Submitted:
11 May 2023
Posted:
12 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Epidemiology
3. Clinical investigations and diagnosis of PICC
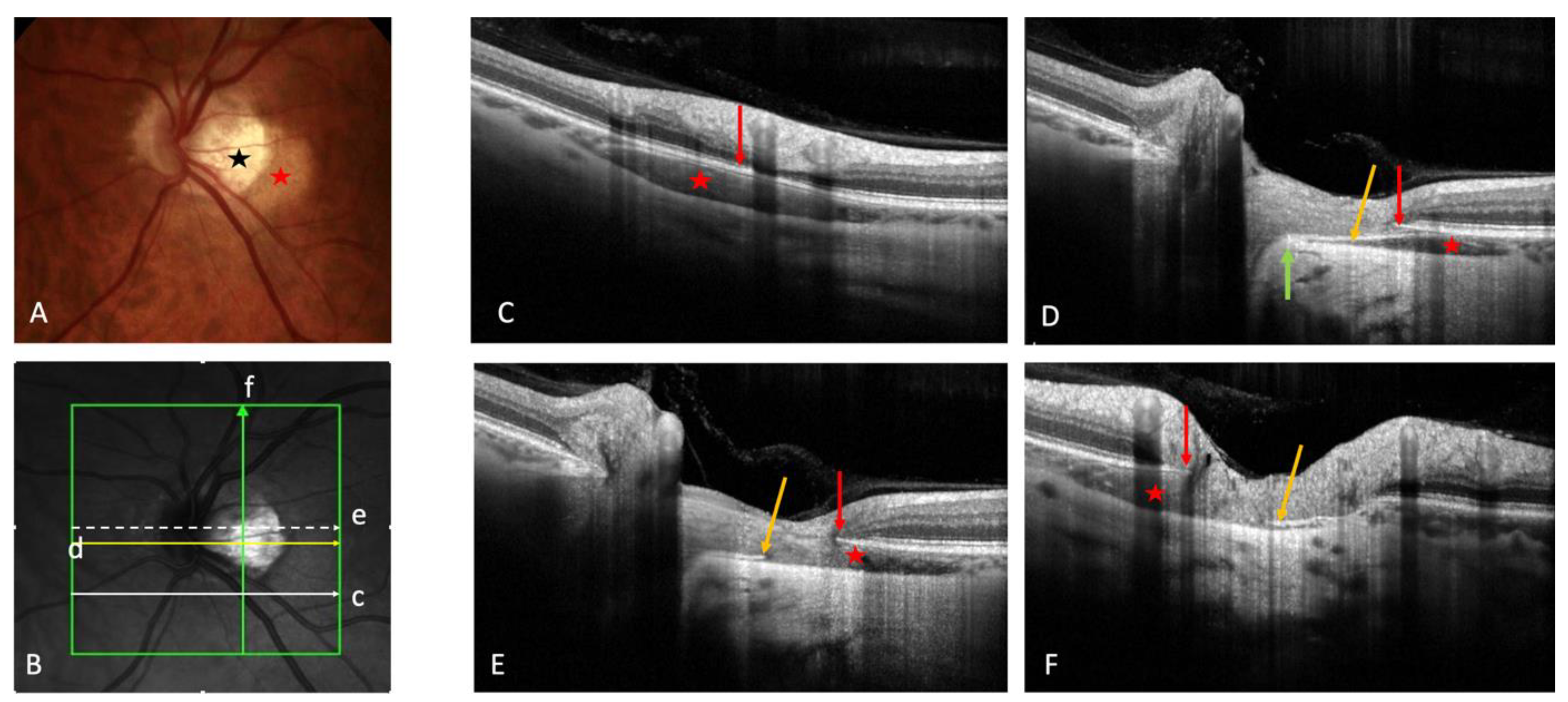
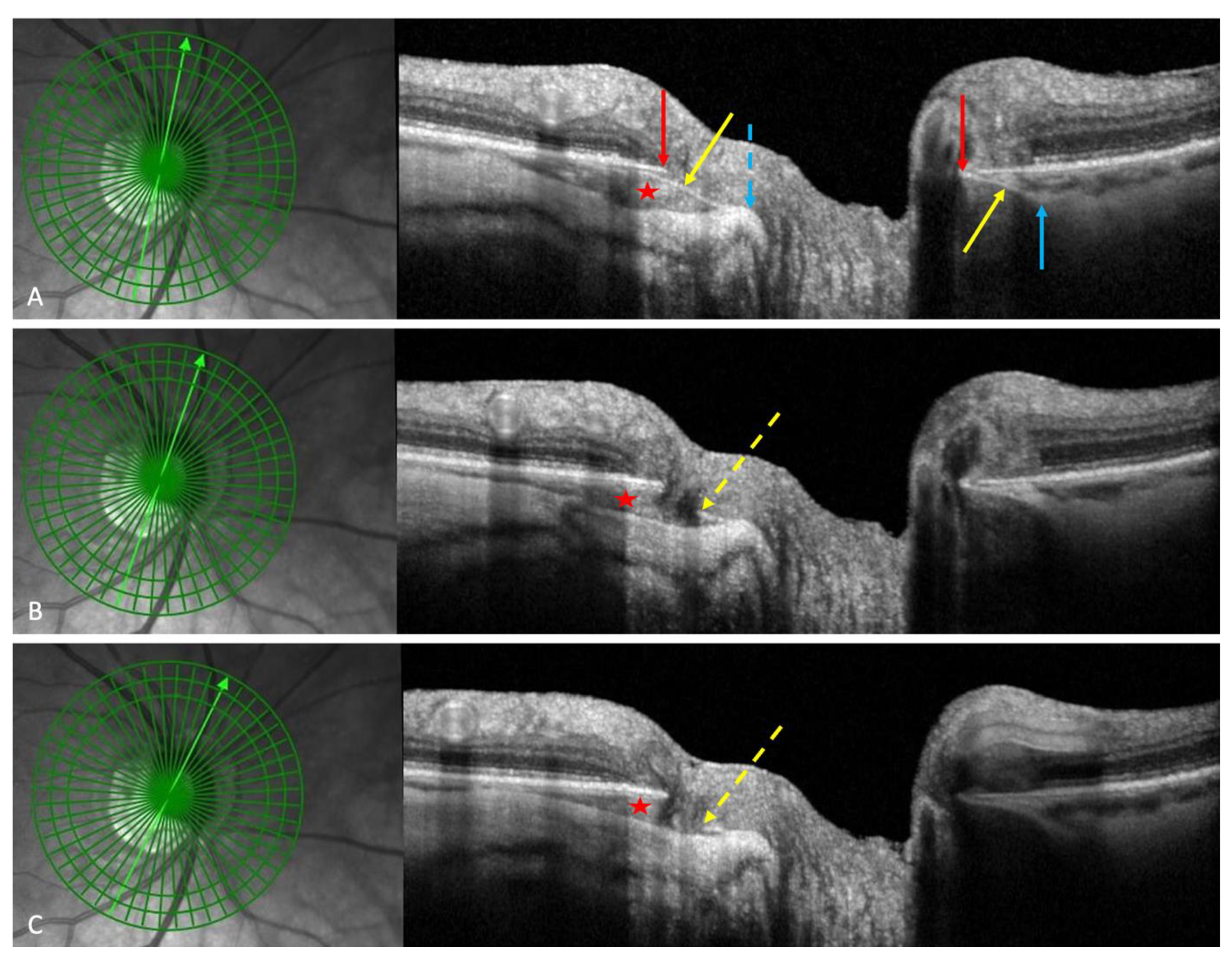
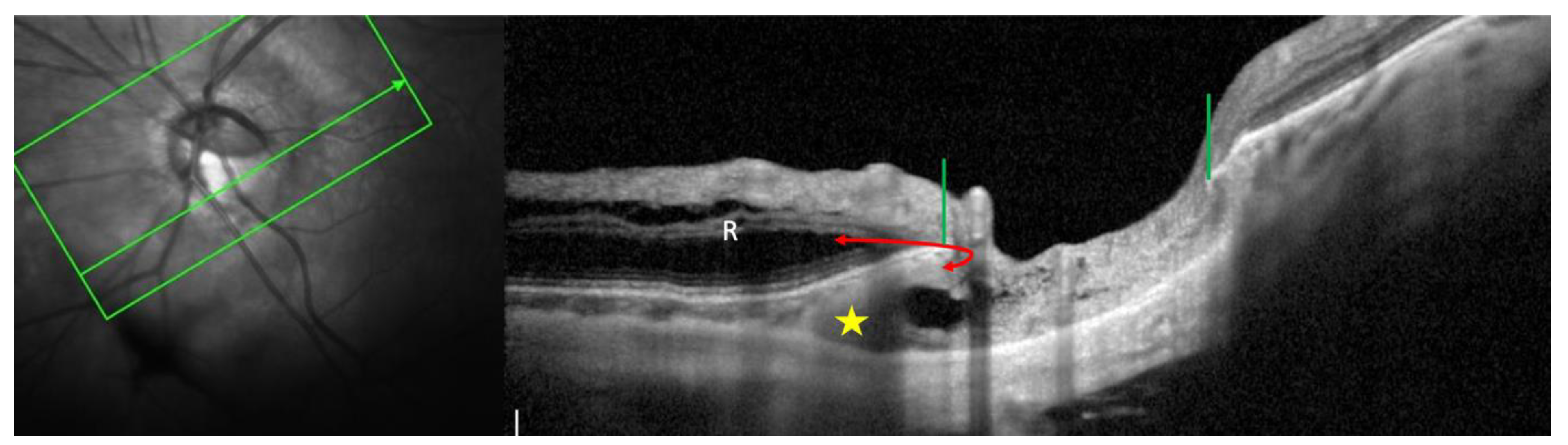
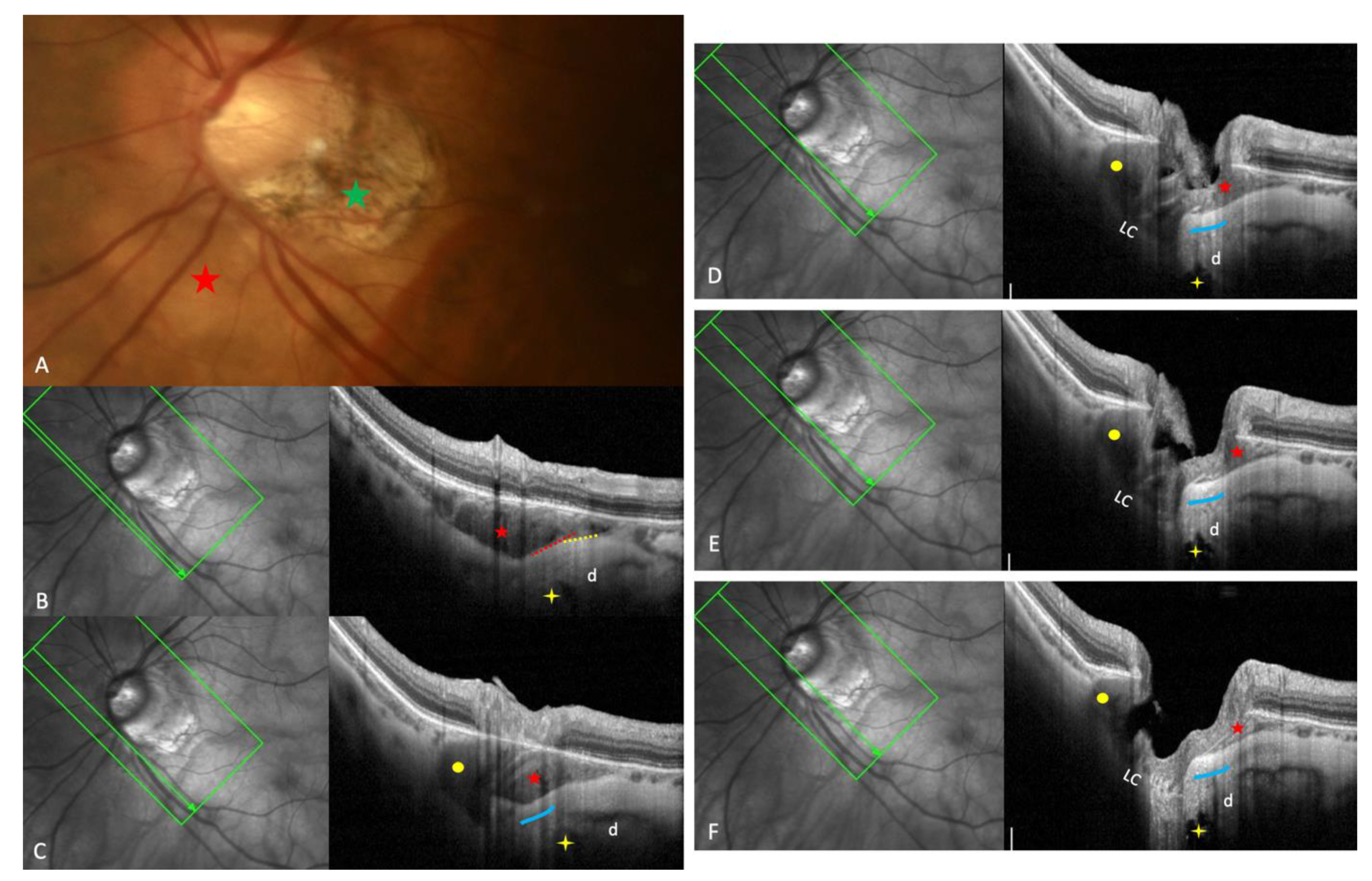
3.1. Fundoscopy and OCT
3.2. Fluorescein angiography
3.3. Indocyanine green angiography
3.4. OCT-Angiography
3.5. Other modalities
4. Association of PICC with other Myopia-related changes
4.1. Gamma peripapillary atrophy
4.2. Tilted disc
4.3. Posterior staphyloma
4.4. Others
5. Structural changes in the vicinity of the PICC
5.1. Peripapillary atrophy
5.2. Choroid
5.3. Posterior scleral curvature
5.4. Border tissue of the choroid
5.5. Vessels
6. Clinical relevance of the PICC
6.1. Visual field defects and PICC
6.2. Macular abnormalities and PICC
7. Differential diagnosis – Natural history of uncomplicated cases of PICC
8. Pathogenetic hypotheses of PICC
8.1. Congenital hypothesis
8.2. Fluidic considerations
8.3. Mechanical considerations
8.3.1. PICC as a complication of peripapillary staphyloma
8.3.2. PICC as a complication of myopic tilted disc and myopic conus
8.3.3. PICC as a complication of the optic nerve sheaths traction
9. Conclusion and perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
|
Year Author [Ref] |
Objective- Material-Methods | Relevant results for PICC | Pathogenetic clues | Strength |
| 2003 Freund [1] |
Objective: To describe a newly recognized fundus lesion in HM Design: retrospective study, Case-series (20 eyes of 15 patients included) Examinations: fundus pictures and OCT (and FA) |
Location: the inferior border of the myopic conus Associations: TD (75%), Myopic conus (100%), PS (90%), Fundus myopic changes (100%) Connection with vitreous at PICC-conus junction in 10% of PICC Stable in the follow-up period (1-15 years) except for one patient with bilateral reduction of PICC. |
Distinct complication of high myopia. Located at the inferior border of the myopic conus. Hypothesis: incomplete form of coloboma or gravitational accumulation of subretinal fluid coming from the optic disc/vitreous. |
First description of PICC and recognition as a distinct fundus anomaly in highly myopic eye Description of a PICC-Vitreous communication in 10% cases. |
| 2005 Toranzo [2] |
Objective: To refine the PICC features Design: Observational case report Examinations: OCT- FA - ICG |
The intrachoroidal location of PICC is revealed (with a preserved RPE plane and no detachment of the RPE) The size of PICC increased during the 10 years follow-up. |
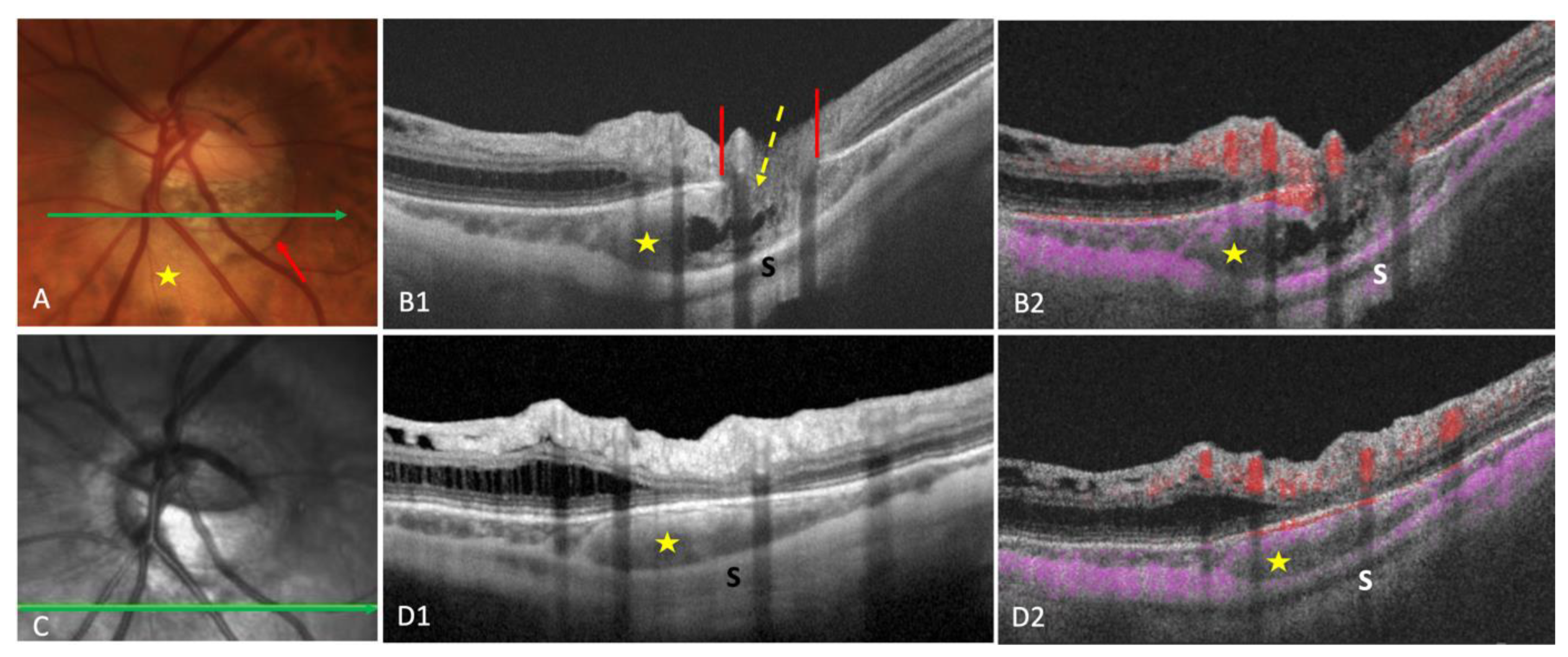
Mechanical hypothesis: progression of posterior staphyloma would stretch and break the BT, resulting in an intrachoroidal cavitation. | Intrachoroidal site of PICC revealed by OCT. Proposition of the current name (PICC) The RPE/BM plane and anterior structures is preserved |
| 2006 Shimada [17] |
Objective: To study the prevalence and clinical characteristics of PICC Analysis of VF in cases of PICC Design: Prospective design (632 eyes with HM included) Comparison between PICC and a control group of HM. Examinations: OCT, FA, ICG and VF |
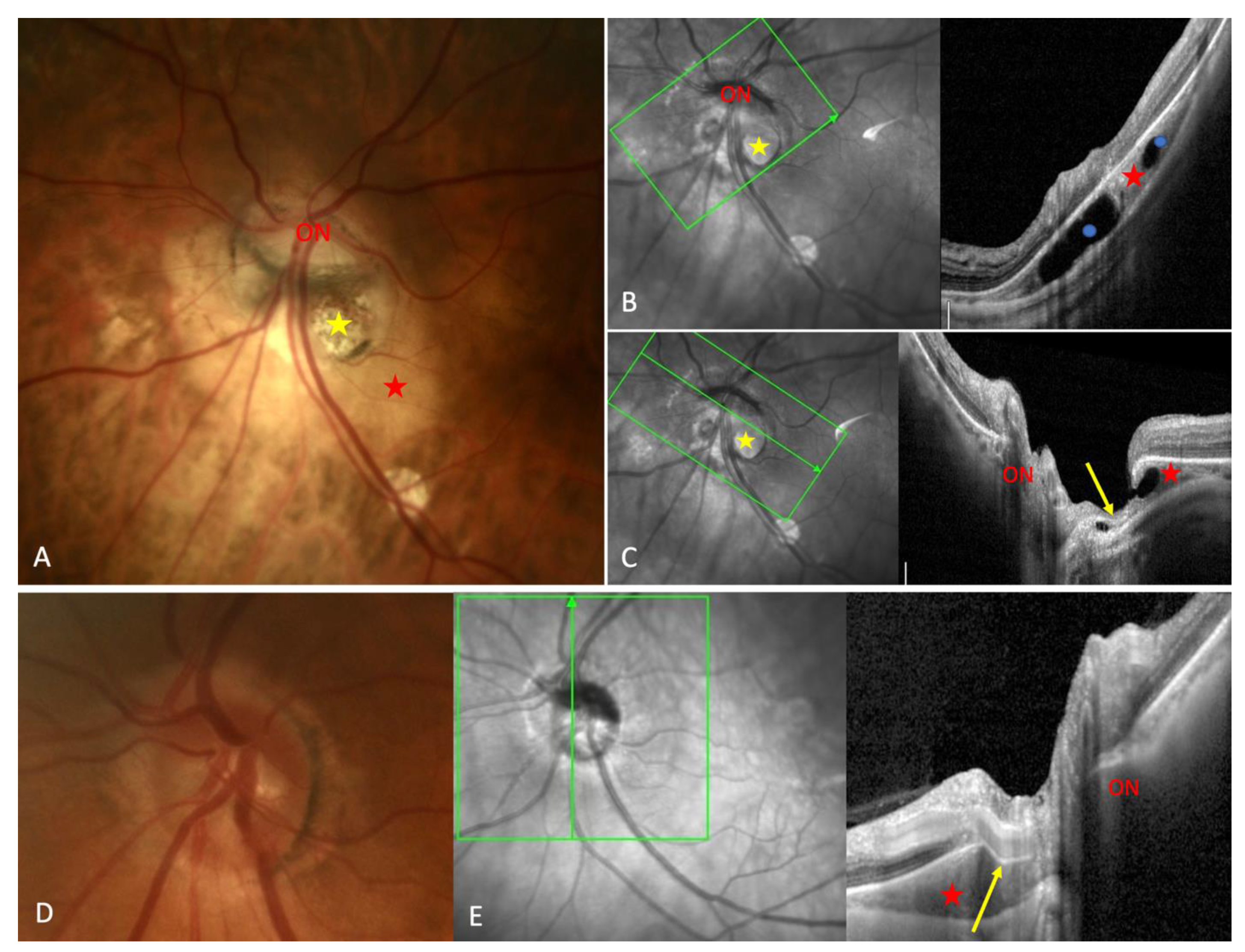
Prevalence:4.9% of PICC in HM (on fundus examination) Location: inferior part in all cases Full thickness defect at the conus-PICC junction in 10% of PICC Marked posterior bowing of the sclera where PICC lies in 83.9% of cases. Association: TD: 93.5%. Myopic conus: 100%. PS: 64.3%. VFD: 71% FA sequence: early hypofluorescence and late hyperfluorescence without dye pooling |
The hypothesis of an incomplete form of coloboma unlikely: Lesion not confined to the inferior sector but can extent around the entire ON. No PICC in young patients: lesion probably acquired. Hypothesis: VFD could be caused by tissular distortion (TD and excavation of the myopic conus) and not by the PICC itself (could explain why the main location of PICC and VF defects don’t always match). |
VFD in 71% of PICC, no consistent correlation with PICC location VFD significantly more frequent in PICC group than in control group. Communication with vitreous cavity in 10%. Marked bending of the infero-temporal vein in 83.9% of PICC. No PICC in subjects under 30 years old. Large series (632 eyes). |
| 2007 Shimada [21] |
Objective: To study peripapillary changes in eyes with HM using OCT. Design: Observational case-series (127 eyes with HM included) Control group with low myopic and emmetropic eyes Examinations: Fundus images, VF, OCT |
Prevalence of PICC in HM on fundus examination: 9.4%. None in controls (emmetropic or low myopia) Prevalence of PICC in HM using OCT: 11%. PICC features on OCT: intrachoroidal hypo-reflective space showing multiple cystic spaces. Retinal full thickness defects: in 7% of PICC. VFD in 64.3% of PICC and in 19.5% of HM without PICC. |
