Submitted:
08 May 2023
Posted:
10 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant collections
2.2. Ploidy level estimation
2.3. Reproductive mode
2.4. Reproductive pathway efficiency
2.5. Mating system and seed fertility
2.6. Statistical analysis
3. Results
3.1. Ploidy estimations
3.2. Embryo sac analysis
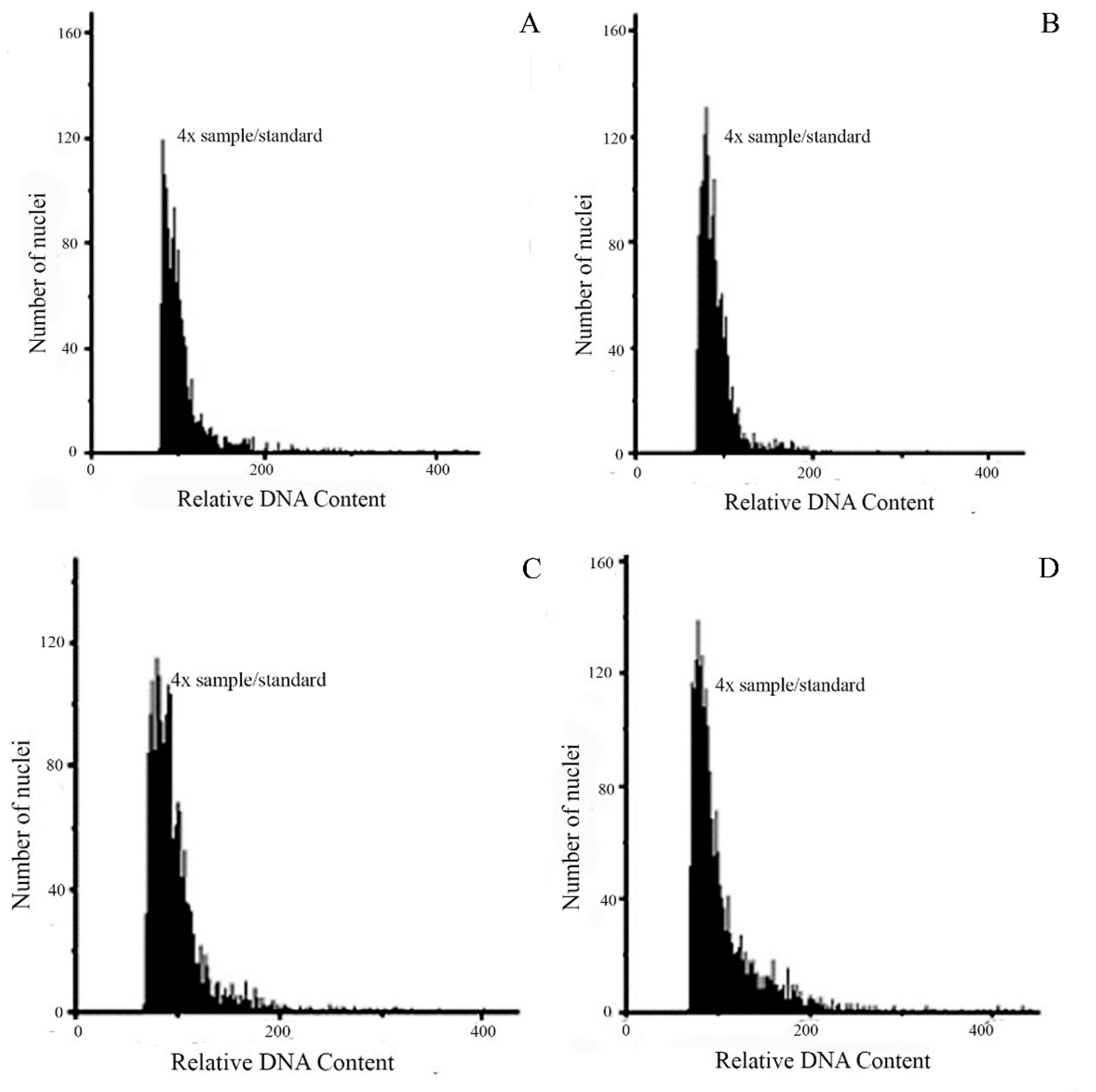
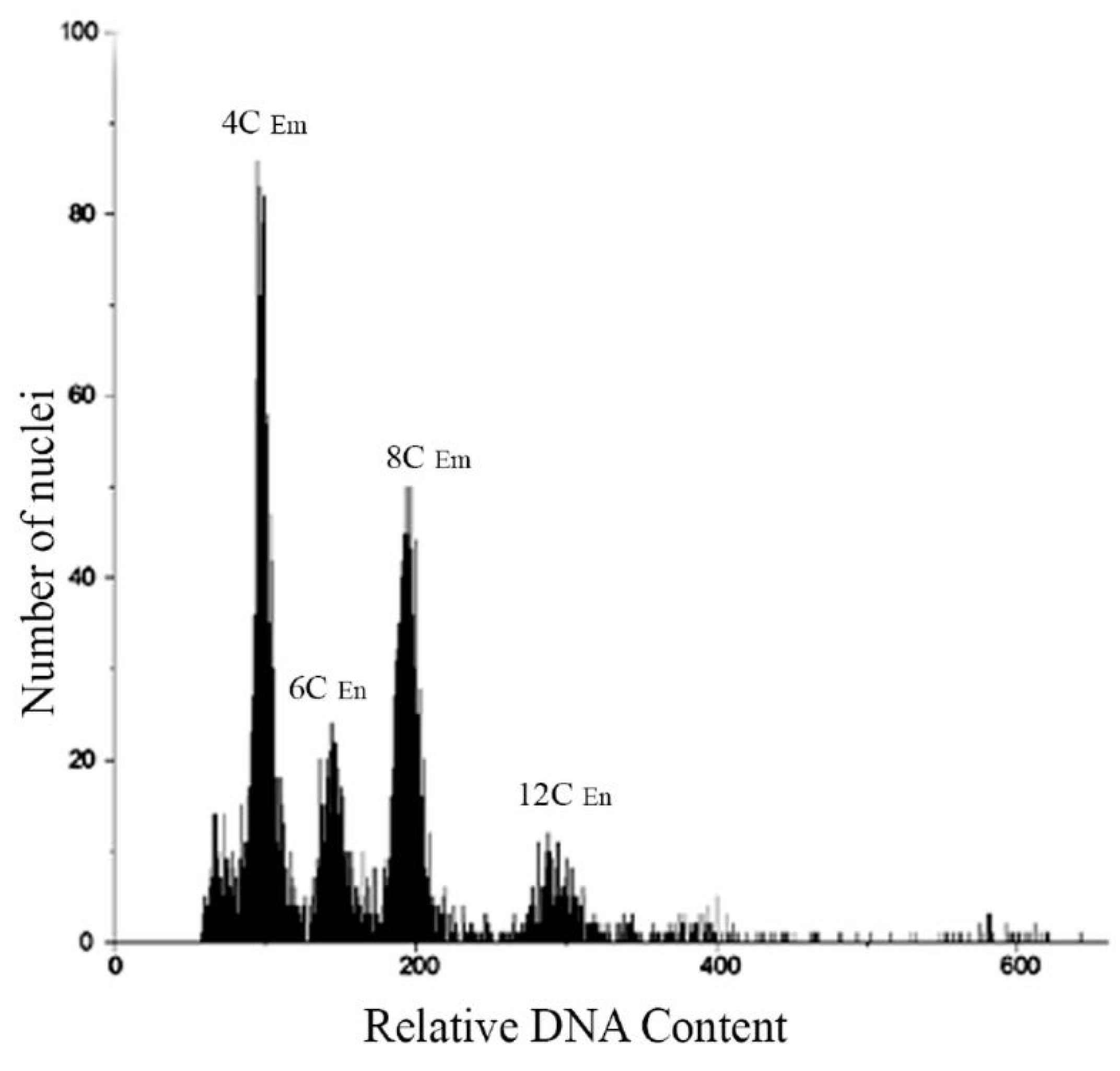
3.3. Flow cytometric seed screen
3.4. Reproductive variability in ovules and seeds
3.5. Mating system and seed fertility
4. Discussion
4.1. Single ploidies featured in all species
4.2. Reproductive pathways and cytotype stability
4.4. Two mating systems with partial breakdown of self-incompatibility
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meirmans, P.G.; Van Tienderen, P.H. The effects of inheritance in tetraploids on genetic diversity and population divergence. Heredity 2013, 110, 131–137. [CrossRef]
- Jiao, Y.; Wickett, N.J.; Ayampalayam, S.; Chanderbali, A.S.; Landherr, L.; Ralph, P.E.; Tomsho, L.P.; Hu, Y.; Liang, H.; Soltis, P.S.; Soltis, D.E.; Clifton, S.W.; Schlarbaum, S.E.; Schuster, S.C.; Ma, H.; Leebens-Mack, J.; de Pamplilis, C.W. Ancestral polyploidy in seed plants and angiosperms. Nature 2011, 473, 97–100. [CrossRef]
