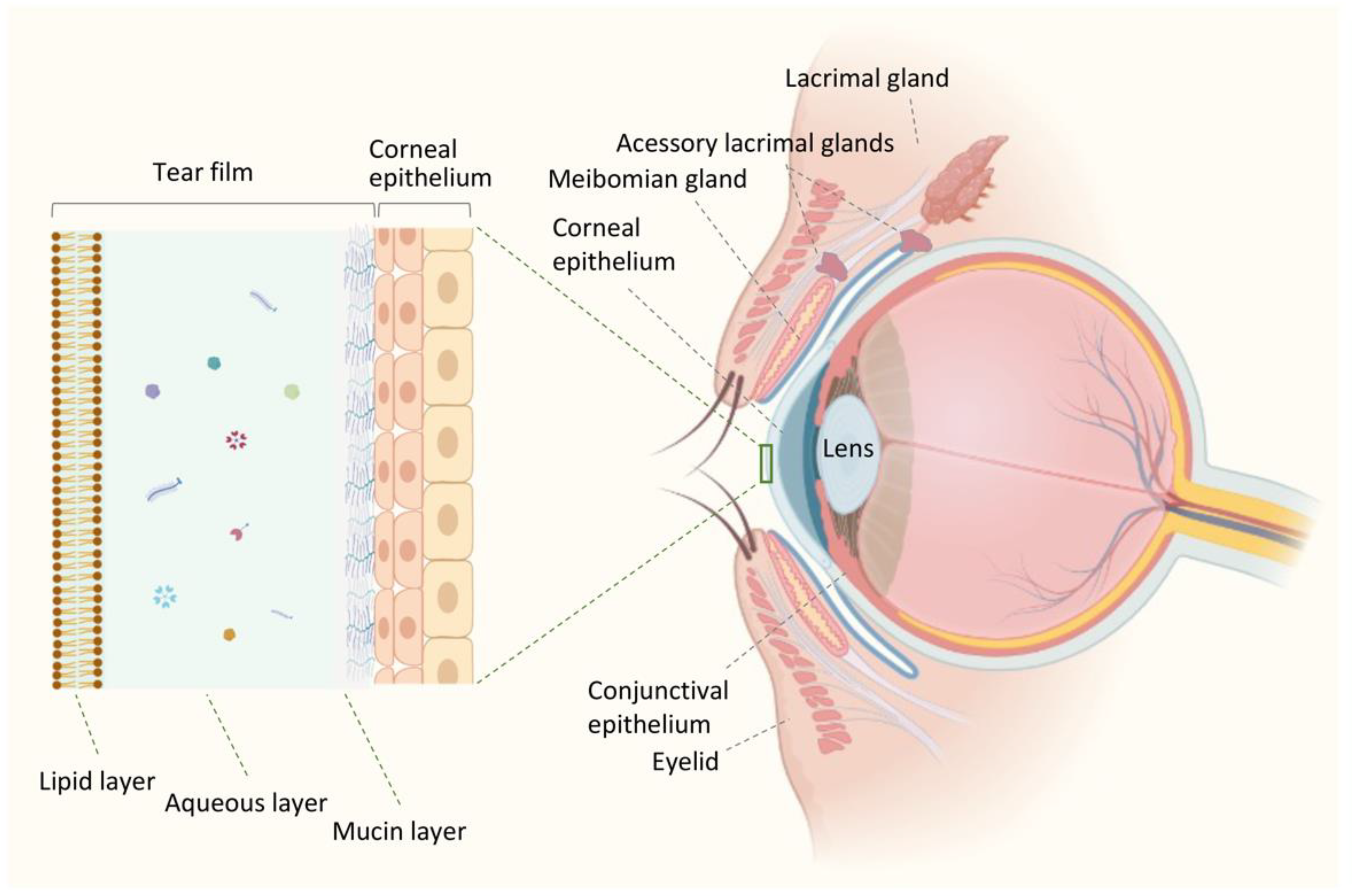
2. Ocular surface environment in health
3. Ocular surface innate immunity
4. Homeostasis of the innate immune network in the retina
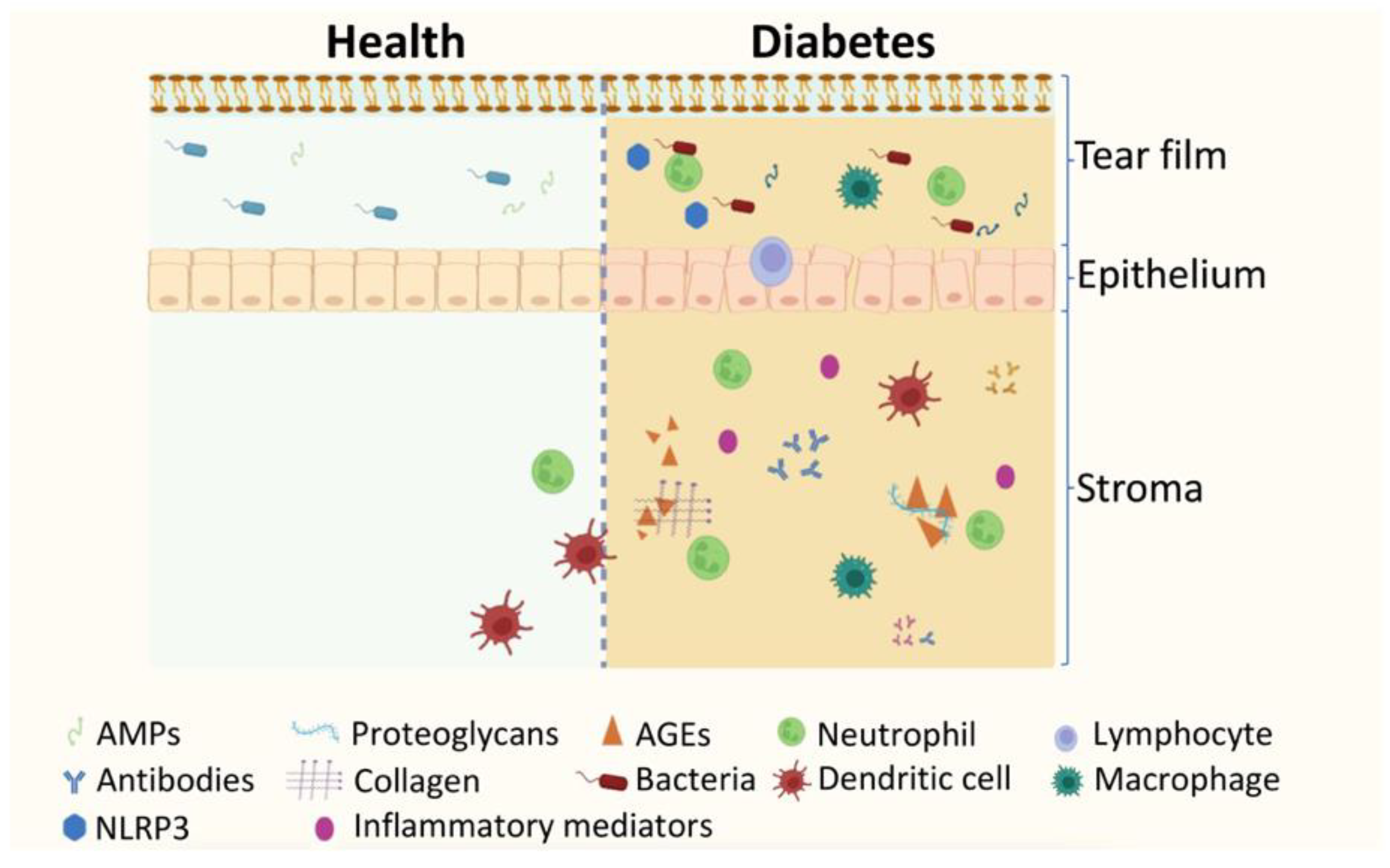
5. The impact of diabetes and DR on ocular surface and tear protein profile
6. Evidence of changes in ocular surface and immune response in experimental models of diabetes
7. Mechanisms of DR neurodegeneration and vascular lesions
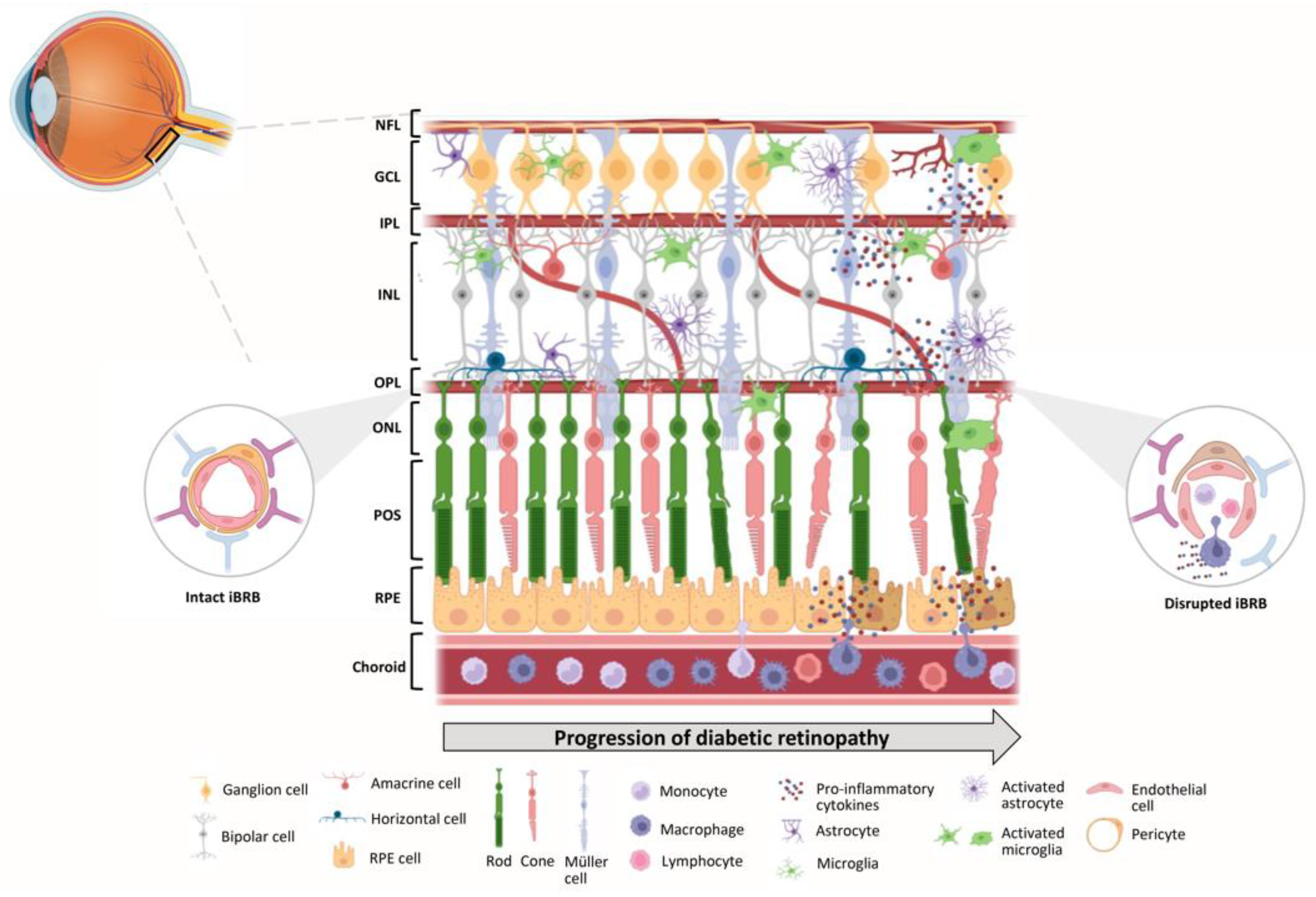
8. The contribution of the immune response to DR in preclinical models
8. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Magliano: D. J.; Boyko, E.J. In IDF DIABETES ATLAS, 10th ed.; Brussels, 2021.
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Groop, P.H.; Handelsman, Y.; Insel, R.A.; Mathieu, C.; et al. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes 2017, 66, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Suwajanakorn, O.; Puangsricharern, V.; Kittipibul, T.; Chatsuwan, T. Ocular surface microbiome in diabetes mellitus. Sci R 2022, 12, 21527. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, L.; Wang, Q.; Li, Y.; Wei, C.; Xie, L. Mechanistic investigations of diabetic ocular surface diseases. Front Endocrinol (Lausanne) 2022, 13, 1079541. [Google Scholar] [CrossRef] [PubMed]
- Ljubimov, A.V. Diabetic complications in the cornea. Vision Res 2017, 139, 138–152. [Google Scholar] [CrossRef]
- Murakami, Y.; Ishikawa, K.; Nakao, S.; Sonoda, K.H. Innate immune response in retinal homeostasis and inflammatory disorders. Prog Retin Eye Res 2020, 74, 100778. [Google Scholar] [CrossRef]
- Roy, S.; Kern, T.S.; Song, B.; Stuebe, C. Mechanistic Insights into Pathological Changes in the Diabetic Retina: Implications for Targeting Diabetic Retinopathy. Am J Pathol 2017, 187, 9–19. [Google Scholar] [CrossRef]
- Kinoshita, N.; Kakehashi, A.; Inoda, S.; Itou, Y.; Kuroki, M.; Yasu, T.; Kawakami, M.; Kanazawa, Y. Effective and selective prevention of retinal leukostasis in streptozotocin-induced diabetic rats using gliclazide. Diabetologia 2002, 45, 735–739. [Google Scholar] [CrossRef]
- Forrester, J.V.; Kuffova, L.; Delibegovic, M. The Role of Inflammation in Diabetic Retinopathy. Front Immunol 2020, 11, 583687. [Google Scholar] [CrossRef]
- Barrett, E.J.; Liu, Z.; Khamaisi, M.; King, G.L.; Klein, R.; Klein, B.E.K.; Hughes, T.M.; Craft, S.; Freedman, B.I.; Bowden, D.W.; et al. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J Clin Endocrinol Metab 2017, 102, 4343–4410. [Google Scholar] [CrossRef]