Posterior excavation of conus related to PICC location. Hypothesis: Mechanical stress at peripapillary tissues associated with posterior excavation of the myopic conus could split the intrachoroidal structures and produce cystoid spaces leading to the PICC by coalescence. Description of retinal full thickness defects leading to PICC-vitreous communication |
Glaucoma-like VFD in cases of PICC, and significantly more frequent than in the control group. OCT recommended for PICC diagnosis. |
| 2008 Forte [23] |
Objective: Evaluation of the thickness and lateral extent of PICC using En-face OCT. Design: prospective case-series: 6 eyes/3 patients. Examinations: En-face OCT, FA, ICG, VF |
Retinal full thickness defect in 2/6 eyes, allowing a PICC-vitreous communication. VFD in 4/4 eyes with anormal central fixation. VFD matching the PICC location in 3 of the 4 cases. |
Hypothesis: steep excavation of the myopic conus stretches the retina/RPE complex, causing a splitting of the choroid and a hypo-reflective intrachoroidal space Gravitational accumulation of fluid from the vitreous cavity through a cleft at the border of the PICC could be an additional factor |
VFD in 4/4 eyes (Humphrey) The VFD matched the PICC location in 3 of the 4 cases. |
| 2009 Wei [22] |
Objective: Evaluation of OCT features and clinical aspects of PICC Design: Observational case series (16 PICC diagnosed on fundus) Examinations: OCT, fundus, FA |
Associations: Myopic conus: 100% Vitreous connection at the PICC-conus junction: 46.2% Intrachoroidal cystic spaces: 19% of PICC (intrachoroidal splitting/schisis without optical empty cavity) Inferior location in 94% of cases HM in all cases of PICC except one (moderate myopia) |
Hypothesis: Vitreous fluid would gain access to the suprachoroidal space through the path caused by the breaking of the BT Fluid accumulation induces a choroidal schisis and splitting of the choroidal structures. BT discontinuity is caused by the PS progression |
PICC and choroidal schisis would be different stages of the same pathologic spectrum. PICC are also in other locations than the inferior border of the optic nerve. History of glaucoma in 37.5% of cases |
| 2009 Shimada [6] |
Objective: To report a case of a macular retinal detachment related to a PICC. Design: case report Examinations: OCT, fundus, FA |
Retinal detachment in a highly myopic eye with PICC. No dye leakage on FA. A PICC-vitreous connection at the PICC-vitreous junction and a PICC- retinal detachment connection through subretinal space at the conus area |
Hypothesis: A retinal detachment can complicate a PICC through a subretinal connection with the full retinal thickness defect located at the PICC-conus margin. | PICC can be complicated by retinal detachment. |
| 2011 Freund [27] |
Objective: To study the PICC characteristics using OCT-EDI. Design: Case series Examinations: fundus, OCT-EDI. |
On OCT-EDI, PICC is characterized by thickening of the choroid with or without hypo-reflective “cavitation”. | PICC shows a choroidal thickening with variable hypo-reflectivity. | PICC has several facets of presentation |
| 2012 Spaide [3] |
Objective: To provide a pathogenic hypothesis based on anatomical characteristics of PICC Design: Case-series, 16 PICC included Examinations: fundus images, FA, ICG, SS-OCT and OCT-EDI. |
Associations: TD: 100%, myopic conus: 100%. Vitreous communication: 25% of cases (associated with more prominent cavitation). Posterior scleral bowing with normal retina-RPE plane in all PICCs. dipping of infero-temporal vein into PICC at the conus edge |
Hypothesis: the posterior displacement of the sclera would be the primary cause of PICC. Rupture of BT by excessive stretching might lead to more prominent PICC. |
PICC would start at suprachoroidal space. The radial section of PICC is triangular in the absence of a full thickness defect and shows a more pronounced and rounded protrusion in its presence. |
| 2012 Akimoto [53] |
Objective: To report of a self-limited recurrent macular detachment associated with PICC Design: case report |
Description of a PICC in a low myopic eye (-1D) associated with a retinoschisis and a macular detachment. | A connection between PICC and retinoschisis may promote retinal detachment | Macular detachment in PICC may occur in non-highly myopic eyes. |
| 2013 Yeh [19] |
Objective: Evaluation of the clinical features of peripapillary area associated with PICC Design: Retrospective observational case-series (inclusion of 122 PICC diagnosed on OCT, no control group) Examinations: OCT, VF, Fundus pictures |
Only 46.7% of PICC diagnosed on OCT are detected on fundus examination. Associations: PS: 40.2%, PPA: 98.4%, TD: 69.7%, VFD: 37.7% Maculopathy :14% Non-highly myopic patients with PICC are older (p<0.05) Only 3 PICC in patients younger than 30 Fragmented aspect of the choroidal cavitation seen in 39% Connection with vitreous cavity detected in 16% of PICC (26.4% in case of marked excavation of the myopic conus) Inferotemporal vein bent at the PICC border in 43.4% |