- Stebbins, G.L. Variation and Evolution in plants. Columbia University Press, New York, 1950.
- Grant, V. Polyploidy. Columbia University Press. New York, 1981.
- Otto, S.P.; Whitton, J. Polyploid incidence and evolution. Ann. Rev. Genet. 2000, 34, 401–437. [CrossRef]
- Levin, D.A. The role of chromosomal change in plant evolution. Oxford University Press, New York, United States, 2002.
- Levin, D.A. Polyploidy and novelty in flowering plants. Amer. Nat. 1983, 122, 1–25. [CrossRef]
- Lewis, W.H. Polyploidy: biological relevance. Plenum Press, New York, 1980.
- Osborne, O.; Simon, S.; Collins, S. Attitudes towards science: A review of the literature and its implications. Int. J. Sci. Educ. 2003, 25, 1049–1079, . [CrossRef]
- Coyne, J.A.; Orr, H.A. Speciation. Sunderland, MA. Sinauer Associates, 2004.
- Suda, J.; Kron, P.; Husband, B.C.; Travnıcek, P. Flow cytometry and ploidy: applications in plant systematics, ecology and evolutionary biology. In J Dolezel, J Greilhuber, J Suda, eds., Flow Cytometry with Plant Cells. Analysis of Genes, Chromosomes and Genomes, Wiley–VCH Verlag GmbH & Co. KGaA, Weinheim, 2007; pp. 103–130.
