Submitted:
11 May 2023
Posted:
12 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Epidemiological Evidence Linking Thyroid Nodules, Thyroid Cancer and Obesity
3. Obesity as a Chronic Inflammatory Condition
4. Gender and Adipose Tissue
5. Leptin and the Hypothalamic—Pituitary—Thyroid (HPT) Axis
6. Metabolic Syndrome, Hyperinsulinemia, and Insulin-like Growth Factor-1
7. Adipose Tissue and TSH Levels
8. Oxidative Stress and Diet
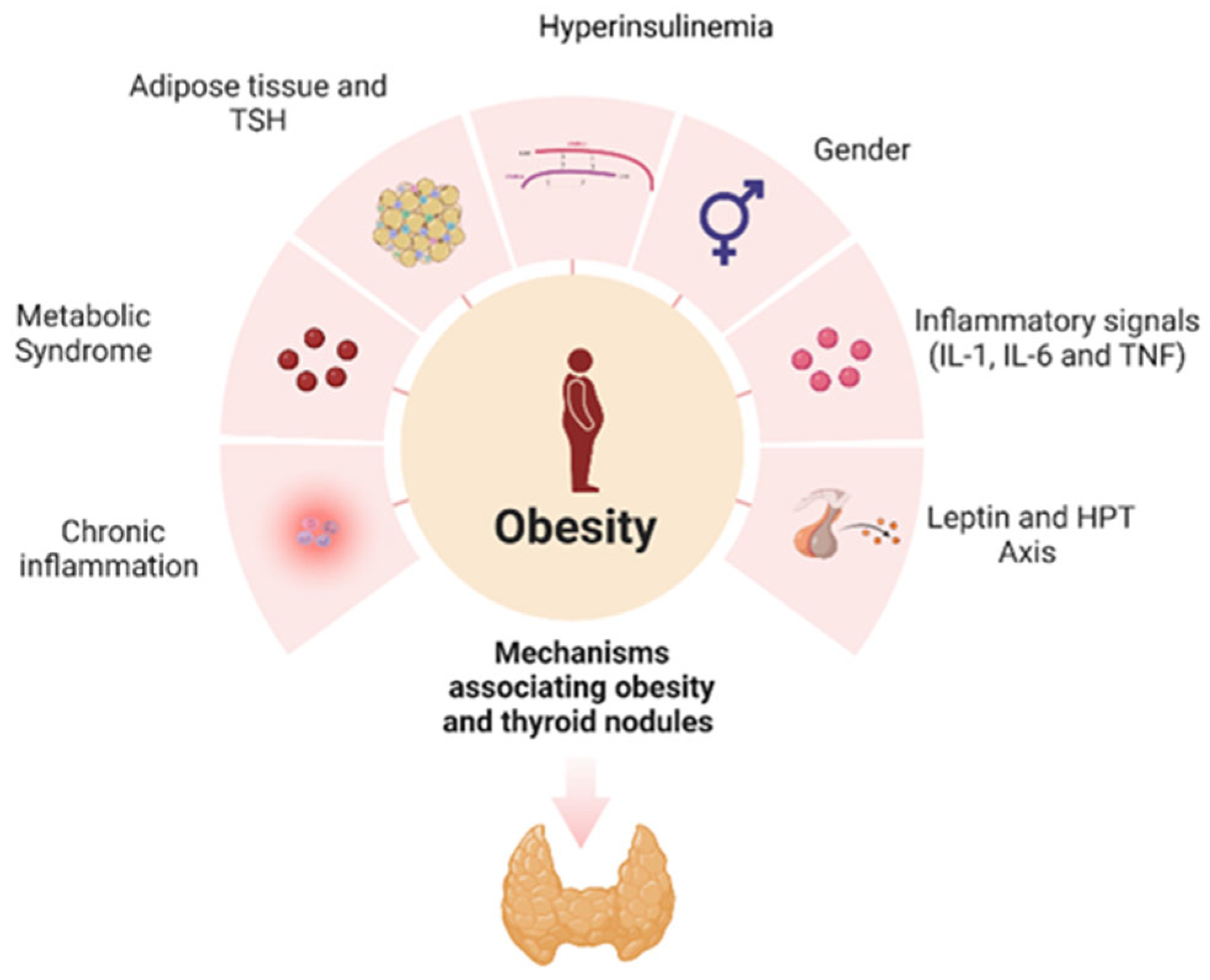
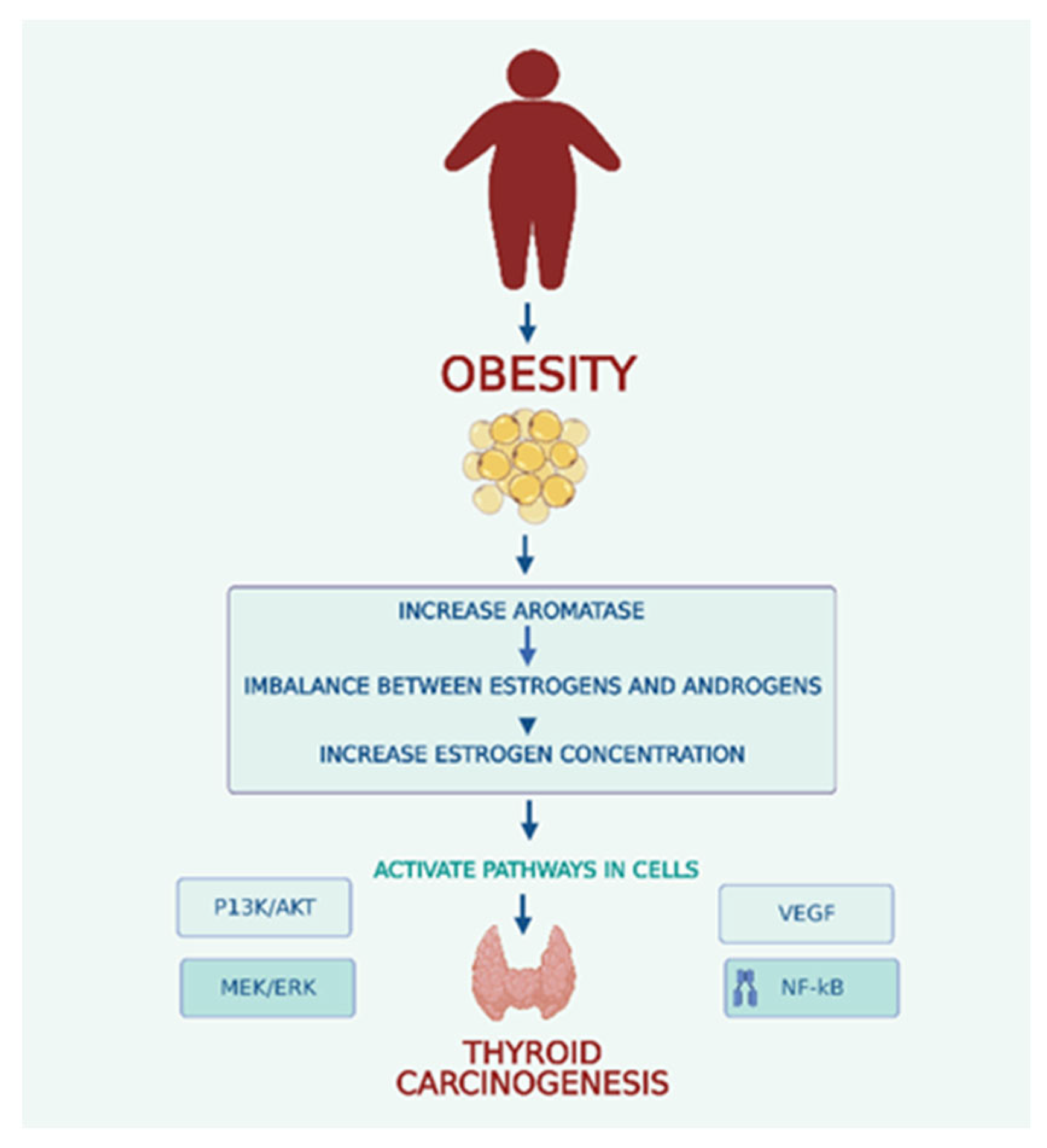
9. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Avgerinos, K. I.; Spyrou, N.; Mantzoros, C. S.; Dalamaga, M. Obesity and Cancer Risk: Emerging Biological Mechanisms and Perspectives. Metabolism: Clinical and Experimental. W.B. Saunders March 1, 2019, pp 121–135. [CrossRef]
- Iyengar, N. M.; Gucalp, A.; Dannenberg, A. J.; Hudis, C. A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. Journal of Clinical Oncology 2016, 34 (35), 4270–4276. [CrossRef]
- Steele, C. B.; Thomas, C. C.; Henley, S. J.; Massetti, G. M.; Galuska, D. A.; Agurs-Collins, T.; Puckett, M.; Richardson, L. C. Morbidity and Mortality Weekly Report Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity-United States, 2005-2014; 2017; Vol. 6.