Hypothesis: possible impact of age in the pathogenesis of PICC (due to age-related reduced resorption of fluids). By gravitational effect, fluids would accumulate and form fluid pocket at the inferior border of the myopic conus This could lead to the onset of PICC | Less than 50% of PICC diagnosed on OCT are detected on the fundus examination. The presence of marked excavation of the myopic conus increases the incidence of PICC communication with the vitreous cavity. Implication of age in the pathogenesis of PICC. |
| 2013 You [4] |
Objective: Determine the prevalence, size, location of PICC and their associations Design: Population-based study, (3468 patients included) Examinations: OCT-EDI, fundus pictures |
PICC prevalence: 16.9% in HM (No PICC found in non-highly myopic eyes). Only 53% of PICC diagnosed on OCT were seen on fundus. Associations with TD and PS Location predominantly the inferior area No association with other ocular or systemic parameters |
Hypothesis: Distortions of the posterior fundus associated with PS and TD could be the primary cause of PICC in highly myopic eyes HM is not the primary cause of PICC (no association between PICC and AL on multivariate analysis) |
Only 50% of PICCs are detected on fundus examination. Only TD and PS were associated with PICC. No association with other ocular or systemic parameters. |
| 2013 Ohno [42] |
Objective: Evaluation of ICC located temporal to the optic disc in highly myopic eyes Design: retrospective design, 125 highly myopic eyes included Examinations: SS-OCT, FA |
Prevalence of temporal ICC in highly myopic eyes: 12.8% Temporal ICC are larger than inferior ones Associations: myopic conus (100%), PS (100%) FA and OCT results are similar than those of PICC. Defects of the border tissue detected in some temporal ICC |
ICC can develop in temporal area only, without involving the inferior peripapillary area. Hypothesis: The separation of the temporal ICC develops at the suprachoroidal level (the entire thickness of the choroid remains attached to the RPE) |
Consistent association between temporal ICC and both PS and myopic conus Temporal ICC is a suprachoroidal lesion |
| 2014 Holak [54] |
Comments on the Yeh et al (2013) article | Similarities exist between morphologic features at the border of optic disc coloboma and PICC | They suggest exploring genetic, environmental, congenital impacts on the pathogenesis of PICC. Hypothesis: cystoid spaces found in coloboma or PICC could be different stages of the same spectrum disease |
Mutations in cell adhesion molecules like cadherin could promote coloboma and PICC formation? |
| 2014 Yoshizawa [8] |
Objective: To report a case of retinoschisis with macular detachment in a PICC treated by vitrectomy and outcome Design: case report Examinations: OCT Intervention: Vitrectomy |
Retinoschisis with macular detachment. A connection between PICC and the schisis cavity was disclosed. Epiretinal membrane was adjacent to the PICC-conus connection. Complete regression of retinoschisis and closure of the PICC-retinoschisis channel |
The connection PICC-retinoschisis was suggested to be promoted by the traction on the PICC | Good outcome of macular detachment associated by PICC when treated by vitrectomy. |
| 2014 Rajagopal [7] |
Objective: To report a case of macular detachment in a PICC Design: retrospective, case report Examinations: OCT, FA |
A PICC-vitreous connection at the PICC-vitreous junction and a PICC- retinal detachment connection through subretinal space at the conus area | / | Confirm that a connection between PICC and subretinal space may promote a macular detachment |
| 2014 Dai [46] |
Objective: To describe the course of the inferotemporal vein into peripapillary region and to evaluate the characteristics of beta and gamma PPA Design: case report Examinations: fundus pictures and OCT |
Description of a case combining PICC, PPA and TD The infero-temporal vein disappeared in the Peripapillary area next to ON. On OCT, the detection of a scleral lamellar defect suggests an intrascleral or extra-scleral pathway of this vein |
/ | Description of the abnormal course of the infero-temporal vein in an eye with PICC and PPA and TD |
| 2015 Chen [9] |
Objective: To investigate clinical characteristics and treatment outcomes of macular detachment associated with PICC. Design: retrospective case-series Examinations: |
Depending on the case, a connection between the subretinal space and PICC or peripapillary area was found. Variable results with gas tamponade, topical carbonic inhibitors |
/ | / |
| 2015 Dai [20] |
Objective: Intra-individual comparative study of ON morphology in unilateral PICC. Design: Hospital-based observational Examinations: OCT-EDI, fundus pictures |