- Lumaret, R. Polyploidy and the critical size of natural populations: the case of cocksfoot (Dactylis glomerata L.), a grass used as a fodder plant. Bocconea 1997, 7, 133–139.
- Karunarathne, P.; Schedler, M.; Martínez, E.J.; Honfi, A.I.; Novichkova, A.; Hojsgaard, D.H. Intraspecific ecological niche divergence and reproductive shifts foster cytotype displacement and provide ecological opportunity to polyploids. Ann. Bot. 2018, 121, 1183–1196. [CrossRef]
- Karunarathne, P.; Reutemann, A. V.; Schedler, M.; Glücksberg, A.; Martínez, E. J.; Honfi, A. I.; Hojsgaard, D. H. Sexual modulation in a polyploidy grass: a reproductive contest between environmentally inducible sexual and genetically dominant apomictic pathways. Sci. Rep. 2020, 10, 8319. [CrossRef]
- Rua, G.H.; Speranza, P.R.; Vaio, M.; Arakaki, M. A phylogenetic analysis of the genus Paspalum (Poaceae) based on cpDNA and morphology. Plant Syst. Evol. 2010, 288, 227–243. [CrossRef]
- Bonasora, M.G.; Pozzobon, M.T.; Honfi, A.I.; Rua, G.H. Paspalum schesslii (Poaceae, Paspaleae), a new species from Mato Grosso (Brazil) with an unusual base chromosome number. Plant Syst. Evol. 2015, 301, 2325–2339. [CrossRef]
- Zuloaga, F.O.; Morrone, O. Revisión de las especies de Paspalum para América del Sur austral (Argentina, Bolivia, sur del Brasil, Chile, Paraguay y Uruguay). Ann. Mo. Bot. Gard. Monogr. Syst. Bot. 2005, 102, 1–297.
- Giussani, L.M.; Zuloaga, F.O.; Quarin, C.L.; Cota-Sánchez, H.; Ubayasena, K.; Morrone, O. Phylogenetic relationships in the genus Paspalum (Poaceae: Panicoideae: Paniceae): an assessment of the Quadrifaria and Virgata informal groups. Syst. Bot. 2009, 34, 32–43. [CrossRef]
- Quarin, C.L. Relaciones citotaxonómicas entre Paspalurn almum Chase y P. hexastachyum Parodi (Gramineae). Bonplandia, 1974, 3, 115-127.
- Davidse, G.; Pohl, R.W. (1974) Chromosome numbers meiotic behaviour and notes on tropical American grasses (Gramineae). Can. J. Bot. 1974, 52, 317–328. [CrossRef]
- Ortiz, J.P.A.; Quarin, C.L., Pessino, S.C.; Acuña, C.A.; Martínez, E.J.; Espinoza, F.; Hojsgaard, D.H.; Sartor, M.E.; Cáceres, M.E.; Pupilli, F. Harnessing apomictic reproduction in grasses: what we have learned from Paspalum. Ann. Bot. 2013, 112, 767–787. [CrossRef]
- Darlington, C.D. Evolution of genetic systems. Cambridge: Cambridge University Press, 1939.
- Hojsgaard, D.H.; Martínez, E.J.; Quarin, C.L. Competition between meiotic and apomictic pathways during ovule and seed development results in clonality. New. Phytol. 2013, 197, 336–347. [CrossRef]
- Norrmann, G.A. Citología y modo de reproducción en dos especies de Paspalum (Gramineae). Bonplandia 1981, 17, 149–158.
- Quarin, C.L.; Norrmann, G.A.; Espinoza, F. Evidence for autoploidy in apomictic Paspalum rufum. Hereditas 1998, 129, 119–124. [CrossRef]
- Hörandl, E.; Paun, O. Patterns and sources of genetic diversity in apomictic plants: implications for evolutionary potentials. In Hörandl, E., Grossniklaus, U., van Dijk, P.J., Sharbel, T.F. (ed.) Apomixis: Evolutions, mechanisms and perspectives, A.R.G. Gantner Verlag, Rugell, Liechtenstein, 2007, pp. 170–194.
- Brugnoli, E.A.; Urbani, M.H., Quarin, C.L.; Zilli, A.L.; Martínez, E.J.; Acuña, C.A. Diversity in apomictic populations of Paspalum simplex Morong. Crop. Sci. 2014, 54, 1656–1664. [CrossRef]
- Daurelio, L.D.; Espinoza, F.; Quarin, C.L.; Pessino, S.C. Genetic diversity in sexual diploid and apomictic tetraploid populations of Paspalum notatum situated in sympatry or allopatry. Plant Syst. Evol. 2004, 244, 189–199. [CrossRef]
- Sartor, M.E.; Quarin, C.L.; Urbani, M.H.; Espinoza, F. Ploidy levels and reproductive behaviour in natural populations of five Paspalum species. Plant Syst. Evol. 2011, 293, 31–41. [CrossRef]
- Urbani, M.H.; Quarin, C.L.; Espinoza, F.; Penteado, M.I.O.; Rodrigues, I.F. Cytogeography and reproduction of the Paspalum simplex polyploid complex. Plant Syst. Evol. 2002, 236, 99–105. [CrossRef]
- Gornall, R.J. Population genetic structure in agamospermous plants. In: Hollingsworth, P.M.; Bateman, R.M.; Gornall, R.J., eds. Mol. Syst. Plant Evol. London: Taylor & Francis, 1999, pp. 118–138.