- Pan, W.W.; Lin, F.; Fort, P.E. The innate immune system in diabetic retinopathy. Prog Retin Eye Res 2021, 84, 100940. [Google Scholar] [CrossRef]
- Gipson, I.K. The ocular surface: the challenge to enable and protect vision: the Friedenwald lecture. Invest Ophthalmol Vis Sci 2007, 48, 4390–4398. [Google Scholar] [CrossRef] [PubMed]
- Bolanos-Jimenez, R.; Navas, A.; Lopez-Lizarraga, E.P.; de Ribot, F.M.; Pena, A.; Graue-Hernandez, E.O.; Garfias, Y. Ocular Surface as Barrier of Innate Immunity. Open Ophthalmol J 2015, 9, 49–55. [Google Scholar] [CrossRef]
- Taylor, A.W. Ocular immune privilege. Eye (Lond) 2009, 23, 1885–1889. [Google Scholar] [CrossRef]
- Caspi, R.R. In this issue: Immunology of the eye--inside and out. Int Rev Immunol 2013, 32, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Adachi, W.; Sotozono, C.; Nishida, K.; Yokoi, N.; Quantock, A.J.; Okubo, K. Characteristics of the human ocular surface epithelium. Prog Retin Eye Res 2001, 20, 639–673. [Google Scholar] [CrossRef] [PubMed]
- Walcott, B.; Moore, L.C.; Birzgalis, A.; Claros, N.; Valiunas, V.; Ott, T.; Willecke, K.; Brink, P.R. Role of gap junctions in fluid secretion of lacrimal glands. Am J Physiol Cell Physiol 2002, 282, C501–C507. [Google Scholar] [CrossRef]
- Williams, K.; Watsky, M. Gap junctional communication in the human corneal endothelium and epithelium. Curr Eye Res 2002, 25, 29–36. [Google Scholar] [CrossRef]
- Mantelli, F.; Mauris, J.; Argueso, P. The ocular surface epithelial barrier and other mechanisms of mucosal protection: from allergy to infectious diseases. Curr Opin Allergy Clin Immunol 2013, 13, 563–568. [Google Scholar] [CrossRef]
- Zhou, L.; Beuerman, R.W. Tear analysis in ocular surface diseases. Prog Retin Eye Res 2012, 31, 527–550. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Hamrah, P.; Shimazaki, J. Bilateral Alterations in Corneal Nerves, Dendritic Cells, and Tear Cytokine Levels in Ocular Surface Disease. Cornea 2016, 35 (Suppl. 1), S65–S70. [Google Scholar] [CrossRef]
- Belmonte, C.; Aracil, A.; Acosta, M.C.; Luna, C.; Gallar, J. Nerves and sensations from the eye surface. Ocul Surf 2004, 2, 248–253. [Google Scholar] [CrossRef]
- Morris, C.A.; Holden, B.A.; Papas, E.; Griesser, H.J.; Bolis, S.; Anderton, P.; Carney, F. The ocular surface, the tear film, and the wettability of contact lenses. Adv Exp Med Biol 1998, 438, 717–722. [Google Scholar] [PubMed]
- Holly, F.J. Basic Aspects of Tear Film Formation and Stability. In Physicochemical Hydrodynamics, Series, N.A. Ed. Springer, Boston, MA: 1988; Vol. 174.