Intra-individual comparison: eyes with PICC have lower ovality index, are more tilted, have a shorter vertical diameter and a shorter minimal diameter of ON compared to the contralateral eye. Only 53% of PICC are detected on fundus examination. |
A shorter ovality index implies a rotation of the optic disc around the vertical or horizontal axis. Hypothesis: PICC is caused by the disruption of BT due to excessive disc tilting |
Optic disc is more tilted in eyes with PICC. 53% of PICC detected on OCT are not seen on fundus |
| 2015 Lee [52] |
Objective: Description of a disc haemorrhage associated with a PICC in the absence of a glaucomatous neuropathy Design: Case report Examinations: OCT-EDI, fundus pictures |
A disc haemorrhage associated with PICC in a non-glaucomatous eye lasted more than a year. It showed a disappearance in conjunction with the reduction in size of the PICC and papillary schisis. | Peripapillary haemorrhages may be a manifestation of peripapillary stress. Hypothesis: Mechanical modifications due to PICC may alter the vessels and cause peripapillary haemorrhages without any glaucoma. |
Impact of mechanical damage to peripapillary structures associated with PICC enlargement. |
| 2015 Azar [29] |
Objective: Multimodal imaging of PICC Design: Case report Examinations: SD-OCT, En-face OCT, ICG, FA, VF |
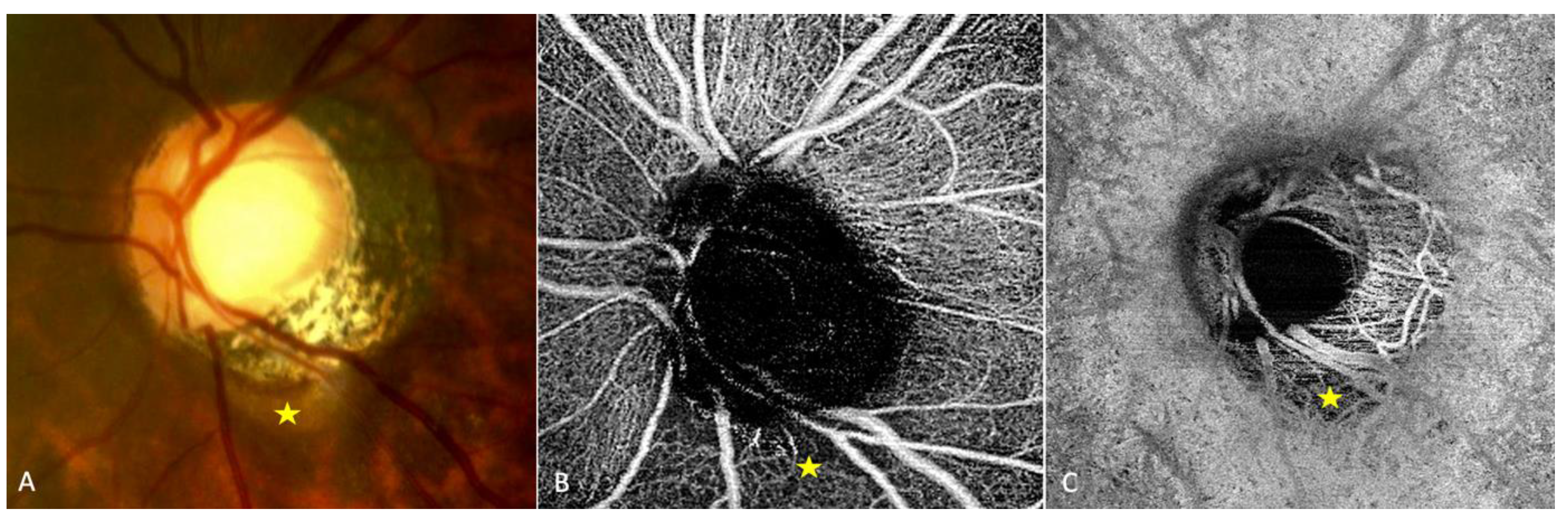
En-face OCT combined with FA show an early hypo fluorescence resulting from the absence of choroidal tissue and a late staining resulting from the scleral impregnation. | / | Early hypo-fluorescence due to choroidal alteration. Late hyper-fluorescence due to scleral impregnation |
| 2015 Ando [10] |
Objective: To report features of a macular detachment associated with PICC and the outcomes of vitrectomy. Design: Retrospective, case-series (3eyes). Examinations: Chart review. OCT. |
All 3 eyes were non highly myopic. No vitreous detachment observed in any case. Definite vitreous-PICC connection in 2 cases. Definite subretinal space-PICC connection in 2 cases. Vitrectomy resolved the macular detachment in all cases. |
/ | Macular detachment can complicate PICC even in non-highly myopic eyes. |
| 2016 Jonas [56] |
Objective: Comment (on Wang’s biomechanical study) | / | Suggestion that the stress exerted by the ON on its head during adduction movement could be part of the pathogenesis of the PICC | Impact of ON biomechanics in the pathogenesis of PICC |
| 2016 Okuma [5] |
Objective: Evaluation of the VF and the macular ganglion cell complex in PICC and evaluation of the similarities between these results and those typical of glaucomatous changes Design: retrospective, 16 eyes affected by PICC included Examinations: OCT, VF |
VF defects were detected in 73.3% of PICC. Good correlation between PICC location and VF defects distribution in 53.3% of cases. Thinning of ganglion cell complex was correlated with PICC location in 66.7%. |
/ | VF defects observed in 75% of PICC. PICC-VF defects correlation present in half of the with a PICC. Ganglion cell complex and VF defects found in PICC are very similar to those observed in early glaucoma. |
| 2016 Kita [25] |
Objective: Study of a PICC associated with a full thickness retinal defect in the papillo-macular bundle Design: Case report Examinations: SS-OCT, VF |
No visual field defect detected in the full-thickness defect located at the papillo-macular. Retina herniated in PICC may preserve some function despite the apparent retinal defect. |
/ | / |
| 2017 Mazzaferro [33] |
Objective: Evaluation of the characteristics of PICC on OCT-A Design: Case report Examinations: OCT-A |
Absence of choroidal and choriocapillary network in the choroidal cavitation PICC associated with a PS and a TD |
/ | Absence of any intra-choroidal vascular tissue in this case of PICC |
| 2017 Chen [30] |
Objective: To study the peripapillary, ONH vasculature by OCT-A in highly myopic eyes with PICC Design: Hospital-based cross-sectional study Examinations: OCT-A |
Highly myopic eyes show a lower peripapillary capillary vessel density than non-highly myopic eyes. The peripapillary vascular density is more reduced in PICC than non-PICC eyes (especially in temporal area) |
/ | The peripapillary vascular density is more reduced in PICC than non-PICC eyes (especially in temporal area) |
| 2018 Chen [34] |
Objective: Multimodal imaging of PICC associated with myopic sinkhole Design: Case report Examinations: fundus image, OCT-EDI US, VF |
Description of a PICC associated with an inferotemporal sinkhole in the myopic conus. Presence of a cleft between the ICC and the vitreous cavity |
Hypothesis: myopic sinkhole could favour the flow of vitreous fluid through the suprachoroidal space and facilitate the formation of a PICC | Possible role of the myopic sinkhole in the physiopathology of PICC |