- Quarin, C.L. The nature of apomixis and its origin in Panicoid grasses. Apomixis Newsl. 1992, 5, 8–15.
- Burson, B.L. Cytology of Paspalum chacoense and P. durifolium and their relationship to P. dilatatum. Bot. Gaz. 1985, 146, 124–129. [CrossRef]
- Burson, B.L.; Bennett, H.W. Cytology and reproduction of three Paspalum species. J. Hered. 1970, 61, 129–132. [CrossRef]
- Honfi, A.I., Quarin, C.L. Citología y modo de reproducción de Paspalum durifolium Mez pentaploide (Poaceae: Panicoideae: Paniceae). Lilloa 2009, 45, 85.
- Moraes Fernandes, M.I.B.; Barreto, I.; Salzano, F.M.; Sacchet, A.M.O.F. Cytological and evolutionary relationships in Brazilian forms of Paspalum (Gramineae). Caryologia 1974, 27, 455–465.
- Quarin, C.L.; Norrmann, G.A. Cytology and reproductive behavior of Paspalum equitans, P. ionanthum, and their hybrids with diploid and tetraploid cytotypes of P. cromyorrhizon. Bot. Gaz. 1987, 148, 386–391. [CrossRef]
- Pozzobon, M.T.; Valls, J.F.M.; Dos Santos, S. Contagens cromossômicas em espécies brasileiras de Paspalum L. (Gramineae). Acta Bot. Bras. 2000, 14, 151–162. [CrossRef]
- Quarin, C.L. A tetraploid cytotype of Paspalum durifolium: Cytology, reproductive behavior and its relationship to diploid P. intermedium. Hereditas 1994, 121, 115–118. [CrossRef]
- Martínez, E.J., Quarin, C.L., Hayward, M.D. Genetic control of apospory in apomictic Paspalum species. Cytologia 1999, 64, 425–433. [CrossRef]
- Pozzobon, M.T.; Carvalho Machado, A.C.; Vaio, M.; Valls, J.F.M.; de Souza Peñaloza, A.D.; dos Santos, S.; Côrtes, A.L.; Rua, G.H. Cytogenetic analyses in Paspalum L. reveal new diploid species and accessions. Ciência Rural 2008, 38, 1292-1298.
- Bashaw, E.C.; Hovin, A.W.; Holt, E.C. Apomixis, its evolutionary significance and utilization in plant breeding. In: Norman MJT ed. Proceed. 11th Intl Grassl Congr. Surfers Paradise, Queensland. University of Queensland Press, St. Lucia, 1970, pp. 245-248.
- Honfi, A.I.; Quarin, C.L.; Valls, J.F.M. Estudios cariológicos en gramíneas sudamericanas. Darwiniana 1990, 30, 87–94.
- Pagliarini, M.S.; Carraro, L.R.; Freitas, P.M.; Adamowsky, E.V.; Rocha Batista, L.A.; Valls, J.F.M. Cytogenetic characterization of Brazilian Paspalum accessions. Hereditas 2002, 135, 27–34. [CrossRef]
- Hojsgaard, D.H.; Honfi, A.I.; Rua, G.H.; Daviña, J.R. Chromosome numbers and ploidy levels of Paspalum species from subtropical South America (Poaceae). Genet. Resour. Crop Evol. 2009, 56, 533–545. [CrossRef]
- Pozzobon, M.T.; Valls, J.F.M. Chromosome number in germplasm accessions of Paspalum notatum (Gramineae). Braz. J. Genet. 1997, 20, 29–34. [CrossRef]
- Brugnoli, A.E.; Urbani, M.H.; Quarin, C.L.; Martínez. E.J.; Acuña, C.A. Diversity in diploid, tetraploid and mixed diploid-tetraploid populations of Paspalum simplex. Crop Sci. 2013, 53, 1509–1516. [CrossRef]
- Hijmans, R.J.; Guarino, L.; Mathur, P.; Jarvis, A. Diva Gis. Version 4. Lizard Tech, Inc and University of California, Patente U.S. No. 5,710,835, 2004.
- Galdeano, F.; Urbani, M.H.; Sartor, M.E.; Honfi, A.I.; Espinoza, F.; Quarin, C.L. Relative DNA content in diploid, polyploid, and multiploid species of Paspalum (Poaceae) with relation to reproductive mode and taxonomy. J. Plant Res. 2016, 129, 697–710. [CrossRef]
- Young, B.A.; Sherwood, R.T.; Bashaw, E.C. Cleared-pistil and thick-sectioning techniques for detecting aposporous apomixis in grasses. Canad. J. Bot. 1979, 57, 1668–1672. [CrossRef]
- Zilli, A.L.; Brugnoli, E.A.; Marcón. F.; Billa, M.B.; Rios, E.F.; Martínez, E.J.; Acuña. C.A. Heterosis and expressivity of apospory in tetraploid Bahiagrass hybrids. Crop Sci. 2015, 55, 1189–1201. [CrossRef]
- Matzk, F.; Meister, A.; Schubert, I. An efficient screen for reproductive pathways using mature seeds of monocots and dicots. Plant J. 2000, 21, 97–108. [CrossRef]
- Siena, L.A.; Sartor, M.E.; Espinoza, F.; Quarin, C.L.; Ortiz, J.P.A. Genetic and embryological evidences of apomixis at the diploid level in Paspalum rufum support recurrent auto-polyploidization in the species. Sex. Plant Reprod. 2008, 21, 205–215. [CrossRef]
- Balzarini, M.; Di Rienzo, J. Info-Gen: Software para análisis estadístico de datos genéticos. Facultad de Ciencias Agropecuarias. Universidad Nacional de Córdoba. Argentina, 2003.