- Willcox, M.D.P.; Argueso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul Surf 2017, 15, 366–403. [Google Scholar] [CrossRef] [PubMed]
- Ambroziak, A.M.; Szaflik, J.; Szaflik, J.P.; Ambroziak, M.; Witkiewicz, J.; Skopinski, P. Immunomodulation on the ocular surface: a review. Cent Eur J Immunol 2016, 41, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Tamhane, M.; Cabrera-Ghayouri, S.; Abelian, G.; Viswanath, V. Review of Biomarkers in Ocular Matrices: Challenges and Opportunities. Pharm Res 2019, 36, 40. [Google Scholar] [CrossRef] [PubMed]
- Albarran, C.; Pons, A.M.; Lorente, A.; Montes, R.; Artigas, J.M. Influence of the tear film on optical quality of the eye. Cont Lens Anterior Eye 1997, 20, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Rentka, A.; Koroskenyi, K.; Harsfalvi, J.; Szekanecz, Z.; Szucs, G.; Szodoray, P.; Kemeny-Beke, A. Evaluation of commonly used tear sampling methods and their relevance in subsequent biochemical analysis. Ann Clin Biochem 2017, 54, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Stahl, U.; Willcox, M.; Stapleton, F. Osmolality and tear film dynamics. Clin Exp Optom 2012, 95, 3–11. [Google Scholar] [CrossRef]
- Bachman, W.G.; Wilson, G. Essential ions for maintenance of the corneal epithelial surface. Invest Ophthalmol Vis Sci 1985, 26, 1484–1488. [Google Scholar]
- King-Smith, P.E.; Hinel, E.A.; Nichols, J.J. Application of a novel interferometric method to investigate the relation between lipid layer thickness and tear film thinning. Invest Ophthalmol Vis Sci 2010, 51, 2418–2423. [Google Scholar] [CrossRef]
- Green-Church, K.B.; Butovich, I.; Willcox, M.; Borchman, D.; Paulsen, F.; Barabino, S.; Glasgow, B.J. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci 2011, 52, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- Van Haeringen, N.J. Clinical biochemistry of tears. Surv Ophthalmol 1981, 26, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Butovich, I.A. Lipidomics of human Meibomian gland secretions: Chemistry, biophysics, and physiological role of Meibomian lipids. Prog Lipid Res 2011, 50, 278–301. [Google Scholar] [CrossRef] [PubMed]
- Carreno, E.; Enriquez-de-Salamanca, A.; Teson, M.; Garcia-Vazquez, C.; Stern, M.E.; Whitcup, S.M.; Calonge, M. Cytokine and chemokine levels in tears from healthy subjects. Acta Ophthalmol 2010, 88, e250–e258. [Google Scholar] [CrossRef] [PubMed]
- Gokcinar, N.B.; Karabulut, A.A.; Onaran, Z.; Yumusak, E.; Budak Yildiran, F.A. Elevated Tear Human Neutrophil Peptides 1-3, Human Beta Defensin-2 Levels and Conjunctival Cathelicidin LL-37 Gene Expression in Ocular Rosacea. Ocul Immunol Inflamm 2019, 27, 1174–1183. [Google Scholar] [CrossRef]
- Mohammed, I.; Said, D.G.; Dua, H.S. Human antimicrobial peptides in ocular surface defense. Prog Retin Eye Res 2017, 61, 1–22. [Google Scholar] [CrossRef]
- Garreis, F.; Gottschalt, M.; Schlorf, T.; Glaser, R.; Harder, J.; Worlitzsch, D.; Paulsen, F.P. Expression and regulation of antimicrobial peptide psoriasin (S100A7) at the ocular surface and in the lacrimal apparatus. Invest Ophthalmol Vis Sci 2011, 52, 4914–4922. [Google Scholar] [CrossRef]
- Garreis, F.; Gottschalt, M.; Paulsen, F.P. Antimicrobial peptides as a major part of the innate immune defense at the ocular surface. Dev Ophthalmol 2010, 45, 16–22. [Google Scholar]
- Findlay, F.; Pohl, J.; Svoboda, P.; Shakamuri, P.; McLean, K.; Inglis, N.F.; Proudfoot, L.; Barlow, P.G. Carbon Nanoparticles Inhibit the Antimicrobial Activities of the Human Cathelicidin LL-37 through Structural Alteration. J Immunol 2017, 199, 2483–2490. [Google Scholar] [CrossRef]
- Sharma, P.; Guha, S.; Garg, P.; Roy, S. Differential expression of antimicrobial peptides in corneal infection and regulation of antimicrobial peptides and reactive oxygen species by type III secretion system of Pseudomonas aeruginosa. Pathog Dis 2018, 76. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am J Transl Res 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Haynes, R.J.; Tighe, P.J.; Dua, H.S. Antimicrobial defensin peptides of the human ocular surface. Br J Ophthalmol 1999, 83, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.; Jean, D.; Proske, R.J.; Reins, R.Y.; McDermott, A.M. Ocular surface expression and in vitro activity of antimicrobial peptides. Curr Eye Res 2007, 32, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, O.E.; Follin, P.; Johnsen, A.H.; Calafat, J.; Tjabringa, G.S.; Hiemstra, P.S.; Borregaard, N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 2001, 97, 3951–3959. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.M.; Redfern, R.L.; Zhang, B.; Pei, Y.; Huang, L.; Proske, R.J. Defensin expression by the cornea: multiple signalling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci 2003, 44, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Akpek, E.K.; Gottsch, J.D. Immune defense at the ocular surface. Eye (Lond) 2003, 17, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Niederkorn, J.Y.; Peeler, J.S.; Mellon, J. Phagocytosis of particulate antigens by corneal epithelial cells stimulates interleukin-1 secretion and migration of Langerhans cells into the central cornea. Reg Immunol 1989, 2, 83–90. [Google Scholar] [PubMed]
- Cubitt, C.L.; Lausch, R.N.; Oakes, J.E. Synthesis of type II interleukin-1 receptors by human corneal epithelial cells but not by keratocytes. Invest Ophthalmol Vis Sci 2001, 42, 701–704. [Google Scholar]
- Hattori, T.; Chauhan, S.K.; Lee, H.; Ueno, H.; Dana, R.; Kaplan, D.H.; Saban, D.R. Characterization of Langerin-expressing dendritic cell subsets in the normal cornea. Invest Ophthalmol Vis Sci 2011, 52, 4598–4604. [Google Scholar] [CrossRef]
- Hamrah, P.; Dana, M.R. Corneal antigen-presenting cells. Chem Immunol Allergy 2007, 92, 58–70. [Google Scholar]
- Forrester, J.V.; Xu, H.; Kuffova, L.; Dick, A.D.; McMenamin, P.G. Dendritic cell physiology and function in the eye. Immunol Rev 2010, 234, 282–304. [Google Scholar] [CrossRef] [PubMed]
- Akhlaq, A.; Colon, C.; Cavalcanti, B.M.; Aggarwal, S.; Qazi, Y.; Cruzat, A.; Jersey, C.; Critser, D.B.; Watts, A.; Beyer, J.; et al. Density and distribution of dendritiform cells in the peripheral cornea of healthy subjects using in vivo confocal microscopy. Ocul Surf 2022, 26, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Sichien, D.; Lambrecht, B.N.; Guilliams, M.; Scott, C.L. Development of conventional dendritic cells: from common bone marrow progenitors to multiple subsets in peripheral tissues. Mucosal Immunol 2017, 10, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Hamrah, P.; Huq, S.O.; Liu, Y.; Zhang, Q.; Dana, M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol 2003, 74, 172–178. [Google Scholar] [CrossRef]
- Mayer, W.J.; Irschick, U.M.; Moser, P.; Wurm, M.; Huemer, H.P.; Romani, N.; Irschick, E.U. Characterization of antigen-presenting cells in fresh and cultured human corneas using novel dendritic cell markers. Invest Ophthalmol Vis Sci 2007, 48, 4459–4467. [Google Scholar] [CrossRef]