- Schmid, D.; Ricci, C.; Behrens, G.; Leitzmann, M. F. Adiposity and Risk of Thyroid Cancer: A Systematic Review and Meta-Analysis. Obesity Reviews. December 1, 2015, pp 1042–1054. [CrossRef]
- Durante, C.; Grani, G.; Lamartina, L.; Filetti, S.; Mandel, S. J.; Cooper, D. S. The Diagnosis and Management of Thyroid Nodules a Review. JAMA - Journal of the American Medical Association. American Medical Association March 6, 2018, pp 919–924.
- Ferrari, S. M.; Fallahi, P.; Galdiero, M. R.; Ruffilli, I.; Elia, G.; Ragusa, F.; Paparo, S. R.; Patrizio, A.; Mazzi, V.; Varricchi, G.; Marone, G.; Antonelli, A. Immune and Inflammatory Cells in Thyroid Cancer Microenvironment. International Journal of Molecular Sciences. MDPI AG September 1, 2019. [CrossRef]
- Masone, S.; Velotti, N.; Savastano, S.; Filice, E.; Serao, R.; Vitiello, A.; Berardi, G.; Schiavone, V.; Musella, M. Clinical Medicine Morbid Obesity and Thyroid Cancer Rate. A Review of Literature. J. Clin. Med 2021, 10, 1894.
- Tumminia, A.; Vinciguerra, F.; Parisi, M.; Graziano, M.; Sciacca, L.; Baratta, R.; Frittitta, L. Adipose Tissue, Obesity and Adiponectin: Role in Endocrine Cancer Risk. International Journal of Molecular Sciences. MDPI AG June 2, 2019. [CrossRef]
- Derwahl, M.; Nicula, D. Estrogen and Its Role in Thyroid Cancer. Endocr Relat Cancer 2014, 21 (5), T273–T283. [CrossRef]
- Bener, A.; Özdenkaya, Y.; Barışık, C. C.; Öztürk, M. The Impact of Metabolic Syndrome on Increased Risk of Thyroid Nodules and Size. Health Serv Res Manag Epidemiol 2018, 5, 233339281877551.
- Buscemi, S.; Massenti, F. M.; Vasto, S.; Galvano, F.; Buscemi, C.; Corleo, D.; Barile, A. M.; Rosafio, G.; Rini, N.; Giordano, C. Association of Obesity and Diabetes with Thyroid Nodules. Endocrine 2018, 60 (2), 339–347. [CrossRef]
- Fernández-Trujillo, C.; Pérez-Zaballos, J.; Rodríguez-Pérez, C. A.; López-Plasencia, Y.; Marrero-Arencibia, D.; Cabrera-Galván, J. J.; Boronat, M. TSH Level and Risk of Malignancy in Patients with Bethesda Category IV Thyroid Nodules. 2020. [CrossRef]
- Jiang, H.; Tian, Y.; Yan, W.; Kong, Y.; Wang, H.; Wang, A.; Dou, J.; Liang, P.; Mu, Y. The Prevalence of Thyroid Nodules and an Analysis of Related Lifestyle Factors in Beijing Communities. Int J Environ Res Public Health 2016, 13 (4). [CrossRef]
- Yao, Y.; Chen, X.; Wu, S.; Guo, L.; Zhang, H.; Zhu, Q.; Tang, J.; Luan, F.; Zhao, Y.; Lv, F.; He, Y. Thyroid Nodules in Centenarians: Prevalence and Relationship to Lifestyle Characteristics and Dietary Habits. Clin Interv Aging 2018, 13, 515–522. [CrossRef]
- Moon, J. H.; Hyun, M. K.; Lee, J. Y.; Shim, J. I.; Kim, T. H.; Choi, H. S.; Ahn, H. Y.; Kim, K. W.; Park, D. J.; Park, Y. J.; Yi, K. H. Prevalence of Thyroid Nodules and Their Associated Clinical Parameters: A Large-Scale, Multicenter-Based Health Check-up Study. Korean Journal of Internal Medicine 2018, 33 (4), 753–762. [CrossRef]
- Kobaly, K.; Kim, C. S.; Mandel, S. J. Annual Review of Medicine Contemporary Management of Thyroid Nodules. 2021.