- Saura, F. Cariología de gramíneas. Revista de la Facultad de Agronomía y Veterinaria, Universidad de Buenos Aires, 1943, 2, 63–74.
- Pagliarini, M.S.; De Freitas, P.M.; Takayama, S.Y.; Batista, L.A.R. An original meiotic mutation in Paspalum regnellii. Sex. Plant Reprod. 1998, 11, 17–21. [CrossRef]
- Burton, G.W. A cytological study of some species in the genus Paspalum. J. Agric. Res. 1940, 60, 193–198.
- Burton, G.W. A cytological study of some species in the tribe Paniceae. Am. J. Bot. 1942, 19, 355–359. [CrossRef]
- Brown, W.V. A cytological study of some Texas gramineae. Bull. Torrey Bot. Club 1950, 77, 63–76. [CrossRef]
- Brown, W.V.; Emery, H.P. Apomixis in the Gramineae: Panicoideae. Am. J. Bot. 1958, 45, 253–263. [CrossRef]
- Nielsen, E.L. Grass studies III. Additional somatic chromosome complements. Am. J. Bot. 1939, 26, 366–372. [CrossRef]
- Reutemann, A. V.; Honfi, A. I.; Karunarathne, P.; Eckers, F.; Hojsgaard, D. H.; Martínez, E. J. Variation of residual sexuality rates along reproductive development in apomictic tetraploids of Paspalum. Plants 2022 11(13): 1639. https://. [CrossRef]
- Delgado, L.; Galdeano, F.; Sartor, M.E.; Quarin, C.L.; Espinoza, F.; Ortiz, J.P. Analysis of variation for apomictic reproduction in diploid Paspalum rufum. Ann. Bot. 2014, 113, 1211–1218. [CrossRef]
- Quarin, C.L. Seasonal changes in the incidences of apomixis of diploid, triploid, and tetraploid plants of Paspalum cromyorrhizon. Euphytica 1986, 35, 515–522. [CrossRef]
- Norrmann, G.A.; Quarin, C.L.; Burson, B.L. Cytogenetics and reproductive behavior of different chromosome races in six Paspalum species. J. Hered. 1989, 80, 24–28. [CrossRef]
- Martínez, E.J.; Urbani, M.H.; Quarin, C.L.; Ortiz, J.P.A. Inheritance of apospory in Bahiagrass Paspalum notatum. Hereditas 2001, 135, 19–25. [CrossRef]
- Naumova, T.N.; Hayward, M.D.; Wagenvoort, M. Apomixis and sexuality in diploid and tetraploid accessions of Brachiaria decumbens. Sex. Plant Rep. 1999, 12, 43–52. [CrossRef]
- Rebozzio, R.N.; Sartor, M.E.; Quarin, C.L.; Espinoza, F. Residual sexuality and its seasonal variation in natural apomictic Paspalum notatum accessions. Biol. Plant. 2011, 55, 391–395. [CrossRef]
- Reutemann, A. V.; Martínez, E. J.; Schedler, M.;Daviña, J. R.; Hojsgaard, D. H.; Honfi, A. I. Uniparentality: Advantages for range expansion in diploid and diploid-autopolyploid species. Bot. J. Linn. Soc. 2022, 200(4): 563-585. https://. [CrossRef]
- Urbani, M.H. Estudios sobre citología, sistema reproductivo y compatibilidad polen–pistilo de Panicum dichotomiflorum y Paspalum fasciculatum (Gramineae:Paniceae). Darwiniana 1996, 34, 193–198.
- Burton, G.W.; Forbes, I. Jr.; Jackson, J. Effect of ploidy on fertility and heterosis in Pensacola Bahiagrass. Crop Sci. 1970, 10, 63–66. [CrossRef]
- Quarin, C.L. Morfología, citología y sistema reproductivo de una nueva especie de gramínea: Paspalum procurrens. Bol. Soc. Arg. Bot. 1993, 29, 73–76.
- Quarin, C.L. Effect of pollen source and pollen ploidy on endosperm formation and seed set in pseudogamous apomictic Paspalum notatum. Sex. Plant Reprod. 1999, 11, 331–335.