| 2018 Choudhury [18] |
Objective: Estimation of the prevalence of myopic degeneration in Chinese Americans Design: Population-based cross-sectional Study |
1523 myopic Chinese included (<-0.5D) Prevalence of ICC: 2.2% overall myopic eyes and in the 22% of HM eyes. |
/ | Population-based study (Chinese Americans) |
| 2019 Venkatesh [50] |
Objective: To study the prevalence and clinical characteristics of PICC Design: Case-series. Retrospective, non-interventional, comparative study Examinations: fundus photography, OCT |
Prevalence of ICC in highly myopic eyes: 55.8% (15.8% of PICC and 84.2% of macular ICC) |
/ | There is not a clear separation between two concepts in this study: PPA and patchy chorioretinal atrophy. |
| 2019 Parlak [32] |
Objective: Description of a case of PICC Design: Case report Examinations: Fundus photography, autofluorescence, OCT, OCT-A |
Reduction of the vessel density at the level of the PICC with OCT-A | / | PICC is characterised by a hypo signal on OCT-A |
| 2020 Markan [26] |
Objective: to describe a case of an acquired PICC secondary to intercalary membrane detachment Design: Case report |
Description of an irido-fundal coloboma with intercalary membrane detachment associated with a ICC at the edge of a coloboma. | Hypothesis of a new pathogenic mechanism of ICC formation: the intercalary membrane detachment allows fluid from the sub-ICM communicating with the choroid space | ICC secondary acquired in a case of irido-fundal coloboma |
| 2020 Comune [31] |
Objective: To analyse the vessel density of radial peripapillary capillary in HM with (32eyes) and without (23 eyes) PICC Design: Prospective. Examinations: OCT-A |
Myopic eyes with PICC had a significantly lower vessel density than eyes without PICC, especially those with choroidal neovascularization. | / | Radial peripapillary capillary vessel density is significantly influenced (reduced) by the presence of PICC. |
| 2021 Kim [24] |
Objective: To study the choroidal microvasculature in glaucomatous eyes with PICC Design: Retrospective Examinations: SD-OCT, OCT-A, SS-OCT, Fundus examination, VF |
PICC showed larger hypovascular area on En-face OCT-A 89.4% of PICC had choroidal microvascular dropout (focal sectoral capillary dropout with no visible microvascular network on deep-layers En-face images) in the area proximal to the PICC. Concordance between location of PICC and area of dropout 98% of PICC had hemifield VF defects corelating PICC hemispheric location |
Hypothesis: common pathogenesis of PICC and microvascular dropout due to their close spatial proximity Distortions of peripapillary tissues (due to tensile stress) induce both PICC and damage of RNFL and micro-vessels leading to microvascular dropout |
98% VFD corresponding to the PICC hemispheric location. Probable common pathogenic mechanisms of PICC and microvascular dropout in glaucoma OCT-A characteristics of PICC |
| 2021 Liu [12] |
Objective: Characterisation of PICC in Chinese highly myopic eyes and its associated risk factors Design: Observational cross-sectional study, 890 patients with HM included Examinations: fundus photography, OCT |
Prevalence of PICC in high myopia 3.6% (diagnosis based on the presence of typical lesion on both the fundus and the OCT) Location mainly inferior (87.5%), multiple (9.4%), superior (3.1%) Association with age, axial length and myopic spherical equivalent (based on the multiple linear logistic regression model) |
Hypothesis: Impact of mechanical forces. Axial elongation during myopia progression stretches the posterior tissues leading to the appearance and progression of PICC. The lack of overlying tissues and relative thinness of the myopic conus leads to more pronounced deformation and is thus more susceptible to mechanical stress. |
Prevalence of PICC in a large highly myopic population with a wide range of age (7-70 years old) PICC more frequent in eyes with severe myopic maculopathy and eyes with PS |
| 2022 Fujimoto [36] |
Objective: To evaluate 3D parameters of PICC using SS-OCT and deep learning and to correlate with VF sensitivity Design: Retrospective Examinations: SS-OCT, deep learning |
The correlation between 3D volume of PICC and VF sensitivity | / | The 3D rendering has a potential to improve detection and pathological understanding of PICC |
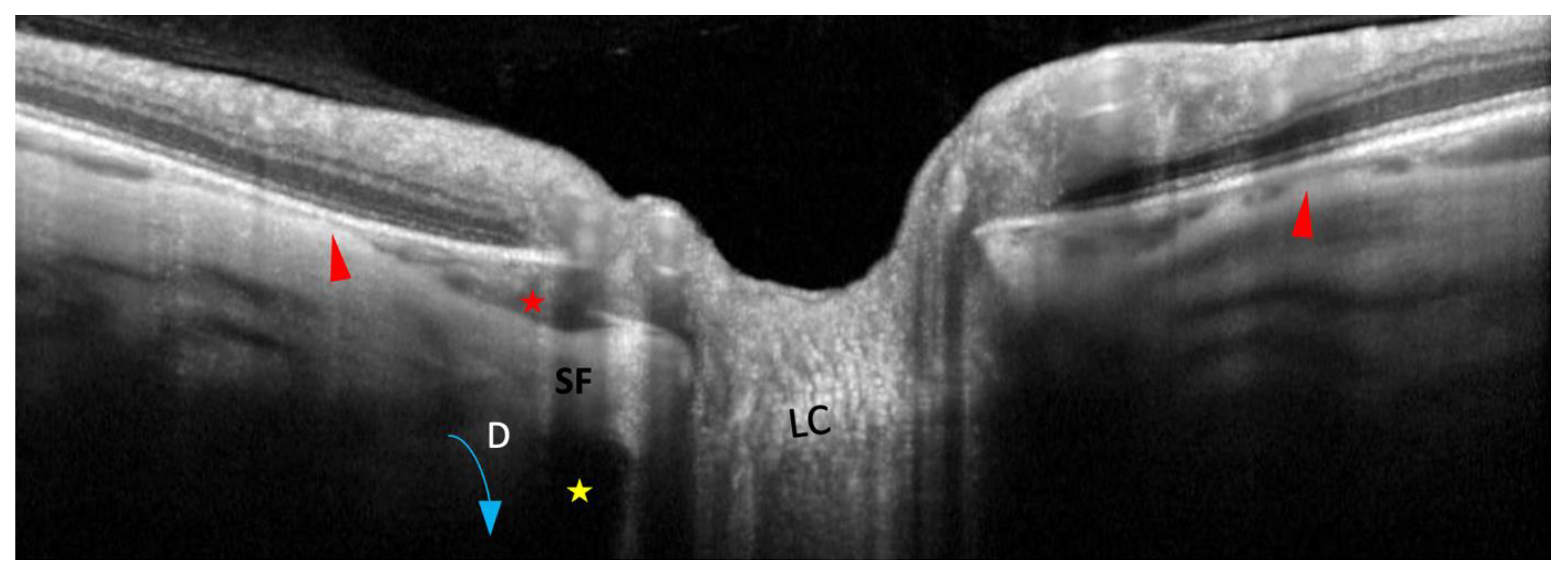
| 2022 Ehongo [16] |
Objective: To compare the peripapillary polar regions in eyes with gamma PPA and PPS in the presence or absence of PICC Design: Observational cross-sectional study, Examinations: fundus pictures, serial SD-OCT |
PICC is a suprachoroidal detachment. PICC is aligned with the subarachnoid space. PICC is detected up on the visualisation of the ON sheaths. |