- Ospina, N. S.; Papaleontiou, M. Thyroid Nodule Evaluation and Management in Older Adults: A Review of Practical Considerations for Clinical Endocrinologists. Endocrine Practice. Elsevier B.V. March 1, 2021, pp 261–268. [CrossRef]
- Xu, L.; Zeng, F.; Wang, Y.; Bai, Y.; Shan, X.; Kong, L. Prevalence and Associated Metabolic Factors for Thyroid Nodules: A Cross-Sectional Study in Southwest of China with More than 120 Thousand Populations. BMC Endocr Disord 2021, 21 (1). [CrossRef]
- Kant, R.; Davis, A.; Verma, V. Thyroid Nodules Advances. Am Fam Physician 2020, 102 (5), 298–304.
- Mu, C.; Ming, X.; Tian, Y.; Liu, Y.; Yao, M.; Ni, Y.; Liu, Y.; Li, Z. Mapping Global Epidemiology of Thyroid Nodules among General Population: A Systematic Review and Meta-Analysis. Frontiers in Oncology. Frontiers Media S.A. November 10, 2022. [CrossRef]
- Dong, X.; Li, Y.; Xie, J.; Li, L.; Wan, Z.; Kang, Y.; Luo, Y.; Wang, J.; Duan, Y.; Ding, S.; Cheng, A. S. K. The Prevalence of Thyroid Nodules and Its Factors among Chinese Adult Women: A Cross-Sectional Study. Front Endocrinol (Lausanne) 2022, 13. [CrossRef]
- Zhang, Y.; Wehbe, A.; Wang, X.; Sun, R.; Zheng, Z.; Zhao, D. The Prevalence of Thyroid Nodules and Risk Factors of Thyroid Nodules with Metabolic Disorder in Beijing: A Cross-Sectional Study. Environ Dis 2022, 7 (1), 22. [CrossRef]
- Hu, L.; Li, T.; Yin, X. L.; Zou, Y. An Analysis of the Correlation between Thyroid Nodules and Metabolic Syndrome. Endocr Connect 2020, 9 (9), 933–938. [CrossRef]
- Liu, J.; Wang, C.; Tang, X.; Fu, S.; Jing, G.; Ma, L.; Sun, W.; Li, Y.; Wu, D.; Niu, Y.; Niu, Q.; Guo, H.; Song, P. Correlation Analysis of Metabolic Syndrome and Its Components with Thyroid Nodules. Diabetes, Metabolic Syndrome and Obesity 2019, 12, 1617–1623. [CrossRef]
- Song, B.; Zuo, Z.; Tan, J.; Guo, J.; Teng, W.; Lu, Y.; Liu, C. Association of Thyroid Nodules with Adiposity: A Community-Based Cross-Sectional Study in China. BMC Endocr Disord 2018, 18 (1). [CrossRef]
- Zhang, C.; Gao, X.; Han, Y.; Teng, W.; Shan, Z. Correlation Between Thyroid Nodules and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2021, 12. [CrossRef]
- Parad, M. T.; Fararouei, M.; Mirahmadizadeh, A. R.; Afrashteh, S. Thyroid Cancer and Its Associated Factors: A Population-Based Case-Control Study. Int J Cancer 2021, 149 (3), 514–521. [CrossRef]
- Zhou, Y.; Yang, Y.; Zhou, T.; Li, B.; Wang, Z. Adiponectin and Thyroid Cancer: Insight into the Association between Adiponectin and Obesity. Aging and Disease. International Society on Aging and Disease April 1, 2021, pp 597–613. [CrossRef]
- Prete, A.; Borges de Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Frontiers in Endocrinology. Frontiers Media S.A. March 13, 2020. [CrossRef]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L. D.; Newbold, K.; Papotti, M. G.; Berruti, A. Thyroid Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Annals of Oncology 2019, 30 (12), 1856–1883. [CrossRef]
- Kitahara, C. M.; Schneider, A. B. Epidemiology of Thyroid Cancer. Cancer Epidemiology Biomarkers and Prevention 2022, 31 (7), 1284–1297.