- Hörandl, E. The evolution of self-fertility in apomictic plants. Sex. Plant Rep. 2010, 23, 73–86. [CrossRef]
- Wilkins, P.W.; Thorogood, D. Breakdown of self-incompatibility in perennial ryegrass at high temperature and its uses in breeding. Euphytica 1992, 64, 65–69. [CrossRef]
- Hirosaki, A.; Niikura, S. Developmental and environmental factors affecting level of self-incompatibility response in Brassica rapa L. Sex. Plant Rep. 2008, 21, 123–132. [CrossRef]
- Zilli, A.L.; Acuña, C.A.; Schulz, R.R.; Brugnoli. E.A.; Guidalevich, V.; Quarin, C.L.; Martínez, E.J. Widening the gene pool of sexual tetraploid Bahiagrass: Generation and reproductive characterization of a sexual synthetic tetraploid population. Crop Sci 2018, 58, 762–772. [CrossRef]
- Yamamoto, M.; Nishimura, K.; Kitashiba, H.; Sakamoto, W.; Takeshi, N. High temperature causes breakdown of S haplotype-dependent stigmatic self-incompatibility in self-incompatible Arabidopsis thaliana. J. Exp. Bot. 2019, 70, 5745–5751. [CrossRef]
- Carter, A.L.; McNeilly, T. Effects of increased seed humidity on pollen tube growth and seed set following self-pollination in Brussels sprout (Brassica oleracea var. gemmifera). Euphytica 1975, 24, 805–813. [CrossRef]
- Ockendon, D.J. Effect of hexane and humidity on self-incompatibility in Brassica oleracea. Theor. Appl. Genet. 1978, 52, 113–117. [CrossRef]
- Barrett, S.C.H.; Harder, L.D. The ecology of mating and its evolutionary consequences in seed plants. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 135–57. [CrossRef]
- Caponio, I.; Quarin, C.L. Intra- and interspecific hybridization between Dallisgrass and Vaseygrass. Crop Sci. 1990, 30, 362–364. [CrossRef]
- Mable, B.K. Polyploidy and self-compatibility: is there an association? New. Phytol. 2004, 162, 803–811. [CrossRef]
- Barringer, B.C. Polyploidy and self-fertilization in flowering plants. Amer. J. Bot. 2007, 94, 1527–1533. [CrossRef]
- Barrett, S.C.H.; Harder, L.D.; Worley, A.C. The comparative biology of pollination and mating in flowering plants. Phil. Trans. Roy. Soc. London B Biol. Sci. 1996, 351, 1271–1280. [CrossRef]
- Bernardello, G.; Anderson, G.J.; Stuessy, T.F.; Crawford, D.J. A survey of floral traits, breeding systems, floral visitors, and pollination systems of the angiosperms of the Juan Fernández Islands (Chile). Bot. Rev. 2001, 67, 255–308. [CrossRef]
- Kao, T.H.; Tsukamoto, T. The molecular and genetic bases of S-RNase-based self-incompatibility. Plant Cell 2004, 16, S72–S83. [CrossRef]
- Charlesworth, D.; Vekemans, X.; Castric, V.; Glemin, S. Plant self-incompatibility systems: a molecular evolutionary perspective. New Phytol. 2005, 168, 61–69. [CrossRef]
- Richards, A.J. Plant breeding systems, 2nd Edn. London: Chapman & Hall. 1997.
- de Nettancourt, D. Incompatibility and incongruity in wild and cultivated plants. 2nd ed. Springer, Berlin. 2001. [CrossRef]
- Stone, J.L. Molecular mechanisms underlying the breakdown of gametophytic self-incompatibility. Quart. Rev. Biol. 2002, 77, 17–32. [CrossRef]

| Species | Population code (Voucher) | Collection sites | Geographical coordinates | No. Plants |
|---|---|---|---|---|
| P. durifolium | PD1 (M6) | Corrientes, Dpto. Ituzaingó, NR 12 | 27°37.622S, 56°44.873W | 19 |
| PD2 (M7) | Corrientes, Dpto. San Miguel, NR 118, and PR 5 | 27°55.901S, 57°30.996W | 18 | |
| PD3 (M16) | Corrientes, Dpto. Santo Tomé, Esteros del Iberá, PR 40 | 28°17.435S, 56°46.219W | 17 | |
| PD4 (M17) | Corrientes, Dpto. Santo Tomé, NR 120 | 27°49.513S, 56°15.294W | 18 | |