Hypothesis: The pulling of ON sheaths on the scleral flange during eye movements would promote PICC. | Confirmation that PICC is as suprachoroidal detachment. Suggestion that it is promoted by tractions of the ON sheaths during eye movement. |
| 2022 Aoki [11] |
Objective: To report a case of macular lamellar hole with retinoschisis in a PICC eye that underwent vitrectomy with gas tamponade Design: Case report Examinations: OCT Intervention: Vitrectomy |
Non-highly myopic eye. Anatomical recovery after vitrectomy with gas tamponade Visual acuity improvement. But a full thickness defect at the PICC-conus junction appeared after vitrectomy. |
/ | Retinoschisis with lamellar macular hole may complicate a PICC even in non-highly myopic eyes. |
| BM: Bruch membrane. BT: border tissue. ICG: indocyanine green angiography. FA: fluorescein angiography. HM: high myopia. OCT: Optical coherence tomography. SD-OCT: spectral-domain OCT. SS-OCT: swept-source OCT. OCT-A: OCT angiography. ON: optic nerve. OHN: optic nerve head. PICC: peripapillary intra-choroidal cavitation. PPA: peripapillary atrophy, PS: posterior staphyloma, RNFL: retinal nerve fibre layer. RPE: retinal pigment epithelium. TD: tilted disc. VF: visual field. VFD: visual field defects. | ||||
References
- Freund KB, Ciardella AP, Yannuzzi LA, Pece A, Goldbaum M, Kokame GT, Orlock D. Peripapillary detachment in pathologic myopia. Arch Ophthalmol. 2003 Feb;121(2):197-204. PMID: 12583785. [CrossRef]
- Toranzo J, Cohen SY, Erginay A, Gaudric A. Peripapillary intrachoroidal cavitation in myopia. Am J Ophthalmol. 2005 Oct;140(4):731-2. PMID: 16226529. [CrossRef]
- Spaide RF, Akiba M, Ohno-Matsui K. Evaluation of peripapillary intrachoroidal cavitation with swept source and enhanced depth imaging optical coherence tomography. Retina. 2012 Jun;32(6):1037-44. PMID: 22466483. [CrossRef]
- You QS, Peng XY, Chen CX, Xu L, Jonas JB. Peripapillary intrachoroidal cavitations. The Beijing eye study. PLoS One. 2013 Oct 24;8(10):e78743. PMID: 24302981; PMCID: PMC3840228. [CrossRef]
- Okuma S, Mizoue S, Ohashi Y. Visual field defects and changes in macular retinal ganglion cell complex thickness in eyes with intrachoroidal cavitation are similar to those in early glaucoma. Clin Ophthalmol. 2016 Jun 29;10:1217-22. PMID: 27418805; PMCID: PMC4935007. [CrossRef]
- Shimada N, Ohno-Matsui K, Iwanaga Y, Tokoro T, Mochizuki M. Macular retinal detachment associated with peripapillary detachment in pathologic myopia. Int Ophthalmol. 2009 Apr;29(2):99-102. Epub 2007 Nov 22. PMID: 18034213. [CrossRef]
- Rajagopal J, H CK 5th, Ganesh S. Macular detachment associated with peripapillary detachment in pathologic myopia. Retin Cases Brief Rep. 2014 Spring;8(2):103-6. PMID: 25372320. [CrossRef]
- Yoshizawa C, Saito W, Noda K, Ishida S. Pars plana vitrectomy for macular schisis associated with peripapillary intrachoroidal cavitation. Ophthalmic Surg Lasers Imaging Retina. 2014 Jul-Aug;45(4):350-3. Epub 2014 Jun 30. PMID: 24972183. [CrossRef]
- Chen TC, Yang CH, Sun JP, Chen MS, Yang CM. Macular retinal detachment associated with intrachoroidal cavitation in myopic patients. Graefes Arch Clin Exp Ophthalmol. 2015 Sep;253(9):1437-46. Epub 2014 Nov 4. PMID: 25367830. [CrossRef]
- Ando Y, Inoue M, Ohno-Matsui K, Kusumi Y, Iida T, Hirakata A. Macular detachment associated with Intrachoroidal Cavitation in nonpathological myopic eyes. Retina. 2015 Oct;35(10):1943-50. PMID: 26035397. [CrossRef]
- Aoki S, Imaizumi H. Vitrectomy for macular retinoschisis associated with peripapillary intrachoroidal cavitations in a moderately myopic eye. Int J Retina Vitreous. 2022 Sep 5;8(1):62. PMID: 36064619; PMCID: PMC9446725. [CrossRef]
- Liu R, Li Z, Xiao O, Zhang J, Guo X, Loong Lee JT, Wang D, Lee P, Jong M, Sankaridurg P, He M.Characteristics of peripapillary intrachoroidal cavitation in highly myopic eyes: The Zhongshan Ophthalmic Center-Brien Holden Vision Institute High Myopia Cohort Study. Retina. 2021 May 1;41(5):1057-1062. PMID: 32833786. [CrossRef]
- Wang X, Rumpel H, Lim WE, Baskaran M, Perera SA, Nongpiur ME, Aung T, Milea D, Girard MJ. Finite Element Analysis Predicts Large Optic Nerve Head Strains During Horizontal Eye Movements. Invest Ophthalmol Vis Sci. 2016 May 1;57(6):2452-62. PMID: 27149695. [CrossRef]
- Demer JL. Optic Nerve Sheath as a Novel Mechanical Load on the Globe in Ocular Duction. Invest Ophthalmol Vis Sci. 2016 Apr;57(4):1826-38. PMID: 27082297; PMCID: PMC4849549. [CrossRef]
- Chang MY, Shin A, Park J et al. Deformation of Optic Nerve Head and Peripapillary Tissues by Horizontal Duction. Am J Ophthalmol. 2017 Feb; 174:85-94. Epub 2016 Oct 15. PMID: 27751810; PMCID: PMC5812679. [CrossRef]
- Ehongo A, Bacq N, Kisma N, Dugauquier A, Alaoui Mhammedi Y, Coppens K, Bremer F, Leroy K. Analysis of Peripapillary Intrachoroidal Cavitation and Myopic Peripapillary Distortions in Polar Regions by Optical Coherence Tomography. Clin Ophthalmol. 2022 Aug 13;16:2617-2629. PMID: 35992567; PMCID: PMC9387167. [CrossRef]