- Li, M.; Zheng, R.; Dal Maso, L.; Zhang, S.; Wei, W.; Vaccarella, S. Mapping Overdiagnosis of Thyroid Cancer in China. The Lancet Diabetes and Endocrinology. Lancet Publishing Group June 1, 2021, pp 330–332. [CrossRef]
- Lincango-Naranjo, E.; Solis-Pazmino, P.; El Kawkgi, O.; Salazar-Vega, J.; Garcia, C.; Ledesma, T.; Rojas, T.; Alvarado-Mafla, B.; Young, G.; Dy, B.; Ponce, O. J.; Brito, J. P. Triggers of Thyroid Cancer Diagnosis: A Systematic Review and Meta-Analysis. Endocrine. Springer June 1, 2021, pp 644–659. [CrossRef]
- de Siqueira, R. A.; Noll, M.; Rodrigues, A. P. dos S.; Silveira, E. A. Factors Associated with the Occurrence of Thyroid Nodules in Severely Obese Patients: A Case-Control Study. Asian Pacific Journal of Cancer Prevention 2019, 20 (3), 693–697. [CrossRef]
- Fussey, J. M.; Beaumont, R. N.; Wood, A. R.; Vaidya, B.; Smith, J.; Tyrrell, J. Does Obesity Cause Thyroid Cancer? A Mendelian Randomization Study. Journal of Clinical Endocrinology and Metabolism 2020, 105 (7). [CrossRef]
- Economides, A.; Giannakou, K.; Mamais, I.; Economides, P. A.; Papageorgis, P. Association Between Aggressive Clinicopathologic Features of Papillary Thyroid Carcinoma and Body Mass Index: A Systematic Review and Meta-Analysis. Frontiers in Endocrinology. Frontiers Media S.A. June 30, 2021. [CrossRef]
- Eissa, M. S.; Abdellateif, M. S.; Elesawy, Y. F.; Shaarawy, S.; Al-Jarhi, U. M. Obesity and Waist Circumference Are Possible Risk Factors for Thyroid Cancer: Correlation with Different Ultrasonography Criteria. Cancer Manag Res 2020, 12, 6077–6089. [CrossRef]
- Jang, Y.; Kim, T.; Kim, B. H. S.; Park, B. Association between Obesity Indexes and Thyroid Cancer Risk in Korean Women: Nested Case–Control Study. Cancers (Basel) 2022, 14 (19). [CrossRef]
- Franchini, F.; Palatucci, G.; Colao, A.; Ungaro, P.; Macchia, P. E.; Nettore, I. C. Obesity and Thyroid Cancer Risk: An Update. International Journal of Environmental Research and Public Health. MDPI February 1, 2022.