| PD5 (M18) | Corrientes, Dpto. Ituzaingó, NR 118 | 27°33.646S, 57°09.184W | 20 | |
| P. ionanthum | PI1 (M4) | Corrientes, Dpto. Ituzaingó, NR 120 | 27°49.265S, 56°16.328W | 20 |
| PI2 (M8) | Corrientes, Dpto. Concepción, Santa Rosa, NR 118 | 28°14.245S, 58°03.252W | 20 | |
| PI3 (M10) | Corrientes, Dpto. General Paz, Paso Florentín, PR 5 | 27°45.205S, 57°45.658W | 16 | |
| PI4 (M11) | Corrientes, Dpto. Mburucuyá, Parque Nacional Mburucuyá. | 28°02.024S, 58°02.172W | 20 | |
| PI5 (M12) | Corrientes, Dpto. Goya, Paraje Marucha, NR 12 | 29°09.906S, 59°05.795W | 20 | |
| P. regnellii | PR1 (M2) | Misiones, Dpto. Montecarlo, Puerto Laharrague, NR 12 | 26°32.492S, 54°43.506W | 20 |
| PR2 (M19) | Misiones, Dpto. San Ignacio, Jardín América, NR 12 | 27°03.170S, 55°15.040W | 19 | |
| PR3 (M20) | Misiones, Dpto. San Pedro, NR 14 | 26°43.626S, 54°14.845W | 20 | |
| PR4 (M21) | Misiones, Dpto. 25 de Mayo, PR 2 | 27°22.441S, 54°25.503W | 18 | |
| PR5 (M22) | Misiones, Dpto. 25 de Mayo, PR 4 | 27°46.628S, 55°14.998W | 19 | |
| P. urvillei | PU1 (M1) | Misiones, Dpto. San Ignacio, 2,2 Km East PR 210 | 27°16.613S, 55°27.735W | 17 |
| PU2 (M5) | Corrientes, Dpto. Ituzaingó, NR 120 | 27°49.265S, 56°16.328W | 20 | |
| PU3 (M14) | Entre Ríos, Dpto. La Paz, PR 6 | 31°01.223S, 59°25.222W | 20 | |
| PU4 (M23) | Santa Fe, Dpto. Gral. Obligado, NR 11 | 28°35.733S, 59°25.005W | 20 | |
| PU5 (M27) | Chaco, Dpto. Gral. San Martin, PR 90 | 26°24.117S, 59°22.726W | 17 |
| Species | Pop | n | Number of ovules bearing (%) | Proportions | |||||
|---|---|---|---|---|---|---|---|---|---|
| MES | AES | MES + AES | AbES | SP | AP | p | |||
| P. durifolium | PD1 | 152 | 127 (83.5) | - | 1 (0.7) | 24 (15.8) | 0.99 | 0.01 | <0.001 |
| PD2 | 150 | 141 (94.0) | - | - | 9 (6.0) | 1.00 | 0.00 | <0.001 | |
| PD3 | 152 | 134 (88.2) | - | 6 (3.9) | 12 (7.9) | 0.96 | 0.04 | <0.001 | |
| PD4 | 153 | 129 (84.3) | - | 10 (6.5) | 14 (9.2) | 0.93 | 0.07 | <0.001 | |
| PD5 | 156 | 124 (79.5) | - | 5 (3.2) | 27 (17.3) | 0.96 | 0.04 | <0.001 | |
| P. ionanthum | PI1 | 158 | 150 (95.0) | - | 4 (2.5) | 4 (2.5) | 0.97 | 0.03 | <0.001 |
| PI2 | 151 | 147 (97.4) | - | 2 (1.3) | 2 (1.3) | 0.99 | 0.01 | <0.001 | |
| PI3 | 150 | 149 (99.3) | - | - | 1 (0.7) | 1.00 | 0.00 | <0.001 | |
| PI4 | 165 | 160 (97.0) | - | - | 5 (3.0) | 1.00 | 0.00 | <0.001 | |
| PI5 | 158 | 156 (98.7) | - | - | 2 (1.3) | 1.00 | 0.00 | <0.001 | |
| P. regnellii | PR1 | 160 | 112 (70.0) | - | - | 48 (30.0) | 1.00 | 0.00 | <0.001 |
| PR2 | 151 | 132 (87.4) | - | - | 19 (12.6) | 1.00 | 0.00 | <0.001 | |
| PR3 | 155 | 148 (95.5) | - | - | 7 (4.5) | 1.00 | 0.00 | <0.001 | |
| PR4 | 170 | 162 (95.3) | - | - | 8 (4.7) | 1.00 | 0.00 | <0.001 | |
| PR5 | 164 | 153 (93.3) | - | - | 11 (6.7) | 1.00 | 0.00 | <0.001 | |
| P. urvillei | PU1 | 167 | 160 (95.8) | - | - | 7 (4.2) | 1.00 | 0.00 | <0.001 |
| PU2 | 158 | 153 (96.8) | - | - | 5 (3.2) | 1.00 | 0.00 | <0.001 | |
| PU3 | 156 | 155 (99.4) | - | - | 1 (0.6) | 1.00 | 0.00 | <0.001 | |
| PU4 | 156 | 151 (96.8) | - | - | 5 (3.2) | 1.00 | 0.00 | <0.001 | |
| PU5 | 155 | 155 (100) | - | - | - | 1.00 | 0.00 | <0.001 | |
| Species | Pop | Sexual pathways | Apomictic pathways | Statistical analysis | Reproductive efficiency | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ei | Oi | Ei | Oi | χ2 | p | Sex | Apo | ||
| P. durifolium | PD1 | 0.99 | 1.00 | 0.01 | 0.00 | 4.67e-31 | 1.00 | 1.01 | - |
| PD2 | 1.00 | 1.00 | 0.00 | 0.00 | - | - | 1.06 | - | |