- Zhivov, A.; Stave, J.; Vollmar, B.; Guthoff, R. In vivo confocal microscopic evaluation of Langerhans cell density and distribution in the normal human corneal epithelium. Graefes Arch Clin Exp Ophthalmol 2005, 243, 1056–1061. [Google Scholar] [CrossRef]
- Chinnery, H.R.; Zhang, X.Y.; Wu, C.Y.; Downie, L.E. Corneal immune cell morphometry as an indicator of local and systemic pathology: A review. Clin Exp Ophthalmol 2021, 49, 729–740. [Google Scholar] [CrossRef]
- Jamali, A.; Kenyon, B.; Ortiz, G.; Abou-Slaybi, A.; Sendra, V.G.; Harris, D.L.; Hamrah, P. Plasmacytoid dendritic cells in the eye. Prog Retin Eye Res 2021, 80, 100877. [Google Scholar] [CrossRef]
- Hamrah, P.; Liu, Y.; Zhang, Q.; Dana, M.R. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci 2003, 44, 581–589. [Google Scholar] [CrossRef]
- Knickelbein, J.E.; Watkins, S.C.; McMenamin, P.G.; Hendricks, R.L. Stratification of Antigen-presenting Cells within the Normal Cornea. Ophthalmol Eye Dis 2009, 1, 45–54. [Google Scholar] [CrossRef]
- Hattori, T.; Takahashi, H.; Dana, R. Novel Insights Into the Immunoregulatory Function and Localization of Dendritic Cells. Cornea 2016, 35 (Suppl. 1), S49–S54. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Yin, J.; Yoon, G.S.; Mi, Q.S.; Yu, F.S. Dendritic cell-epithelium interplay is a determinant factor for corneal epithelial wound repair. Am J Pathol 2011, 179, 2243–2253. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, C.; Lee, I.X.Y.; Lin, M.T.Y.; Liu, Y.C. Corneal dendritic cells in diabetes mellitus: A narrative review. Front Endocrinol (Lausanne) 2023, 14, 1078660. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.J.; Liu, J. Human Microbiota and Ophthalmic Disease. Yale J Biol Med 2016, 89, 325–330. [Google Scholar] [PubMed]
- Dong, Q.; Brulc, J.M.; Iovieno, A.; Bates, B.; Garoutte, A.; Miller, D.; Revanna, K.V.; Gao, X.; Antonopoulos, D.A.; Slepak, V.Z.; et al. Diversity of bacteria at healthy human conjunctiva. Invest Ophthalmol Vis Sci 2011, 52, 5408–5413. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Iovieno, A. The role of microbial flora on the ocular surface. Curr Opin Allergy Clin Immunol 2009, 9, 466–470. [Google Scholar] [CrossRef]
- van der Meulen, I.J.; van Rooij, J.; Nieuwendaal, C.P.; Van Cleijnenbreugel, H.; Geerards, A.J.; Remeijer, L. Age-related risk factors, culture outcomes, and prognosis in patients admitted with infectious keratitis to two Dutch tertiary referral centers. Cornea 2008, 27, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: a source of disease or defence? Br J Dermatol 2008, 158, 442–455. [Google Scholar] [CrossRef]
- Dai, D.F.; Chiao, Y.A.; Marcinek, D.J.; Szeto, H.H.; Rabinovitch, P.S. Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan 2014, 3, 6. [Google Scholar] [CrossRef]
- Joyal, J.S.; Sun, Y.; Gantner, M.L.; Shao, Z.; Evans, L.P.; Saba, N.; Fredrick, T.; Burnim, S.; Kim, J.S.; Patel, G.; et al. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat Med 2016, 22, 439–445. [Google Scholar] [CrossRef]
- Kooragayala, K.; Gotoh, N.; Cogliati, T.; Nellissery, J.; Kaden, T.R.; French, S.; Balaban, R.; Li, W.; Covian, R.; Swaroop, A. Quantification of Oxygen Consumption in Retina Ex Vivo Demonstrates Limited Reserve Capacity of Photoreceptor Mitochondria. Invest Ophthalmol Vis Sci 2015, 56, 8428–8436. [Google Scholar] [CrossRef] [PubMed]
- Damani, M.R.; Zhao, L.; Fontainhas, A.M.; Amaral, J.; Fariss, R.N.; Wong, W.T. Age-related alterations in the dynamic behavior of microglia. Aging Cell 2011, 10, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Karlstetter, M.; Scholz, R.; Rutar, M.; Wong, W.T.; Provis, J.M.; Langmann, T. Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res 2015, 45, 30–57. [Google Scholar] [CrossRef] [PubMed]
- Dando, S.J.; Naranjo Golborne, C.; Chinnery, H.R.; Ruitenberg, M.J.; McMenamin, P.G. A case of mistaken identity: CD11c-eYFP(+) cells in the normal mouse brain parenchyma and neural retina display the phenotype of microglia, not dendritic cells. Glia 2016, 64, 1331–1349. [Google Scholar] [CrossRef] [PubMed]
- Rathnasamy, G.; Foulds, W.S.; Ling, E.A.; Kaur, C. Retinal microglia - A key player in healthy and diseased retina. Prog Neurobiol 2019, 173, 18–40. [Google Scholar] [CrossRef] [PubMed]
- Uckermann, O.; Wolf, A.; Kutzera, F.; Kalisch, F.; Beck-Sickinger, A.G.; Wiedemann, P.; Reichenbach, A.; Bringmann, A. Glutamate release by neurons evokes a purinergic inhibitory mechanism of osmotic glial cell swelling in the rat retina: activation by neuropeptide Y. J Neurosci Res 2006, 83, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Biernacki, K.; Wosik, K.; Antel, J.P. Glial cell influence on the human blood-brain barrier. Glia 2001, 36, 145–155. [Google Scholar] [CrossRef]
- Bu, Y.; Shih, K.C.; Tong, L. The ocular surface and diabetes, the other 21st Century epidemic. Exp Eye Res 2022, 220, 109099. [Google Scholar] [CrossRef]
- Amorim, M.; Martins, B.; Caramelo, F.; Goncalves, C.; Trindade, G.; Simao, J.; Barreto, P.; Marques, I.; Leal, E.C.; Carvalho, E.; et al. Putative Biomarkers in Tears for Diabetic Retinopathy Diagnosis. Front Med (Lausanne) 2022, 9, 873483. [Google Scholar] [CrossRef]
- Naik, K.; Magdum, R.; Ahuja, A.; Kaul, S.; S, J.; Mishra, A.; Patil, M.; Dhore, D.N.; Alapati, A. Ocular Surface Diseases in Patients with Diabetes. Cureus 2022, 14, e23401. [Google Scholar] [CrossRef]
- Han, J.X.; Wang, H.; Liang, H.H.; Guo, J.X. Correlation of the retinopathy degree with the change of ocular surface and corneal nerve in patients with type 2 diabetes mellitus. Int J Ophthalmol 2021, 14, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Markoulli, M.; Flanagan, J.; Tummanapalli, S.S.; Wu, J.; Willcox, M. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul Surf 2018, 16, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Lee, A.; Lo, A.C.Y.; Kwok, J. Diabetic Corneal Neuropathy: Pathogenic Mechanisms and Therapeutic Strategies. Front Pharmacol 2022, 13, 816062. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, H.; Malik, T.G.; Mazhar, A.; Ali, A. Association of Dry Eye Disease with Diabetic Retinopathy. J Coll Physicians Surg Pak 2020, 30, 493–497. [Google Scholar] [PubMed]
- Csosz, E.; Deak, E.; Kallo, G.; Csutak, A.; Tozser, J. Diabetic retinopathy: Proteomic approaches to help the differential diagnosis and to understand the underlying molecular mechanisms. J Proteomics 2017, 150, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Amil-Bangsa, N.H.; Mohd-Ali, B.; Ishak, B.; Abdul-Aziz, C.N.N.; Ngah, N.F.; Hashim, H.; Ghazali, A.R. Total Protein Concentration and Tumor Necrosis Factor alpha in Tears of Nonproliferative Diabetic Retinopathy. Optom Vis Sci 2019, 96, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Y.; Ru, Y.S.; Wang, X.W.; Yang, J.Z.; Li, C.H.; Wang, H.X.; Li, X.R.; Li, B. Ocular surface changes in type II diabetic patients with proliferative diabetic retinopathy. Int J Ophthalmol 2015, 8, 358–364. [Google Scholar]
- K Co Shih, KS-L Lam, L Tong, A systematic review on the impact of diabetes mellitus on the. 2017.