- Shimada N, Ohno-Matsui K, Yoshida T, Yasuzumi K, Kojima A, Kobayashi K, Futagami S, Tokoro T, Mochizuki M. Characteristics of peripapillary detachment in pathologic myopia. Arch Ophthalmol. 2006 Jan;124(1):46-52. PMID: 16401784. [CrossRef]
- Choudhury F, Meuer SM, Klein R, Wang D, Torres M, Jiang X, McKean-Cowdin R, Varma R; Chinese American Eye Study Group. Prevalence and Characteristics of Myopic Degeneration in an Adult Chinese American Population: The Chinese American Eye Study. Am J Ophthalmol. 2018 Mar;187:34-42. Epub 2017 Dec 27. PMID: 29288031; PMCID: PMC5837945. [CrossRef]
- Yeh SI, Chang WC, Wu CH, Lan YW, Hsieh JW, Tsai S, Chen LJ. Characteristics of peripapillary choroidal cavitation detected by optical coherence tomography. Ophthalmology. 2013 Mar;120(3):544-552. Epub 2012 Dec 1. PMID: 23207174. [CrossRef]
- Dai Y, Jonas JB, Ling Z, Wang X, Sun X. Unilateral peripapillary intrachoroidal cavitation and optic disk rotation. Retina. 2015 Apr;35(4):655-9. PMID: 25299968. [CrossRef]
- Shimada N, Ohno-Matsui K, Nishimuta A, Tokoro T, Mochizuki M. Peripapillary changes detected by optical coherence tomography in eyes with high myopia. Ophthalmology. 2007 Nov;114(11):2070-6. Epub 2007 Jun 1. PMID: 17543388. [CrossRef]
- Wei YH, Yang CM, Chen MS, Shih YF, Ho TC. Peripapillary intrachoroidal cavitation in high myopia: reappraisal. Eye (Lond). 2009 Jan;23(1):141-4. Epub 2007 Aug 24. PMID: 17721499. [CrossRef]
- Forte R, Pascotto F, Cennamo G, de Crecchio G. Evaluation of peripapillary detachment in pathologic myopia with en face optical coherence tomography. Eye (Lond). 2008 Jan;22(1):158-61. Epub 2006 Dec 15. PMID: 17173013. [CrossRef]
- Kim J, Kim J, Lee EJ, Kim TW. Parapapillary Intrachoroidal Cavitation in Glaucoma: Association with Choroidal Microvasculature Dropout. Korean J Ophthalmol. 2021 Feb;35(1):44-50. Epub 2020 Dec 11. PMID: 33307621; PMCID: PMC7904409. [CrossRef]
- Kita Y, Inoue M, Hollό G, Kita R, Sano M, Hirakata A. Preserved retinal sensitivity in spatial correspondence to an intrachoroidal cavitation area with full thickness retinal defect: a case report. BMC Ophthalmol. 2016 Oct 26;16(1):186. PMID: 27784274; PMCID: PMC5081664. [CrossRef]
- Markan A, Jain M, Singh R. Secondary intrachoroidal cavitation in a case of iridofundal coloboma. Med Hypotheses. 2020 Oct;143:110085. Epub 2020 Jul 9. PMID: 32721794. [CrossRef]
- Freund KB, Mukkamala SK, Cooney MJ. Peripapillary choroidal thickening and cavitation. Arch Ophthalmol. 2011 Aug;129(8):1096-7. PMID: 21825199. [CrossRef]
- Marticorena-Álvarez P, Clement-Fernández F, Iglesias-Ussel L. Peripapillary intrachoroidal cavitation in pathological myopia. Arch Soc Esp Oftalmol. 2014 Aug;89(8):316-9. English, Spanish. Epub 2013 Aug 3. PMID: 24269422. [CrossRef]
- Azar G, Leze R, Affortit-Demoge A, Faure C. Peripapillary Intrachoroidal Cavitation in Myopia Evaluated with Multimodal Imaging Comprising "En-Face" Technique. Case Rep Ophthalmol Med. 2015;2015:890876. Epub 2015 Oct 12. PMID: 26543655; PMCID: PMC4620261. [CrossRef]
- Chen Q, He J, Hua Y, Fan Y. Exploration of peripapillary vessel density in highly myopic eyes with peripapillary intrachoroidal cavitation and its relationship with ocular parameters using optical coherence tomography angiography. Clin Exp Ophthalmol. 2017 Dec;45(9):884-893. Epub 2017 Jun 15. PMID: 28494517. [CrossRef]
- Comune C, Montorio D, Cennamo G. Optical coherence tomography angiography in myopic peripapillary intrachoroidal cavitation complicated by choroidal neovascularization. Eur J Ophthalmol. 2021 Jul;31(4):1920-1924. Epub 2020 Jul 16. PMID: 32674595. [CrossRef]
- Parlak M, Ipek SC, Saatci AO. Peripapilläre Aufhellung bei hoher Myopie: nur ein Staphyloma posticum? [Peripapillary whitening in high myopia: only a staphyloma posticum?]. Ophthalmologe. 2020 Apr;117(4):379-383. German. PMID: 31511965. [CrossRef]
- Mazzaferro A, Carnevali A, Zucchiatti I, Querques L, Bandello F, Querques G. Optical coherence tomography angiography features of intrachoroidal peripapillary cavitation. Eur J Ophthalmol. 2017 Mar 10;27(2):e32-e34. PMID: 28233894. [CrossRef]
- Chen Y, Ma X, Hua R. Multi-modality imaging findings of huge intrachoroidal cavitation and myopic peripapillary sinkhole. BMC Ophthalmol. 2018 Feb 2;18(1):24. PMID: 29394916; PMCID: PMC5797380. [CrossRef]
- Chawla R, Kumar A, Mandal S. Three-Dimensional Reconstruction Imaging of Peripapillary Intrachoroidal Cavitation in a Myopic Patient. Ophthalmol Retina. 2019 Nov;3(11):928. PMID: 31699309. [CrossRef]
- Fujimoto S, Miki A, Maruyama K, Mei S, Mao Z, Wang Z, Chan K, Nishida K. Three-Dimensional Volume Calculation of Intrachoroidal Cavitation Using Deep-Learning-Based Noise Reduction of Optical Coherence Tomography. Transl Vis Sci Technol. 2022 Jul 8;11(7):1. PMID: 35802370; PMCID: PMC9279919. [CrossRef]