- Yang, H. X.; Zhong, Y.; Lv, W. H.; Zhang, F.; Yu, H. Association of Adiposity with Thyroid Nodules: A Cross-Sectional Study of a Healthy Population in Beijing, China. BMC Endocr Disord 2019, 19 (1). [CrossRef]
- Ahmadi, S.; Pappa, T.; Kang, A. S.; Coleman, A. K.; Landa, I.; Marqusee, E.; Kim, M.; Angell, T. E.; Alexander, E. K. Point of Care Measurement of Body Mass Index and Thyroid Nodule Malignancy Risk Assessment. Front Endocrinol (Lausanne) 2022, 13. [CrossRef]
- Yildirim Simsir, I.; Cetinkalp, S.; Kabalak, T. Review of Factors Contributing to Nodular Goiter and Thyroid Carcinoma. Medical Principles and Practice. S. Karger AG January 1, 2020, pp 1–5. [CrossRef]
- Chen, X.; Wang, J. juan; Yu, L.; Wang, H. yu; Sun, H. The Association between BMI, Smoking, Drinking and Thyroid Disease: A Cross-Sectional Study in Wuhan, China. BMC Endocr Disord 2021, 21 (1). [CrossRef]
- Lai, X.; Zhang, B.; Wang, Y.; Jiang, Y.; Li, J.; Gao, L.; Wang, Y. Adiposity and the Risk of Thyroid Nodules with a High-Suspicion Sonographic Pattern: A Large Cross-Sectional Epidemiological Study. J Thorac Dis 2019, 11 (12), 5014–5022. [CrossRef]
- Power, M. L.; Schulkin, J. Sex Differences in Fat Storage, Fat Metabolism, and the Health Risks from Obesity: Possible Evolutionary Origins. British Journal of Nutrition. 2008, pp 931–940. [CrossRef]
- Chen, Y.; Zhu, C.; Chen, Y.; Wang, N.; Li, Q.; Han, B.; Zhao, L.; Chen, C.; Zhai, H.; Lu, Y. The Association of Thyroid Nodules with Metabolic Status: A Cross-Sectional SPECT-China Study. Int J Endocrinol 2018. [CrossRef]
- Pazaitou-Panayiotou, K.; Polyzos, S. A.; Mantzoros, C. S. Obesity and Thyroid Cancer: Epidemiologic Associations and Underlying Mechanisms. Obesity Reviews. December 2013, pp 1006–1022. [CrossRef]
- Walczak, K.; Sieminska, L. Obesity and Thyroid Axis. International Journal of Environmental Research and Public Health. MDPI September 1, 2021.
- Shin, J.; Kim, M. H.; Yoon, K. H.; Kang, M. Il; Cha, B. Y.; Lim, D. J. Relationship between Metabolic Syndrome and Thyroid Nodules in Healthy Koreans. Korean Journal of Internal Medicine 2016, 31 (1), 98–105. [CrossRef]
- Ma, S.; Jing, F.; Xu, C.; Zhou, L.; Song, Y.; Yu, C.; Jiang, D.; Gao, L.; Li, Y.; Guan, Q.; Zhao, J. Thyrotropin and Obesity: Increased Adipose Triglyceride Content Through Glycerol-3-Phosphate Acyltransferase 3. Sci Rep 2015, 5. [CrossRef]
- Ayturk, S.; Gursoy, A.; Kut, A.; Anil, C.; Nar, A.; Tutuncu, N. B. Metabolic Syndrome and Its Components Are Associated with Increased Thyroid Volume and Nodule Prevalence in a Mild-to-Moderate Iodine-Deficient Area. Eur J Endocrinol 2009, 161 (4), 599–605. [CrossRef]
- Li, Z.; Zhang, L.; Huang, Y.; Yang, P.; Xu, W.; Faustini-Fustini, M. A Mechanism Exploration of Metabolic Syndrome Causing Nodular Thyroid Disease; 2019. [CrossRef]
- Luo, J.; Hendryx, M.; Manson, J. A. E.; Figueiredo, J. C.; LeBlanc, E. S.; Barrington, W.; Rohan, T. E.; Howard, B. V.; Reding, K.; Ho, G. Y. F.; Garcia, D. O.; Chlebowski, R. T. Intentional Weight Loss and Obesity-Related Cancer Risk. JNCI Cancer Spectr 2019, 3 (4). [CrossRef]
- Youssef, M. R.; Reisner, A. S. C.; Attia, A. S.; Hussein, M. H.; Omar, M.; LaRussa, A.; Galvani, C. A.; Aboueisha, M.; Abdelgawad, M.; Toraih, E. A.; Randolph, G. W.; Kandil, E. Obesity and the Prevention of Thyroid Cancer: Impact of Body Mass Index and Weight Change on Developing Thyroid Cancer – Pooled Results of 24 Million Cohorts. Oral Oncol 2021, 112. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