- Rubinstein, M.P.; Parrish, S.T.; Vernon, S.A. Corneal epithelial oxygen uptake rate in diabetes mellitus. Eye (Lond) 1990, 4 Pt 5, 757–759. [Google Scholar] [CrossRef]
- Shih, K.C.; Lam, K.S.; Tong, L. A systematic review on the impact of diabetes mellitus on the ocular surface. Nutr Diabetes 2017, 7, e251. [Google Scholar] [CrossRef]
- GM, I. Ocular problems in diabetes mellitus. Sudanese J Ophthalmol 2014, 6, 43–48. [Google Scholar]
- He, F.; Zhao, Z.; Liu, Y.; Lu, L.; Fu, Y. Assessment of Ocular Surface Damage during the Course of Type 2 Diabetes Mellitus. J Ophthalmol 2018, 2018, 1206808. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, X.; Qin, G.; Xie, H.; Lv, P. Tear film function in type 2 diabetic patients with retinopathy. Ophthalmologica 2008, 222, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, J.; Shi, B.; He, S.; Yao, X.; Willcox, M.D. Advanced glycation end product (AGE) modified proteins in tears of diabetic patients. Mol Vis 2010, 16, 1576–1584. [Google Scholar] [PubMed]
- Sandra Johanna, G.P.; Antonio, L.A.; Andres, G.S. Correlation between type 2 diabetes, dry eye and Meibomian glands dysfunction. J Optom 2019, 12, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res 2007, 2007, 95103. [Google Scholar] [CrossRef]
- Messmer, E.M.; Schmid-Tannwald, C.; Zapp, D.; Kampik, A. In vivo confocal microscopy of corneal small fiber damage in diabetes mellitus. Graefes Arch Clin Exp Ophthalmol 2010, 248, 1307–1312. [Google Scholar] [CrossRef]
- Beckman, K.A. Characterization of dry eye disease in diabetic patients versus nondiabetic patients. Cornea 2014, 33, 851–854. [Google Scholar] [CrossRef]
- Alves Mde, C.; Carvalheira, J.B.; Modulo, C.M.; Rocha, E.M. Tear film and ocular surface changes in diabetes mellitus. Arq Bras Oftalmol 2008, 71, 96–103. [Google Scholar] [CrossRef]
- Herber, S.; Grus, F.H.; Sabuncuo, P.; Augustin, A.J. Two-dimensional analysis of tear protein patterns of diabetic patients. Electrophoresis 2001, 22, 1838–1844. [Google Scholar] [CrossRef]
- Nguyen-Khuong, T.; Everest-Dass, A.V.; Kautto, L.; Zhao, Z.; Willcox, M.D.; Packer, N.H. Glycomic characterization of basal tears and changes with diabetes and diabetic retinopathy. Glycobiology 2015, 25, 269–283. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Salvayre, R.; Auge, N.; Pamplona, R.; Portero-Otin, M. Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal 2009, 11, 3071–3109. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ghosh, S.; Azharuddin, M.; Bera, S.; Datta, H.; Dasgupta, A. Change in tear protein profile in diabetic retinopathy with duration of diabetes. Diabetes Metab Syndr 2014, 8, 233–235. [Google Scholar] [CrossRef]
- Chung, Y.R.; Kim, Y.H.; Ha, S.J.; Byeon, H.E.; Cho, C.H.; Kim, J.H.; Lee, K. Role of Inflammation in Classification of Diabetic Macular Edema by Optical Coherence Tomography. J Diabetes Res 2019, 2019, 8164250. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.Z.; Guo, X.; Klein, R.; Klein, B.E.; Cui, J.; Rotter, J.I.; Ipp, E.; Chen, Y.D. Systemic soluble tumor necrosis factor receptors 1 and 2 are associated with severity of diabetic retinopathy in Hispanics. Ophthalmology 2012, 119, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Kim, S.S.; Kim, J.C.; Kim, H.C.; Im, Y.S.; Ahn, C.W.; Lee, H.K. Serum and tear levels of nerve growth factor in diabetic retinopathy patients. Am J Ophthalmol 2008, 145, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Csosz, E.; Boross, P.; Csutak, A.; Berta, A.; Toth, F.; Poliska, S.; Torok, Z.; Tozser, J. Quantitative analysis of proteins in the tear fluid of patients with diabetic retinopathy. J Proteomics 2012, 75, 2196–2204. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, P.K.; Yoo, H.S.; Kim, C.W. Comparison of tear proteins between healthy and early diabetic retinopathy patients. Clin Biochem 2012, 45, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Yu, F.S. Impaired epithelial wound healing and EGFR signaling pathways in the corneas of diabetic rats. Invest Ophthalmol Vis Sci 2011, 52, 3301–3308. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Y.; Sullivan, D.A. Effects of Insulin and High Glucose on Human Meibomian Gland Epithelial Cells. Invest Ophthalmol Vis Sci 2015, 56, 7814–7820. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, H.; Zhao, Z.; Luo, X.; Zhang, M.; Bu, J.; Liang, M.; Wu, H.; Yu, J.; He, H.; et al. Hyperglycemia Induces Meibomian Gland Dysfunction. Invest Ophthalmol Vis Sci 2022, 63, 30. [Google Scholar] [CrossRef]
- Yin, J.; Huang, J.; Chen, C.; Gao, N.; Wang, F.; Yu, F.S. Corneal complications in streptozocin-induced type I diabetic rats. Invest Ophthalmol Vis Sci 2011, 52, 6589–6596. [Google Scholar] [CrossRef] [PubMed]
- Dota, A.; Sakamoto, A.; Nagano, T.; Murakami, T.; Matsugi, T. Effect of Diquafosol Ophthalmic Solution on Airflow-Induced Ocular Surface Disorder in Diabetic Rats. Clin Ophthalmol 2020, 14, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sheng, M.; Liu, Y.; Wang, P.; Chen, Y.; Chen, L.; Wang, W.; Li, B. Expression of SIRT1 and oxidative stress in diabetic dry eye. Int J Clin Exp Pathol 2015, 8, 7644–7653. [Google Scholar] [PubMed]
- Bettahi, I.; Sun, H.; Gao, N.; Wang, F.; Mi, X.; Chen, W.; Liu, Z.; Yu, F.S. Genome-wide transcriptional analysis of differentially expressed genes in diabetic, healing corneal epithelial cells: hyperglycemia-suppressed TGFbeta3 expression contributes to the delay of epithelial wound healing in diabetic corneas. Diabetes 2014, 63, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Gao, N.; Sun, H.; Yin, J.; Lee, P.; Zhou, L.; Fan, X.; Yu, F.S. Targeting Imbalance between IL-1beta and IL-1 Receptor Antagonist Ameliorates Delayed Epithelium Wound Healing in Diabetic Mouse Corneas. Am J Pathol 2016, 186, 1466–1480. [Google Scholar] [CrossRef] [PubMed]