| PD3 | 0.96 | 1.00 | 0.04 | 0.00 | 0.333 | 0.564 | 1.04 | - | |
| PD4 | 0.93 | 1.00 | 0.07 | 0.00 | 1.049 | 0.3055 | 1.07 | - | |
| PD5 | 0.96 | 1.00 | 0.04 | 0.00 | 0.237 | 0.626 | 1.04 | - | |
| P. ionanthum | PI1 | 0.97 | 1.00 | 0.03 | 0.00 | - | - | 1.03 | - |
| PI2 | 0.99 | 1.00 | 0.01 | 0.00 | - | - | 1.01 | - | |
| PI3 | 1.00 | 1.00 | 0.00 | 0.00 | - | - | 1.00 | - | |
| PI4 | 1.00 | 1.00 | 0.00 | 0.00 | - | - | 1.00 | - | |
| PI5 | 1.00 | 1.00 | 0.00 | 0.00 | - | - | 1.00 | - | |
| P. regnellii | PR1 | 1.00 | 1.00 | 0.00 | 0.00 | - | - | 1.00 | - |
| PR2 | 1.00 | 1.00 | 0.00 | 0.00 | - | - | 1.00 | - | |
| PR3 | 1.00 | 1.00 | 0.00 | 0.00 | - | - | 1.00 | - | |
| PR4 | 1.00 | 1.00 | 0.00 | 0.00 | - | - | 1.00 | - | |
| PR5 | 1.00 | 1.00 | 0.00 | 0.00 | - | - | 1.00 | - | |
| P. urvillei | PU1 | 1.00 | 1.00 | 0.00 | 0.00 | - | - | 1.00 | - |
| PU2 | 1.00 | 1.00 | 0.00 | 0.00 | - | - | 1.00 | - | |
| PU3 | 1.00 | 1.00 | 0.00 | 0.00 | - | - | 1.00 | - | |
| PU4 | 1.00 | 1.00 | 0.00 | 0.00 | - | - | 1.00 | - | |
| PU5 | 1.00 | 1.00 | 0.00 | 0.00 | - | - | 1.00 | - | |
| Species | Pop | Seed set (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Self-pollination | Open pollination | Self-pollination | Open pollination | |||||||
| 1st period | 2nd period | p valueα | 1st period | 2nd period | p valueα | Total† | Total† | p valueβ | ||
| P. durifolium | PD1 | 0.27a | 0.61a | 0.281 | 28.09a | 29.58a | 0.839 | 0.44a | 28.83b | <0.001 |
| PD2 | 0.35a | 0.65a | 0.333 | 46.30a | 51.54a | 0.326 | 0.50a | 48.92a | <0.001 | |
| PD3 | 0.16a | 0.65a | 0.262 | 57.90a | 45.16a | 0.242 | 0.41a | 51.53a | <0.001 | |
| PD4 | 1.24a | 0.47a | 0.409 | 45.52a | 42.70a | 0.881 | 0.86a | 44.11ab | <0.001 | |
| PD5 | 0.17a | 1.18a | 0.119 | 33.17a | 27.97a | 0.507 | 0.68a | 30.57b | <0.001 | |
| p valueγ | 0.284 | 0.701 | 0.173 | 0.058 | 0.74 | 0.006 | ||||
| P. ionanthum | PI1 | 1.00a | 1.00a | 0.995 | 35.60a | 23.20a | 0.168 | 1.04a | 29.41c | <0.001 |
| PI2 | 0.26a | 1.22a | 0.418 | 60.25a | 31.27b | 0.002 | 0.75a | 45.80ab | <0.001 | |
| PI3 | 4.26a | 1.36a | 0.337 | 49.66a | 58.88a | 0.661 | 2.79a | 54.27a | <0.001 | |
| PI4 | 0.16a | 1.10a | 0.164 | 52.80a | 39.22a | 0.092 | 0.61a | 46.02ab | <0.001 | |
| PI5 | 2.85a | 0.71a | 0.089 | 45.53a | 27.82a | 0.036 | 1.80a | 36.69bc | <0.001 | |
| p valueγ | 0.565 | 0.972 | 0.191 | 0.004 | 0.574 | 0.017 | ||||
| P. regnellii | PR1 | 8.51a | 4.96a | 0.44 | 23.47a | 34.19a | 0.360 | 6.73b | 28.83b | <0.001 |
| PR2 | 25.93a | 6.19b | 0.018 | 38.14a | 44.17a | 0.602 | 16.06ab | 41.16ab | 0.001 | |
| PR3 | 12.44a | 9.74a | 0.704 | 24.06a | 28.70a | 0.682 | 11.09ab | 26.38b | 0.031 | |
| PR4 | 10.48a | 2.34a | 0.16 | 35.83a | 20.77a | 0.144 | 6.41b | 28.30b | <0.001 | |
| PR5 | 25.16a | 12.47a | 0.055 | 62.20a | 37.35a | 0.103 | 18.81a | 49.77a | 0.001 | |
| p valueγ | 0.059 | 0.142 | 0.006 | 0.426 | 0.039 | 0.026 | ||||
| P. urvillei | PU1 | 44.61a | 40.75a | 0.828 | 80.15a | 59.76a | 0.12 | 42.68a | 69.96a | 0.018 |
| PU2 | 30.76a | 52.99a | 0.071 | 86.87a | 74.41a | 0.269 | 41.88a | 80.64a | <0.001 | |
| PU3 | 38.47a | 32.75a | 0.671 | 92.01a | 64.01b | 0.007 | 35.61a | 78.01a | <0.001 | |
| PU4 | 33.34a | 52.22a | 0.224 | 76.71a | 69.56a | 0.428 | 42.78a | 73.32a | 0.004 | |
| PU5 | 31.80a | 54.44a | 0.057 | 74.00a | 66.05a | 0.391 | 43.12a | 70.03a | 0.002 | |
| p valueγ | 0.906 | 0.200 | 0.091 | 0.767 | 0.893 | 0.483 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