- Kim TW, Kim M, Weinreb RN, Woo SJ, Park KH, Hwang JM. Optic disc change with incipient myopia of childhood. Ophthalmology. 2012 Jan;119(1):21-6.e1-3. Epub 2011 Oct 5. PMID: 21978594. [CrossRef]
- Kim M, Choung HK, Lee KM, Oh S, Kim SH. Longitudinal Changes of Optic Nerve Head and Peripapillary Structure during Childhood Myopia Progression on OCT: Boramae Myopia Cohort Study Report 1. Ophthalmology. 2018 Aug;125(8):1215-1223. Epub 2018 Mar 14. PMID: 29550000. [CrossRef]
- Jonas JB, Jonas SB, Jonas RA, Holbach L, Dai Y, Sun X, Panda-Jonas S. Parapapillary atrophy: histological gamma zone and delta zone. PLoS One. 2012;7(10):e47237. Epub 2012 Oct 18. PMID: 23094040; PMCID: PMC3475708. [CrossRef]
- Dai Y, Jonas JB, Huang H, Wang M, Sun X. Microstructure of parapapillary atrophy: beta zone and gamma zone. Invest Ophthalmol Vis Sci. 2013 Mar 19;54(3):2013-8. PMID: 23462744. [CrossRef]
- Tay E, Seah SK, Chan SP, Lim AT, Chew SJ, Foster PJ, Aung T. Optic disk ovality as an index of tilt and its relationship to myopia and perimetry. Am J Ophthalmol. 2005 Feb;139(2):247-52. PMID: 15733984. [CrossRef]
- Ohno-Matsui K, Shimada N, Akiba M, et al. Characteristics of intrachoroidal cavitation located temporal to optic disc in highly myopic eyes. Eye (Lond) 2013; 27:630–638.
- Shinohara K, Moriyama M, Shimada N, Yoshida T, Ohno-Matsui K. Characteristics of Peripapillary Staphylomas Associated With High Myopia Determined by Swept-Source Optical Coherence Tomography. Am J Ophthalmol. 2016 Sep;169:138-144. Epub 2016 Jun 27. PMID: 27365146. [CrossRef]
- Ohno-Matsui K. Proposed classification of posterior staphylomas based on analyses of eye shape by three-dimensional magnetic resonance imaging and wide-field fundus imaging. Ophthalmology. 2014 Sep;121(9):1798-809. Epub 2014 May 9. PMID: 24813630. [CrossRef]
- Wang X, Fisher LK, Milea D, Jonas JB, Girard MJ. Predictions of Optic Nerve Traction Forces and Peripapillary Tissue Stresses Following Horizontal Eye Movements. Invest Ophthalmol Vis Sci. 2017 Apr 1;58(4):2044-2053. PMID: 28384725. [CrossRef]
- Dai Y, Jonas JB, Ling Z, Sun X. Temporal inferior vein submerging into intrachoroidal cavitation and gamma zone. Retin Cases Brief Rep. 2014 Spring;8(2):110-2. PMID: 25372322. [CrossRef]
- Anderson DR. Ultrastructure of human and monkey lamina cribrosa and optic nerve head. Arch Ophthalmol. 1969 Dec;82(6):800-14. PMID: 4982225. [CrossRef]
- Reis AS, Sharpe GP, Yang H, Nicolela MT, Burgoyne CF, Chauhan BC. Optic disc margin anatomy in patients with glaucoma and normal controls with spectral domain optical coherence tomography. Ophthalmology. 2012 Apr;119(4):738-47. Epub 2012 Jan 4. PMID: 22222150; PMCID: PMC3319857. [CrossRef]
- Sawada Y, Araie M, Shibata H, Ishikawa M, Iwata T, Yoshitomi T. Optic Disc Margin Anatomic Features in Myopic Eyes with Glaucoma with Spectral-Domain OCT. Ophthalmology. 2018 Dec;125(12):1886-1897. Epub 2018 Aug 23. PMID: 30144950. [CrossRef]
- Venkatesh R, Jain K, Aseem A, Kumar S, Yadav NK. Intrachoroidal cavitation in myopic eyes. Int Ophthalmol. 2020 Jan;40(1):31-41. Epub 2019 Jul 12. PMID: 31300972. [CrossRef]
- Belamkar AV, Dolan J, Olatunji S, Bhatti MT, Chen JJ, Mansukhani SA. The 'Fault' Lies in the Choroid: Peripapillary Intrachoroidal Cavitation Presenting with Progressive Vision Loss. Neuroophthalmology. 2022 Jan 25;46(4):254-257. PMID: 35859631; PMCID: PMC9291674. [CrossRef]
- Lee KM, Lee EJ, Lee SH, Kim TW. Disc haemorrhage associated with an enlarged peripapillary intrachoroidal cavitation in a non-glaucomatous myopic eye: a case report. BMC Ophthalmol. 2015 Oct 29;15:145. PMID: 26511202; PMCID: PMC4625871. [CrossRef]
- Akimoto M, Akagi T, Okazaki K, Chihara E. Recurrent macular detachment and retinoschisis associated with intrachoroidal cavitation in a normal eye. Case Rep Ophthalmol. 2012 May;3(2):169-74. Epub 2012 May 16. PMID: 22679435; PMCID: PMC3369248. [CrossRef]
- Holak SA, Holak N, Holak HM. Peripapillary choroidal cavitation. Ophthalmology. 2014 Jan;121(1):e6-e7. Epub 2013 Nov 16. PMID: 24252822. [CrossRef]
- Wang X, Beotra MR, Tun TA, Baskaran M, Perera S, Aung T, Strouthidis NG, Milea D, Girard MJ. In Vivo 3-Dimensional Strain Mapping Confirms Large Optic Nerve Head Deformations Following Horizontal Eye Movements. Invest Ophthalmol Vis Sci. 2016 Oct 1;57(13):5825-5833. PMID: 27802488. [CrossRef]
- Jonas JB, Dai Y, Panda-Jonas S. Peripapillary Suprachoroidal Cavitation, Parapapillary Gamma Zone and Optic Disc Rotation Due to the Biomechanics of the Optic Nerve Dura Mater. Invest Ophthalmol Vis Sci. 2016 Aug 1;57(10):4373. PMID: 27557435. [CrossRef]
- Ehongo A, Dugauquier A, Alaoui Mhammedi Y. General shape of the optic nerve head in one high myopic glaucomatous patient before and after trabeculectomy. Int J Ophthalmol. 2020 Nov 18;13(11):1836-1838. PMID: 33215019; PMCID: PMC7590865. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