- Alba-Loureiro, T.C.; Hirabara, S.M.; Mendonca, J.R.; Curi, R.; Pithon-Curi, T.C. Diabetes causes marked changes in function and metabolism of rat neutrophils. J Endocrinol 2006, 188, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Yan, C.; Lee, P.; Sun, H.; Yu, F.S. Dendritic cell dysfunction and diabetic sensory neuropathy in the cornea. J Clin Invest 2016, 126, 1998–2011. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Lee, P.S.Y.; Yang, L.; Gao, N.; Zhang, Y.; Ljubimov, A.V.; Yang, E.; Zhou, Q.; Xie, L. The impact of sensory neuropathy and inflammation on epithelial wound healing in diabetic corneas. Prog Retin Eye Res 2022, 89, 101039. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Shi, D.; Chen, P.; Yu, Y.; Yang, L.; Xie, L. Overexpression of SIRT1 promotes high glucose-attenuated corneal epithelial wound healing via p53 regulation of the IGFBP3/IGF-1R/AKT pathway. Invest Ophthalmol Vis Sci 2013, 54, 3806–3814. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Salimian, M.; Konduru, S.R.; Choi, J.; Kumar, P.; Long, A.; Klimova, N.; Ho, C.Y.; Kristian, T.; Russell, J.W. Overexpression of Sirtuin 1 protein in neurons prevents and reverses experimental diabetic neuropathy. Brain 2019, 142, 3737–3752. [Google Scholar] [CrossRef]
- Wan, L.; Bai, X.; Zhou, Q.; Chen, C.; Wang, H.; Liu, T.; Xue, J.; Wei, C.; Xie, L. The advanced glycation end-products (AGEs)/ROS/NLRP3 inflammasome axis contributes to delayed diabetic corneal wound healing and nerve regeneration. Int J Biol Sci 2022, 18, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Therapeutic regulation of the NLRP3 inflammasome in chronic inflammatory diseases. Arch Pharm Res 2021, 44, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.H.; Adijiang, A.; Vandanmagsar, B.; Burk, D.; Ravussin, A.; Dixit, V.D. Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage. Endocrinology 2011, 152, 4039–4045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fu, Y.; Li, H.; Shen, L.; Chang, Q.; Pan, L.; Hong, S.; Yin, X. H3 relaxin inhibits the collagen synthesis via ROS- and P2X7R-mediated NLRP3 inflammasome activation in cardiac fibroblasts under high glucose. J Cell Mol Med 2018, 22, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, X.; Zong, B.; Yuan, H.; Wang, Z.; Wei, Y.; Wang, X.; Liu, G.; Zhang, J.; Li, S.; et al. Gypenosides improve diabetic cardiomyopathy by inhibiting ROS-mediated NLRP3 inflammasome activation. J Cell Mol Med 2018, 22, 4437–4448. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.W.; Zhang, J.; Li, X.; Wang, Y.; Fu, Y.H.; Gao, X.Y. A new research hot spot: The role of NLRP3 inflammasome activation, a key step in pyroptosis, in diabetes and diabetic complications. Life Sci 2020, 240, 117138. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Fei, Y.; Qin, Y.; Luo, D.; Yang, S.; Kou, X.; Zi, Y.; Deng, T.; Jin, M. Bacterial Flora Changes in Conjunctiva of Rats with Streptozotocin-Induced Type I Diabetes. PLoS One 2015, 10, e0133021. [Google Scholar] [CrossRef]
- Li, X.; Yu, Z.W.; Wang, Y.; Fu, Y.H.; Gao, X.Y. MicroRNAs: Potential Targets in Diabetic Retinopathy. Horm Metab Res 2020, 52, 142–148. [Google Scholar] [CrossRef]
- Fernandes, R.; Girao, H.; Pereira, P. High glucose down-regulates intercellular communication in retinal endothelial cells by enhancing degradation of connexin 43 by a proteasome-dependent mechanism. J Biol Chem 2004, 279, 27219–27224. [Google Scholar] [CrossRef]
- Fernandes, R.; Carvalho, A.L.; Kumagai, A.; Seica, R.; Hosoya, K.; Terasaki, T.; Murta, J.; Pereira, P.; Faro, C. Downregulation of retinal GLUT1 in diabetes by ubiquitinylation. Mol Vis 2004, 10, 618–628. [Google Scholar]
- Bento, C.F.; Fernandes, R.; Matafome, P.; Sena, C.; Seica, R.; Pereira, P. Methylglyoxal-induced imbalance in the ratio of vascular endothelial growth factor to angiopoietin 2 secreted by retinal pigment epithelial cells leads to endothelial dysfunction. Exp Physiol 2010, 95, 955–970. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Hosoya, K.; Pereira, P. Reactive oxygen species downregulate glucose transport system in retinal endothelial cells. Am J Physiol Cell Physiol 2011, 300, C927–C936. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Marques, C.; Leal, E.; Ribeiro, C.F.; Reis, F.; Ambrosio, A.F.; Fernandes, R. Dipeptidyl peptidase-IV inhibition prevents blood-retinal barrier breakdown, inflammation and neuronal cell death in the retina of type 1 diabetic rats. Biochim Biophys Acta 2014, 1842, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, C.; Lu, L.; Tian, H.; Liu, K.; Luo, D.; Qiu, Q.; Xu, G.T.; Zhang, J. Melatonin Maintains Inner Blood-Retinal Barrier by Regulating Microglia via Inhibition of PI3K/Akt/Stat3/NF-kappaB Signaling Pathways in Experimental Diabetic Retinopathy. Front Immunol 2022, 13, 831660. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Xie, H.; Zhang, C.; Wang, T.; Tian, H.; Lu, L.; Xu, J.Y.; Xu, G.T.; Liu, L.; Zhang, J. Enhancing fractalkine/CX3CR1 signalling pathway can reduce neuroinflammation by attenuating microglia activation in experimental diabetic retinopathy. J Cell Mol Med 2022, 26, 1229–1244. [Google Scholar] [CrossRef]
- Jackson, G.R.; Barber, A.J. Visual dysfunction associated with diabetic retinopathy. Curr Diab Rep 2010, 10, 380–384. [Google Scholar] [CrossRef]
- Trento, M.; Durando, O.; Lavecchia, S.; Charrier, L.; Cavallo, F.; Costa, M.A.; Hernandez, C.; Simo, R.; Porta, M.; investigators, E. t. Vision related quality of life in patients with type 2 diabetes in the EUROCONDOR trial. Endocrine 2017, 57, 83–88. [Google Scholar] [CrossRef]
- Wolff, B.E.; Bearse, M.A. Jr.; Schneck, M.E.; Dhamdhere, K.; Harrison, W.W.; Barez, S.; Adams, A.J. Color vision and neuroretinal function in diabetes. Doc Ophthalmol 2015, 130, 131–139. [Google Scholar] [CrossRef]
- Chhablani, J.; Sharma, A.; Goud, A.; Peguda, H.K.; Rao, H.L.; Begum, V.U.; Barteselli, G. Neurodegeneration in Type 2 Diabetes: Evidence From Spectral-Domain Optical Coherence Tomography. Invest Ophthalmol Vis Sci 2015, 56, 6333–6338. [Google Scholar] [CrossRef]
- Di Leo, M.A.; Caputo, S.; Falsini, B.; Porciatti, V.; Greco, A.V.; Ghirlanda, G. Presence and further development of retinal dysfunction after 3-year follow up in IDDM patients without angiographically documented vasculopathy. Diabetologia 1994, 37, 911–916. [Google Scholar] [CrossRef]
- Juen, S.; Kieselbach, G.F. Electrophysiological changes in juvenile diabetics without retinopathy. Arch Ophthalmol 1990, 108, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A 2016, 113, E2655–64. [Google Scholar] [CrossRef] [PubMed]
- Tyrberg, M.; Lindblad, U.; Melander, A.; Lovestam-Adrian, M.; Ponjavic, V.; Andreasson, S. Electrophysiological studies in newly onset type 2 diabetes without visible vascular retinopathy. Doc Ophthalmol 2011, 123, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.G.; Lee, J.Y.; Kim, C.; Park, Y.H. Early Microglial Changes Associated with Diabetic Retinopathy in Rats with Streptozotocin-Induced Diabetes. J Diabetes Res 2021, 2021, 4920937. [Google Scholar] [CrossRef] [PubMed]
- Harrison, W.W.; Bearse, M.A. Jr.; Ng, J.S.; Jewell, N.P.; Barez, S.; Burger, D.; Schneck, M.E.; Adams, A.J. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Invest Ophthalmol Vis Sci 2011, 52, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Dodo, Y.; Murakami, T.; Uji, A.; Yoshitake, S.; Yoshimura, N. Disorganized retinal lamellar structures in nonperfused areas of diabetic retinopathy. Invest Ophthalmol Vis Sci 2015, 56, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, F.S.; Matteini, S.; Bonifazzi, C.; Sebastiani, A.; Parmeggiani, F. Diabetic retinopathy and endothelin system: microangiopathy versus endothelial dysfunction. Eye (Lond) 2018, 32, 1157–1163. [Google Scholar] [CrossRef]
- Rodriguez, M.L.; Perez, S.; Mena-Molla, S.; Desco, M.C.; Ortega, A.L. Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxid Med Cell Longev 2019, 2019, 4940825. [Google Scholar] [CrossRef]
- Santiago, A.R.; Boia, R.; Aires, I.D.; Ambrosio, A.F.; Fernandes, R. Sweet Stress: Coping With Vascular Dysfunction in Diabetic Retinopathy. Front Physiol 2018, 9, 820. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Ahmad, A.; Siddiquei, M.M.; De Zutter, A.; Allegaert, E.; Gikandi, P.W.; De Hertogh, G.; Van Damme, J.; Opdenakker, G.; Struyf, S. The Proinflammatory and Proangiogenic Macrophage Migration Inhibitory Factor Is a Potential Regulator in Proliferative Diabetic Retinopathy. Front Immunol 2019, 10, 2752. [Google Scholar] [CrossRef]
- Martins, B.; Amorim, M.; Reis, F.; Ambrosio, A.F.; Fernandes, R. Extracellular Vesicles and MicroRNA: Putative Role in Diagnosis and Treatment of Diabetic Retinopathy. Antioxidants (Basel) 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Aouiss, A.; Anka Idrissi, D.; Kabine, M.; Zaid, Y. Update of inflammatory proliferative retinopathy: Ischemia, hypoxia and angiogenesis. Curr Res Transl Med 2019, 67, 62–71. [Google Scholar] [CrossRef]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Aboualizadeh, E.; Ranji, M.; Sorenson, C.M.; Sepehr, R.; Sheibani, N.; Hirschmugl, C.J. Retinal oxidative stress at the onset of diabetes determined by synchrotron FTIR widefield imaging: towards diabetes pathogenesis. Analyst 2017, 142, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.L.; Lin, C.H.; Li, C.H.; Tsai, C.H.; Ho, J.D.; Chiou, G.C.; Kang, J.J.; Cheng, Y.W. Anti-inflammatory properties of shikonin contribute to improved early-stage diabetic retinopathy. Sci Rep 2017, 7, 44985. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Lai, T.T.; Li, C.J. The Oxidative Stress and Mitochondrial Dysfunction during the Pathogenesis of Diabetic Retinopathy. Oxid Med Cell Longev 2018, 2018, 3420187. [Google Scholar] [CrossRef] [PubMed]
- Al-Shabrawey, M.; Zhang, W.; McDonald, D. Diabetic retinopathy: mechanism, diagnosis, prevention, and treatment. Biomed Res Int 2015, 2015, 854593. [Google Scholar] [CrossRef]
- Al-Kharashi, A.S. Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi J Ophthalmol 2018, 32, 318–323. [Google Scholar] [CrossRef]
- Yue, T.; Shi, Y.; Luo, S.; Weng, J.; Wu, Y.; Zheng, X. The role of inflammation in immune system of diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Front Immunol 2022, 13, 1055087. [Google Scholar] [CrossRef]
- Miyamoto, K.; Khosrof, S.; Bursell, S.E.; Rohan, R.; Murata, T.; Clermont, A.C.; Aiello, L.P.; Ogura, Y.; Adamis, A.P. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A 1999, 96, 10836–10841. [Google Scholar] [CrossRef]
- Ma, Y.; Ashander, L.M.; Appukuttan, B.; Ryan, F.J.; Tan, A.C.R.; Matthews, J.M.; Michael, M.Z.; Lynn, D.J.; Smith, J.R. Selective Transcription Factor Blockade Reduces Human Retinal Endothelial Cell Expression of Intercellular Adhesion Molecule-1 and Leukocyte Binding. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, S.S.; Lim, R.R.; Parikh, B.H.; Wey, Y.S.; Tun, B.B.; Wong, T.Y.; Luu, C.D.; Agrawal, R.; Ghosh, A.; Mortellaro, A.; et al. The NLRP3 Inflammasome May Contribute to Pathologic Neovascularization in the Advanced Stages of Diabetic Retinopathy. Sci Rep 2018, 8, 2847. [Google Scholar] [CrossRef] [PubMed]
- Yumnamcha, T.; Devi, T.S.; Singh, L.P. Auranofin Mediates Mitochondrial Dysregulation and Inflammatory Cell Death in Human Retinal Pigment Epithelial Cells: Implications of Retinal Neurodegenerative Diseases. Front Neurosci 2019, 13, 1065. [Google Scholar] [CrossRef] [PubMed]
- Adhya, P.; Sharma, S.S. Redox TRPs in diabetes and diabetic complications: Mechanisms and pharmacological modulation. Pharmacol Res 2019, 146, 104271. [Google Scholar] [CrossRef] [PubMed]
- Rossino, M.G.; Casini, G. Nutraceuticals for the Treatment of Diabetic Retinopathy. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, M. Diabetic retinopathy and dysregulated innate immunity. Vision Res 2017, 139, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Cho, C.L.; Liang, C.L.; Chen, S.D.; Liliang, P.C.; Wang, S.Y.; Chen, H.J. Inhibition of the MEK/ERK pathway reduces microglial activation and interleukin-1-beta expression in spinal cord ischemia/reperfusion injury in rats. J Thorac Cardiovasc Surg 2007, 133, 934–941. [Google Scholar] [CrossRef]
- Altmann, C.; Schmidt, M.H.H. The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef]
- Krady, J.K.; Basu, A.; Allen, C.M.; Xu, Y.; LaNoue, K.F.; Gardner, T.W.; Levison, S.W. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes 2005, 54, 1559–1565. [Google Scholar] [CrossRef]
- Othman, R.; Vaucher, E.; Couture, R. Bradykinin Type 1 Receptor - Inducible Nitric Oxide Synthase: A New Axis Implicated in Diabetic Retinopathy. Front Pharmacol 2019, 10, 300. [Google Scholar] [CrossRef]
- Berezin, A. Neutrophil extracellular traps: The core player in vascular complications of diabetes mellitus. Diabetes Metab Syndr 2019, 13, 3017–3023. [Google Scholar] [CrossRef]
- Cecilia, O.M.; Jose Alberto, C.G.; Jose, N.P.; Ernesto German, C.M.; Ana Karen, L.C.; Luis Miguel, R.P.; Ricardo Raul, R.R.; Adolfo Daniel, R.C. Oxidative Stress as the Main Target in Diabetic Retinopathy Pathophysiology. J Diabetes Res 2019, 2019, 8562408. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Long, P.; Guo, L.; Zhang, M.; Wang, S.; He, H. Fushiming Capsule Attenuates Diabetic Rat Retina Damage via Antioxidation and Anti-Inflammation. Evid Based Complement Alternat Med 2019, 2019, 5376439. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Y.; Steinle, J.J. Glycyrrhizin Protects the Diabetic Retina against Permeability, Neuronal, and Vascular Damage through Anti-Inflammatory Mechanisms. J Clin Med 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Fresta, C.G.; Fidilio, A.; Caruso, G.; Caraci, F.; Giblin, F.J.; Leggio, G.M.; Salomone, S.; Drago, F.; Bucolo, C. A New Human Blood-Retinal Barrier Model Based on Endothelial Cells, Pericytes, and Astrocytes. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H. Effects of Propolis Extract and Propolis-Derived Compounds on Obesity and Diabetes: Knowledge from Cellular and Animal Models. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Picconi, F.; Parravano, M.; Ylli, D.; Pasqualetti, P.; Coluzzi, S.; Giordani, I.; Malandrucco, I.; Lauro, D.; Scarinci, F.; Giorno, P.; et al. Retinal neurodegeneration in patients with type 1 diabetes mellitus: the role of glycemic variability. Acta Diabetol 2017, 54, 489–497. [Google Scholar] [CrossRef]
- Rasmussen, K.L.; Nordestgaard, B.G.; Nielsen, S.F. Complement C3 and Risk of Diabetic Microvascular Disease: A Cohort Study of 95202 Individuals from the General Population. Clin Chem 2018, 64, 1113–1124. [Google Scholar] [CrossRef]
- Zhang, J.; Gerhardinger, C.; Lorenzi, M. Early complement activation and decreased levels of glycosylphosphatidylinositol-anchored complement inhibitors in human and experimental diabetic retinopathy. Diabetes 2002, 51, 3499–3504. [Google Scholar] [CrossRef]
- Amadi-Obi, A.; Yu, C.R.; Dambuza, I.; Kim, S.H.; Marrero, B.; Egwuagu, C.E. Interleukin 27 induces the expression of complement factor H (CFH) in the retina. PLoS One 2012, 7, e45801. [Google Scholar] [CrossRef]
- Ciecko, A.E.; Foda, B.; Barr, J.Y.; Ramanathan, S.; Atkinson, M.A.; Serreze, D.V.; Geurts, A.M.; Lieberman, S.M.; Chen, Y.G. Interleukin-27 Is Essential for Type 1 Diabetes Development and Sjogren Syndrome-like Inflammation. Cell Rep 2019, 29, 3073–3086.e5. [Google Scholar] [CrossRef] [PubMed]
- Houssen, M.E.; El-Hussiny, M.A.B.; El-Kannishy, A.; Sabry, D.; El Mahdy, R.; Shaker, M.E. Serum and aqueous humor concentrations of interleukin-27 in diabetic retinopathy patients. Int Ophthalmol 2018, 38, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.R.P.; Boia, R.; Campos, E.J.; Martins, J.; Nunes, S.; Madeira, M.H.; Santiago, A.R.; Pereira, F.C.; Reis, F.; Ambrosio, A.F.; et al. Subtle thinning of retinal layers without overt vascular and inflammatory alterations in a rat model of prediabetes. Mol Vis 2018, 24, 353–366. [Google Scholar] [PubMed]
- van Dijk, H.W.; Verbraak, F.D.; Stehouwer, M.; Kok, P.H.; Garvin, M.K.; Sonka, M.; DeVries, J.H.; Schlingemann, R.O.; Abramoff, M.D. Association of visual function and ganglion cell layer thickness in patients with diabetes mellitus type 1 and no or minimal diabetic retinopathy. Vision Res 2011, 51, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest 1998, 102, 783–791. [Google Scholar] [CrossRef]
- Brown, G.C.; Neher, J.J. Microglial phagocytosis of live neurons. Nat Rev Neurosci 2014, 15, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Asare-Bediko, B.; Harbour, A.; Floyd, J.L.; Chakraborty, D.; Duan, Y.; Lamendella, R.; Wright, J.; Grant, M.B. Microbial Signatures in The Rodent Eyes With Retinal Dysfunction and Diabetic Retinopathy. Invest Ophthalmol Vis Sci 2022, 63, 5. [Google Scholar] [CrossRef]
- Taylor, A.W.; Kaplan, H.J. Ocular immune privilege in the year 2010: ocular immune privilege and uveitis. Ocul Immunol Inflamm 2010, 18, 488–492. [Google Scholar] [CrossRef]
- Fernandes, R.; Viana, S.D.; Nunes, S.; Reis, F. Diabetic gut microbiota dysbiosis as an inflammaging and immunosenescence condition that fosters progression of retinopathy and nephropathy. Biochim Biophys Acta Mol Basis Dis 2019, 1865, 1876–1897. [Google Scholar] [CrossRef]
- Madaan, A.; Chaudhari, P.; Nadeau-Vallee, M.; Hamel, D.; Zhu, T.; Mitchell, G.; Samuels, M.; Pundir, S.; Dabouz, R.; Howe Cheng, C.W.; et al. Muller Cell-Localized G-Protein-Coupled Receptor 81 (Hydroxycarboxylic Acid Receptor 1) Regulates Inner Retinal Vasculature via Norrin/Wnt Pathways. Am J Pathol 2019, 189, 1878–1896. [Google Scholar] [CrossRef]
- Coburn, P.S.; Wiskur, B.J.; Astley, R.A.; Callegan, M.C. Blood-Retinal Barrier Compromise and Endogenous Staphylococcus aureus Endophthalmitis. Invest Ophthalmol Vis Sci 2015, 56, 7303–7311. [Google Scholar] [CrossRef] [PubMed]
- Manfredo Vieira, S.; Hiltensperger, M.; Kumar, V.; Zegarra-Ruiz, D.; Dehner, C.; Khan, N.; Costa, F.R.C.; Tiniakou, E.; Greiling, T.; Ruff, W.; et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 2018, 359, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Talreja, D.; Singh, P.K.; Kumar, A. In Vivo Role of TLR2 and MyD88 Signaling in Eliciting Innate Immune Responses in Staphylococcal Endophthalmitis. Invest Ophthalmol Vis Sci 2015, 56, 1719–1732. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.K.; Yu, F.S.; Kumar, A. Targeting toll-like receptor signaling as a novel approach to prevent ocular infectious diseases. Indian J Med Res 2013, 138, 609–619. [Google Scholar]
- Singh, P.K.; Shiha, M.J.; Kumar, A. Antibacterial responses of retinal Muller glia: production of antimicrobial peptides, oxidative burst and phagocytosis. J Neuroinflammation 2014, 11, 33. [Google Scholar] [CrossRef]
- Shamsuddin, N.; Kumar, A. TLR2 mediates the innate response of retinal Muller glia to Staphylococcus aureus. J Immunol 2011, 186, 7089–7097. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




